Introduction
Gastric cancer is one of the most commonly diagnosed
cancers worldwide, and it is projected that it ranks fourth in
incidence and is the third leading cause of death worldwide
(1). Although producing low pH
gastric acid, gastric tissue can still be infected with
Helicobacter pylori, leading to gastritis (2,3).
Stimulations by certain food, such as spicy and hot temperature
food and alcohol, often contribute to tissue inflammation and
accumulation of reactive oxygen species (ROS) (4). Chronic inflammation along with altered
expression of various genes and abnormal local microenvironment
leads to the occurrence of gastric cancer (3). Heme oxygenase-1 (HO-1) is one of the
predominant genes to reduce inflammation and oxidation in the body
(5). HO-1 regulates the expression
of many genes and important molecules, and these have been involved
in regulation of cell proliferation, migration, and invasion in
many types of cancers, including colon (6), lung (7) and breast cancer (8).
HO-1 and its two isomers, HO-2 and HO-3, have an
extremely low expression in the physiological state (5,9). HO-1
plays a vital role in the anti-oxidation and anti-inflammation
system and is mainly responsible for suppressing inflammation and
removing ROS in the tissue microenvironment (10). In the presence of inflammation,
including gastritis, HO-1 expression was upregulated in local
tissues (11). HO-1 decomposes heme
into CO, Fe2+, and biliverdin. CO may decrease
inflammation and inhibit apoptosis by regulating the P38-MPAK
signaling pathway and increasing the expression of BCL-2 (12). The conversion of Fe2+ to
Fe3+ could reduce the accumulation of ROS via Fenton
reaction (13,14). Biliverdin has strong anti-oxidative
properties, which could inhibit angiogenesis by suppressing the
VEGF and HIF-1 signaling pathways (15). Since HO-1 and its downstream
signaling mitigate inflammation and repair oxidative stress injury,
we hypothesize that HO-1 may play an important role in the
occurrence and development of gastric cancer.
In this study, we examined the expression of HO-1 in
gastric cancer tissue compared with the peritumoral tissue by
immunochemistry. The correlation of HO-1 expression with clinical
characteristics and prognosis was evaluated in gastric cancer
patients. Moreover, the effects of downregulation of HO-1 by two
different strands of siRNAs were studied in gastric cancer cell
lines. Our results demonstrated the critical functions of HO-1 in
gastric cancer.
Materials and methods
Patients and tissue specimens
The tissue microarray, containing 89 gastric cancer
tissues and matched adjacent normal tissues, was purchased from
Shanghai Outdo Biotech (Shanghai, China). Clinical characteristics
and survival data were obtained from the 89 patients with gastric
cancer. The survival data were obtained between October 2008 and
July 2015. The gastric cancer patients for the tissue microarray
were all diagnosed by history and pathology. The local ethics
committee approved this study, and informed consent was obtained
from each patient.
Immunohistochemical assay
Primary antibodies against HO-1 (1:500 dilution;
Abnova Corp., Taipei, Taiwan) were used in the present experiment.
Immunohistochemical staining was carried out as described in our
previous study (16). The
immunohistochemistry results were evaluated based on positive cell
numbers in the cytoplasmic and nuclear staining. The following
score rank system for immunohistochemical staining was used: I, no
staining; II, 10% of cells with nuclear staining and/or weak
cytoplasmic staining; III, 10–50% of cells with nuclear staining
and/or distinct cytoplasmic staining; and IV, >50% of cells with
nuclear staining and/or strong cytoplasmic staining. The scores I
and II were considered negative expression, and the scores III and
IV were considered as positive expression.
Cell culture and siRNA
transfection
The two human gastric cancer cell lines, MKN-28 and
SGC7901, and the immortalized normal gastric epithelial cell line,
GES-1, were gifts from the Cancer Centre at Sun Yat-Sen University.
Commercially available HO-1 siRNAs, the empty vector containing a
nonsense RNA sequence, and the RNA transfection kit were obtained
from Guangzhou Ribo Bio (Guangzhou, China). All cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM, Life
Technologies, Carlsbad, CA, USA) with 10% fetal calf serum (Gibco,
Carlsbad, CA, USA). The cell lines at the harvesting logarithmic
growth phase were plated on six-well plates at a density of
2×105 and cultured in a 37°C humidified incubator with a
5% CO2 atmosphere. Each cell line was treated with
either the nonsense RNA sequence (negative control), HO-1 siRNA-1,
or HO-1 siRNA-2. Transfection was carried out with siRNA at a
concentration of 100 nM according to the instruction of the kit.
The mRNA expression of HO-1 was evaluated by quantitative real-time
PCR (qPCR).
MTT assay
MKN-28 and SGC7901 cells at the logarithmically
growing phase were seeded at a density of 1×104 on
96-well plates and transfected with either nonsense RNA, HO-1
siRNA-1, or HO-1 siRNA-2 after overnight culture. At 24, 48, and 72
h post-transfection, a mixture of 20 µl MTT and 180 µl DMED was
added to each well. The cells were cultured for another 4 h. The
supernatant was removed, and 150 µl DMSO was added to each well.
The absorbance was measured at 490 nm.
Flow cytometric analysis
MKN-28 and SGC7901 cells at the logarithmically
growing phase were seeded at a density of 1.5×105 on
6-well plates and transfected with either nonsense RNA, HO-1
siRNA-1, or HO-1 siRNA-2 after overnight culture. The cells were
harvested at 24 h and evaluated for apoptosis by flow cytometry
using propidium iodide (PI) and Annexin V, as previously described
(17).
Cell invasion assay
MKN-28 and SGC7901 cells were harvested and seeded
at a density of 1×104/100 µl DMEM in the upper chamber.
