Introduction
Lung cancer is one of the most serious causes of
cancer-related deaths worldwide (1). It affects human life and health
intimately, and accounts for 19.4% of all cancer deaths (2). Non-small cell lung cancer (NSCLC)
accounts for up to 85% of lung cancers (3–5), which
includes adenocarcinoma, squamous cell carcinoma, and large cell
carcinoma. The morbidity of lung adenocarcinoma has increased
steadily in the past 30–40 years (6,7). The
overall 5-year survival rate for a patient with lung cancer is no
more than 20%. Approximately 33% of patients with lung cancer are
diagnosed at an advanced stage and not suitable for surgery
(8–10). Radiotherapy is considered to be one
of the most effective and commonly applied treatment to human
malignancies. However, radioresistance of tumor cells often become
an obstruction in lung cancer treatment. How to reduce the
resistance to radiation or improve the radiosensitivity is under
active study in lung cancer radiotherapy (11,12).
c-Myc, n-Myc, and l-Myc are the members of the Myc
oncoprotein superfamily, they have similar functions, but are
expressed differently in various cancer types. c-Myc is usually
overexpressed in both blood-borne and solid tumors. n-Myc is mainly
expressed in neural tumors, and l-Myc is generally upregulated in
small cell lung cancer (13–15).
As a heterodimer partner, MYC-associated factor X (MAX) helps c-Myc
to regulate at least 15% of gene transcription of human genome
(16). c-Myc plays an important
role in the broad spectrum of cell functions, including cell
proliferation, metabolism, and differentiation. It can enhance the
sensitization of cells to apoptotic stimuli to further promote cell
apoptosis (17,18).
Fas signaling pathway is one of the most classical
mechanisms inducing apoptosis (19). As a 45-kDa type I transmembrane
protein, Fas (CD95, APO-1) is a member of the tumor necrosis factor
receptor family (20). Its natural
ligand, FasL, is only expressed in the lung, small intestine,
testes, and anterior chamber of the eye (21,22).
Once FasL combined with Fas to form the death-inducing signaling
complex, it initiates caspase-8 cleavage, which activates the
downstream effector caspases (e.g., caspase-3), resulting in
apoptosis.
Although it has been demonstrated that modulation of
FAS and c-Myc altered radiation response in radioresistant cell
lines, less is known about the relationship between c-Myc and Fas.
In this study, we established radiation-resistant A549 cell model,
and investigated the roles of c-Myc and Fas in radiation induced
cytotoxicity of A549 cells. We found that inhibition of c-Myc
expression had an important role in irradiation-mediated apoptosis
in A549/R cells, by upregulation of Fas and activation of caspase-8
which is associated with the Bid-mediated mitochondrial pathway of
apoptosis.
Materials and methods
Cell culture and treatment
A549 and LTEP-α-2 cells were obtained from Shanghai
Institute of Cell Biology, China. A549/R cells were cultured
through X-rays irradiation of up to 68 Gy by using X-RAD 320 (PXi,
North Branford, CT, USA) as previously described (23). The cells were cultured in Roswell
Park Memorial Institute medium (RPMI-1640; Hyclone; GE Healthcare,
Logan, UT, USA) mixing together with 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare) at 37°C with 5% CO2.
EdU proliferation assay
Cell replication activity was dectected using
5-ethynyl-2-deoxyuridine (EdU) test kits (EdU; RiboBio Co. Ltd.,
Guangzhou, China) according to the manufacturer's protocol.
Briefly, the cells were exposed to 50 µm of EdU for 2 h at 37°C.
Then, the cells were fixed with 4% paraformaldehyde for 20 min and
wash with 0.5% Triton X-100 in PBS five times. Afterwards, the
cells were reacted with 100 µl of 1X Apollo® reaction
cocktail for 30 min. Following wash with PBS, the cells were
stained with 100 µl of Hoechst 33258 (5 µg/ml) for 20 min and
visualized under a fluorescent microscope (Leica DFC450 C; Leica
Microsystems GmbH, Wetzlar, Germany).
Cell cycle analysis
A549, LTEP-α-2 or A549/R cells were seeded in the
6-well plate and irradiated from 0 to 10 Gy 24 h later. Cells were
then incubated for 24 h and collected for cell cycle analysis as
previous described (24).
Apoptosis detection
The cells were stained with Annexin V-FITC/propidium
iodide (PI) (BD Pharmingen, San Diego, CA, USA) according to the
manufacturer's instructions as previously reported (23). The cell apotosis was anazlyed using
flow cytometry (FACSCalibur; Becton-Dickinson; BD Biosciences,
Franklin Lakes, NJ, USA).
MTT assay
Cells were seeded at a density of 1×104
cells/well into a 96-well plate and grown in 5% CO2 at
37°C for 24 h. The cells were then transfected with DNA or RNA and
their negative controls or irradiated. After transfection or
irradiation, each well was added with 10 µl MTT (MTT;
Sigma-Aldrich, St. Louis, MO, USA) and incubated with cells for 4 h
in an incubator. The formazan was dissolved in 150 µl dimethyl
sulfoxide (DMSO) and the optical density was measured using
enzyme-linked immunosorbent assay reader (ELx800; USA) at an
absorption wavelength of 570 nm.
Transfection
The c-Myc plasmid was purchased from GeneChem, Inc.
(Shanghai, China). The siRNA against c-Myc was obtained from
GenePharma. Transfection of DNA (plasmids) and RNA (siRNA) was
performed using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
instructions.
Western blot analysis
Cells were harvested and lysed as per a previous
study (24). The primary antibodies
against c-Myc, Fas, Bcl-2, Bax, Bid, caspase-3, caspase-8 and
caspase-9 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were
used according to the manufacturer's instructions, and GAPDH
(Bioworld Technology, Inc., St. Louis Park, MN USA) was used as the
control. Protein bands were detected by incubation with horseradish
peroxidase-conjugated secondary antibody (Beijing Zhongshan Golden
Bridge Technology Co., Ltd., Beijing, China) at room temperature
for 2 h, and visualized with Fluor Chem FC2 gel imaging system.
