Introduction
Colorectal cancer (CRC) is a major public health
problem and the leading cause of death in the economically
developed and developing countries, and is a growing burden
worldwide due to increasing of the aging population (1,2).
According to the statistics, CRC is the third most commonly
diagnosed and deadly cancer both in males and females, with an
estimated 69,090 and 63,610 new cases, and 26,100 and 23,600
deaths, respectively (1). The
incidence rates of CRC are much higher in the developed countries
such as Northern America, Europe, and Australia, while they are
lower in economically underdeveloped areas for instance
South-Central Asia and Africa (2,3). In
the recent decades, the incidence rate is increasing notably in
Asia, South America and Eastern Europe, which may reflect the
adoption of lifestyle behaviors that increase the colorectal cancer
risk, including smoking, unhealthy diet, obesity, and physical
inactivity (3,4).
On the contrary, decreasing morbidity and mortality
rates have been observed in a large number of developed countries
worldwide which may due to a higher cancer screening rates, reduced
prevalence of risk factors, as well as the improved comprehensive
treatment (4–6). As far as we know, surgical resection,
adjuvant chemotherapy, radiation therapy and targeted therapy are
treatments that may offer curative potential for patients with CRC
(7). Based on the scientific
advances in affected gene detection by different technologies
including gene expression tools, CRC could be classified into
different molecular subtypes (7).
Though improvement of comprehensive treatment has been made, CRC is
clinically resistant to conventional chemotherapy treatments
(8,9). Consequently, there is an urgent need
to develop natural anticancer compounds which have higher efficacy
and lower toxicity in the treatment of CRC.
Sanggenon C
(C40H36O12), one of the most
abundant flavonoids isolated from the stem bark of Morus cathayana,
is a natural chemical entity of benzopyrone derivative, belonging
to the class of flavonoids (10).
Flavonoids are widely distributed in the plants, which possess
various biological and pharmacological properties, including
anticancer (11), anti-inflammatory
(12), antimicrobial, antiviral
(13), and immune-modulatory
(14) activities. As a flavanone
Diel-Alder adduct compound, sanggenon C has been reported to
possess some biological and pharmacological effects, such as
anti-oxidant and anti-inflammation activities, according to the few
reports up to now (10,15,16).
Further study demonstrated that sanggenon C could inhibit
TNF-α-stimulated cell adhesion and decrease the expression of
VCAM-1 through suppressing the activation of NF-κB (15). Thereafter, sanggenon C has also been
proposed to decrease the ICAM-1 expression at a post-translational
level. Moreover, due to the potent cell toxicity of sanggenon C, it
could serve as an effective candidate anticancer agent. These
reports, although invaluable, are invariably incomplete, as the
precise molecular mechanisms underlying sanggenon C-induced
apoptosis still remains to be delineated.
To date, there is no available information about the
anticancer effects and the mechanism of sanggenon C on human
colorectal cancer cells. Therefore, this study was undertaken to
address the effects of sanggenon C on CRC cells in vitro and
clarify its potential mechanism.
Materials and methods
Chemicals and cell culture
Sanggenon C (>98% purity) was purchased from
Biorbyt Ltd. (orb105480; Biorbyt Ltd., Cambridge, UK). The
sanggenon C stock solution was prepared at 1 mM in dimethyl
sulfoxide (DMSO) and stored at −20°C. Human colon cancer cell lines
(LoVo, HT-29 and SW480) were obtained from the Shanghai Cell
Collection (Shanghai, China) and cultured in DMEM medium (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS,
Gibco), 1% antibiotics (100 IU penicillin and 100 µg/ml
streptomycin), then maintained in a humidified incubator at 37°C
with a 5% CO2/95% air atmosphere.
Cell proliferation assay
Cell proliferation and viability was determined
using the WST-8 tetrazolium salt assay (Cell Counting Kit-8;
Beyotime Institute of Biotechnology, Wuhan, China) to quantify the
amount of formazan dye formed when a tetrazolium salt is cleaved by
cellular mitochondrial dehydrogenase. Cells were seeded in 96-well
culture plates with a density of 5×103/well, and allowed
to adhere for 12 h. Then the supernatant was replaced and cells
were incubated in fresh medium containing sanggenon C (0, 5, 10,
20, 40 and 80 µM) diluted from the stock solution for 0, 12, 24,
48, or 72 h. At 2 h before the end of the specified incubation
periods, 10 ml CCK-8 reagent was added to each well. The absorbance
at 450 nm was determined using a micro-plate reader (Victor3 1420
Multilable Counter; Perkin-Elmer, Waltham, MA, USA). DMEM
containing 10% CCK-8 solution was used as a control. The percentage
of cell survival representing the function of drug concentration
was then plotted to determine the IC50 value.
Hoechst 33258 staining
Colon cancer cells in exponential growth were
modulated at a final concentration of 1×105 cells per
well on sterile cover glasses in the 6-well plates. After
adherence, the supernatant was replaced and cells were cultured in
fresh medium containing sanggenon C (0, 10, 20, and 40 µM) for 24
h. After exposure, the cells were subsequently fixed, washed three
times with PBS and stained with 0.5 ml Hoechst 33258 staining
solution (Sigma-Aldrich, St. Louis, MO, USA) for 5 min at room
temperature in the dark. Then washed twice with PBS and the stained
nuclei with apoptotic features were scored and categorized
according to the condensation and staining characteristics of
chromatin under a fluorescence microscope (BX51; Olympus Co.,
Tokyo, Japan). Ten random fields per dish were observed and
counted.
Measurement of reactive oxygen species
(ROS)
The intracellular generation of ROS was measured
using a 20, 70-dichlorofluorescin diacetate (DCFH-DA,
Sigma-Aldrich). The adjusted concentration of colon cancer cells
was 5×103 cells/well in 96-well culture plates or
1×105 cells/well in 6-well culture plates. After
adherence, the supernatant was replaced and cells in 96-well
culture plates were then cultured in fresh medium containing
sanggenon C (0, 10, 20, and 40 µM) for 12, 24 or 48 h, while cells
in 6-well culture plates were only cultured for 24 h. After
exposure, the supernatant was removed, and the cells were washed
with PBS for three times followed by maintaining with DCFH-DA (10
µM) in DMEM (without FBS) for 20 min and then washed again. The
intracellular ROS detection of cells in 96-well culture plates was
determined by the fluorescent absorbance value using a microplate
reader (Victor3 1420 Multilabel Counter; Perkin-Elmer). The levels
of intracellular ROS of cells in 6-well culture plates were
verified by a fluorescence microscope (BX51; Olympus Co.).
