Introduction
Gastric cancer (GC) is one of the most common human
cancers and the second leading cause of cancer-related mortality
worldwide (1). The major cause of
death is metastasis, which greatly hinders treatment success
(2). Human gastric cancer tissue
contains cancer initiating cells (CIC) (3). CIC are defined as the unique
subpopulation in the tumors that possess the ability to initiate
tumor growth and sustain self-renewal as well as metastatic
potential (4). Elucidating
molecular mechanism of formation of gastric CIC will not only help
us to further understand the pathogenesis and progression of the
disease, but also will offer new targets for effective
therapies.
EHF/ESE-3 is a new member of the ETS transcription
factors which is exclusively expressed in a subset of epithelial
cells (5). Aberrent expression of
EHF may affect the normal process of epithelial cell
differentiation and contribute to cell transformation (5–7).
Moreover, EHF may regulate epithelial growth and differentiation
and have an important role in oncogenesis of epithelium-derived
tumors (5,8). EHF is overexpressed in ovarian and
mammary cancers and may be a predictive marker for poor survival in
ovarian cancer (9). Recently, it
has been reported that increased expression of EHF via gene
amplification contributes to the activation of HER family signaling
and associates with poor survival in gastric cancer (10).
miRNAs are regulatory, non-coding RNAs ~18–25
nucleotides in length and are expressed at specific stages of
tissue development or cell differentiation, and have large-scale
effects on the expression of a variety of genes at the
post-transcriptional level. Through base-pairing with its targeted
mRNAs, a miRNA induces RNA degradation or translational suppression
of the targeted transcripts (11–15).
Some miRNAs can function either as oncogenes or tumor suppressors
(16–18) and expression profiling analyses have
revealed characteristic miRNA signatures in certain human cancers
(19–21). miR-206 was confirmed to be
downregulated in gastric cancer specimens (22). Restoration of miR-206 can inhibit
gastric cancer progression (22,23).
However, its role in regulating formation of CIC has not been
reported. In this study, we showed that miR-206 inhibits cancer
initiating cells by targeting EHF in gastric cancer.
Materials and methods
Gastric cancer tissues
Gastric cancer tissues and adjacent normal tissues
were obtained from Department of Gastrointestinal Surgery, Shandong
Provincial Qianfoshan Hospital. All tissues were examined
histologically, and pathologists confirmed the diagnosis. Medical
ethics committee of Qianfoshan Hospital approved the experiments
undertaken. The use of human tissue samples followed
internationally recognized guidelines as well as local and national
regulations. Informed consent was obtained from each
individual.
Human gastric cancer cell lines
NCI-N87 and GES-1 cell lines were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). They
were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (Wisent, Canada) and antibiotics (1%
penicillin/streptomycin; Gibco, USA). All cell lines were grown in
a humidified chamber supplemented with 5% CO2 at
37°C.
MTT assay
The proliferation of cells was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay
(Sigma, St. Louis, MO, USA). The MTT analysis was performed as
described previously (24–29). In brief, the cells were plated in
96-well plates in Dulbecco's modified Eagle's medium containing 10%
fetal bovine serum at a density of 8×103 cells per well
at 37°C in a 5% CO2 incubator for 12 h. Cells were
transfected with EHF expressing plasmids or empty vectors and then
were treated with fluorouracil or DMSO (10 µM) for 24 h. Then MTT
(5 mg/ml) was added to the wells (20 µl per well). The plates were
incubated in a cell incubator for 4 h, then the supernatant was
removed and 150 µl of dimethyl sulfoxide was added to each well.
After incubation for 10 min, the absorbance of each well was
measured using a Synergy™ 4 (BioTek Instruments, Winooski, VT, USA)
with a wavelength of 570 nm, with the reference wavelength set at
630 nm. Absorbance was directly proportional to the number of
survival cells.
Colony formation
The assay was performed as described (16). For colony formation assay, cells
were transfected as indicated, and then seeded in a 6-well plate.
FBS (0.2 ml) was added per well on day 5. After 9-10-day
incubation, plates were washed with PBS and cells were fixed with
4% paraformaldehyde and stained with 1% crystal violet. Colonies
with >50 cells were manually counted.
Western blotting
Protein extracts were resolved through
SDS-polyacrylamide gel electrophoresis, transferred to PVDF
membranes (Bio-Rad, Berkeley, CA, USA), probed with antibodies
against EHF, ALDH1, CD44, CD133, c-MET, PAX3, cyclin D2 and CDK4 or
β-actin (Abcam, Cambridge, MA, USA) and then with secondary
antibodies (Abcam).
Sphere formation assay
Cells (103/ml) in serum-free RPMI-1640/1
mM Na-pyruvate were seeded on 0.5% agar precoated 6-well plates.
After 10 days, half the medium was exchanged every third day.
Single spheres were picked out and counted.
Quantitative reverse transcription PCR
detection for miR-206
It was performed as described previously (30).
Methods of bioinformatics
The analysis of potential microRNA target sites was
by the prediction algorithms - miRanda (http://www.microrna.org/).
Immunofluorescence staining
The staining was performed as described (31). Cells were stained for
immunofluorescence on coverslips. After fixation and
permeabilization, the cells were incubated with primary antibodies
against EHF (ab172730; 1:200 dilution; Abcam) and then incubated
with the secondary antibodies. The coverslips were counterstained
with 4′, 6- diamidino-2-phenyl indole and imaged under a confocal
microscope TCS SP5 (Lecia, Solms, Germany).
Statistical analysis
Data are presented as mean ± SEM. Student's t-test
(two-tailed) was used to compare two groups. Spearman correlation
was used to analyze correlation between miR-206 and EHF. P<0.05
was considered significant.