The lower chamber was loaded with 600 µl DMEM with 10% FBS. Both
cell lines were transfected with either nonsense RNA, HO-1 siRNA-1,
or HO-1 siRNA-2. After incubation for 24 h, crystal violet was used
to stain the cells in the lower membrane of the chamber. Cells in
five randomly selected fields were counted under the
microscope.
RNA isolation and reverse
transcription PCR
MKN-28 and SGC7901 cells were harvested after being
transfected with either nonsense RNA, HO-1 siRNA-1, or HO-1 siRNA-2
for 48 h. TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used
to extract total RNA from cells. These procedures were carried out
on ice to prevent RNA degradation. The Nanodrop spectrophotometer
(ND-2000; Nanodrop Technology, Wilmington, DE, USA) was used to
evaluate the purity and concentration of RNA. The reverse
transcription reagent (Takara Bio, Inc., Kusatsu, Shiga, Japan) was
used to transcribe RNA to cDNA. The SYBR-Green Prime Script RT-PCR
kit (Takara Bio, Inc.) and the Real-time PCR detection system
(Applied Biosystems, Foster City, CA, USA) were used to perform
qPCR. The internal control was GAPDH, and the primers were:
forward, 5′-TCCCATCACCATCTTCCAG-3′ and reverse,
5′-GAGCCCCAGCCTTCTCCAT-3′. Three pairs of primers were used to
quantitate HO-1 and obtain independent results. The first pair was
5′-GGAGAUUGAGCGCAACAAG-3′ (forward) and 5′-CUUGUUGCGCUAAAUCUCC-3′
(reverse). The second pair was 5′-UGAUAGAAGAGGCCAAGAC-3′ (forward)
and 5′-GUCUUGGCCUCUUCUAUCA-3′ (reverse). The third pair was 11
5′-CUGCGUUCCUGCUCAACAU-3′ (forward) and 5′-AUGUUGAGCAGGAACGCAG-3′
(reverse). The 2−∆∆Ct method was used for fold change
calculation.
Western blot analysis
The GES-1, MKN-28, and SGC7901 cells at the
logarithmically growing stage were harvested for protein isolation
using the protein lysis solution. The protein from each cell line
was loaded into 12% sodium dodecyl sulfate (SDS) polyacrylamide
gels and transferred to polyvinylidene difluoride (PVDF) membranes.
The membranes were blocked with 5% milk for 1.5 h, and incubated
with a 1:500 dilution of primary antibody against HO-1 (Abnova
Corp.) overnight at 4°C. The primary antibody was visualized with
an enhanced chemiluminescence reagent (Beyotime) against the
peroxidase-conjugated secondary antibody. The results were analyzed
by densitometry analysis. β-actin was used as an internal
control.
Statistical analysis
Statistical analysis was conducted using the SPSS
19.0 software (IBM, Chicago, IL, USA). Each experiment was
conducted three times. Experimental results were demonstrated as
mean ± standard deviation (mean ± SD). Comparisons of
clinicopathological parameters between high and low HO-1 expression
patients were performed using a two-sided and unpaired t-test.
Kaplan-Meier analysis was used to evaluate the survival curves, and
the log-rank test was used to analyze prognostic significance. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
HO-1 expression in gastric cancer
tissues and matched adjacent non-cancer tissues
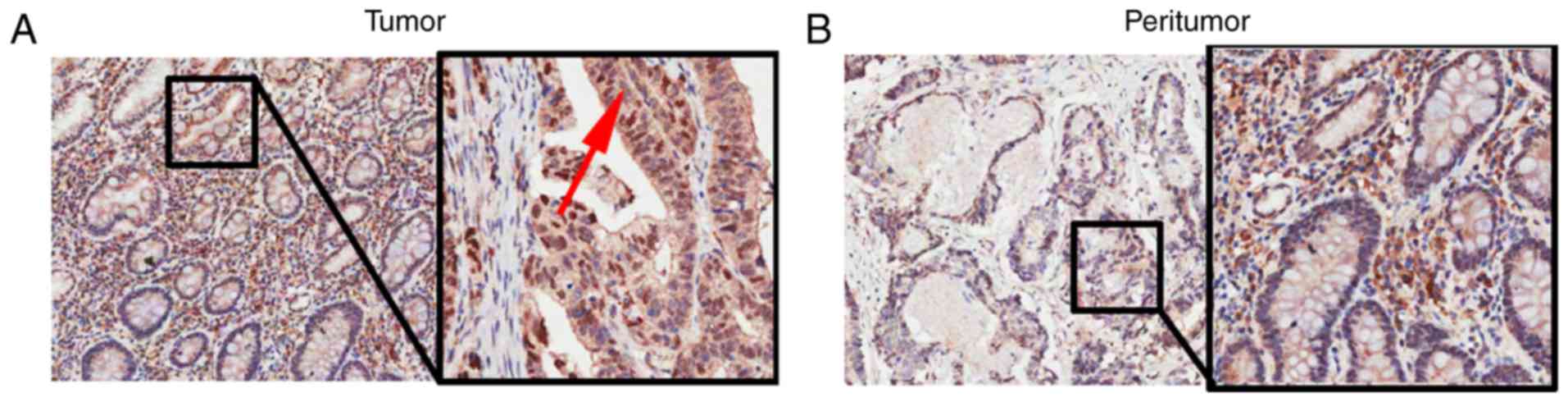
The immunohistochemical assay showed that high
expression of HO-1 was present in 10 of 89 (11.2%) gastric cancer
patient tissues. The expression of HO-1 was mainly located in the
cytoplasm of gastric cancer cells (Fig.
1A). In contrast, low expression of HO-1 was observed in 1 of
89 (1.1%) matched adjacent non-cancer tissues, with HO-1 expression
primarily located in the cytoplasm of adjacent non-cancer cells
(Fig. 1B).
