Introduction
Glioblastoma (GBM) is the most common and aggressive
glioma, representing 50% of all gliomas and more than 40% of all
central nervous system (CNS) tumors. The standard GBM treatment,
which consists of surgical resection, radiation and/or
chemotherapy, is rarely curative. The location of GBM in the
central nervous system and its characteristics of a diffuse pattern
of growth (diffuse glioma) in the majority of adult patients
prevent complete resection of the tumor, requiring additional
therapy. Regardless of the initial response to radiation and
chemotherapy, the tumor generally recurs within a year after these
treatments (1). Tumor cell
resistance to chemo-radiation contributes to the poor prognosis of
the disease. Indeed, use of chemotherapeutic agents alone or in
combination with radiotherapy is unable to improve significantly
the median survival time of GBM patients (2). Thus, changes in the outcome of GBM
patients remain a challenge.
Several chemotherapeutic drugs act by inducing
apoptosis of cancer cells. The apoptotic process is characterized
by a series of morphological alterations and biochemical reactions
leading to DNA fragmentation and breakdown of the cell into
apoptotic bodies. Activation of initiator caspases (caspase-8 and
−9), triggered either by activation of death receptors on the
plasma membrane (extrinsic pathway) or by stress
signals/alterations of the mitochondrial membrane potential
(intrinsic pathway), induces the activation of effector caspases
(caspase-3, −6 and −7), the main executors of apoptosis (3). Additionally, chemotherapeutic drugs
may also act by increasing the generation of reactive oxygen
species (ROS) in the target cell. Since mitochondria are the main
source of intracellular ROS it has been suggested that the elevated
levels of ROS produced in response to stress signals/alterations of
mitochondrial membrane potential are relevant for drug-induced
apoptosis (4).
Drug resistance remains the major cause of death of
cancer patients. Among the several mechanisms used by cancer cells
to escape death, great attention has been focused on the
transporter proteins of the ABC cassette family, such as ABCB1
(P-glycoprotein), the ABCC (multidrug resistance-associated
protein) family and ABCG2 (breast cancer resistance protein). These
proteins, which actively remove drugs from cells decreasing their
intracellular concentration and preventing death (5), are recognized as an important
mechanism of chemo-resistance in tumor cells, including GBM
(6,7). Although all transporter proteins are
present in glioblastoma, members of the MRP family seem to be
important for GBM drug resistance as their expression is correlated
with a poor patient prognosis (8).
Additionally, inhibition of MRP1 increases drug cytotoxicity in GBM
(9–11).
Another important factor that contributes to death
of GBM patients is the tumor invasiveness. Due to their rapid
growth and infiltrative characteristics, malignant gliomas are
largely incurable even with a combination therapy of surgery,
irradiation and chemotherapy (12).
Thus, in addition to its resistance to regular chemotherapics, the
aggressive proliferation, vascularization and diffuse invasiveness
of GBM contribute to its poor prognosis (13). As the tumor infiltrating edges
prevent the complete removal of the tumor, leading to recurrence,
there is a great interest in therapies to inhibit tumor
migration.
Bioactive products of natural origin constitute a
source for new agents with pharmacological potential. Among these
products, the pentacyclic triterpenes are emerging as a group of
substances with many interesting biological effects (14). We focused on pomolic acid (PA), a
pentacyclic triterpene isolated from a broad spectrum of plants
that show several pharmacological activities (15–17).
Pomolic acid inhibits breast cancer cell migration (18) and shows cytotoxicity towards
different types of neoplastic cells such as leukemia (19,20),
human gastric adenocarcinoma and uterine carcinoma and, murine
melanoma (21), human breast cancer
(22) and human ovarian
adenocarcinoma (23). It is also
active against leukemia multidrug resistance cell lines
overexpressing P-glycoprotein (Pgp) or Bcl-2 (19,24).
However, there are no reports on its activity towards glioblastoma
(GBM), a very aggressive brain tumor. This study investigated the
in vitro antitumoral activity of PA on human GBM cell lines
and explored the mechanisms for its effectiveness. Pomolic acid
activates apoptosis through the intrinsic pathway, with an
important role of ROS production in the process. Moreover, PA
down-modulates the activity of the transporter protein MRP1/ABCC1
and inhibits the migration of GBM cells, two important factors of
tumor drug resistance and progression. These results together with
previous studies show that PA is also able to act on different
pathways of apoptosis resistance, support the potential usefulness
of this triterpene for the development of novel therapies to treat
patients with GBM and MDR tumors.
Materials and methods
Cells and reagents
Human glioblastoma cell lines A172, U87 and GBM-1, a
cell line established from a GBM tumor biopsy (25) were grown in Dulbeccos modified
Eagles medium (DMEM) supplemented with 10% heat-inactivated fetal
calf serum (FCS), 100 U/ml penicillin and 100 mg/ml streptomycin in
disposable plastic bottles at 37°C with 5% CO2. Cells
were sub-cultured using trypsin-EDTA every 3–4 days.
3-(4,5dimethylthiozol-2-yl)-2,5-diphenyl-tetrazolium
bromide (MTT), verapamil, penicillin, streptomycin,
N-acetyl-L-cysteine (NAC), propidium iodide (PI), sodium fluoride
(NaF), phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate,
RIPA buffer, rhodamine 123 (Rho123) and FITC-labeled goat anti-rat
IgG antibody were purchased from Sigma-Aldrich (St. Louis, MO,
USA). 5-carboxifluorescein diacetate (5-CFDA) and
2,7-dichlorofluorescein diacetate (H2-DCFDA) were
obtained from Calbiochem (San Diego, CA, USA).
DiOC6(3) was from
Molecular Probes (Eugene, OR, USA). MK-571 and anti-MRP1 (MRPr1)
were provided by Enzo Life Sciences, Inc., (Farmingdale, NY, USA).
DMEM, FCS and trypsin-EDTA were from Gibco-BRL (Carlsbad, CA, USA).
Caspase-3 and −9 assay kits (CaspGLOW) were from BioVision, Inc.,
(Mountain View, CA, USA). Pomolic acid (MW 472.71) obtained from
BioBioPha Co., Ltd., (Kunming, China) was dissolved in dimethyl
sulfoxide (DMSO), stored at −20°C and diluted in culture medium for
use. 4,6-Diamidino-2-phenylindole dihydrochloride (DAPI) was from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell cytotoxicity assay
The MTT assay was used to measure the effect of
pomolic acid (PA) on cell viability of glioblastoma cell lines.
Cells were plated at a density of 1×104/well in 96-well
plate overnight and then treated with medium or different
concentrations of PA (7.5, 10.0, 12.5, 15.0, 17.5 or 20.0 µg/ml
equivalent to 15.86, 21.15, 26.44, 31.73, 37.02 or 42.30 µM,
respectively). DMSO at the higher concentration carried by PA, had
no effect on cell viability. Four hours before the end of the
treatment, cells were incubated with MTT (2.5 mg/ml) and kept in
the dark at 37°C until the end of the treatment. The formazan
produced by the reduction of MTT by viable cells was dissolved in
DMSO, and the optical density was measured with an ELISA reader
(BenchMark; Bio-Rad Laboratories, Hercules, CA, USA) at 570 nm
(reference filter 630 nm). Experiments were repeated at least three
times. The results were expressed as percentage of the control,
considered to be 100%.