Measurement of ATP
ATP assay kit (S0026, Beyotime Institute of
Biotechnology) was chosen to detect the levels of ATP. Colon cancer
cells (5×103 cells/well) seeded in 96-well culture
plates were cultured and treated with sanggenon C (0, 5, 10, 20, 40
and 80 µM) for 0, 12, 24, 48 or 72 h as that in the measurement of
intracellular ROS. After exposure, the supernatant was replaced,
and cells were then broken by the lysis buffer in the kit at 4°C.
Subsequently, the suspension of lysates was gathered, diluted and
then mixed with the ATP detecting solution (100 µl). The levels of
ATP were determined by the fluorescent absorbance value using a
microplate reader with a standard curve which was calculated using
the standard ATP concentration and the corresponding absorbance
value as a control.
Measurement of intracellular
Ca2+
The level of intracellular Ca2+ was
assessed using Fluo-3/AM (S1056, Beyotime Institute of
Biotechnology). Cells (5×103 cells/well) seeded in
96-well culture plates were cultured and treated with sanggenon C
(0, 5, 10, 20, 40 and 80 µM) for 0, 12, 24, 48, or 72 h as that in
the measurement of intracellular ROS and ATP. After exposure, the
supernatant was removed, and cells were then washed three times
using D-Hanks balanced salt solution (D-HBBS; Jinuo, Hangzhou,
China) without Ca2+, Mg2+ and phenol red.
Following, 5 µM Fluo-3/AM diluted by D-Hanks was applied to
maintain cells for 60 min at 37°C. Subsequently, cells were then
washed using D-Hanks further three times and balanced in D-Hanks
for 30 min at 37°C. Finally, the levels of intracellular
Ca2+ were determined by the fluorescent absorbance value
using a microplate reader.
Measurement of nitric oxide (NO)
production
Griess method was adopted to detect the production
of NO using an assay kit (S0021, Beyotime Institute of
Biotechnology) based on the chemical diazotization reaction, which
uses sulfanilamide and N-1-napthylethylenediamine dihydrochloride
(NED) under acidic (phosphoric acid) conditions. Under the above
condition, two stable breakdown products, nitrate (NO3−)
and nitrite (NO2−) can be easily detected by photometric
means instead of NO with transient and volatile nature. Cells
(5×103 cells/well) seeded in 96-well culture plates were
cultured and treated with sanggenon C (0, 5, 10, 20, 40 and 80 µM)
for 0, 12, 24, 48, or 72 h according to the aforementioned
grouping. Then the production of NO in cells was measured by Griess
method in accordance with the directions of the NO assay kit.
Western blot analysis
Colon cancer cell lysates were prepared with RIPA
lysis buffer (Beyotime Institute of Biotechnology) and
phenylmethylsulfonyl fluoride (PMSF, Beyotime Institute of
Biotechnology). The whole extracted cellular proteins were
subjected to SDS-polyacrylamide gels and electro-transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Transfer efficiency and homogeneous loading could be assessed
by Ponceau stain. Membranes were blocked with 5% non-fat milk in
TBST, then immunoblotted with several titrated primary antibodies
(all from Cell Signaling Technology, Beverly, MA, USA) including
anti-Bcl-2, anti-cleaved caspase-9, anti-cytochrome C (Cyt C),
anti-inducible nitric oxide synthase (iNOS) or anti-GAPDH,
overnight at 4°C. After washing with TBST three times, the
membranes were labeled with a 1:10,000 diluted secondary antibody
(LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room temperature
before they were washed further three times. Finally, the membranes
were scanned using a two-color infrared imaging system (Odyssey,
Lincoln, NE, USA). Membranes were probed for GAPDH as an additional
loading control, and the protein band intensity was also
determined.
Xenograft tumor model
Male BALB/c-nu/nu nude mice (4–6 weeks old) were
purchased from Beijing HFK Bioscience and housed in specific
pathogen-free conditions under approved institutional animal care
and use protocols. All animal experiments were approved by the
Committee on the Use of Live Animals in Teaching and Research
(CULATR), the First Affiliated Hospital of Zhengzhou University.
Colon cancer cells, harvested from subconfluent cultures, washed in
serum-free medium, then suspended in 100 µl PBS, were
subcutaneously inoculated into the dorsal area of the nude mice.
The tumor size was measured every 3 day, and when reached
approximately 150 mm3 the nude mice were divided into
four groups (six in each group): a control group, a low-dose group
(2.5 mg/kg), a mid-dose group (5 mg/kg), and a high-dose group (10
mg/kg). Sanggenon C was administered via intraperitoneal injection
every other day. Following, the mice were weighed, and the size of
each tumor and its central necrotic area were monitored every 3
days. At the end of the experiment, tumors were harvested, weighed
and then analyzed using hematoxylin and eosin H&E staining and
TUNEL assay.
H&E staining and TUNEL assay
For histologic analysis, tumor tissue was fixed in
4% formaldehyde, dehydrated with an ethanol gradient, and embedded
in paraffin. Then the paraffin tumor tissue sections (4 µm) were
obtained and stained with H&E. The TUNEL assay for apoptosis
analysis was performed using an apoptosis detection kit (Roche
Diagnostics, Branchburg, NJ, USA) according to the manufacturer's
instructions. Positive cells were identified, counted (six random
fields per slides), and analyzed by light microscopy (BX51; Olympus
Co.).