Correlations between HO-1 expression
and clinicopathological characteristics in gastric cancer
patients
We next explored the possible correlations between
the expression of HO-1 and clinicopathological characteristics in
gastric cancer patients. As shown in Table I, there was no statistical
significance between HO-1 expression and the pathological grade of
gastric cancer (P=0.052), and no significant correlations between
HO-1 expression and the other clinicopathological characteristics,
including age, sex, tumor size, clinicopathologic classifications
(T, N, M), and clinical staging among the 89 gastric cancer
patients (P>0.05).
 | Table I.Correlations of HO-1 protein
expression with clinicopathological characteristics in the tissue
specimens from gastric cancer patients. |
Table I.
Correlations of HO-1 protein
expression with clinicopathological characteristics in the tissue
specimens from gastric cancer patients.
|
|
| HO-1 expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Patient
characteristics | No. | Low (n=79) | High (n=10) | χ2 | P-value |
|---|
| Age (years) |
|
|
| 0.664 | 0.497 |
| ≤65 | 55 | 50 | 5 |
|
|
|
>65 | 34 | 29 | 5 |
|
|
| Sex |
|
|
| 0.621 | 0.513 |
| Male | 52 | 45 | 7 |
|
|
|
Female | 37 | 34 | 3 |
|
|
| Tumor size |
|
|
| 0.348 | 0.503 |
| ≤5
cm | 48 | 44 | 4 |
|
|
| >5
cm | 41 | 35 | 6 |
|
|
| Pathological
grade |
|
|
| 4.357 | 0.052 |
| Grade
II | 21 | 16 | 5 |
|
|
| Grade
III | 68 | 63 | 5 |
|
|
| T
classification |
|
|
| 1.731 | 0.189 |
|
T1-T2 | 14 | 11 | 3 |
|
|
|
T3-T4 | 75 | 68 | 7 |
|
|
| N
classification |
|
|
| 0.321 | 0.736 |
|
N0-N1 | 34 | 31 | 3 |
|
|
|
N2-N3 | 55 | 48 | 7 |
|
|
| M
classification |
|
|
| 0.530 | 1.000 |
| M0 | 85 | 75 | 10 |
|
|
| M1 | 4 | 4 | 0 |
|
|
| Clinical stage |
|
|
| 0.411 | 0.734 |
|
I–II | 35 | 32 | 3 |
|
|
|
III–IV | 54 | 47 | 7 |
|
|
Low expression of HO-1 is associated
with an improved prognosis in gastric cancer patients
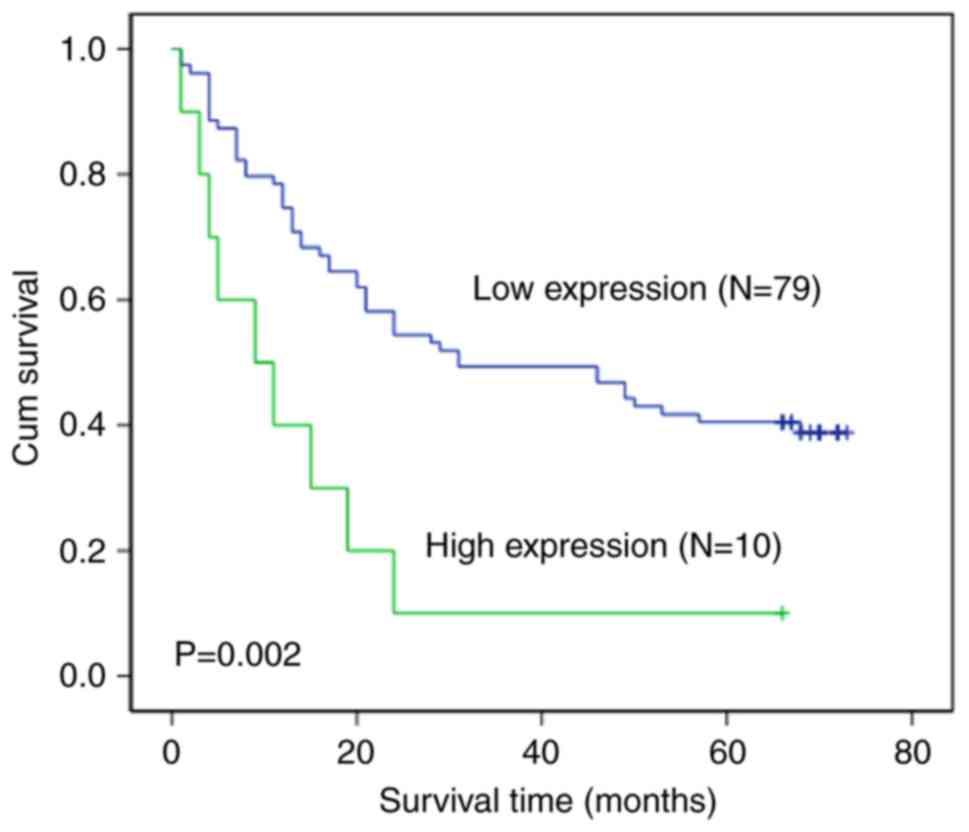
We next investigated the relationship between HO-1
expression in gastric cancer tissue and patient survival. Our
results indicated that there was a significant relationship between
HO-1 expression and the overall survival time in gastric cancer
patients (P=0.002). The Kaplan-Meier curves demonstrated that the
gastric cancer patients with low expression of HO-1 had a longer
overall survival time (median, 33.4 months) compared with patients
expressing a high level of HO-1 (median, 11.2 months, P=0.002). The
cumulative 5-year survival rate was 39.2% (31/79) for gastric
cancer patients with low HO-1 expression. The survival rate
decreased to 10% (1/10) for patients with high expression of HO-1
(Fig. 2).
