Introduction
It has been well documented that exercise has many
potential benefits in chronic metabolic diseases including type II
diabetes mellitus and metabolic syndrome, cardiovascular disease
and cancer (1–4). Recently, studies have focused on the
benefits of exercise in various types of cancers, such as breast,
lung and gynaecological cancer (5–7).
However the potential underlying mechanisms of exercise on cancer
treatment and improved quality of life are not well
established.
In recent years, skeletal muscle is gaining
increased attention as an endocrine organ (8). Various myokines which regulate
beneficial effects have been identified, such as irisin, a newly
discovered myokine, which is secreted from the skeletal muscles
following exercise (9). Irisin is
believed to be a bridge that links exercise with increased energy
expenditure. Studies have documented that irisin is released upon
cleavage of the membrane of fibronectin type III domain-containing
protein 5 (FNDC5) and is increased with exercise (9,10).
Previous studies have demonstrated the role of irisin in body
energy expenditure and in insulin sensitivity (9,10).
Furthermore, irisin has been demonstrated to play a role in the
mediation of H19-7 hippocampal neuronal cell proliferation
(11) and in the differentiation of
adipocytes and osteoblasts (12–14).
In addition to these studies, irisin has been demonstrated to have
a protective role in vascular pathology by regulating endothelial
cell proliferation, apoptosis and migration, as well as smooth
muscle cell phenotype modulation (15–18).
Notably, recent studies have focused on the relationship between
irisin and cancer. A recent study found that serum irisin levels
were lower in patients with breast cancer (19), while other studies revealed that
irisin was significantly increased in gastrointestinal cancer
tissues (20,21). In addition, studies have revealed
increased irisin immunoreactivity in tissues which were obtained
from ovary, cervix and breast carcinomas and endometrial
hyperplasias (22). Moreover, a
recent study demonstrated that irisin significantly inhibited the
viability and migration and enhanced the tumor sensitivity to
doxorubicin (DOX) in malignant breast cancer cells (23). Additionally another study revealed
the suppressive effect of irisin in lung cancer cell migration and
invasion (24). These studies
underlined the critical role of irisin in carcinogenesis.
Furthermore, these findings may offer therapeutic benefits for
cancer prevention and treatment.
At present, osteosarcoma, the most common primary
bone malignant tumor, has a poor prognosis due to distal metastases
(25). Although chemotherapy is
combined with surgery to improve the prognosis, the survival rate
is still low (26). Therefore it is
urgent to identify novel drugs for a better treatment outcome in
osteosarcoma therapy.
Osteosarcoma cells possess highly invasive
properties by undergoing a unique phenotypic switch,
epithelial-mesenchymal transition (EMT) (27). EMT is thought to be a highly
conserved cellular process which is characterized by inhibition of
epithelial molecule E-cadherin and gaining of mesenchymal markers,
such as N-cadherin, vimentin and fibronectin (28). Studies have revealed the critical
role of EMT in carcinoma metastasis (28,29).
It has been well established that IL-6 promotes the
proliferation, metastasis and angiogenesis of osteosarcoma
(30,31). Studies further demonstrated that
IL-6 induced EMT in various types of cancer cells, such as
pancreatic (32), lung (33), hepatocellular (34) and colorectal cancer cells (35). The IL-6 downstream signals included
STAT3, Akt and ERK1/2 MAPK (30,36,37).
Among them, STAT3 has been demonstrated to exhibit an important
role in IL-6-modulated EMT (33,38).
Previous studies have revealed the critical role of STAT3 in
modulating angiogenesis and metastasis of cancer (39,40).
Furthermore, studies have revealed that the Snail family members,
including Snail, ZEB1, Twist, Slug and SIP1, played a crucial role
in regulating EMT (41).
Thus, the present study evaluated the effect of
irisin on osteosarcoma cell migration, invasion and EMT and
explored the mechanisms involved.
Materials and methods
Cell lines and reagents
The U2OS and MG-63 osteosarcoma cell lines were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and were routinely cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA) containing 10% FBS (Invitrogen Life
Technologies), 100 U/ml penicillin, and 100 µg/ml streptomycin in
5% CO2 at 37°C. Rabbit anti-STAT3, phosphor-STAT3,
rabbit anti-ERK1/2, phosphor-ERK1/2, rabbit anti-p38, phosphor-p38,
rabbit anti-MMP-2, rabbit anti-MMP-9 and rabbit anti-Snail
antibodies were all purchased from Cell Signaling Technology
(Danvers, MA, USA). Rabbit anti-E-cadherin, vimentin, N-cadherin,
fibronectin and rabbit anti-MMP-7 antibodies were purchased from
Abcam (Cambridge, UK). Alexa Fluor 488-conjugated goat anti-rabbit
IgG was purchased from Invitrogen Life Technologies. IL-6 was
purchased from PeproTech (Rocky Hill, NJ, USA). Irisin was
purchased from Phoenix Pharmaceuticals (Burlingame, CA, USA).
WP1066, a selective inhibitor of STAT3, was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay was used to assess the cell proliferation and
viability. The U2OS and MG-63 osteosarcoma cells (5,000 cells/well)
in 100 µl medium were seeded into 96-well plates. After being
stimulated with irisin at different doses (0, 25, 50, 100 and 200
ng/ml) for different time-points (12, 24 and 48 h), 20 µl MTT
solution (5 mg/ml) was added into each well. After incubation for 4
h, 100 µl of dimethyl sulfoxide (DMSO) was added to each well for
another 15 min. Then, the absorbance values were determined using a
microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 490 nm.
Scratch wound healing assay
To evaluate the migration of the U2OS and MG-63
osteosarcoma cells, a scratch wound healing assay was used.
Briefly, the U2OS and MG-63 cells (1×106/well) were
seeded in 6-well plates and cultured with DMEM supplemented with
10% FBS. When reaching confluency, each well was directly scratched
with a 200 µl pipette tip. To detect the effect of irisin on the
migration of U2OS and MG-63 cells, 100 ng/ml irisin was added to
each well. After 24 h of incubation, the wound healing areas were
photographed and the distance between the two cell edges was
analyzed by ImageJ software.
In vitro invasion assay
The Transwell system was used to evaluate the effect
of irisin on the invasion of U2OS and MG-63 osteosarcoma cells. The
U2OS and MG-63 cells were cultured in Boyden chambers, with 8-µm
pore filter inserts, in 24-well plates (Corning Costar, Corning,
NY, USA). The pore inserts were pre-coated with Matrigel (BD
Biosciences, San Jose, CA, USA) overnight. The U2OS and MG-63 cells
(1×105 cells/well) were suspended in 100 µl DMEM
supplemented with 1% FBS and were added to the upper chamber. DMEM
supplemented with 10% FBS and irisin (100 ng/ml) were added to the
lower chamber. After 24 h of incubation, the cells that had
attached to the lower surface were fixed with methanol and stained
with 0.1% crystal violet. Then, 5 random high-power fields
(magnification, ×200) of each sample were chosen and counted to
evaluate the average number of invasive cells.
