Introduction
Mesenchymal stem cells (MSCs) are adult stem cells
with self-renewal and multi-directional differentiation abilities.
MSCs can be recruited to inflammatory and tumor sites by
microenvironmental signals, where they undergo phenotypic and
functional changes (1). For
example, LPS-treated MSCs show increased expression of NO and IL-6
and decreased expression of TNF-α (2). MSCs pre-stimulated with IFN-γ and
TNF-α express a higher level of VEGF via the activation of the
HIF-1α signaling pathway, accelerating colon cancer growth and
tumor angiogenesis in vivo (3). Although the plasticity of MSCs has
been identified, the exact roles of MSCs in cancer and the
mechanisms that mediate their response to microenvironmental
signals have not been well studied.
Gastric cancer is the third most frequent cause of
cancer-related death in China and the mortality rate for gastric
carcinoma remains >50% worldwide (4,5).
Normal gastric mucosa is subjected to multiple stages of malignant
transformation, including different types of gastritis, varying
degrees of atypical hyperplasia, finally evolving into gastric
cancer. Cells at the early stage of carcinogenesis including the
periods of simple and atypical hyperplasia, are reversible.
Therefore, studies on the transformation from gastritis to cancer
may have important implications for the control of gastric
cancer.
Among all the established animal models of gastric
cancer (6–8), the rat model induced with chemical
carcinogens such as
N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) is
widely used due to the simple operation, cheap cost, easy keep and
lower natural cancer rate (9). It
has been shown that MNNG treatment results in the mutation of DNA
and increased oxidative injury, which is critical to gastric
carcinogenesis (10,11). Furthermore, the various stages
before gastric cancer in Wistar rats are similar to the tissue
morphology of humans (12).
We previously reported the isolation of MSCs from
human gastric cancer tissues and demonstrated that gastric
cancer-derived MSCs promote gastric cancer growth and metastasis.
However, the mechanism responsible for the transition of MSCs in
gastric cancer remains unknown. We hypothesized that the
inflammatory microenvironment may induce changes in MSCs, promoting
the transformation from gastritis to gastric cancer. To this end,
we established a gastric cancer model in rats by the administration
of MNNG in drinking water and a high-salt diet. MSCs were isolated
from the gastric tissues of normal (RGN-MSCs) and MNNG-induced
(RGI-MSCs) rats. We found that MSCs from the MNNG-induced rat
gastric tissues had stronger abilities to proliferate and migrate.
In addition, MSCs from the MNNG-induced rat gastric tissues
produced higher levels of pro-inflammatory factors and showed more
profound effects in promoting gastric mucosa epithelial cell
migration. We further demonstrated that the increased expression of
miR-374 may be associated with the phenotypic and functional
changes in RGI-MSCs.
Materials and methods
Animal model
Four-week-old male Wistar rats (Laboratory Animal
Center of Shanghai, Academy of Science, Shanghai, China) were kept
at 23±3°C and 55±5% humidity. The control group was administered a
normal diet and regular drinking water. The treatment group was fed
with water containing 100 mg/l of MNNG [Tokyo Chemical Industry
(TCI) Tokyo, Japan] for 40 weeks. A high-salt diet containing 8%
sodium chloride was administered starting from the 5th week for 15
weeks. The body weight of each rat was weighed regularly every 4
weeks.
Histopathological staining
Gastric tissues were collected at different time
points (0, 12, 24, 36 and 48 weeks), processed in 4.0%
paraformaldehyde, embedded with paraffin and sectioned into 4-µm
sections. The sections were subjected to hematoxylin and eosin
(H&E) staining and Alcian blue-periodic acid Schiff (AB-PAS)
staining as previously described (13).
MSC isolation and cell culture
The fresh stomach tissues were cut into
1-mm3 sized pieces and cultured in low-glucose Dulbeccos
modified Eagles medium (LG-DMEM) supplemented with 10% fetal bovine
serum (FBS) (both from Life Technologies, Grand Island, NY, USA)
and 1% penicillin/streptomycin at 37°C in humid air with 5%
CO2. The medium was replaced every 3 days until adherent
fibroblast-like cells appeared. Then, the cells were trypsinized
and passaged into a new flask for further expansion. The cells in
passage 3 were used for all the experiments. MSCs from normal
gastric tissues were termed as RGN-MSCs and those from gastric
tissues at 24 weeks after exposure to MNNG were termed as RGI-MSCs.
The gastric mucosa epithelial cell line GES-1 (Institute of
Biochemistry and Cell Biology at the Chinese Academy of Sciences,
Shanghai, China) was cultured in RPMI-1640 medium (Life
Technologies) with 10% FBS. Cells were incubated at 37°C in a
humidified cell culture incubator with 5% CO2.
Osteogenic and adipogenic
differentiation in vitro
RGN-MSCs and RGI-MSCs were seeded into 6-well plates
at 1×105 cells/well and cultured in 10% FBS-containing
DMEM with either osteogenic (0.1 µM dexamethasone, 10 µM
β-glycerophosphate, 50 µM ascorbate-phosphate; Sigma, St. Louis,
MO, USA) or adipogenic supplements (Cyagen, Guangzhou, China). The
medium was changed every 3 days and the cells were induced for 2
weeks. At the end of induction, the cells were subjected to
neutrophil alkaline phosphatase (NAP) staining or Oil Red O
staining.
Cell transfection
miRNA mimics and inhibitors were synthesized by
GenePharma (Shanghai, China). RGN-MSCs and RGI-MSCs
(2×105 cells/well) were plated in 6-well plate and
transfected with miRNA mimics (5 nM), inhibitors (200 nM) or
negative controls using Lipofectamine 2000 (Life Technologies).
Flow cytometry
RGN-MSCs and RGI-MSCs (1×106 cells) were
trypsinized, washed twice in phosphate-buffered saline (PBS), and
stained for 30 min on ice in fluorescein isothiocyanate
(FITC)-conjugated or phycoerythrin (PE)-conjugated antibodies:
CD29, CD44H, CD45 and CD90 (Becton-Dickinson, San Jose, CA, USA).
Mouse PE-IgG and FITC-IgG were used as the isotype control.
Genetics and DNA contents
RGN-MSCs and RGI-MSCs were incubated with colchicine
for 4 h during the logarithmic growth phase. The cells were
collected, treated with KCl (0.075 M) for 30 min, and fixed in
methanol/acetic acid (1:1 v/v). Cell smears were subjected to
Giemsa staining for chromosome analysis. For DNA content analysis,
the cells were harvested, washed with PBS twice, and stained with
10 µg/ml propidium iodide (Sigma) in 500 µl PBS (containing 100
µg/ml RNase) for 30 min in the dark at room temperature. The
distribution of cells at different phases of the cell cycle was
analyzed using a flow cytometer (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA).