Statistical analysis
All data were expressed as the mean ± standard
deviation (SD). The difference among groups was determined by
ANOVA, while the difference between groups was analyzed by the
Student's t-test using SPSS 17.0 for Microsoft Windows (SPSS Inc.,
Chicago, IL, USA). A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Sanggenon C inhibits the proliferation
of colon cancer cells
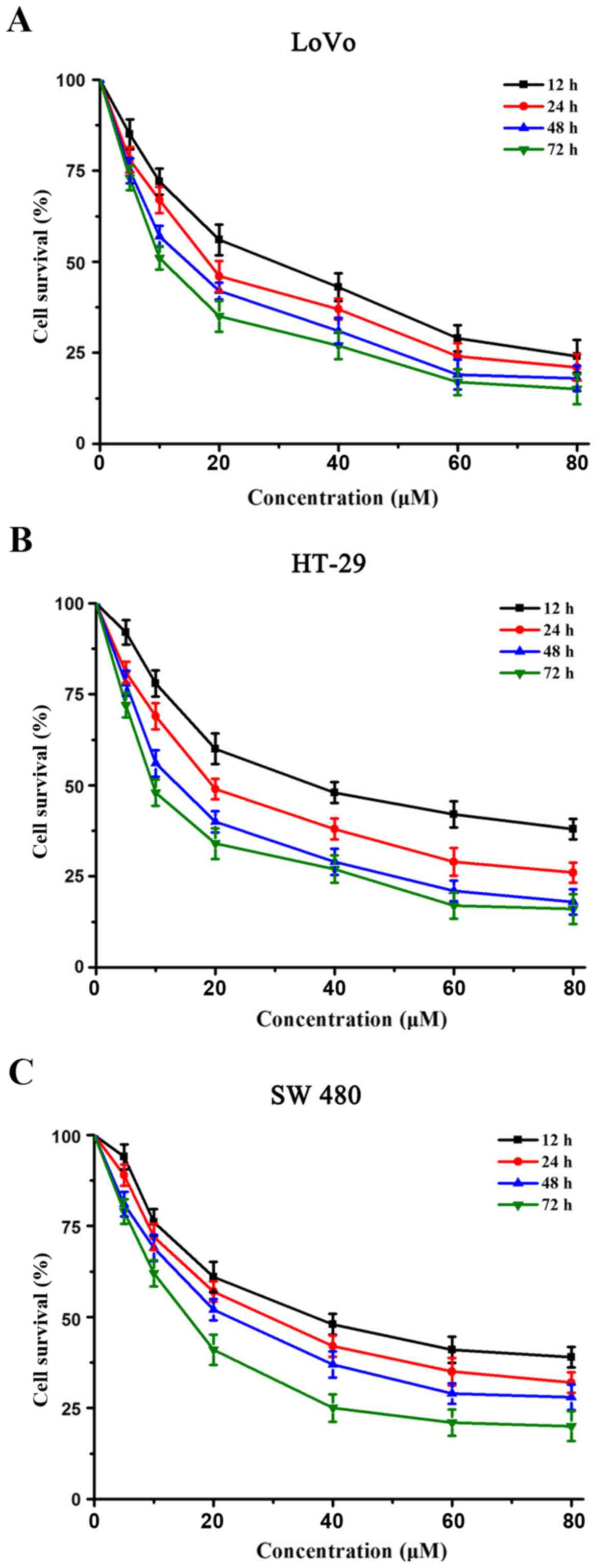
The inhibition of human colon cancer cell (LoVo,
HT-29 and SW480) proliferation by sanggenon C was determined by
CCK-8 cell viability assay after drug exposure maintained above,
following 24 h culture in a drug-free medium. As shown in Fig. 1, sanggenon C could significantly
inhibit the proliferation of colon cancer cells in a dose- and
time-dependent manner. Of the three colon cancer cell lines, LoVo
was the most sensitive to sanggenon C, while SW480 was the least
sensitive. Therefore, HT-29 was chosen for the following
experiments.
Sanggenon C induces apoptosis of colon
cancer cells
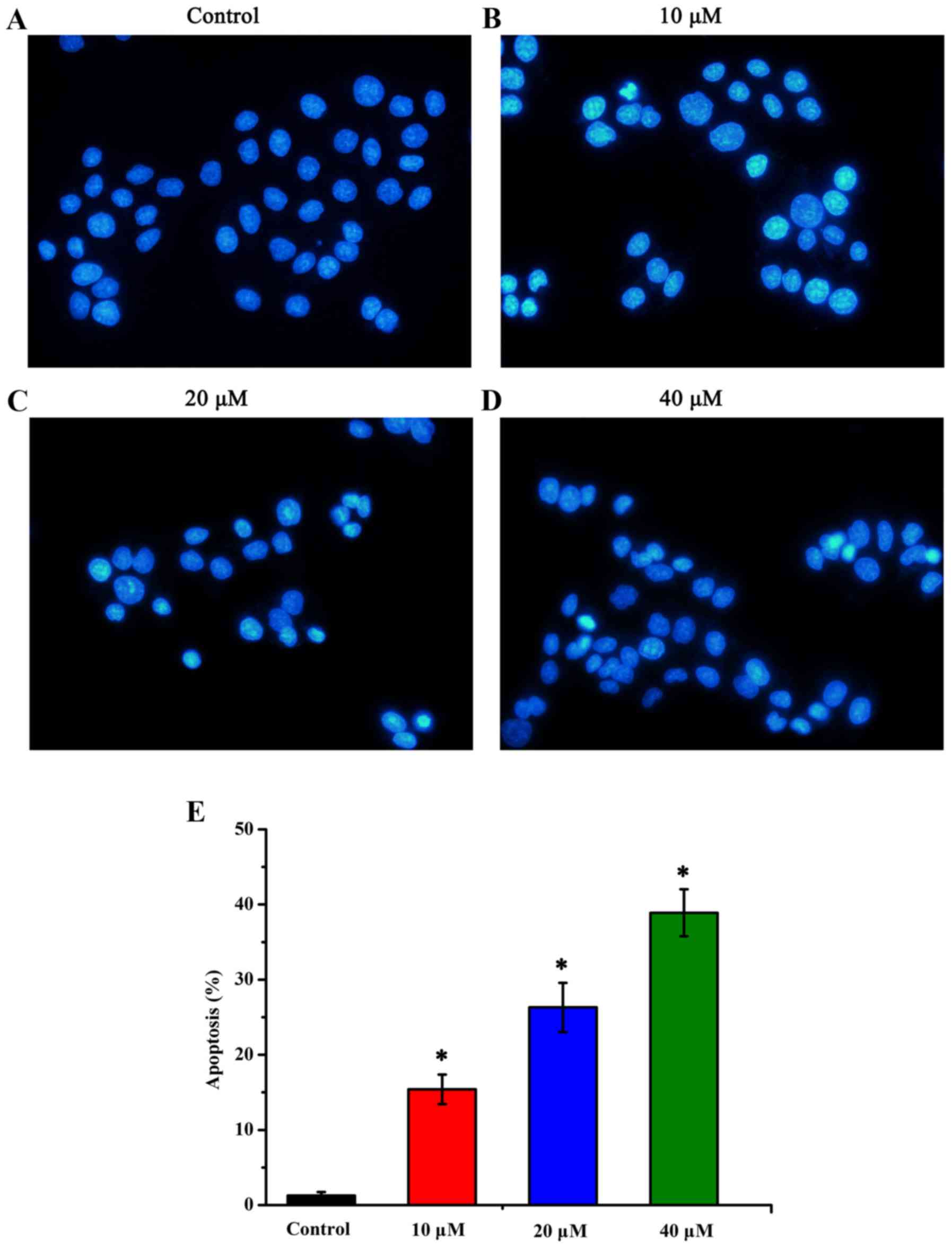
Apoptosis of HT-29 human colon cancer cells induced
by sanggenon C was assessed by using the Hoechst 33258 staining.