DNA fragmentation assays
DNA fragmentation was evaluated by cell cycle
analysis using flow cytometry. Twenty-four hours after plating, the
cells (5×104 cells/well) were treated with medium
(control) or 15 µg/ml (31.73 µM) PA and incubated for different
times (1, 2, 4, 6, 8 or 24 h). Next, cells were harvested,
resuspended in HFS, a hypotonic fluorescent solution (50 mg/ml PI
and 0.1% Triton X-100 in 0.1% Na citrate buffer) for 1 h in the
dark at 4°C. The cell cycle was analyzed by flow cytometry (5,000
events, FL-2 channel) (FACSCalibur; Becton-Dickinson, San Jose, CA,
USA) to determine the sub-G0/G1 DNA content. Sub-diploid
populations were considered to be apoptotic. Data acquisition and
analysis were controlled by CellQuest software, version 3.1f. The
results are presented as representative histograms and as the mean
± SD of the percentage of the fragmented DNA.
Measurement of mitochondrial
transmembrane potential
Variations of mitochondrial membrane potential (MMP)
were assessed using the fluorochrome DiOC6(3) (40 nM), a compound that accumulates in
viable mitochondria due to its electro-chemical gradient and leaks
in response to loss of transmembrane potential. Cells were plated
and treated or not with 15 µg/ml (31.73 µM) PA for the same periods
of time described for detection of apoptosis. Then, cells were
harvested, resuspended in 200 µl DiOC6(3) for 30 min and analyzed by flow
cytometry (10,000 events, FL-1 channel). The results represent the
average ± SD of three experiments.
Caspase activation assay
Activation of caspase-3 and −9 were assayed using
CaspGLOW commercial kits according to the instructions of the
manufacturer (BioVision). The CaspGLOW is a sensitive method used
to detect activated caspases in apoptotic cells. In brief, cells
(5×104/well) were incubated with medium (control) or
with 15 µg/ml (31.73 µM) PA for 8 or 24 h before being harvested,
centrifuged and suspended in the caspase assay solution. This
solution contained the caspase-3 inhibitor, DEVD-FMK, or caspase-9
inhibitor LEHD-FMK conjugated to FITC as a marker. These FITC
conjugated molecules are cell permeable, non-toxic, and
irreversibly bind to activated caspase-3 or caspase-9 in apoptotic
cells allowing for direct detection of activated caspases in
apoptotic cells by flow cytometry. After 1 h of incubation (37°C,
5% CO2), cells were washed twice with washing buffer,
and the percentage of caspase-activated cells was analyzed by flow
cytometry (FL-1). The results are presented as representative
histograms and as the mean of fluorescence intensity (MFI) ±
SD.
Quantification of reactive oxygen
species (ROS)
ROS was determined by flow cytometry in cells
treated with H2-DCFDA. GBM-1 cells seeded in 24-well
plates (5×104 cells/well) were incubated with medium or
15 µg/ml (31.73 µM) PA for 1, 2, 4, 6, 8 and 24 h. After the
desired time, cells were harvested, washed with phosphate-buffered
saline (PBS), pH 7.4, and re-suspended in 0.16 ml PBS containing 20
µM H2-DCFDA. After 15 min of incubation at 37°C, the
production of ROS was evaluated by flow cytometry (FL-1 channel).
Estimates of ROS following drug treatment were determined by
measuring the change in mean fluorescence intensity using only live
cells, which was calculated by CellQuest software. For each sample,
10,000 events were collected. To assess the role of ROS in PA
cytotoxicity cells were treated for 24 h with media, pre-treated or
not for 1 h with the ROS inhibitor N-acetyl-L-cysteine (NAC, 10 mM)
and then incubated with 15 µg/ml (31.73 µM) PA. ROS production and
DNA fragmentation were evaluated after 6 and 8 h, respectively.
Activity and expression of MDR
transporter proteins
The functional activity of ABC proteins was
determined based on the intracellular accumulation of specific
substrates. For each experiment, GBM-1 cells
(1×105/well) were seeded into 24-well plates and
pre-incubated for 24 h at 37°C/5% CO2 to allow
stabilization of the culture. Platted cells were then incubated for
30 min with substrates specific for Pgp (200 ng/ml rhodamine 123),
MRP1 (5 µM CFDA) or BCRP (3 µM mitoxantrone) in presence of medium
or the conventional inhibitor of these proteins, verapamil (50 µM),
MK571 (50 µM) and fumitremogin C (10 µM), respectively. Next, cells
were washed in PBS, harvested and intracellular fluorescence was
evaluated in a FACSCalibur, Beckton-Dickinson cytometer (10,000
events; channels FL-1 or FL-3). The mean fluorescence intensity
(MFI) associated with intracellular fluorescence which reflects the
transporter activity of the proteins, was used to quantify their
activity. The results are presented as the mean ± SD of arbitrary
units of mean fluorescence intensity.
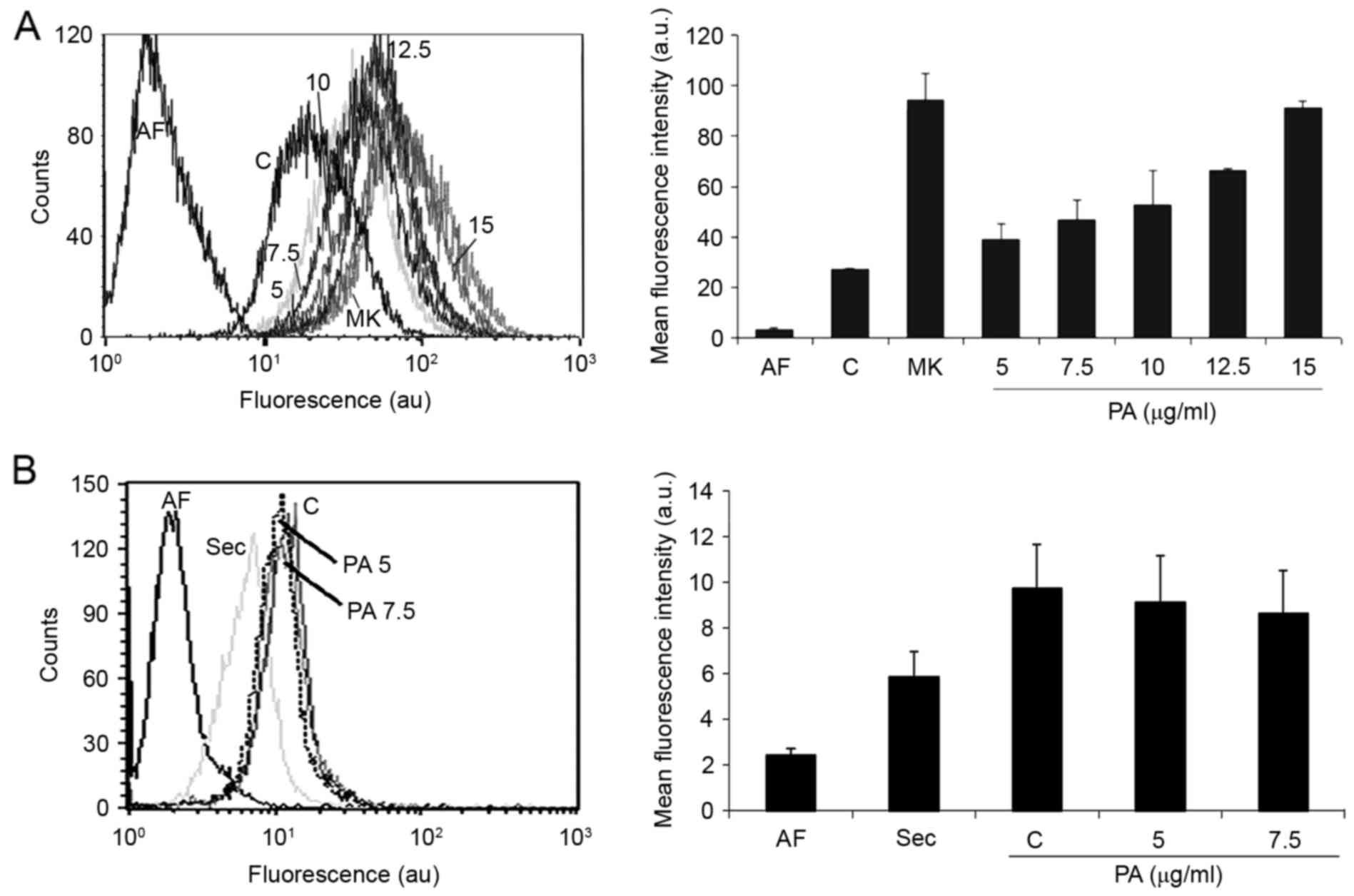
To assess the effect of PA on MRP1 activity, platted
cells were incubated for 30 min with medium (autofluorescence) or
with 5 µM CFDA in the presence of medium, MRP1 inhibitor (50 µM
MK-571) or the desired concentrations of PA (5, 7.5, 10. 12.5 or 15
µg/ml correspondent to 10.57, 15.86, 21.15, 26.44 or 31.73 µM).
