Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer and the third most common cause of cancer-related
death worldwide (1,2). Surgical resection, liver
transplantation, percutaneous ethanol injection (PEI), and
radiofrequency ablation (RFA) are considered to be the primary
treatments for patients with possibly curable disease. Sorafenib is
another currently available treatment option for HCC, but its
efficacy is not satisfactory due to insufficient anticancer
properties in a large portion of HCC patients (3).
Radiotherapy is one of the most effective tools in
the clinical treatment of many types of solid tumors; however, its
use for HCC has been limited due to the intrinsic tolerance of the
liver and the development of radiation-induced liver disease
(4,5). As an increase in the radiation dose
may lead to severe damage to normal tissues around the tumor and
could potentially result in wound complications and transformation
of tumor to radio-resistant ones (6,7), this
cannot serve as an option for enhancing the response of cancers to
radiation therapy. Ideally, effective radiation therapy should
eliminate cancer cells in the tumor and simultaneously protect
normal tissues and organs (8).
Recent studies have shown that drug delivery systems, such as
radiosensitizer-incorporated nanocarriers capable of targeting
tumors, can enhance radiosensitivity (9–11).
Murine monoclonal anti-EGFR antibody-conjugated gold nanoparticle
(AuNP) containing β-lapachone, an anticancer drug that induces
direct cytotoxic effects and loss of telomerase activity, appears
to enhance radiosensitivity through active targeting of the tumor
in vitro and in vivo (9). Polymeric nanoparticles containing
taxanes, an anti-neoplastic agent, or sirolimus, an mTOR inhibitor
(PNP-taxanes or PNP-sirolimus, respectively), reportedly enhanced
the efficacy of chemoradiation therapy in non-small cell lung
cancer (10,11).
Cis-diamminedichloroplatinum (II) (cisplatin,
CDDP) is a chemotherapeutic drug used for the treatment of various
solid tumors and is a well-known radiosensitizing agent (12–14).
CDDP binds to DNA through crosslinking, induces DNA damage and then
triggers apoptosis, if the damage is not repaired. Although CDDP is
preferentially used for HCC treatment, it also evokes side-effects
such as nephrotoxicity, hematopoietic injury, and deafness through
a biotransformation pathway (15,16).
Nephrotoxicity is the most severe among these side-effects and is
responsible for the dose limitation of this drug. Although the
incorporation of CDDP into nanoparticles to overcome this
limitation has been proposed (17,18),
this option has not been tested for HCC or as an active targeting
drug delivery system.
We recently created a bio-nanocapsule (BNC), a
hollow particle ~50 nm in diameter, that contained the hepatitis B
virus surface antigen (HBsAg) L protein and displayed a human
hepatocyte-recognizing molecule (pre-S1 peptide), and reported on
its effectiveness for active targeting to human liver (19). BNC binds well with the surface of
the liposome (LP), probably due to the spontaneous fusion of the
lipid bilayer of the BNC with the LP (20,21).
Since LPs can adjust both the pharmacokinetics and biodistribution
of the drugs by modifying the size, surface charge, and membrane
lipid packing, it has become widely accepted as a
well-characterized, classical carrier for drug delivery (22). A size-controlled LP can efficiently
deliver a drug to the tumor site through the enhanced permeability
and retention (EPR) effect and can protect the drug from metabolic
processes that could clear it from the body (23). However, LPs may not be able to
actively target a specific site or ensure long-term circulation in
the bloodstream due to its rapid elimination through the
reticuloendothelial system (RES) (24). Therefore, we used BNC and LP as a
fused complex to achieve active targeting drug delivery
specifically aimed at HCC based on the characteristics of BNC and
LP.
In the present study, we introduced a BNC-LP complex
that incorporates CDDP (BNC-LP-CDDP) for clinical application in
human HCC treatment, and preclinically proved its effectiveness
through in vitro and in vivo analysis.
Materials and methods
Preparation and characterization of
BNC-LP-CDDP
BNC was prepared as previously described (21,25).
In brief, BNCs were purified from S. cerevisiae AH22R cells
harboring the BNC expression plasmid, pGLDLIIP39-RcT. Protein
concentrations were determined with a bicinchoninic acid (BCA)
protein assay kit (Thermo Fisher Scientific, Inc., Rockford, IL,
USA) using bovine serum albumin (BSA) as a control protein. To
remove contaminated materials, all samples were precipitated with
ice-cold acetone containing 10% (w/v) trichloroacetic acid and
0.07% (v/v) 2-mercaptoethanol at −20°C for 1 h. The precipitated
samples were washed with ice-cold acetone and then tested using the
BCA protein assay kit. CDDP for the preparation of LP-CDDP was
purchased from JW Pharmaceutical Co. (Seoul, Republic of Korea).
LP-CDDP was prepared by Katayama Chemical Industries, Co., Ltd.,
(Osaka, Japan) on a contract basis, and was formulated using
dipalmitoylphosphatidylcholine, cholesterol, ganglioside, diacetyl
phosphate and dipalmitoylphosphatidyl ethanolamine in the molar
ratio of 35:40:15:5:5. The lipid concentrations of LP-CDDP were
measured as total cholesterol with 0.5% Triton X-100, using a
Deternuber TC555 kit. The total lipid concentration was calculated
by multiplying 2.5 by the cholesterol concentration. LP-CDDP was
diluted to 10,000-fold with distilled water, and the concentration
of platinum was measured using an automatic flameless atomic
absorption spectrophotometer (FAAS) (Model AA-6700; Shimadzu,
Kyoto, Japan) (26). Our previous
studies demonstrated that, among the BNC-LP complexes prepared at
70°C and pH 3.0, the highest yield of BNCs was obtained at a BNC:LP
ratio of 1:20 [molar ratio of BNC (as particle):LP (as lipids) =
1:1.6×104 or 1:1.6×105, respectively]
(21). To produce the BNC-LP-CDDP
complex, LP-CDDP was incubated in Britton-Robinson buffer (0.1 M
H3BO3, 0.1 M CH3COOH, 0.1 M
H3PO4 and 0.5 M NaOH; pH 3.0) at 70°C for 5
min, mixed with BNC, and then kept at 70°C for 60 min with gentle
stirring. To exchange the buffer, the solutions were passed through
a Sephadex G-25 (GE Healthcare, Buckinghamshire, UK) gel-filtration
column with phosphate-buffered saline (PBS) at pH 7.4. The sizes
and ζ-potentials of the LP-CDDP and BNC-LP-CDDP were measured by
dynamic light scattering (DLS) using a Zetasizer Nano ZS device
(Malvern Instruments Ltd., Worcestershire, UK). The polydispersity
index (PDI) is a width parameter for the Z-average as an intensity
mean.
