Introduction
Prostate cancer (PCa) is the second most commonly
diagnosed malignancy all over the world, and one of the most common
leading cause of cancer-specific mortality in men, which causes
913,000 new cases and >261,000 deaths worldwide each year
(1,2). As the development of prostate-specific
antigen (PSA) serum level screening and the prostate biopsy through
transrectal ultrasound-guided method, the detection rate and
incidence of PCa is markedly increased (3). Currently, therapeutic schedules for
this disease in a localized stage or when it is still
castration-sensitive would have good results in most of the
patients. However, such handlings are only palliative in the
advanced stage (4). Therefore, new
biomarkers and therapeutic targets should be found to get effective
treatments for more aggressive stages in prostate cancer.
miRNAs are a kind of small, 20–22 nucleotides
non-coding RNAs that regulate the expression of hundreds of target
genes through binding to the 3′-untranslated region (3′-UTR) of
their target mRNAs (5). miRNAs play
critical roles in many kinds of physiological and pathological
processes, such as apoptosis, cell proliferation, differentiation,
development, stress response and migration (6,7). A
growing number of evidence has revealed that miRNAs act as either
tumor suppressors or promoters in the process of many kinds of
tumors and play important roles in regulating the
posttranscriptional gene expression (8–10).
miR-212 which is located on chromosome 17p13.3 and share an
identical 5′-seed sequence (11).
It has been demonstrated that miR-212 is markedly overexpressed in
tongue squamous cell carcinoma (12) and colorectal carcinoma (13), but low-expressed in gastric cancer
(14) and non-small cell lung
cancers (15). Moreover, studies
have shown that dysregulated miR-212 plays a critical role in the
proliferation and invasion of many solid malignancies, which makes
it a potential target for tumor treatment (16). However, the exact function of
miR-212 in PCa has not yet been studied.
Engrailed-2 (EN-2), which is a
homeodomain-containing transcription factor, was recently verified
to be involved in the development of prostate cancer (17) and is also confirmed to express
abnormally in prostate cancer cell lines (18). Recent studies had shown that EN-2 is
a potential biomarker for prostate cancer (19,20).
While the regulatory mechanism and the role in progress of prostate
cancer still almost known.
In this study, we aimed to detect the expression of
miR-212 in PCa tissues and cell lines as well as to illustrate the
role of miR-212 in PCa cell line proliferation, colony formation
and migration. Importantly, we also illustrate EN-2 as a potential
target gene of miR-212.
Materials and methods
Collection and cell lines
Prostate cancer samples and the adjacent normal
tissues were collected from 58 patients who underwent
tumor-resection surgery in Peking Union Medical College Hospital
from September 1, 2014 to January 1, 2016. Patients signed an
informed consent form. The tissues were separately frozen in liquid
nitrogen for further study. Protocols were approved by the
institutional ethics committee of Peking Union Medical College
Hospital and carried out in accordance with the guidelines for
ethical management. PCa cell lines, PC3 and DU145, and prostate
epithelial cells were purchased from American Type Culture
Collection (ATCC, USA). PC3 was incubated in F-12 medium, and DU145
was grown in DMEM medium (Life Technologies, Inc., USA), and all
the mediums were supplemented with penicillin/streptomycin (100
U/ml and 100 mg/ml, respectively) and 10% fetal bovine serum (Life
Technologies Inc.). The human prostate epithelial cells (hPrEC)
were cultured in prostate epithelium basal media. All the cells
were incubated at 37°C in a humidified atmosphere of 5%
CO2.
Quantitative real-time PCR
Total RNA was extracted from the tissues or cells
via TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions and quantified with UV absorbancy at
260 and 280 nm (A260/280). Subsequently the RNA was
reverse-transcribed into cDNA by using reverse transcription system
(Thermo Scientific, CA, USA). The expression level of miR-212 was
detected by the Applied Biosystems 7500 Real-time PCR system (ABI)
using the TaqMan MicroRNA assay kits (Applied Biosystems, CA, USA).
U6 was used as the control normalizer. The gene expression of EN-2
was also analyzed by SYBR Green and normalized with GAPDH. The
specificity of primer sequences was analyzed by dissociation curve,
2−ΔΔCt (cycle threshold) was used to calculate the
relative gene expression levels.
Western blotting
Protein from tissues or cells was collected by using
RIPA buffer which contain a protease inhibitor cocktail and
phosphatase inhibitors (Sigma, St. Louis, MO, USA), according to
the manufacturer's instructions and quantified via bicinchoninic
acid (BCA) kit (Thermo Scientific). Protein samples (30 µg) were
separated by SDS-PAGE and then transferred onto the polyvinylidene
difluoride (PVDF, Millipore, Bedford, MA, USA) membranes. Western
blotting was performed using anti-β-actin, anti-EN-2, anti-Bcl-2
and anti-Bax (CST, Denver, CO, USA). The protein levels were
detected with an ECL kit (Thermo Scientific) according to the
manufacturer's instructions.
CCK-8 assay measurements of the
proliferation of PCa cells
CCK-8 assay was performed to evaluate cell
proliferation according to the manufacturer's instructions. For
experiments the cells were seeded at a density of 5×103
cells per well in 96-well plates and cultured for various periods
of time (0, 24, 48 and 72 h). The absorbance at 450 nm was measured
using an electroluminescence immunosorbent assay reader (Thermo
Fisher Scientific, Waltham, MA, USA).
Apoptosis of PCa cells were detected
by flow cytometry
Cells were collected and washed twice with cold
phosphate-buffered saline solution (PBS) to remove floating cells
then labeled with Annexin V-FITC (BD Biosciences, San Jose, CA,
USA). Apoptosis were evaluated with a flow cytometry analyzer. Data
were analyzed by Flowjo 7.6 software.
TUNEL detection of apoptosis of PCa
cells
Cells were fixed with 4% paraformaldehyde,
permeabilized in 0.2% Triton X-100, and incubated with the TUNEL
detection kit from Roche (Roche, Shanghai, China) at 37°C for 1 h.
The samples were mounted in mounting media containing DAPI.
Fluorescent images were captured using a fluorescence microscope.
The total number of DAPI positive cells and total number of TUNEL
positive cells were counted.