After harvesting, cells were analyzed as described above. CFDA
(5-carboxyfluorescein diacetate), a non-fluorescent molecule that
is converted into fluorescent carboxy-fluorescein (CF) by
intracellular esterases, was used. Several studies have shown that
cells exhibiting high levels of the MRP1 protein actively exclude
carboxy-fluorescein (CF); CF can therefore be used as an indicator
of MRP1 pump activity.
Expression of MRP1 protein was analyzed by flow
cytometry. Cells were harvested, permeabilized with FACS lysing
solution for 30 min and incubated for 10 min with a blocking
solution (PBS with 5% BFS). The cells were then centrifuged (1,400
rpm/5 min) and resuspended in PBS solution with an anti-MRP1
antibody (1:20 dilution) for 60 min at room temperature. After two
washes with PBS, the cells were incubated with a FITC-labeled goat
anti-rat IgG antibody (1:1,000) from Sigma-Aldrich for 30 min.
After washing with PBS, the cells were resuspended in PBS, and
their fluorescence was evaluated by flow cytometry (FACSCalibur;
Beckton-Dickinson cytometer, FL-1). The results are presented as
representative histograms or mean ± SD of arbitrary units of mean
fluorescence intensity.
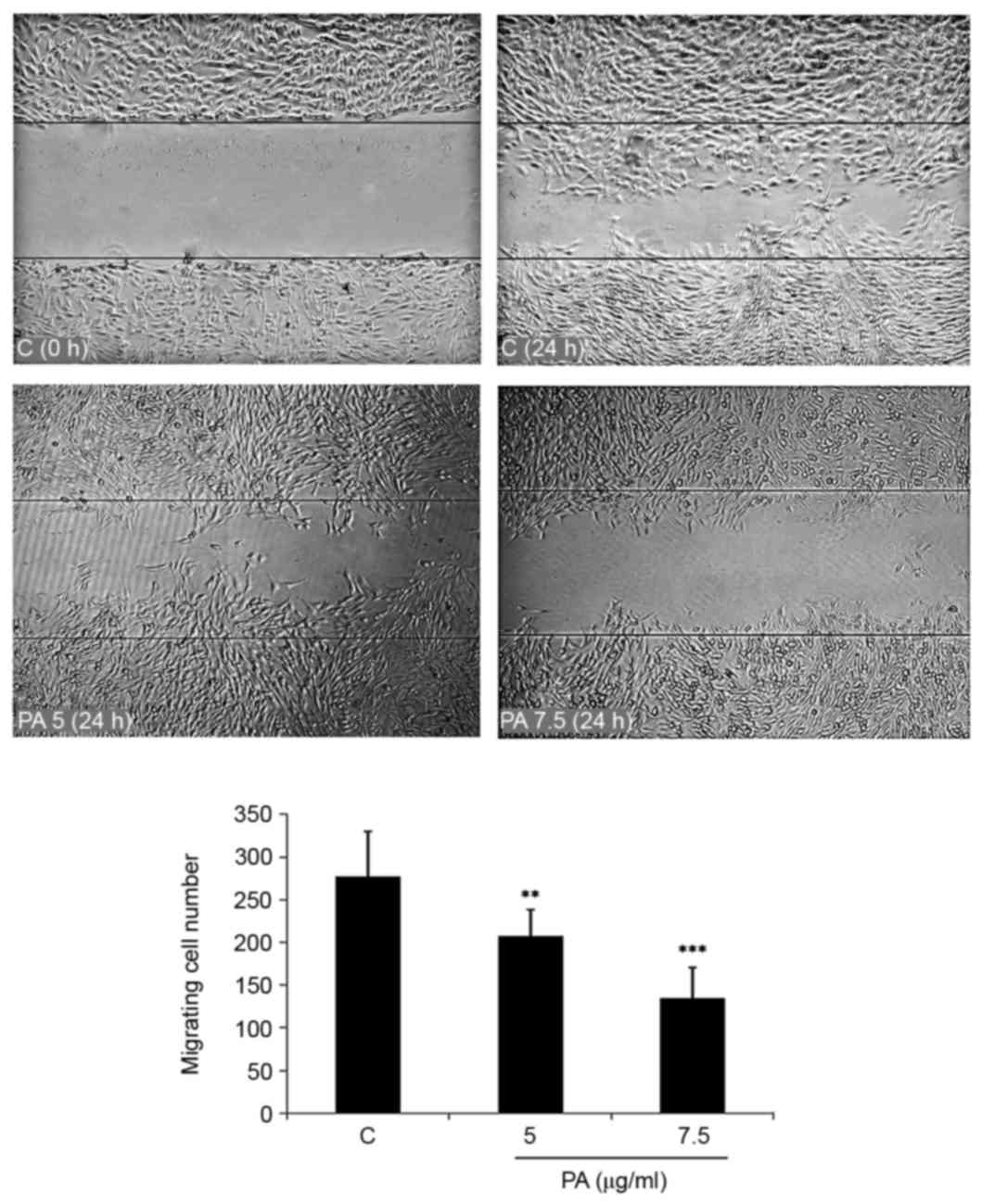
Wound healing assay
GBM-1 cells were seeded in a 24-well tissue culture
plate and grown to 90% confluence. After removing the medium, a
scratch was made in the middle of the well with a P200 pipette tip.
Subsequently, the debris was washed with PBS and new media was
added to the wells containing 5.0 or 7.5 µg/ml equivalent to 10.57
or 15.86 µM PA. Wound closure was monitored and photographed at 0
and 24 h under the inverted microscope. In order to quantify the
migrated cells, pictures of the initial wounded monolayers were
compared with the corresponding pictures of cells at the end of the
incubation. Artificial lines fitting the cutting edges were drawn
on pictures of the original wounds and overlaid on the pictures of
cultures after incubation. Cells that had migrated across the white
area were counted in random fields from each triplicate treatment.
Results were expressed as mean ± SD from four individual
experiments.
Statistical analysis
All data reported here are expressed as the mean ±
SD from three independent experiments. A significant difference
from the respective control for each experimental test condition
was assessed by one-way analysis of variance (ANOVA) using GraphPad
Prism 4.0 software. Values of P<0.05 were considered
statistically significant.