Protein expression of HO-1 in GES-1,
MKN-28, and SGC7901 cells and the effects of knockdown of HO-1
expression on MKN-28 and SGC7901 cells
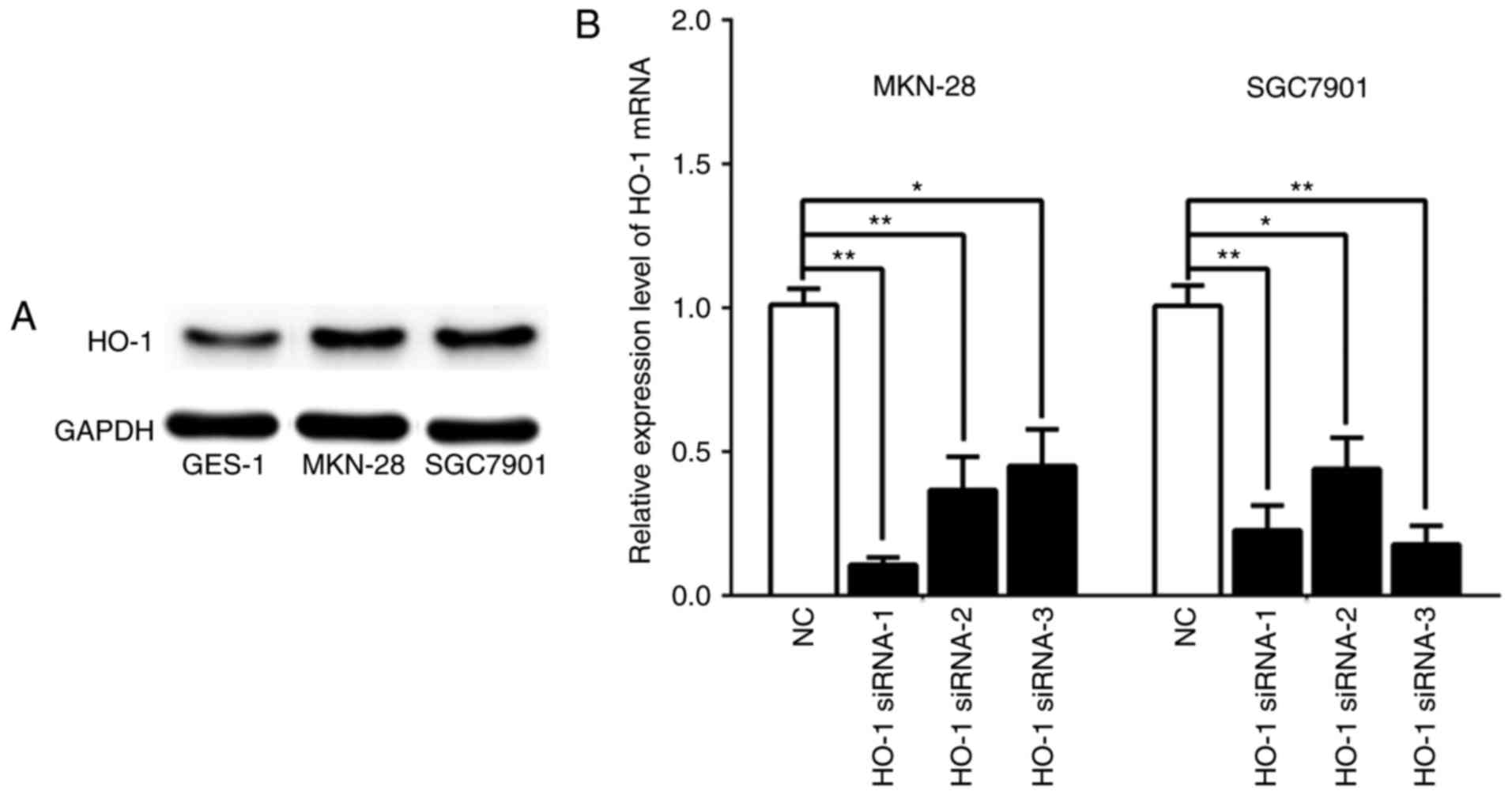
Western blot results indicated that the protein
expression of HO-1 was markedly higher in MKN-28 and SGC7901 cell
lines compared with GES-1 cells (Fig.
3A). After confirming HO-1 expression in MKN-28 and SGC7901
cells, we downregulated the expression of HO-1 in these two cell
lines using three different strands of siRNAs, namely HO-1 siRNA-1,
HO-1 siRNA-2, and HO-1 siRNA-3. At 48 h post-transfection, the qPCR
results demonstrated that the mRNA expression of HO-1 in both cell
lines was significantly decreased by each strand of siRNA when
compare with the respective negative control (NC) group (Fig. 3B, P<0.05).