Statistical analysis
All experiments were repeated three times
independently and the data are presented as the mean ± standard
deviation (SD) from triplicate parallel experiments. PRISM
(GraphPad Software, San Diego, CA, USA) was performed to analyze
the data. Group mean comparisons were compared using an unpaired,
two-sided, Student's t-test. ANOVA was used to compare different
groups with respect to continuous variables. P-value <0.05 was
considered to be significant.
Results
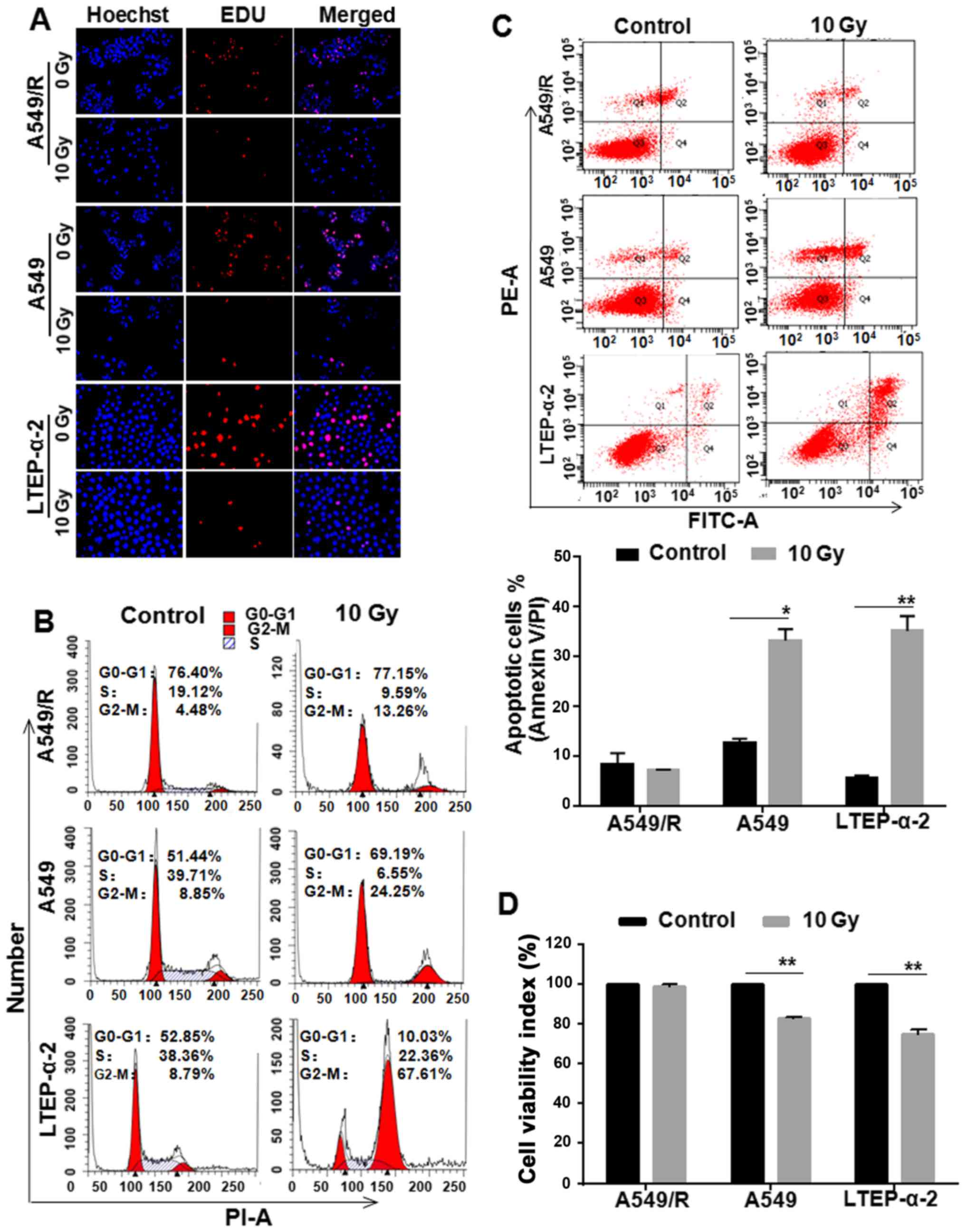
Apoptosis and proliferation changes to
radiation in A549/Rcells
To establish radiation-resistant A549 (A549/R)
cells, parental A549 cells were prolonged exposed to X-rays for 68
Gy (2 Gy/day, 5 days/week). EdU detection showed fewer cells
proliferated in A549/R, A549 and LTEP-α-2 cells treated with 10 Gy
X-rays compared with respective control cells (Fig. 1A). Similarly, more cells were
blocked in G2-M phase in A549/R and NSCLC cells (Fig. 1B). However, apoptotic detection
showed that there were fewer apoptotic cells in A549/R cells
treated with radiation than that in A549 and LTEP-α-2 cells
(Fig. 1C). MTT assay further proved
that the number of viable cells was much more in the A549/R cell
cultures exposed to 10 Gy of X-rays than those in A549 and LTEP-α-2
cells (Fig. 1D). These data
suggested that the resistance of radiation-induced apoptosis is
involved in A549/R cells compared with NSCLC A549 and LTEP-α-2
cells.