Cell growth curve and cell colony
formation
RGN-MSCs and RGI-MSCs were seeded into 24-well
plates (5×103 cells/well). The cell numbers were counted
for each group at indicated time points. RGN-MSCs and RGI-MSCs were
resuspended in 6-well plates (1×105 cells/well) and
incubated for 14 days. Colonies were fixed with methanol, stained
with crystal violet and counted. For miRNA transfection, the cells
were collected at 24 h after transfection and seeded in the 24-well
plates (2×105 cells/well). All the experiments were
carried out in triplicate.
Western blotting
Cells were lysed in RIPA buffer supplemented with
proteinase inhibitors. A total of 200 µg proteins was loaded and
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The proteins were transferred to a
polyvinylidene difluoride (PVDF) membrane, blocked in 5% (w/v)
non-fat milk and incubated with the primary antibodies at 4°C
overnight. The sources of primary antibodies were as follows:
anti-Oct4, anti-Sox2, anti-Lin28B, anti-Kif4, anti-vimentin (Cell
Signaling Technology, Danvers, MA, USA), anti-E-cadherin and
anti-N-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-GAPDH (Kangcheng Biotechnology, Shanghai, China). After
incubation with the secondary antibodies (Kangcheng Biotechnology)
at 37°C for 1 h, the signals were visualized using a
chemiluminescent imaging system (Beijing Sage Creation Science,
Beijing, China).
Transwell migration assay
For RGN-MSCs and RGI-MSCs, cells (5×104
cells/well) were plated into the top chamber of Transwell plates
and medium containing 10% FBS was placed into the bottom chamber of
Transwell plates (8-µm pore size; Corning Inc., Corning, NY, USA).
For GES-1, cells (5×104 cells/well) were plated into the
top chamber of Transwell plates and the supernatant of RGN-MSCs or
RGI-MSCs was placed in the bottom chamber. After incubation at 37°C
in 5% CO2 for 10 h, the cells remaining on the upper
surface of the membrane were removed with a cotton swab. Cells on
the lower surface of the membrane were fixed and stained with
crystal violet. The migratory ability of the cells was determined
by counting the cells in at least 6 fields for each assay.
Luminex assay
The serum of rats in the control and the treatment
group were collected at different time points (0, 12, 24, 36 and 48
weeks). The supernatant from the RGN-MSCs and RGI-MSCs was also
collected. The Rat Cytokine and Chemokine Magnetic Bead Panel kit
(cat. #RECYTMAG-65K; Merck Millipore, Darmstadt, Germany) was
designed to detect granulocyte macrophage colony-stimulating factor
(GM-CSF), granulocyte colony stimulating factor (G-CSF), IL-10,
IL-6, IL-4, IL-1β, monocyte chemoattractant protein-1 (MCP-1),
tumor necrosis factor-α (TNF-α) and vascular endothelial growth
factor (VEGF). All procedures were processed according to the
manufacturer's instructions. The signal was detected and analyzed
using the Luminex 200 System (Merck Millipore).
RNA isolation and reverse
transcription-quantitative PCR
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Serum miRNAs were
extracted from 400 µl of serum using the miRNeasy Mini kit and
reversely transcribed using the miScript II RT kit (both from
Qiagen, Hilden, Germany). The levels of miRNAs were detected using
the miScript SYBR-Green PCR kit (Qiagen) in a Bio-Rad fluorescence
thermal cycler (Bio-Rad, Hercules, CA, USA). The relative
expression levels of the miRNAs were normalized to that of U6.
Statistical analyses
All data are expressed as mean ± SD. SPSS software
was used to analyze all the data (SPSS, Inc., Chicago, IL, USA).
The means of different treatment groups were compared by two-way
ANOVA, LSD-t test. A P-value <0.05 was considered as
statistically significant.
Results
MNNG exposure increases the expression
of inflammatory factors in rats
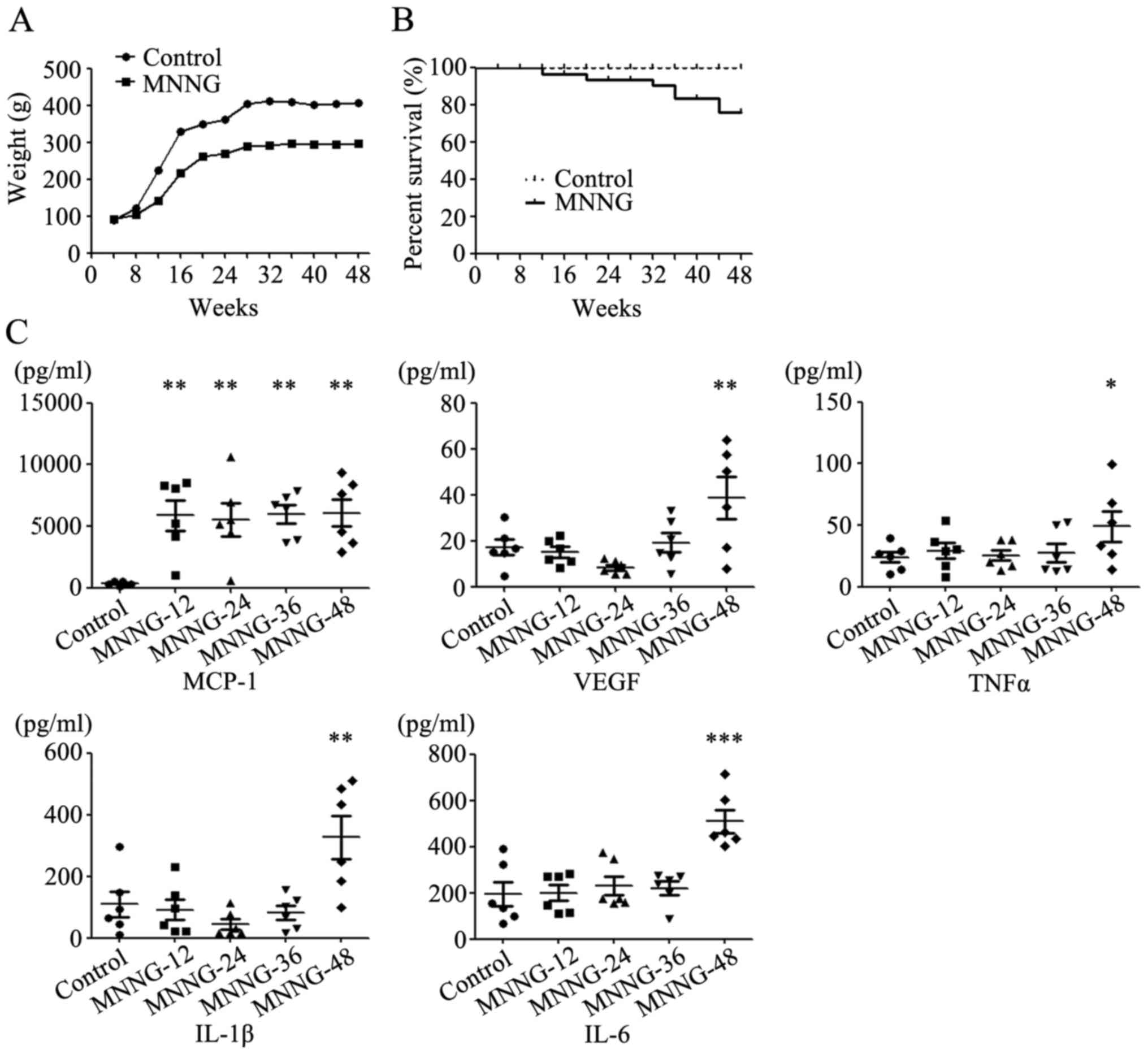
The weight of rats in the control group increased
rapidly from the 4th week, and became stabilized in the 24th week.
