Introduction
Glioma is the most frequent intracranial tumor in
adult humans (1). Despite numerous
clinical advances, the median survival time for high-grade glioma
remains poor (2), primarily due to
the invasive nature of glioma within the central nervous system
(CNS). Therefore, the mechanisms regulating the aggressive
characteristics of glioma are logical therapeutic targets.
The invasiveness of glioma cells is partly regulated
by the interaction of cells with the extracellular matrix (ECM),
followed by the degradation of the ECM by tumor cell-derived
proteases, particularly matrix metalloproteinases (MMPs) (3–6). MMPs
are the principal secreted proteinases required for ECM degradation
during tumor invasion and metastasis (7,8). The
overexpression of MMPs is well documented in malignant gliomas.
Among the MMPs, the present study focused on MMP2 and MMP9, which
are considered to enhance the invasion of glioma (9–11).
However, the molecular regulation of MMP2 and MMP9 in glioblastoma
has not been fully clarified.
The glioma microenvironment, including glial cells
and their inflammatory products, regulates tumor development and
progression (12). As a member of
the interleukin (IL)-1 superfamily of cytokines, IL-33 is a
multi-functional pro-inflammatory cytokine that is released upon
cell damage and serves as an ‘alarm’ (13). Live cells can also produce IL-33
under biomechanical stress conditions (14). IL-33 is initially generated as a
precursor protein that can be digested into a secreted and mature
form by caspase-1 (15). IL-33 can
bind to the receptor ST2 and induce the activation of various
signaling proteins, including p38, nuclear factor-κB (NF-κB), c-Jun
N-terminal kinase (JNK) and extracellular signal-regulated kinase
1/2 (ERK1/2), which promote the production of pro-inflammatory
cytokines, such as IL-1, IL-6, tumor necrosis factor (TNF) and
chemokine (C-C motif) ligand 2 (CCL2) (16–18).
In addition to the pro-inflammatory cytokines, the IL-33/ST2 axis
can also increase the expression of MMP2, MMP3 and MMP9 (19,20).
Recent studies have reported that a high level of IL-33 is a
diagnostic and prognostic marker for hepatocellular carcinoma and
non-small cell lung cancer (21,22).
The IL-33/ST2 axis can also facilitate gastric cancer migration and
invasion by inducing the secretion of IL-6 and MMP3 (20). Accumulating data indicate the
importance of the IL-33/ST2 axis in cancer growth. In the CNS,
IL-33 was reported to be highly expressed in mature
oligodendrocytes and gray matter astrocytes, and released at sites
of injury to promote recovery (23). More recently, it was reported that
IL-33 is highly expressed in glioma cells and facilitates cell
proliferation and migration via the regulation of growth factor and
chemokine expression (24).
Previously, we detected the overexpression of IL-33 in glioma
tissues compared with that in normal brain tissues, and identified
IL-33 overexpression as an independent factor that predicted a poor
prognosis in patients with glioma (25). However, the underlying molecular
mechanisms of the IL-33/ST2 axis during glioma development remain
to be elucidated.
In the present study, the effects of IL-33 on glioma
cell invasion and migration were investigated, and the underlying
mechanisms of its function were examined. The results revealed that
IL-33 and ST2 expression levels were positively correlated with the
tumor grade. In addition, IL-33 promoted glioma cell migration and
invasion via ST2. Furthermore, we found that the IL-33/ST2 axis
induced increases in MMP2 and MMP9 through activation of NF-κB
signaling. Collectively, these findings suggest that IL-33
increases glioma cell migration and invasion by stimulating the
secretion of MMP2 and MMP9 via the ST2-NF-κB pathway. Therefore,
IL-33 may be an effective therapeutic target for the treatment of
glioma.
Materials and methods
Cell culture, reagents and tumor
samples
U87 and U251 cell lines were cultivated in DMEM
(Gibco, UK) supplemented with 10% fetal bovine serum (FBS; Hyclone,
Logan, UT, USA) and 0.05% (v/v) gentamycin (Gibco) in petri dishes
at 37°C and 6% CO2. Recombinant human IL-33 was
purchased from R&D Systems (Minneapolis, MN, USA). Anti-IL-33
antibody (#ABF108) was purchased from Millipore (Billerica, MA,
USA), and anti-ST2 antibody (#ab25877) was purchased from Abcam
(Cambridge, MA, USA). A specific antibody against phospho-NF-κB
(#3033) was obtained from Cell Signaling Technology, Inc. (Beverly,
MA, USA). An inhibitor of the NF-κB signaling pathway (BAY 11–7082)
was obtained from Abcam.
Glioma tissue sections were obtained from the
Division of Neurosurgery, Wuxi Second People's Hospital Affiliated
to Nanjing Medical University (Wuxi, Jiangsu, China). The glioma
tissue collection was approved by the Institutional Review Board of
Wuxi Second People's Hospital Affiliated to Nanjing Medical
University. According to the WHO criteria, the pathological grades
of the glioma specimens were grouped into the following two
categories: low-grade (grades I–II; 18 cases); and high-grade
(grades III–IV; 28 cases). In addition, 15 tumor-adjacent brain
tissue samples and 16 normal brain tissue samples were included.
This study was approved by the Ethics Review Committee of Wuxi
Second People's Hospital Affiliated to Nanjing Medical University
(permission no.: WXEY2013-0218).
Immunohistochemistry
According to the standard method, all tumor tissue
sections were dewaxed. After preconditioning, slides were incubated
with IL-33 and ST2 primary antibodies at 4°C overnight. The slides
were subsequently incubated with secondary antibodies at 37°C for
30 min. Distinct cytoplasmic staining of ST2 and IL-33 were
considered to indicate positive immunoreactivity.
Immunofluorescent staining
The experimental procedure of immunofluorescent
staining was performed as previously described (26). Glioma tissues were acquired
following surgery and frozen immediately. The slices were incubated
with the anti-IL-33 antibody at 4°C overnight, followed by nuclear
counter staining with DAPI (1:5,000, Sigma). Images were
subsequently observed and photographed with a fluorescent
microscope (Nikon, Tokyo, Japan).
Migration assay
Cell migration was determined using Transwell
chambers (Corning Costar, San Diego, CA, USA). In brief, 200 µl of
cell suspension (1×105 cells) was added into the upper
wells, and the lower wells were filled with DMEM as a
chemoattractant. After incubation for 12 h at 37°C, cells on the
top surface were removed with a cotton swab, and cells on the lower
surface were fixed in methanol, stained with crystal violet and
counted under the microscope. Five predetermined fields were
counted for each membrane, and the mean values from three
independent experiments, performed in duplicate, were used. The
data were presented as the mean ± standard deviation.
Invasion assay
Cell invasion was also performed using Transwell
chambers. Briefly, 1×105 cells in 0.2 ml of serum-free
media were seeded into the upper chambers of Matrigel-coated
filters. The lower chambers were filled with 0.5 ml of complete
medium as a chemoattractant. After incubation at 37°C for 12 h,
cells in the upper chamber were removed with a cotton swab, and
cells on the lower side of the chamber were fixed with methanol,
stained with crystal violet, and counted under the microscope. The
mean values from three independent experiments performed in
duplicate were used.
