Introduction
Glioblastoma multiforme (GBM), which develops from
astrocytes, is the most malignant primary brain tumor in adults.
Classified as a grade IV (most serious) astrocytoma, it has one of
the worst prognoses among all human tumors, with a median survival
of approximately 12 months (1).
Chemoprevention to prevent glioma progression in patients after
surgery is an important strategy, but glioma resistance to
chemotherapy has been one of the major obstacles to successful
anticancer therapy (2).
Chemotherapeutic agents, such as doxorubicin, paclitaxel and
5-fluorouracil, clearly have some degree of activity in tumor
treatment, but did not increase the median overall survival in
patients with gliomas (3). Thus, it
is clearly necessary to determine the molecular mechanisms
underlying malignant tumor progression and resistance to treatment,
with the goal of developing chemotherapies based on more effective
clinical therapies.
Pin1 is a peptidylprolylcis/transisomerase (PPIase)
that catalyzes the isomerization of the peptide bond between
phospho-serine/threonine and proline (4,5).
Previous reports have indicated that it plays a critical role in
Alzheimers disease, adipogenesis and malignant tumor formation
(4). Recently, it was reported that
Pin1 protein expression was upregulated in human GBM specimens
(6,7); thus, it is necessary to determine if
Pin1 inhibitors have chemotherapeutic potential in this disease.
Juglone (5-hydroxy-1,4-napthoquinone) is a natural compound
isolated from Juglans mandshurica Maxim (8). Several studies have shown that it has
various pharmacological effects such as anti-viral, anti-bacterial
and anticancer properties (9,10).
Moreover, many experiments have demonstrated that it irreversibly
inhibits the enzymatic activity of Pin1 (8,11,12).
Thus, we hypothesized that juglone exerts its antitumor effects in
glioma cells by suppressing Pin1-mediated signaling.
Materials and methods
Cell culture
The human glioblastoma cell line, U251, and human
umbilical vein endothelial cells (HUVECs) were obtained from the
China Academia Sinica Cell Repository (Shanghai, China). The cells
were maintained in RPMI-1640 (Gibco, Grand Island, NY, USA)
supplemented with 15% fetal bovine serum (FBS; Gibco), 2 mM
glutamine (Sigma-Aldrich, St. Louis, MO USA), 100 units/ml
penicillin (Sigma-Aldrich), and 100 µg/ml streptomycin
(Sigma-Aldrich) and were incubated at 37°C with 5%
CO2.
Transfection
Cells were transfected with 1-µg/ml pc-DNA3.1-hPin1
plasmid (Genechem, Shanghai, China) using Lipofectamine™ 2000 and
were lysed after 48 h.
MTT assay
U251 cells were seeded in a 96-well plate at a
density of 2.5×104 cells/well and were allowed to adhere
to the bottom of each well for 24 h. After treatment, the medium in
each well was removed and replaced with a phosphate-buffered saline
(PBS) solution containing 5 mg/ml MTT, after which the plate was
incubated at 37°C for 3 h. Then, the remaining supernatant was
removed, and 100 µl dimethyl sulfoxide (DMSO) was added to each
well and mixed thoroughly to dissolve the formazan crystals that
developed. After 10 min of incubation to ensure, cell viability was
determined by measuring the absorbance of each well at a wavelength
of 570 nm. Relative cell viability was expressed as the percentage
of the treatment group relative to that of the control group.
Fluorescent microscopy
measurements
U251 cells were seeded on a glass coverslip. After
juglone (Sigma-Aldrich) treatment, the coverslips were treated with
20 µl freshly prepared acridine orange/ethidium bromide (AO/EB)
solution (100 µg/ml AO and 100 µg/ml EB in PBS) and viewed under a
fluorescence microscope (Olympus Corp., Tokyo, Japan). Cell images
were captured with a charge-coupled device (CCD) digital camera
(CoolSNAPcf; Roper Scientific, Tucson, AZ, USA). Apoptosis was
identified using morphological criteria, including nuclear
condensation and/or fragmentation.
Electron microscopy
To perform electron microscopy analysis, the U251
cells were fixed in 2.5% glutaraldehyde in PBS for 2 h at 4°C and
post-fixed in 1% osmium tetroxide. After dehydration in a series of
graded ethanol baths (30–100%) and propylene oxide, cells were
embedded in Epon. Cell sections (80–200 nm) were obtained using a
Reichert UltraCut E microtome and stained with uranyl acetate.
Grids were examined using a JEOL 1200 EXII electron microscope
(JEOL Ltd., Tokyo, Japan).
Caspase-3 activity assay
Caspase-3 activity was determined with colorimetric
assay kits (Sigma-Aldrich) according to the manufacturers
instructions. U251 cells were scraped from the plates in ice-cold
PBS and lysed in 160 µl ice-cold cell lysis buffer for 30 min. The
lysate was centrifuged at 13,000 × g for 30 min at 4°C, and the
supernatant was used for subsequent assays. The fluorogenic
substrates for caspase-3 were labeled with the fluorochrome
7-amino-methyl coumarin (AMC), which was released from these
substrates upon cleavage by caspase-3. Enzyme activity was
determined by monitoring the fluorescence produced by free AMC
using a spectrofluorophotometer (RF-5301PC; Shimadzu Co., Kyoto,
Japan) at 360/460 nm. Caspase-3 activity was expressed in pmole AMC
liberated per minute per microgram of protein.
Transwell migration assay
The Transwell migration and invasion assay was
performed using 24-well cell culture inserts without Matrigel (8 µm
pore; BD Biosciences, San Jose, CA, USA). Briefly, 5×104
cells were re-suspended in 250 µl serum-free RPMI-1640 and added to
the inserts. A total volume of 500 µl Dulbeccos modified Eagles
medium (DMEM) with 10% FBS was added to the lower chamber. After
allowing cells to migrate for 4 h or invade for 22 h, cells on the
upper surface of the membrane were removed using a cotton swab, and
the membranes were fixed in methanol and stained with crystal
violet. The number of migrating or invading cells was determined by
averaging the cell counts from nine randomly selected ×100
fields.