Results
Pomolic acid induces apoptosis of GBM
cells
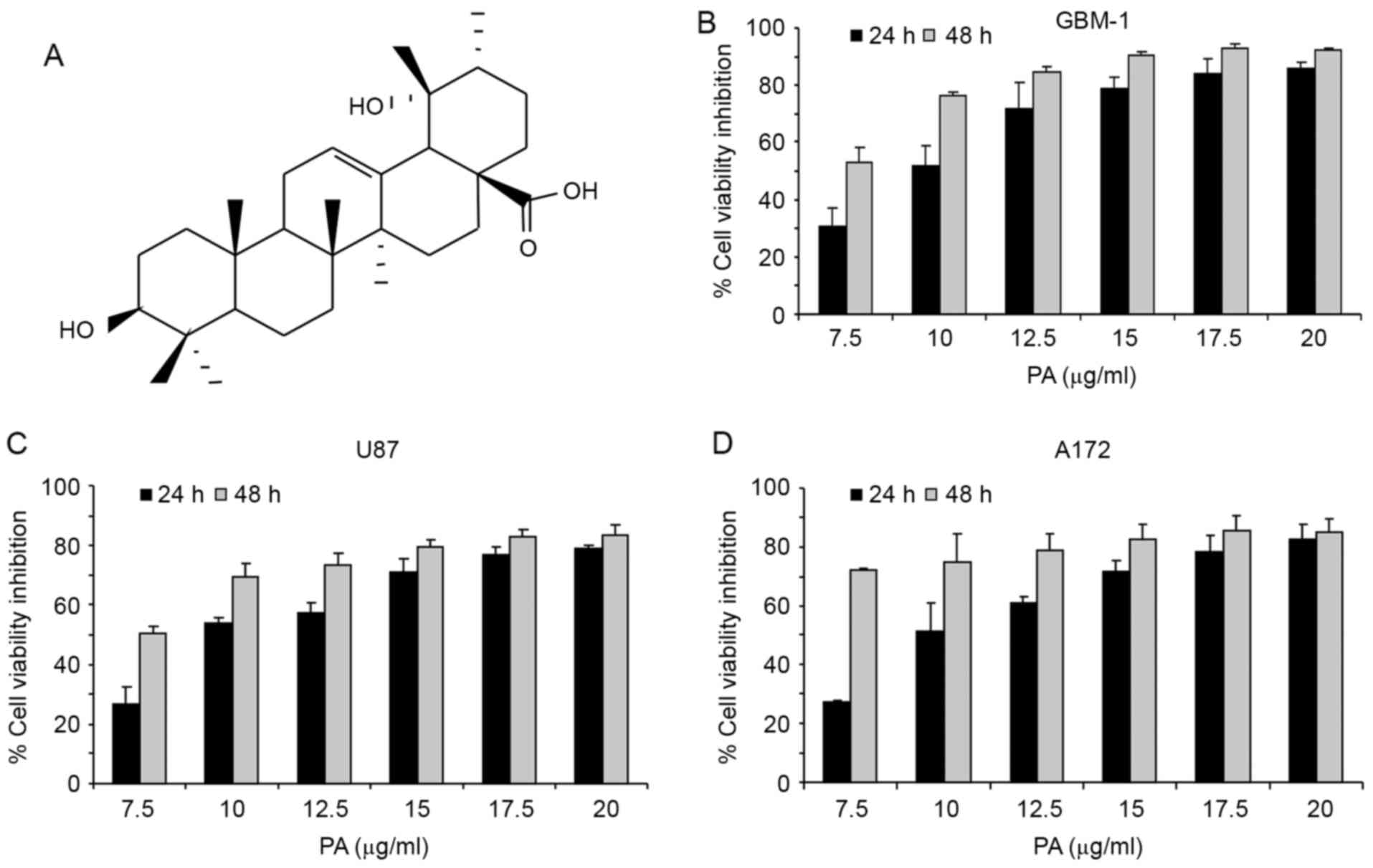
We used MTT assay to evaluate the effects of pomolic
acid (PA; Fig. 1A) on the viability
of the GBM cell lines U87, A172 and GBM-1. Cells were treated with
different concentrations of the triterpene for 24 or 48 h. PA
inhibits cell viability of all GBM cell lines dose- and
time-dependently (Fig. 1B-D). After
24-h treatment, the IC50 for U87, A172 and GBM-1 was
11.09±1.075, 8.82±0.205 and 9.72±0.8294 µg/ml, respectively. At
this incubation time, 15 µg/ml PA decreases the viability of the
cells in 70–90%, indicating the high antineoplastic potential of
this compound. As the activity of PA on these cells was quite
similar, IC50 varying from 8.8 to 11.0 µg/ml after 24-h
treatment, the next experiments were performed on the GBM-1 cell
line. This cell line was recently established from a GBM biopsy
(25) and can better reproduce
glioblastoma characteristics.
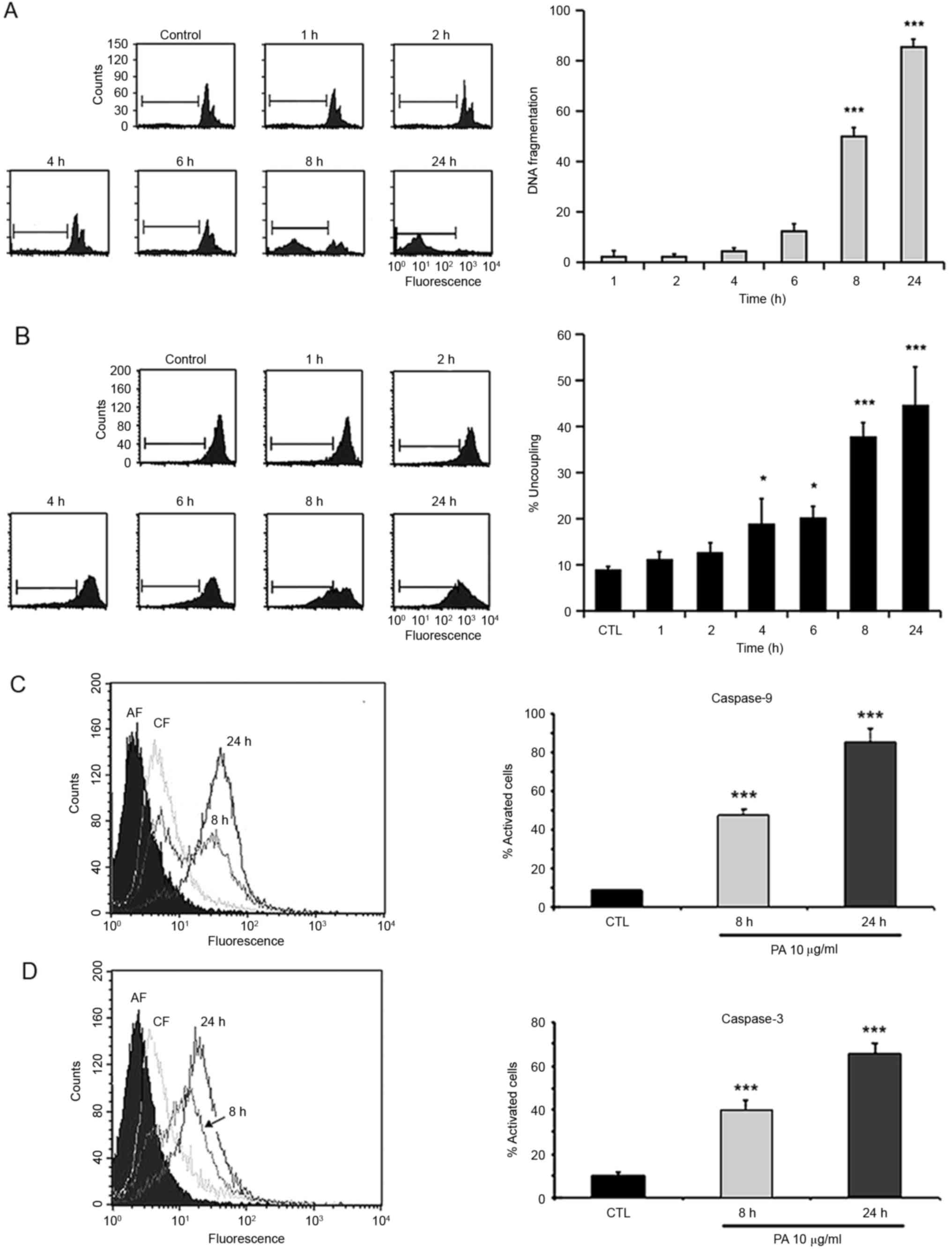
Next, we investigated if the cytotoxic activity of
PA would be mediated by induction of apoptosis. In order to test
that, GBM-1 cells were treated with 15.0 µg/ml PA for different
time intervals (1, 2, 4, 6, 8 or 24 h) and the percentage of
hypodiploid nuclei in the subG1 peak of the cell cycle, indicative
of apoptosis, was determined. PA induced a time-dependent increase
in DNA fragmentation. After 8-h treatment, this effect is ~50%
(Fig. 2A). Caspase-3 activation is
a characteristic of the apoptosis process. To confirm that cell
death is being achieved by induction of apoptosis, activation of
caspase-3 was assessed. Cells were treated with PA for 8 and 24 h
and analyzed by the CaspGLOW assay. Results in Fig. 2D show a time-dependent activation of
caspase-3.