Transfection of siRNA
Cells were transfected with siRNA using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The ST2 siRNA
was designed and obtained from Invitrogen with the following
sequence: 5′-CCAGAAAGGCCUCUAGUUUUU-3′. For transfection with ST2
siRNA, cells were plated in 12-well plates. At 6 h after
transfection, the medium was removed and replaced with fresh medium
supplemented with 10% FBS. Cells were further incubated for 24
h.
Western blot analyses
An equal amount of protein from each protein sample
was loaded onto an SDS-PAGE gel, separated by electrophoresis, and
transferred to a PVDF membrane (Bio-Rad, Hercules, CA, USA). The
membranes were blocked for 1 h in 1% milk (Bio-Rad) in PBST (0.1%
Tween-20 in PBS), then incubated with the primary antibodies
overnight at 4°C. After three washes with PBST, the membranes were
incubated with peroxidase-conjugated secondary IgG (H+L) antibodies
for 1 h at room temperature and washed three times with PBST. The
bands were then detected using Supersignal West Pico
chemiluminescent substrate (Thermo Scientific).
Enzyme-linked immunosorbent assay for
the determination of MMP2 and MMP9
Cell supernatant was centrifuged at 12,000 g for 15
min at 4°C. MMP2 ELISA kits (RAB0365; Sigma, USA) and MMP9 ELISA
kits (RAB0372; Sigma, Nanjing, China) were used to measure the
levels of protein secretion of MMP2 and MMP9 by glioma cells,
respectively, according to the manufacturer's instructions.
Statistical analysis
All data were presented as the mean ± standard
deviation. A Student's t-test was used to assess the statistical
differences between two groups, and statistical differences among
multiple groups were calculated by one-way analysis of variance.
All data were analyzed using the statistical package GraphPad Prism
version 5.0 (GraphPad Software Inc., La Jolla, CA, USA). p<0.05,
p<0.01 and p<0.001 were considered statistically significant.
All experiments were repeated at least three times.
Results
IL-33 and ST2 levels are elevated in
glioma tissues and are associated with glioma grade
To determine the role of the IL-33/ST2 pathway in
glioma tumorigenesis, we performed an immunohistochemical analysis
of IL-33 and ST2 expression on samples of 15 tumor-adjacent normal
brain tissues, 16 normal brain tissues, 18 low-grade glioma tissues
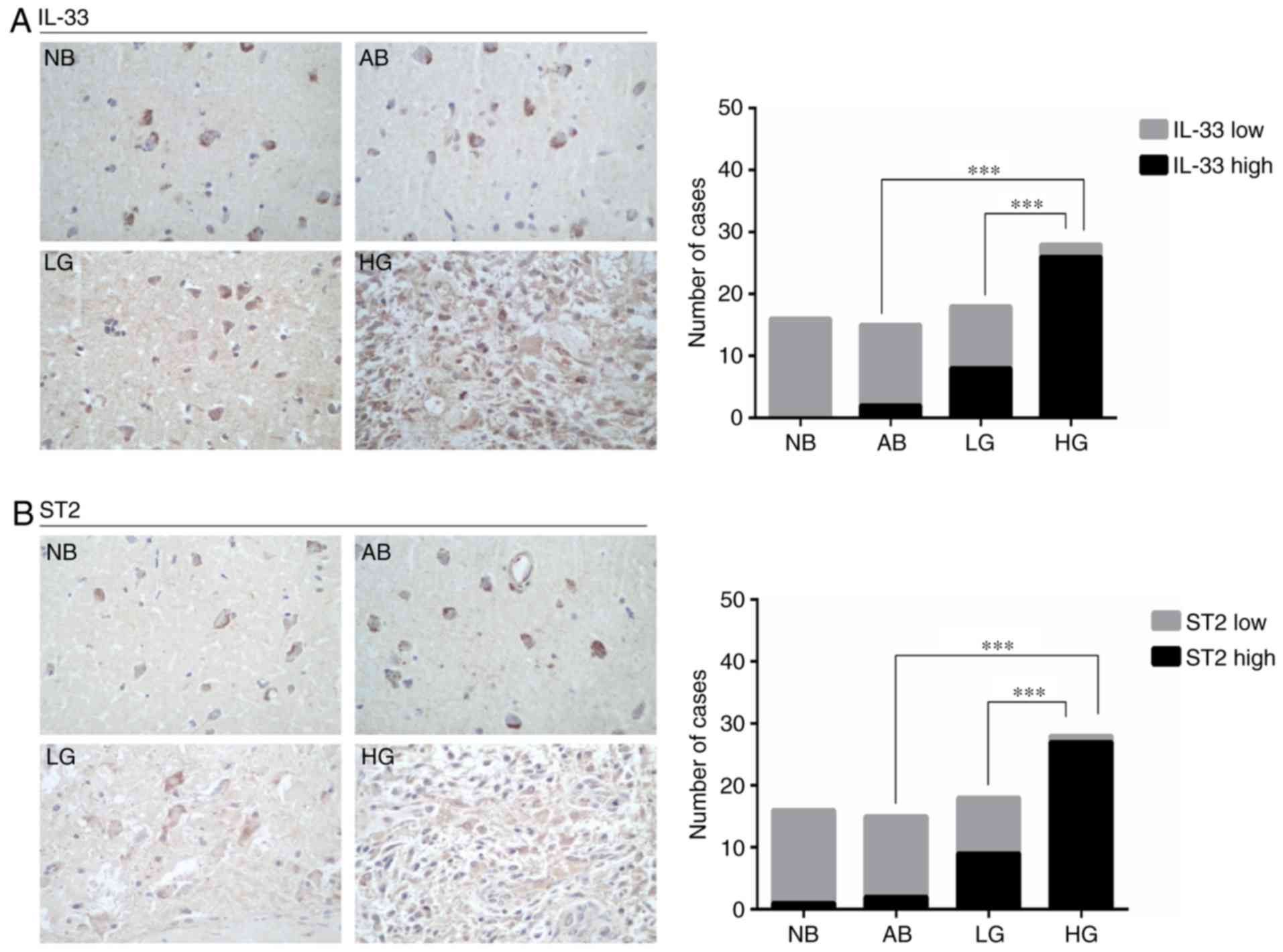
and 28 high-grade glioma tissues. As shown in Fig. 1A, low IL-33 expression was observed
in all 16 normal brain specimens and in 13 of the 15 tumor-adjacent
normal brain specimens, whereas 8 of the 18 low-grade glioma
specimens and 26 of the 28 high-grade glioma tissues expressed high
levels of IL-33. ST2 expression was positively correlated with
IL-33 expression (Fig. 1B).