Results
EHF promotes formation of CIC
phenotypes and clonogenic ability in gastric cancer NCI-N87
cells
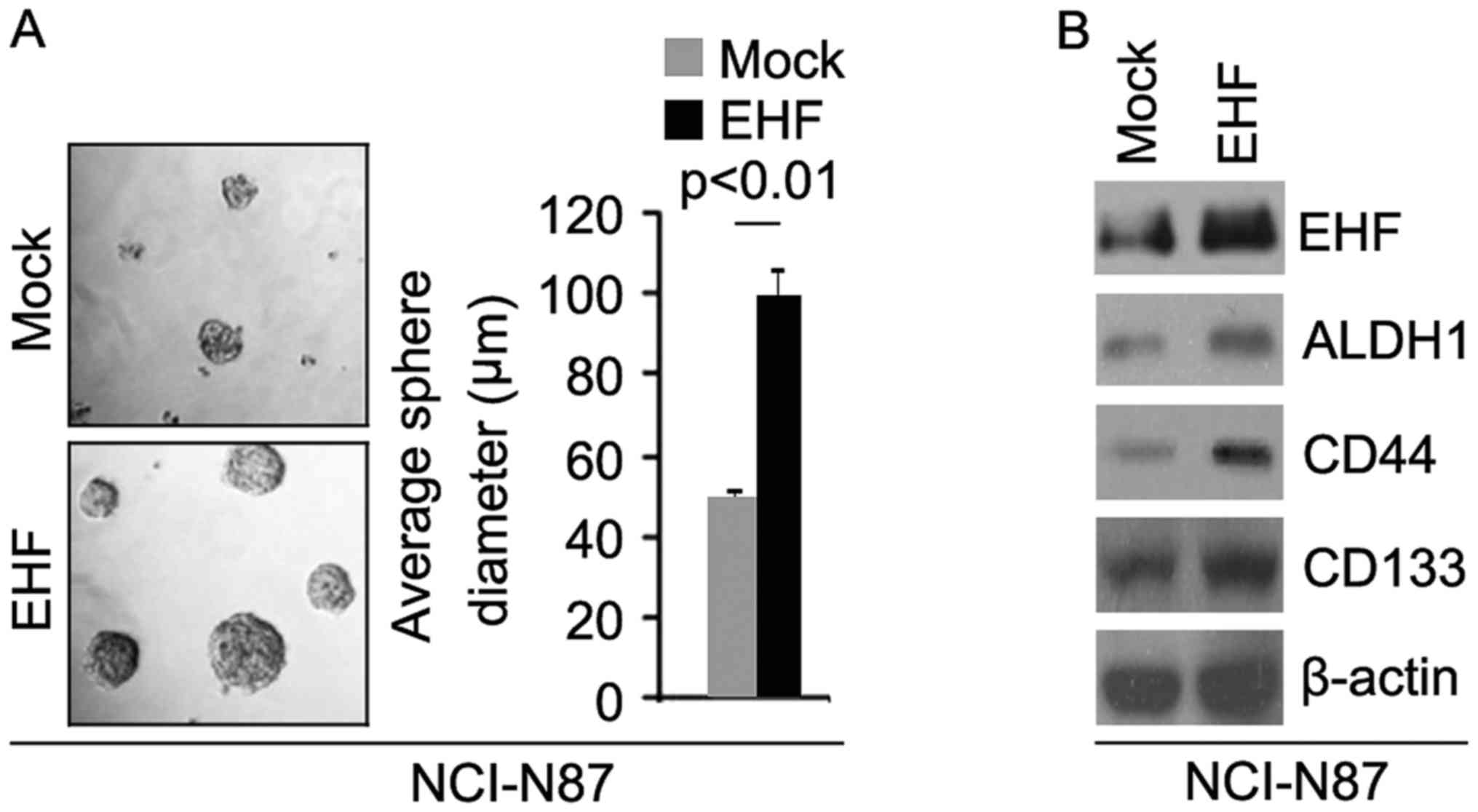
To identify the role of EHF, we tested whether EHF
expressing plasmids could stably express EHF protein in NCI-N87
cells. The results showed that EHF protein could be significantly
increased by EHF expressing plasmids in the cells (Fig. 1B). In order to identify whether EHF
can affect CIC traits in NCI-N87 cells, we performed sphere forming
assay to assess the capacity of CIC or CIC-like cell self renewal
in NCI-N87 cells. Sphere forming assay showed EHF overexpressing
cells formed much bigger spheres after 14 days of culture as
compared with control cells, indicating markedly increased CIC
traits by EHF (Fig. 1A). To
identify whether EHF can regulate ALDH1, CD133 and CD44 protein
expression, we performed western blotting in NCI-N87 cells
transfected with EHF expressing plasmids and empty vectors. The
results showed that ALDH1, CD44 and CD133 protein are upregulated
in NCI-N87 cells transfected with EHF expressing plasmids (Fig. 1B).
Clonogenic ability was increased by
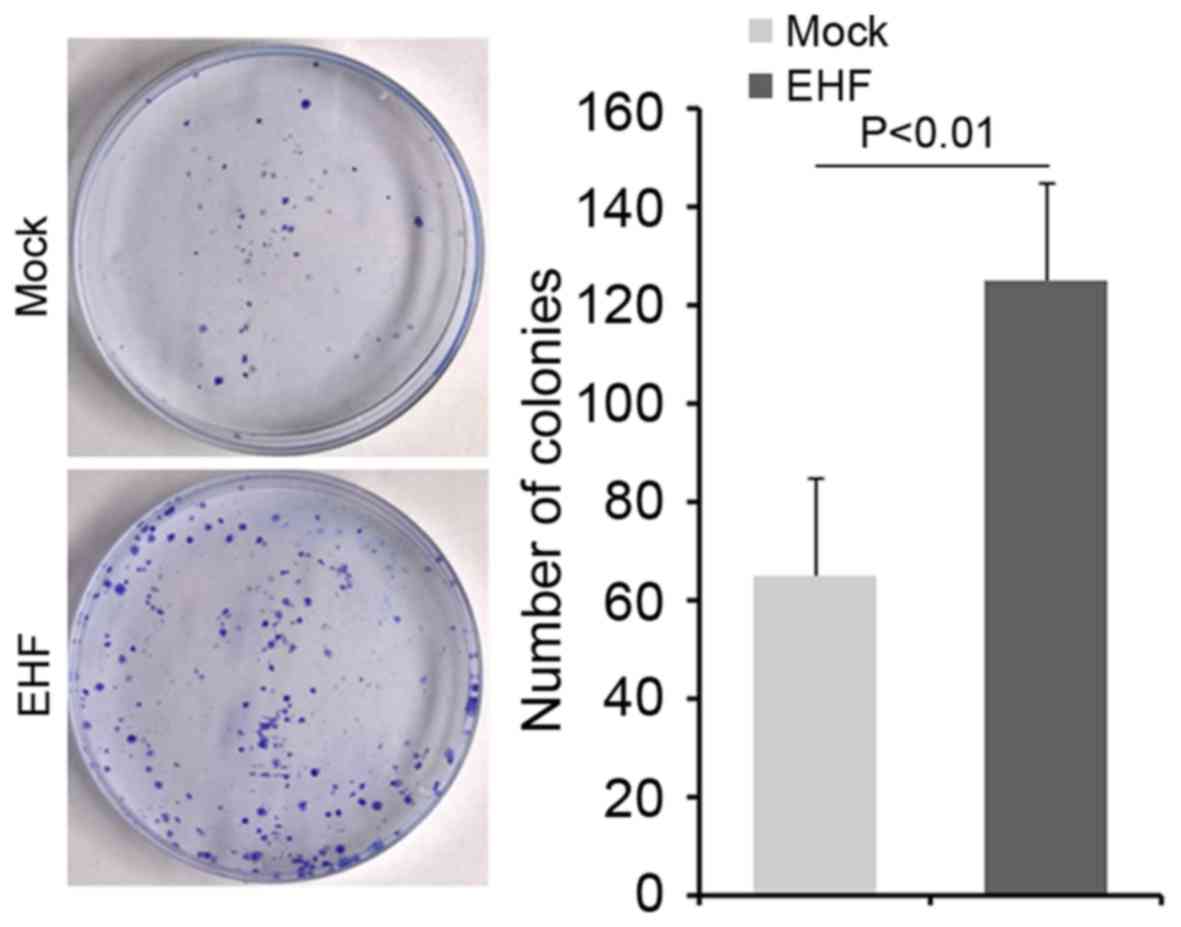
EHF in gastric cancer NCI-N87 cells
To determine whether cells with elevated stem-like
cell characteristics could have increased clonogenic ability in
NCI-N87 cells, we performed clonogenic assay. We found that
clonogenic ability was significantly increased in NCI-N87 cells
transfected with EHF expressing plasmids compared with NCI-N87
cells transfected with empty vectors (Fig. 2).