Cell invasion assays
Cell invasion assays were performed by using 24-well
Transwell plates (BD Biocoat, USA). Cells transfected with the
miR-212 mimics and mimic control for 24 h. Then the cells were
plated onto the 24-well upper chamber with a membrane that was
pre-treated with Matrigel (100 µg per well; BD Biosciences). In the
lower portion of the chamber, fresh medium containing 10% FBS was
added. After the cells were incubated for 24 h at 37°C, the cells
in the upper chamber were removed. Invaded cells were fixed with 4%
formaldehyde, stained with 0.5% crystal violet, and counted under a
microscope.
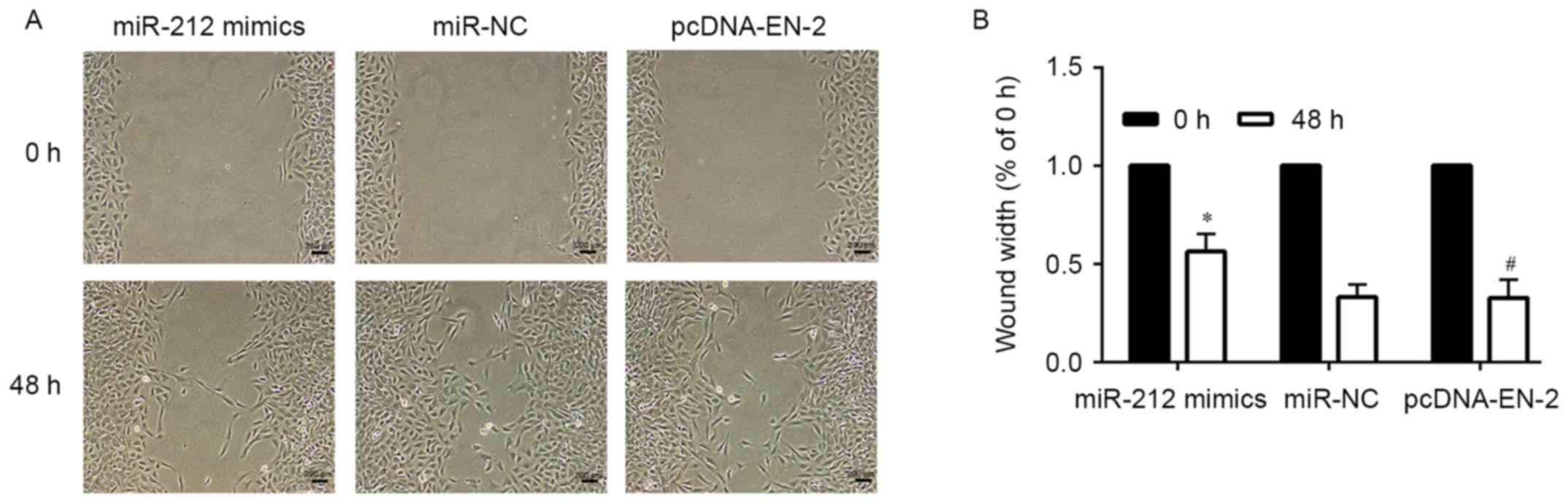
Wound healing assay
Cells were seeded into 6-well plates. Wounds were
created in the confluent cell monolayer using a 200-µl sterile
pipette tip, and the other free-floating cells and debris were
removed by washing with PBS three times. Medium was then added, and
the culture plates were incubated at 37°C for another 24 or 48 h.
Wound healing within the scraped wound line was measured at a 48-h
time-point by using microscope (Nikon, Tokyo, Japan).
Dual luciferase reporter assay
TargetScan software (http://www.targetscan.org/) was used to identify the
potential target gene of miR-212. The putative miR-212 binding
sites or mutant at the 3′-UTR of EN-2 were cloned into a vector and
EN-2 3′-UTR WT or EN-2 3′-UTR Mut, was generated, respectively.
EN-2 3′-UTR WT or EN-2 3′-UTR Mut was transfected into PCa cells
which stably overexpressed miR-212 and transfected with negative
control. Dual-Luciferase Reporter assay system (Promega, Madison,
WI, USA) was used to detect the luciferase activity 36 h after
transfection.
Lentivirus production and
transfection
The LV-miR-212 mimics and mimics control were
purchased from Gene-Chem (Shanghai, China). Cells were seed into
24-well plates at a density of 30% and cultured overnight before
transfection. Cells were transfected with miR-212 mimics or mimics
control by using Lipofectamine 2000 reagent (Invitrogen) according
to the manufacturer's protocols.
Animals and xenograft
In this study all the animal procedures and
experiments were performed in accordance with the National
Institutes of Health Guide for Care and Use of Laboratory Animals.
The 6–8-week-old nude male mice [BALB/c A-nu (nu/nu)] were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China) and housed in the Animal Resource Facility at Laboratory
Animal Centre. Cells stably expressing either miR-212
overexpression (LV-miR-212) or miRNA control were injected
subcutaneously into the mice. The volume of the tumor was recorded
for 7 weeks (once each week).
Statistical analysis
All quantitative data presented are the mean ± SD
from at least three independent experiments. The relationship
between the expression level of miR-212 and clinicopathological
parameters was examined using Fisher's exact test or χ2
test. One-way ANOVA test or Student's t-test was used to analyze
the other date. The significant statistical differences level was
set at P<0.05. SPSS 18.0 and Graph Pad Prism 6.0 software were
used for date analysis.