Cell culture
Human HCC Hep3B cells (ATCC no. HB-8064) were
maintained in Dulbeccos modified Eagles medium (Gibco-Invitrogen,
Carlsbad, CA, USA). Human colorectal carcinoma HCT116 cells (ATCC
no. CCL-247) were maintained in RPMI-1640 medium
(Gibco-Invitrogen). Both media were supplemented with 10% fetal
bovine serum (FBS; Gibco-Invitrogen) and 1% penicillin/streptomycin
(Gibco-Invitrogen) in a humidified atmosphere of 5% CO2
at 37°C.
Analysis of cell proliferation and
cell cycle distribution
Hep3B and HCT116 cells in an exponential growth
phase were harvested and plated in 96-well plates (5×103
cells/well in 100 µl of growth medium). Each experiment was
performed in triplicate. Cells were exposed to CDDP, LP-CDDP, or
BNC-LP-CDDP at concentrations of 0, 5, 10 and 15 µg/ml for 4 h and
further incubated for 48 h without any drug. Cell proliferation
assays were performed using the CCK-8 assay kit (Dojindo Molecular
Technologies, Gaithersburg, MD, USA) according to the manufacturers
instructions. For the cell cycle distribution assay, Hep3B cells
were plated in a 60-mm tissue culture dish at a density of
2×105 and incubated overnight. Cells were treated with 5
µg/ml of CDDP, LP-CDDP or BNC-LP-CDDP for 4 h and further incubated
for 48 h without any drug. The cells were collected and suspended
in 100% cold-ethanol for fixation. The fixed cells were stained
with propidium iodide (PI), and the cell cycle distribution was
then analyzed via flow cytometry (BD FACSCancto II).
Clonogenic survival assay
A clonogenic assay to determine the eternal
proliferative potential of cells after treatment was performed by
quantifying the colonies formed from single cells. Cells were
plated in a 6-well tissue culture plate at a density of 400, 800,
2,000 and 4,000 cells/well and were exposed to IR of 0, 2, 4 and 8
Gy, respectively. Cells were incubated in the presence of drugs
such as CDDP, LP-CDDP, BNC-LP-CDDP, or BNC (equivalent to a CDDP
concentration of 0.5 µg/ml, 1.667 µM) for 4 h, and were exposed IR
in the absence of drugs. IR was delivered to the cell on plate
using a 6-MV photon beam linear accelerator (CL/1800; Varian
Medical Systems, Inc., Palo Alto, CA, USA). The cells were further
incubated for ~20 days at 37°C for colony formation. Cells were
stained with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO,
USA) in 20% methanol. Stained colonies were washed with water,
air-dried, and counted when they comprised >50 cells. The
surviving fraction was calculated as mean colonies counted/(cells
plated × plating efficiency), where plating efficiency was defined
as mean colonies counted/cells plated. All values were normalized
to untreated cells. The mean number of colonies from triplicate
tests, and their standard deviations were calculated. The
sensitizer enhancement ratio (SER) was calculated as the radiation
dose needed for radiation alone divided by the dose needed for
various concentrations of drug plus radiation at a survival
fraction of 0.5%.
In vivo tumor growth delay
All animal experiments were performed following the
protocol approved by the Institutional Animal Care and Use
Committee of the Asan Institute for Life Science. The Hep3B
cell-derived xenograft tumor model from male athymic nude mice
(BALB/c-nude; 6-weeks old; Japan SLC Inc., Hamamatsu, Japan) was
used to assess the in vivo therapeutic efficacy. To
establish the xenograft model, a suspension of 3×106
cells was implanted subcutaneously (s.c) into the right hind leg of
the mouse. The mice bearing xenograft tumors grown from 80 to 120
mm3 were pair-matched according to the tumor volume into
experimental groups (n=5 in each group). Drugs were intravenously
(i.v.) administered through the tail vein 2 h before IR treatment.
A single dose of CDDP included 2 mg/kg, and the doses of all other
drugs were adjusted equivalent to the CDDP dose. The drug for
administration was prepared at the concentration of 0.3 mg/ml and
the volume given a mouse that was usually ~150 µl/mouse was
proportional to the body weight. IR of 3 Gy was locally delivered
to the tumor using a 6-MV photon beam linear accelerator, when mice
were held in a mouse restrainer. The tumor volume and body weight
were monitored during the entire experimental period. Tumor volume
was calculated using the formula: Volume = (length ×
width2) × 0.5. The results are expressed as the mean ±
standard deviation.
Histological and immunohistochemical
analysis
For immunohistochemical analysis of activated
caspase-3, the Hep3B cell-derived xenograft mouse was i.v. injected
with BNC, CDDP, LP-CDDP, or BNC-LP-CDDP at doses of 10 mg/kg
depending on the amount of CDDP. After 2 h, tumors were irradiated
with 3 Gy using a 6-MV photon beam linear accelerator. Tumor
tissues were collected after 24 h and fixed with 4%
paraformaldehyde. The fixed tumor tissues were kept frozen in OCT
compound and then cryosectionized using a cryotome.
Immunohistochemical staining was performed with
anti-cleaved-caspase-3 (#9661; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and was analyzed under a microscope (DP71;
Olympus, Tokyo, Japan). The tumor tissues were counter-stained with
hematoxylin. To assess nephrotoxicity, the mice were treated with
CDDP, LP-CDDP, or BNC-LP-CDDP (equivalent to CDDP dose of 10 mg/kg;
n=3) and were sacrificed after 4 days. The kidney tissue was
harvested, fixed in 4% paraformaldehyde, embedded in paraffin and
sliced. The sections at a 5 µm-thickness were observed under a
microscope (DP71; Olympus, Tokyo, Japan) after staining with
hematoxylin and eosin.