The rats in the MNNG treatment group were lighter than the normal
rats at all time periods. The average weight of the normal adult
male Wistar rats was ~400 g, while the average weight of rats in
the MNNG treatment group was only ~300 g at the 48th week (Fig. 1A). Some rats in the MNNG treatment
group could not tolerate the exposure to MNNG and started to die at
the 24th week after MNNG treatment. The survival rate in the MNNG
treatment group was ~75% at the end of the experiment (Fig. 1B).
We then detected the expression of inflammatory
factors including GM-CSF, IL-1β, IL-4, IL-6, IL-10, MCP-1, TNF-α
and VEGF in the serum of rats using Luminex assay. The expression
of GM-CSF, IL-4 and IL-10 was undetectable in the serum of rats.
The expression level of MCP-1 in the serum of the MNNG-treated rats
increased notably at the 12th week after MNNG exposure. However,
there was no further change in the expression level of MCP-1 over
time. The expression levels of VEGF, TNF-α, IL-1β and IL-6 in the
serum of the MNNG-treated rats were higher than those in control
group at the 48th week after MNNG exposure (Fig. 1C).
Establishment of the MNNG-induced
gastric cancer model in rats
As shown in Fig. 2A,
the structure of the gastric mucosa of the normal rats was
complete. The boundaries of the glandular and glandular epithelium
were clear. The dense glands had uniform size and the shape of the
glandular lumen was regular. At 12 weeks after MNNG exposure, the
mucosal layer of the gastric tissues became thinner and displayed
focal or diffuse lesions, which manifested as superficial gastritis
(Fig. 2A). The gastric mucosa of
the rats treated with MNNG for 24 weeks became even thinner with
visible inflammatory cell infiltration. The size of the glands
became smaller and the number of the glands was reduced. The normal
gastric pit became shallower, which could be diagnosed as atrophic
gastritis (Fig. 2A). The inner
gastric mucosa of rats treated with MNNG for 36 weeks had fibroid
tissue. There was inflammatory granuloma or ulcer-like pathology,
namely the surface was covered by inflammatory exudates (white
blood cells, cellulose), under which were necrotic tissue layer,
granulation tissue layers and layers of old scar tissue. The glands
were shrinking with vacuole goblet cells. Therefore, the
pathological changes at this stage were a mixture of atrophic