Results
The expression level of miR-212 and
EN-2 in human PCa tissues and PCa cell lines
In order to confirm the molecular mechanisms
underlying PCa, we analyzed the expression level of miR-212 and
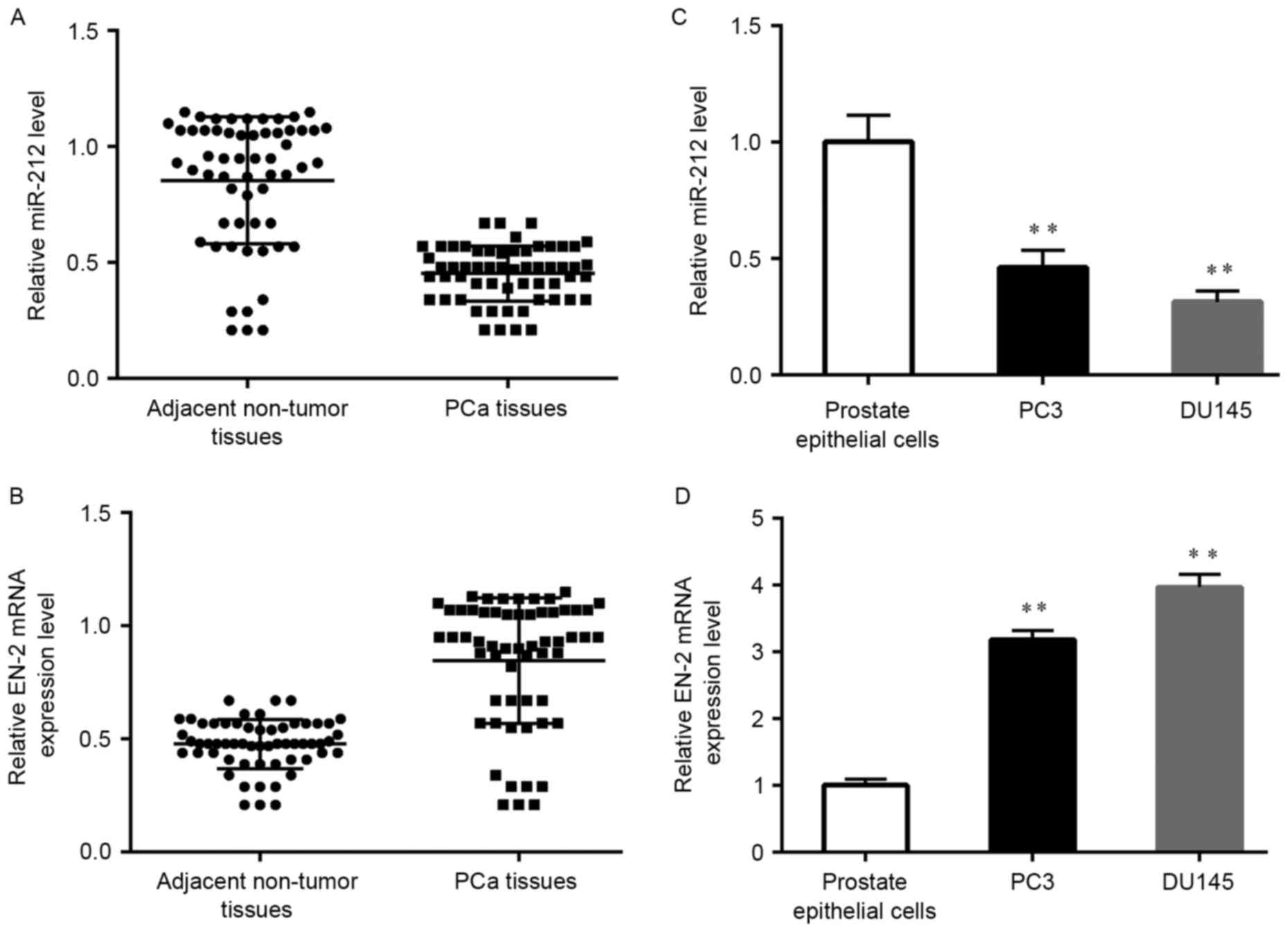
EN-2 in a series of clinical PCa tissues. Results showed that
miR-212 was markedly decreased in cancer samples when compared with
the adjacent normal tissues (Fig.
1A). In contrary, the level of EN-2 mRNA was over-expressed
(Fig. 1B). Subsequently, we
measured the miR-212 and EN-2 level in PCa cell lines and the human
prostate epithelial cells (hPrEC) by RT-qPCR. As shown in Fig. 1C, the expression level of miR-212 in
PCa cells was significantly lower than that in hPrEC cells. While
the expression level of EN-2 had the opposite trend (Fig. 1D). The relationship between miR-212
expression level and clinicopathological features in our PCa cases
is presented in Table I. The
decreased miR-212 was significantly associated with high Gleason
scores (P<0.05). As shown in Table
I, the decreased levels of miR-212 was present in 3 of 11
(27.3%) cases with low Gleason score (<7), 8 of 17 (47.1%) cases
with Gleason score 7, but 21 of 30 (70.0%) cases with high Gleason
score (>7). The expression level of miR-212 was obviously
decreased in PCa patients with distant metastasis (P<0.05).
There were no statistically significant difference between miR-212
level and the age of the patients, PSA levels or tumor stage.
 | Table I.The relationship between miR-212
expression level and clinicopathological features in our PCa
cases. |
Table I.
The relationship between miR-212
expression level and clinicopathological features in our PCa
cases.