Results
HCC-specific cytotoxicity of
BNC-LP-CDDP
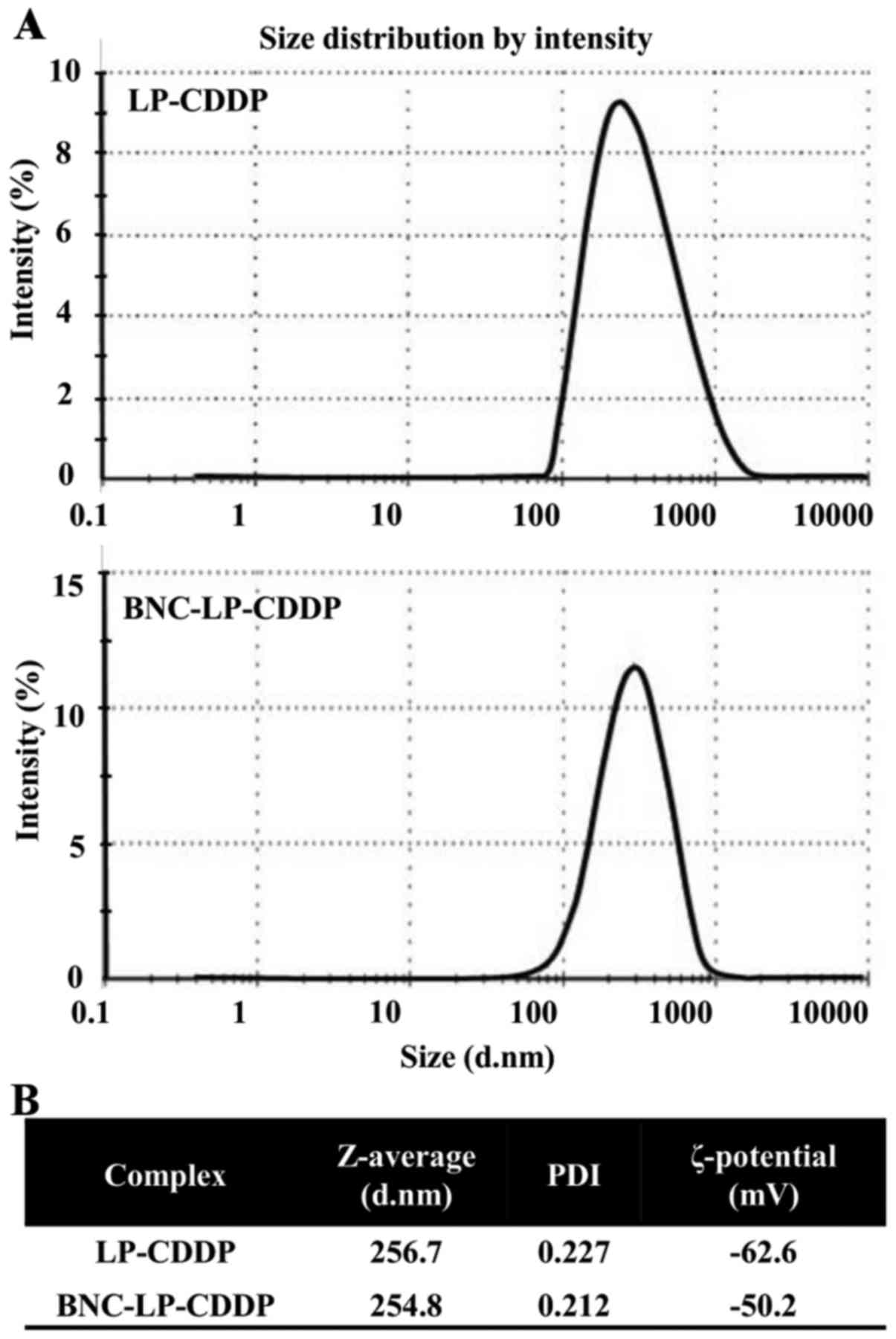
The size distribution of LP-CDDP and BNC-LP-CDDP was
first analyzed using the DLS method, which indicated a value of
~250 nm for both complexes (Fig.
1A). The material contents of LP-CDDP included 13.9 mg of LP
and 2.8 mg of CDDP per ml, whereas that of BNC-LP-CDDP included
0.58 mg of BNC, 7.6 mg of LP and 1.5 mg of CDDP per ml (Fig. 1B).
To ensure that BNC-LP-CDDP produces HCC-specific
cytotoxicity, human HCC Hep3B cells and human colon cancer HCT116
cells were treated with BNC-LP-CDDP, and their viabilities were
assessed. The viability of Hep3B treated with CDDP at 0, 5, 10 and
15 µg/ml was 100, 61 and 43%, respectively, whereas that of HCT116
in the same conditions was 100, 57 and 43%, respectively, thus,
indicating that CDDP induced equivalent cytotoxicity in both cell
lines (Fig. 2A and B). When the
cells were treated with BNC-LP-CDDP, the Hep3B cells were more
likely to die as compared to the HCT116 cells. The viability of
Hep3B cells was reduced to 34% following BNC-LP-CDDP treatment at
15 µg/ml, in contrast to the viability of 76% in HCT116 cells
following BNC-LP-CDDP treatment. These results suggest that
BNC-LP-CDDP specifically targets and kills HCC cells.
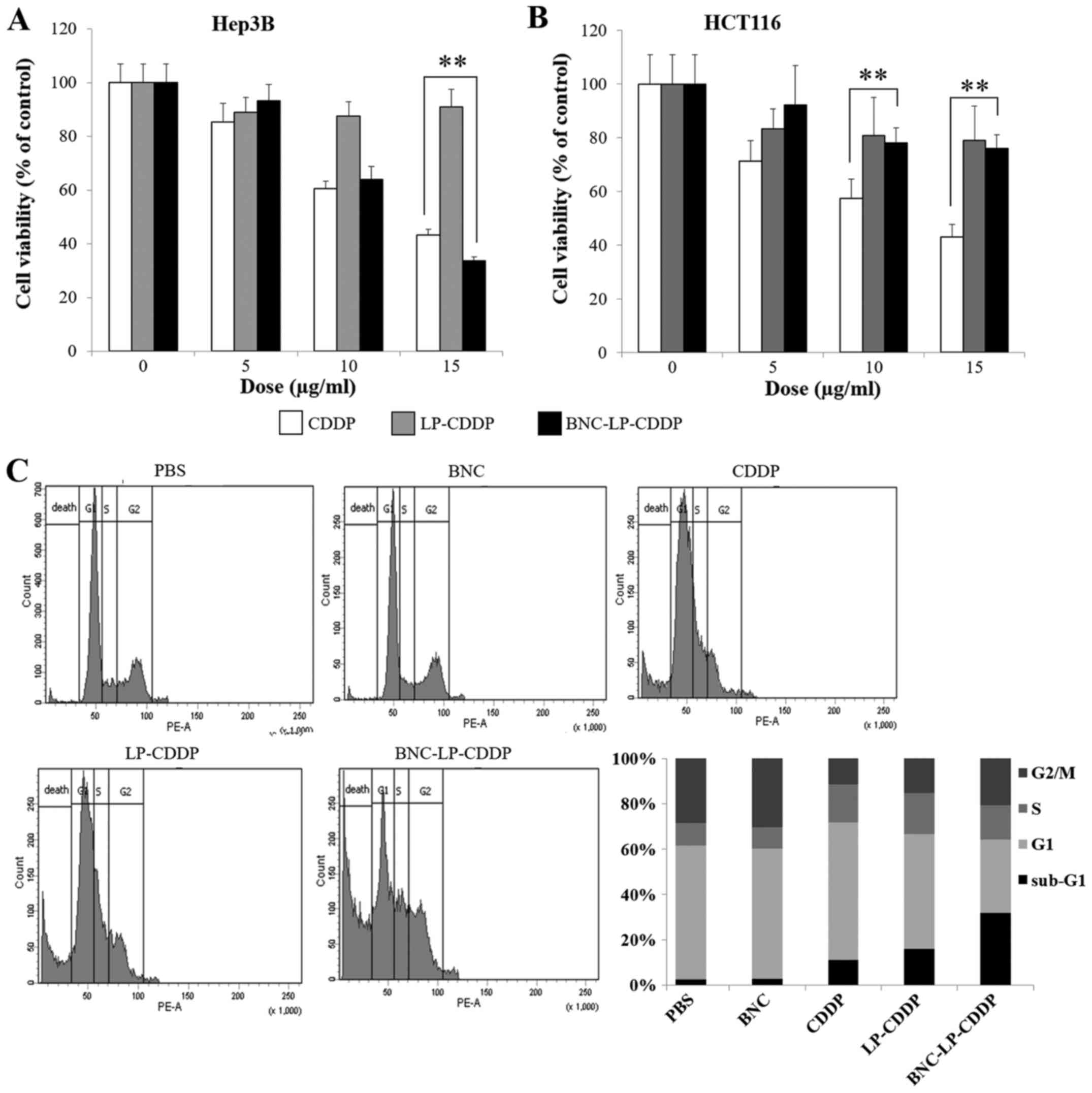 | Figure 2.HCC-specific cytotoxicity of
BNC-LP-CDDP. (A and B) Cell viability assay. Hep3B (HCC) (A) and
HCT116 (colon cancer) (B) cells were treated with
cis-diamminedichloroplatinum (II) (CDDP), liposomal CDDP
(LP-CDDP), or BNC-LP-CDDP at different concentrations (0, 10 or 15
µg/ml) for 4 h. After incubation for 48 h without any drug,
cellular viability was measured using the Cell Counting kit-8. Data
are presented as mean ± standard deviation. **P<0.001. (C) Cell