Silencing EHF inhibits formation of
CIC phenotypes and clonogenic ability in gastric cancer NCI-N87
cells
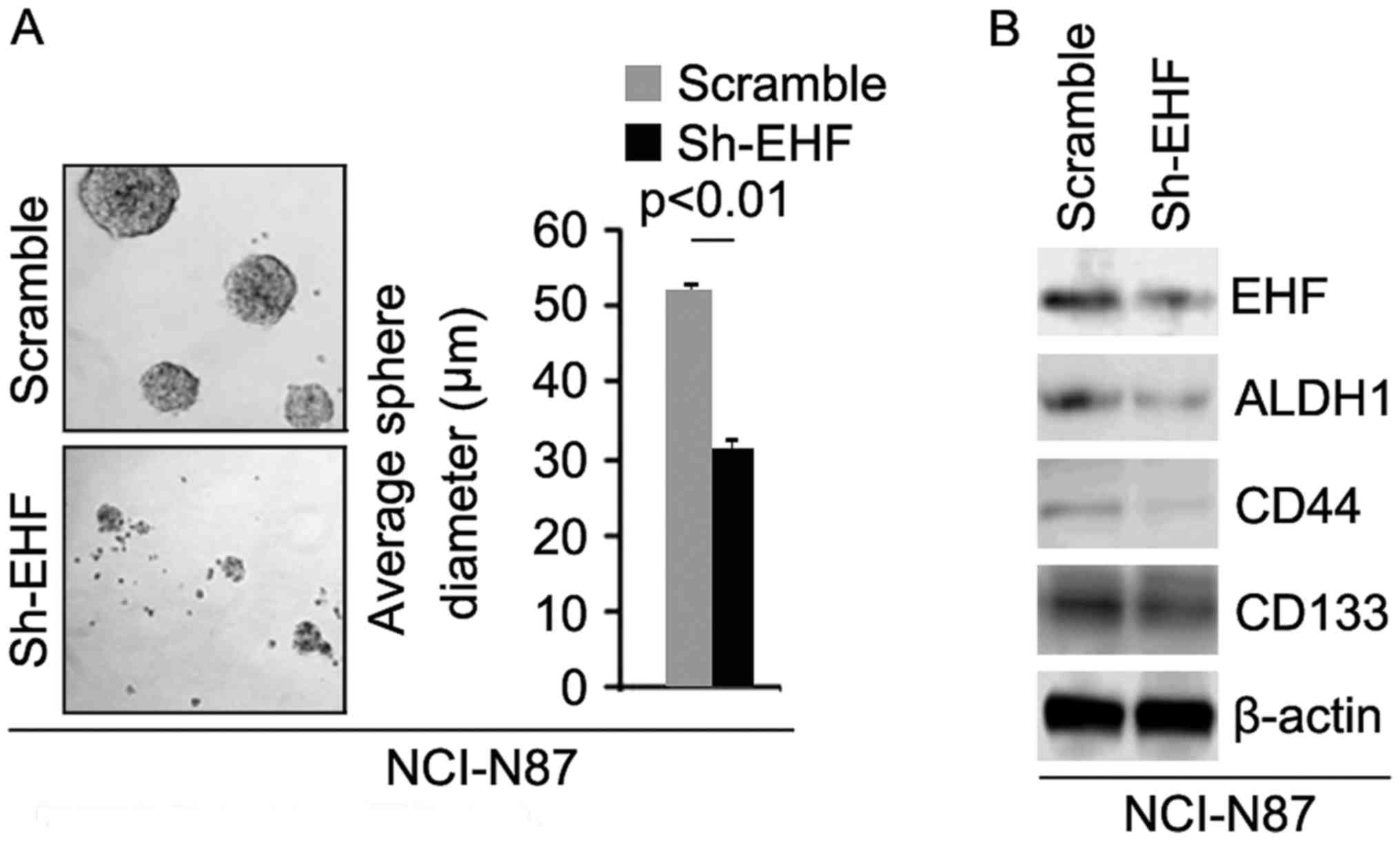
To identify the role of sh-EHF, we tested whether
sh-EHF plasmids could stably downregulate EHF protein in NCI-N87
cells. The results showed that EHF protein could be significantly
decreased by sh-EHF plasmids in the cells (Fig. 3B). In order to identify whether
sh-EHF can affect CIC traits in NCI-N87 cells, we performed sphere
forming assay to assess the capacity of CIC or CIC-like cell self
renewal in NCI-N87 cells. Sphere forming assay showed silencing of
EHF formed much smaller spheres after 14 days of culture as
compared with control cells transfected with scrambles, indicating
markedly decreased CIC traits by sh-EHF (Fig. 3A). To identify whether EHF can
regulate ALDH1, CD133 and CD44 protein expression, we performed
western blotting in NCI-N87 cells transfected with sh-EHF plasmids
and scrambles. The results showed that ALDH1, CD44 and CD133
protein are downregulated in NCI-N87 cells transfected with sh-EHF
plasmids (Fig. 3B).
Clonogenic ability was attenuated by
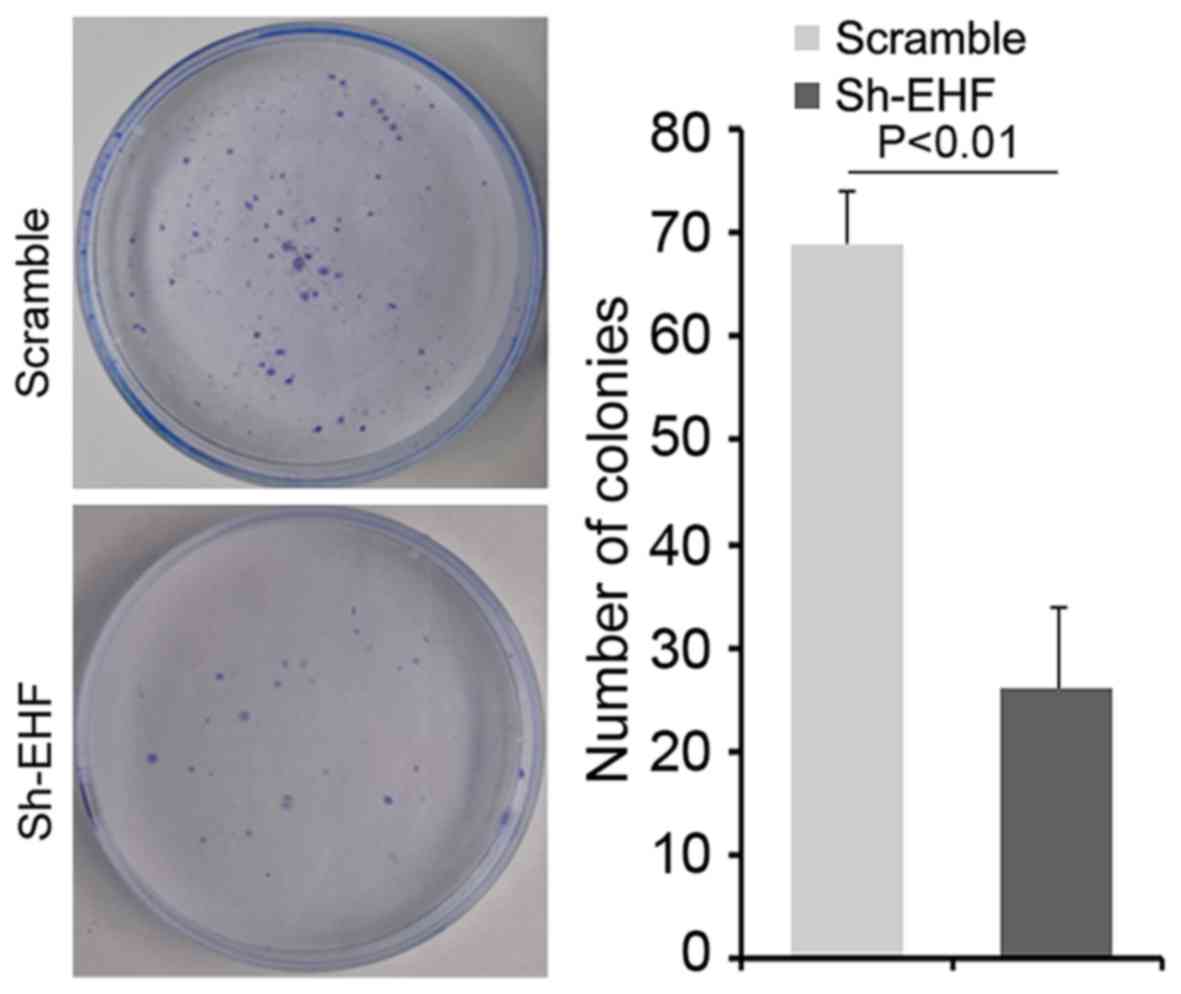
silencing EHF in gastric cancer NCI-N87 cells
To further determine whether silencing EHF could
affect clonogenic ability in NCI-N87 cells, we performed a
clonogenic assay. We found that clonogenic ability was
significantly decreased in NCI-N87 cells transfected with sh-EHF
plasmids compared with NCI-N87 cells transfected with scrambels
(Fig. 4).