gastritis (Fig. 2A), ulcers
(Fig. 2B), and intestinal metaphase
(Fig. 2C). After exposure to MNNG
for 48 weeks, the glandular structure disappeared and showed
disorders with irregular shape. There were a large number of
inflammatory cells with disorganized size surrounding the basement
membrane, which was judged as atypical hyperplasia (Fig. 2A). The cardiac part of the
cauliflower uplift was identified to have benign esophageal-like
sarcoma lesions (Fig. 2D). Normal
gastric epithelial cells secrete neutral mucous and are positive
for periodic acid-Schiff (PAS) reaction (shown in red). Small
intestinal epithelial cells secrete acid mucus and are positive for
alcian blue reaction (shown in blue). The mucous mixed with neutral
and acidic mucous are shown in purple. To determine the subtype of
gastric intestinal metaplasia, the stomach tissue sections were
used for Alcian blue-Schiff (AB-PAS) staining. The mucous glands
were mixed with blue and purple nuclei, indicating that the subtype
of intestinal metaplasia was small intestinal type (Fig. 2E). The incidence of atrophic
gastritis, atypical hyperplasia, appaloosa and adenocarcinoma was
100, 85.4, 14.6 and 0%, respectively at 48 weeks in the MNNG
treatment group.
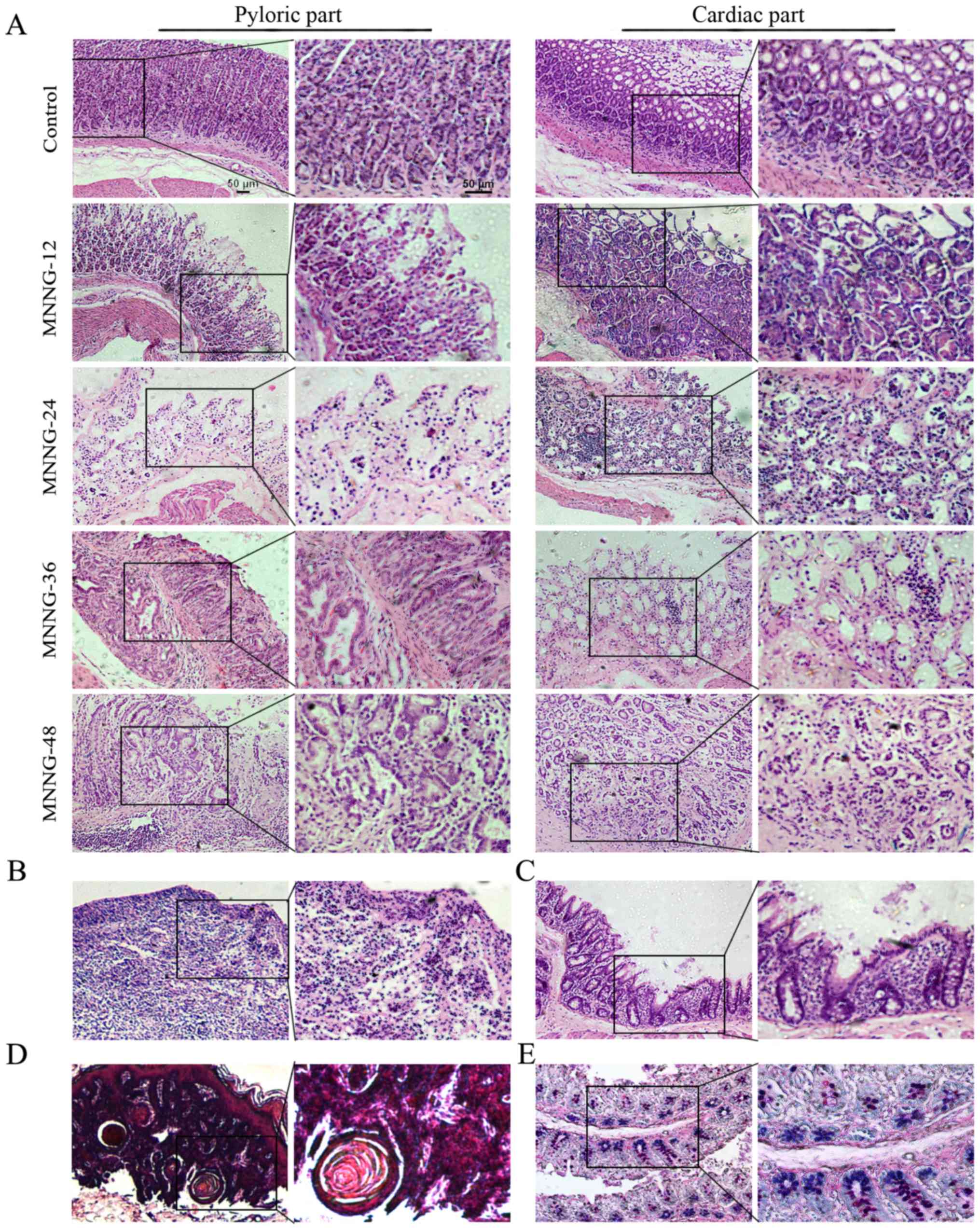 | Figure 2.Histology of the control and
MNNG-induced rat gastric tissues. (A) Superficial gastritis was
noted in the MNNG-12 group, atrophic gastritis in the MNNG-24 group
and the cardiac part of the MNNG-36 group, and atypical hyperplasia
in the pyloric part of the MNNG-36 and MNNG-48 groups. (B) H&E
staining of ulcers in the MNNG-36 group. (C) H&E staining of
gastric tissues in the MNNG-36 group. Intestinal metaphase changes
were observed. Goblet cells were noted in the gastric mucosa. (D)
H&E staining for gastric tissuse in the MNNG-48 group.
Esophageal-like sarcoma lesions were observed. (E) Small intestinal
type metaplasia was detected using AB-PAS staining. MNNG-12, 24, 36
and 48 represent the rats treated with MNNG for 12, 24, 36 and 48
weeks, respectively. Original magnification of the left panel,
×100; the right panel, ×200; scale bar, 50 µm. |
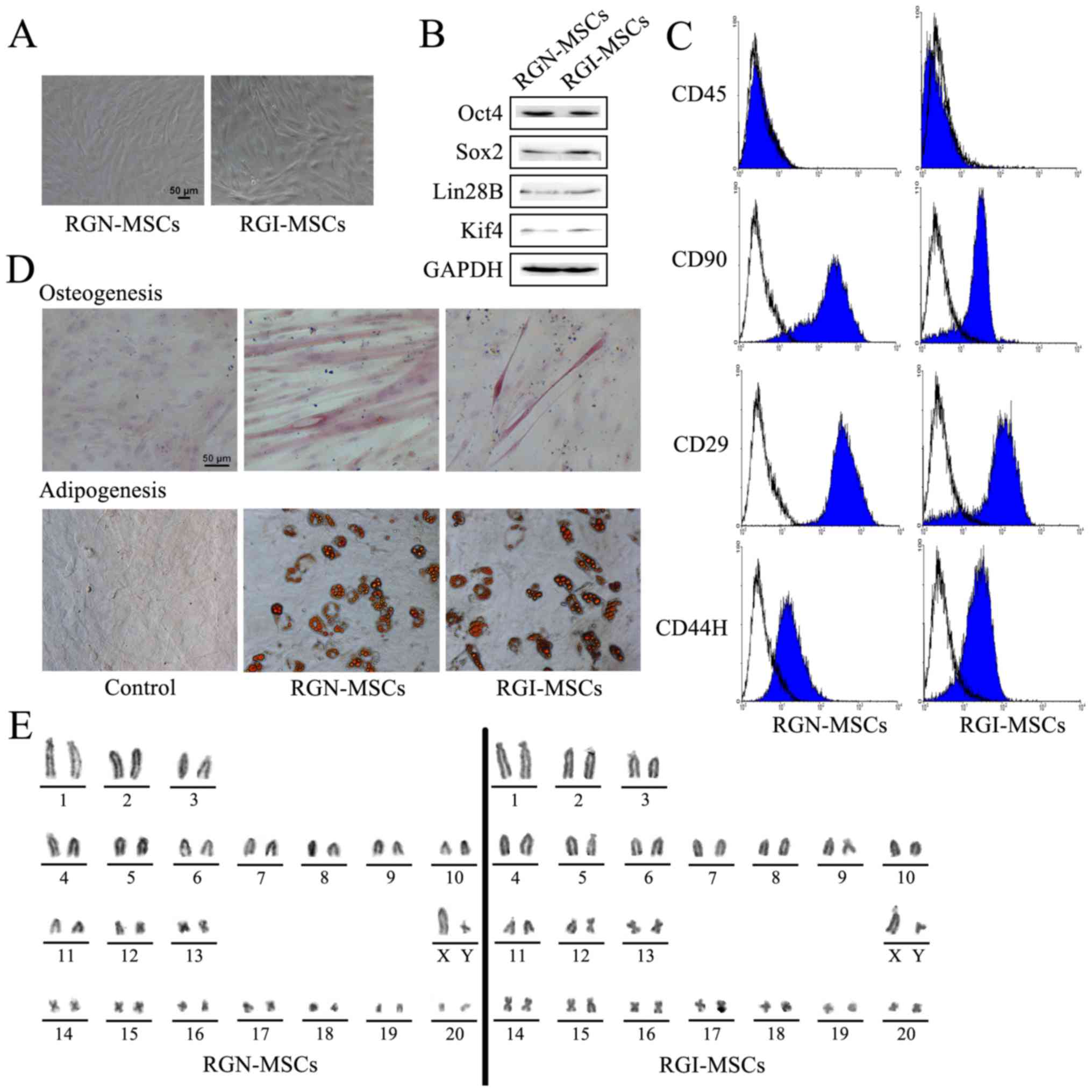
Isolation and characterization of MSCs
from the gastric tissues of normal and MNNG-treated rats
After primary culture for 14 days, RGN-MSCs and
RGI-MSCs adhered to the plastic surface of the culture plates.