cycle distribution. Hep3B cells were treated with PBS, BNC, CDDP,
LP-CDDP, or BNC-LP-CDDP at 5 µg/ml for 4 h. After incubation for 48
h without any drug, cells were subjected to FACS analysis and were
stained with propidium iodide (PI), and the DNA contents were
measured using flow cytometry. |
To examine the effect of BNC-LP-CDDP on cell cycle
distribution and the sub-G1 population of Hep3B cells, the cells
were exposed to a lower concentration (5 µg/ml) of drugs and were
subjected to analysis of the DNA contents using flow cytometry. We
found that BNC-LP-CDDP treatment markedly increased the sub-G1
population of the cells to 31.3%, whereas CDDP and LP-CDDP
treatment resulted in a lower sub-G1 population. These results
indicate that BNC-LP-CDDP strongly induces cell death in human HCC
cells.
Radiosensitization of HCC cells by
BNC-LP-CDDP
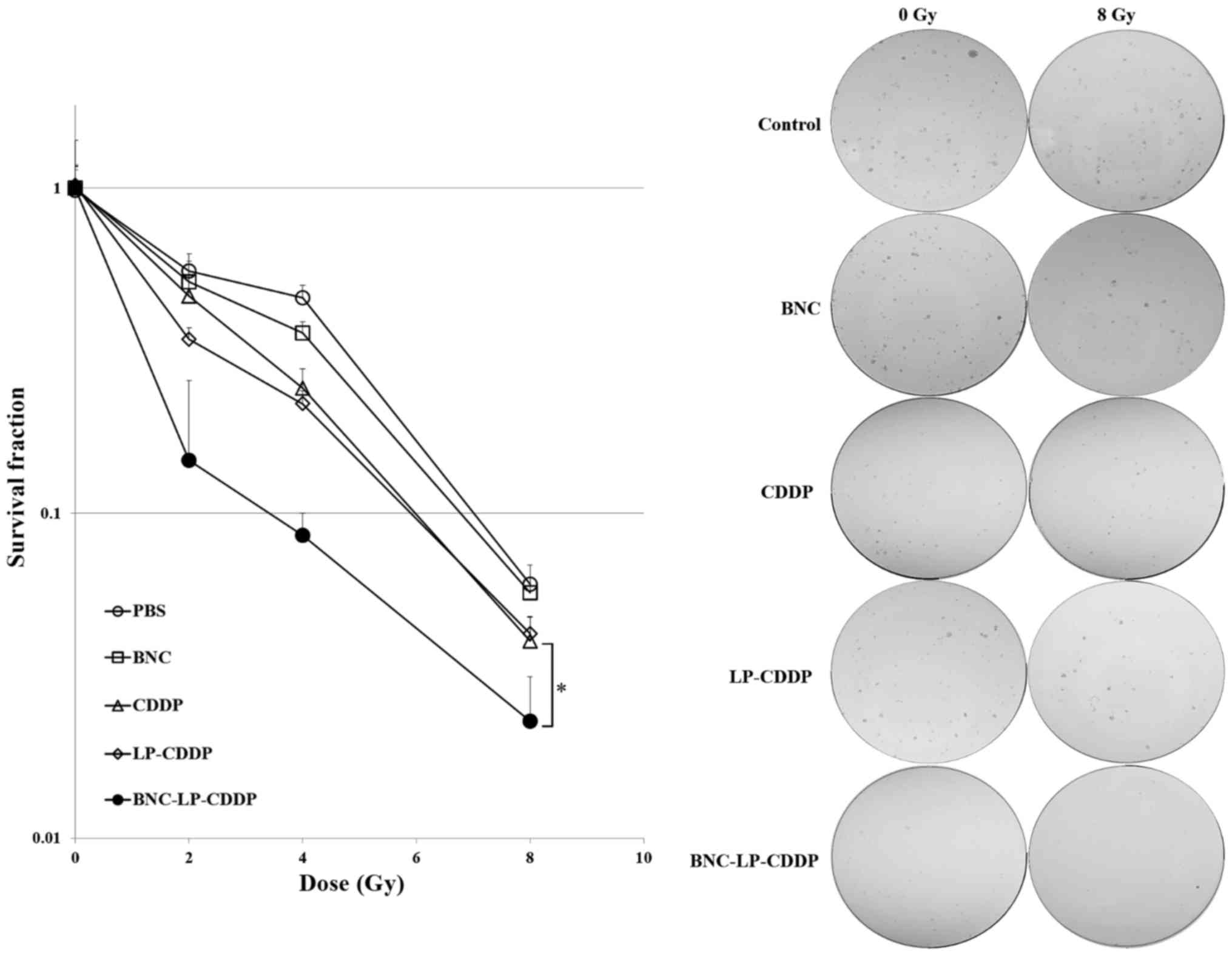
To assess the effect of BNC-LP-CDDP on the in
vitro chemoradiotherapeutic efficacy, Hep3B cells were treated
with BNC, CDDP, LP-CDDP, or BNC-LP-CDDP at a lower concentration
(such as 0.5 µg/ml) and were exposed to IR at 0, 2, 4 or 8 Gy. In
the clonogenic assay, the survival fractions of the cells without
drug treatment were estimated as follows: 1, 0.56, 0.46 and 0.06 at
radiation doses of 0, 2, 4 and 8 Gy, respectively (Fig. 3). The survival fraction of cells was
most effectively reduced when the cells were pre-treated with
BNC-LP-CDDP, which led to survival fractions of 1, 0.15, 0.09 and
0.02 at 0, 2, 4, and 8 Gy, respectively. The sensitizer enhancement
ratio (SER) of BNC-LP-CDDP, LP-CDDP and CDDP were 5.83, 2.12 and
1.41, respectively, compared to that with IR alone. These results
suggest that BNC-LP-CDDP potentially exerted radiosensitization
effects in HCC cells.