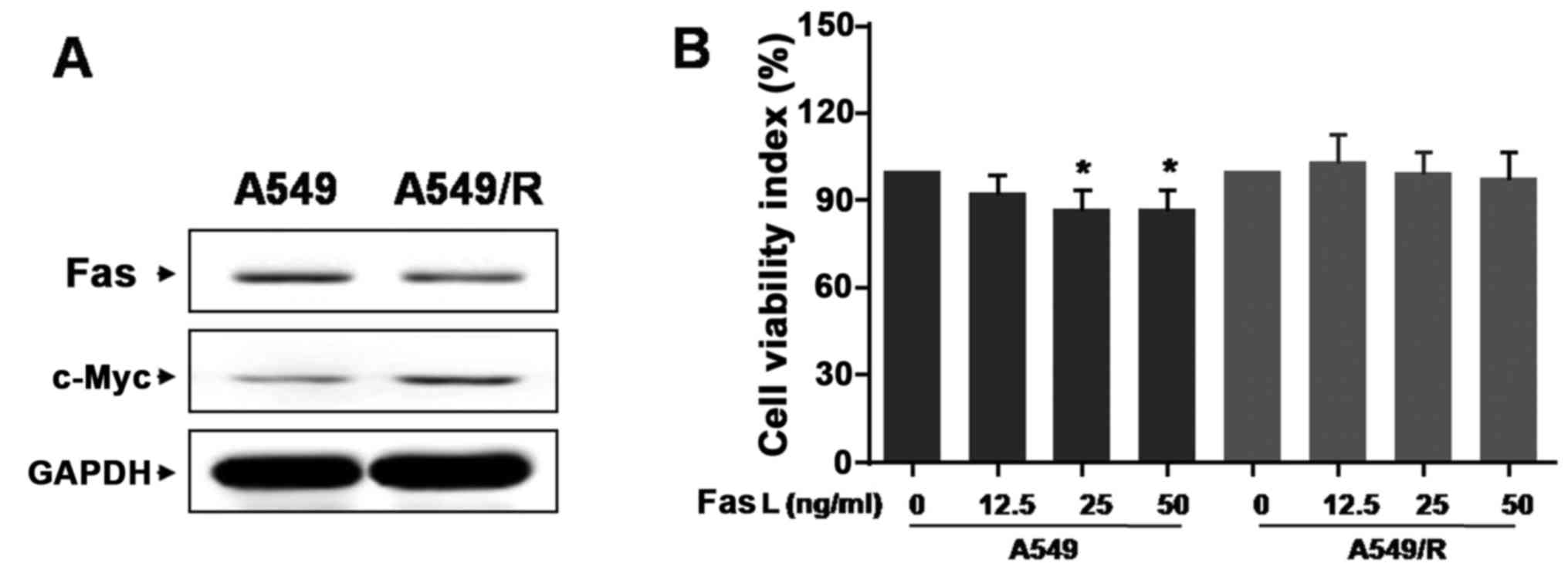
Resistance of A549/R cells to
radiation involves c-Myc and Fas
Next, we studied whether c-Myc and Fas play
important roles in the resistance of A549/R cells to irradiation,
both of which have been implicated in tumor aggression and
radiation therapy (25,26). Intriguingly, Fas expression notably
decreased in A549/R cells compared to A549 cells, while c-Myc was
highly expressed (Fig. 2A),
indicating that there is an inverse expression pattern between
c-Myc and Fas in A549 and A549/R cells.
Only the Fas ligand (FasL), binds to their cell
surface death receptor Fas, the extrinsic pathway of apoptosis is
activated. To determine if FasL was involved in the radioresistance
of A549/R cells, we used the exogenously sufficient FasL to
activate the Fas-meditated signals. We found that 25 ng/ml FasL
significantly inhibited A549 cell growth compared with untreated
cells. Nevertheless, 25 ng/ml FasL could not obviously suppress