RNA extraction and quantitative
reverse transcription-PCR
Total RNA was extracted from the U2OS and MG-63
osteosarcoma cells using TRIzol reagent (Invitrogen Life
Technologies) and then reverse transcribed to cDNA with the
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
qPCR analysis was performed using a LightCycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The primer sequences used
for qPCR were as follows: E-cadherin forward,
5′-CGAGAGCTACACGTTCACGG-3′ and reverse,
5′-GGGTGTCGAGGGAAAAATAGG-3′; N-cadherin forward,
5′-TTTGATGGAGGTCTCCTAACACC-3′ and reverse,
5′-ACGTTTAACACGTTGGAAATGTG-3′; vimentin forward,
5′-TGCCGTTGAAGCTGCTAACTA-3′ and reverse,
5′-CCAGAGGGAGTGAATCCAGATTA-3′; fibronectin forward,
5′-TCTCCTGCCTGGTACAGAATATGTAGTGAG-3′ and reverse,
5′-GGTCGCAGCAACAACTTCCAGGT-3′; Snail forward,
5′-TCGGAAGCCTAACTACAGCGA-3′ and reverse,
5′-AGATGAGCATTGGCAGCGAG-3′; MMP-2 forward,
5′-TTGATGGCATCGCTCAGATC-3′ and reverse, 5′-TTGTCACGTGGCGTCACAGT-3′;
MMP-9 forward, 5′-GACGCAGACATCGTCATCCA-3′ and reverse,
5′-CACAACTCGTCATCGTCGAAA-3′; MMP-7 forward,
5′-GAGTGAGCTACAGTGGGAACA-3′ and reverse,
5′-CTATGACGCGGGAGTTTAACAT-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. Melting curves were assessed to
confirm the specificity of the products generated for each set of
primers. Following amplification, the ΔΔCt comparative method was
used to normalize the relative levels of gene expression. The
experiments were performed in triplicate.
Western blot analysis
After being treated with and without IL-6 and/or
irisin, the U2OS and MG-63 osteosarcoma cells were collected and
lysed. Total cell protein concentrations were assessed using the
BCA protein assay kit (Pierce, Rockford, IL, USA). Equal protein
from the cell lysates was loaded onto 12% SDS-PAGE. After
electrophoresis, the proteins were transferred onto PVDF membranes
(Millipore, Billerica, MA, USA) and then blocked with 5% fat-free
milk at room temperature for 1 h and incubated with primary
antibodies overnight at 4°C. Then, the membranes were washed with
TBST and incubated with HRP-conjugated secondary antibodies for 1 h
at room temperature. Immune complexes were detected with ECL
reagents (Millipore, USA), and the blots were quantified by
densitometric analysis using the Alpha Imager 2200.
Immunofluorescence staining
After being treated with and without IL-6 and/or
irisin, the U2OS osteosarcoma cells were fixed with 4%
paraformaldehyde, permeabilized with 0.5% Triton X-100 and then
incubated with 5% normal goat serum for 1 h. Subsequently, the U2OS
cells were incubated with rabbit anti-E-cadherin and rabbit
anti-vimentin overnight at 4°C. This step was followed by
incubation with Alexa Fluor 488-conjugated goat anti-rabbit IgG for
1 h at room temperature. Then the cells were incubated with
4′,6-diamidino-2-phenylindole (DAPI)/PBS (1:5,000; Sigma-Aldrich)
for 5 min at room temperature. Finally, the images were captured
using a Nikon Eclipse 80i fluorescence microscope.
Statistical analysis
The data are expressed as the mean ± standard
deviation (SD). All of the experiments were repeated at least three
times. A Student's t-test was used to compare differences between
different groups. Comparisons among values of multiple groups were
performed by one-way analysis of variance (ANOVA). Differences were
considered to be statistically significant at P<0.05.
Results
Irisin treatment inhibits the
proliferation, migration and invasion of osteosarcoma cells
To explore the effect of irisin on the proliferation
of osteosarcoma cells, an MTT assay was applied as previously
described in Materials and methods. As shown in Fig. 1A, various doses (0, 25, 50, 100 and
200 ng/ml) of irisin were added into the cultured U2OS and MG-63
osteosarcoma cells. After 24 h of incubation, irisin significantly
suppressed the viability of U2OS and MG-63 osteosarcoma cells at a
concentration of 200 ng/ml. After a 48-h incubation, irisin
significantly inhibited the proliferation of U2OS and MG-63
osteosarcoma cells at a dose above 25 ng/ml (50, 100 and 200
ng/ml). Therefore, irisin inhibited the proliferation of U2OS and
MG-63 osteosarcoma cells in a dose- and time-dependent manner.
Based on these results, we selected irisin at a concentration of
100 ng/ml for 24 h and conducted the following migration and
invasion experiments, so as to eliminate the effect of irisin on
U2OS and MG-63 osteosarcoma cell proliferation.