| Parameters | N (case) | Low (%) | High (%) | P-value |
|---|
| Age |
|
|
| 0.168 |
|
<65 | 13 | 7
(53.8) | 6
(46.2) |
|
|
≥65 | 45 | 21 (46.7) | 24 (53.3) |
|
| Gleason scores |
|
|
| 0.005 |
|
<7 | 11 | 3
(27.3) | 8
(72.7) |
|
| 7 | 17 | 8
(47.1) | 9
(52.9) |
|
|
>7 | 30 | 21 (70.0) | 9
(30.0) |
|
| Tumor stage |
|
|
| 0.067 |
|
≤T2b | 43 | 21 (48.8) | 22 (51.2) |
|
|
≥T3b | 15 | 4
(26.7) | 11 (73.3) |
|
| Preoperative PSA
level (ng/ml) |
|
|
| 0.076 |
|
≤10 | 11 | 8
(72.7) | 3
(27.3) |
|
|
>10 | 47 | 20 (42.6) | 27 (57.4) |
|
| Distant
metastasis |
|
|
| 0.002 |
| M0 | 45 | 28 (62.2) | 17 (37.8) |
|
| M1 | 13 | 2
(15.4) | 11 (84.6) |
|
Upregulation of miR-212 inhibits PCa
cell survival, proliferation, invasion and migration
Next, in order to verify the role of miR-212 in
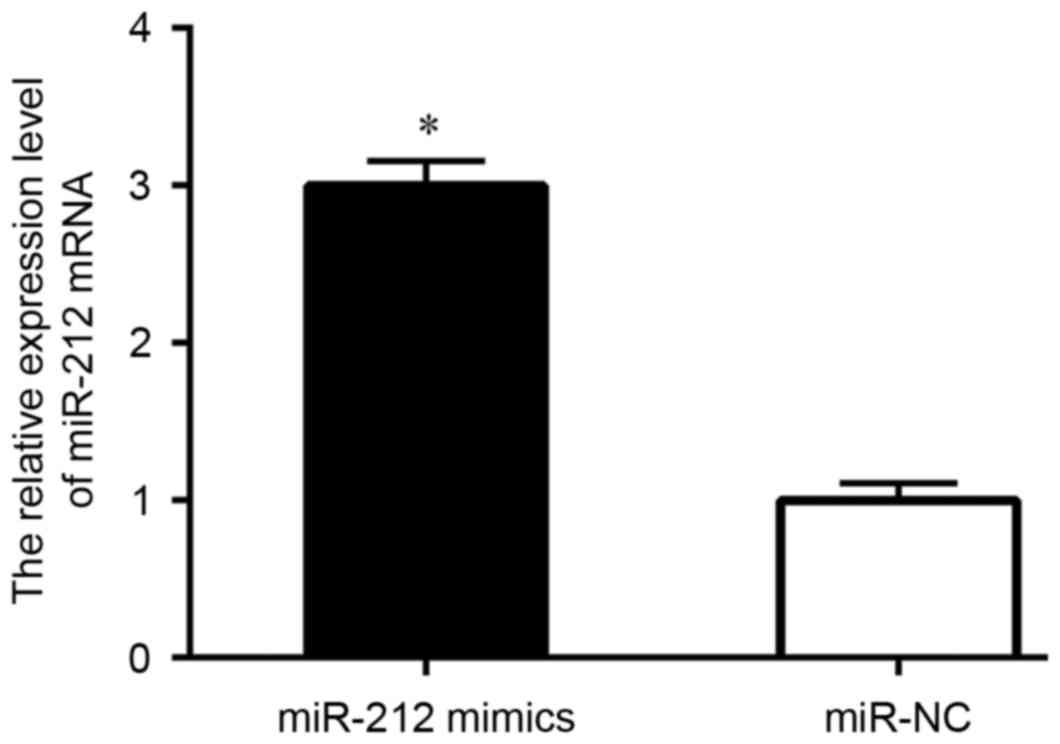
control PCa cell survival and progress, miR-212 mimics and miR-NC
were transfected into DU145 cells, then cells were collected for
analysis. The level of miR-212 was detected by qRT-PCR. Results
showed that the expression level of miR-212 in the mimic group was
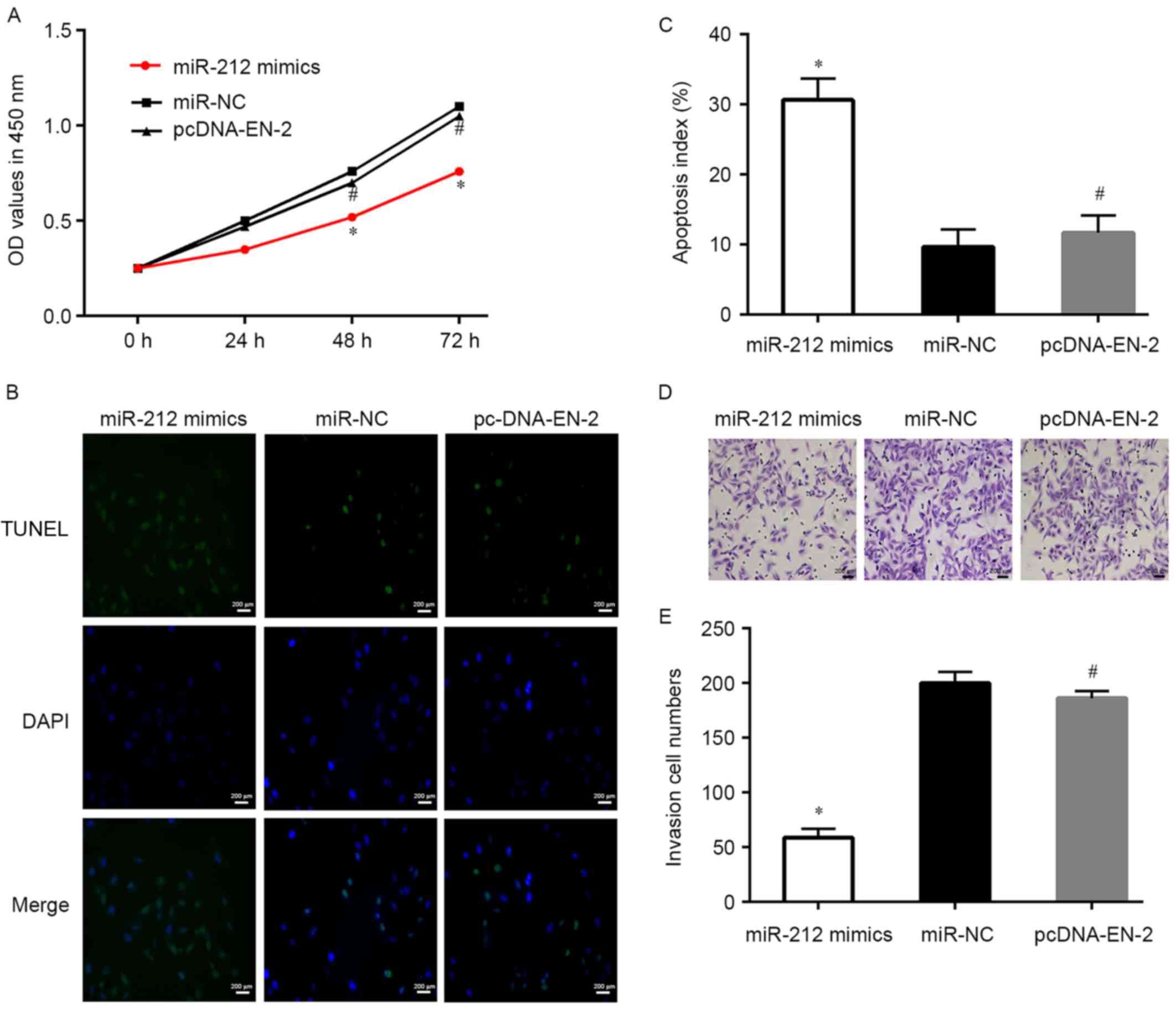
markedly increased when compared with the control (Fig. 2). CCK-8 assay demonstrated that
miR-212 upregulation significantly suppressed DU145 cells
proliferation (Fig. 3A). Moreover,
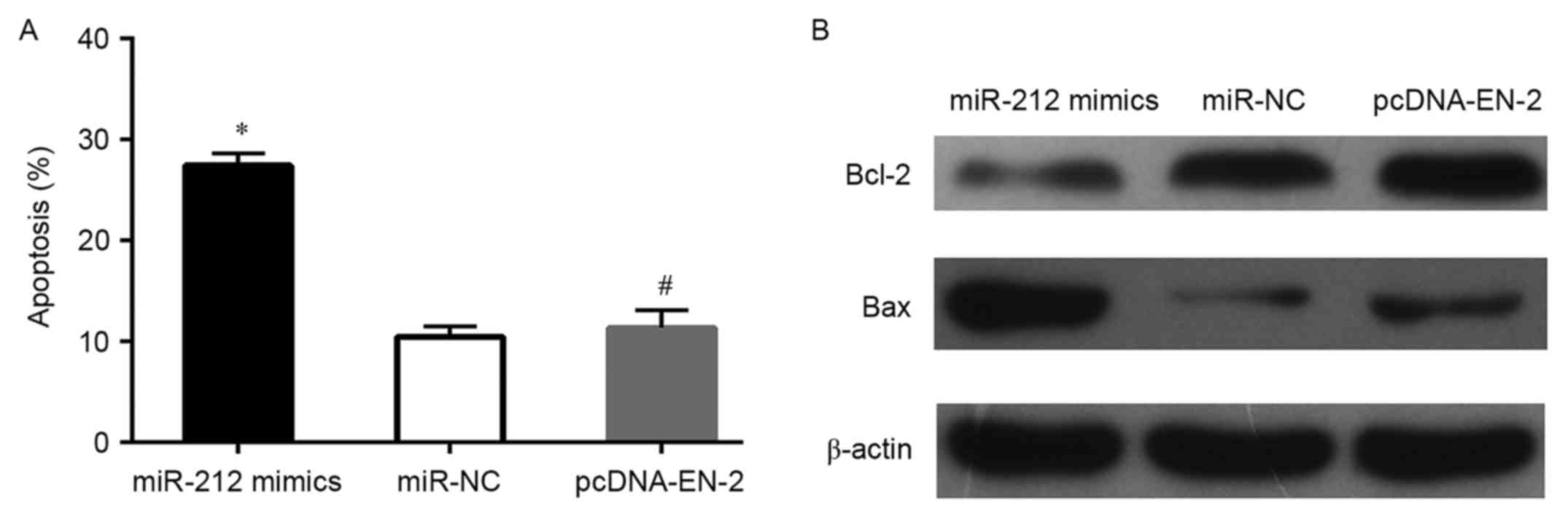
the TUNEL and flow cytometry assays demonstrated that the apoptosis