Since several drugs induce apoptosis through
activation of the intrinsic pathway, the involvement of this
pathway in PA-induced cell death was analyzed. GBM-1 cells treated
in the same conditions used to measure DNA fragmentation were
incubated with the fluorescent dye DiOC6(3) and alterations of the transmembrane
potential (PTP) were evaluated by cytometry. Pomolic acid induces a
time-dependent decrease in mitochondria membrane potential
(Fig. 2B) suggesting that the
apoptotic process is mediated by activation of the intrinsic
pathway. To reinforce these observations, activation of caspase-9
was assessed. For this, cells were treated with PA for 8 and 24 h
and analyzed by the CaspGLOW assay. Results in Fig. 2C show a time-dependent activation of
caspase-9, confirming that PA induces apoptosis of GBM cells by the
activation of the intrinsic pathway.
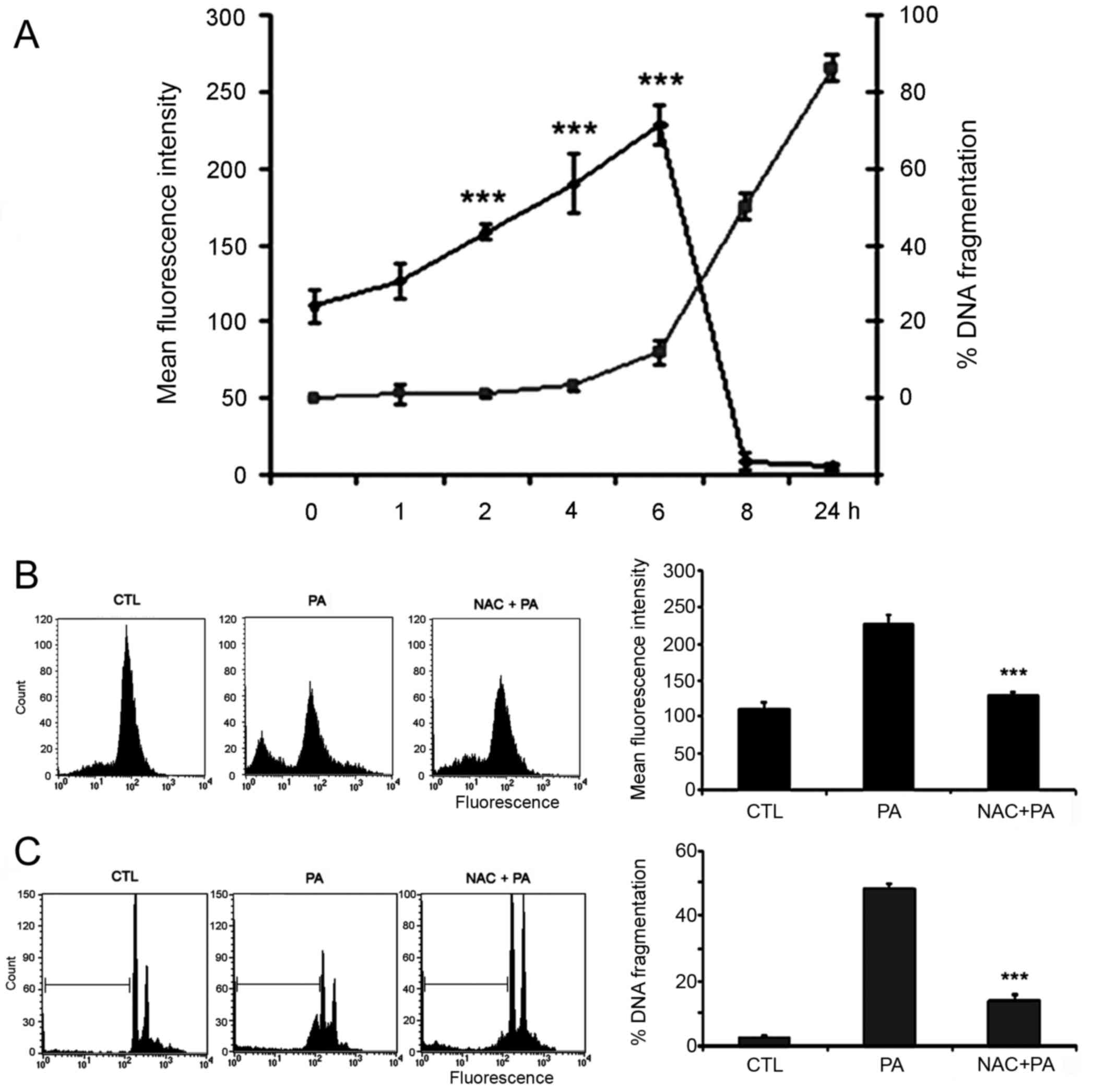
We then investigated if the oxidative stress,
resulting from alterations of the mitochondria membrane
permeability, was involved in PA-induced apoptotic death. For this,
DNA fragmentation and ROS production were measured in cells
incubated with PA for different times. Observation that DNA
fragmentation starts when production of ROS reach a peak (6 h)
suggests its involvement on PA-induced apoptosis (Fig. 3A). To further analyze this
possibility, cells were pre-incubated for 1 h with the anti-oxidant
NAC then treated with PA and the production of ROS and DNA
fragmentation was evaluated. Pre-treatment with NAC inhibits both,
the production of ROS (Fig. 3B) and
the fragmentation of DNA (Fig. 3C);
reinforcing the role of ROS in PA-induced apoptosis.