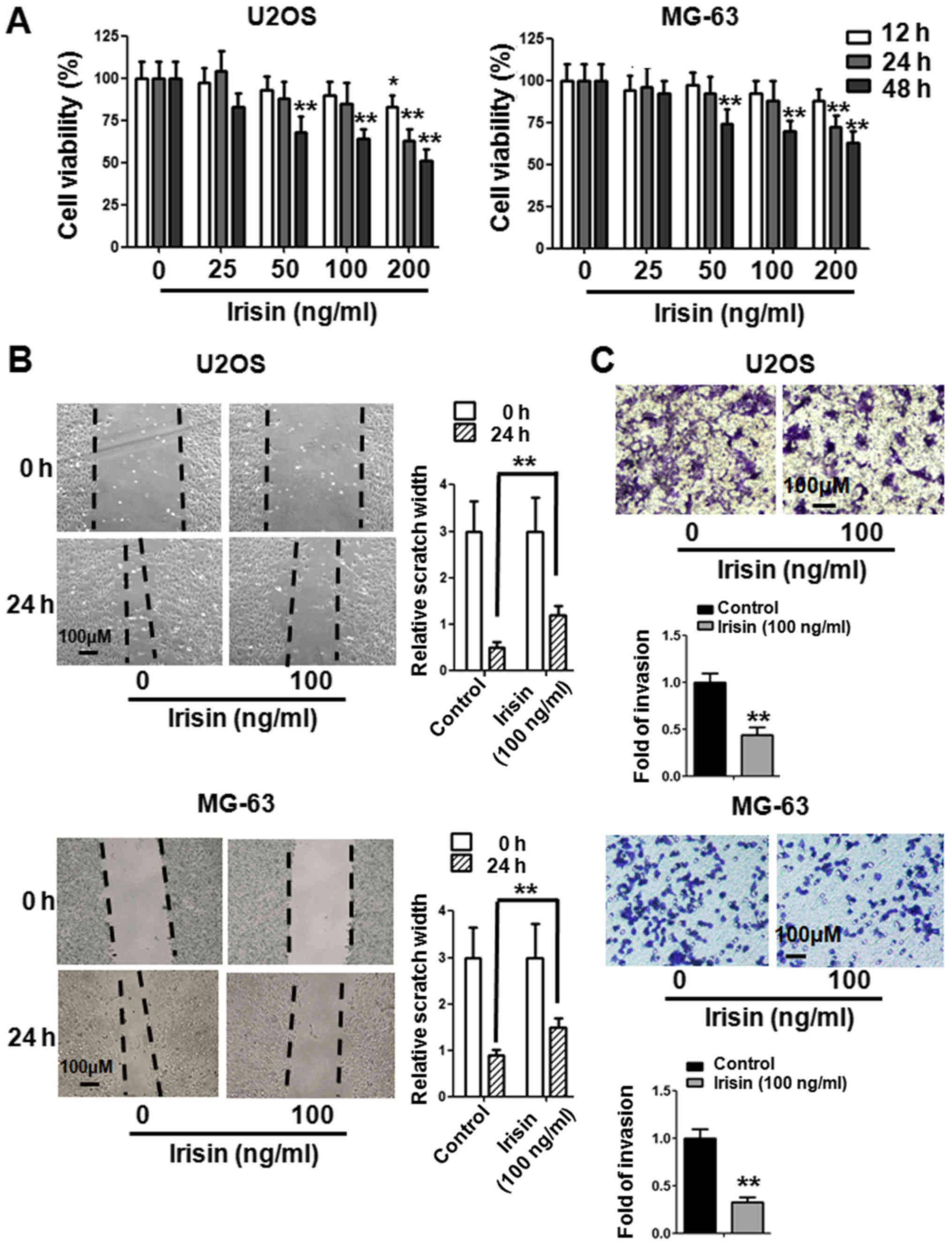 | Figure 1.Irisin inhibits the proliferation,
migration and invasion of U2OS and MG-63 osteosarcoma cells. (A)
After treatment with irisin for various time-points (12, 24 and 48
h) and at different concentration gradients (0, 25, 50, 100 and 200
ng/ml), the viability of the U2OS and MG-63 osteosarcoma cells was
assessed by MTT assay. (B) The U2OS and MG-63 osteosarcoma cells
were treated with irisin at a concentration of 100 ng/ml for 24 h
and then assessed by a wound healing assay. (C) The U2OS and MG-63
osteosarcoma cells were treated with irisin at a concentration of
100 ng/ml for 24 h. The effect of irisin on the invasion of U2OS
and MG-63 osteosarcoma cells was examined by a Transwell assay. The
bar graph represents the results of three independent experiments.
*P<0.05, **P<0.01 vs. the control group. The results
represent the mean ± SD from three independent experiments. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
The wound healing assay was applied to detect the
effect of irisin on the migration of osteosarcoma cells. The U2OS
and MG-63 osteosarcoma cells were treated with 100 ng/ml irisin for
24 h. As shown in Fig. 1B, the
migration of U2OS and MG-63 cells was suppressed by 100 ng/ml
irisin. The results of the wound healing assay revealed that the
relative scratch width was significantly decreased when treated
with irisin. To further explore the effect of irisin on
osteosarcoma cell invasion, a Transwell assay was used. The U2OS
and MG-63 cells were treated with 100 ng/ml irisin for 24 h. As
shown in Fig. 1C, the invasion of
U2OS and MG-63 cells was inhibited by 100 ng/ml irisin. The results
of the Transwell assay revealed that irisin may inhibit the
invasive ability of osteosarcoma cells.
Irisin treatment reverses the
IL-6-induced EMT in osteosarcoma cells
Evidence that EMT plays an important role in tumor
invasion and metastasis have been well demonstrated (42,43).
In addition the role of IL-6 in the induction of EMT has been
established (44). To further
explore the effect of irisin on osteosarcoma cell EMT, the
expression of EMT markers (E-cadherin, N-cadherin, vimentin,
fibronectin, MMP-2, MMP-7 and MMP-9) when treated with IL-6 (100
ng/ml) and irisin (0, 50 and 100 ng/ml) was detected using qPCR and
western blot analysis. As illustrated in Fig. 2A and B, IL-6 inhibited E-cadherin
protein expression and irisin restored the effect of IL-6 in a
dose-dependent manner. The expression of N-cadherin, vimentin,
fibronectin, MMP-2, MMP-7 and MMP-9 was upregulated when treated
with IL-6 and irisin suppressed the effect in a dose-dependent
manner. We also used immunofluorescence staining to evaluate the
effect of irisin on the expression of EMT markers. After being
treated with IL-6 (100 ng/ml) and irisin (100 ng/ml) for 24 h, the
U2OS cells were stained with E-cadherin and vimentin and were
analyzed by fluorescence microscopy. As shown in Fig. 2C, IL-6 downregulated E-cadherin
expression and upregulated vimentin expression, while irisin
effectively reversed the effect of IL-6. Collectively, these
results indicated that irisin treatment prevented IL-6-induced EMT.
Furthermore they revealed that the suppressive effect of irisin on
osteosarcoma cell invasion and migration may be related to EMT.