A549/R cell growth (Fig. 2B). Such
finding suggests that A549/R cell radio-resistance is associated
with Fas downregulation rather than FasL.
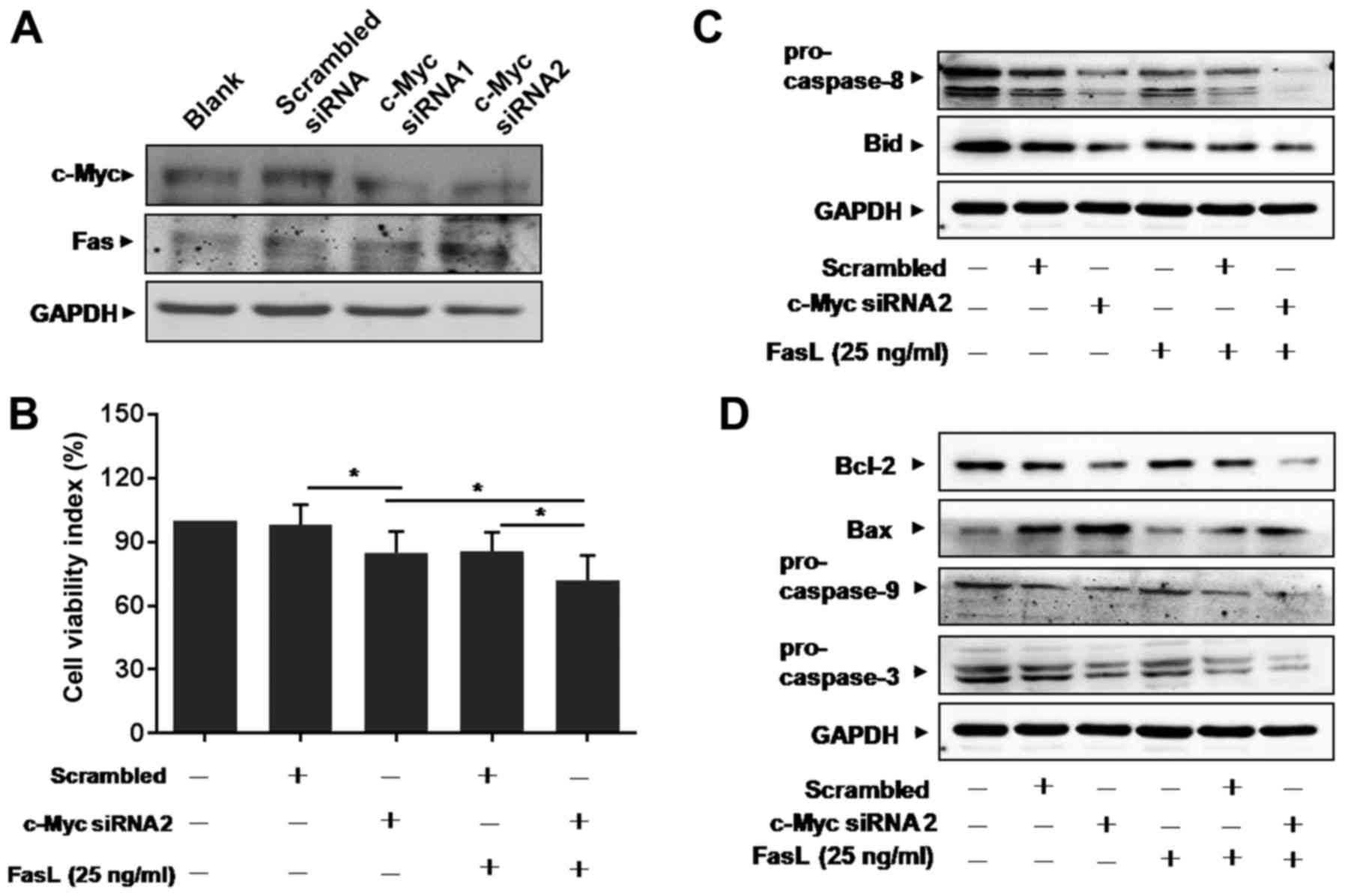
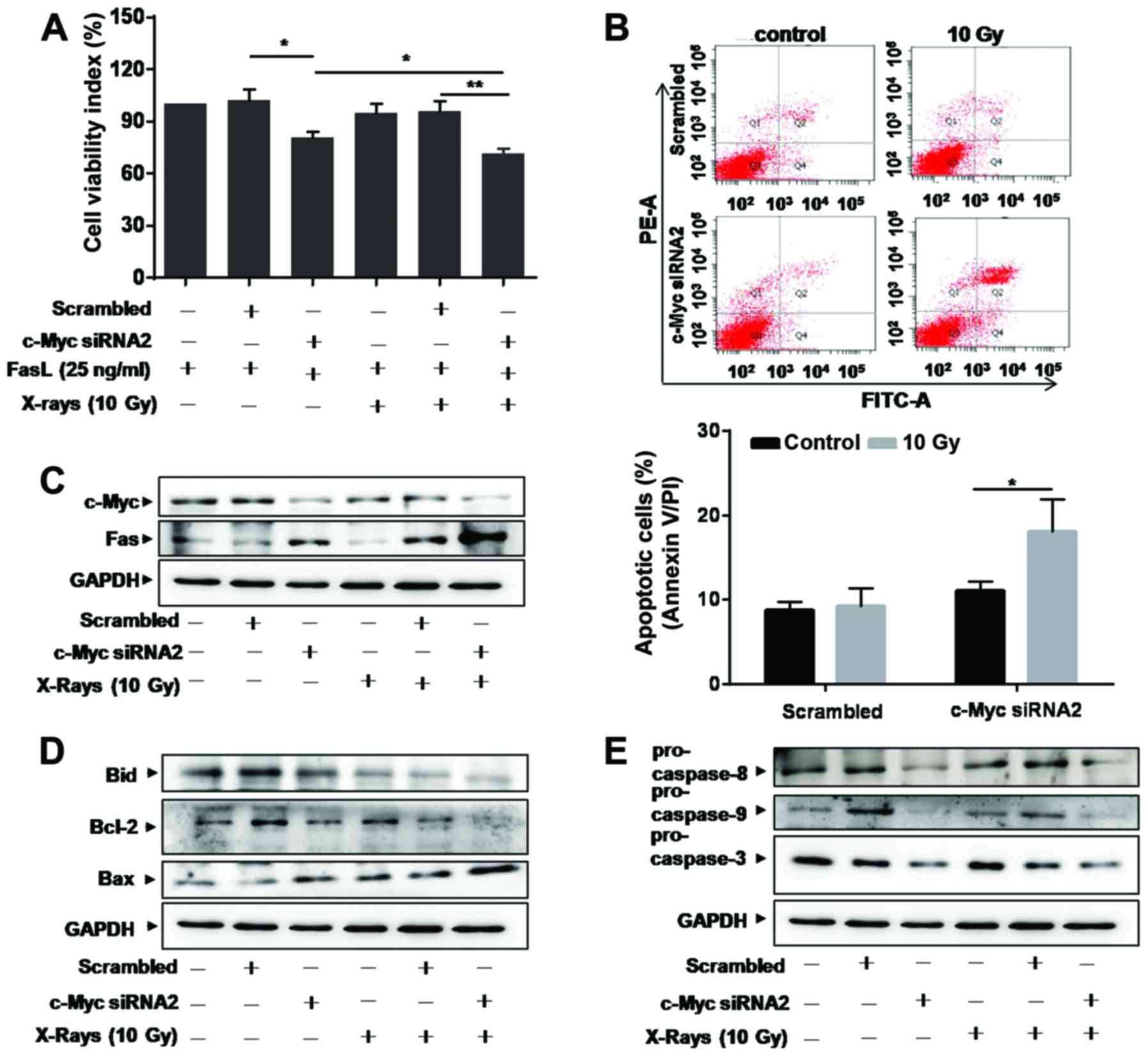
Knockdown of c-Myc enhances
Fas-mediated apoptosis
Little is known about the relationship between c-Myc
and Fas in lung cancer up to now. We supposed that c-Myc could
affect Fas signals and designed siRNA specific to c-Myc to
transfect A549/R cells. The efficacy of siRNA to inhibit c-Myc is
shown in Fig. 3A. After
transfection, the expression level of Fas increased in c-Myc
siRNA-transfected cells, compared with scrambled siRNA-transfected
cells (Fig. 3A). MTT results showed
that the number of viable A549/R cells decreased in c-Myc siRNA
treated cultures (Fig. 3B). We
found that FasL treatment further decreased the number of viable
cells in c-Myc siRNA-transfected A549/R cells (Fig. 3B).