of DU145 cells was increased when transfected with miR-212 mimics
(Figs. 3B and C and 4A), whereas, the protein expression levels
of Bcl-2 decreased and Bax markedly increased in DU145 cells after
transfected with miR-212 mimics (Fig.
4B). Subsequently, we measured the effect of miR-212 on cell
invasion, the Transwell assays indicated that miR-212 mimics
transfected DU145 cells markedly suppressed cell invasion ability
when compared to the mimic control cells (Fig. 3D and E). A similar tendency was
discovered in the wound healing assays (Fig. 5). These outcomes clearly suggested
that restoration of the expression of miR-212 inhibits PCa cell
proliferation, invasion and migration.
miR-212 decreases EN-2 expression by
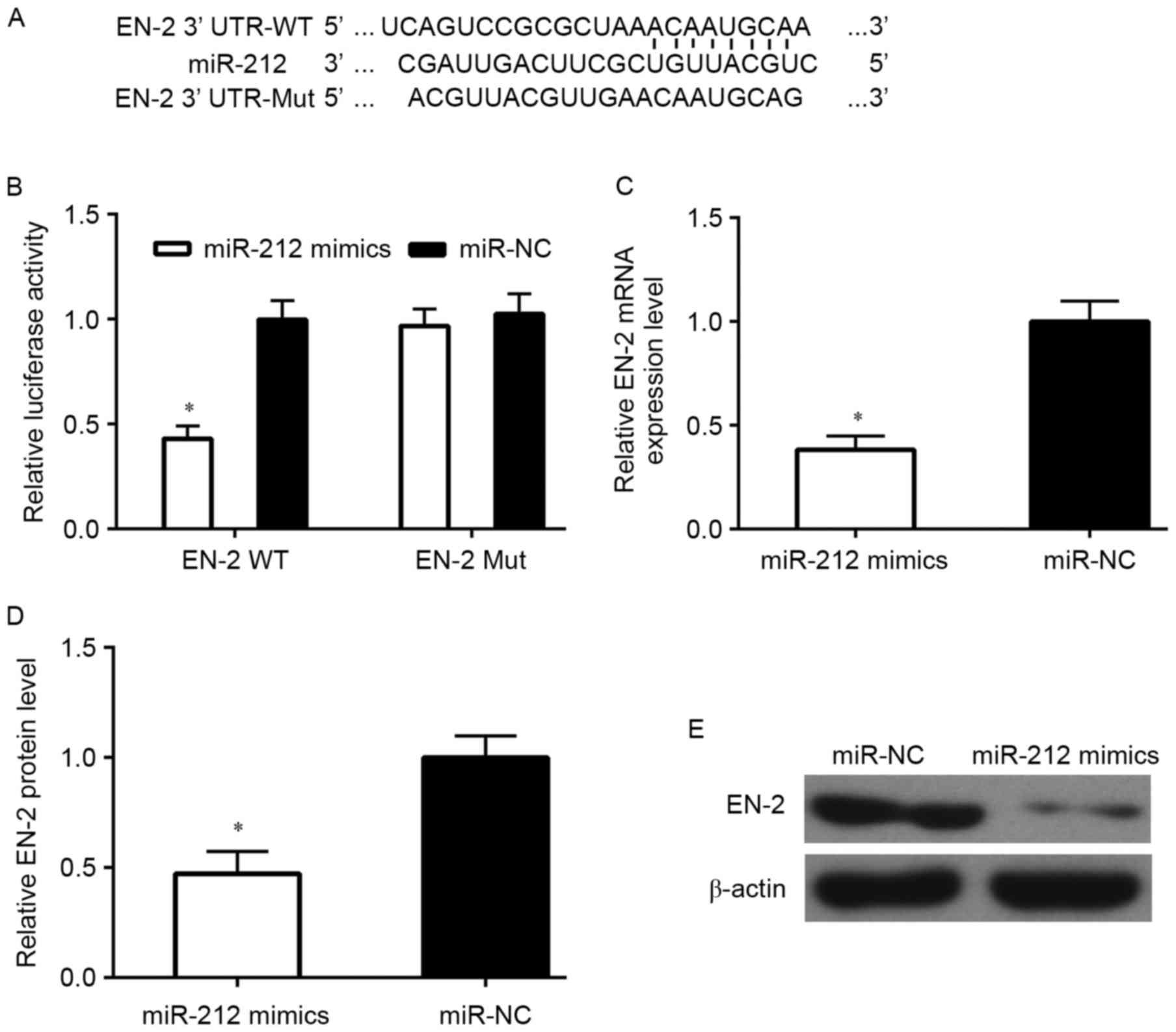
binding to 3′-UTR of its mRNA
In order to illustrate that EN-2 is a direct target
of miR-212, EN-2 wild-type (WT) or mutant 3′-UTR (Mut) (Fig. 6A) were subcloned into a luciferase
reporter vector and co-transfected with miR-212 mimics or negative
control (NC) into PCa cells. Then, luciferase activity was detected
after 48-h transfection. The results demonstrated that miR-212
markedly inhibited luciferase activity of the EN-2 WT 3′-UTR but
had no influence on the mutant (Fig.
6B). To verify whether miR-212 can regulate the expression of
EN-2, miR-212 mimics was transfected into DU145 cells for analysis.