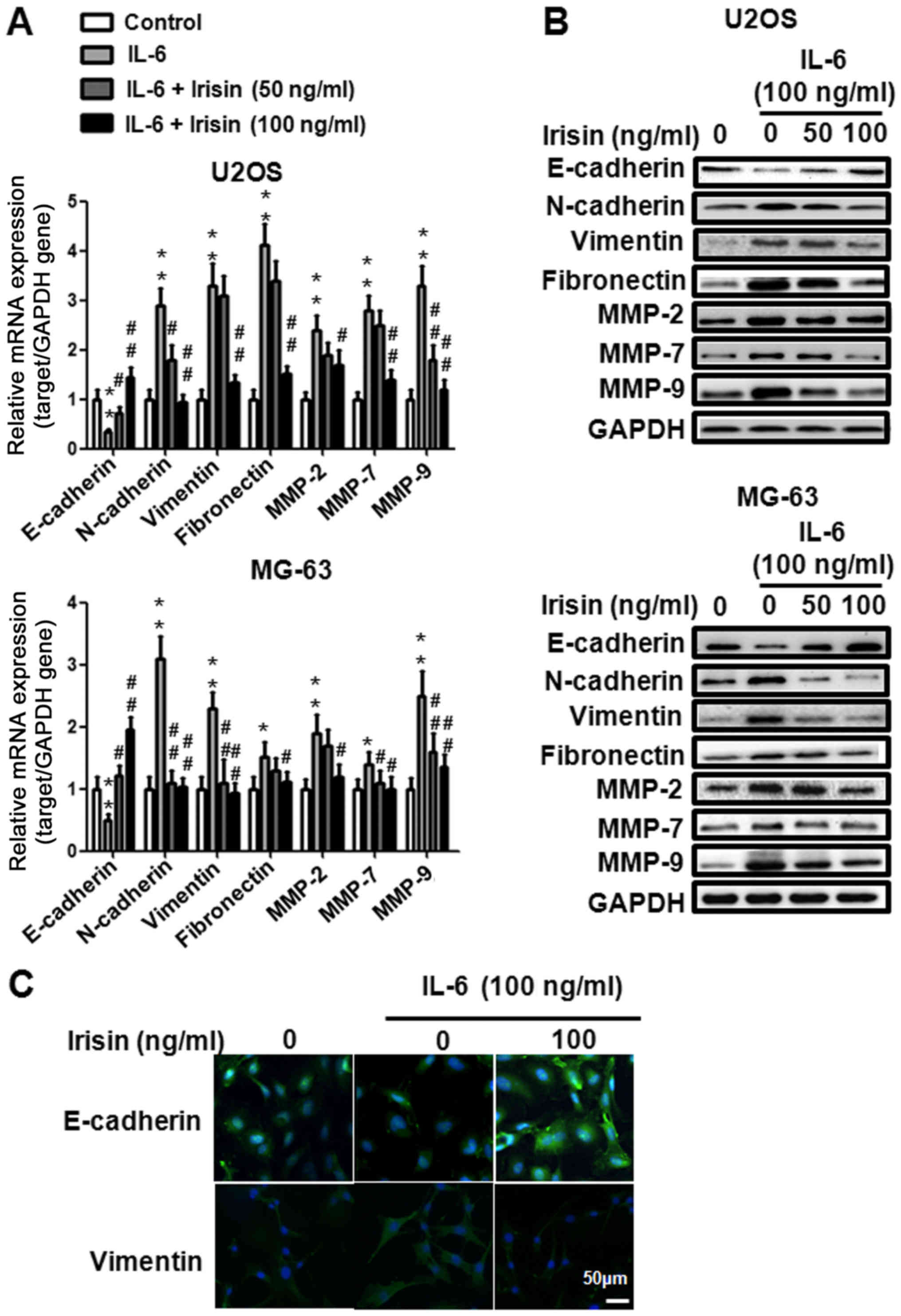 | Figure 2.Irisin treatment reverses
IL-6-mediated EMT in U2OS and MG-63 osteosarcoma cells. (A and B)
The U2OS and MG-63 osteosarcoma cells were treated with or without
IL-6 (100 ng/ml) and/or irisin at the indicated concentrations (0,
50 and 100 ng/ml) for 24 h and the expression level of E-cadherin,
N-cadherin, vimentin, fibronectin, MMP-2, MMP-7 and MMP-9 mRNA and
the protein expression was examined by qPCR analysis and western
blot analysis. (C) The U2OS osteosarcoma cells were treated with or
without IL-6 (100 ng/ml) and/or irisin at the indicated
concentrations (100 ng/ml) for 24 h and the expression of
E-cadherin and vimentin was detected by immunofluorescence
staining. *P<0.05, **P<0.01 vs. the control group;
#P<0.05, ##P<0.01, compared with the
cells treated with IL-6. Data shown are expressed as the means ± SD
from three independent experiments. EMT, epithelial-mesenchymal
transition. |
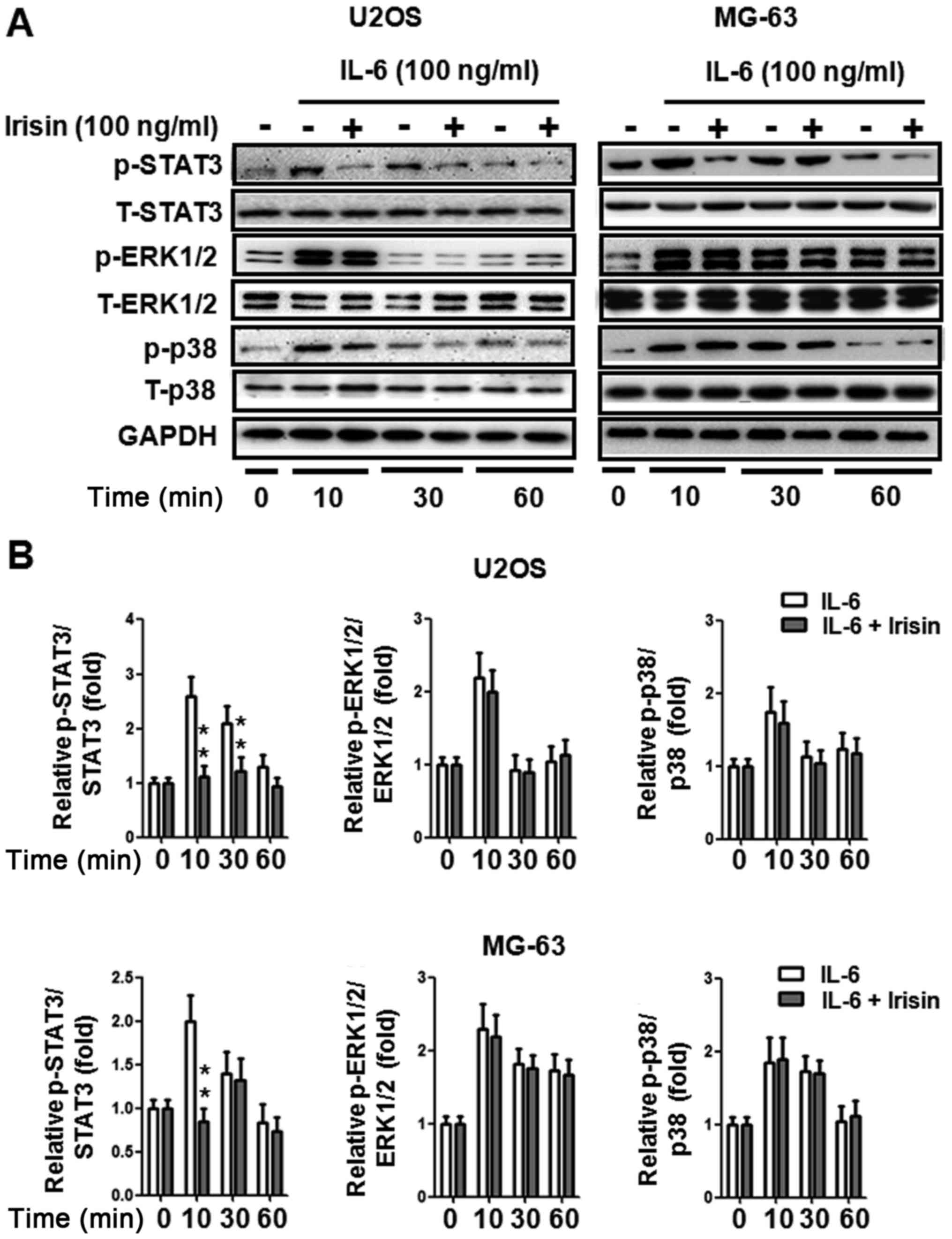
Irisin inhibits IL-6-induced STAT3
phosphorylation in osteosarcoma cells
The MAPK and STAT3 signaling pathways play a crucial
role in IL-6-induced cancer cell EMT (44–46).