Pomolic acid downmodulates activity of
MRP1
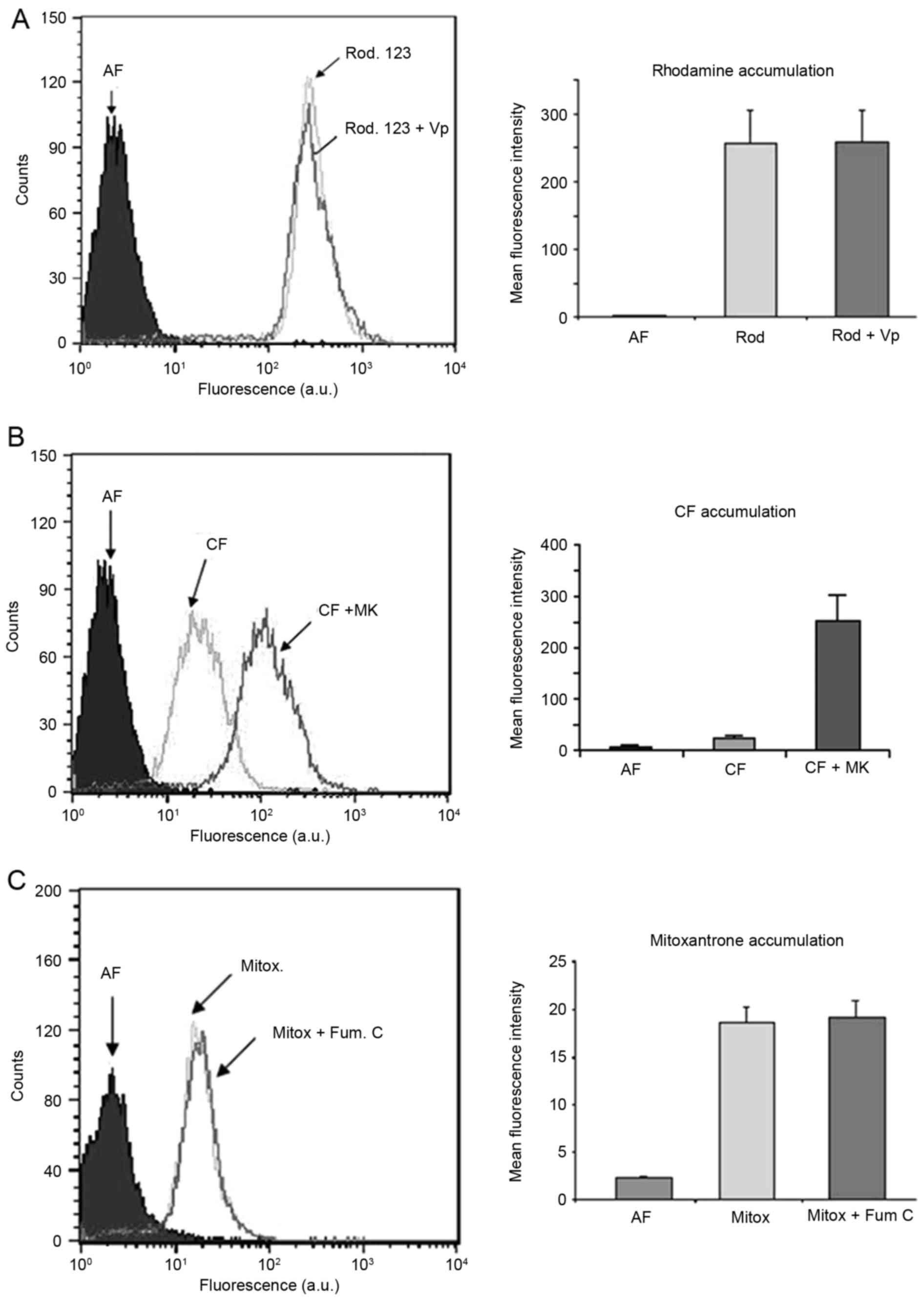
ABC transporters are involved in the acquisition of
the multidrug-resistance (MDR) phenotype in cancer cells (5). The expression and activity of these
transporters contribute to drug resistance in GBM cells (6). The expression of ABC transporter
proteins in GBM cells led us to probe if they were involved in
PA-induced cell death. Initially, we investigate if these proteins
were functional in GBM-1 cells. Pgp and ABCG2 inhibitors were
unable to block the transport of a fluorescent probe in GBM-1 cells
(Fig. 4A and C) while changes were
observed when cells were incubated with an MRP1 inhibitor (Fig. 4B). These data demonstrated that MRP1
is active in GBM-1 cells, while Pgp and ABCG2 are not.
Due to the important role played by MRP1 in GBM
resistance (9,11), we then investigated the effects of
PA on the activity of this protein. For this, MRP1 activity was
analyzed in the presence or absence of different concentrations of
PA (5.0.7.5, 10.0, 12.5 and 15.0 µg/ml). The results show that PA
down-modulates MRP1 activity dose-dependently. Additionally, we
observed that PA (15 µg/ml) inhibits MRP1 activity in a similar way
as the commercial inhibitor MK571 (Fig.
5A). Assessment of effects of PA treatment in MRP1 expression
revealed that down-modulation of MRP1 activity is not due to
inhibition of protein expression (Fig.
5B).
Pomolic acid inhibits migration of GBM
cells
Glioblastomas are highly invasive tumors. Tumor cell
migration and diffuse infiltration into brain parenchyma are known
mechanisms of tumor recurrence (13). To further explore the potential of
PA as a tool to treat GBM, we used wound healing assay to
investigate the effect of this triterpene on the migration of GBM-1
cells. The treatment of glioblastoma cells with low concentrations
of PA (5.0 or 7.5 µg/ml) for 24 h significantly inhibited migration
(Fig. 6).
Discussion
Malignant central nervous system neoplasms,
particularly GBMs, are included among the most lethal and
intractable human tumors defying all current therapeutic modalities
and presenting a poor patient prognosis. Almost all GBM patients
undergo tumor recurrence and only a small percentage of the
patients survived 2 years after the treatment. The standard GBM
treatment, which consists of surgical resection, radiation and/or
chemotherapy, is rarely curative. The diffuse growth pattern of GBM
prevents complete resection of the tumor, requiring additional
therapy. Moreover, tumor cell resistance to chemo-radiation
contributes to the poor prognosis of the disease (1,2). Thus,
despite the massive research efforts, the outcome of these tumors
remain dismal stressing the need for new drugs or strategies able
to improve the patient survival.
In the last few years, natural products have been
recognized as an important source of novel antineoplastic drugs.
Accumulating evidence on the antitumor activity of triterpenes
supports these materials as one of these drug sources (14,26,28).
However, although the antitumoral activities of pomolic acid
(Fig. 1A) against different cancer
cell lines have been described (19,21–23),
there is no previous information on its effect on GBM cell lines.
Data presented in the present study (Fig. 1B-D) show that PA has a potent
antitumoral activity against GBM cell lines (U87, A172 and GBM-1)
decreasing their viability up to 70–90% after 24-h treatment.
The apoptotic cell death program is compromised in
GBM, leading to a survival advantage of the tumor cells.
Alterations of pathways that control apoptosis make GBM virtually
resistant to apoptotic stimuli. Results showing that PA induces
apoptosis by activation of the intrinsic/mitochondrial pathway are
in agreement with previous literature data showing that PA induced
uncoupling of mitochondria membrane potential (20) and apoptosis of different tumor cell
lines (19,23,29).
Accumulating data from several reports described the susceptibility
of tumor cells to increased oxidative stress (30,31)
and showed ROS as an important mediator of the cytotoxic activity
of several drugs (4). The present
study demonstrates that treatment with PA doubles the level of ROS
in the GBM cells. Addition of NAC inhibits both ROS and DNA
fragmentation (Fig. 3B and C)
demonstrating the dependence of PA-induced cell death on ROS
production. Moreover, as GBM resistance to temozolomide (TMZ) has
been associated with increased mitochondria coupling and reduction
of ROS production (32), it is
possible that a pro-oxidant therapy may also work as an anti-MDR
strategy to evercome drug-resistance in GBM.
Extrusion of drugs by plasma membrane transporters
of the ABC family of proteins is a well recognized mechanism of
chemoresistance (5). In gliomas,
expression of these proteins has been correlated with the tumor
grade, being higher in GBMs (33).