These cells began to form colonies and appeared like long
spindle-shaped fibroblastic cells in the initial plating for ~20 to
30 days (Fig. 3A). The results of
western blotting showed that both RGN-MSCs and RGI-MSCs expressed
stem cell markers including Oct4, Sox2, Lin28B, and Klf4 (Fig. 3B). RGN-MSCs and RGI-MSCs were
positive for CD25, CD44H and CD90, but negative for CD45 (Fig. 3C). RGN-MSCs and RGI-MSCs could be
induced to differentiate into osteocytes and adipocytes at 2 weeks
after induction (Fig. 3D). The
karyotypes of the RGN-MSCs and RGI-MSCs were normal, with 20 pairs
of autosomes and one pair of sex chromosomes. No deletions or
translocations in chromosomes were detected (Fig. 3E).
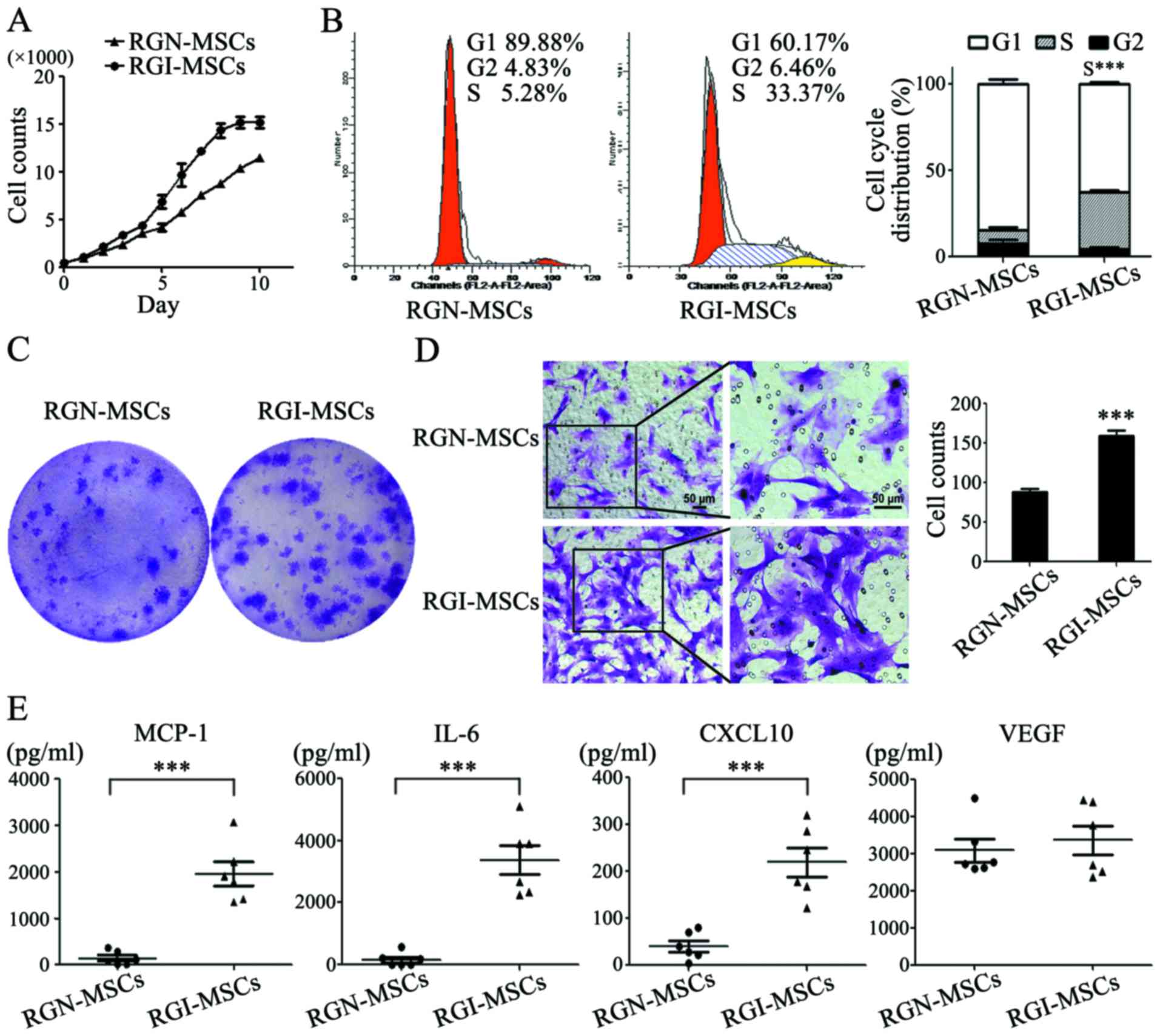
RGI-MSCs show increased proliferative
and migratory abilities and produce higher levels of
pro-inflammatory factors than RGN-MSCs
The results of the cell growth curves showed that
the RGI-MSCs proliferated more quickly than that noted for the
RGN-MSCs. The number of RGN-MSCs was ~3/4 that of the RGI-MSCs at
10 days of culture (Fig. 4A). The
results of cell cycle analysis showed that the percentage of
RGI-MSCs at the S phage (32.8±1.39%) was higher than that of the
RGN-MSCs (7.75±2.23%) (Fig. 4B).
The number of cell colonies formed by RGI-MSCs was significantly
higher than that of the RGN-MSCs. The average diameter of the cell
colonies formed by the RGI-MSCs was larger than that of the
RGN-MSCs after culture for 14 days (Fig. 4C). In the cell Transwell migration
assay, the number of migrated RGI-MSCs was 2-fold higher than that
of the RGN-MSCs (Fig. 4D). To
analyze the cytokine profiles of RGN-MSCs and RGI-MSCs, we
performed Luminex assay to determine the levels of several
pro-inflammation factors, including G-SCF, IL-10, IL-6, IL-1β,
MCP-1, TNF-α and VEGF, in the supernatant of the RGN-MSCs and
RGI-MSCs. We observed that the levels of IL-6, CXCL-10 and MCP-1
were markedly upregulated in the supernatant of the RGI-MSCs. The
level of VEGF had no significant difference between the RGN-MSCs
and RGI-MSCs (Fig. 4E). The
expression of other factors (G-CSF, IL-10, IL-1β and TNF-α) were
undetectable in both the RGN-MSCs and RGI-MSCs.