Caspase-8 is a major initiator caspase in the death
receptor-dependent apoptotic pathway and a known mediator of Bid
(27). Our results showed that the
expression of pro-caspase-8 and total Bid decreased in c-Myc
siRNA-transfected A549/R cells (Fig.
3C). FasL treatment further decreased the levels of
pro-caspase-8 and total Bid (Fig.
3C). Furthermore, Bid is known as a critical mediator of the
mitochondrial pathway of apoptosis following death receptor
activation (27). We next
investigated the expression of anti-apoptotic protein Bcl-2 and
pro-apoptotic protein Bax, both of which are critical mediators of
the mitochondrial pathway of apoptosis. Confirming our hypothesis,
Bcl-2 expression decreased, while Bax increased in cells
transfected with c-Myc siRNA (Fig.
3D). The expression of pro-caspase-9 and −3 was also
downregulated significantly in c-Myc siRNA-transfected A549/R cells
(Fig. 3D), which lead to increase
in cell apoptosis. These results indicate that Fas-mediated
apoptosis is required for radiation induced apoptosis in A549/R
cells.
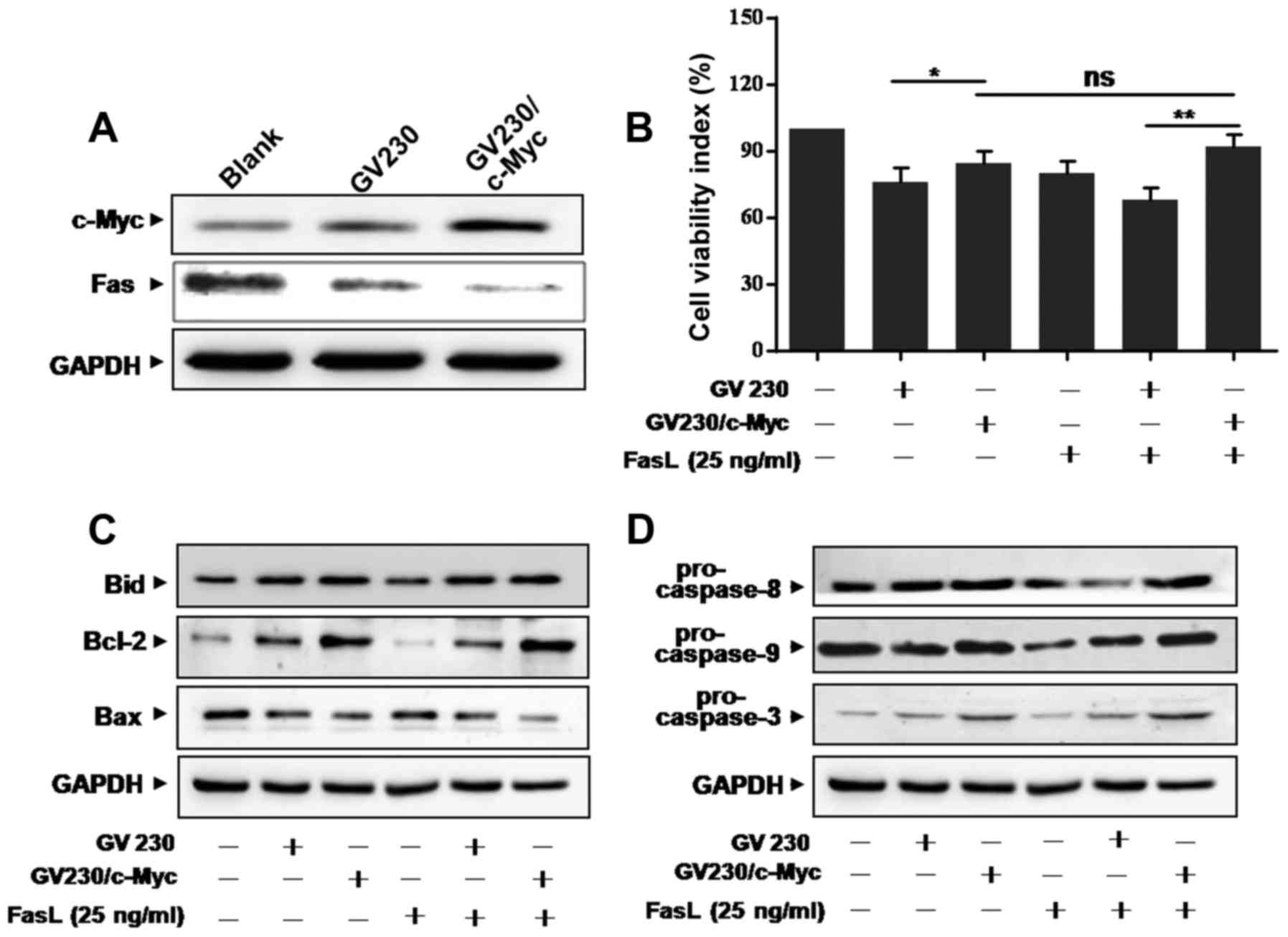
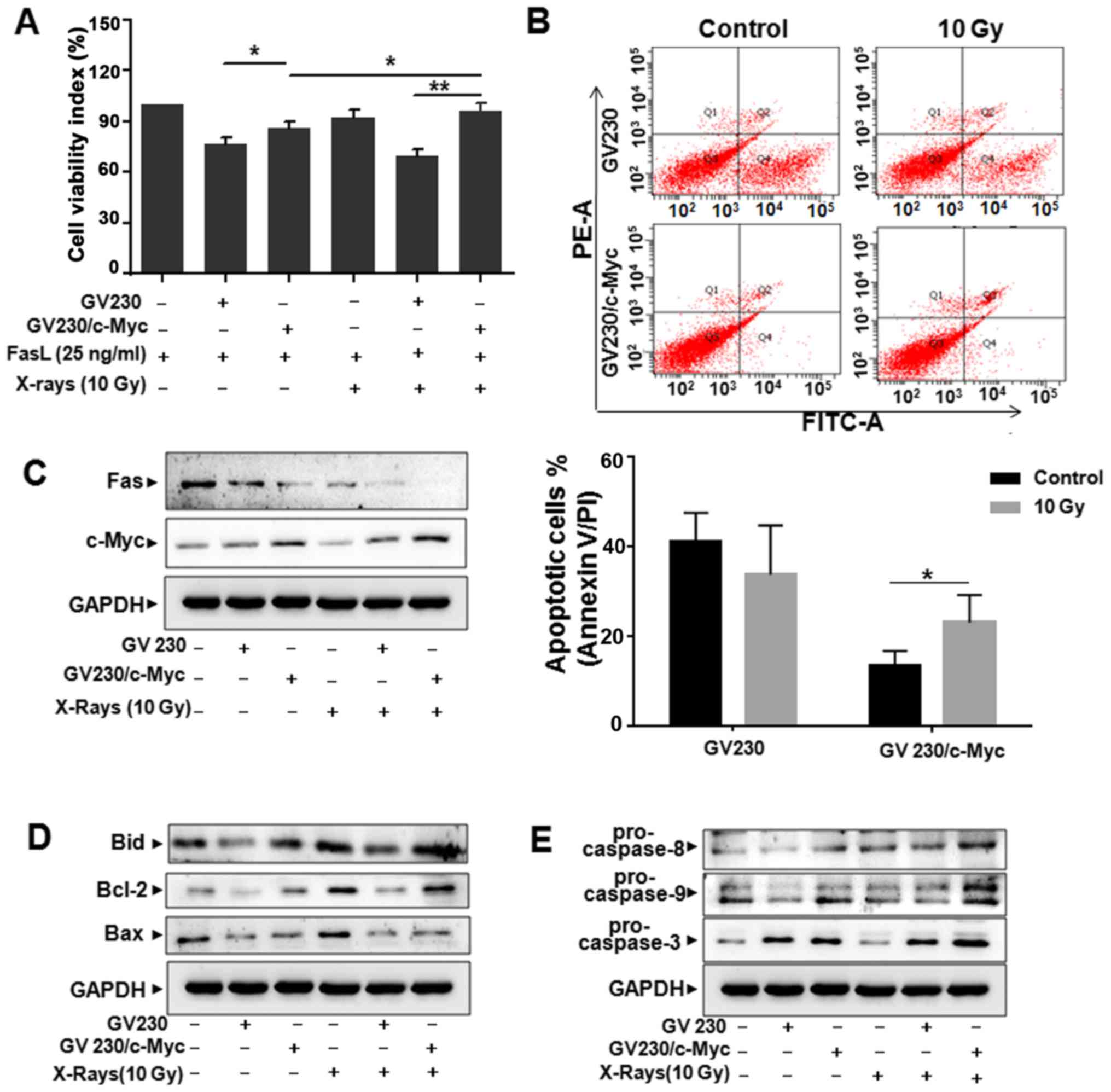
c-Myc overexpression attenuates
Fas-mediated apoptosis
To further investigate the effects of c-Myc on
Fas-mediated apoptosis in A549 cells, c-Myc overexpression plasmid
(GV230/c-Myc) was employed. First, the overexpression efficiency
was confirmed by western blotting (Fig.
4A). Interestingly, the expression level of Fas was attenuated
by ectopic expression of c-Myc in A549 cells (Fig. 4A). MTT assay showed that the reduced
number of viable cells by FasL was further attenuated by
overexpression of c-Myc in both GV230/c-Myc- and FasL-treated A549
cells (Fig. 4B). We then determined
whether overexpression of c-Myc could affect Fas-mediated apoptosis
pathway. Results showed that the levels of Bid and Bcl-2 were
upregulated, while Bax was downregulated in GV230/c-Myc-transfected
A549 cells treated with FasL (Fig.
4C). Also, the levels of pro-caspase-8, −9 and −3 increased in
GV230/c-Myc-transfected A549 cells (Fig. 4D). These results supported that
upregulation of c-Myc resulted in decreasing Fas expression level,