Increased expression of ATP-dependent drug efflux pumps was
observed after chemotherapy (34)
in glioma cancer stem cells (CSC) from GBM patients (35) and have been associated with TMZ
resistance (36,37). Thus, drug resistance mediated by
these proteins, decreases the efficacy of treatment, favoring the
maintenance of a residual disease from which the tumor grows.
Amongst the ABC proteins, members of the MRP family seem to be
particularly important for GBM drug resistance as expression of
these proteins increase in high-grade gliomas (9,33), in
recurrent GBM (12), and correlate
with poor patient prognosis (8,9). In
the present study, we show that PA induces apoptosis of GBM cell
lines expressing an active MRP1/ABCC1 (Fig. 4B) and down-modulates the activity of
this protein without altering its expression (Fig. 5A and B). These results support
previous literature data showing that inhibition of MRP1 activity
chemosensitize GBM cells leading to increased drug cytotoxicity
(13,38,39).
Previous results from our group demonstrated that PA is effective
against cancer MDR cells whose resistance mechanism is mediated by
overexpression of the MDR protein Pgp/ABCB1 (19) and cells expressing, simultaneously,
different MDR mechanisms (29).
Thus, the ability of PA to overcome resistance mediated by
different ABC transporters may be useful for GBM control and also
to other tumor types expressing this family of transporters.
However, in addition to drug resistance mechanisms,
the high invasive nature of glioblastoma cells has also been
indicated as an important factor to the failure of current
therapeutic approaches (13) and as
a result, to tumor recurrence. Thus, identifying new agents that
target migration and invasion processes can be of great importance
for the prognosis of GBM. Cell invasion is a complex process with
multiple biologic features (40),
in which cell migration is one of the first steps. Results in the
present study showing that PA dose-dependently decreased GBM cell
invasiveness indicate a possible role for this triterpene in
reducing recurrence and metastasis. Indeed an inhibitory effect of
PA on breast cancer cell migration was also reported (18).
Despite the advances on GBM therapy there is still
an urgent need for treatments that are more effective and able to
bypass the tumor mechanisms of drug resistance. Natural products
are emerging as an important source of new potential candidates for
the treatment or as adjuvant therapy for glioblastoma (41–44).
In GBM, natural triterpenes were shown to induce cell arrest and/or
apoptosis both in vitro (45,46)
and in vivo (47), to
inhibit migration and invasion (48) and to attenuate TMZ resistance
(49). Results presented in this
study show that the pentacyclic triterpene pomolic acid (PA) is
cytotoxic to GBM cell lines and that this effect is associated with
induction of apoptosis, uncoupling of mitochondria potential,
increase in ROS production and modulation of MDR pump, MRP1/ABCC1
activity. The mechanism of PA drug action appears to be a
combination of increased redox mediated cytotoxicity and modulation
of MDR mechanisms. The ability of PA to cross the
blood-brain-barrier was not investigated; however, since PA is able
to bypass resistance mechanisms mediated by Pgp (19) and down-modulates MRP1 activity, this
possibility cannot be discarded yet. Together, the results present
herein and in other studies (19,24,29),
showing that PA bypass different mechanisms of cell death
resistance and inhibits tumor cell migration, demonstrate the
potential of this compound to control tumor progression. They also
call attention to PA as a possible new strategy to improve cancer
therapy protocols for GBM.
Acknowledgements
The present study was supported by grants from
Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ),
Instituto Nacional para Pesquisa Translacional em Saúde e Ambiente
na Região Amazônica/Conselho Nacional de Desenvolvimento Científico
e Tecnológico/MCT, Brazil (INCT-INPeTAm/CNPq/MCT) and Fundação do
Câncer. The authors wish to thank the Conselho Nacional de
Desenvolvimento Científico e Tecnológico (CNPq) and Conselho de
Aperfeiçoamento do Pessoal de Nível Superior (CAPES) for the
graduate fellowships awarded to Lívia Paes Tavares Pacheco
Guimarães, Rafaela Muniz de Queiroz and Carollina de Araújo
Martins.
References
|
1
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharom FJ: ABC multidrug transporters:
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Declèves X, Amiel A, Delattre JY and
Scherrmann JM: Role of ABC transporters in the chemoresistance of
human gliomas. Curr Cancer Drug Targets. 6:433–445. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu C and Shervington A: Chemoresistance in
gliomas. Mol Cell Biochem. 312:71–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagawa T, Ido K, Sakuma T, Takeuchi H,
Sato K and Kubota T: Prognostic significance of the
immunohistochemical expression of O6-methylguanine-DNA
methyltransferase, P-glycoprotein, and multidrug resistance
protein-1 in glioblastomas. Neuropathology. 29:379–388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Declèves X, Fajac A, Lehmann-Che J, Tardy
M, Mercier C, Hurbain I, Laplanche JL, Bernaudin JF and Scherrmann
JM: Molecular and functional MDR1-Pgp and MRPs expression in human
glioblastoma multiforme cell lines. Int J Cancer. 98:173–180. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tivnan A, Zakaria Z, OLeary C, Kögel D,
Pokorny JL, Sarkaria JN and Prehn JHM: Inhibition of multidrug
resistance protein 1 (MRP1) improves chemotherapy drug response in
primary and recurrent glioblastoma multiforme. Front Neurosci.
9:2182015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peigñan L, Garrido W, Segura R, Melo R,
Rojas D, Cárcamo JG, San Martín R and Quezada C: Combined use of
anticancer drugs and an inhibitor of multiple drug
resistance-associated protein-1 increases sensitivity and decreases
survival of glioblastoma multiforme cells in vitro. Neurochem Res.
36:1397–1406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demuth T and Berens ME: Molecular
mechanisms of glioma cell migration and invasion. J Neurooncol.
70:217–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salvador JAR, Moreira VM, Gonçalves BMF,
Leal AS and Jing Y: Ursane-type pentacyclic triterpenoids as useful
platforms to discover anticancer drugs. Nat Prod Rep. 29:1463–1479.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banno N, Akihisa T, Tokuda H, Yasukawa K,
Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J and
Nishino H: Triterpene acids from the leaves of Perilla
frutescens and their anti-inflammatory and antitumor-promoting
effects. Biosci Biotechnol Biochem. 68:85–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee MS and Thuong PT: Stimulation of
glucose uptake by triterpenoids from Weigela subsessilis.
Phytother Res. 24:49–53. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alvarado-Castillo C, Estrada O and
Carvajal E: Pomolic acid, triterpenoid isolated from Licania
pittieri, as competitive antagonist of ADP-induced aggregation
of human platelets. Phytomedicine. 19:484–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Cho YY, Yoon SW and Park B:
Suppression of MMP-9 and FAK expression by pomolic acid via
blocking of NF-κB/ERK/mTOR signaling pathways in growth
factor-stimulated human breast cancer cells. Int J Oncol.
49:1230–1240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernandes J, Castilho RO, da Costa MR,
Wagner-Souza K, Kaplan Coelho MA and Gattass CR: Pentacyclic
triterpenes from Chrysobalanaceae species: Cytotoxicity on
multidrug resistant and sensitive leukemia cell lines. Cancer Lett.
190:165–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernandes J, Weinlich R, Castilho RO,
Kaplan MA, Amarante-Mendes GP and Gattass CR: Pomolic acid triggers
mitochondria-dependent apoptotic cell death in leukemia cell line.
Cancer Lett. 219:49–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida M, Fuchigami M, Nagao T, Okabe H,
Matsunaga K, Takata J, Karube Y, Tsuchihashi R, Kinjo J, Mihashi K,
et al: Antiproliferative constituents from Umbelliferae plants VII.