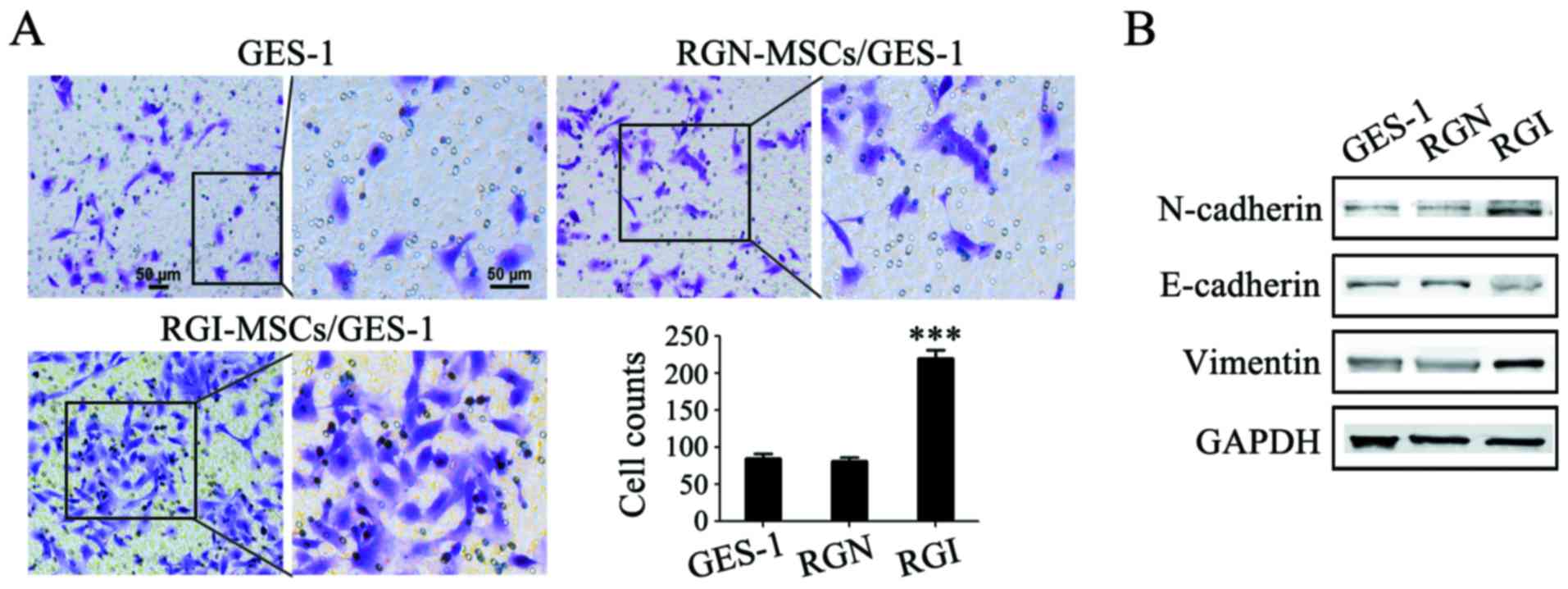
RGI-MSCs promote the migration of
gastric mucosa epithelial cells more profoundly than RGN-MSCs
The gastric mucosal epithelial cells were treated
with the supernatant of RGN-MSCs and RGI-MSCs and the migratory
abilities of the treated cells were determined using Transwell
migration assay. The number of GES-1 cells migrating through the
Transwell membrane was higher than that in the control group
(~2.5-fold increase). Whereas, there was only a minimal increase in
the number of migrated GES-1 cells in the RGN-MSC group compared
that in the control group (Fig.
5A). The results of western blotting showed that the expression
of E-cadherin expression decreased while that of N-cadherin and
vimentin increased after treatment with the supernatant of the
RGI-MSCs for 48 h (Fig. 5B). No
significant changes in the expression of E-cadherin, N-cadherin and
vimentin were observed in GES-1 cells treated with the supernatant
of the RGN-MSCs.
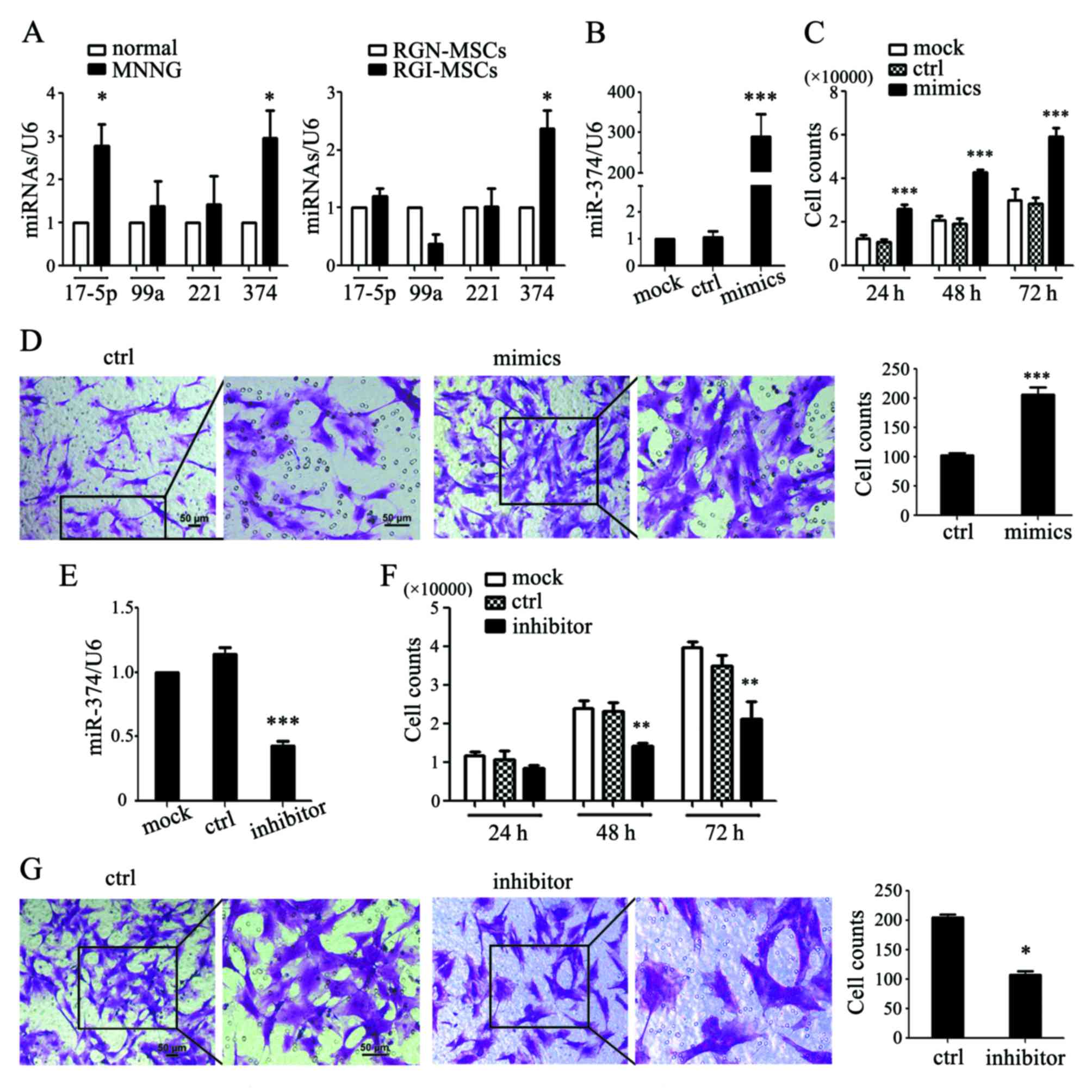
miR-374 is upregulated in RGI-MSCs and
is responsible for the increased proliferative and migratory
abilities of RGI-MSCs
In our preliminary study, we used an Exiqon miRCURY
LNA Array to evaluate the expression profile of miRNAs between MSCs
from human gastric cancer and non-cancerous gastric tissues. We
identified 177 upregulated and 160 downregulated miRNAs, in which
the expression of miR-17-5p, miR-99a, miR-221 and miR-374 was
differentially significant (15).