Scratch wound healing assay
For the scratch wound healing assay, U251 cells were
seeded at a density of 1×105 cells/6-well plate and
allowed to grow overnight. After a 24-h treatment with juglone, a
scratch wound was applied using a pipette tip, and a baseline image
was obtained. Scratch wound closure was monitored over a 24-h
period. Wound healing and cell migration were quantified by
measuring the distance between edges of the wound.
Chick chorioallantoic membrane
assay
Chicken eggs were purchased from Heilongjiang
Institute of Veterinary Science (Harbin, China) and maintained in a
humidified 39°C incubator (Lyon Electric, Chula Vista, CA, USA).
Pellets containing 0.5% methylcellulose plus juglone (200 µg) or
control saline were placed onto the chick chorioallantoic membrane
(CAM) of 10-day-old chick embryos. Eggs were subsequently incubated
at 39°C and on day 13, the CAMs were fixed, excised, and imaged
using a digital camera (Canon PowerShot 6) attached to a stereo
microscope (Carl Zeiss, Oberkochen, Germany). Angiogenesis was
quantified by counting the branch points arising from tertiary
vessels from a minimum of eight specimens from the three separate
experiments.
Tube formation
To evaluate in vitro angiogenesis activity,
tube formation assays were performed with HUVECs. Twenty-four-well
plates were coated with 300 µl Matrigel (Becton-Dickinson, Suzhou,
China). HUVECs (5×104 cells) were suspended in 500 µl
medium containing various concentrations of juglone, and added to
the polymerized Matrigel. After incubation at 37°C for 6 h, cells
were fixed and stained with Diff-Quik II reagents (Dade Behring AG,
Marburg, Germany), photographed and counted.
Western blot analysis
U251 cells were lysed in a buffer containing 50 mM
Tris-HCl (pH 7.4), 0.1 mM phenylmethylsulfonyl fluoride and 5 mM
EGTA for extraction of cellular proteins. Proteins (100 µg/lane)
were resolved on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) gels, and then electrophoretically
transferred to nylon membranes. After blocking, the membranes were
incubated overnight at 4°C with the appropriate rabbit polyclonal
TGF-β1, p-Smad2/3, Smad2/3 (1:500 dilution; Abcam, Cambridge, UK),
rabbit anti-VEGF antibody, or rabbit anti-CD31 antibodies
(Proteintech, Wuhan, China). The goat anti-rabbit secondary
antibody was from Invitrogen (Carlsbad, CA, USA). Western blot
bands were quantified using Odyssey v1.2 software by measuring the
band intensity (area × OD) for each group and normalizing to GAPDH
(anti-GAPDH antibody from Shanghai Kangcheng, Co., Ltd., Shanghai,
China) served as the internal control.
TaqMan quantitative real-time PCR
analysis of mature miRNA-21
Total RNA isolated by TRIzol reagent (Invitrogen)
was treated with the Turbo DNA-Free kit (Ambion, Austin, TX, USA)
to eliminate genomic DNA contamination. TaqMan stem-loop real-time
PCR was used to assess the expression of miR-21 using kits from
Applied Biosystems (Foster City, CA, USA). In each sample, we
calculated the ΔCt (target-reference). The fold-change between
juglone-treated samples and the normal control for miR-21 was
calculated with the 2−ΔΔCt method, in which ΔΔCT = ΔCT
(target-reference) (in juglone-treated samples) - ΔCT (target
reference) (in untreated samples). Quantitative real-time PCR
(qPCR) was repeated in triplicate for each sample, and the average
2−ΔΔCt value and its standard deviation (SD) were
calculated for each sample relative to the normal control for
expression of miR-21. U6 and geNorm were used as reference genes to
which the expression of miR-21 was normalized.
Statistical analysis
Data are presented as mean ± SD. Statistical
comparison was performed by the Students t-test and analysis of
variance (ANOVA). P<0.05 were considered statistically
significant.
Results
Juglone suppresses cell viability and
induces apoptotic cell death of cultured U251 cells
After U251 glioma cells were treated with 5–100 µM
juglone for 24, 48 and 72 h, juglone gradually attenuated cell
viability in a concentration- and time-dependent manner (Fig. 1A). To determine if the reduction in
juglone-induced cell viability was due to apoptosis, AO/EB staining
and electron microscopy were performed to confirm the apoptotic
changes. Fluorescence microscopic analysis showed that untreated
U251 cells were stained with uniform green fluorescence (Fig. 1B, upper panel). Cells treated with
20 µM juglone for 48 h showed clear morphological changes in the
nucleolus, such as nuclear condensation and/or fragmentation and
orange apoptotic cells (indicated by arrows in Fig. 1B, upper panel). Juglone induced
apoptosis of U251 cells in a time-dependent manner, and the number
of apoptotic cells increased by 26% when cells were treated with 20
µM of juglone for 48 h (Fig. 1C).
We also examined the micromorphological changes induced by juglone
using electron microscopy at an original magnification of ×8,000 as
an alternative indication of apoptosis. As shown in Fig. 1B, the microstructure of the cell
appeared normal under control conditions. However, cells treated
with 20 µM juglone for 48 h exhibited morphological changes in
microstructure including chromosomal DNA condensation, segmentation
of the nucleus, a sunken nucleus membrane and loss of microvilli.
In addition, as shown in Fig. 1D
(black bars), caspase-3 activity significantly increased after the
treatment of U251 cells with 20 µM juglone for 24, 48 and 72 h.