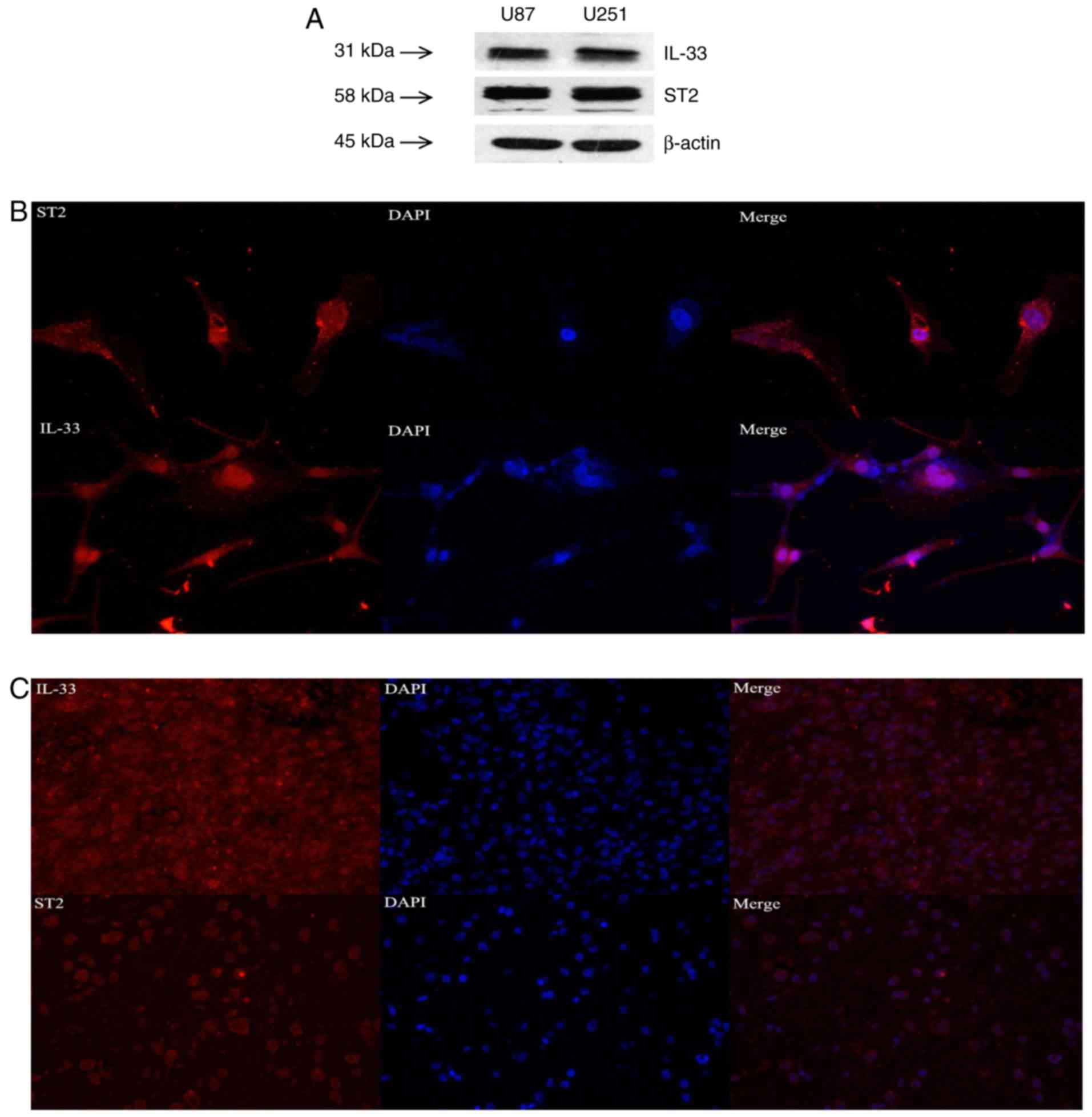
Moreover, IL-33 and ST2 proteins were detected in U251 and U87
cells by western blot analysis (Fig.
2A), and immunofluorescent staining revealed that IL-33 and ST2
were widely expressed in U87 glioma cells and glioma tissues
(Fig. 2B and C). Collectively,
these results indicate that the expression levels of IL-33 and ST2
receptor in glioma tissues are higher than those in normal tissues,
and that their expression levels are positively correlated with
glioma grade.
IL-33 facilitates the invasion and
migration of glioma cells in vitro
The association between IL-33/ST2 expression levels
and glioma grade suggested that IL-33/ST2 may be important in
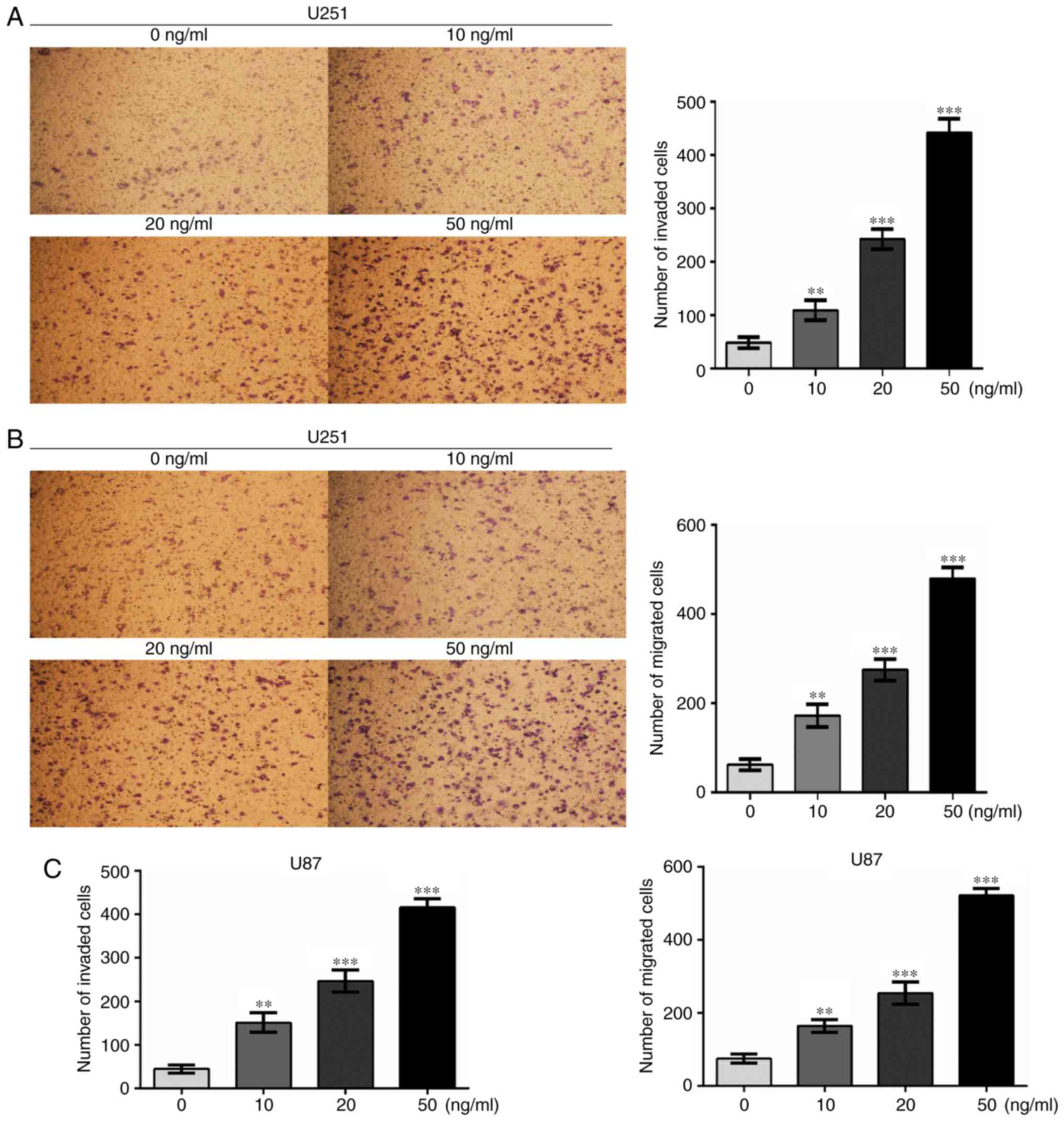
migration and invasion. To examine the effects of IL-33 on cell
invasion and migration, Transwell assays were performed. Glioma
cells were treated with various concentrations of recombinant human
IL-33 (0, 10, 20 and 50 ng/ml), then subjected to invasion and
migration assays. As shown in Fig.