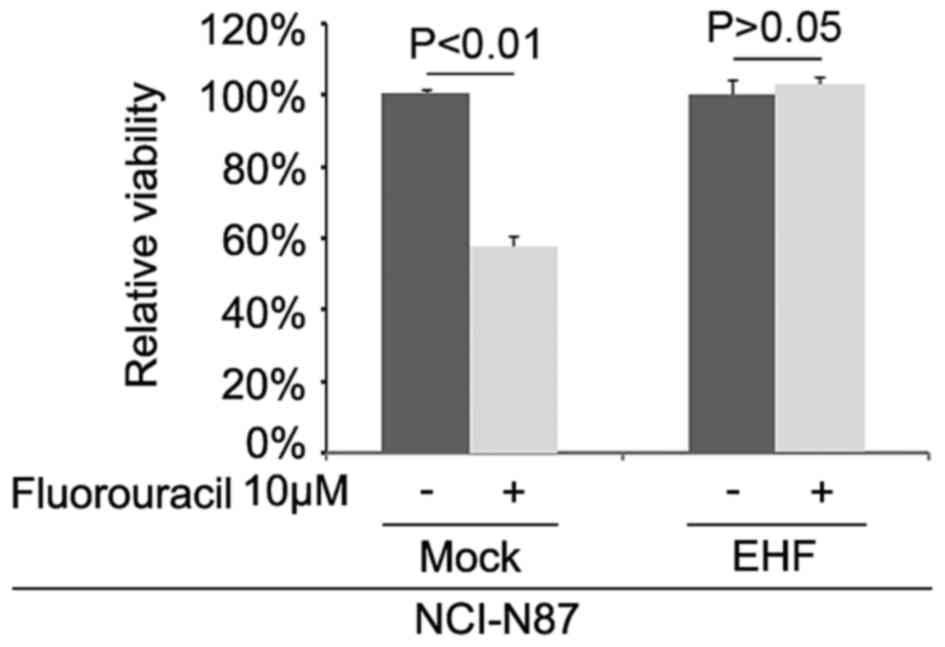
EHF promotes fluorouracil-resistance
in gastric cancer NCI-N87 cells
To further identify whether EHF can affect
fluorouracil efficacy in NCI-N87 cells, we transfected NCI-N87
cells with EHF expressing plasmids. Then we performed MTT assay in
the cells transfected with EHF expressing plasmids and empty
vectors. The results showed that overexpressing EHF could transform
fluorouracil sensitive NCI-N87 cells to fluorouracil-resistant
cells (Fig. 5), suggesting that its
overexpression promoted fluorouracil-resistance.
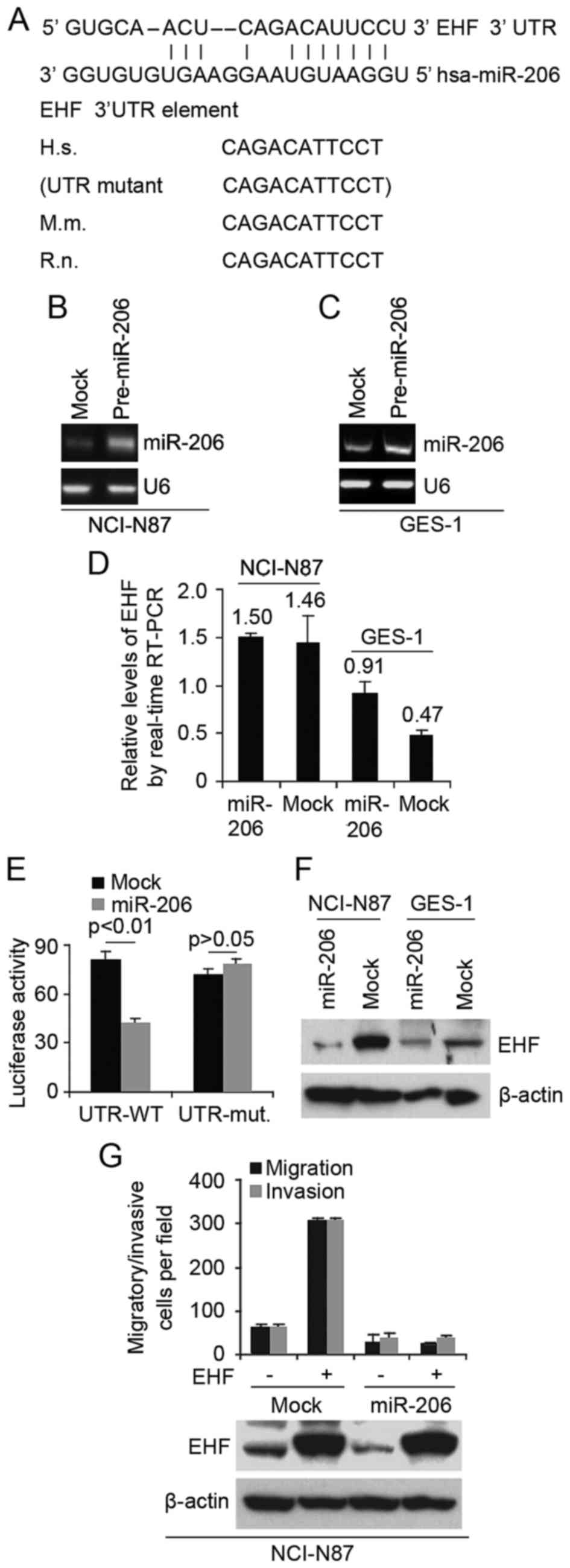
miR-206 inhibits EHF expression in
immortalized human gastric epithelial mucosa GES-1 cells and
gastric cancer NCI-N87 cells
Having demonstrated that EHF is associated with
formation of CICs phenotypes and increased clonogenic ability in
NCI-N87 cells, next we studied the mechanisms regulating EHF
expression in GES-1 cells and NCI-N87 cells. MicroRNAs (miRs) are a
class of small non-coding RNAs (~22 nucleotides) and negatively
regulate protein-coding gene expression by targeting mRNA
degradation or translation inhibition (12,32).