Enhancement of radiotherapeutic
efficacy by BNC-LP-CDDP in vivo
As BNC-LP-CDDP showed in vitro
radiosensitization effects in HCC cells, we then examined the in
vivo effect of BNC-LP-CDDP on the chemoradiotherapeutic
efficacy in mice bearing Hep3B xenograft tumors, compared with that
of CDDP or LP-CDDP. The mice were i.v. injected with CDDP, LP-CDDP,
or BNC-LP-CDDP (single dose, 2 mg/kg; equivalent to the amount of
CDDP) 2 h before IR at 3 Gy. Tumor growth in the CDDP-, LP-CDDP- or
BNC-LP-CDDP-treated mice without IR irradiation was delayed, as
compared to that in the control group (Fig. 4A). On day 32, the treated/control
tumor size (T/C) percentage values of the control, CDDP-, LP-CDDP-
and BNC-LP-CDDP-treated groups were 100, 66, 73.6 and 56%,
indicating that BNC-LP-CDDP exerted better in vivo
therapeutic efficacy than CDDP or LP-CDDP, even without combination
treatment with IR (Table I). When
the treatment was combined with IR, the T/C percentage values of IR
alone, CDDP, LP-CDDP and BNC-LP-CDDP treatment on day 32 were
estimated as 34.4, 24.6, 26.4 or 4.3%, respectively (Table I). These results suggest that
BNC-LP-CDDP displays potent radiosensitization effects in
vivo and significantly enhances the chemoradiotherapeutic
efficacy. An equal amount of BNC and BNC-LP-CDDP was tested under
similar experimental conditions to confirm the effect on
radiotherapy. BNC did not affect tumor growth or radiosensitivity
(Fig. 4C). Considerable changes in
body weight were not observed in this experiment, indicating that
none of the mice suffered from severe toxicity (Fig. 4B and D). Thus, these results clearly
demonstrate that BNC-LP-CDDP effectively enhances the
chemoradiotherapeutic efficacy in vivo without severe
toxicity.
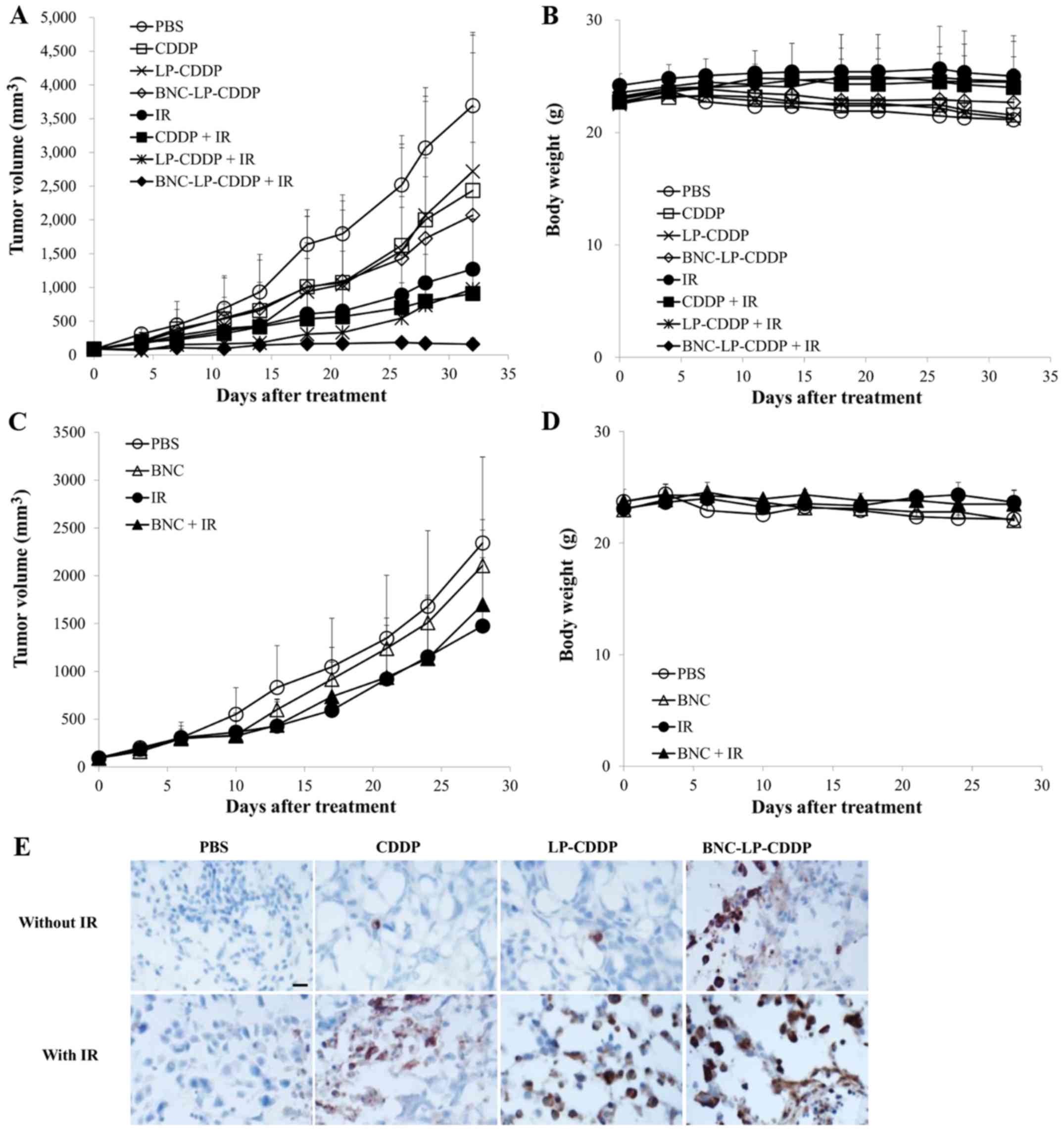 | Figure 4.In vivo radiosensitization
effect and apoptosis induction by BNC-LP-CDDP. (A-D) Tumor growth
delay curves (A, C) and changes in body weight (B and D). Mice
bearing the Hep3B tumor were intravenously administered with CDDP,
liposomal CDDP (LP-CDDP), BNC-LP-CDDP (A), or BNC (C) at a single 2
mg/kg dose based on the amount of CDDP. After 2 h, tumors were
irradiated with IR at 3 Gy, and the tumor volumes and body weights
were monitored during the experimental course. Data are presented
as the mean ± standard deviation. (E) Mice bearing the Hep3B tumor
were treated intravenously with CDDP, liposomal CDDP (LP-CDDP), and
BNC-LP-CDDP at a single 10 mg/kg dose based on the amount of CDDP.