Results showed that the mRNA and protein level of EN-2 was markedly
suppressed by miR-212 upregulation (Figs. 6C-E and 8).
EN-2 re-introduction reverses the
anti-cell survival and metastasis role of miR-212
In order to study the effect of EN-2 in PCa survival
and invasion, miR-212 mimics and pcDNA-EN-2 were co-transfected
into PCa cell lines, and after transfected for 48 h, cell survival
and the ability of invasion were measured. Results showed that
restoration of EN-2 reversed the anti-cell survival actions of
miR-212 and the cell proliferation ability also recovered in
comparison to miR-212 mimics (Figs.
3A-C and 4A). Moreover, the
capability of invasion of PCa cells was significantly restored via
upregulation of EN-2 when compared with miR-212 mimics (Fig. 3D and E), the migration ability of
PCa cells was markedly increased by EN-2 restoration when compared
with miR-212 mimics (Fig. 5).
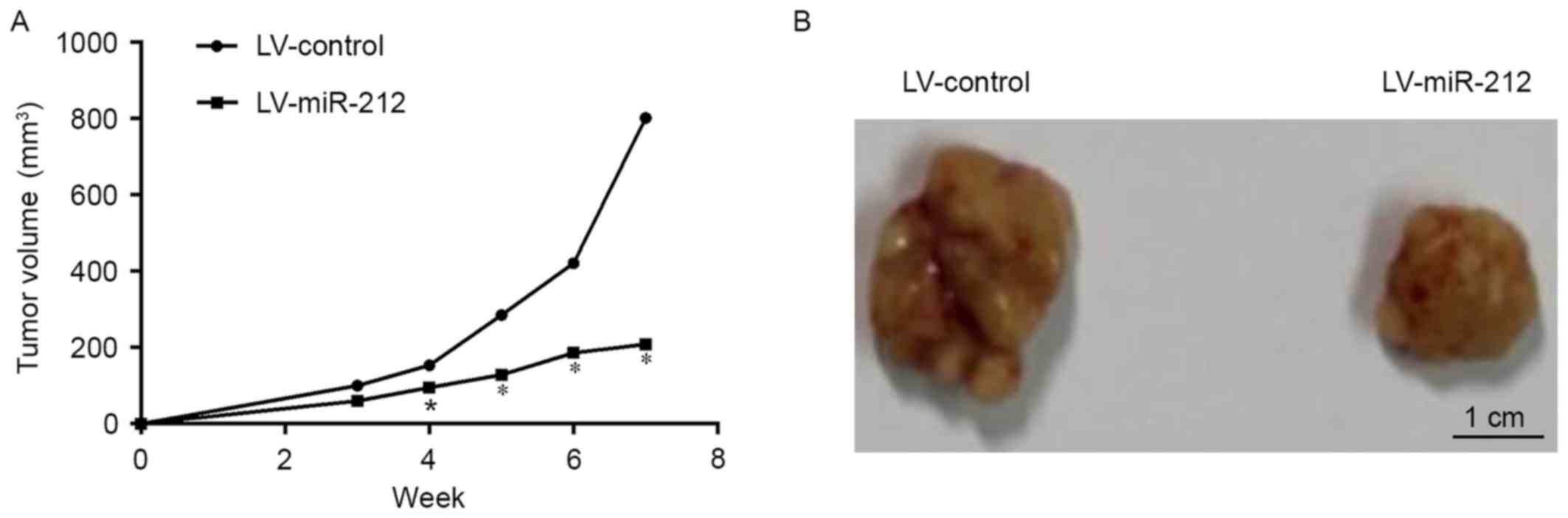
Upregulation of miR-212 suppresses PCa
growth in vivo
In order to confirm the role of miR-212 against
tumor growth in vivo, an animal model was made by nude mice
subcutaneously injected with LV-miR-212. The tumor volume of
subcutaneous xenograft tumors in nude mice apparently decreased
with miR-212 upregulation when compared with the LV-control and the
significant difference emerged from 4-week post-tumor
transplantation (Fig. 7).