3, IL-33 stimulation promoted the invasion and migration of
U251 and U87 cells in a dose-dependent manner. These results
illustrate that IL-33 may be involved in glioma cell invasion and
migration. U251 cells were selected for subsequent study.
ST2 mediates the invasion and
migration of glioma cells induced by IL-33
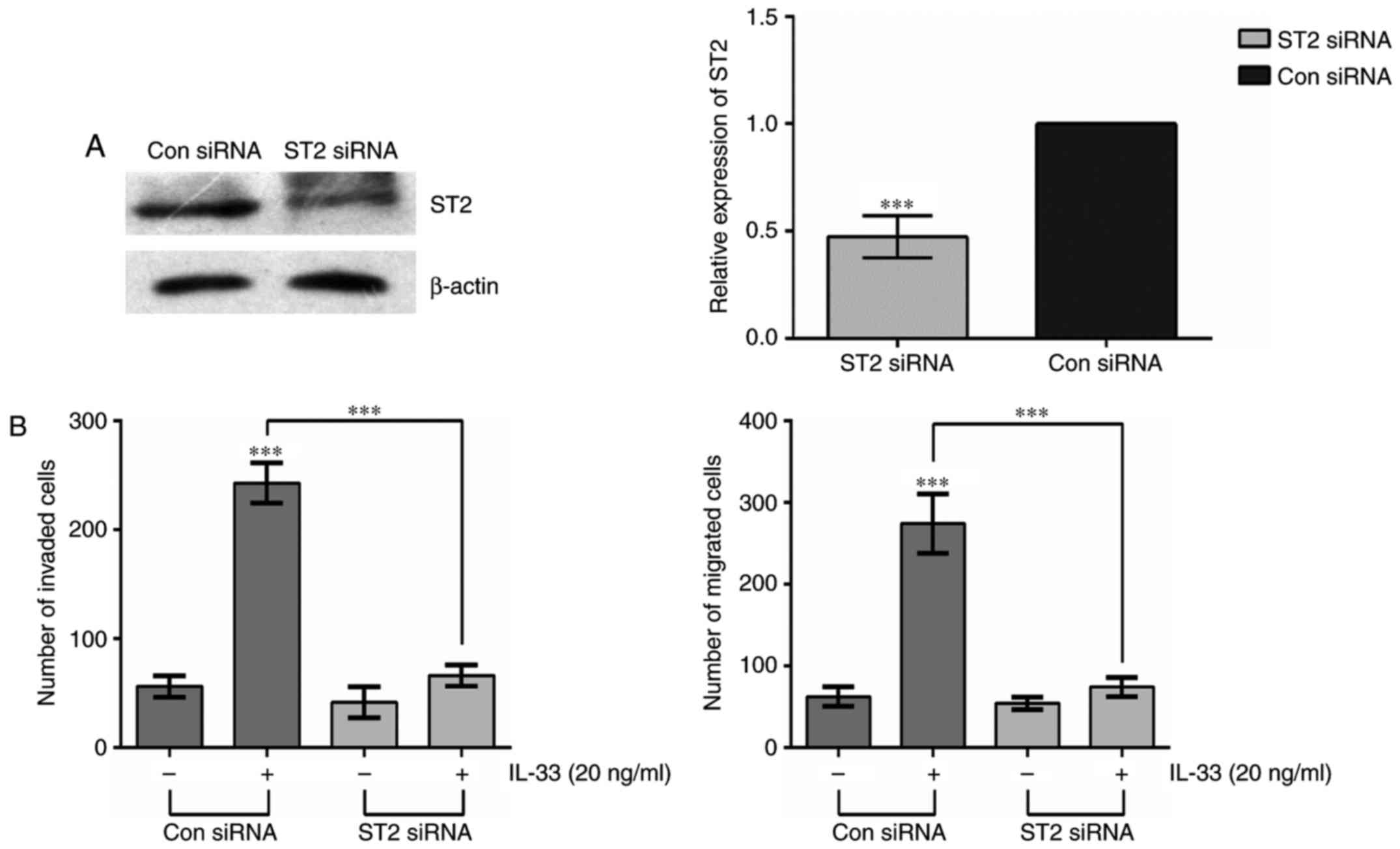
In order to investigate the relationship between ST2
and IL-33-mediated invasion and migration in glioma cells, siRNA
technology was used. U251 glioma cells were transiently transfected
with ST2 siRNA or control siRNA (Con siRNA). Compared with Con
siRNA-transfected cells, the expression of ST2 was dramatically
inhibited in ST2 siRNA-transfected cells (Fig. 4A). Furthermore, the cells
transfected with ST2 siRNA exhibited a distinct reduction in
IL-33-induced invasion and migration compared with Con siRNA cells
(Fig. 4B). These data suggest that
ST2 plays an important role in IL-33-induced cell invasion and
migration.