The apoptotic nuclei were identified by changes revealed in
condensed chromatin as well as nuclear fragmentation with formation
of apoptotic bodies. In the control group, the nuclei were stained
lightly homogeneous blue, while in those treated with sanggenon C,
bright condensed chromatin and nuclear fragmentation could be
observed (Fig. 2A-D). Ten random
fields per dish were counted and the mean values ± SD were
expressed as the percentage of apoptotic cells. Significant changes
were detected in the number of apoptotic cells, and the percentages
of apoptotic cells in the control group, low-dose sanggenon C group
(10 µM), mid-dose sanggenon C group (20 µM) and high-dose sanggenon
C group (40 µM) were 1.27±0.46, 15.4±1.97, 26.3±3.26 and
38.9±3.13%, respectively (Fig.
2E).
Sanggenon C induces the generation of
intracellular ROS
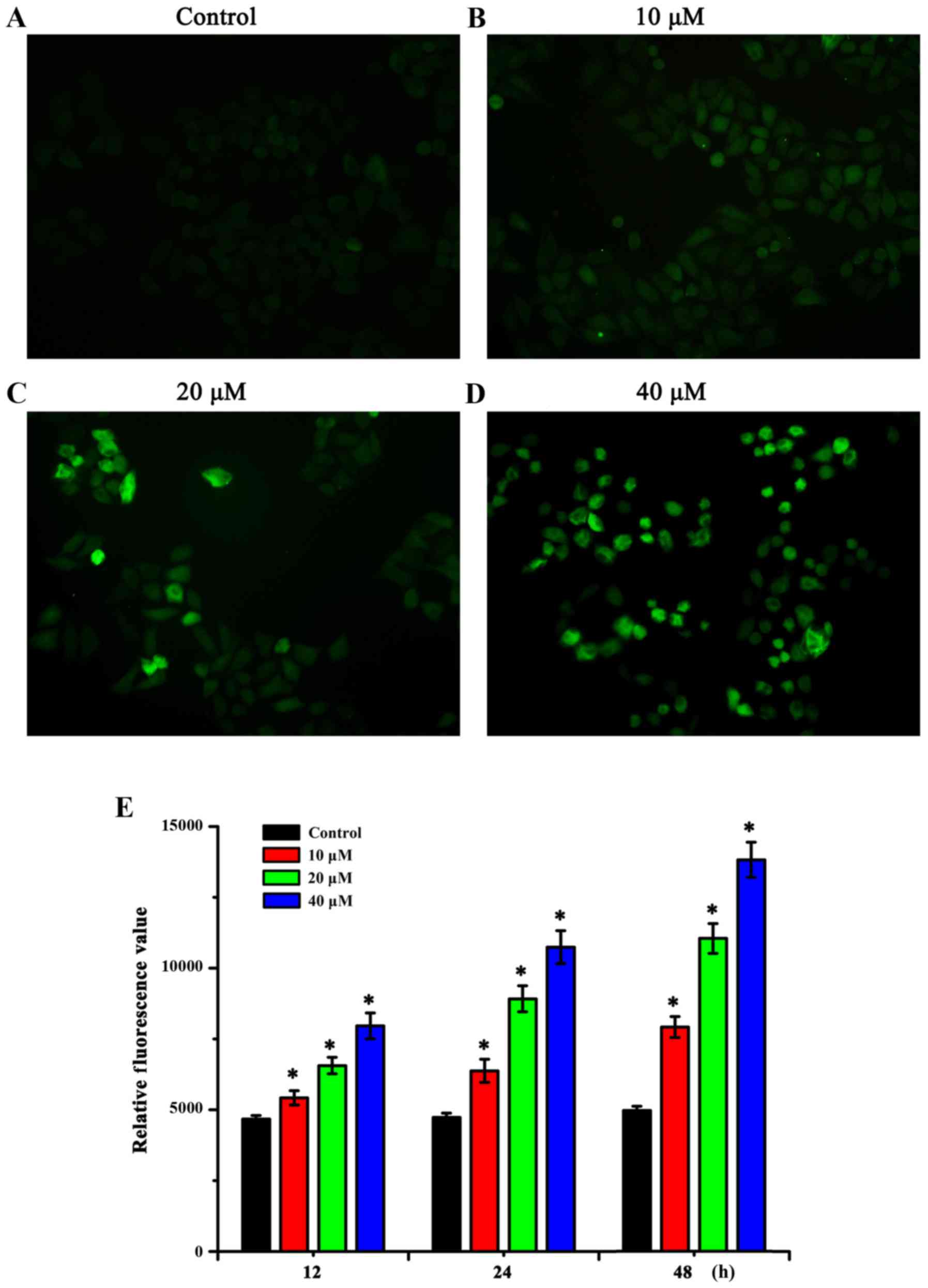
To determine whether the effect of sanggenon C on
the proliferation and apoptosis of human colon cancer cells is
bound to the deregulation of cellular redox status, the
intracellular generation of ROS was detected using DCFH-DA. The
fluorescence intensity was reflected by a fluorescence microscope
which showed that sanggenon C could increase the levels of
intracellular ROS in human colon cancer cells, and this
accumulation was enhanced when the dose was increased (Fig. 3A-D). The fluorescent absorbance
results determined by a microplate reader displayed that the
relative fluorescence value changed following the changes of the
dose and treatment time of sanggenon C (Fig. 3E), which indicated that sanggenon C
could interfere with the levels of intracellular ROS.