Discussion
PCa is the most frequently diagnosed malignance in
men, and it is the second cause of tumor-related death in developed
countries (1,21). Therefore, the early diagnosis is of
great importance to allow successful treatment. Currently, the main
methods for PCa diagnosis are PSA testing, digital rectal
examination (DRE), PCA3, multiparametric magnetic resonance image
(mpMRI) and Gleason score (22).
However, the therapeutic approaches are relatively limited. miRNAs
are a series of small non-coding RNAs that are widely involved in
both physiological and pathological condition (23–25).
For example, miR-135a and miR-21 are separately taken part in the
cancer pathological process and insulin resistance (26,27).
At the same time, there are studies also reporting that miR-125b
and miR-449a are respectively, participating in the physiological
condition of apoptosis and autophagy (28,29).
It has been widely spread that dysregulation of miRNAs may cause
cells dysfunction and further give rise to oncogenesis (30). Therefore, research on miRNAs may
illustrate the understanding of PCa development and be a great help
for recognizing potential biomarkers.
miR-212 is one of the most important miRNAs that has
been studied in many kinds of cancers, including hepatocellular
carcinoma and pancreatic cancer (31,32).
In our study, we firstly investigated the expression level of
miR-212 in PCa patients and found that it was markedly decreased in
PCa patient tissues than in the adjacent normal tissues. In PCa
cell lines, the level of miR-212 also decreased compared to the
hPrEC. Importantly, the low level of miR-212 has a significant
correlation with high Gleason score and distant metastasis. These
results suggested that miR-212 play an important role in the
pathogenesis of PCa.
Previous research has revealed that miR-212
negatively regulates starvation-induced autophagy in PCa cells by
inhibiting SIRT1, upregulation of miR-212 also leads to suppression
of angiogenesis and cellular senescence (33). In this study, overexpression of
miR-212 significantly suppressed cell proliferation, invasion and
migration, and markedly promoted apoptosis of PCa cells. Bcl-2 and
Bax are apoptosis-related genes. In this study, we found that
upregulation of miR-212, regulates Bcl-2 family members, the level
of Bax increased and the Bcl-2 decreased, while the recovery of
EN-2 promoted the Bcl-2 resistance to apoptosis and inhibited Bax
promoting apoptosis. Which was consistent with the above previous
study. In order to further research the function of miR-212 in PCa
cells, we detected the role of miR-212 in the growth of
subcutaneous xenograft tumors in nude mice. In accordance with the
in vitro study, upregulation of the expression of miR-212
suppressed the growth of xenograft tumors in vivo.
Collectively, these results sustained the concept that miR-212 may
act as a tumor suppressor in PCa.
Many target genes for miR-212 have been found in
various kinds of malignancies such as Lin28B in PCa (34). There are also some other targets for
miR-212, including RB, SMAD2, FOXA1 and SGK3 have been reported in
some other cancers (11,32,35,36).
Although the role of miR-212 has been studied in many tumors, its
mechanistic role in PCa is still unclear. Increasing evidence has
recognized that EN-2 acts as a novel well-known biomarker for
diagnosing PCa (37,38), while its effect on PCa oncogenesis
has not been defined. There are also studies demonstrating that the
expression of EN-2 dysregulated in many human cancers, and
inhibiting EN-2 expression can suppress the development of bladder
cancer and chronic myelogenous leukemia (39,40),
which suggested that EN-2 play an important role in the progression
of cancer. In order to study the involvement of EN-2 and the role
of interaction between miR-212 and EN-2 in oncogenesis of PCa, we
studied PCa cell activity in DU145 cells co-transfected with
miR-212 mimics and pcDNA-EN-2. Results demonstrated that EN-2
restoration effectively reversed repressive effect of miR-212 on
cell survival and progression. In this study, we also confirmed
that EN-2 was as a direct target gene of miR-212 in PCa cells.
As demonstrated above, the level of miR-212 was
downregulated in PCa patients and the cell lines. Upregulation of
miR-212 markedly suppressed PCa cell proliferation and promoted
apoptosis and it may be via targeting EN-2. Our findings introduced
a better understanding of miR-212 in PCa progression, which is
likely to provide more precise targets for diagnostic and
therapeutics against PCa, and we will carry out further study to
confirm this suggestion.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
prostate cancer
|
|
EN-2
|
Engrailed-2
|
|
PSA
|
prostate specific antigen
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cuzick J, Thorat MA, Andriole G, Brawley
OW, Brown PH, Culig Z, Eeles RA, Ford LG, Hamdy FC, Holmberg L, et
al: Prevention and early detection of prostate cancer. Lancet
Oncol. 15:e484–e492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen FZ and Zhao XK: Prostate cancer:
Current treatment and prevention strategies. Iran Red Crescent Med
J. 15:279–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinfeld I, Navon R, Ach R and Yakhini Z:
miRNA target enrichment analysis reveals directly active miRNAs in
health and disease. Nucleic Acids Res. 41:e452013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Medina PP and Slack FJ: microRNAs and
cancer: An overview. Cell Cycle. 7:2485–2492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalmay T: Mechanism of miRNA-mediated
repression of mRNA translation. Essays Biochem. 54:29–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JK, Henry JC, Jiang J, Esau C, Gusev
Y, Lerner MR, Postier RG, Brackett DJ and Schmittgen TD: miR-132
and miR-212 are increased in pancreatic cancer and target the
retinoblastoma tumor suppressor. Biochem Biophys Res Commun.
406:518–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scapoli L, Palmieri A, Lo Muzio L,
Pezzetti F, Rubini C, Girardi A, Farinella F, Mazzotta M and
Carinci F: MicroRNA expression profiling of oral carcinoma
identifies new markers of tumor progression. Int J Immunopathol
Pharmacol. 23:1229–1234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wada R, Akiyama Y, Hashimoto Y, Fukamachi
H and Yuasa Y: miR-212 is downregulated and suppresses
methyl-CpG-binding protein MeCP2 in human gastric cancer. Int J
Cancer. 127:1106–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Incoronato M, Garofalo M, Urso L, Romano
G, Quintavalle C, Zanca C, Iaboni M, Nuovo G, Croce CM and
Condorelli G: miR-212 increases tumor necrosis factor-related
apoptosis-inducing ligand sensitivity in non-small cell lung cancer
by targeting the antiapoptotic protein PED. Cancer Res.