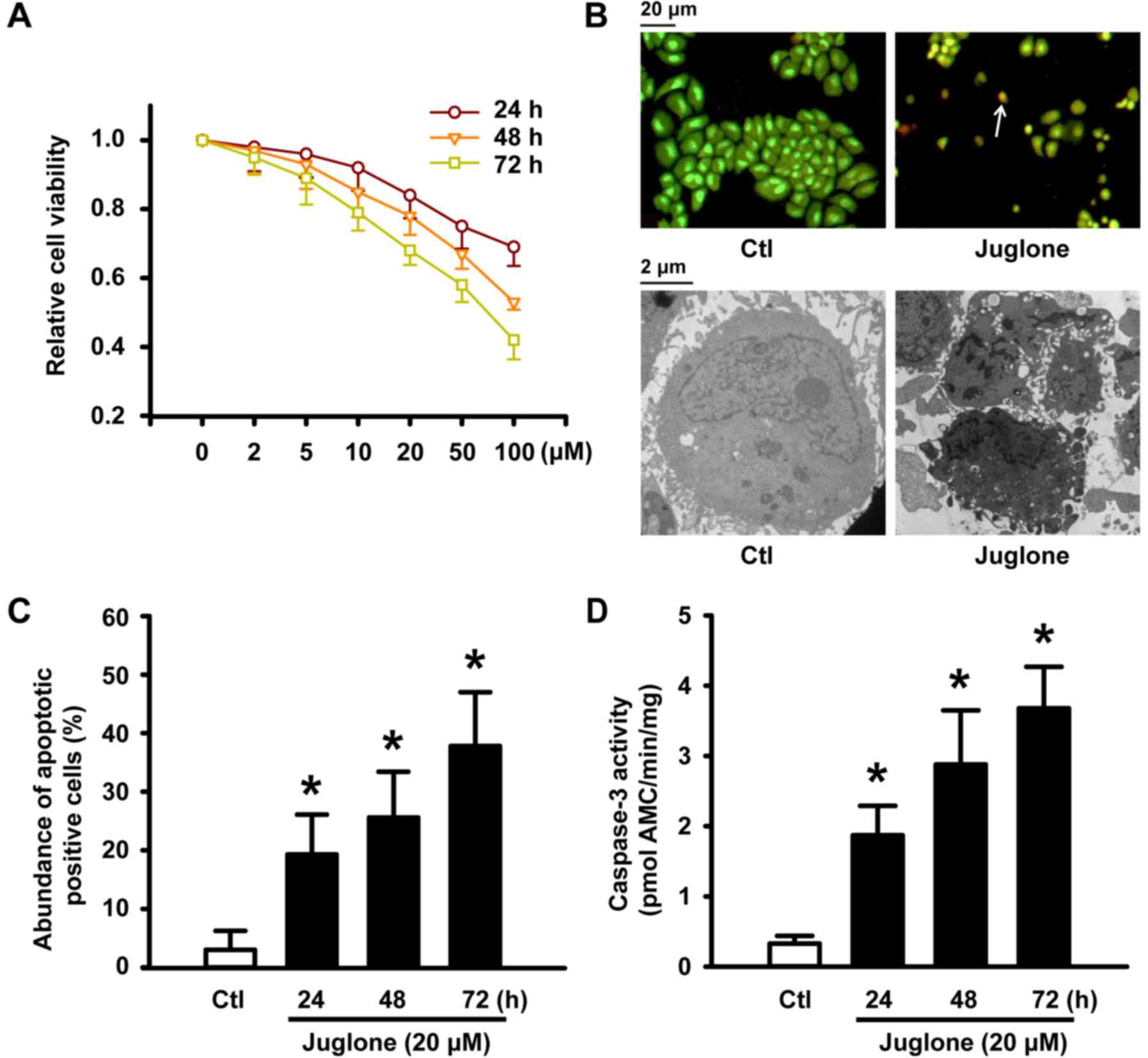 | Figure 1.Juglone-induced reduction in cell
viability and apoptosis in U251 cells. (A) U251 cells were treated
with 2, 5, 10, 20, 50 and 100 µM juglone for 24, 48 or 72 h. Cell
viability was detected by MTT assays. All of the assays were
conducted in six replicates (n=6) for each treatment. Data are
shown as mean ± SD from three independent experiments. (B) U251
cells after exposure to 20 µM juglone for 24, 48 and 72 h.
Representative image of AO/EB staining (upper panel) and
transmission electron microscopy (lower panel) of U251 cells
treated with 20 µM juglone for 48 h. White arrows indicate
apoptotic cells with morphological characteristics (chromatin
condensation and nuclear shrinkage, original magnification, ×100).
Transmission electron microscopy showed characteristic changes of
apoptosis in U251 cells treated with 20 µM juglone for 48 h
(chromosomal DNA condensation, segmentation of the nucleus, sunken
nucleus membrane and loss of microvilli, original magnification,
×8000). (C) Statistical bar graph of the abundance of apoptotic
cells induced by juglone according to AO/EB staining. (D) Juglone
enhanced the activity of caspase-3 by 7-amino-methyl coumarin
assay. Data are shown as mean ± SD of three independent
experiments, *P<0.05 vs. control. |