Sanggenon C induces an increase in the
level of intracellular Ca2+ and ATP
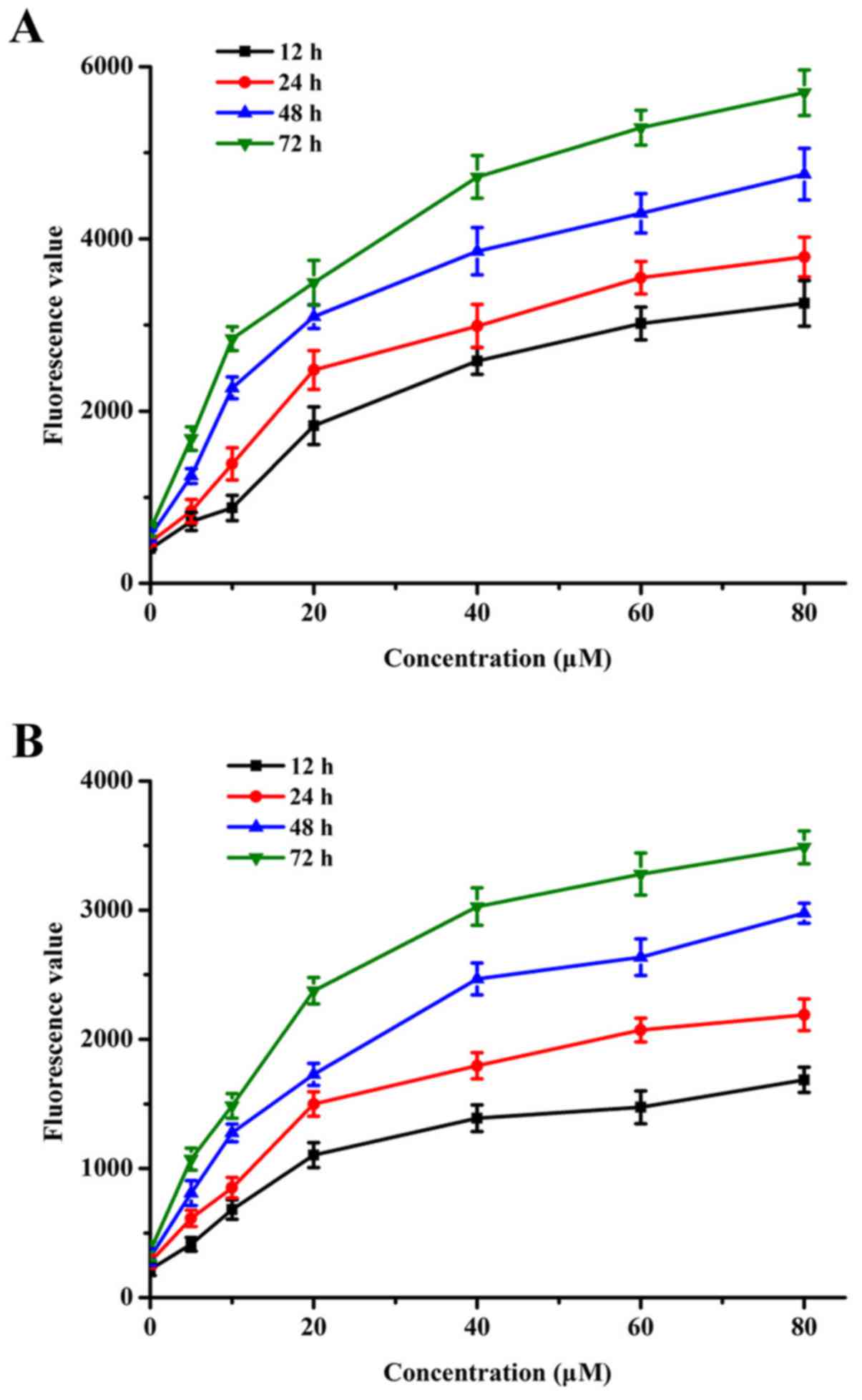
For the sake of understanding whether the effect of
sanggenon C on the proliferation and apoptosis of human colon
cancer cells is related to the fluxion of Ca2+ and the
changes of generation of ATP, we assessed the levels of
intracellular Ca2+ and ATP by the fluorescent absorbance
value using a microplate reader. The detection results showed that
sanggenon C could interfere and increase the levels of
intracellular Ca2+ and ATP, both in a time-dependent
manner, and this accumulation was enhanced when the dose was
increased as shown in Fig. 4.
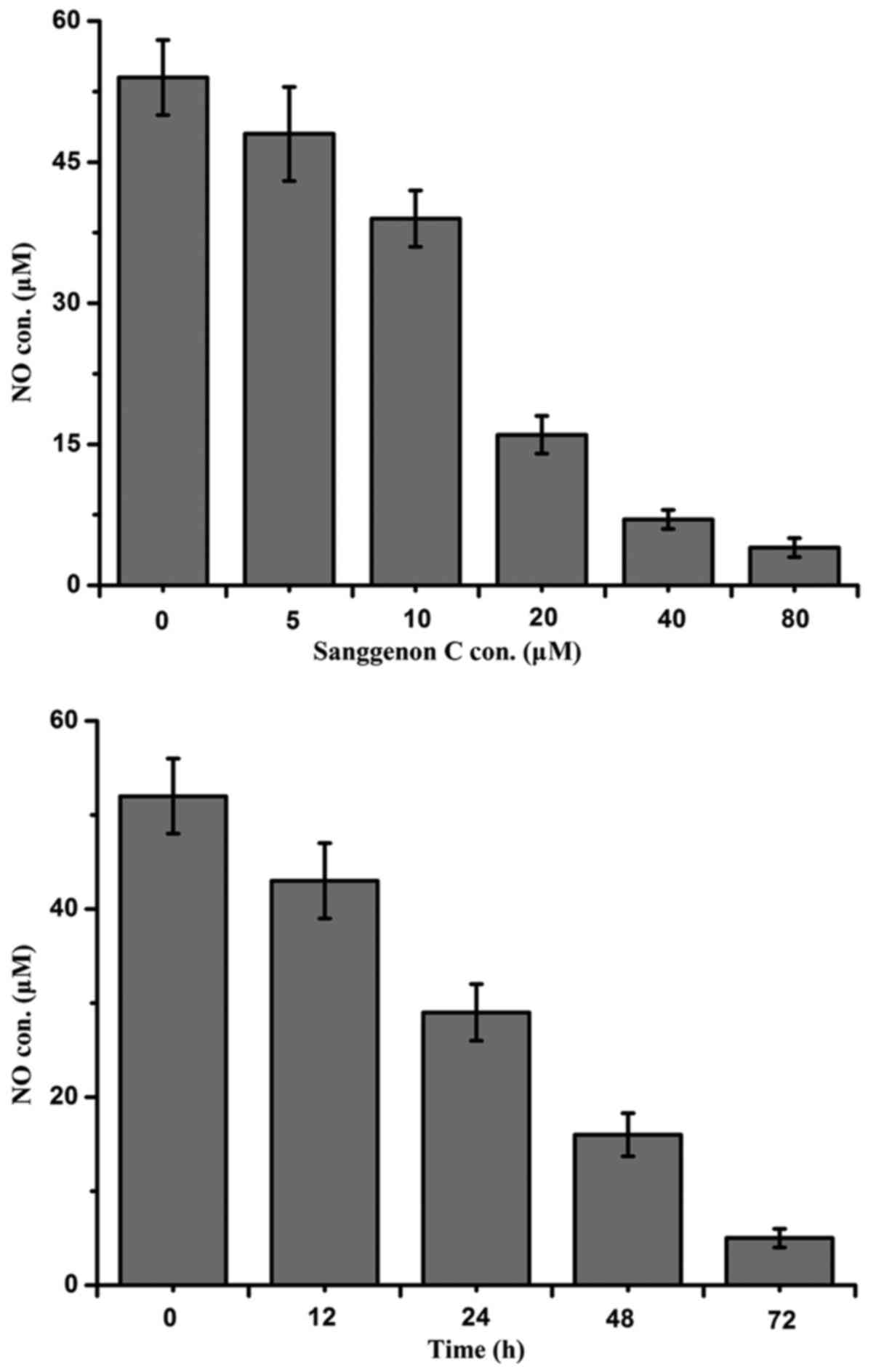
Sanggenon C inhibits the production of
intracellular NO
The inhibitory effect on the production of NO
induced by sanggenon C which plays a significant role on the
proliferation and apoptosis of human colon cancer cells was
evaluated by Griess method as described above using a microplate
reader. The intracellular NO levels in the culture media were
measured as nitrite concentration and the detection results showed
that sanggenon C could notably interfere and inhibit the NO
production both in a dose- and time-dependent manner as shown in
Fig. 5.