IL-33 upregulates the expression of
MMP2 and MMP9 in glioma cells via the IL-33/ST2 pathway
IL-33 stimulation can induce the expression of MMPs
(19,20). In the present study, the expression
levels of MMP2 and MMP9 were detected in glioma cells by ELISA
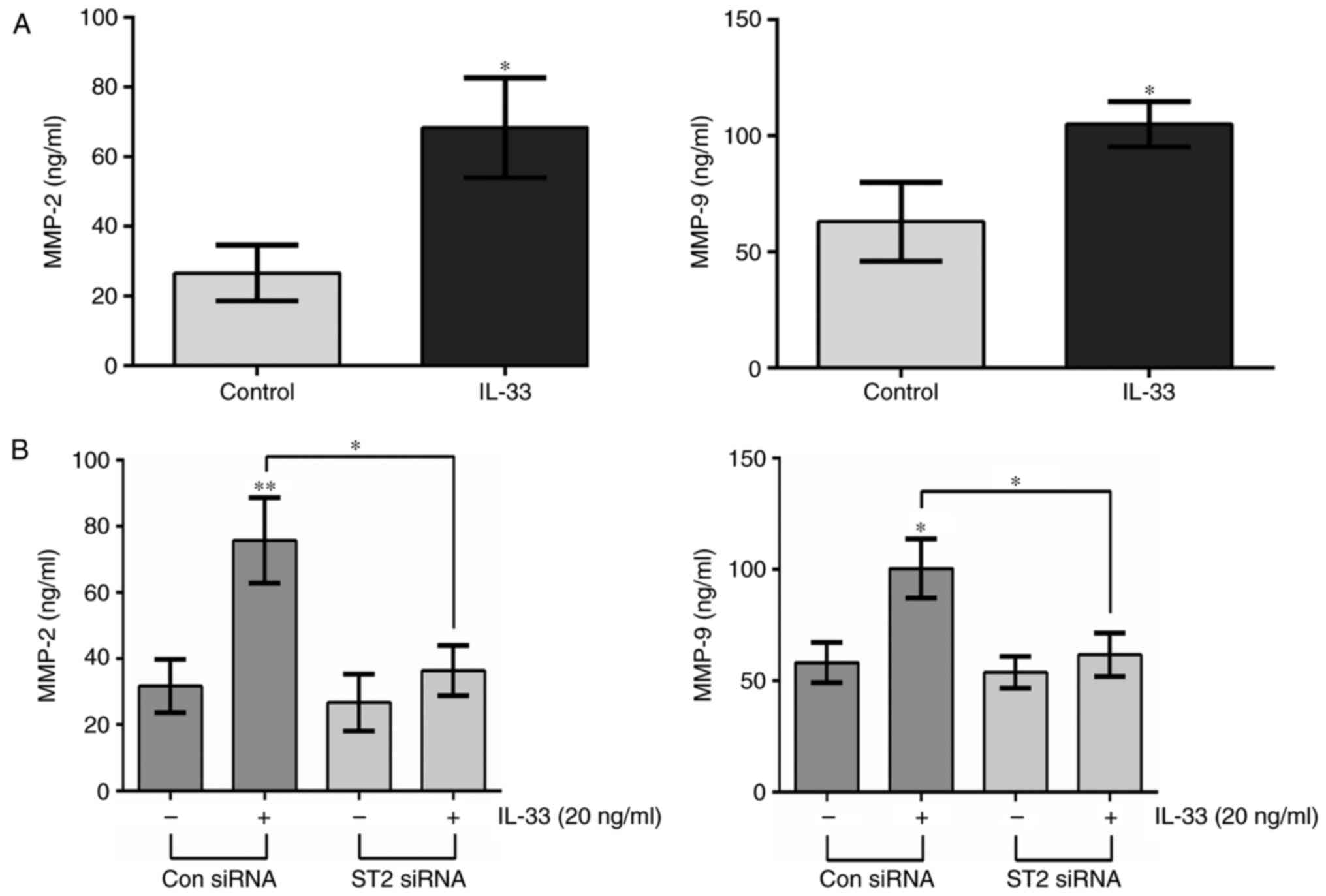
following IL-33 treatment. The data indicated that IL-33 treatment
led to significant increases in MMP2 and MMP9 levels in the culture
supernatant of U251 cells (Fig.
5A). Furthermore, to determine the involvement of ST2 in
IL-33-stimulated secretion of MMP2 and MMP9, the protein levels of
MMP2 and MMP9 in ST2-knockdown cells were examined after IL-33
stimulation for 20 h, which illustrated that downregulation of ST2
decreased the expression of MMP2 and MMP9 compared with IL-33
stimulation alone (Fig. 5B). These
results suggested that IL-33-mediated expression of MMP2 and MMP9
may depend on the IL-33/ST2 pathway.
IL-33 induces activation of the NF-κB
pathway via the ST2 receptor
IL-33 can activate various signaling pathways,
including NF-κB, PI3K/AKT and MAPK/ERK, of which NF-κB was of
particular interest in the present study. Recent studies have
demonstrated that IL-33 can activate an NF-κB kinase, leading to
NF-κB phosphorylation, and drive the production of Th2 cytokines
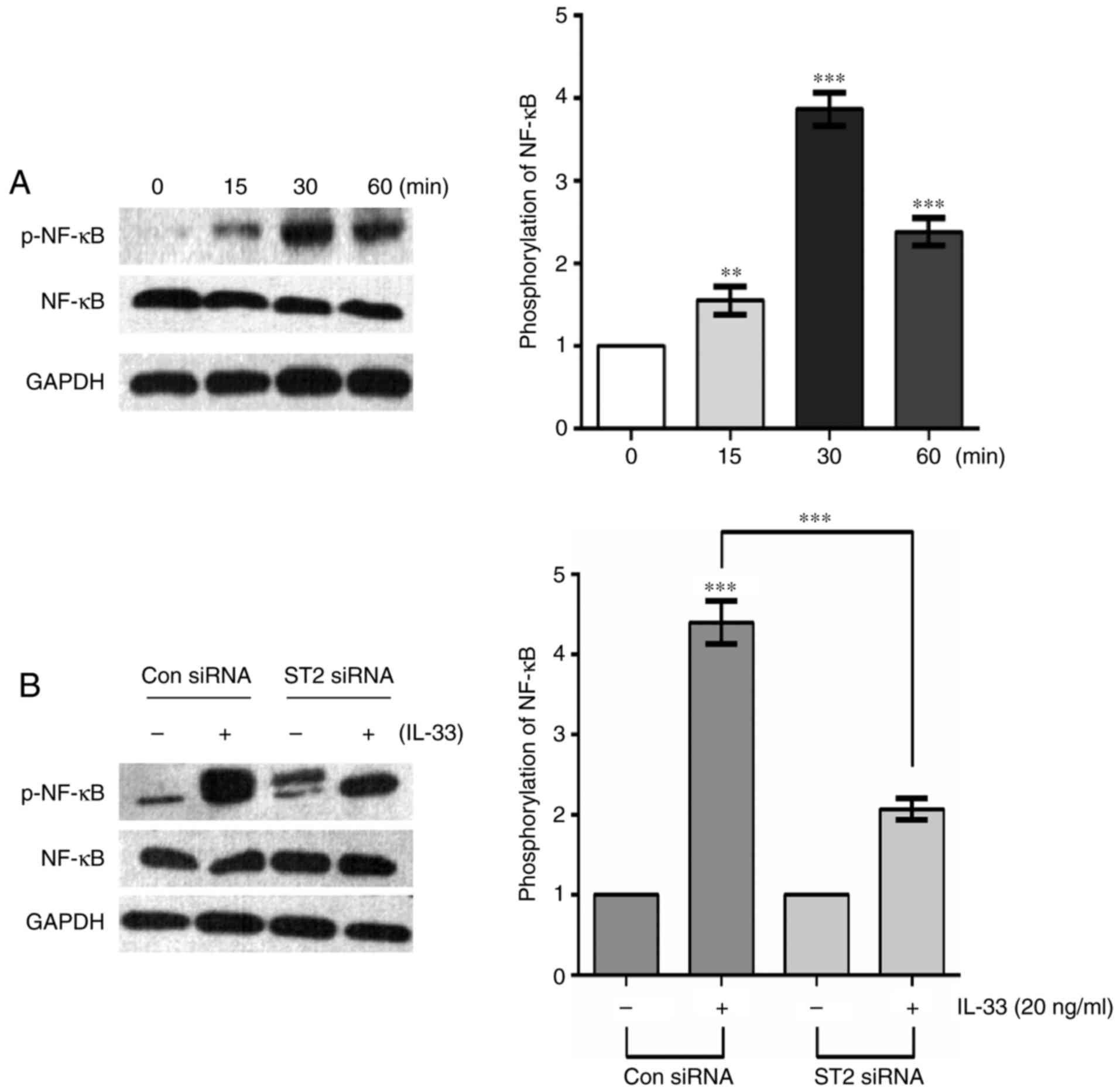
via the ST2 receptor (15,27). To investigate whether IL-33 may
induce NF-κB activation in glioma cells, U251 cells were treated
with 20 ng/ml IL-33 for various durations. Western blot analysis
indicated that IL-33 stimulation time-dependently enhanced the
phosphorylation of NF-κB, with peak phosphorylation occurring at 30
min and decreasing levels at 60 min (Fig. 6A). Moreover, IL-33-induced NF-κB
kinase activation was significantly repressed in ST2
siRNA-transfected cells (Fig. 6B).