which is related to Fas-mediated apoptosis.
Knockdown of c-Myc sensitizes A549/R
cells to radiation
We supposed that downregulation of c-Myc could
sensitize A549/R cells to radiation. To address this hypothesis, we
treated A549/R cells with c-Myc siRNA for 24 h before subjecting to
radiation. MTT assay showed that cell viability markedly decreased
in cells transfected with c-Myc siRNA compared with scrambled siRNA
when A549/R cells were exposed to FasL alone or combined with 10 Gy
of X-rays (Fig. 5A). Apoptosis
assay showed that more apoptotic cells were found in c-Myc siRNA
transfected A549/R cells exposed to radiation (Fig. 5B). These results indicated that
knockdown of c-Myc significantly enhanced the sensitivity of A549/R
cells to radiation.
We further evaluated the potential mechanisms of
c-Myc siRNA on sensitivity of A549/R cells to radiation. When c-Myc
expression decreased after siRNA treatment, Fas was upregulated,
especially in 10 Gy of X-ray-treated cells (Fig. 5C). Simultaneously, total Bid and
anti-apoptotic protein Bcl-2 decreased, while Bax was upregulated
in c-Myc siRNA-transfected A549/R cells (Fig. 5D). Western blotting demonstrated
that the levels of pro-caspase-8, −9 and −3 were decreased, which
are related to Fas-meditated apoptosis (Fig. 5E). These results provided evidence
for the mechanisms of c-Myc and Fas in the sensitivity of A549/R
cells to radiation.
Upregulation of c-Myc reduces the
sensitivity of A549 cells to radiation
To further investigate the effects of c-Myc
deregulation on lung cancer cells to irradiation. The plasmid of
GV230/c-Myc or GV230 was transfected into A549 cells, respectively.
Compared with that of GV230-transfected cells, the cell viability
significantly increased in GV230/c-Myc-transfected A549 cells when
exposed to FasL (25 ng/ml) or irradiation (10 Gy) (Fig. 6A). Likewise, apoptosis of
GV230/c-Myc-transfected A549 cells was significantly decreased
compared with GV230-transfected cells when treated with radiation
(10 Gy) (Fig. 6B). These data
indicated that the expression level of c-Myc played a critical role
in radioresistance of A549 cells.