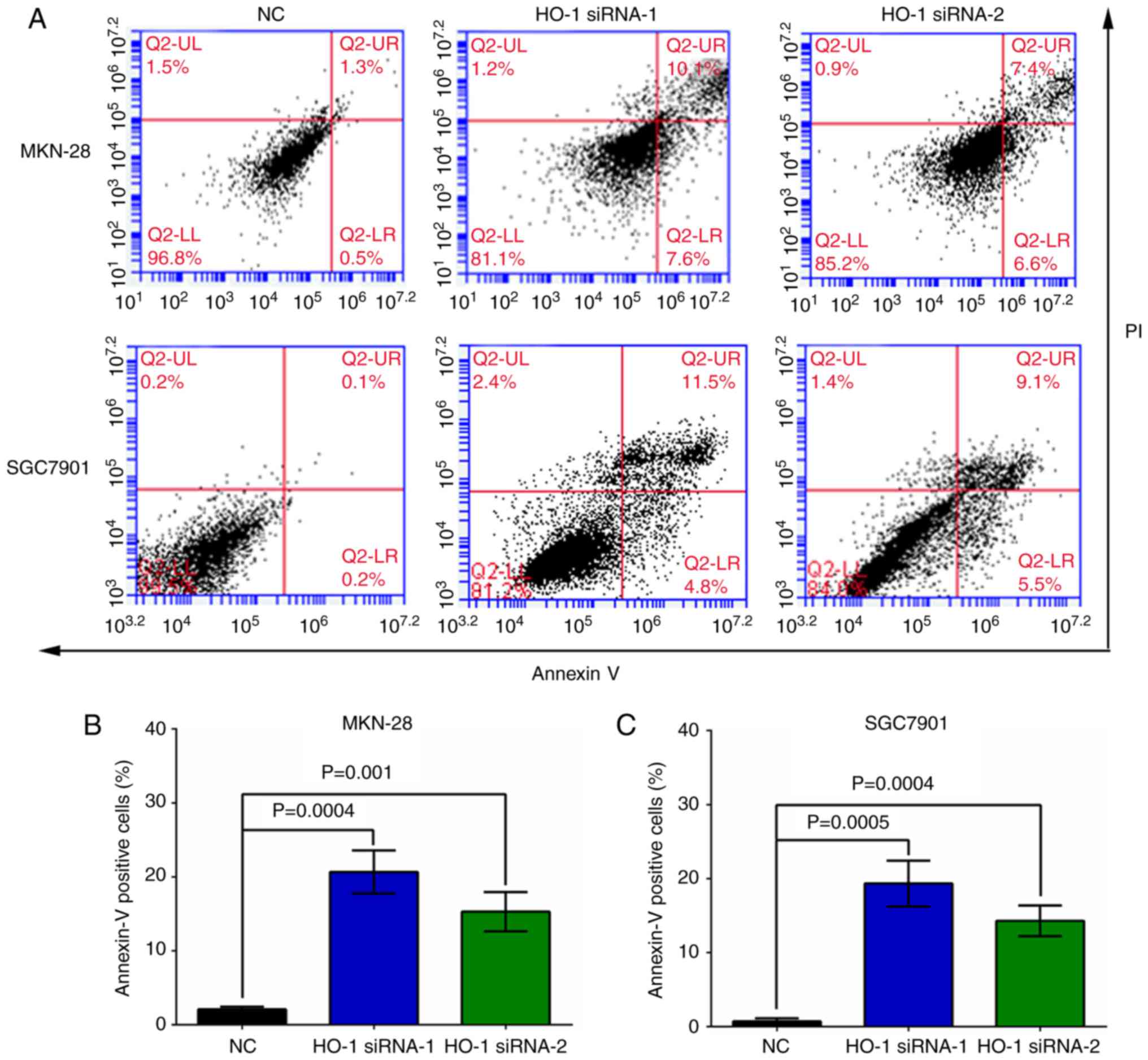
Knockdown of HO-1 expression inhibits
apoptosis in gastric cancer cells
At 24 h post-transfection, flow cytometry analysis
demonstrated that the apoptosis rates were 1.2±0.2%, 10.8±1.4%, and
7.5±1.9% in the MKN-28 cells transfected with NC, HO-1 siRNA-1, and
HO-1 siRNA-2, respectively (Fig.
4A, upper panel). Similarly, at the same time point, the
apoptosis rates were 0.4±0.2%, 11.8±1.1%, and 8.5±1.7% in the
SGC7901 cells transfected with NC, HO-1 siRNA-1, and HO-1 siRNA-2,
respectively (Fig. 4A, lower
panel). The knockdown of HO-1 expression by siRNAs significantly
increased the apoptosis rates in MKN-28 (Fig. 4B, P<0.001) and SGC7901 cells
(Fig. 4C, P<0.001) when compared
to their respective NC group.
Knockdown of HO-1 expression reduces
the viability of gastric cancer cells
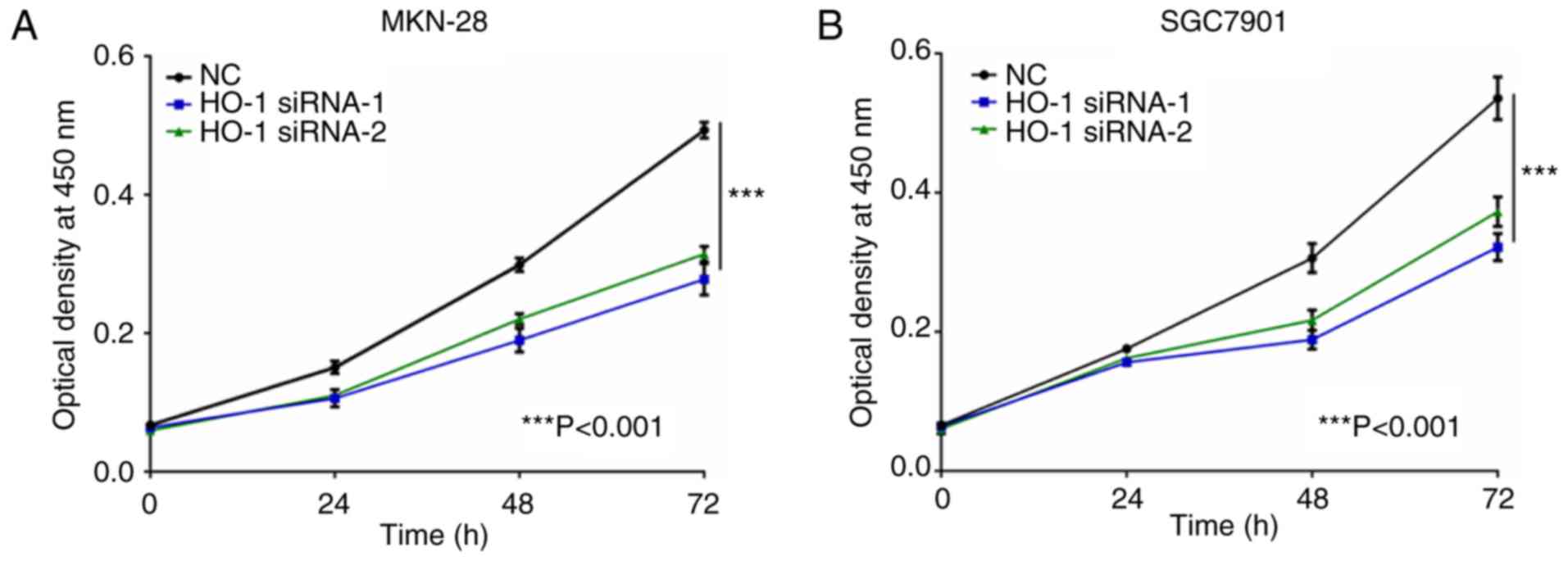
At 24, 48, and 72 h post-transfection, the MTT assay
was used to measure cell viability in MKN-28 and SGC7901 cells
transfected with either NC-RNA, HO-1 siRNA-1, or HO-1 siRNA-2
(Fig. 5). At 72 h
post-transfection, the MKN-28 and SGC7901 cells transfected with
HO-1 siRNAs had less viable cells compared with their respective NC
group (P<0.001).