After 1 day, the tumors were harvested and cryo-sectioned. DAB
images of immunohistochemically stained activated caspase-3 were
obtained under a microscope. Scale bar, 20 µm. |
 | Table I.The treated/control tumor size (T/C)
percentage values. |
Table I.
The treated/control tumor size (T/C)
percentage values.
| Treatment | TGI % (Day 32) |
|---|
| PBS | 100 |
| CDDP | 66.0 |
| LP-CDDP | 73.6 |
| BNC-LP-CDDP | 56.0 |
| IR | 34.4 |
| CDDP + IR | 24.6 |
| LP-CDDP + IR | 26.4 |
| BNC-LP-CDDP +
IR | 4.3 |
Increased apoptosis induction by
BNC-LP-CDDP in vivo
To determine whether BNC-LP-CDDP induces more
apoptosis than other drug, the levels of activated caspase-3 in
tumor tissue were estimated via immunohistochemistry. As shown in
Fig. 4E, BNC-LP-CDDP without IR
induced greater apoptosis in tumor tissue as compared to CDDP alone
or LP-CDDP alone. Notably, BNC-LP-CDDP with IR significantly
increased the cleavage of caspase-3 as compared to other drugs.
These results suggest that BNC-LP-CDDP effectively induced
apoptosis in tumor tissue, which led to greater enhancement of the
in vivo therapeutic efficacy.
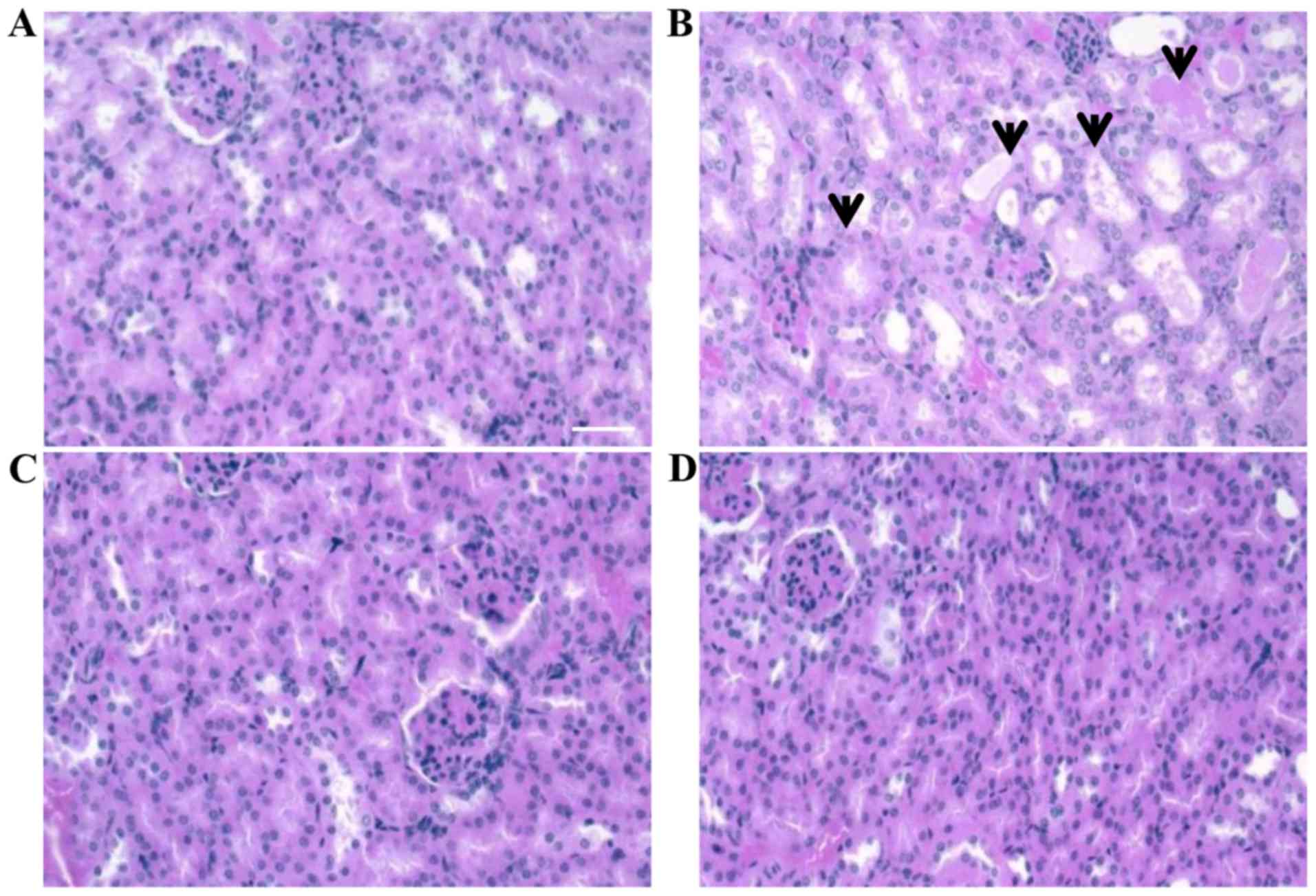
Withdrawal of nephrotoxicity by
BNC-LP-CDDP
The side-effects of CDDP, including nephrotoxicity,
are well known, and hence, we investigated whether BNC-LP-CDDP led
to the withdrawal of CDDP-induced nephrotoxicity through a
histopathological examination in the mice. Mice were injected with
a single 10-mg/kg dose of CDDP, LP-CDDP, or BNC-LP-CDDP. After 4
days, the kidney tissue was collected and stained with hematoxylin
and eosin. Acute cortical tubular degeneration and regeneration
were observed in the kidney in CDDP-treated animals (Fig. 5B). In contrast, the kidneys of mice
treated with BNC-LP-CDDP did not show any observable toxic damage
(Fig. 5D), similar to those treated
with LP-CDDP (Fig. 5C). These
results indicate that BNC-LP-CDDP did not exhibit the nephrotoxic
properties of CDDP, potentially due to the encapsulation of CDDP
into the LP.