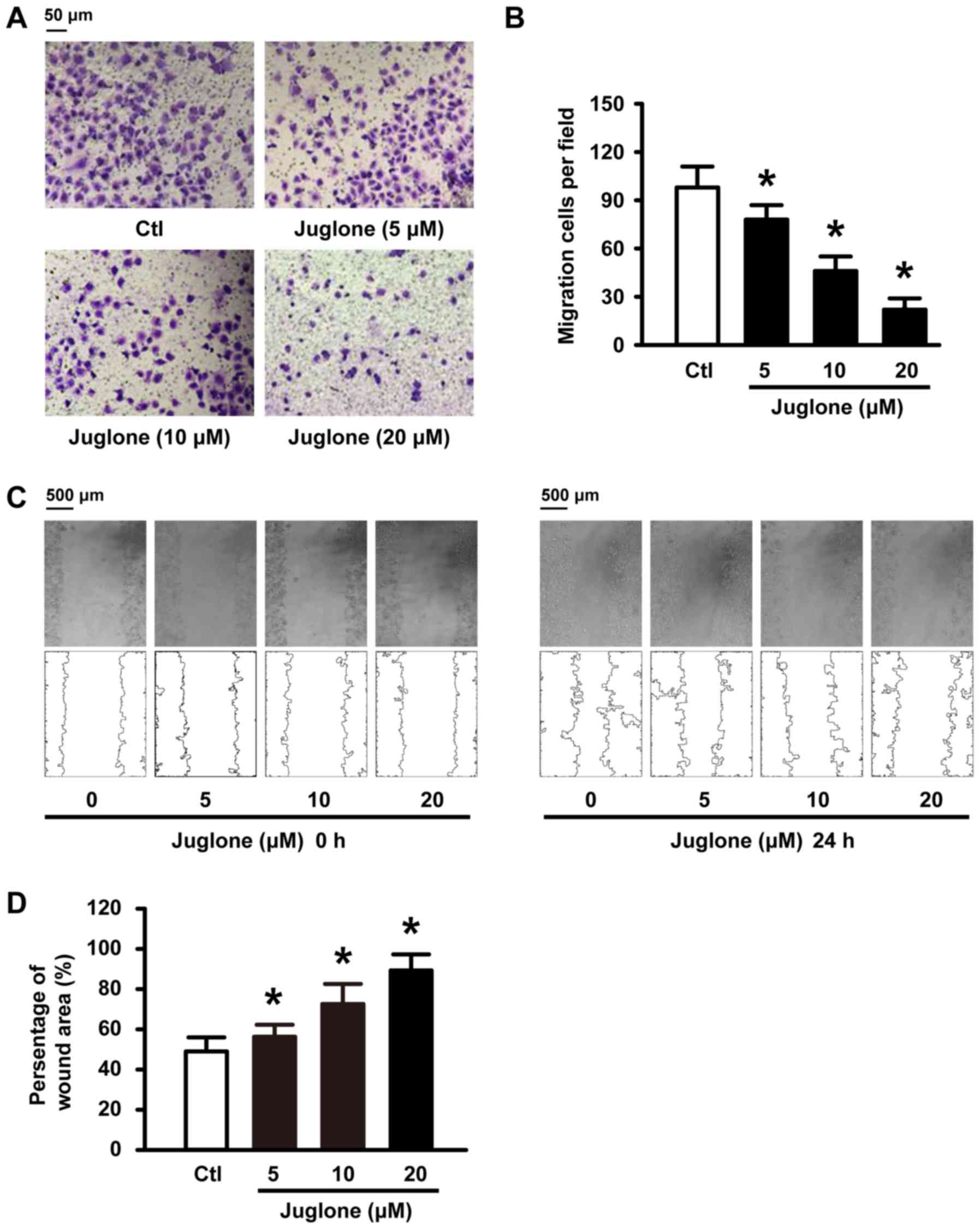
Juglone delays and decreases U251 cell
migration in vitro
We further examined whether juglone affects cell
migration ability in vitro. To this end, U251 cells were
subjected to 5, 10 and 20 µM juglone and their ability to migrate
was evaluated with Transwell and wound healing assays. As shown in
Fig. 2A and C, juglone
significantly inhibited cellular transmigration ability in a
dose-dependent manner compared to the controls. Quantitative
analysis using ImageJ software also confirmed the significant
anti-migratory effects of juglone at 24 h (Fig. 2B and D). These results strongly
indicate that juglone may regulate the migration ability of the
glioma cells.
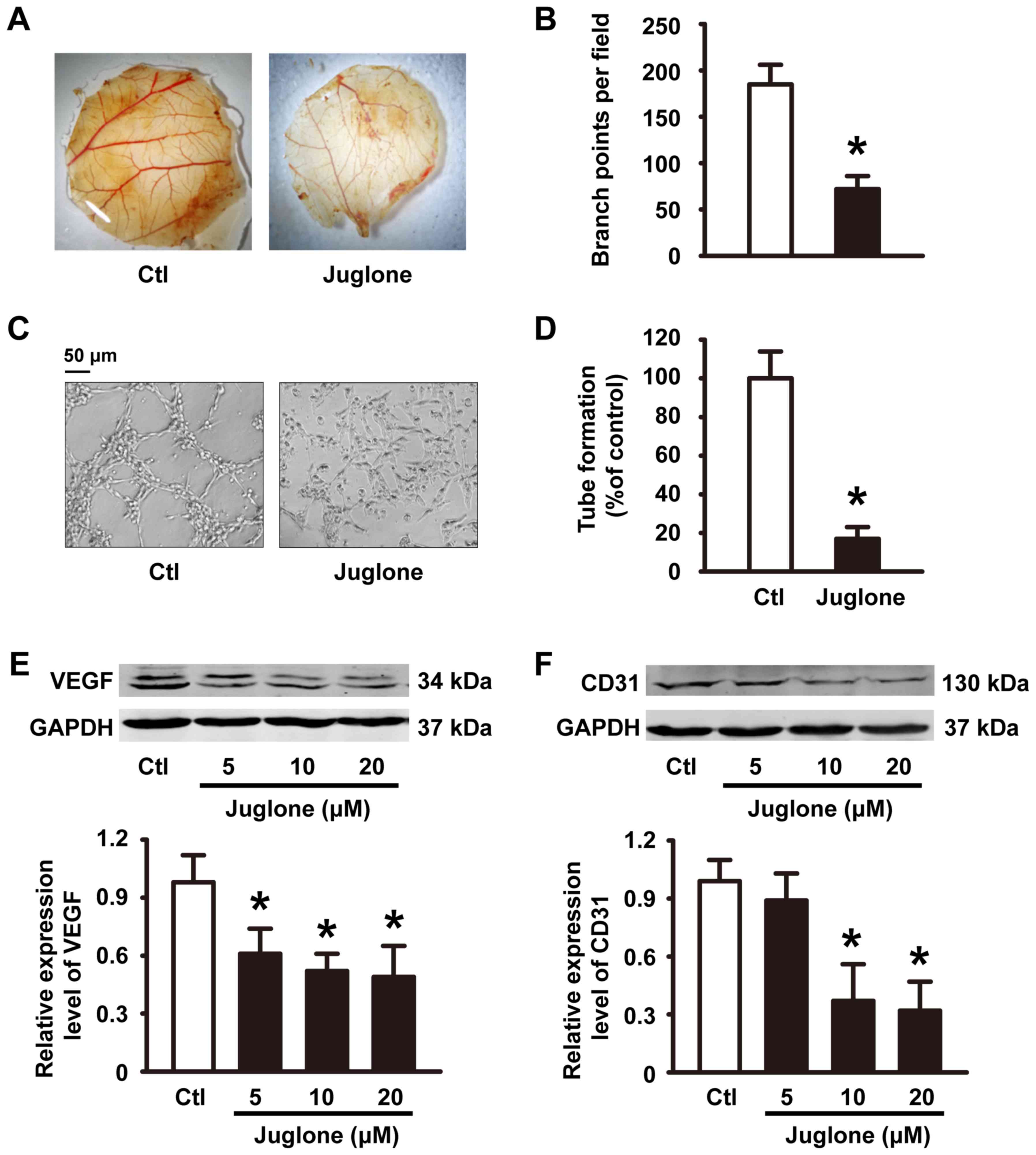
The anti-angiogenesis role of
juglone
Angiogenesis is a prerequisite for glioma tumor
growth (13). The sprouting of
endothelial cells and formation of tubes are crucial steps in the
angiogenic process (14). We
examined angiogenesis using the CAM assay. Compared with the
controls, incubation with 200 µg juglone directly inhibited
formation of new blood vessels in the CAM (Fig. 3A and B). Control HUVECs spread and
aligned with each other and formed a rich meshwork of branching
anastomosing capillary-like tubules with multicentric junctions.