Knockdown of HO-1 expression
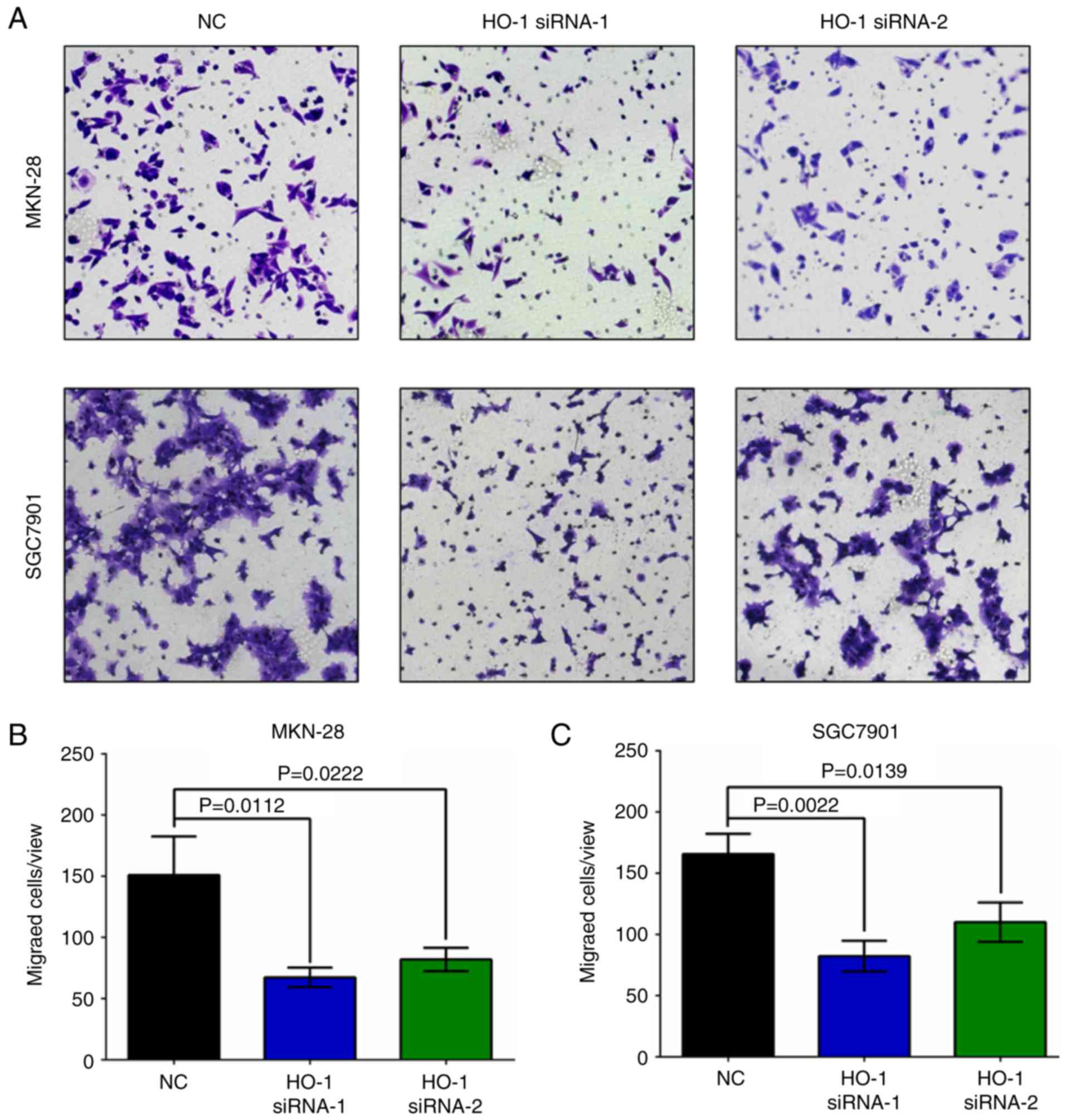
attenuates invasion of gastric cancer cells
The invasive ability of MKN-28 and SGC7901 cells was
evaluated at 24 h post-HO-1 siRNA transfection. Knockdown of HO-1
significantly attenuated cell invasion in both cell lines when
compared to their respective NC group (Fig. 6, P<0.05).