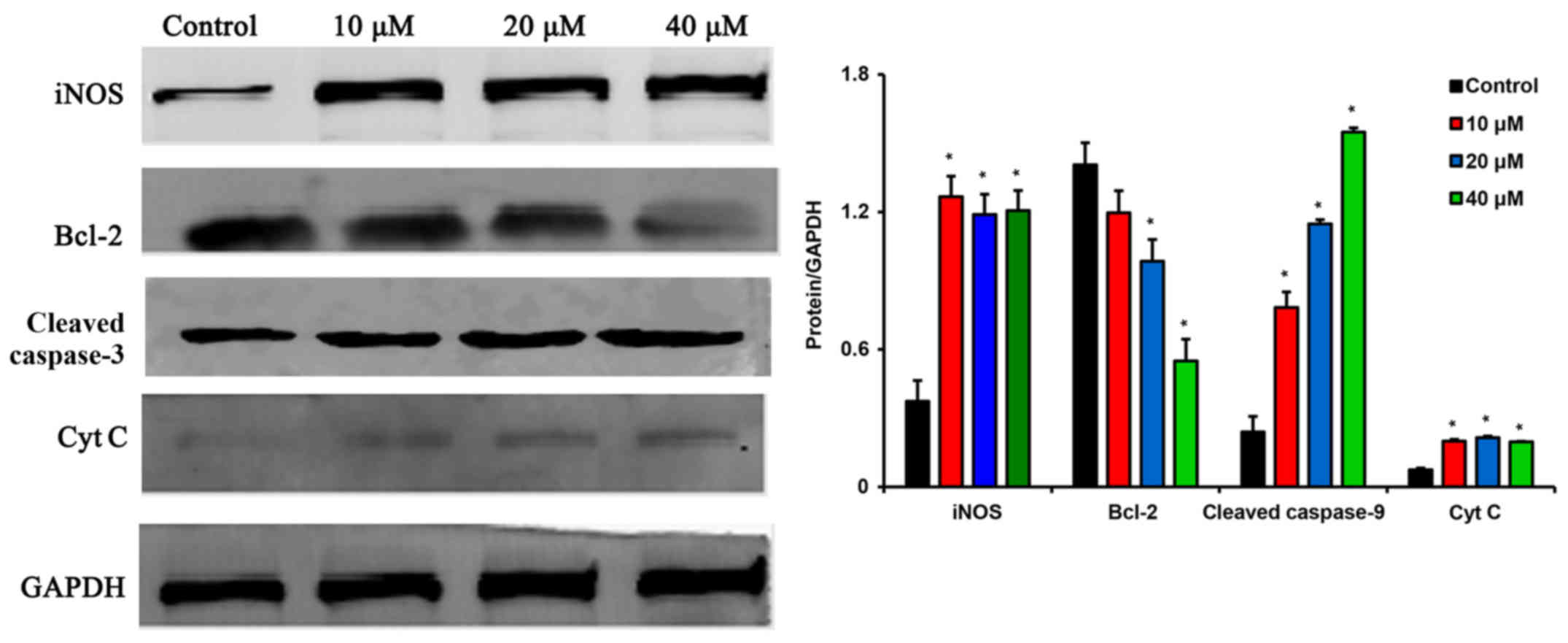
Sanggenon C induces apoptosis of colon
cancer cells via inhibiting iNOS expression and triggering the
activation of the mitochondrial pathway
To further study the detailed mechanism underlying
sanggenon C-induced apoptosis, we examined the effect of sanggenon
C on iNOS expression and the key protein levels of mitochondrial
pathway using western blotting. Sanggenon C could inhibit iNOS
expression and lead to an increase in the levels of Cyt C, which is
known as the critical role that activate caspase-9 and the
subsequent mitochondria-mediated apoptosis. Sanggenon C caused a
decrease in Bcl-2 levels as shown in Fig. 6.