70:3638–3646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehta A, Mann M, Zhao JL, Marinov GK,
Majumdar D, Garcia-Flores Y, Du X, Erikci E, Chowdhury K and
Baltimore D: The microRNA-212/132 cluster regulates B cell
development by targeting Sox4. J Exp Med. 212:1679–1692. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgan R, Boxall A, Bhatt A, Bailey M,
Hindley R, Langley S, Whitaker HC, Neal DE, Ismail M, Whitaker H,
et al: Engrailed-2 (EN2): A tumor specific urinary biomarker for
the early diagnosis of prostate cancer. Clin Cancer Res.
17:1090–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bose SK, Bullard RS and Donald CD:
Oncogenic role of engrailed-2 (en-2) in prostate cancer cell growth
and survival. Transl Oncogenomics. 3:37–43. 2008.PubMed/NCBI
|
|
19
|
Marszałł MP, Sroka W, Adamowski M, Słupski
P, Jarzemski P, Siódmiak J and Odrowąż-Sypniewska G: Engrailed-2
protein as a potential urinary prostate cancer biomarker: A
comparison study before and after digital rectal examination. Eur J
Cancer Prev. 24:51–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGrath SE, Michael A, Morgan R and Pandha
H: EN2: A novel prostate cancer biomarker. Biomarkers Med.
7:893–901. 2013. View Article : Google Scholar
|
|
21
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs as biomarkers for diagnosis, prognosis and theranostics
in prostate cancer. Int J Mol Sci. 17:4212016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ha TY: MicroRNAs in human diseases: From
cancer to cardiovascular disease. Immune Netw. 11:135–154. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
26
|
Zhang C, Chen X, Chen X, Wang X, Ji A,
Jiang L, Sang F and Li F: miR-135a acts as a tumor suppressor in
gastric cancer in part by targeting KIFC1. Onco Targets Ther.
9:3555–3563. 2016.PubMed/NCBI
|
|
27
|
Zhao XY and Shao K: Roles of MicroRNA-21
in the pathogenesis of insulin resistance and diabetic
mellitus-induced non-alcoholic fatty liver disease. Zhongguo Yi Xue
Ke Xue Yuan Xue Bao. 38:144–149. 2016.(In Chinese). PubMed/NCBI
|
|
28
|
Yu G, Zhan X, Zhang Z and Li Y:
Overexpression of miR-125b promotes apoptosis of macrophages. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 32:958–962. 2016.(In Chinese).
PubMed/NCBI
|
|
29
|
Han R, Ji X, Rong R, Li Y, Yao W, Yuan J,
Wu Q, Yang J, Yan W, Han L, et al: miR-449a regulates autophagy to
inhibit silica-induced pulmonary fibrosis through targeting Bcl2. J
Mol Med (Berl). 94:1267–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bouyssou JM, Manier S, Huynh D, Issa S,
Roccaro AM and Ghobrial IM: Regulation of microRNAs in cancer
metastasis. Biochim Biophys Acta. 1845:255–265. 2014.PubMed/NCBI
|
|
31
|
Ma C, Nong K, Wu B, Dong B, Bai Y, Zhu H,
Wang W, Huang X, Yuan Z and Ai K: miR-212 promotes pancreatic
cancer cell growth and invasion by targeting the hedgehog signaling
pathway receptor patched-1. J Exp Clin Cancer Res. 33:542014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng
C, Zhang L, Feng Y, Zhou H, Zhou B, et al: MicroRNA-212 inhibits
hepatocellular carcinoma cell proliferation and induces apoptosis
by targeting FOXA1. Onco Targets Ther. 8:2227–2235. 2015.PubMed/NCBI
|
|
33
|
Ramalinga M, Roy A, Srivastava A,
Bhattarai A, Harish V, Suy S, Collins S and Kumar D: MicroRNA-212
negatively regulates starvation induced autophagy in prostate
cancer cells by inhibiting SIRT1 and is a modulator of angiogenesis
and cellular senescence. Oncotarget. 6:34446–34457. 2015.PubMed/NCBI
|
|
34
|
Borrego-Diaz E, Powers BC, Azizov V,
Lovell S, Reyes R, Chapman B, Tawfik O, McGregor D, Diaz FJ, Wang
X, et al: A potential regulatory loop between Lin28B:miR-212 in
androgen-independent prostate cancer. Int J Oncol. 45:2421–2429.
2014.PubMed/NCBI
|
|
35
|
Zhao JL, Zhang L, Guo X, Wang JH, Zhou W,
Liu M, Li X and Tang H: miR-212/132 downregulates SMAD2 expression
to suppress the G1/S phase transition of the cell cycle and the
epithelial to mesenchymal transition in cervical cancer cells.
IUBMB Life. 67:380–394. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Li C, Shen C, Yin F, Wang K, Liu Y,
Zheng B, Zhang W, Hou X, Chen X, et al: miR-212-3p inhibits
glioblastoma cell proliferation by targeting SGK3. J Neurooncol.
122:431–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Killick E, Morgan R, Launchbury F,
Bancroft E, Page E, Castro E, Kote-Jarai Z, Aprikian A, Blanco I,
Clowes V, et al: Role of Engrailed-2 (EN2) as a prostate cancer
detection biomarker in genetically high risk men. Sci Rep.
3:20592013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee S, Jo H, Her J, Lee HY and Ban C:
Ultrasensitive electrochemical detection of engrailed-2 based on
homeodomain-specific DNA probe recognition for the diagnosis of
prostate cancer. Biosens Bioelectron. 66:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Liu H, Lai C, Su Z, Heng B and Gao
S: Repression of engrailed 2 inhibits the proliferation and
invasion of human bladder cancer in vitro and in
vivo. Oncol Rep. 33:2319–2330. 2015.PubMed/NCBI
|
|
40
|
Abollo-Jiménez F, Campos-Sánchez E,
Toboso-Navasa A, Vicente-Dueñas C, González-Herrero I,
Alonso-Escudero E, González M, Segura V, Blanco O, Martínez-Climent
JA, et al: Lineage-specific function of Engrailed-2 in the
progression of chronic myelogenous leukemia to T-cell blast crisis.
Cell Cycle. 13:1717–1726. 2014. View
Article : Google Scholar : PubMed/NCBI
|