To further confirm whether EHF could be regulated by
microRNA, we used the common prediction algorithm - miRanda
(http://www.microrna.org/microrna/home.do) to analyze
3′UTR of EHF. A dozen of microRNAs were found by the algorithm.
However, we are interested in miR-206, because it has been reported
that miR-206 is significantly down-regulated in gastric carcinoma
(22,23). Target sites on 3′UTR of EHF are
shown in Fig. 6A and it carries the
identical sequence in the human, mouse (M.m.) and rat (R.n.) mRNA
orthologues (Fig. 6A).
In an attempt to identify the role of miR-206 in
regulating EHF expression in GES-1 cells and NCI-N87 cells, we
transfected GES-1 cells and NCI-N87 cells with pre-miR-206 and
control miR. After transfection, miR-206 expression was detected by
real-time PCR and the results showed that miR-206 was significantly
increased by pre-miR-206 in the cells (Fig. 6B and C). miR-206 overexpression did
not cause degradation of EHF mRNA (Fig.
6D), however, it reduced the activity of a luciferase reporter
gene fused to the wild-type EHF 3′UTR (Fig. 6E), indicating that miR-206 targets
EHF through translational inhibition.
The action of miR-206 on EHF depends on the presence
of miR-206 binding sites on the 3′UTR of EHF, because the activity
of a luciferase reporter with a mutant 3′UTR of EHF was not reduced
by expression of miR-206 (Fig. 6E).
To support the results, we performed western blotting to detect EHF
protein in NCI-N87 and GES-1 cells. We observed an evident
reduction for EHF protein in miR-206-overexpressing cells (Fig. 6F). In order to detect whether EHF
and miR-206 can affect abilities of migration and invasion, we
performed migration and invasion assay in NCI-N87 cells. Evidently,
re-expression of miR-206 completely abrogated EHF-induced cell
motility and invasiveness (Fig.
6G), suggesting that this EHF is indeed a functionally
important target of miR-206.
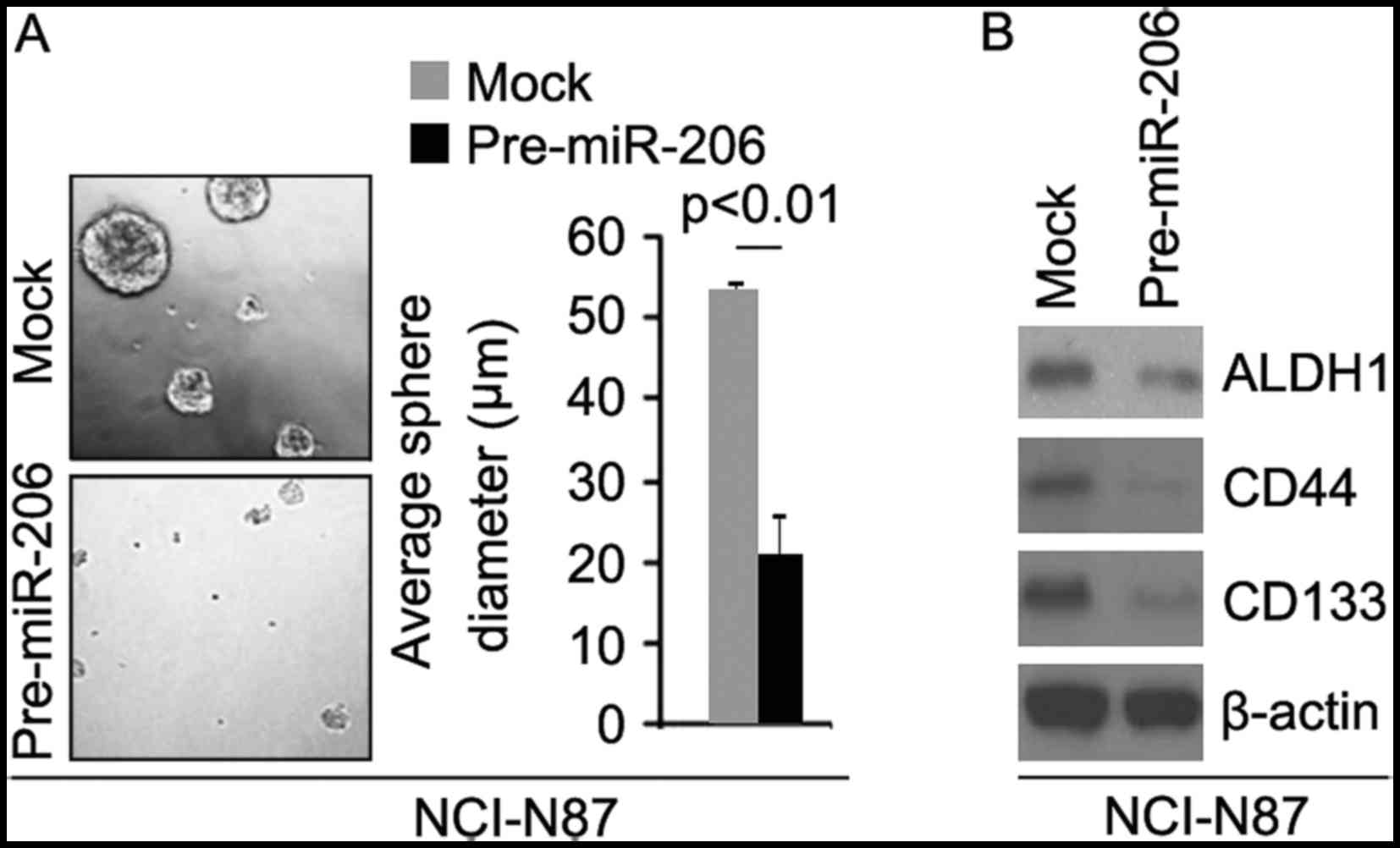
miR-206 inhibits formation of CIC
phenotypes and clonogenic ability in gastric cancer NCI-N87
cells
In order to identify whether pre-miR-206 can affect
CIC traits in NCI-N87 cells, we performed sphere forming assay to
assess the capacity of CIC or CIC-like cell self renewal in NCI-N87
cells. Sphere forming assay showed miR-206 overexpressing cells
formed much smaller spheres after 14 days of culture as compared
with control cells, indicating markedly decreased CIC traits by
miR-206 (Fig. 7A). To identify
whether miR-206 can regulate ALDH1, CD133 and CD44 protein
expression, we performed western blotting in NCI-N87 cells
transfected with pre-miR-206 and control miR. The results showed
that ALDH1, CD44 and CD133 protein are downregulated in NCI-N87
cells transfected with pre-miR-206 (Fig. 7B).