This process was hardly influenced by 20 µM juglone (Fig. 3C and D). Moreover, vascular
endothelial growth factor (VEGF) and CD31, angiogenic markers, were
detected by western blotting. Our experiments showed that treatment
of HUVECs with juglone (0, 5, 10 and 20 µM) decreased VEGF and CD31
expression in a dose-dependent manner (Fig. 3E and F).
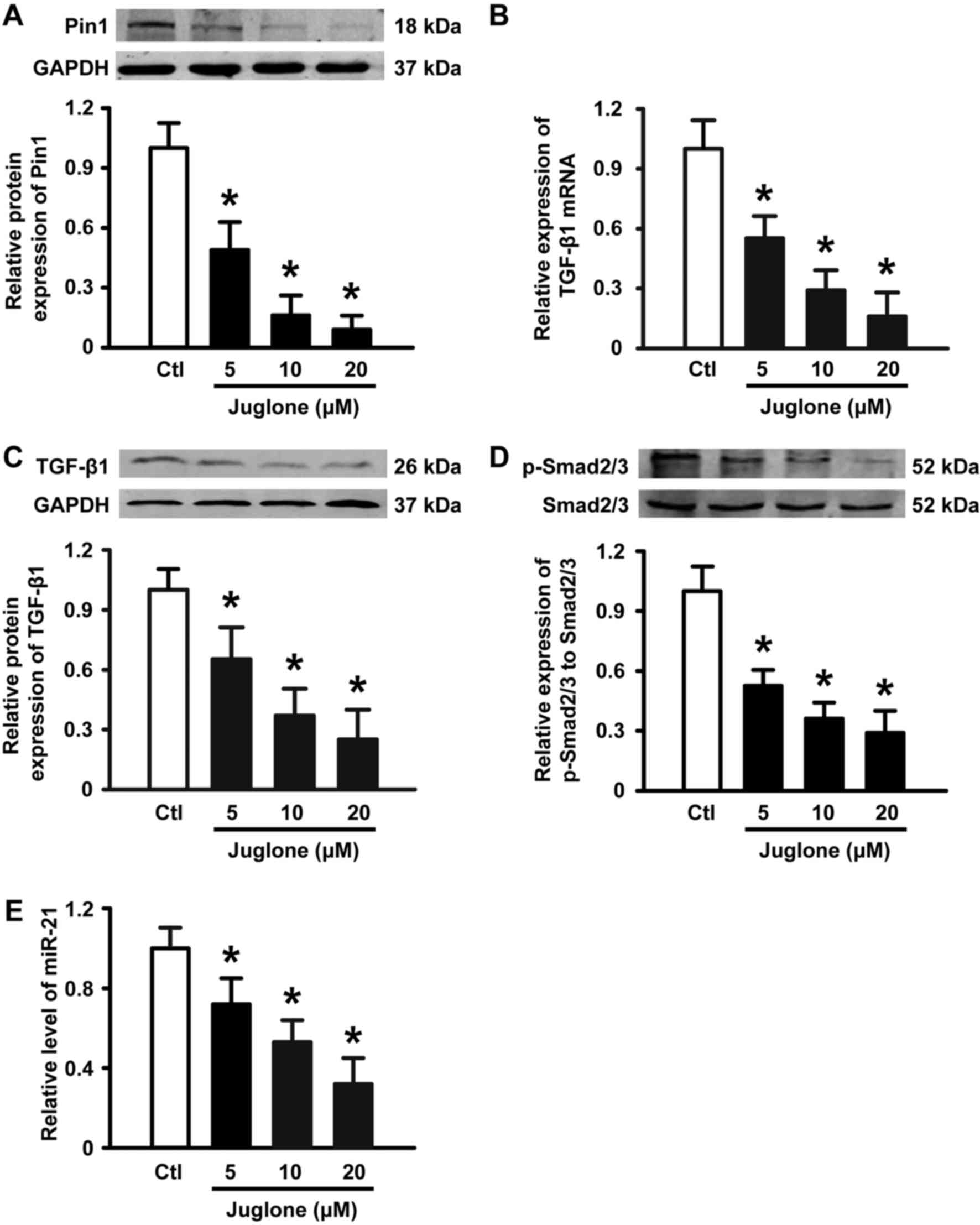
Juglone downregulates Pin1 expression
and inhibits the TGF-β1/Smad/miR-21 axis in U251 glioma cells
It has been suggested that Pin1 overexpression is
associated with the development of glioma. Next, we performed
western blotting to examine the expression of Pin1 in the absence
and presence of juglone. As illustrated in Fig. 4A, 5–20 µM juglone led to a gradual
decrease in Pin1 protein expression in a dose-dependent manner.
Previous studies have shown that the TGF-β/Smad/miR-21 axis is
implicated in glioma growth, migration and angiogenesis (15,16).