These results indicate that IL-33/ST2 markedly induces the
activation of NF-κB in glioma cells.
Effects of NF-κB pathway on
IL-33-mediated glioma cell invasion, migration and secretion of
MMP2 and MMP9
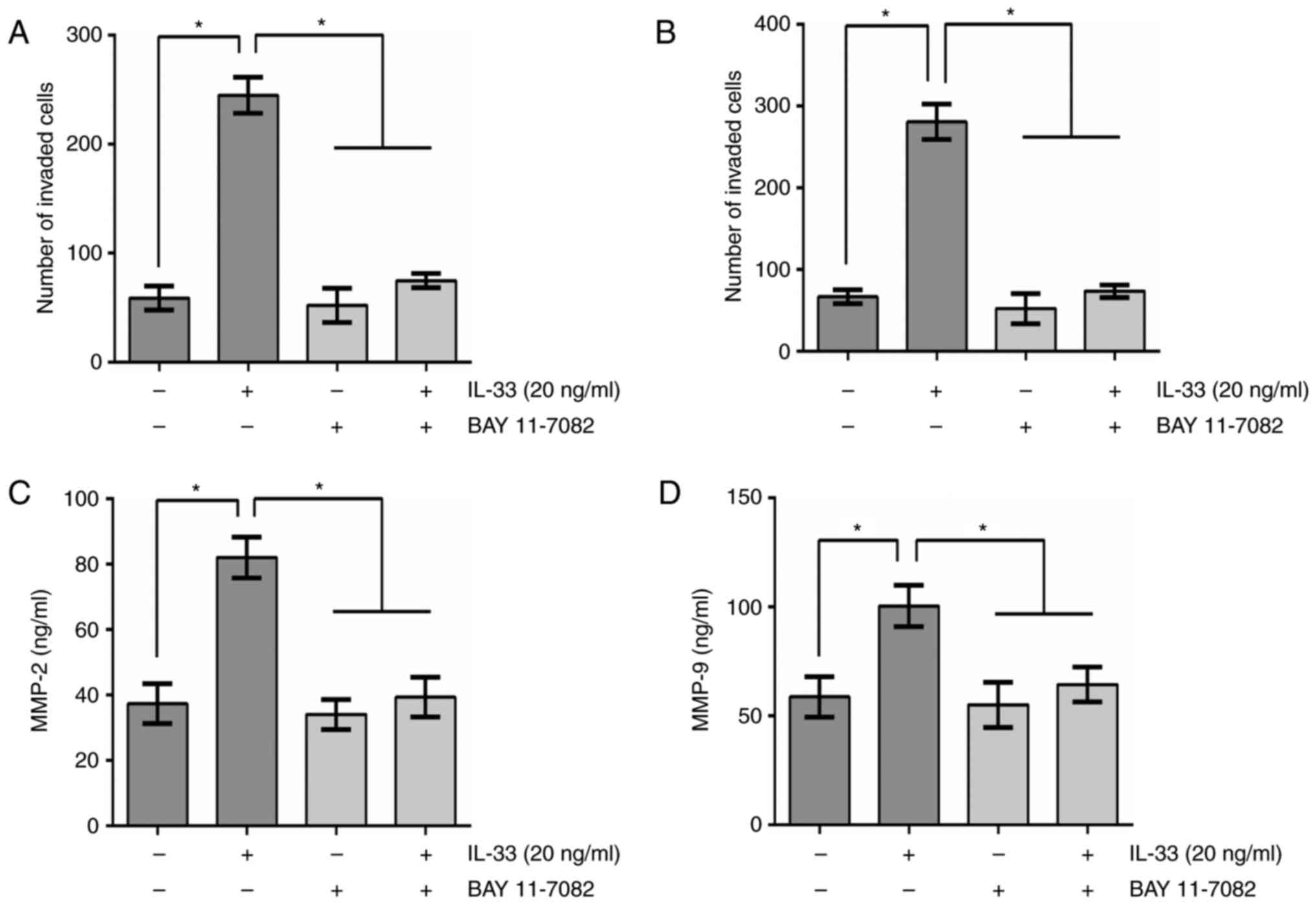
To further elucidate the involvement of NF-κB
signaling in the stimulation of MMP2 and MMP9 expression in glioma
cells, the effect of a specific signal transduction inhibitor on
glioma cells was examined. U251 cells were pretreated with BAY
11–7082 (an NF-κB-specific inhibitor) for 30 min prior to IL-33
stimulation. Invasion and migration assays revealed that BAY
11–7082 suppressed IL-33-induced increases in the invasion and
migration of U251 cells, indicating that the NF-κB pathway is
required for the induction of invasion and migration by IL-33
(Fig. 7A and B). Furthermore, ELISA
revealed that BAY 11–7082 decreased IL-induced secretion of MMP2
and MMP9, suggesting an involvement of the NF-κB pathway in
regulating IL-33-induced secretion of MMP2 and MMP9 (Fig. 7C and D).
Discussion
The tumor microenvironment serves a crucial role in
tumor development and progression (28). The tumor microenvironment consists
of a variable combination of tumor cells, infiltrating leukocytes
and endothelial cells, which produce cytokines and other substances
that drive tumor development (29,30).
As a member of the IL-1 cytokine family, IL-33 has been shown to be
involved in the progression and development of cancer (31). Elevated expression of IL-33 has been
found in many types of tumors, including human colorectal, gastric
and breast cancers (19,20,32).
Further studies have demonstrated that IL-33 increases the
migration and invasion of gastric carcinoma (20). In the present study, the
overexpression of IL-33 and ST2 was observed in glioma tissues.
Importantly, we found that IL-33 can enhance the invasion and
migration of glioma cell lines. These results suggest that the
IL-33/ST2 signaling pathway may play an important role in glioma
cells.
IL-33 acts via its receptor, ST2. Asa member of the
IL-1 receptor family, ST2 has two types: a soluble secreted form
(sST2) and a transmembrane, full-length form (ST2L) (33). Certain clinical studies have
confirmed that serum sST2 is associated with the progression of
hepatocellular carcinoma (21).
Moreover, activation of ST2 by IL-33 enhances the metastasis of
breast cancer (34). Recently, Fang
et al (24) found that IL-33
was a crucial factor in tumorigenic glioma C6 cells, and inhibition
of IL-33 gene expression suppressed the growth rate and colony
formation of C6 cells. Furthermore, IL-33 was associated with C6
cell migration and the regulation of the expression of several
chemokines and growth factors (24). In accordance with previous reports,
the data from the present study demonstrated that IL-33 promoted
the migration and invasion of glioma cells, while ST2 knockdown
attenuated this effect, further verifying the involvement of ST2 in
cancer.
Aside from its roles in promoting cancer
progression, IL-33 has been shown to be associated with antitumor
immune responses during cancer development (8,31,35,36).
In animal models, IL-33 could activate CD8+ T and
natural killer (NK) cells, and inhibit the progression and
migration of tumors (8,36). Conversely, other studies suggested
that IL-33 could enhance type-2 immune responses, suppress NK cells
and promote tumor development in tumor-bearing animals (34,37–40).
These data suggest that IL-33 may have both pro-tumor and antitumor
effects during the development of different tumors.