To further explore the potential mechanism of irisin on
osteosarcoma cell EMT, the STAT3, ERK1/2 and p38 signaling pathways
were detected. As shown in Fig. 3A,
STAT3, ERK1/2 and p38 phosphorylation was upregulated while being
treated with IL-6 (100 ng/ml). STAT3 phosphorylation was suppressed
when treated with irisin (100 ng/ml). The suppression was
statistically significant, as quantified by densitometry (Fig. 3B). However, irisin did not change
the phosphorylation of ERK1/2 and p38. These results revealed that
the STAT3 signaling pathway may be involved in irisin-mediated
osteosarcoma cell EMT.
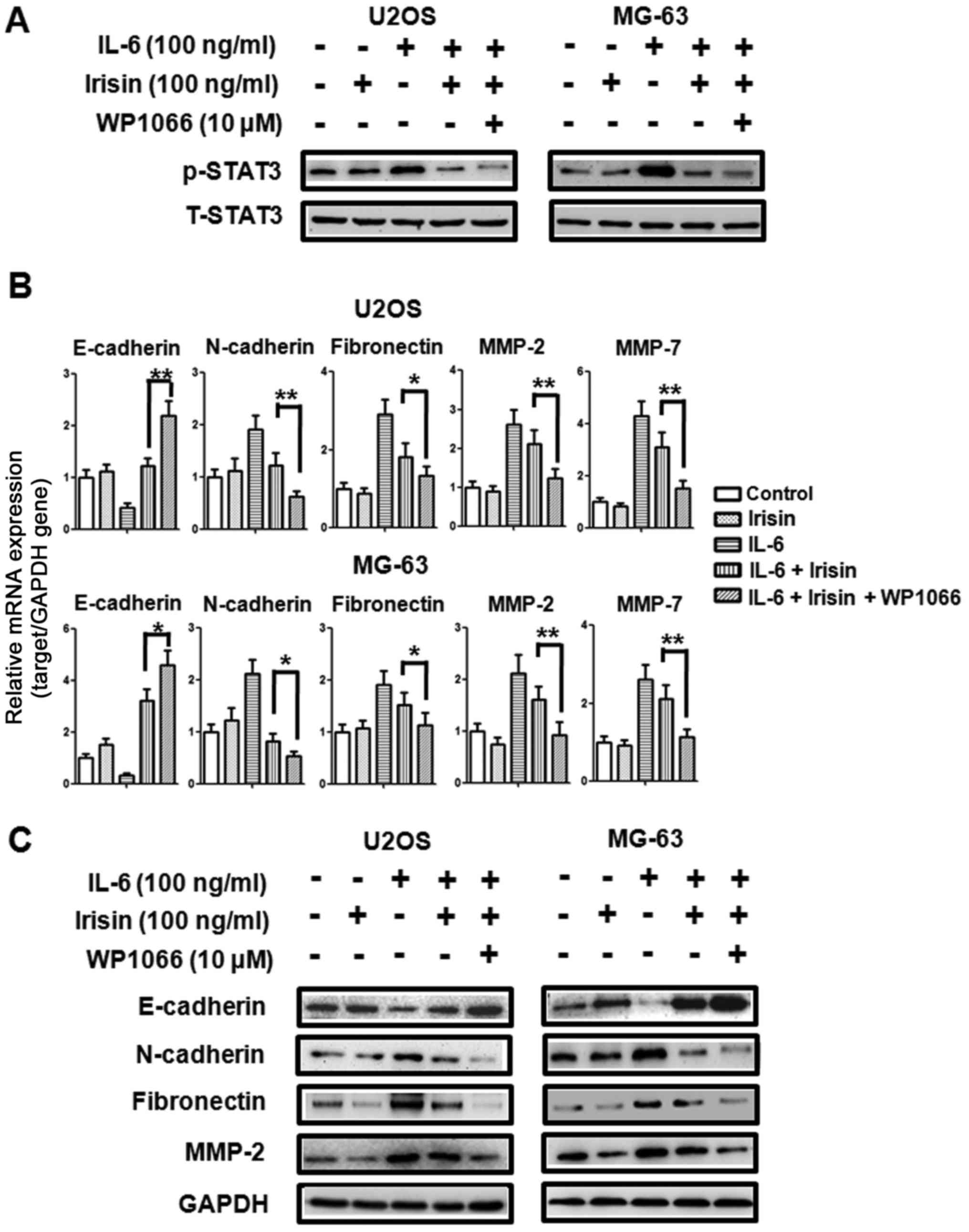
The STAT3 pathway is involved in
irisin-modulated EMT in osteosarcoma cells
To further demonstrate whether STAT3 is involved in
irisin-regulated osteosarcoma cell EMT, we selected WP1066 (a STAT3
inhibitor) to block STAT3 phosphorylation (Fig. 4A). As shown in Fig. 4B and C, at the mRNA and protein
level, the suppression of STAT3 phosphorylation with WP1066 (10 µM)
enhanced the expression of E-cadherin and further downregulated the
expression of some EMT markers (N-cadherin, fibronectin, MMP-2 and
MMP-7) which were induced by irisin. These results revealed that
irisin-mediated osteosarcoma cell EMT may be STAT3-dependent.
Blockade of STAT3 activation could further enhance irisin-mediated
osteosarcoma cell EMT.