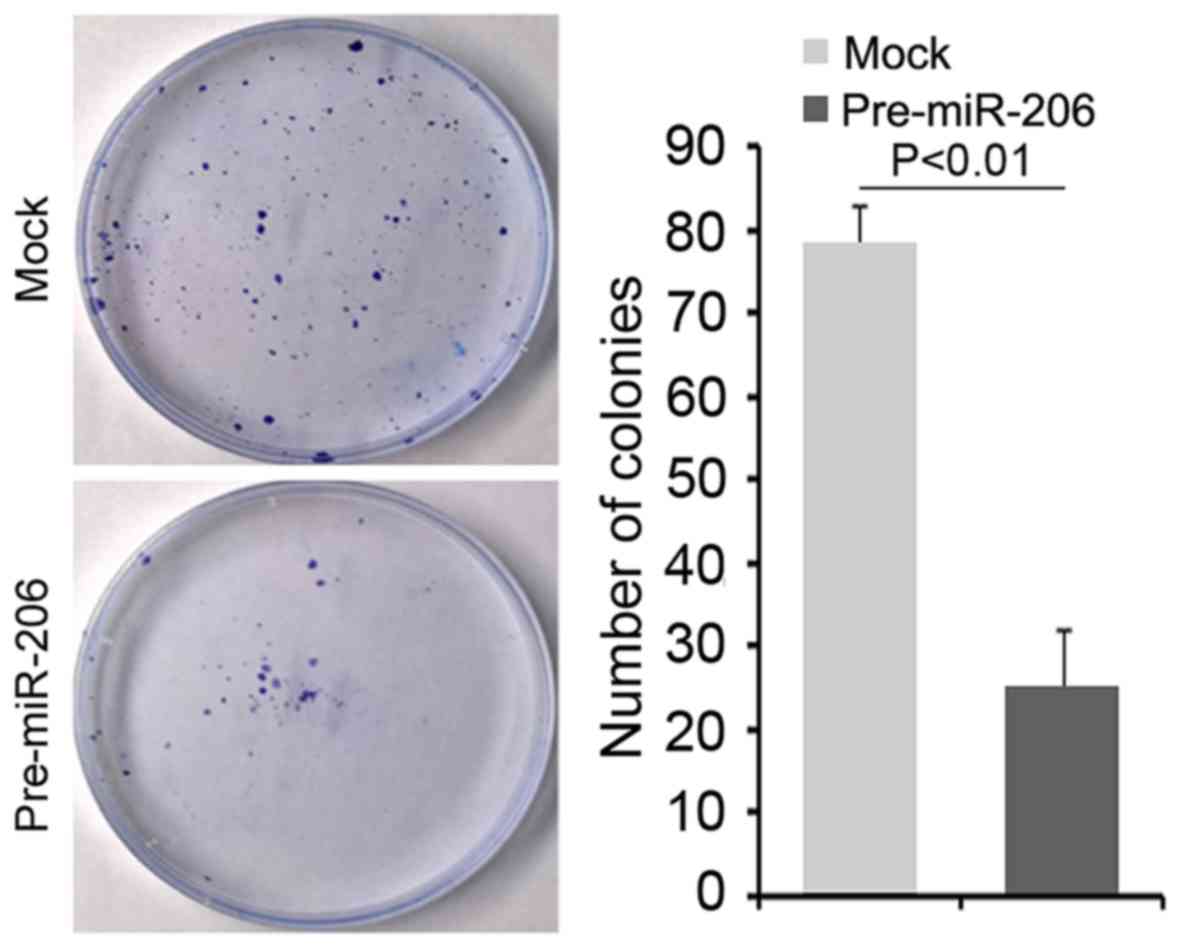
Clonogenic ability was attenuated by
miR-206 in gastric cancer NCI-N87 cells
To further determine whether overexpressing miR-206
could affect clonogenic ability in NCI-N87 cells, we performed a
clonogenic assay. We found that clonogenic ability was
significantly decreased in NCI-N87 cells transfected with
pre-miR-206 compared with NCI-N87 cells transfected with control
miR (Fig. 8).
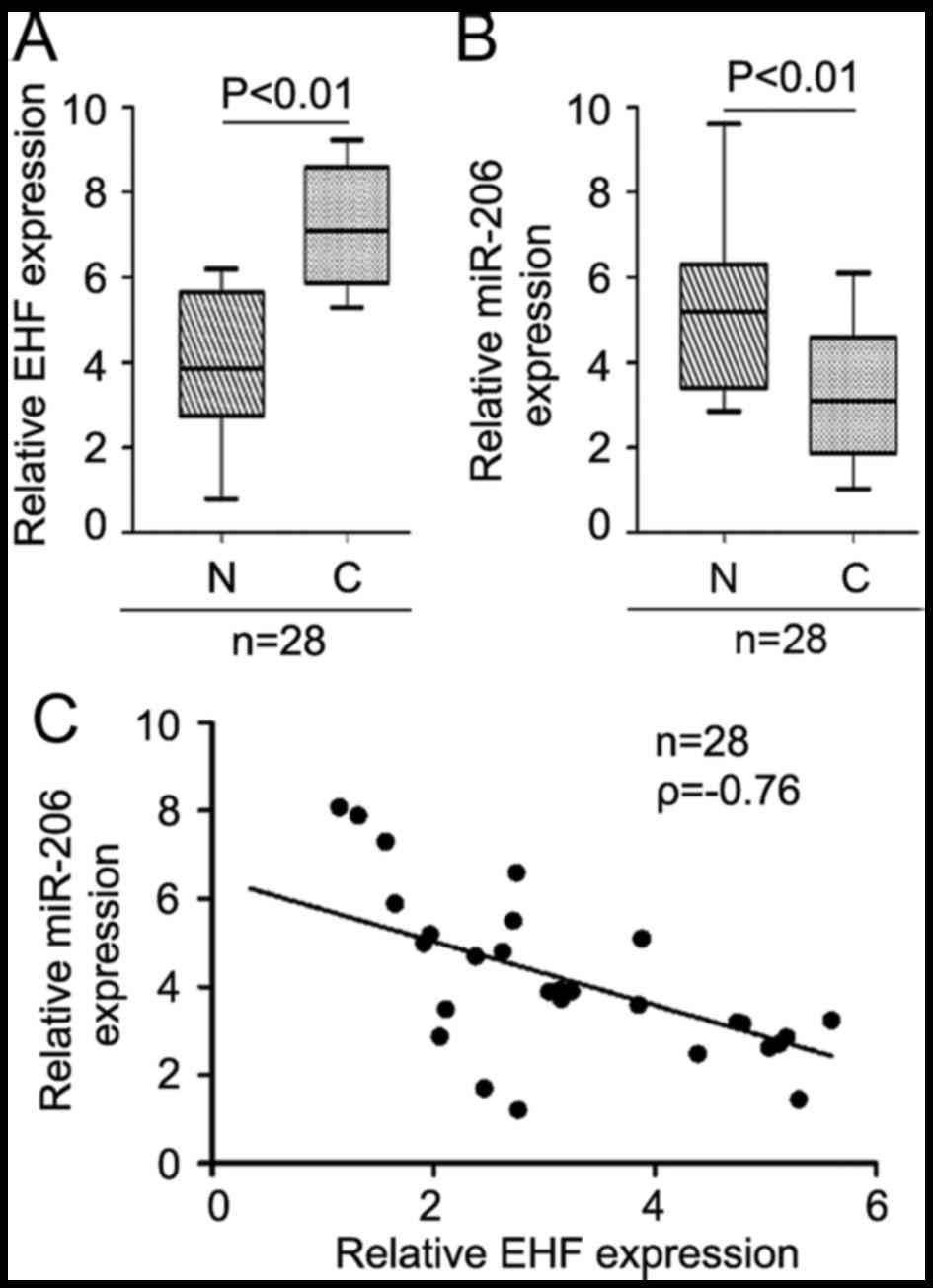
miR-206 is inversely correlated with
EHF mRNA expression in gastric cancer tissues
Furthermore, we detected the endogenous mRNA level
of EHF in GC tissues. The results showed EHF is upregulated
(Fig. 9A) and miR-206 is
downregulated (Fig. 9B) and we
observed a negative correlation between miR-206 mRNA levels and EHF
mRNA expression in GC tissue samples (n=28) (Fig. 9C, ρ=−0.76).
miR-206 inhibits c-MET, PAX3, cyclin
D2, and CDK4 protein in NCI-N87 cells
In order to detect whether miR-206 can inhibit
c-MET, PAX3, cyclin D2, and CDK4 protein expression, we transfected
NCI-N87 cells with pre-miR-206 and control miR. Then western
blotting was performed to detect the proteins. The results showed
that c-MET, PAX3, cyclin D2 and CDK4 protein were downregulated in
NCI-N87 cells transfected with pre-miR-206 (Fig. 10A).