Discussion
The expression and function of HO-1, a novel spy
gene, are variable in the physiological, inflammatory, and
cancerous states. HO-1 removes the toxic heme by breaking it down
to biliverdin, iron ions, and carbon monoxide (9). In the physiological state, the HO-1
expression is extremely low and maintains homeostasis in the
microenvironment of local tissues. Upon injury, inflammation, and
other external insults in local tissues, the expression of HO-1 is
elevated and serves as a protective mechanism for the body to fight
against inflammation, oxidation, and apoptosis (10,18).
When the harmful stimulations subside, the HO-1 expression returns
to baseline (19). Chronic
inflammation may lead to the development of various types of
cancers, including gastric cancer (3). High expression of HO-1 has been found
in various cancers, with correlations with the clinical
characteristics and prognosis in patients. The prolonged high
expression of HO-1 is harmful to the cancerous tissues and is
associated with a poor prognosis in numerous cancers, including
breast (8), colorectal (6), and prostate cancers (20). However, the role of HO-1 in gastric
cancer has yet to be determined.
Previous studies demonstrated that upregulation of
HO-1 leads to resistance of apoptosis in gastric cancer cells
(21). In addition, the DNA
polymorphism in the HO-1 promoter is associated with the risk of
developing gastric adenocarcinoma (22). Expression of HO-1 was detected in 62
gastric cancer tissues of 74 cases (83.8%) (23). This evidence suggests that HO-1 may
play a vital role in gastric carcinogenesis. At present, scarce
data have shown whether the expression of HO-1 is higher in gastric
cancer compared to matched adjacent non-cancer tissues or
investigated the relationship between HO-1 expression and overall
survival of gastric cancer patients.
Our present study found that 11% gastric cancer
tissues had high expression of HO-1 compared to only 1% matched
adjacent normal tissues had HO-1 expression. We are the first to
demonstrate that low HO-1 expression in gastric cancer patients was
positively correlated with their overall survival. This result is
consistent with findings from previous studies that found HO-1
expression is elevated in breast cancer tissues and associated with
the prognosis of breast cancer patients (8). However, our analysis did not find
correlations between HO-1 expression with either the pathological
grade or clinical characteristics of gastric cancer. This result
differs from previous studies and may be due to a limited number of
cases or demographical reasons.
Several studies have reported that low expression of
HO-1 promotes apoptosis in acute myeloid leukemia cells and that
increased HO-1 expression inhibits the chemotherapy-induced
apoptosis (24,25). HO-1 influences apoptosis in human
acute myeloid leukemia by regulating the transcription factors
Nrf2, NF-κB, and AP-1. In contrast, knockdown of HO-1 expression in
gastric cancer cells by siRNA sensitizes the cells to chemotherapy
(23). In this study, we found that
the two gastric cancer lines, MKN-28 and SGC7901 cells, had higher
protein expression of HO-1 compared with the immortalized normal
gastric epithelial cell line GES-1. For more reliable results, we
used two strands of siRNAs targeting HO-1 to independently knock
down HO-1 expression in MKN-28 and SGC7901 cells. Knockdown of HO-1
significantly increased the apoptosis rate in gastric cancer cells,
which is consistent with previous studies.
An increasing number of studies have identified the
correlation between HO-1 expression and cancer cell proliferation.
For example, cell proliferation is inhibited in HO-1 siRNA-treated
human pancreatic cancer cells and bladder cancer cell lines
(26,27). In addition, downregulation of HO-1
in human urothelial cancer cell lines decreases the expression of
Ki-67, a marker for cell proliferation (28). Our data are consistent with these
findings. In gastric cancer cell lines, the proliferation was
significantly decreased at 72 h after HO-1 siRNA transfection. To
our knowledge, little is known about how HO-1 regulates cell
proliferation so far, and more research is needed to investigate
this mechanism.
It has been reported that increased expression of
HO-1 promotes the invasive ability of lung adenocarcinoma cell
lines, while downregulation of HO-1 expression by siRNA inhibits
their invasive ability (29,30).
ROS and the TGF-β1/PI3K/Akt signaling pathway may be the mechanism
involved in HO-1 regulation of cell invasion. In another study,
upregulation of HO-1 expression in gastric cancer cells increased
the expression of MMP, a biomarker for cell invasion and migration,
and vice versa (23). In this
study, we found that knockdown of HO-1 expression attenuated
gastric cancer cell invasion, strongly agreeing with the previous
studies.
In conclusion, our results demonstrated that low
expression of HO-1 in gastric cancer tissues correlated with a
better prognosis in patients and that knockdown of the HO-1
expression inhibited gastric cancer cell apoptosis, proliferation,
and invasion. More research is needed to elucidate the molecular
mechanisms, but our findings suggest that the HO-1 gene can be
targeted to treat gastric cancer.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Korwin J-D: Epidemiology of
Helicobacter pylori infection and gastric cancer. Rev Prat.
64:189–193. 2014.(In French). PubMed/NCBI
|
|
3
|
Munn LL: Cancer and inflammation. Wiley
Interdiscip Rev Syst Biol Med. 9:e13702017. View Article : Google Scholar
|
|
4
|
Vohlonen I, Pukkala E, Malila N, Härkönen
M, Hakama M, Koistinen V and Sipponen P: Risk of gastric cancer in
Helicobacter pylori infection in a 15-year follow-up. Scand J
Gastroenterol. 51:1159–1164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jozkowicz A, Was H and Dulak J: Heme
oxygenase-1 in tumors: Is it a false friend? Antioxid Redox Signal.