As a multi-functional pro-inflammatory cytokine,
IL-33 stimulation can induce the activation of various signaling
pathways, including the PI3K/AKT, ERK1/2, and MAPK pathways, and
this has been shown to be involved in the progression and
development of cancer (20,41). NF-κB is a key factor in inflammation
and innate immunity. A recent study showed that IL-33 could induce
the activation of the NF-κB pathway to enhance the invasiveness of
decidual stromal cells via the upregulation of CCL2/CCR2 (17). Another study showed that NF-κB
activation could mediate the effect of IL-33 on cytokine production
in pancreatic carcinoma cells (42). Concordantly, the results from the
present study demonstrated that IL-33 activates the ST2/NF-κB
pathway, which contributes to glioma cell motility and
invasion.
Although growing evidence indicates a role for IL-33
in tumor progression, the potential underlying mechanism by which
IL-33 facilitates cancer cell growth and invasion remains unclear.
Glioma invasiveness is mediated in part by MMPs (43–45).
Secreted proteases, such as MMP2, MMP7, MMP9, can digest the ECM,
basal lamina, and cell adhesion proteins, thereby promoting cell
invasion and tumor growth (3,9). IL-33
stimulation can induce a train of pro-destructive molecules,
including IL-6, CXCR4, MMP2, MMP3 and MMP9. In the present study,
the results demonstrated that IL-33 induced the expression of MMP2
and MMP9 in glioma cells, whereas the silencing of ST2 or
inhibition of the NF-κB pathway could strongly suppress the
IL-33-induced expression of MMP2 and MMP9, indicating that the
ST2-NF-κB pathway is required for IL-33-mediated MMP2 and MMP9
production. As MMP2 and MMP9 are necessary for tumor invasion and
migration, it is possible that IL-33 facilitates glioma cell
invasion and migration via upregulating MMP2 and MMP9
production.
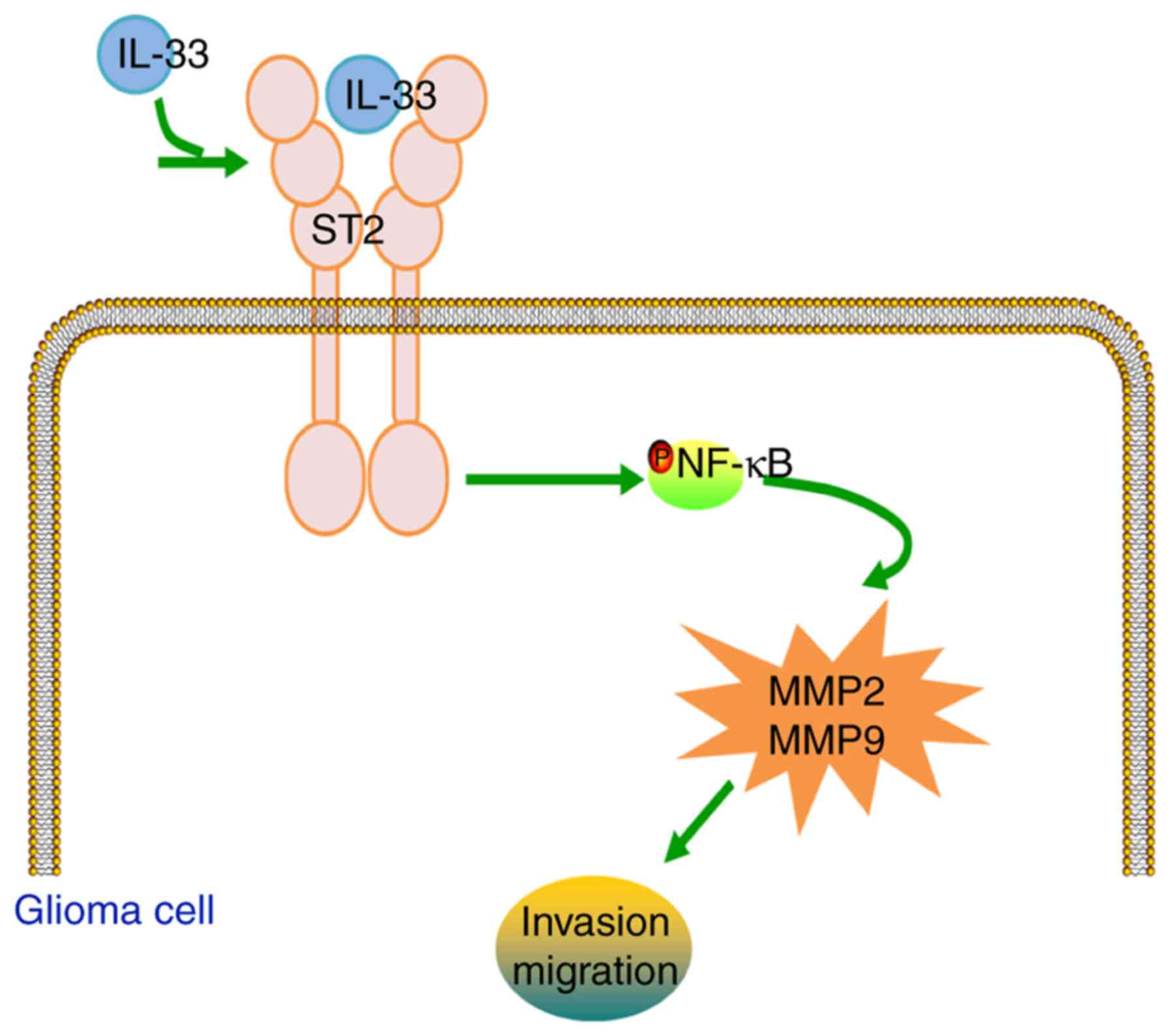
In conclusion, the present data demonstrate that
IL-33 stimulation facilitates the invasion and migration of glioma
cells through the ST2-NF-κB pathway in vitro. Activation of
the ST2-NF-κB pathway, and subsequently of MMP2 and MMP9
production, may potentiate the biological effects of IL-33 on
glioma cells (Fig. 8). These
results suggest that IL-33 plays a significant role in glioma cell
invasion and migration, which could contribute to the elucidation
of glioma pathogenesis and the development of novel
therapeutics.
References
|
1
|
Robins HI, Peterson CG and Mehta MP:
Combined modality treatment for central nervous system
malignancies. Semin Oncol. 30 Suppl 9:11–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Meter TE, Rooprai HK, Kibble MM,
Fillmore HL, Broaddus WC and Pilkington GJ: The role of matrix
metalloproteinase genes in glioma invasion: Co-dependent and
interactive proteolysis. J Neurooncol. 53:213–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yong VW: Metalloproteinases: Mediators of
pathology and regeneration in the CNS. Nat Rev Neurosci. 6:931–944.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yong VW, Power C, Forsyth P and Edwards
DR: Metallo-proteinases in biology and pathology of the nervous
system. Nat Rev Neurosci. 2:502–511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
8
|
Gao X, Wang X, Yang Q, Zhao X, Wen W, Li
G, Lu J, Qin W, Qi Y, Xie F, et al: Tumoral expression of IL-33
inhibits tumor growth and modifies the tumor microenvironment
through CD8+ T and NK cells. J Immunol. 194:438–445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forsyth PA, Wong H, Laing TD, Rewcastle
NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM,
Sutherland G, et al: Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and
membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in
different aspects of the pathophysiology of malignant gliomas. Br J
Cancer. 79:1828–1835. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kargiotis O, Chetty C, Gondi CS, Tsung AJ,
Dinh DH, Gujrati M, Lakka SS, Kyritsis AP and Rao JS:
Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in
impaired invasion and tumor-induced angiogenesis, induces apoptosis
in vitro and inhibits tumor growth in vivo in glioblastoma.