Discussion
HCC is the most common cause of cancer-related
death, particularly in Asia. Moreover, HCC is known to be resistant
to the currently available chemotherapeutic agents and radiotherapy
(1,2,27). In
the present study, we introduce BNC-LP-CDDP as a candidate for
overcoming the limitations in the current treatment of human HCC.
The use of LPs as carriers of drugs or genes has been attempted in
anticancer therapy (22,23). For instance, Doxil, wherein
doxorubicin is incorporated in an LP, was the first nano anticancer
drug to be approved by the FDA (28). Although several nanodrugs have been
approved since then, no agent that specifically targets HCC has
been identified. We previously developed a BNC and reported on its
ability to target liver as well as HCC, as LPs serve as a versatile
form of drug delivery system for carrying genes or drugs (29–31).
Furthermore, hepatitis B virus (HBV) infects liver cells only in
humans and chimpanzees, and not in other animals. The
hepatophilicity is determined by the pre-S regions (pre-S1, 2) at
the N-terminal half of the HBV surface antigen L-protein.
Furthermore, pre-S1 is indispensable for the specific binding of
HBV to hepatocytes, whereas pre-S2 in necessary for both the
poly-albumin-mediated cell attachment of HBV and cell permeability
of HBV (30). In the present study,
we described the properties of BNC-LP-CDDP along with pre-S1 in
L-protein as a promising treatment strategy and the potency of
BNC-LP-CDDP as radiosensitizer for HCC through in vitro and
in vivo experiments in human HCC models. In results,
BNC-LP-CDDP targeted specifically to human HCC Hep3B cells both
in vitro (Figs. 2 and
3) and in vivo (Fig. 4), and induced potent apoptosis that
led to a marked enhancement of therapeutic efficacy, of which
results were consistently observed in other hepatoma cell lines
including Huh7 or HepG2 as reported previously by us (29,31).
According to the reports, we concluded through repeated experiment
that BNC targeted HCC better than normal liver tissue (29). Most importantly, BNC-LP-CDDP
displayed great radiosensitization effects via the active-targeted
delivering of CDDP, thus simultaneously eliminating the
nephrotoxicity induced by CDDP (Fig.
5). Given that CDDP is both a competent radiosensitizer and
toxic to the kidney, BNC-LP-CDDP could serve as a promising
therapeutic option and present a new conceptual modality, such as
chemoradiotherapy with active targeting nanomedicine, for human HCC
treatment.
With regard to diverse utilizations of BNC for
targeting other sites, BNC has been modified as zz-BNC harboring
the protein-A Z domain (Staphylococcus aureus), LL-BNC
harboring the protein-L B1 domain (Finegoldia magna), or
LG-BNC harboring the protein-L B1 domain and the protein-G C2
domain (Streptococcus species) by replacing the domain
indispensable for the human hepatotrophic property of BNC. Thus,
BNC can be modified to recognize specific target cells in an
antibody-dependent manner by displaying IgGs such as human IgG3,
human IgM, mouse IgG1 and rat IgG on the modified BNC (29,32,33).
Thus, BNC may represent a promising active targeting-based drug
delivery system for not only HCC, but also other types of human
cancers.
Radiotherapy is a common strategy that is used for
the treatment of diverse cancer types. Above all, radiotherapy for
liver tumor has been performed historically and currently through
diverse strategy including three-dimensional conformal radiation
therapy (3D-CRT), stereotactic body radiation therapy (SBRT) and
transarterial radioembolization (TARE) (34). Concurrent chemoradiotherapy can also
enhance the cure rate of patients resistant to other therapies.
Doxorubicin, gemcitabine, docetaxel, paclitaxel and CDDP are widely
known drugs for chemoradiotherapy. Among these drugs, CDDP is known
to be the most potent radiosensitizer in a wide array of cancer
types, although it cannot be currently used as chemoradiotherapy
for HCC due to the accompanied side-effects. We have confirmed that
BNC-LP-CDDP overcame the original nephrotoxicity due to CDDP via
histopathological analysis. A preclinical toxicology study
regarding the successful clinical application of BNC-LP-CDDP is
currently ongoing.
Acknowledgements
The present study was supported by grants from the
Korean Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI), funded by the Ministry for
Health & Welfare, Republic of Korea (HI06C0868 and HI15C0972);
the National R&D Program for Cancer Control, Ministry of Health
& Welfare, Republic of Korea (15201101); and the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (NRF-2017R1D1A1B03034359
and NRF-2017R1D1A1B03035167).
References
|
1
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yau T, Chan P, Ng KK, Chok SH, Cheung TT,
Fan ST and Poon RT: Phase 2 open-label study of single-agent
sorafenib in treating advanced hepatocellular carcinoma in a
hepatitis B-endemic Asian population: Presence of lung metastasis
predicts poor response. Cancer. 115:428–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Romero Mendez A and Høyer M: Radiation
therapy for liver metastases. Curr Opin Support Palliat Care.