Importantly, Pin1 is positive regulator of TGF-β1 mRNA expression
(17,18) and the TGF-β signaling pathway has
been suggested to increase the level of miR-21 in a variety of cell
types (19,20). Thus, we tested the effects of
juglone on TGF-β, Smad2/3 and miR-21 expression in U251 glioma
cells. As expected, juglone significantly induced a decrease in
TGF-β1 mRNA (Fig. 4B) and protein
expression (Fig. 4C), and the
phosphorylation of its downstream signaling molecule Smad2/3
(Fig. 4D). Accordingly, qPCR
analysis revealed that miR-21, as a TGF-β downstream molecule, was
downregulated in a dose-dependent manner with juglone treatment
(Fig. 4E). These results suggest
that the Pin1-associated TGF-β1/Smad2/3/miR-21 axis may play a role
in juglone-mediated antitumor activity in cultured U251 glioma
cells.
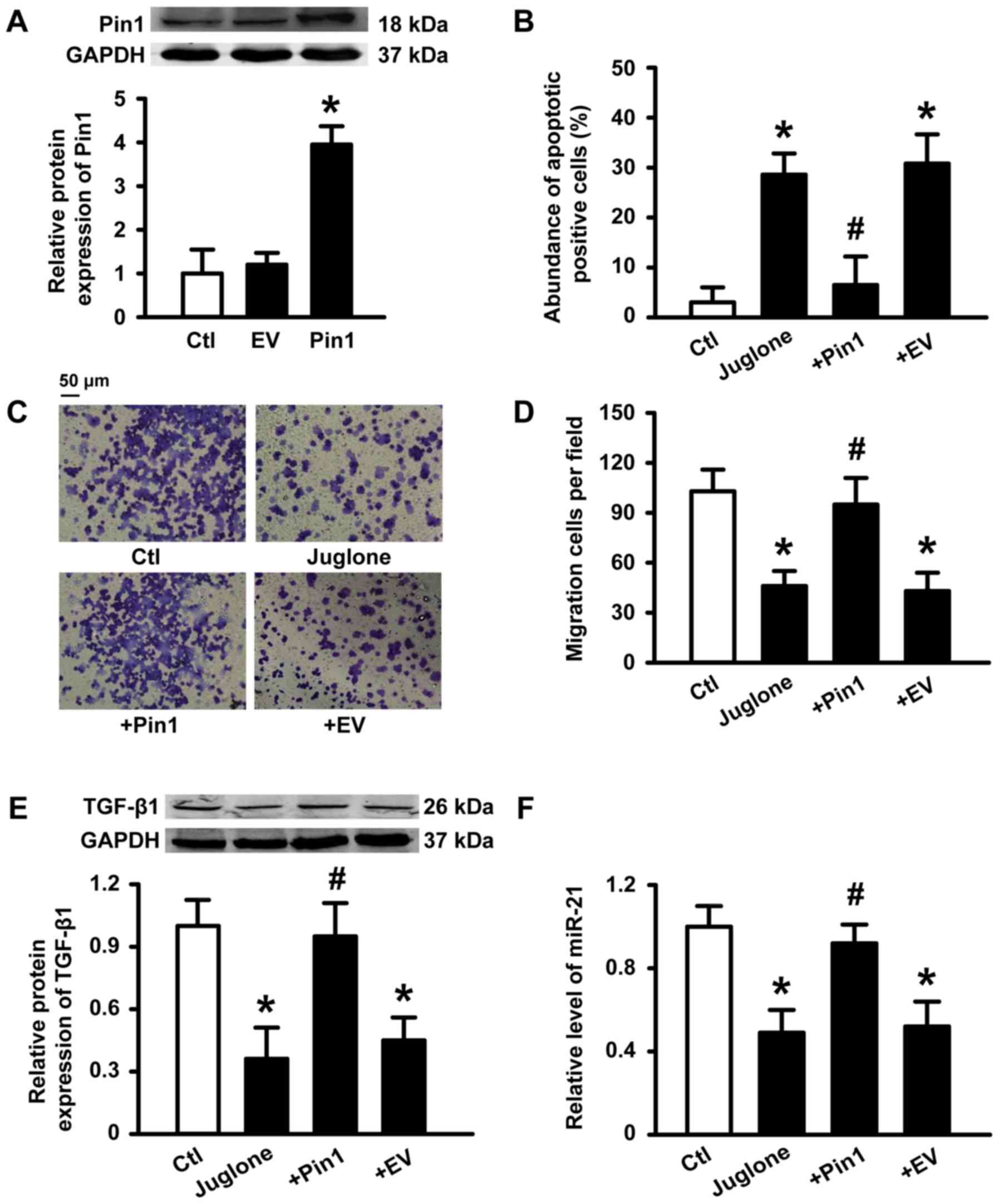
Transient overexpression of Pin1
prevents julgone-induced antitumor effects
To further determine if Pin1 plays a critical role
in the antitumor activity induced by juglone, we transfected human
Pin1 plasmid into U251 cells. Successful transfection of Pin1 was
verified (Fig. 5A). A statistical
graph of apoptosis levels as determined by AO/EB staining is shown
in Fig. 5B. Incubation with 10 µM
juglone for 48 h caused an increase in the number of apoptotic
cells compared to control cells. However, juglone-induced apoptosis
was inhibited by Pin1 overexpression. Similarly, Pin1
overexpression also reduced the ability of juglone to block U251
migration (Fig. 5C and D).
Accordingly, the inhibitory effects of juglone on TGF-β1 and miR-21
expression were also reversed by Pin1 overexpression (Fig. 5E and F). These results suggest that
Pin1 is a key target for juglone-mediated antitumor effects.
Discussion
The present study clearly demonstrates that juglone,
a Pin1 inhibitor, suppresses proliferation, induces apoptosis and
migration of U251 glioma cells, and exerts anti-angiogenesis
effects, thereby highlighting its therapeutic potential in glioma
disease. The antitumor mechanisms of this naturally-occurring
compound was associated with the downregulation of Pin1 expression
and inhibition of downstream signaling molecules including TGF-β1,
Smad2/3 and miR-21, suggesting that Pin1 inhibition may be an
attractive therapeutic target for glioma.