Oncogene. 27:4830–4840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shastry AH, Thota B, Arimappamagan A and
Santosh V: P53 stratification reveals the prognostic utility of
matrix metalloproteinase-9 protein expression in glioblastoma.
Neurol India. 63:399–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heimberger AB and Sampson JH:
Immunotherapy coming of age: What will it take to make it standard
of care for glioblastoma? Neuro-oncol. 13:3–13. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palmer G and Gabay C: Interleukin-33
biology with potential insights into human diseases. Nat Rev
Rheumatol. 7:321–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kakkar R, Hei H, Dobner S and Lee RT:
Interleukin 33 as a mechanically responsive cytokine secreted by
living cells. J Biol Chem. 287:6941–6948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ali S, Huber M, Kollewe C, Bischoff SC,
Falk W and Martin MU: IL-1 receptor accessory protein is essential
for IL-33-induced activation of T lymphocytes and mast cells. Proc
Natl Acad Sci USA. 104:18660–18665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu WT, Li MQ, Liu W, Jin LP, Li DJ and Zhu
XY: IL-33 enhances proliferation and invasiveness of decidual
stromal cells by up-regulation of CCL2/CCR2 via NF-κB and ERK1/2
signaling. Mol Hum Reprod. 20:358–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moulin D, Donzé O, Talabot-Ayer D, Mézin
F, Palmer G and Gabay C: Interleukin (IL)-33 induces the release of
pro-inflammatory mediators by mast cells. Cytokine. 40:216–225.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W
and Qin Q: IL-33/ST2 pathway contributes to metastasis of human
colorectal cancer. Biochem Biophys Res Commun. 453:486–492. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ and
Hu WH: IL-33 Promotes gastric cancer cell invasion and migration
via ST2-ERK1/2 pathway. Dig Dis Sci. 60:1265–1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bergis D, Kassis V, Ranglack A, Koeberle
V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O and Radeke HH:
High Serum levels of the interleukin-33 receptor soluble ST2 as a
negative prognostic factor in hepatocellular carcinoma. Transl
Oncol. 6:311–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu LA, Fu Y, Zhang DN and Zhang J: Serum
IL-33 as a diagnostic and prognostic marker in non-small cell lung
cancer. Asian Pac J Cancer Prev. 14:2563–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gadani SP, Walsh JT, Smirnov I, Zheng J
and Kipnis J: The glia-derived alarmin IL-33 orchestrates the
immune response and promotes recovery following CNS injury. Neuron.
85:703–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang KM, Yang CS, Lin TC, Chan TC and
Tzeng SF: Induced interleukin-33 expression enhances the
tumorigenic activity of rat glioma cells. Neuro-oncol. 16:552–566.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Wang P, Ji W, Ding Y and Lu X:
Overexpression of interleukin-33 is associated with poor prognosis
of patients with glioma. Int J Neurosci. 127:210–217. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ii M, Nishimura H, Iwakura A, Wecker A,
Eaton E, Asahara T and Losordo DW: Endothelial progenitor cells are
rapidly recruited to myocardium and mediate protective effect of
ischemic preconditioning via ‘imported’ nitric oxide synthase
activity. Circulation. 111:1114–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Funakoshi-Tago M, Tago K, Hayakawa M,
Tominaga S, Ohshio T, Sonoda Y and Kasahara T: TRAF6 is a critical
signal transducer in IL-33 signaling pathway. Cell Signal.
20:1679–1686. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radisky DC and Bissell MJ: Cancer. Respect
thy neighbor! Science. 303:775–777. 2004.PubMed/NCBI
|
|
29
|
Balkwill F, Charles KA and Mantovani A:
Smoldering and polarized inflammation in the initiation and
promotion of malignant disease. Cancer Cell. 7:211–217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Milovanovic M, Volarevic V, Radosavljevic
G, Jovanovic I, Pejnovic N, Arsenijevic N and Lukic ML: IL-33/ST2
axis in inflammation and immunopathology. Immunol Res. 52:89–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JY, Lim SC, Kim G, Yun HJ, Ahn SG and
Choi HS: Interleukin-33/ST2 axis promotes epithelial cell
transformation and breast tumorigenesis via upregulation of COT
activity. Oncogene. 34:4928–4938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bergers G, Reikerstorfer A, Braselmann S,
Graninger P and Busslinger M: Alternative promoter usage of the
Fos-responsive gene Fit-1 generates mRNA isoforms coding for either
secreted or membrane-bound proteins related to the IL-1 receptor.
EMBO J. 13:1176–1188. 1994.PubMed/NCBI
|
|
34
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Pantic JM, Milovanovic MZ, Arsenijevic NN and Lukic ML:
Interleukin-33/ST2 axis promotes breast cancer growth and
metastases by facilitating intratumoral accumulation of
immunosuppressive and innate lymphoid cells. Int J Cancer.
134:1669–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jovanovic I, Radosavljevic G, Mitrovic M,
Juranic VL, McKenzie ANJ, Arsenijevic N, Jonjic S and Lukic ML: ST2
deletion enhances innate and acquired immunity to murine mammary
carcinoma. Eur J Immunol. 41:1902–1912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao K, Li X and Zhang L, Bai L, Dong W,
Gao K, Shi G, Xia X, Wu L and Zhang L: Transgenic expression of
IL-33 activates CD8(+) T cells and NK cells and inhibits tumor
growth and metastasis in mice. Cancer Lett. 335:463–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller AM, Xu D, Asquith DL, Denby L, Li
Y, Sattar N, Baker AH, McInnes IB and Liew FY: IL-33 reduces the
development of atherosclerosis. J Exp Med. 205:339–346. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barbour M, Allan D, Xu H, Pei C, Chen M,
Niedbala W, Fukada SY, Besnard AG, Alves-Filho JC, Tong X, et al:
IL-33 attenuates the development of experimental autoimmune
uveitis. Eur J Immunol. 44:3320–3329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Besnard AG, Togbe D, Guillou N, Erard F,
Quesniaux V and Ryffel B: IL-33-activated dendritic cells are
critical for allergic airway inflammation. Eur J Immunol.
41:1675–1686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schmieder A, Multhoff G and Radons J:
Interleukin-33 acts as a pro-inflammatory cytokine and modulates
its receptor gene expression in highly metastatic human pancreatic
carcinoma cells. Cytokine. 60:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Markovic DS, Vinnakota K, Chirasani S,
Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, van
Rooijen N, et al: Gliomas induce and exploit microglial MT1-MMP
expression for tumor expansion. Proc Natl Acad Sci USA.
106:12530–12535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mentlein R, Hattermann K and Held-Feindt
J: Lost in disruption: Role of proteases in glioma invasion and
progression. Biochim Biophys Acta. 1825:178–185. 2012.PubMed/NCBI
|
|
45
|
Zheng X, Chopp M, Lu Y, Buller B and Jiang
F: MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis
via NRP-2 and MMP-3. Cancer Lett. 329:146–154. 2013. View Article : Google Scholar : PubMed/NCBI
|