We performed qRT-PCR to determine the expression of several
microRNAs in the serum of normal and MNNG-induced rats. As shown in
Fig. 6A, the expression levels of
miR-17-5p and miR-374 were significantly upregulated in the serum
of MNNG-induced rats compared to that in normal rats. The
expression levels of miR-99a and miR-221 had no significant
differences between MNNG-induced rats and normal rats. We also
detected the expression levels of these miRNAs in the RGN-MSCs and
RGI-MSCs. We found that only the expression level of miR-374 was
notably increased in the RGI-MSCs compared to that in the RGN-MSCs
(Fig. 6A). We overexpressed miR-374
in the RGN-MSCs and found that miR-347-transfected RGN-MSCs
proliferated more quickly than the cells transfected with the
control mimics (Fig. 6B and C).
miR-374 overexpression also significantly promoted the migration of
RGN-MSCs (Fig. 6D). We inhibited
miR-374 expression in the RGI-MSCs using miRNA inhibitors and found
that the inhibition of miR-374 retarded the proliferation of
RGI-MSCs at 48 and 72 h after transfection (Fig. 6E and F). The number of migrated
RGI-MSCs was also decreased by transfection with an miR-374
inhibitor (Fig. 6G).
Discussion
Many factors are involved in the development of
gastric cancer, including high-salt diet, acid secretion,
inflammatory cytokines, virulence and colonization of
Helicobacter pylori, and host genetic background (14). The establishment of authentic animal
models is critical for the prospective study of the pathogenesis of
gastric cancer. It is generally believed that gastric stem cells
are mainly located in the isthmus of the gastric glands, moving
downward or upward to form mature gastric epithelial cells. In our
previous study, we reported the isolation of MSCs from human
gastric cancer tissues, which provide direct evidence for the
presence of MSCs in the gastric cancer microenvironment (15). Whether MSCs are involved in the
formation and regulation of the inflammatory microenvironment in
gastric cancer was the focus of the present study. To observe the
transformation from gastritis to gastric cancer, we established a
gastritis cancer transformation model. Wistar rats were exposed to
carcinogen MNNG and fed a high-salt diet which induced the
occurrence of gastric cancer.
The histological analyses of the rat stomach tissues
at different time points indicated that the gastric mucosa
experienced superficial gastritis, atrophic gastritis, intestinal
metaplasia and dysplasia, which was similar to the pathological
process of human gastric cancer. We detected the expression of
several pro-inflammatory factors in the serum of rats collected at
different time points using Luminex assay. The expression of MCP-1
was maintained at a high level during the whole process of MNNG
induction, which is consistent with our previous study showing that
MCP-1 plays an important role in the inflammatory microenvironment
(16). In addition, the expression
levels of IL-1β and IL-6 markedly increased at the 48th week after
MNNG induction, suggesting that IL-1β and IL-6 function at the late
stage of malignant transformation. These findings indicate that
inflammatory factors are critically involved in the formation of
the tumor microenvironment.
We isolated MSCs from the gastric tissues of normal
and MNNG-induced rats. RGN-MSCs and RGI-MSCs showed similar stem
cell characteristics to that of normal rat bone marrow: mesenchymal
cell-like (MSC) morphology, normal karyotype, capabilities of
differentiating into osteoblasts and adipocytes, expression of stem
cell factors; positive for the mesenchymal cell markers CD29, CD90
and CD44 while negative for the hematopoietic cell surface marker
CD45. We further compared the biological characteristics of
RGN-MSCs and RGI-MSCs to better understand the effects of the
inflammatory microenvironment on MSCs. The proliferation rate of
RGI-MSCs was higher than that of the RGN-MSCs due to an increase in
the cell population in the S phase. The increased proliferative
ability of the RGI-MSCs may reflect an accelerated formation of
tumor stroma. RGI-MSCs also had an increased migratory ability than
the RGN-MSCs. This finding is consistent with that reported by Park
et al who demonstrated that the migratory capacity of MSCs
from inflamed periodontal ligament tissues was higher than that
from healthy control tissues (17).
RGI-MSCs produced higher levels of IL-6, CXCL10 and MCP-1 than
RGN-MSCs. The previous study showed that the increased secretion of
CCL5 by MSCs resulted in the acceleration of metastatic potential
of breast cancer cells (18). Thus,
activated MSCs in an inflamed microenvironment may secrete
bioactive molecules to promote tumor development and
progression.
Epithelial-mesenchymal transition (EMT) is
considered to be one of the key steps in tumor initiation, growth
and metastasis. Previous studies have demonstrated that direct and
indirect interactions with MSCs induce the occurrence of EMT in
tumor cells (19–21). We found that RGI-MSCs could induce
EMT in gastric epithelial cells more profoundly than RGN-MSCs.
Therefore, our findings, along with the findings of previous
studies, suggest that MSCs may promote tumor development and
progression by the induction of EMT.
We further revealed that the expression of miR-374
was significantly higher in the RGI-MSCs, which positively
regulated the proliferation and migration of RGI-MSC. It has been
reported that miR-374 promoted the development of gastric cancer
(22,23). In addition, miR-374 was highly
expressed in the tissues or serum of cancer patients (24–26).
We previously reported the upregulation of miR-221 in gastric
cancer-derived MSCs, which could promote gastric cancer growth and
metastasis (15). Therefore,
miR-374 may also act as an oncogene in gastric cancer, providing a
potential target for the diagnosis and treatment of gastric
cancer.
In conclusion, we established a rat model for the
transformation of gastritis to gastric cancer using MNNG induction
method. MSCs from the inflammatory gastric tissues display
increased abilities to proliferate, migrate and produce
pro-inflammatory factors. MSCs from inflammatory gastric tissues
were found to have more profound effects in promoting the migration
of gastric mucosal epithelial cells. The upregulation of miR-374
may be associated with the phenotypic and functional changes in
MSCs in gastric cancer. These findings not only help to better
understand the role and mechanism of MSCs in gastric cancer, but
also provide new diagnostic and therapeutic targets.