The unique role of Pin1 as a molecular switch that
impacts multiple downstream pathways necessitates the evaluation of
a highly specific Pin1 inhibitor to aid in potential therapeutic
drug discovery. Recent data have indicated that juglone may be a
useful antitumor agent for some cancer types. Accumulating data
have indicated that juglone inhibits tumor cell growth through
multiple functions such as cytotoxicity, induction of apoptosis and
prevention of angiogenesis. For instance, Hu et al (11) revealed that juglone can effectively
inhibit the proliferation, migration and angiogenic ability of
MCF7Adr cells. Moreover, Fang et al (21) found that juglone induces apoptosis
via the mitochondrial pathway and reduces cell invasiveness by
decreasing matrix metalloproteinase (MMP) expression. Avcı et
al (22) demonstrated that
juglone inhibits cell invasion and metastasis in a pancreatic
cancer line. A report by Jha et al (23) indicated that juglone induces cell
death of Acanthamoeba through increased production of
reactive oxygen species. In the present study, we found that
juglone (5–20 µM) decreased the viability of cultured U251 glioma
cells in a concentration- and time-dependent manner, which was
accompanied by apoptosis, inhibition of cell migration and
anti-angiogenesis activity. These results are consistent with
previously published results showing that juglone inhibits the
survival of many types of cultured cancer cells.
Many studies have shown that Pin1 is a major
signaling molecule involved in the regulation of cellular
proliferation, apoptosis, migration and angiogenesis (4). Notably, some studies have also shown
that it is significantly overexpressed in glioma and correlates
with malignant tumor progression and resistance to chemotherapy.
For example, a report by Atabay et al (24) indicated that knockdown of Pin1
decreased the levels of VEGF and MMP-9 and reduced the angiogenic
potential and tumorigenicity of glioblastoma cells. Similarly, Ryo
et al (25) demonstrated
that the targeted inhibition of Pin1 by small interfering RNA in
A172 glioblastoma cells significantly enhanced the apoptotic
response induced by hydrogen peroxide. These studies suggest that
juglone may have chemotherapeutic potential in glioblastoma. To
explain these observations at the molecular level, we evaluated the
effects of juglone on Pin1 expression, and found that juglone
reduced Pin1 protein expression in a dose-dependent manner,
consistent with previous investigations. Shen et al
(18) confirmed that Pin1 promotes
the stability of TGF-β1 mRNA by modulating the RNA-binding protein
including AUF1, HuR and TIA-1 interaction with TGF-β1 mRNA.
Accordingly, in this context, after 24 h of juglone treatment,
TGF-β1 mRNA and protein levels were significantly reduced. These
phenomena suggest that juglone can inhibit TGF-β expression,
probably due to its inhibitory effects on Pin1 activity.
In addition to tumor cells, juglone also affects
other cells because Pin-1 has a key role in the regulation of many
cell processes. For example, juglone can cause cell death in human
fibroblasts (26), stimulate
suicidal erythrocyte death or eryptosis (27), promote skin cell migration (9), alleviate myocardial fibrosis (28) and suppress cell adhesion to
endothelial cells (29). In
addition, juglone can also exert its effects in a Pin-1-independent
manner, as was shown in a unilateral ureteral obstruction rat model
in which juglone attenuated fibrogenesis. These antifibrotic
effects may have resulted from the inhibition of Smad2 and
oxidative stress (28).
It is generally recognized that miR-21 is a critical
regulator for promoting the progression of malignant glioma. Recent
results have indicated that it is upregulated in glioma vessels and
subsets of glioma cells and exerts its anti-apoptotic effects by
regulating programmed cell death (31). Hermansen et al (32) found that miR-21 co-localizes with
the angiogenesis markers, HIF-1α and VEGF, suggesting that it is
correlated with glioma angiogenesis. We also confirmed that both
VEGF and CD31 expression was downregulated in HUVECs upon exposure
to juglone. Notably, Smad2/3, a downstream component of the TGF-β
signaling pathway, also controls DROSHA (also known as RNASEN)
complex-mediated miR-21 maturation (19). Juglone also causes the
downregulation of phospho-Smad2/3 protein expression. Notably, a
report by Yang et al (17)
demonstrated that TGF-β1-induced Smad2/3 phosphorylation is
inhibited by Pin1 knockdown, indicating that decreased miR-21
expression induced by juglone might be due to inhibition of
Pin1-related TGF-β signaling. Most importantly, the transient
overexpression of Pin1 inhibited the antitumor effects of juglone
on U251 cells and reversed changes in the TGF-β1/miR-21 signaling
pathway that were induced by juglone. These data demonstrate that
Pin1 plays a critical role in the antitumor effects of juglone.
In summary, the present study demonstrated that
juglone inhibits Pin1 expression, thereby blocking
TGF-β1/Smad/miR-21 signaling, suppressing U251 glioma cell growth
and migration, and disrupting angiogenesis. These findings support
the potential therapeutic effects of juglone in the treatment of
glioma, although in vivo studies in animal models are
needed.
References
|
1
|
Chen R, Cohen AL and Colman H: Targeted
therapeutics in patients with high-grade gliomas: past, present,
and future. Curr Treat Options Oncol. 17:422016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levin VA, Tonge PJ, Gallo JM, Birtwistle
MR, Dar AC, Iavarone A, Paddison PJ, Heffron TP, Elmquist WF,
Lachowicz JE, et al: CNS Anticancer Drug Discovery and Development
Conference White Paper. Neuro oncol. 17 Suppl 6:vi1–vi26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taal W, Bromberg JE and van den Bent MJ:
Chemotherapy in glioma. CNS Oncol. 4:179–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu Z and Hunter T: Prolyl isomerase Pin1
in cancer. Cell Res. 24:1033–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
La Montagna R, Caligiuri I, Giordano A and
Rizzolio F: Pin1 and nuclear receptors: A new language? J Cell
Physiol. 228:1799–1801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Atkinson GP, Nozell SE, Harrison DK,
Stonecypher MS, Chen D and Benveniste EN: The prolyl isomerase Pin1
regulates the NF-kappaB signaling pathway and interleukin-8
expression in glioblastoma. Oncogene. 28:3735–3745. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Niu CS and Cheng CD: Pin1-Nanog
expression in human glioma is correlated with advanced tumor
progression. Oncol Rep. 30:560–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costantino S, Paneni F, Lüscher TF and
Cosentino F: Pin1 inhibitor Juglone prevents diabetic vascular
dysfunction. Int J Cardiol. 203:702–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wahedi HM, Park YU, Moon EY and Kim SY:
Juglone ameliorates skin wound healing by promoting skin cell
migration through Rac1/Cdc42/PAK pathway. Wound Repair Regen.