Next, western blot assay was performed to detect the
expression of Fas. As shown in Fig.
6C, when combined with irradiation treatment (10 Gy), c-Myc
overexpression significantly decreased Fas expression compared with
GV230 group. The expression of Bid and Bcl-2 increased obviously in
GV230/c-Myc-transfected A549 cells compared with the cells
transfected with GV230 after exposure to 10 Gy of X-rays, but the
expression of Bax decreased (Fig.
6D). Also, western blotting results indicated that the
expression of pro-caspase-8, −9 and −3 protein in c-Myc
overexpression A549 cells increased in comparison with those
derived from GV230-transfected A549 cells, when treated with
radiation (Fig. 6E). Collectively,
upregulation of c-Myc could lead to the decreased radiation-induced
Fas-mediated apoptosis in A549 cells.
Discussion
A series of reports have demonstrated mechanisms of
radioresistance, but the relationship of c-Myc and Fas in
radiotherapy of lung adenocarcinoma remains unclear. In the present
study, we established radiation-resistant A549 cell models
(A549/R), and showed that there is an inverse expression pattern
between c-Myc and Fas in A549/R cells. Notably, this study
demonstrates for the first time that upregulation of Fas through
inhibition of expression of c-Myc has an important role in
irradiation-mediated lethality in A549/R cells. In addition, our
results suggest that caspase-8-mediated Bid cleavage and the
subsequent association with the mitochondrial pathway of apoptosis,
which is primarily controlled by the Bcl-2 family proteins, are two
critical events that are responsible for induction of apoptosis
during irradiation-mediated cell death in A549 cells.
c-Myc is a common transcriptional factor. The
amplification of c-Myc consequently results in an increased
expression, which not only leads to the occurance of human cancers,
but also influences the effect of cancer therapy (28). As a multifunctional protein, c-Myc
has two distinct characteristics. One is the proto-oncoprotein
property and the other is the tumor suppressor property, which
leads to apoptosis, cellular senescence, and DNA damage responses
through triggering the intrinsic barrier of cancer proliferation
(29). As an oncogenic
transcription factor, c-Myc can effectively master the balance
point, as well as control accurately cell proliferation and cell
death. In this study, we found that the expression level of c-Myc
was associated with Fas/FasL-meditated apoptosis. We also found
that c-Myc affected the sensitivity of lung cancer cells to
radiation, which was supported by previous studies (30–33),
demonstrating that cancer radioresistance is also associated with
the overexpression of c-Myc.
Fas death signaling pathway, as an important
extrinsic apoptosis signaling pathways, is triggered by the
interaction of FasL and Fas (34),
which plays an important pro-apoptotic role in the apoptosis of
receptor-mediated manner (35).
Binding intimately with FasL, Fas is awakened to activate
caspase-8, which forms the death inducing signaling complex (DISC).
Caspase-8 oligomerizes its cleaved fragment release from the DISC
to initiate the apoptotic program (36). Several lines of evidence showed that
apoptosis is mediated by receptor or mitochondrial pathway leading
to the successive activation of a series of caspases (37). Indeed, we found that FasL treatment
induced the changes of pro-caspases, which further participated in
c-Myc- and Fas-mediated lung cancer cell apoptosis.
Bcl-2 family is made up of anti- and pro-apoptotic
members. The anti-apoptotic proteins include Bcl-2, Bcl-xL, Bcl-w
and Mcl-1, and the pro-apoptotic family members contains Bax, Bak,
Bid, Bad and Bim (38). Bid (a
cytosolic, globular shaped protein) mainly consists of eight α
helices and two central hydrophobic helices. Caspase-8 can cleave
full length Bid into truncated fragment (tBid), which links
caspase-8 activation together with mitochondrial death machinery to
act as an intracellular bridge (39). tBid further actives Bax or Bak to
increase the apoptotic activity (40,41).
In our study we found upregulation of anti-apoptotic protein Bcl-2,
while Bax was downregulated in c-Myc overexpression plasmid-treated
cells compared with control treatment, supporting that Bcl-2 and
Bax play important roles in c-Myc and Fas-mediated apoptosis.
In conclusion, this study proposed a novel
relationship between c-Myc and Fas in A549 cell apoptosis induced
by radiation. Ectopic expression of c-Myc substantially attenuates
Fas-mediated lethality in A549 cells, whereas knockdown of c-Myc
through siRNA significantly enhances Fas-mediated lethality in
A549/R cells. The findings of this study will help in further
understanding the molecular mechanism of radioresistance of lung
carcinogenesis.
Acknowledgements
The authors thank Zheng-Ping Hu (Medical Center,
Binzhou Medical University) for kind help in treating cells with
X-rays irradiation by using X-RAD 320. This study was supported by
the National Natural Science Foundation (nos. 31371321 and
31440061), Shandong Provincial Natural Science Foundation (nos.
ZR2015PH002 and ZR2014HL056), Shandong province Taishan Scholar
Program and Shandong science and Technology Committee (no.
2015GSF118073), Project of Shandong Province Higher Educational
Science and Technology Program (no. J15LM51), the Medicine and
Health and Scientific Development Programme of Shandong Province
(no. 2015WSB30011), and Scientific Development Programme of Binzhou
Medical University (no. BY2014KJ45).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayes DN, Monti S, Parmigiani G, Gilks CB,
Naoki K, Bhattacharjee A, Socinski MA, Perou C and Meyerson M: Gene
expression profiling reveals reproducible human lung adenocarcinoma
subtypes in multiple independent patient cohorts. J Clin Oncol.