6:97–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gallicchio R, Nardelli A, Mainenti P,
Nappi A, Capacchione D, Simeon V, Sirignano C, Abbruzzi F, Barbato
F, Landriscina M, et al: Therapeutic strategies in HCC: Radiation
modalities. BioMed Res Int. 2016:12953292016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris AL: Hypoxia: a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basu Jothy KS, Bahl A, Subramani V, Sharma
DN, Rath GK and Julka PK: Normal tissue complication probability of
fibrosis in radiotherapy of breast cancer: Accelerated partial
breast irradiation vs conventional external-beam radiotherapy. J
Cancer Res Ther. 4:126–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong SY1, Park SJ, Yoon SM, Jung J, Woo
HN, Yi SL, Song SY, Park HJ, Kim C, Lee JS, et al: Systemic
delivery and preclinical evaluation of Au nanoparticle containing
beta-lapachone for radiosensitization. J Control Release.
139:239–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung J, Park SJ, Chung HK, Kang HW, Lee
SW, Seo MH, Park HJ, Song SY, Jeong SY and Choi EK: Polymeric
nanoparticles containing taxanes enhance chemoradiotherapeutic
efficacy in non-small cell lung cancer. Int J Radiat Oncol Biol
Phys. 84:e77–e83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woo HN, Chung HK, Ju EJ, Jung J, Kang HW,
Lee SW, Seo MH, Lee JS, Lee JS, Park HJ, et al: Preclinical
evaluation of injectable sirolimus formulated with polymeric
nanoparticle for cancer therapy. Int J Nanomed. 7:2197–2208.
2012.
|
|
12
|
Yoshikawa M, Ono N, Yodono H, Ichida T and
Nakamura H: Phase II study of hepatic arterial infusion of a
fine-powder formulation of cisplatin for advanced hepatocellular
carcinoma. Hepatol Res. 38:474–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pottier A, Borghi E and Levy L: New use of
metals as nanosized radioenhancers. Anticancer Res. 34:443–453.
2014.PubMed/NCBI
|
|
14
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao X, Panichpisal K, Kurtzman N and
Nugent K: Cisplatin nephrotoxicity: A review. Am J Med Sci.
334:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: Mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Yang H, Gu K, Chen J, Rui M and
Jiang GL: In vitro and in vivo study of a nanoliposomal cisplatin
as a radiosensitizer. Int J Nanomed. 6:437–444. 2011. View Article : Google Scholar
|
|
18
|
Guo S, Wang Y, Miao L, Xu Z, Lin CM, Zhang
Y and Huang L: Lipid-coated Cisplatin nanoparticles induce
neighboring effect and exhibit enhanced anticancer efficacy. ACS
Nano. 7:9896–9904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada T, Iwabuki H, Kanno T, Tanaka H,
Kawai T, Fukuda H, Kondo A, Seno M, Tanizawa K and Kuroda S:
Physicochemical and immunological characterization of hepatitis B
virus envelope particles exclusively consisting of the entire L
(pre-S1 + pre-S2 + S) protein. Vaccine. 19:3154–3163. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagaoka T, Fukuda T, Yoshida S, Nishimura
H, Yu D, Kuroda S, Tanizawa K, Kondo A, Ueda M, Yamada H, et al:
Characterization of bio-nanocapsule as a transfer vector targeting
human hepatocyte carcinoma by disulfide linkage modification. J
Control Release. 118:348–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Jung J, Somiya M, Iijima M,
Yoshimoto N, Niimi T, Maturana AD, Shin SH, Jeong SY, Choi EK, et
al: Virosomes of hepatitis B virus envelope L proteins containing
doxorubicin: Synergistic enhancement of human liver-specific
antitumor growth activity by radiotherapy. Int J Nanomed.
10:4159–4172. 2015.
|
|
22
|
Allen TM and Cullis PR: Liposomal drug
delivery systems: From concept to clinical applications. Adv Drug
Deliv Rev. 65:36–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drummond DC, Meyer O, Hong K, Kirpotin DB
and Papahadjopoulos D: Optimizing liposomes for delivery of
chemotherapeutic agents to solid tumors. Pharmacol Rev. 51:691–743.
1999.PubMed/NCBI
|
|
24
|
Deshpande PP, Biswas S and Torchilin VP:
Current trends in the use of liposomes for tumor targeting.
Nanomedicine (Lond). 8:1509–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung J, Iijima M, Yoshimoto N, Sasaki M,
Niimi T, Tatematsu K, Jeong SY, Choi EK, Tanizawa K and Kuroda S:
Efficient and rapid purification of drug- and gene-carrying
bio-nanocapsules, hepatitis B virus surface antigen L particles,
from Saccharomyces cerevisiae. Protein Expr Purif. 78:149–155.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirai M, Minematsu H, Hiramatsu Y,
Kitagawa H, Otani T, Iwashita S, Kudoh T, Chen L, Li Y, Okada M, et
al: Novel and simple loading procedure of cisplatin into liposomes
and targeting tumor endothelial cells. Int J Pharm. 391:274–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dawidczyk CM, Kim C, Park JH, Russell LM,
Lee KH, Pomper MG and Searson PC: State-of-the-art in design rules
for drug delivery platforms: Lessons learned from FDA-approved
nanomedicines. J Control Release. 187:133–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung J, Matsuzaki T, Tatematsu K, Okajima
T, Tanizawa K and Kuroda S: Bio-nanocapsule conjugated with
liposomes for in vivo pinpoint delivery of various materials. J
Control Release. 126:255–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kasuya T, Yamada T, Uyeda A, Matsuzaki T,
Okajima T, Tatematsu K, Tanizawa K and Kuroda S: In vivo protein
delivery to human liver-derived cells using hepatitis B virus
envelope pre-S region. J Biosci Bioeng. 106:99–102. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kasuya T, Jung J, Kinoshita R, Goh Y,
Matsuzaki T, Iijima M, Yoshimoto N, Tanizawa K and Kuroda S:
Chapter 8 - Bio-nanocapsule-liposome conjugates for in vivo
pinpoint drug and gene delivery. Methods Enzymol. 464:147–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tatematsu K, Iijima M, Yoshimoto N, Nakai
T, Okajima T and Kuroda S: Bio-nanocapsules displaying various
immunoglobulins as an active targeting-based drug delivery system.
Acta Biomater. 35:238–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsutsui Y, Tomizawa K, Nagita M, Michiue
H, Nishiki T, Ohmori I, Seno M, Matsui H, et al: Development of
bionanocapsules targeting brain tumors. J Control Release.
122:159–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanguturi SK, Wo JY, Zhu AX, Dawson LA and
Hong TS: Radiation therapy for liver tumors: Ready for inclusion in
guidelines? Oncologist. 19:868–879. 2014. View Article : Google Scholar : PubMed/NCBI
|