Active triterpenes and rosmarinic acid from Centella
asiatica. Biol Pharm Bull. 28:173–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Youn SH, Lee JS, Lee MS, Cha EY, Thuong
PT, Kim JR and Chang ES: Anticancer properties of pomolic
acid-induced AMP-activated protein kinase activation in MCF7 human
breast cancer cells. Biol Pharm Bull. 35:105–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo KH, Park JH, Lee DK, Fu YY, Baek NI
and Chung IS: Pomolic acid induces apoptosis in SK-OV-3 human
ovarian adenocarcinoma cells through the mitochondrial-mediated
intrinsic and death receptor-induced extrinsic pathways. Oncol
Lett. 5:386–390. 2013.PubMed/NCBI
|
|
24
|
Fernandes J, Weinlich R, Castilho RO,
Amarante-Mendes GP and Gattass CR: Pomolic acid may overcome
multidrug resistance mediated by overexpression of anti-apoptotic
Bcl-2 proteins. Cancer Lett. 245:315–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernandes J, da Fonseca CO, Teixeira A and
Gattass CR: Perillyl alcohol induces apoptosis in human
glioblastoma multiforme cells. Oncol Rep. 13:943–947.
2005.PubMed/NCBI
|
|
26
|
Shanmugam MK, Nguyen AH, Kumar AP, Tan BK
and Sethi G: Targeted inhibition of tumor proliferation, survival,
and metastasis by pentacyclic triterpenoids: Potential role in
prevention and therapy of cancer. Cancer Lett. 320:158–170. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patlolla JM and Rao CV: Triterpenoids for
cancer prevention and treatment: Current status and future
prospects. Curr Pharm Biotechnol. 13:147–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gheorgheosu D, Duicu O, Dehelean C, Şoica
C and Muntean D: Betulinic acid as a potent and complex antitumor
phytochemical: A minireview. Anticancer Agents Med Chem.
14:936–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vasconcelos FC, Gattass CR, Rumjanek VM
and Maia RC: Pomolic acid-induced apoptosis in cells from patients
with chronic myeloid leukemia exhibiting different drug resistance
profile. Invest New Drugs. 25:525–533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ravindran J, Gupta N, Agrawal M, Bhaskar
Bala AS and Rao Lakshmana PV: Modulation of ROS/MAPK signaling
pathways by okadaic acid leads to cell death via, mitochondrial
mediated caspase-dependent mechanism. Apoptosis. 16:145–161. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oliva CR, Moellering DR, Gillespie GY and
Griguer CE: Acquisition of chemoresistance in gliomas is associated
with increased mitochondrial coupling and decreased ROS production.
PLoS One. 6(1–10): e246652011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Faria GP, de Oliveira JA, de Oliveira
JG, Romano SO, Neto VM and Maia RC: Differences in the expression
pattern of P-glycoprotein and MRP1 in low-grade and high-grade
gliomas. Cancer Invest. 26:883–889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abe T, Mori T, Wakabayashi Y, Nakagawa M,
Cole SP, Koike K, Kuwano M and Hori S: Expression of multidrug
resistance protein gene in patients with glioma after chemotherapy.
J Neurooncol. 40:11–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin F, Zhao L, Guo YJ, Zhao WJ, Zhang H,
Wang HT, Shao T, Zhang SL, Wei YJ, Feng J, et al: Influence of
Etoposide on anti-apoptotic and multidrug resistance-associated
protein genes in CD133 positive U251 glioblastoma stem-like cells.
Brain Res. 1336:103–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Happold C, Roth P, Wick W, Schmidt N,
Florea AM, Silginer M, Reifenberger G and Weller M: Distinct
molecular mechanisms of acquired resistance to temozolomide in
glioblastoma cells. J Neurochem. 122:444–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perazzoli G, Prados J, Ortiz R, Caba O,
Cabeza L, Berdasco M, Gónzalez B and Melguizo C: Temozolomide
resistance in glioblastoma cell lines: Implication of MGMT, MMR,
P-Glycoprotein and CD133 expression. PLoS One. 10:e01401312015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benyahia B, Huguet S, Declèves X and
Mokhtari K: Crinière1 E, Bernaudin JF, Scherrmann JM and Delattre
JY: Multidrug resistance-associated protein MRP1 expression in
human gliomas: chemosensitization to vincristine and etoposide by
indomethacin in human glioma cell lines overexpressing MRP. J
Neurooncol. 66:65–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garrido W, Muñoz M, San Martín R and
Quezada C: FK506 confers chemosensitivity to anticancer drugs in
glioblastoma multiforme cells by decreasing the expression of the
multiple resistance-associated protein-1. Biochem Biophys Res
Commun. 411:62–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lefranc F, Brotchi J and Kiss R: Possible
future issues in the treatment of glioblastomas: Special emphasis
on cell migration and the resistance of migrating glioblastoma
cells to apoptosis. J Clin Oncol. 23:2411–2422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Queiroz RM, Takiya CM, Guimarães LPTP,
Rocha GG, Alviano DS, Blank AF, Alviano CS and Gattass CR:
Apoptosis-inducing effects of Melissa officinalis L.
essential oil in glioblastoma multiforme cells. Cancer Invest.
32:226–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Santos BL, Oliveira MN, Coelho PL, Pitanga
BP, da Silva AB, Adelita T, Silva VD, Mde Costa F, El-Bachá RS,
Tardy M, Chneiweiss H, Junier MP, Moura-Neto V and Costa SL:
Flavonoids suppress human glioblastoma cell growth by inhibiting
cell metabolism, migration, and by regulating extracellular matrix
proteins and metalloproteinases expression. Chem Biol Int.
242:123–138. 2015. View Article : Google Scholar
|
|
43
|
da Fonseca CO, Simão M, Lins IR, Caetano
RO, Futuro D and Quirico-Santos T: Efficacy of monoterpene perillyl
alcohol upon survival rate of patients with recurrent glioblastoma.
J Cancer Res Clin Oncol. 137:287–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cho HY, Wang W, Jhaveri N, Torres S, Tseng
J, Leong MN, Lee DJ, Goldkorn A, Xu T, Petasis NA, et al: Perillyl
alcohol for the treatment of temozolomide-resistant gliomas. Mol
Cancer Ther. 11:2462–2472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jeremias I, Steiner HH, Benner A, Debatin
KM and Herold-Mende C: Cell death induction by betulinic acid,
ceramide and TRAIL in primary glioblastoma multiforme cells. Acta
Neurochir (Wien). 146:721–729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li S, Zhu JH, Cao LP, Sun Q, Liu HD, Li
WD, Li JS and Hang CH: Growth inhibitory in vitro effects of
glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci.
35:1115–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kavitha CV, Jain AK, Agarwal C, Pierce A,
Keating A, Huber KM, Serkova NJ, Wempe MF, Agarwal R and Deep G:
Asiatic acid induces endoplasmic reticulum stress and apoptotic
death in glioblastoma multiforme cells both in vitro and in vivo.
Mol Carcinog. 54:1417–1429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo G, Yao W, Zhang Q and Bo Y: Oleanolic
acid suppresses migration and invasion of malignant glioma cells by
inactivating MAPK/ERK signaling pathway. PLoS One. 8:e720792013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Z, Du S, Ding F, Guo S, Ying G and Yan
Z: Ursolic acid attenuates temozolomide resistance in glioblastoma
cells by downregulating O6-methylguanine-DNA
methyltransferase (MGMT) expression. Am J Transl Res. 8:3299–3308,
eCollection. 2016.PubMed/NCBI
|