24:5079–5090. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: Recent
advances and future directions. Oncologist. 13 Suppl 1:5–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type:
male:female differences diminishing and adenocarcinoma rates
rising. Int J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morita T: A statistical study of lung
cancer in the annual of pathological autopsy cases in Japan, from
1958 to 1997, with reference to time trends of lung cancer in the
world. Jpn J Cancer Res. 93:15–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackson SP and Bartek J: The DNA-damage
response in human biology and disease. Nature. 461:1071–1078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnett GC, West CM, Dunning AM, Elliott
RM, Coles CE, Pharoah PD and Burnet NG: Normal tissue reactions to
radiotherapy: Towards tailoring treatment dose by genotype. Nat Rev
Cancer. 9:134–142. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Osti MF, Agolli L, Valeriani M, Falco T,
Bracci S, De Sanctis V and Enrici RM: Image guided hypofractionated
3-dimensional radiation therapy in patients with inoperable
advanced stage non-small cell lung cancer. Int J Radiat Oncol Biol
Phys. 85:e157–e163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oshiro Y, Mizumoto M, Okumura T, Hashimoto
T, Fukumitsu N, Ohkawa A, Kanemoto A, Hashii H, Ohno T, Sakae T, et
al: Results of proton beam therapy without concurrent chemotherapy
for patients with unresectable stage III non-small cell lung
cancer. J Thorac Oncol. 7:370–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dang CV, Le A and Gao P: MYC-induced
cancer cell energy metabolism and therapeutic opportunities. Clin
Cancer Res. 15:6479–6483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen BJ, Wu YL, Tanaka Y and Zhang W:
Small molecules targeting c-Myc oncogene: Promising anti-cancer
therapeutics. Int J Biol Sci. 10:1084–1096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Riggelen J, Yetil A and Felsher DW:
MYC as a regulator of ribosome biogenesis and protein synthesis.
Nat Rev Cancer. 10:301–309. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ponzielli R, Katz S, Barsyte-Lovejoy D and
Penn LZ: Cancer therapeutics: Targeting the dark side of Myc. Eur J
Cancer. 41:2485–2501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blander JM: A long-awaited merger of the
pathways mediating host defence and programmed cell death. Nat Rev
Immunol. 14:601–618. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HO and Ferguson TA: Biology of FasL.
Cytokine Growth Factor Rev. 14:325–335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Algeciras-Schimnich A, Shen L, Barnhart
BC, Murmann AE, Burkhardt JK and Peter ME: Molecular ordering of
the initial signaling events of CD95. Mol Cell Biol. 22:207–220.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferguson TA and Green DR: Fas-ligand and
immune privilege: The eyes have it. Cell Death Differ. 8:771–772.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang HH, Pang M, Dong W, Xin JX, Li YJ,
Zhang ZC, Yu L, Wang PY, Li BS and Xie SY: miR-511 induces the
apoptosis of radioresistant lung adenocarcinoma cells by triggering
BAX. Oncol Rep. 31:1473–1479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang PY, Sun YX, Zhang S, Pang M, Zhang
HH, Gao SY, Zhang C, Lv CJ and Xie SY: Let-7c inhibits A549 cell
proliferation through oncogenic TRIB2 related factors. FEBS Lett.
587:2675–2681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gravina GL, Festuccia C, Popov VM, Di
Rocco A, Colapietro A, Sanità P, Monache SD, Musio D, De Felice F,
Di Cesare E, et al: c-Myc sustains transformed phenotype and
promotes radioresistance of embryonal rhabdomyosarcoma cell lines.
Radiat Res. 185:411–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horton JK, Siamakpour-Reihani S, Lee CT,
Zhou Y, Chen W, Geradts J, Fels DR, Hoang P, Ashcraft KA, Groth J,
et al: FAS death receptor: A breast cancer subtype-specific
radiation response biomarker and potential therapeutic target.
Radiat Res. 184:456–469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang K, Zhang J, O'Neill KL, Gurumurthy
CB, Quadros RM, Tu Y and Luo X: Cleavage by caspase 8 and
mitochondrial membrane association activate the BH3-only protein
Bid during TRAIL-induced apoptosis. J Biol Chem. 291:11843–11851.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X,
Zhang QB and Yao KT: MYC regulation of CHK1 and CHK2 promotes
radioresistance in a stem cell-like population of nasopharyngeal
carcinoma cells. Cancer Res. 73:1219–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Larsson LG and Henriksson MA: The Yin and
Yang functions of the Myc oncoprotein in cancer development and as
targets for therapy. Exp Cell Res. 316:1429–1437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiang CS, Sawyers CL and Mcbride WH:
Oncogene expression and cellular radiation resistance: A modulatory
role for c-myc. Mol Diagn. 3:21–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim BY, Kwak SY, Yang JS and Han YH:
Phosphorylation and stabilization of c-Myc by NEMO renders cells
resistant to ionizing radiation through up-regulation of γ-GCS.
Oncol Rep. 26:1587–1593. 2011.PubMed/NCBI
|
|
32
|
Papanikolaou V, Iliopoulos D, Dimou I,
Dubos S, Kappas C, Kitsiou-Tzeli S and Tsezou A: Survivin
regulation by HER2 through NF-κB and c-myc in irradiated breast
cancer cells. J Cell Mol Med. 15:1542–1550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung J, Kim EJ, Chung HK, Park HJ, Jeong
SY and Choi EK: c-Myc down-regulation is involved in proteasome
inhibitor-mediated enhancement of radiotherapeutic efficacy in
non-small cell lung cancer. Int J Oncol. 40:385–390.
2012.PubMed/NCBI
|
|
34
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Griffith TS, Brunner T, Fletcher SM, Green
DR and Ferguson TA: Fas ligand-induced apoptosis as a mechanism of
immune privilege. Science. 270:1189–1192. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peter ME, Legembre P and Barnhart BC: Does
CD95 have tumor promoting activities? Biochim Biophys Acta.
1755:25–36. 2005.PubMed/NCBI
|
|
37
|
Zhang H, Xu Q, Krajewski S, Krajewska M,
Xie Z, Fuess S, Kitada S, Pawlowski K, Godzik A and Reed JC: BAR:
An apoptosis regulator at the intersection of caspases and Bcl-2
family proteins. Proc Natl Acad Sci USA. 97:2597–2602. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome
c release from mitochondria in response to activation of
cell surface death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|