Acknowledgements
The present study was supported by the Major
Research Plan of the National Natural Science Foundation of China
(grant no. 91129718), the National Natural Science Foundation of
China (grant no. 81572075), the Zhenjiang Science and Technology
Program (grant no. SH2016047), the Project of Major Research and
Development, Jiangsu Province (grant no. BE2015667), Jiangsu
Province for Outstanding Sci-tech Innovation Team in Colleges and
Universities (grant no. SJK2013-10), the Doctoral Program
Foundation of China (grant nos. 2016M591791 and 2016M591792), the
Doctoral Program Foundation, Jiangsu Province (grant no. 1501071C),
and the Project Funded by the Priority Academic Program Development
of Jiangsu Higher Education Institutions.
References
|
1
|
Norozi F, Ahmadzadeh A, Shahrabi S,
Vosoughi T and Saki N: Mesenchymal stem cells as a double-edged
sword in suppression or progression of solid tumor cells. Tumour
Biol. 37:11679–11689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahmat Z, Jose S, Ramasamy R and
Vidyadaran S: Reciprocal interactions of mouse bone marrow-derived
mesenchymal stem cells and BV2 microglia after lipopolysaccharide
stimulation. Stem Cell Res Ther. 4:122013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX,
Wang CY, Sun K, Jiang GC, Zhao X, Li R, et al: Effects of
inflammatory factors on mesenchymal stem cells and their role in
the promotion of tumor angiogenesis in colon cancer. J Biol Chem.
286:25007–25015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Zeng H and Zhang S: The
incidence and mortality of major cancers in China, 2012. Chin J
Cancer. 35:732016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu S, Yang M and Nam KT: Mouse models of
gastric carcinogenesis. J Gastric Cancer. 14:67–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayakawa Y, Fox JG, Gonda T, Worthley DL,
Muthupalani S and Wang TC: Mouse models of gastric cancer. Cancers.
5:92–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng MJ, Wang J, Chen YW, Xu L, Xue DD,
Fu W, Zhang YF, Du Q, Zhao Y, Ling LJ, et al: A novel mouse model
of gastric cancer with human gastric microenvironment. Cancer Lett.
325:108–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Liu H, Wang X, Zeng Z, Xie LQ, Sun
ZG and Wei MX: Preventive effect of Actinidia valvata Dunn extract
on N-methyl-N'-nitro-N-nitrosoguanidine-induced gastrointestinal
cancer in rats. Asian Pac J Cancer Prev. 15:6363–6367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gunassekaran GR, Priya DK, Gayathri R and
Sakthisekaran D: In vitro and in vivo studies on antitumor effects
of gossypol on human stomach adenocarcinoma (AGS) cell line and
MNNG induced experimental gastric cancer. Biochem Biophys Res
Commun. 411:661–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bai H, Gu L, Zhou J and Deng D: p16
hypermethylation during gastric carcinogenesis of Wistar rats by
N-methyl-N'-nitro-N-nitrosoguanidine. Mutat
Res. 535:73–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miri H, Bathaie SZ, Mohagheghi MA,
Mokhtari-Dizaji M and Shahbazfar AA: A noninvasive method for early
detection of MNNG-induced gastric cancer of male Wistar rat:
Ultrasonic study. Ultrasound Med Biol. 37:780–787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamabayashi S: Periodic acid-Schiff-alcian
blue: A method for the differential staining of glycoproteins.
Histochem J. 19:565–571. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song B, Du J, Feng Y, Gao YJ and Zhao JS:
Co-expressed differentially expressed genes and long non-coding
RNAs involved in the celecoxib treatment of gastric cancer: An RNA
sequencing analysis. Exp Ther Med. 12:2455–2468. 2016.PubMed/NCBI
|
|
15
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai J, Wang M, Zhu M, Zhang Q, Zhang X,
Yan Y, Qian H and Xu W: N-methyl-N-nitro-N'-nitrosoguanidine
induces the expression of CCR2 in human gastric epithelial cells
promoting CCL2-mediated migration. Mol Med Rep. 13:1083–1090.
2016.PubMed/NCBI
|
|
17
|
Park JC, Kim JM, Jung IH, Kim JC, Choi SH,
Cho KS and Kim CS: Isolation and characterization of human
periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed
PDL tissue: In vitro and in vivo evaluations. J Clin Periodontol.
38:721–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Melzer C, Yang Y and Hass R: Interaction
of MSC with tumor cells. Cell Commun Signal. 14:202016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
So KA, Min KJ, Hong JH and Lee JK:
Interleukin-6 expression by interactions between gynecologic cancer
cells and human mesenchymal stem cells promotes
epithelial-mesenchymal transition. Int J Oncol. 47:1451–1459.
2015.PubMed/NCBI
|
|
20
|
Wu C, Zhuang Y, Jiang S, Liu S, Zhou J, Wu
J, Teng Y, Xia B, Wang R and Zou X: Interaction between
Wnt/β-catenin pathway and microRNAs regulates
epithelial-mesenchymal transition in gastric cancer (Review). Int J
Oncol. 48:2236–2246. 2016.PubMed/NCBI
|
|
21
|
Mele V, Muraro MG, Calabrese D, Pfaff D,
Amatruda N, Amicarella F, Kvinlaug B, Bocelli-Tyndall C, Martin I,
Resink TJ, et al: Mesenchymal stromal cells induce
epithelial-to-mesenchymal transition in human colorectal cancer
cells through the expression of surface-bound TGF-β. Int J Cancer.
134:2583–2594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie J, Tan ZH, Tang X, Mo MS, Liu YP, Gan
RL, Li Y, Zhang L and Li GQ: MiR-374b-5p suppresses RECK expression
and promotes gastric cancer cell invasion and metastasis. World J
Gastroenterol. 20:17439–17447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu X, Wang W, Su N, Zhu X, Yao J, Gao W,
Hu Z and Sun Y: miR-374a promotes cell proliferation, migration and
invasion by targeting SRCIN1 in gastric cancer. FEBS Lett.
589:407–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Merhautova J, Hezova R, Poprach A,
Kovarikova A, Radova L, Svoboda M, Vyzula R, Demlova R and Slaby O:
miR-155 and miR-484 are associated with time to progression in
metastatic renal cell carcinoma treated with sunitinib. Biomed Res
Int. 2015:9419802015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tölle A, Jung M, Rabenhorst S, Kilic E,
Jung K and Weikert S: Identification of microRNAs in blood and
urine as tumour markers for the detection of urinary bladder
cancer. Oncol Rep. 30:1949–1956. 2013.PubMed/NCBI
|
|
26
|
Zhao Q, Li T, Qi J, Liu J and Qin C: The
miR-545/374a cluster encoded in the Ftx lncRNA is
overexpressed in HBV-related hepatocellular carcinoma and promotes
tumorigenesis and tumor progression. PLoS One. 9:e1097822014.
View Article : Google Scholar : PubMed/NCBI
|