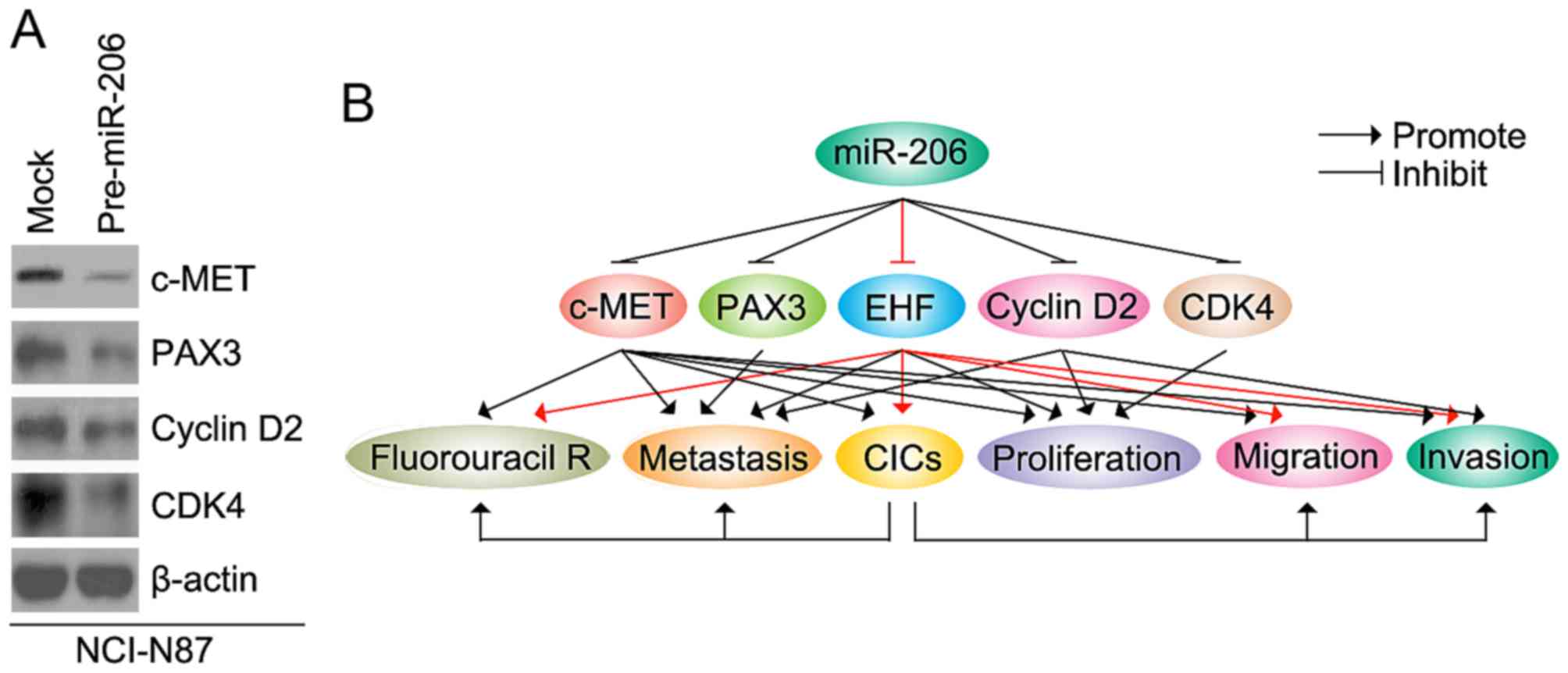 | Figure 10.miR-206 inhibits c-MET, PAX3, cyclin
D2, and CDK4 protein in NCI-N87 cells. (A) Western blotting for
c-MET, PAX3, cyclin D2, and CDK4 protein in NCI-N87 cells
transfected with pre-miR-206 and control miR (mock). (B) miR-206
regulates invasion, migration, proliferation, CICs and metastasis
by targeting c-MET, PAX3, EHF, cyclin D2, and CDK4 protein in
gastric cancer. |
Discussion
In this study, we first provided strong evidence
supporting miR-206 inhibits cancer initiating cells (CIC) by
targeting EHF in gastric cancer. Oncogenic activities of EHF have
been reported in gastric cancer (10). EHF was frequently overexpressed and
amplified in gastric cancers compared with matched non-cancerous
gastric tissues (10). Consistent
with previous reports (10), we
found that EHF mRNA expression was upregulated in gastric cancer
tissues. EHF amplification or overexpression was significantly
associated with poor clinical outcomes and may be used as a
potential prognostic marker for gastric cancer patients (10). CIC were found to possess the ability
to sustain tumor self-renewal, initiate tumor progression, and
possibly also contribute to metastasis and poor prognosis in
gastric cancer (33). We showed
that EHF could promote formation of CIC in gastric cancer NCI-N87
cells. Clonogenic ability was significantly increased with
formation of CIC in various cancers (34–36).
In line with the reports (34–36),
we observed that overexpressing EHF promoted abilities of colony
formation while it induced formation of CIC.
5-Fluorouracil chemotherapy is the first treatment
of choice for advanced gastric cancer; however its effectiveness is
limited by drug resistance. Emerging evidence suggests that the
existence of CIC contributes to 5-Fluorouracil resistance (37,38).
Our results showed that EHF overexpression promoted 5-fluorouracil
resistance. In this study, we only used MTT to analyze
5-fluorouracil resistance. Apoptosis assay and cell cycle analysis
can be performed to further confirm the roles of EHF in NCI-N87
cells.
miR-206 can inhibit progression by downregulating
c-MET, PAX3, cyclin D2 and CDK4 in gastric cancer (Fig. 10B) (22,23,39).
Its expression is downregulated in gastric cancer. miR-206 was
downregulated in gastric cancer cells especially in high metastatic
cell lines and its expression was significantly decreased in
metastatic lymph nodes, compared with their corresponding primary
tumor samples (23). We found that
miR-206 is downregulated in gastric cancer, compared with adjacent
normal tissues. Its restoration inhibited CIC traits in gastric
cancer cells (Fig. 10B). In
addition, we identified that EHF is one of target genes of miR-206
and it plays an important role in the network regulated by miR-206.
miR-206 inhibits the expression of PAX3, cyclin D2, c-Met and
cycle-related proteins CDK4 in gastric cancer cells. Consistent
with previous report, we found that PAX3, cyclin D2, c-Met and CDK4
were downregulated by miR-206 in gastric cancer cells (Fig. 10B). Elucidating miR-206-mediated
molecular mechanism responsible for CIC traits will help us to
further understand the pathogenesis and progression of the disease
and offer new targets for effective therapies.