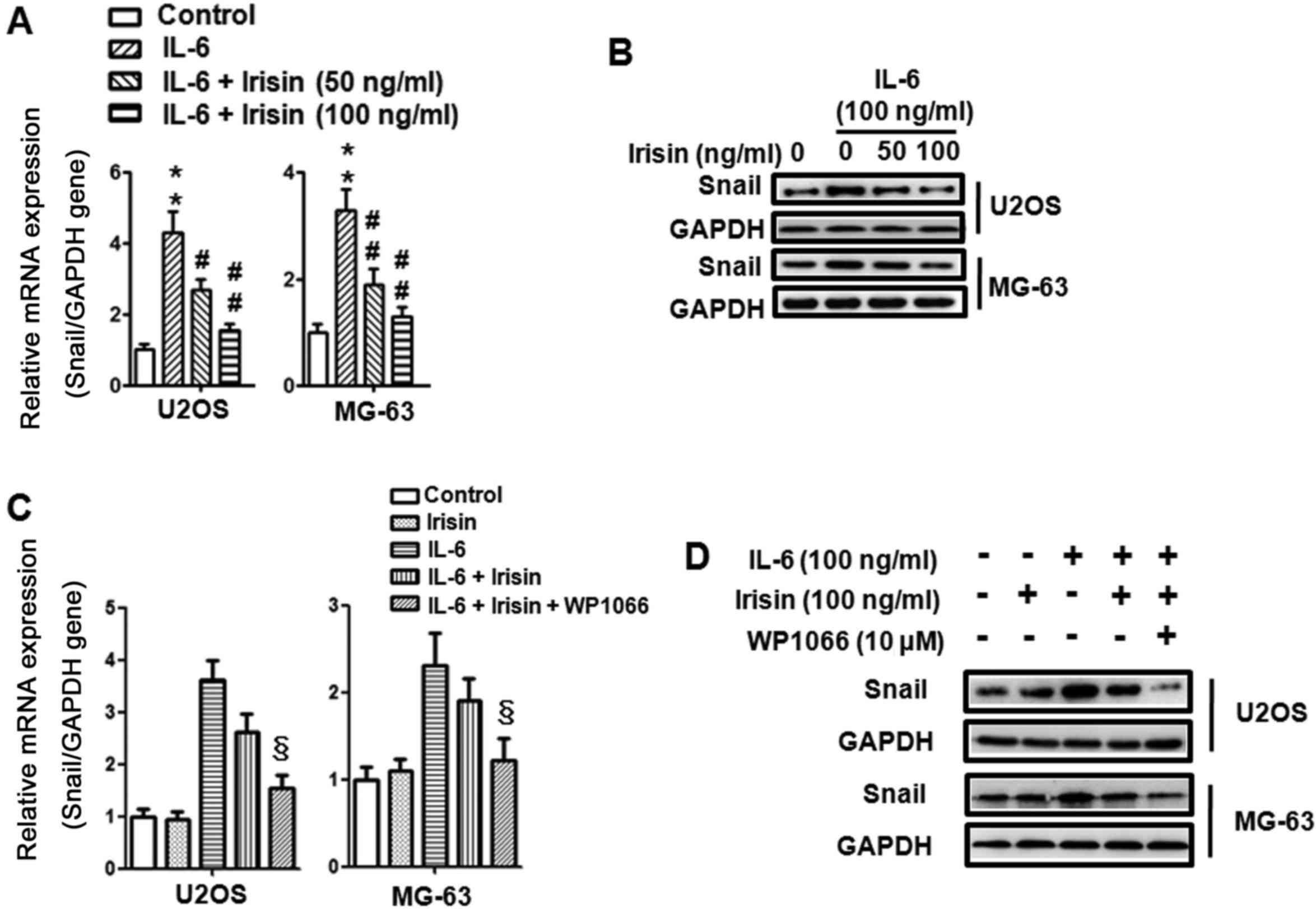
Irisin inhibits IL-6-induced Snail
upregulation via the STAT3 pathway in osteosarcoma cells
Evidence has established the expression and
activation of Snail transcriptional factor during EMT in E-cadherin
suppression and cancer progression (47). In the present study we further
explored the effects of irisin on the IL-6-induced Snail expression
and the relationship between STAT3 and Snail. Firstly, U2OS and
MG-63 osteosarcoma cells were treated with IL-6 (100 ng/ml) and
irisin (0, 50 and 100 ng/ml) for 24 h and the expression of Snail
was detected using qPCR and western blot analysis. As shown in
Fig. 5A and B, IL-6 induced Snail
mRNA and protein expression and irisin inhibited the effect in a
dose-dependent manner. Then, to further evaluate whether STAT3
participated in irisin-regulated Snail expression, U2OS and MG-63
osteosarcoma cells were also pretreated with WP1066 for 30 min
before IL-6 and irisin were added. As displayed in Fig. 5C and D, inhibition of STAT3
phosphorylation with WP1066 further suppressed the mRNA and protein
expression of Snail which was induced by irisin. These results
indicated the crucial role of STAT3 in regulating Snail expression
and irisin inhibition of the expression of Snail via the STAT3
pathway. Collectively, these results demonstrated that the
suppression of metastasis by irisin was mediated by the inhibition
of EMT via the STAT3/Snail signaling pathway in osteosarcoma
cells.
Discussion
The current study revealed several new findings
about irisin. Irisin can inhibit the proliferation, migration and
invasion of osteosarcoma cells. Irisin can also suppress
IL-6-induced EMT in osteosarcoma cells. The beneficial effects of
irisin in the EMT of osteosarcoma cells were observed through the
STAT3/Snail signaling pathway.
Recently, much attention has been paid on the
potential benefits of exercise in chronic metabolic diseases,
cardiovascular diseases and cancer (1–4). As a
novel discovered myokine, irisin is released from the skeletal
muscles following exercise (9).
Previous studies have demonstrated that irisin modulated body
energy expenditure by promoting brown adipocyte thermogenesis in
mice (9,10). Meanwhile, recent studies have
revealed the beneficial effect of irisin in neuronal cells
(11), adipocytes (12), osteoblasts (13,14),
as well as vascular cells, such as endothelial cells (15,18)
and smooth muscle cells (17).
Furthermore, recent studies revealed the direct suppressive effect
of irisin on malignant breast and lung cancer cell proliferation
and migration (23,24). In addition with other findings
concerning the expression of irisin in various cancer tissues
(20–22), these findings revealed the close
association between irisin and cancer. The present study is the
first to investigate the role of irisin on osteosarcoma cell
migration and invasion, as well as its detailed molecular
mechanisms. Firstly, we evaluated the effect of irisin on the
proliferation of osteosarcoma cells. We found that 100 ng/ml of
irisin significantly suppressed cell proliferation after 48 h,
while there was no significant effect before 24 h. We selected 24 h
as the stimulation time-point and 100 ng/ml as the stimulation
concentration in the following migration and invasion experiments,
so that the influence of cell proliferation was excluded. Our study
indicated that irisin effectively suppressed the migration and
invasion of osteosarcoma cells. These finding demonstrated the
anti-metastatic property of irisin.
Metastasis is considered as the primary cause of
mortality in most cancer patients. Studies have well established
that EMT is a distinctive phenotypic switch by which epithelial
cells lose their polarity and acquire the invasive properties of
mesenchymal cells in the process of metastasis (48). Furthermore, studies have
demonstrated that IL-6 could induce EMT in pancreatic, lung,
hepatocellular and colorectal cancer cells (32–35).
In the present study, we revealed the role of irisin in
IL-6-induced EMT in osteosarcoma cells. Our study found that irisin
could significantly reverse the IL-6-induced downregulation of the
epithelial marker (E-cadherin) and upregulation of mesenchymal
markers (N-cadherin, vimentin, fibronectin, MMP-2, MMP-7 and MMP-9)
in osteosarcoma cells. Therefore, for the first time, our study
demonstrated that irisin could effectively reverse the IL-6-induced
EMT of osteosarcoma cells.