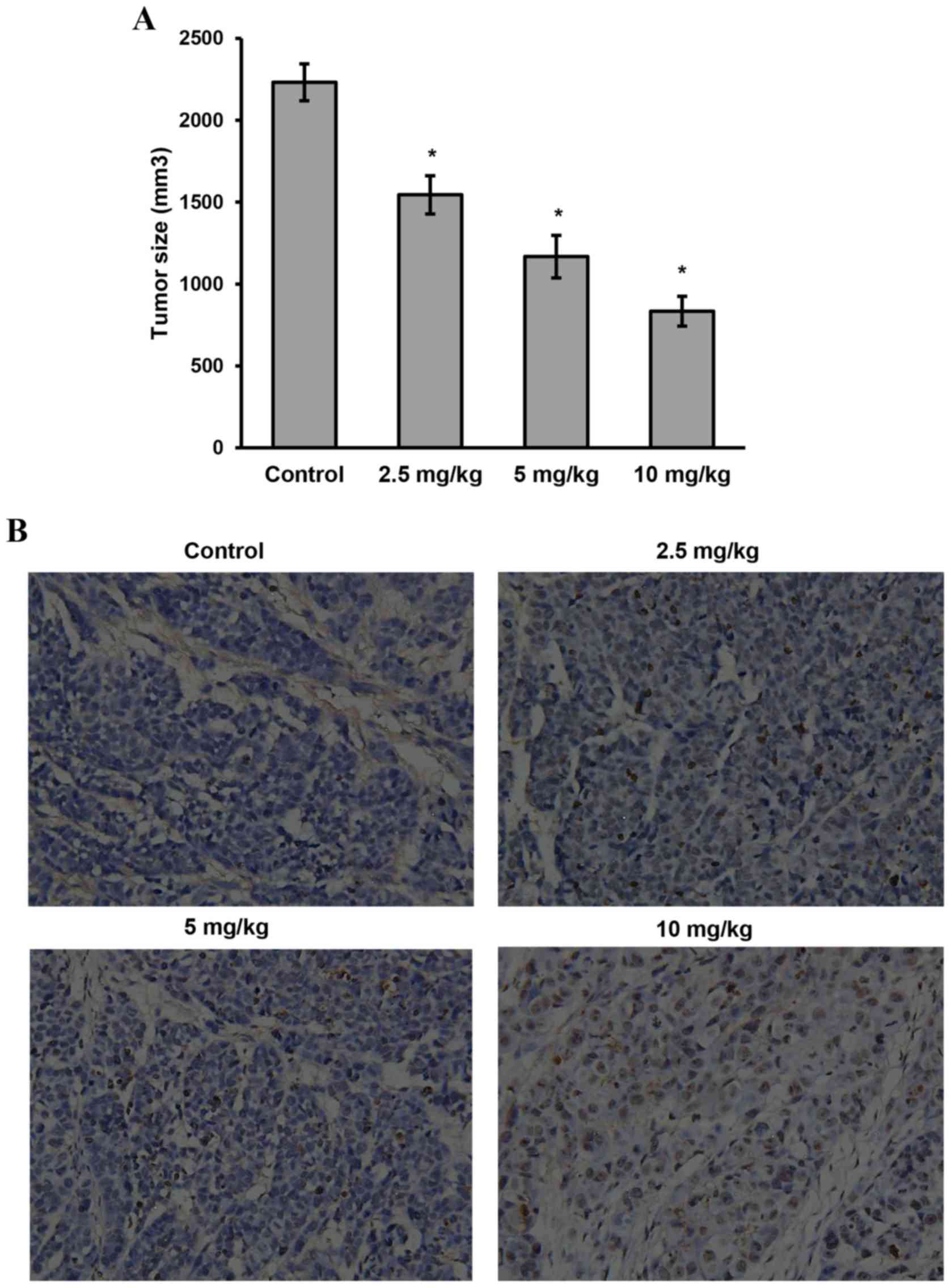
Sanggenon C inhibits the growth of
colon xenograft tumors in vivo
On the basis of the data above, we further
investigated the effects of sanggenon C on xenograft colon tumor
in vivo. As shown in Fig.
7A, tumors grew rapidly in the control group (2231.8±112.7
mm3), while the growth was significantly suppressed in
the disposed groups in a dose-dependent manner (1544.7±116.8
mm3 in low-dose group, 1167.4±129.6 mm3 in
mid-dose group and 833.8±91.3 mm3 in high-dose group).
At the end of the experiment, the weight of the harvested tumors in
control group was heavier than that of the tumors in high dose
group, mid-dose group and even the low-dose group (P<0.05).
Tumor tissues isolated from the xenograft mice of
the four groups were then processed for H&E staining (not
shown) and TUNEL assay as described. It demonstrated that sanggenon
C produced significant cell apoptosis in the tumor mass (Fig. 7B), whereas little apoptosis was
observed in the control group (P<0.05).
Discussion
Redox regulation has been demonstrated to play a
critical role in physiopathological contexts and the free radicals,
from NO and superoxide anion (O2−) to other
forms of ROS, have been proved to be involved in different
biological and regulatory processes (17–19).
ROS, generated from various extracellular and intracellular
actions, have drawn attention as novel host defending molecular and
play critical roles promoting cancer development by inducing
genomic instability, modifying gene expression, as second
messengers, in various signaling pathways (20,21).
Mitochondrial oxidative metabolism, which release ROS as a
byproduct or a waste product of normal mitochondrial metabolism and
homeostasis, is the main resource of cellular ROS; while the other
response to xenobiotics, cytokines or bacterial invasion which
generate ROS either in molecular synthesis or in breakdown, is
another resource of ROS (22,23).
Sanggenon C, a natural chemical entity of
benzopyrone derivative, possesses various biological and
pharmacological properties, including anticancer,
anti-inflammatory, antimicrobial, antiviral, antithrombotic, and
immune-modulatory activities. Previous study reported that
sanggenon C induced death of human K562 cancer cells and primary
cells isolated from leukemic patients via induction of cell cycle
arrest and cell death (15). Our
in vitro data demonstrated that sanggenon C inhibit the
proliferation of colon cancer cells including LoVo, SW480, and
HT-29 cells in a dose- and time-dependent manner. Sanggenon C
treatment at the concentration of 10, 20, and 40 µM resulted in an
induction of HT-29 cell apoptosis. We also found that sanggenon C
could increase the levels of intracellular ROS in HT-29 colon
cancer cells. These results were further confirmed in our in
vivo study, as sanggenon C treatment suppressed xenograft colon
tumor growth and enhanced xenograft colon tumor apoptosis.
Mitochondrial membrane permeabilization (MMP) is
classically considered as a point of no return in multiple forms of
cell death which is involved in diseases including cancer. With
many different stimuli, mitochondrial Ca2+ overload was
confirmed to drive ROS generation, which makes it the main
triggering signal as well as ROS that leads to MMP and releases of
proapoptotic factors, which initiates cell apoptosis and death
(24,25). As a key signaling molecule involved
in the regulation of multiple aspects of mitochondrial
respiration/oxidative phosphorylation, NO is known to play a vital
role in various physiological conditions, and the overproduction of
NO is responsible for the pathophysiological development of cancer.
The expression of NO depend on a medically relevant dual regulation
pathway mediated by traditionally characterized NO synthases (NOS)
including inducible (iNOS, NOS2), neuronal (nNOS, NOS1) and
endothelial (eNOS, NOS3) types and by enzymatic reduction of
available cellular nitrite pools by a diverse class of cytosolic
and mitochondrial nitrite reductases (26,27).