24:786–794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Cheng Y, Wu R, Jiang D, Bai B, Tan
D, Yan T, Sun X, Zhang Q and Wu Z: Antibacterial activity of
Juglone against Staphylococcus aureus: From apparent to
proteomic. Int J Mol Sci. 17:pii: E965. doi:
10.3390/ijms17060965.
|
|
11
|
Hu YG, Shen YF and Li Y: Effect of Pin1
inhibitor juglone on proliferation, migration and angiogenic
ability of breast cancer cell line MCF7Adr. J Huazhong Univ Sci
Technolog Med Sci. 35:531–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanaoka R, Kushiyama A, Seno Y, Nakatsu Y,
Matsunaga Y, Fukushima T, Tsuchiya Y, Sakoda H, Fujishiro M,
Yamamotoya T, et al: Pin1 inhibitor juglone exerts anti-oncogenic
effects on LNCaP and DU145 cells despite the patterns of gene
regulation by Pin1 differing between these cell lines. PLoS One.
10:e01274672015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schnoor R, Maas SL and Broekman ML:
Heparin in malignant glioma: Review of preclinical studies and
clinical results. J Neurooncol. 124:151–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Rhun E and Perry JR: Vascular
complications in glioma patients. Handb Clin Neurol. 134:251–266.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu J, Cai X, He J, Zhao W, Wang Q and Liu
B: Microarray-based analysis of gene regulation by transcription
factors and microRNAs in glioma. Neurol Sci. 34:1283–1289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han J, Alvarez-Breckenridge CA, Wang QE
and Yu J: TGF-β signaling and its targeting for glioma treatment.
Am J Cancer Res. 5:945–955. 2015.PubMed/NCBI
|
|
17
|
Yang JW, Hien TT, Lim SC, Jun DW, Choi HS,
Yoon JH, Cho IJ and Kang KW: Pin1 induction in the fibrotic liver
and its roles in TGF-β1 expression and Smad2/3 phosphorylation. J
Hepatol. 60:1235–1241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen ZJ, Esnault S, Rosenthal LA, Szakaly
RJ, Sorkness RL, Westmark PR, Sandor M and Malter JS: Pin1
regulates TGF-beta1 production by activated human and murine
eosinophils and contributes to allergic lung fibrosis. J Clin
Invest. 118:479–490. 2008.PubMed/NCBI
|
|
19
|
Davis BN, Hilyard AC, Lagna G and Hata A:
SMAD proteins control DROSHA-mediated microRNA maturation. Nature.
454:56–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zavadil J, Narasimhan M, Blumenberg M and
Schneider RJ: Transforming growth factor-beta and microRNA:mRNA
regulatory networks in epithelial plasticity. Cells Tissues Organs.
185:157–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang F, Qin Y, Qi L, Fang Q, Zhao L, Chen
S, Li Q, Zhang D and Wang L: Juglone exerts antitumor effect in
ovarian cancer cells. Iran J Basic Med Sci. 18:544–548.
2015.PubMed/NCBI
|
|
22
|
Avcı E, Arıkoğlu H and Kaya Erkoç D:
Investigation of juglone effects on metastasis and angiogenesis in
pancreatic cancer cells. Gene. 588:74–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jha BK, Jung HJ, Seo I, Suh SI, Suh MH and
Baek WK: Juglone induces cell death of Acanthamoeba through
increased production of reactive oxygen species. Exp Parasitol.
159:100–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atabay KD, Yildiz MT, Avsar T, Karabay A
and Kiliç T: Knockdown of Pin1 leads to reduced angiogenic
potential and tumorigenicity in glioblastoma cells. Oncol Lett.
10:2385–2389. 2015.PubMed/NCBI
|
|
25
|
Ryo A, Hirai A, Nishi M, Liou YC, Perrem
K, Lin SC, Hirano H, Lee SW and Aoki I: A suppressive role of the
prolyl isomerase Pin1 in cellular apoptosis mediated by the
death-associated protein Daxx. J Biol Chem. 282:36671–36681. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paulsen MT and Ljungman M: The natural
toxin juglone causes degradation of p53 and induces rapid H2AX
phosphorylation and cell death in human fibroblasts. Toxicol Appl
Pharmacol. 209:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calabrò S, Alzoubi K, Bissinger R, Jilani
K, Faggio C and Lang F: Enhanced eryptosis following juglone
exposure. Basic Clin Pharmacol Toxicol. 116:460–467. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Liang E, Song X, Du Z, Zhang Y and
Zhao Y: Inhibition of Pin1 alleviates myocardial fibrosis and
dysfunction in STZ-induced diabetic mice. Biochem Biophys Res
Commun. 479:109–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu M, Yu P, Jiang H, Yang X, Zhao J, Zou
Y and Ge J: The essential role of Pin1 via NF-kappaB signaling in
vascular inflammation and atherosclerosis in ApoE−/−
mice. Int J Mol Sci. 18:6442017. View Article : Google Scholar :
|
|
30
|
Reese S, Vidyasagar A, Jacobson L, Acun Z,
Esnault S, Hullett D, Malter JS and Djamali A: The Pin 1 inhibitor
juglone attenuates kidney fibrogenesis via Pin 1-independent
mechanisms in the unilateral ureteral occlusion model. Fibrogenesis
Tissue Repair. 3:12010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang G, Wang JJ, Tang HM and To SS:
Targeting strategies on miRNA-21 and PDCD4 for glioblastoma. Arch
Biochem Biophys. 580:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hermansen SK, Nielsen BS, Aaberg-Jessen C
and Kristensen BW: miR-21 is linked to glioma angiogenesis: A
co-localization study. J Histochem Cytochem. 64:138–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|