Acknowledgements
This study was supported by Natural Science
Foundation of Shandong Province: ZR2015HL081; Science and
Technology Development Project of Shandong Province: 2011YD21035;
Natural Science Foundation of Shandong Province: ZR2015HL070.
References
|
1
|
Network CGAR, . Cancer Genome Atlas
Research Network: Comprehensive molecular characterization of
gastric adenocarcinoma. Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takaishi S, Okumura T and Wang TC: Gastric
cancer stem cells. J Clin Oncol. 26:2876–2882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kas K, Finger E, Grall F, Gu X, Akbarali
Y, Boltax J, Weiss A, Oettgen P, Kapeller R and Libermann TA:
ESE-3, a novel member of an epithelium-specific ets transcription
factor subfamily, demonstrates different target gene specificity
from ESE-1. J Biol Chem. 275:2986–2998. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albino D, Longoni N, Curti L, Mello-Grand
M, Pinton S, Civenni G, Thalmann G, D'Ambrosio G, Sarti M, Sessa F,
et al: ESE3/EHF controls epithelial cell differentiation and its
loss leads to prostate tumors with mesenchymal and stem-like
features. Cancer Res. 72:2889–2900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stephens DN, Klein RH, Salmans ML, Gordon
W, Ho H and Andersen B: The Ets transcription factor EHF as a
regulator of cornea epithelial cell identity. J Biol Chem.
288:34304–34324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tugores A, Le J, Sorokina I, Snijders AJ,
Duyao M, Reddy PS, Carlee L, Ronshaugen M, Mushegian A, Watanaskul
T, et al: The epithelium-specific ETS protein EHF/ESE-3 is a
context-dependent transcriptional repressor downstream of MAPK
signaling cascades. J Biol Chem. 276:20397–20406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brenne K, Nymoen DA, Hetland TE, Trope' CG
and Davidson B: Expression of the Ets transcription factor EHF in
serous ovarian carcinoma effusions is a marker of poor survival.
Hum Pathol. 43:496–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi J, Qu Y, Li X, Sui F, Yao D, Yang Q,
Shi B, Ji M and Hou P: Increased expression of EHF via gene
amplification contributes to the activation of HER family signaling
and associates with poor survival in gastric cancer. Cell Death
Dis. 7:e24422016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Farh KK-H, Grimson A, Jan C, Lewis BP,
Johnston WK, Lim LP, Burge CB and Bartel DP: The widespread impact
of mammalian MicroRNAs on mRNA repression and evolution. Science.
310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ, et al: A microRNA polycistron as a potential human
oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roldo C, Missiaglia E, Hagan JP, Falconi
M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, et
al: MicroRNA expression abnormalities in pancreatic endocrine and
acinar tumors are associated with distinctive pathologic features
and clinical behavior. J Clin Oncol. 24:4677–4684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Z, Yan D, Chen X, Huang H, Chen K,
Li G, Zhou L, Zheng D, Tu L and Dong XD: MicroRNA-206: Effective
Inhibition of Gastric Cancer Progression through the c-Met Pathway.
PLoS One. 10:e01287512015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Xia L, Zhao L, Chen Z, Shang X,
Xin J, Liu M, Guo X, Wu K, Pan Y, et al: Activation of PAX3-MET
pathways due to miR-206 loss promotes gastric cancer metastasis.
Carcinogenesis. 36:390–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao XH, Li YQ, Wang N, Zheng L, Xing WJ,
Zhao DW, Yan TB, Wang Y, Liu LY, Sun XG, et al: Re-expression and
epigenetic modification of maspin induced apoptosis in MCF-7 cells
mediated by myocardin. Cell Signal. 26:1335–1346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao XH, Wang Y, Wang N, Yan TB, Xing WJ,
Zheng L, Zhao DW, Li YQ, Liu LY, Sun XG, et al: Human chorionic
gonadotropin decreases human breast cancer cell proliferation and
promotes differentiation. IUBMB Life. 66:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao XH, Xiang Y, Yu CX, Li JP, Li H, Nie
Q, Hu P, Zhou J and Zhang TC: STAT3 is required for
miR-17-5p-mediated sensitization to chemotherapy-induced apoptosis
in breast cancer cells. Oncotarget. 8:15763–15774. 2017.PubMed/NCBI
|
|
27
|
Liao XH, Li JY, Dong XM, Wang X, Xiang Y,
Li H, Yu CX, Li JP, Yuan BY, Zhou J, et al: ERα inhibited
myocardin-induced differentiation in uterine fibroids. Exp Cell
Res. 350:73–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao XH, Lu DL, Wang N, Liu LY, Wang Y, Li
YQ, Yan TB, Sun XG, Hu P and Zhang TC: Estrogen receptor α mediates
proliferation of breast cancer MCF-7 cells via a
p21/PCNA/E2F1-dependent pathway. FEBS J. 281:927–942. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang Y, Lu DL, Li JP, Yu CX, Zheng DL,
Huang X, Wang ZY, Hu P, Liao XH and Zhang TC: Myocardin inhibits
estrogen receptor alpha-mediated proliferation of human breast
cancer MCF-7 cells via regulating MicroRNA expression. IUBMB Life.
68:477–487. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kondo N, Toyama T, Sugiura H, Fujii Y and
Yamashita H: miR-206 Expression is down-regulated in estrogen
receptor α-positive human breast cancer. Cancer Res. 68:5004–5008.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren Z-G, Dong S-X, Han P and Qi J: miR-203
promotes proliferation, migration and invasion by degrading SIK1 in
pancreatic cancer. Oncol Rep. 35:1365–1374. 2016.PubMed/NCBI
|
|
32
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y,
Uraoka N, Anami K, Sentani K, Oue N and Yasui W: Expression of
cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and
lymph node metastasis of gastric cancer. Pathol Int. 62:112–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z,
Sethi S and Sarkar FH: Epithelial to mesenchymal transition is
mechanistically linked with stem cell signatures in prostate cancer
cells. PLoS One. 5:e124452010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shidal C, Al-Rayyan N, Yaddanapudi K and
Davis KR: Lunasin is a novel therapeutic agent for targeting
melanoma cancer stem cells. Oncotarget. 7:84128–84141.
2016.PubMed/NCBI
|
|
36
|
Gao Y, Liu T, Cheng W and Wang H:
Isolation and characterization of proliferative, migratory and
multidrug-resistant endometrial carcinoma-initiating cells from
human type II endometrial carcinoma cell lines. Oncol Rep.
28:527–532. 2012.PubMed/NCBI
|
|
37
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu ZY, Tang JN, Xie HX, Du YA, Huang L, Yu
PF and Cheng XD: 5-Fluorouracil chemotherapy of gastric cancer
generates residual cells with properties of cancer stem cells. Int
J Biol Sci. 11:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang L, Liu X, Jin H, Guo X, Xia L, Chen
Z, Bai M, Liu J, Shang X, Wu K, et al: miR-206 inhibits gastric
cancer proliferation in part by repressing cyclinD2. Cancer Lett.
332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|