The role of the calcium-independent iNOS in
tumorigenesis is highly complex and quite perplexing, with both
pro- and anti-neoplastic functions having been described. The dual
actions of iNOS-derived NO show that the mount of NO, cell types,
cellular microenvironment, its interaction with other reactive
species and presence of metals are contributing factors to these
observations and ultimately to cellular outcomes (28,29).
Sanggenon C was studied for the mechanism of its action in the ROS
generation and apoptotic pathway in the HT-29 colon cancer cells.
Treatment of Sanggenon C resulted in increased levels of
intracellular Ca2+ and ATP. In addition, there was a
decrease in the cellular levels of NO production in the sanggenon C
treated HT-29 colon cancer cells. Furthermore, our study
demonstrated that the cytotoxic effect of sanggenon C on the HT-29
appeared to be through inhibition of iNOS expression, which is
known to activate the activity of caspase-9 and the subsequent
mitochondria-mediated apoptosis. Thus, sanggenon C may represent a
novel chemotherapeutic agent worth continued investigation in the
treatment of CRC.
In this study, we evaluated sanggenon C-induced
apoptosis in HT-29 colon cancer cells as a result of the increased
ROS generation and activation of the mitochondrial pathway by the
inhibition of the iNOS and decreased NO production. These results
lead us to speculate that sanggenon C may play a role in an
apoptotic cascade in HT-29 colon cancer cells via mitochondrial
pathway. This study is potentially interesting with regard to the
antitumor effect of sanggenon C as it relates to the development of
novel chemotherapeutic approaches in the treatment of malignant
CRC.
References
|
1
|
Siegel RLMK, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosetti C, Levi F, Rosato V, Bertuccio P,
Lucchini F, Negri E and La Vecchia C: Recent trends in colorectal
cancer mortality in Europe. Int J Cancer. 129:180–191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: A
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aran V, Victorino AP, Thuler LC and
Ferreira CG: Colorectal cancer: Epidemiology, disease mechanisms
and interventions to reduce onset and mortality. Clin Colorectal
Cancer. 15:195–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Xu S, Huang X, Li L, Zhang C, Pan
Q, Ren Z, Zhou R, Ren Y, Zi J, et al: Drug resistance in colorectal
cancer cell lines is partially associated with aneuploidy status in
light of profiling gene expression. J Proteome Res. 15:4047–4059.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y, Huang XX, Chen GB, Wang Y, Zhi Q,
Liu YS, Wu XL, Wang LF, Yang B, Xiao CX, et al: Dragon (RGMb)
induces oxaliplatin resistance in colon cancer cells. Oncotarget.
7:48027–48037. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dat NTBP, Binh PT, Quynh TP, Huong HT and
Minh CV: Sanggenon C and O inhibit NO production, iNOS expression
and NF-κB activation in LPS-induced RAW264.7 cells. Immunopharmacol
Immunotoxicol. 34:84–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobral F, Calhelha RC, Barros L, Dueñas M,
Tomás A, Santos-Buelga C, Vilas-Boas M and Ferreira I: Flavonoid
composition and antitumor activity of bee bread collected in
northeast Portugal. Molecules. 22:pii: E248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren J, Zheng Y, Lin Z, Han X and Liao W:
Macroporous resin purification and characterization of flavonoids
from Platycladus orientalis (L.) Franco and their effects on
macrophage inflammatory response. Food Funct. 8:86–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong JJ and Kim DH:
5,7-Dihydroxy-6-methoxy-flavonoids eliminate HIV-1 D3-transfected
cytoprotective macrophages by inhibiting the PI3K/Akt signaling
pathway. Phytother Res. Jun 22–2015.(Epub ahead of print).
View Article : Google Scholar
|
|
14
|
Liu XY, Xu L, Wang Y, Li JX, Zhang Y,
Zhang C, Wang SS and Zhang XM: Protective effects of total
flavonoids of Astragalus against adjuvant-induced arthritis in rats
by regulating OPG/RANKL/NF-κB pathway. Int Immunopharmacol.
44:105–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang H, Liu N, Zhao K, Zhu C, Lu X, Li S,
Lian W, Zhou P, Dong X, Zhao C, et al: Sanggenon C decreases tumor
cell viability associated with proteasome inhibition. Front Biosci
(Elite Ed). 3:1315–1325. 2011.PubMed/NCBI
|
|
16
|
Li LCSF, Shen F, Hou Q and Cheng GF:
Inhibitory effect and mechanism of action of sanggenon C on human
polymorphonuclear leukocyte adhesion to human synovial cells. Acta
Pharmacol Sin. 23:138–142. 2002.PubMed/NCBI
|
|
17
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nickel A, Kohlhaas M and Maack C:
Mitochondrial reactive oxygen species production and elimination. J
Mol Cell Cardiol. 73:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaludercic N, Deshwal S and Di Lisa F:
Reactive oxygen species and redox compartmentalization. Front
Physiol. 5:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chong SJLI, Low IC and Pervaiz S:
Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS
regulator. Mitochondrion. 19:39–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pereira EJ, Smolko CM and Janes KA:
Computational models of reactive oxygen species as metabolic
byproducts and signal-transduction modulators. Front Pharmacol.
7:4572016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy MP: Understanding and preventing
mitochondrial oxidative damage. Biochem Soc Trans. 44:1219–1226.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carraro M and Bernardi P: Calcium and
reactive oxygen species in regulation of the mitochondrial
permeability transition and of programmed cell death in yeast. Cell
Calcium. 60:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Görlach A, Bertram K, Hudecova S and
Krizanova O: Calcium and ROS: A mutual interplay. Redox Biol.
6:260–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou L, Zhang H and Wu J: Effects of
nitric oxide on the biological behavior of HepG2 human
hepatocellular carcinoma cells. Exp Ther Med. 11:1875–1880. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Astuti RI, Watanabe D and Takagi H: Nitric
oxide signaling and its role in oxidative stress response in
Schizosaccharomyces pombe. Nitric Oxide. 52:29–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, He P, Gaida M, Yang S, Schetter
AJ, Gaedcke J, Ghadimi BM, Ried T, Yfantis H, Lee D, et al:
Inducible nitric oxide synthase enhances disease aggressiveness in
pancreatic cancer. Oncotarget. 7:52993–53004. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vannini F, Kashfi K and Nath N: The dual
role of iNOS in cancer. Redox Biol. 6:334–343. 2015. View Article : Google Scholar : PubMed/NCBI
|