9:2099–2117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin H, Fang J, Liao L, Maeda H and Su Q:
Upregulation of heme oxygenase-1 in colorectal cancer patients with
increased circulation carbon monoxide levels, potentially affects
chemotherapeutic sensitivity. BMC Cancer. 14:4362014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Degese MS, Mendizabal JE, Gandini NA,
Gutkind JS, Molinolo A, Hewitt SM, Curino AC, Coso OA and
Facchinetti MM: Expression of heme oxygenase-1 in non-small cell
lung cancer (NSCLC) and its correlation with clinical data. Lung
Cancer. 77:168–175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noh SJ, Bae JS, Jamiyandorj U, Park HS,
Kwon KS, Jung SH, Youn HJ, Lee H, Park BH, Chung MJ, et al:
Expression of nerve growth factor and heme oxygenase-1 predict poor
survival of breast carcinoma patients. BMC Cancer. 13:5162013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wegiel B, Nemeth Z, Correa-Costa M, Bulmer
AC and Otterbein LE: Heme oxygenase-1: A metabolic nike. Antioxid
Redox Signal. 20:1709–1722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lundvig DM, Immenschuh S and Wagener FA:
Heme oxygenase, inflammation, and fibrosis: The good, the bad, and
the ugly? Front Pharmacol. 3(81)2012.PubMed/NCBI
|
|
11
|
Ivanov AV, Valuev-Elliston VT, Tyurina DA,
Ivanova ON, Kochetkov SN, Bartosch B and Isaguliants MG: Oxidative
stress, a trigger of hepatitis C and B virus-induced liver
carcinogenesis. Oncotarget. 8:3895–3932. 2017.PubMed/NCBI
|
|
12
|
Liao YF, Zhu W, Li DP and Zhu X: Heme
oxygenase-1 and gut ischemia/reperfusion injury: A short review.
World J Gastroenterol. 19:3555–3561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strasser-Weippl K and Ludwig H: Ferritin
as prognostic marker in multiple myeloma patients undergoing
autologous transplantation. Leuk Lymphoma. 55:2520–2524. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng J, Nagda DA, Lajud SA, Kumar S,
Mouchli A, Bezpalko O, O'Malley BW Jr and Li D: Biliverdin's
regulation of reactive oxygen species signalling leads to potent
inhibition of proliferative and angiogenic pathways in head and
neck cancer. Br J Cancer. 110:2116–2122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ
and Wu C: The correlation of expression levels of HIF-1α and HIF-2α
in hepatocellular carcinoma with capsular invasion, portal vein
tumor thrombi and patients' clinical outcome. Jpn J Clin Oncol.
44:159–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu JL, Xiao L, Li ZY, Wang Q, Chang Y and
Jin Y: Upregulation of HO-1 is accompanied by activation of p38MAPK
and mTOR in human oesophageal squamous carcinoma cells. Cell Biol
Int. 37:584–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furfaro AL, Traverso N, Domenicotti C,
Piras S, Moretta L, Marinari UM, Pronzato MA and Nitti M: The
Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med
Cell Longev. 2016:19581742016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alaoui-Jamali MA, Gupta A, Szarek WA,
Bismar TA, Gheorghe R and Schipper HM: A novel selective
therapeutic targeting heme oxygenase-1 revealed a potent
antimetastatic activity in androgen-refractory human prostate
cancer models. J Clin Oncol. 27:e160902009.doi:
10.1200/jco.2009.27.15s.e16090.
|
|
21
|
Liu ZM, Chen GG, Ng EKW, Leung WK, Sung
JJY and Chung SCS: Upregulation of heme oxygenase-1 and p21 confers
resistance to apoptosis in human gastric cancer cells. Oncogene.
23:503–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Motovali-Bashi M and Hamidy M: Association
between GT-repeat polymorphism at heme oxygenase-1 gene promoter
and gastric cancer and metastasis. Tumour Biol. 36:4757–4762. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin Y, Liu Q, Wang B, Chen G, Xu L and
Zhou H: Expression and function of heme oxygenase-1 in human
gastric cancer. Exp Biol Med (Maywood). 237:362–371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayerhofer M, Florian S, Krauth MT,
Aichberger KJ, Bilban M, Marculescu R, Printz D, Fritsch G, Wagner
O, Selzer E, et al: Identification of heme oxygenase-1 as a novel
BCR/ABL-dependent survival factor in chronic myeloid leukemia.
Cancer Res. 64:3148–3154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heasman SA, Zaitseva L, Bowles KM,
Rushworth SA and Macewan DJ: Protection of acute myeloid leukaemia
cells from apoptosis induced by front-line chemotherapeutics is
mediated by haem oxygenase-1. Oncotarget. 2:658–668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berberat PO, Dambrauskas Z, Gulbinas A,
Giese T, Giese N, Künzli B, Autschbach F, Meuer S, Büchler MW and
Friess H: Inhibition of heme oxygenase-1 increases responsiveness
of pancreatic cancer cells to anticancer treatment. Clin Cancer
Res. 11:3790–3798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyake M, Ishii M, Kawashima K, Kodama T,
Sugano K, Fujimoto K and Hirao Y: siRNA-mediated knockdown of the
heme synthesis and degradation pathways: Modulation of treatment
effect of 5-aminolevulinic acid-based photodynamic therapy in
urothelial cancer cell lines. Photochem Photobiol. 85:1020–1027.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyake M, Fujimoto K, Anai S, Ohnishi S,
Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, Okajima E, et
al: Inhibition of heme oxygenase-1 enhances the cytotoxic effect of
gemcitabine in urothelial cancer cells. Anticancer Res.
30:2145–2152. 2010.PubMed/NCBI
|
|
29
|
Jeon WK, Hong HY, Seo WC, Lim KH, Lee HY,
Kim WJ, Song SY and Kim BC: Smad7 sensitizes A549 lung cancer cells
to cisplatin-induced apoptosis through heme oxygenase-1 inhibition.
Biochem Biophys Res Commun. 420:288–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Qiao T and Zha L: Inhibition of
heme oxygenase-1 enhances the radiosensitivity in human nonsmall
cell lung cancer a549 cells. Cancer Biother Radiopharm. 26:639–645.
2011. View Article : Google Scholar : PubMed/NCBI
|