IL-6, a major cytokine in the tumor
microenvironment, has long been documented in tumorigenesis in the
regulation of proliferation, apoptosis, angiogenesis and metastasis
of various types of cancer cells (49). Studies have demonstrated that the
MAPK and STAT3 pathways were involved in IL-6-regulation of various
cancer cell functions, such as proliferation and metastasis
(30,36–38).
Notably, STAT3 has been documented to play a crucial role in
IL-6-induced EMT (33,38). In the present study, the expression
of STAT3, ERK1/2 and p38 in osteosarcoma cells was evaluated. We
found that the expression of phosphor-STAT3, phosphor-ERK1/2 and
phosphor-p38 was increased when treated with IL-6, while only the
phosphorylation of STAT3 was inhibited by irisin treatment. To
further examine whether the STAT3 pathway was involved in
irisin-regulated EMT in osteosarcoma cells induced by IL-6, STAT3
was blocked using WP1066 (a STAT3 inhibitor). We found that
inhibition of the STAT3 pathway significantly further enhanced
irisin-mediated EMT of osteosarcoma cells. These results
demonstrated that STAT3 is essential for IL-6-induced EMT in
osteosarcoma cells. These results also revealed that the effect of
irisin may be STAT3-dependent.
Finally, we explored the effect of irisin and STAT3
on the transcription factor Snail. Snail, a zinc finger
transcription factor, has been demonstrated to modulate the
transcriptional suppression of E-cadherin (50). Previous studies revealed that the
expression of Snail was modulated by STAT3 (51). Another study further demonstrated
the crucial role of the STAT3/Snail pathway in regulating EMT in
hepatocellular carcinoma cells (52). In the current study, we found that
irisin suppressed the upregulation of Snail which was induced by
IL-6, while blockade of STAT3 further downregulated irisin-mediated
Snail expression in osteosarcoma cells. These results revealed that
STAT3 was upstream of Snail. Furthermore, the aforementioned
results demonstrated that irisin suppressed the expression of Snail
via the STAT3 pathway.
In conclusion, for the first time, our study
revealed that irisin suppressed the migration and invasion of
osteosarcoma cells. Moreover, irisin reversed the EMT induced by
IL-6 in osteosarcoma cells. Finally, the inhibitory role of irisin
in IL-6-induced EMT was modulated via the STAT3/Snail pathway.
Collectively, irisin reversed IL-6-induced EMT of osteosarcoma
cells through the STAT3/Snail signaling pathway. Thus, our research
indicated that irisin is a promising agent in osteosarcoma
treatment. Therefore further in vivo research needs to be
performed.
References
|
1
|
De Sousa SM and Norman RJ: Metabolic
syndrome, diet and exercise. Best Pract Res Clin Obstet Gynaecol.
37:140–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Francesconi C, Lackinger C, Weitgasser R,
Haber P and Niebauer J: Physical activity and exercise training in
the prevention and therapy of type 2 diabetes mellitus. Wien Klin
Wochenschr. 128:S141–S145. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Endes S: Physical activity reduces
cardiovascular disease risk in older adults. Evid Based Med.
21:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Navigante A and Morgado PC: Does physical
exercise improve quality of life of advanced cancer patients? Curr
Opin Support Palliat Care. 10:306–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nyrop KA, Deal AM, Williams GR, Guerard
EJ, Pergolotti M and Muss HB: Physical activity communication
between oncology providers and patients with early-stage breast,
colon or prostate cancer. Cancer. 122:470–476. 2007. View Article : Google Scholar
|
|
6
|
Michaels C: The importance of exercise in
lung cancer treatment. Transl Lung Cancer Res. 5:235–238. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin KY, Frawley HC, Denehy L, Feil D and
Granger CL: Exercise interventions for patients with gynaecological
cancer: A systematic review and meta-analysis. Physiotherapy.
102:309–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karstoft K and Pedersen BK: Skeletal
muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr
Metab Care. 19:270–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boström P, Wu J, Jedrychowski MP, Korde A,
Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al: A
PGC1-α-dependent myokine that drives brown-fat-like development of
white fat and thermogenesis. Nature. 481:463–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Boström P, Sparks LM, Ye L, Choi JH,
Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al:
Beige adipocytes are a distinct type of thermogenic fat cell in
mouse and human. Cell. 150:366–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon HS, Dincer F and Mantzoros CS:
Pharmacological concentrations of irisin increase cell
proliferation without influencing markers of neurite outgrowth and
synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism.
62:1131–1136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huh JY, Dincer F, Mesfum E and Mantzoros
CS: Irisin stimulates muscle growth-related genes and regulates
adipocyte differentiation and metabolism in humans. Int J Obes
(Lond). 38:1538–1544. 2014.PubMed/NCBI
|
|
13
|
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin
W, Hu Y, Xu W and Xu L: Irisin promotes osteoblast proliferation
and differentiation via activating the MAP kinase signaling
pathways. Sci Rep. 6:187322016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colaianni G, Cuscito C, Mongelli T,
Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M,
Mori G, et al: The myokine irisin increases cortical bone mass.
Proc Natl Acad Sci USA. 112:pp. 12157–12162. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song H, Wu F, Zhang Y, Zhang Y, Wang F,
Jiang M, Wang Z, Zhang M, Li S, Yang L, et al: Irisin promotes
human umbilical vein endothelial cell proliferation through the ERK
signaling pathway and partly suppresses high glucose-induced
apoptosis. PLoS One. 9:e1102732014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Xiang G, Liu M, Mei W, Xiang L and
Dong J: Irisin protects against endothelial injury and ameliorates
atherosclerosis in apolipoprotein E-Null diabetic mice.
Atherosclerosis. 243:438–448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song H, Xu J, Lv N, Zhang Y, Wu F, Li H,
Shao L, Mu Q, Wang F, Tang D, et al: Irisin reverses platelet
derived growth factor-BB-induced vascular smooth muscle cells
phenotype modulation through STAT3 signaling pathway. Biochem
Biophys Res Commun. 479:139–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Song H, Zhang Y, Wu F, Mu Q,
Jiang M, Wang F, Zhang W, Li L, Shao L, et al: Irisin inhibits
atherosclerosis by promoting endothelial proliferation through
microRNA126-5p. J Am Heart Assoc. 5:52016. View Article : Google Scholar
|
|
19
|
Provatopoulou X, Georgiou GP, Kalogera E,
Kalles V, Matiatou MA, Papapanagiotou I, Sagkriotis A, Zografos GC
and Gounaris A: Serum irisin levels are lower in patients with
breast cancer: Association with disease diagnosis and tumor
characteristics. BMC Cancer. 15:8982015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aydin S, Kuloglu T, Ozercan MR, Albayrak
S, Aydin S, Bakal U, Yilmaz M, Kalayci M, Yardim M, Sarac M, et al:
Irisin immunohistochemistry in gastrointestinal system cancers.
Biotech Histochem. 91:242–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Us Altay D, Keha EE, Yaman S Ozer, Ince I,
Alver A, Erdogan B, Canpolat S, Cobanoglu U and Mentese A:
Investigation of the expression of irisin and some cachectic
factors in mice with experimentally induced gastric cancer. QJM.
109:785–790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuloglu T, Celik O, Aydin S, Ozercan I
Hanifi, Acet M, Aydin Y, Artas G, Turk A, Yardim M, Ozan G, et al:
Irisin immunostaining characteristics of breast and ovarian cancer
cells. Cell Mol Biol (Noisy-le-grand). 62:40–44. 2016.PubMed/NCBI
|
|
23
|
Gannon NP, Vaughan RA, Garcia-Smith R,
Bisoffi M and Trujillo KA: Effects of the exercise-inducible
myokine irisin on malignant and non-malignant breast epithelial
cell behavior in vitro. Int J Cancer. 136:197–202. 2015. View Article : Google Scholar
|
|
24
|
Shao L, Li H, Chen J, Song H, Zhang Y, Wu
F, Wang W, Zhang W, Wang F, Li H and Tang D: Irisin suppresses the
migration, proliferation, and invasion of lung cancer cells via
inhibition of epithelial-to-mesenchymal transition. Biochem Biophys
Res Commun. 485:598–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaffney R, Unni KK, Sim FH, Slezak JM,
Esther RJ and Bolander ME: Follow-up study of long-term survivors
of osteosarcoma in the prechemotherapy era. Hum Pathol.
37:1009–1014. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosano L, Cianfrocca R, Spinella F, Di
Castro V, Nicotra MR, Lucidi A, Ferrandina G, Natali PG and Bagnato
A: Acquisition of chemoresistance and EMT phenotype is linked with
activation of the endothelin A receptor pathway in ovarian
carcinoma cells. Clin Cancer Res. 17:2350–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davidson B, Tropé CG and Reich R:
Epithelial-mesenchymal transition in ovarian carcinoma. Front
Oncol. 2:332012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tu B, Du L, Fan QM, Tang Z and Tang TT:
STAT3 activation by IL-6 from mesenchymal stem cells promotes the
proliferation and metastasis of osteosarcoma. Cancer Lett.
325:80–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang
SW, Hwang WL and Tang CH: Interleukin-6 induces vascular
endothelial growth factor expression and promotes angiogenesis
through apoptosis signal-regulating kinase 1 in human osteosarcoma.
Biochem Pharmacol. 85:531–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Wang S, Su J, Chu G, You H, Chen
Z, Sun H, Chen B and Zhou M: Interleukin-32α inactivates JAK2/STAT3
signaling and reverses interleukin-6-induced epithelial-mesenchymal
transition, invasion, and metastasis in pancreatic cancer cells.
Onco Targets Ther. 9:4225–4237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng J, Zhang XT, Liu XL, Fan L, Li C, Sun
Y, Liang XH, Wang JB, Mei QB, Zhang F, et al: WSTF promotes
proliferation and invasion of lung cancer cells by inducing EMT via
PI3K/Akt and IL-6/STAT3 signaling pathways. Cell Signal.
28:1673–1682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W
and Zhang L: Long noncoding RNA lncTCF7, induced by IL-6/STAT3
transactivation, promotes hepatocellular carcinoma aggressiveness
through epithelial-mesenchymal transition. J Exp Clin Cancer Res.
34:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu H, Ren G, Wang T, Chen Y, Gong C, Bai
Y, Wang B, Qi H, Shen J, Zhu L, et al: Aberrantly expressed Fra-1
by IL-6/STAT3 transactivation promotes colorectal cancer
aggressiveness through epithelial-mesenchymal transition.
Carcinogenesis. 36:459–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dou L, Wang S, Sui X, Meng X, Shen T,
Huang X, Guo J, Fang W, Man Y, Xi J and Li J: MiR-301a mediates the
effect of IL-6 on the AKT/GSK pathway and hepatic glycogenesis by
regulating PTEN expression. Cell Physiol Biochem. 35:1413–1424.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Che Q, Liu BY, Wang FY, He YY, Lu W, Liao
Y, Gu W and Wan XP: Interleukin 6 promotes endometrial cancer
growth through an autocrine feedback loop involving ERK-NF-κB
signaling pathway. Biochem Biophys Res Commun. 446:167–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu YS, Chung I, Wong WF, Masamune A, Sim
MS and Looi CY: Paracrine IL-6 signaling mediates the effects of
pancreatic stellate cells on epithelial-mesenchymal transition via
Stat3/Nrf2 pathway in pancreatic cancer cells. Biochim Biophys
Acta. 1861:296–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan J, Lee Y, Zhang Q, Xiong D, Wan TC,
Wang Y and You M: Honokiol decreases lung cancer metastasis through
inhibition of the STAT3 signaling pathway. Cancer Prev Res (Phila).
10:133–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xue D, Yang Y, Liu Y, Wang P, Dai Y, Liu
Q, Chen L, Shen J, Ju H, Li Y, et al: MicroRNA-206 attenuates the
growth and angiogenesis in non-small cell lung cancer cells by
blocking the 14-3-3ζ/STAT3/HIF-1α/VEGF signaling. Oncotarget.
7:79805–79813. 2016.PubMed/NCBI
|
|
41
|
Yuan H, Kajiyama H, Ito S, Yoshikawa N,
Hyodo T, Asano E, Hasegawa H, Maeda M, Shibata K, Hamaguchi M, et
al: ALX1 induces snail expression to promote
epithelial-to-mesenchymal transition and invasion of ovarian cancer
cells. Cancer Res. 73:1581–1590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356(2 Pt B): 1–331. 2015.
|
|
44
|
Wu D, Cheng J, Sun G, Wu S, Li M, Gao Z,
Zhai S, Li P, Su D and Wang X: p70S6K promotes IL-6-induced
epithelial-mesenchymal transition and metastasis of head and neck
squamous cell carcinoma. Oncotarget. 7:36539–36550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee SO, Yang X, Duan S, Tsai Y, Strojny
LR, Keng P and Chen Y: IL-6 promotes growth and
epithelial-mesenchymal transition of CD133+ cells of
non-small cell lung cancer. Oncotarget. 7:6626–6638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Papiewska-Pająk I, Kowalska MA and Boncela
J: Expression and activity of SNAIL transcription factor during
epithelial to mesenchymal transition (EMT) in cancer progression.
Postepy Hig Med Dosw (Online). 70:968–980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Turley EA, Veiseh M, Radisky DC and
Bissell MJ: Mechanisms of disease: Epithelial-mesenchymal
transition - does cellular plasticity fuel neoplastic progression?
Nat Clin Pract Oncol. 5:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saitoh M, Endo K, Furuya S, Minami M,
Fukasawa A, Imamura T and Miyazawa K: STAT3 integrates cooperative
Ras and TGF-β signals that induce Snail expression. Oncogene.
35:1049–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015. View Article : Google Scholar : PubMed/NCBI
|