Introduction
Papillary thyroid cancer (PTC) ranks the first among
endocrine tumors and head and neck tumors. According to statistics
in recent years, the morbidity of PTC shows an increasing and
younger trend, which has become one of the common malignancies
(1). In the USA, PTC accounts for
7.5–10% of all malignancies in population aged 15–24 years
(1). Differentiated thyroid cancer
(DTC) includes PTC and follicular thyroid cancer (FTC), which has
the highest morbidity in PTC, accounting for 95% of all PTCs
(2).
The association of miRNA with tumor has been
explained gradually accompanied by increase in research on microRNA
(miRNA) (3). It is indicated in
research that miRNA is closely related to tumor genesis,
metastasis, as well as sensitivity of tumor cells to antitumor
drugs (4). Therefore, research on
miRNA is promising to achieve breakthroughs in terms of non-131I
uptake in PTC lung metastasis and loss of differentiation (4).
miRNAs are a class of non-coding single-stranded
small RNA molecules with the length of 19–23 nucleotides, which
locate in the non-coding region of the genome (3). miRNAs, which are highly conserved in
evolution, can regulate gene expression at translation level.
Moreover, they can participate in multiple physiological metabolic
processes, including development, cell differentiation, cell
apoptosis, and cell energy metabolism (5). With the advancement in research on
molecular biology and molecular genetics, increasing studies have
demonstrated that miRNAs are related to tumor genesis, development,
metastasis and prognosis (5). Feng
et al showed that microRNA-148a suppressed cell
proliferation and migration of pancreatic cancer cells (6). Zhang et al showed that
microRNA-148a suppressed cell proliferation and invasion potential
through regulation of STAT3 in non-small cell lung carcinomas
(7).
Signal transducer and activator of transcription
(STAT) is a kind of signal transduction and transcriptional
activator, which is also a type of DNA binding protein (8). As the important JAK substrates in the
JAK-STAT pathway, STATs play critical roles in the signal
transduction of cytokines (CKs). STAT3 gene is a member of the STAT
family, which is referred to as the statistical gene (9). Such gene is a part of the basic
intracellular chemical signaling pathway, which gives commands to
the proteins (9). STAT3 gene has
become the hotspot of oncogene research at present since it
participates in cell growth and proliferation (10). STAT3 is one of the important members
among the signal transcription and activating factors (11). STAT3 has been indicated in
substantial research to be closely related to tumor cell
proliferation, differentiation, cell apoptosis, angiogenesis,
invasion, metastasis, and immune escape (12). Abnormal STAT3 expression and
activation has been verified to exist in multiple tumor tissues and
cell lines, including malignant melanoma, breast cancer, prostate
cancer, gastric cancer and colorectal cancer (10). Zhang et al showed that
microRNA-148a suppressed cell proliferation and invasion potential
through regulation of STAT3 in non-small cell lung carcinomas
(7).
Research on the correlation of PI3K/Akt/mTOR
signaling pathway with malignant tumor has become the focus in
recent years (13). The
PI3K/Akt/mTOR signaling pathway not only plays a vital role in the
growth and proliferation of normal cells, but is also closely
related to the growth of cancer cells. It is the central controller
of cell growth and differentiation that is involved in biological
processes such as gene transcription, protein translation, and
ribosome synthesis; in addition, it plays an extremely important
role in cell growth and apoptosis (13,14).
Therefore, investigating the correlation of PI3K/Akt/mTOR signaling
pathway with PTC can provide a brand-new angle of view for
illustrating the pathogenesis and diagnosis of PTC, and probing
into the application of its inhibitor can usher in a new path for
the targeted therapy of PTC (15).
In this study, we tested the function of miRNA-148a in lymphatic
metastases of papillary thyroid cancer and its mechanism.
Materials and methods
Ethics statement and patients
Written informed consent in the study was obtained
from all patients before surgery and volunteer at Department of
General Surgery, The Second Affiliated Hospital of Wenzhou Medical
University. Peripheral blood (5 ml) of patients with lymphatic
metastases of papillary thyroid cancer (n=6) and volunteers (n=6)
were selected into this study and serum were collected after
centrifuge at 2,000 × g for 10 min and saved at −80°C. This study
was approved by the ethics committee of The Second Affiliated
Hospital of Wenzhou Medical University. Samples were immediately
snap-frozen in liquid nitrogen for store.
RNA isolation and qRT-PCR
Total RNA was extracted from serum by using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's protocol. Genomic DNA was removed using RNase-free
DNase RQ1 (10 U; Promega). Total RNA (100 ng) was used to reverse
transcripted cDNA and qRT-PCR was performed with the Bio-Rad
Real-Time PCR Detection system (CFX Connect™; Bio-Rad) using iQ™
SYBR® Green Supermix (CFX Connect; Bio-Rad). The
relative expression of miRNA-148a was calculated using the
2−∆CT method.
Cell culture and transfection
Human PTC-derived TPC-1 cells were cultured in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
FBS (Gibco) in a 5% CO2 atmosphere at 37°C. miRNA-148a
(50 nM), si-STAT3 (50 nM) and negative control mimics (50 nM) were
purchased from and transfected into TPC-1 cell using Lipofectamine
3000 (Invitrogen, Guangzhou, China). After transfected with
miRNA-148a for 4 h, 100 nM of LY294002 was added into cells and
cultured for 48 h.
Cell proliferation assay
After transfection for 24, 48 and 72 h, cells were
placed in 96-well polystyrene tissue culture plates and 50 µl
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT,
1 µg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added and cultured
for 4 h at 37°C. Medium was discarded and 150 µl dimethylsulfoxide
(DMSO; Invitrogen Co., Australia) were loaded in each well for 20
min at 37°C. Absorbancy was measured at 492 nm with Microplate
Reader Model 550 (Bio-Rad Laboratories, Japan).
Transwell cell migration assay
After transfection for 24 h, TPC-1 cells were seeded
into 24-well Transwell plates (Corning, Corning, NY, USA). After
incubation for 24 h, cells were fixed and stained with 2% crystal
violet solution in ethanol (Beyotime Biotechnology). Cell was
observed using an IX71 fluorescence microscope (Olympus, Tokyo,
Japan).
Assessment of apoptosis
After transfection for 48 h, 1×106
cells/ml were counted and washed in PBS, re-suspended in binding
buffer. Cells were stained with FITC-V and PI (Pharmingen,
Becton-Dickinson Co., San Diego, CA, USA), and incubated for 15 min
in the dark at room temperature. Cell apoptosis were analyzed by
flow cytometry (FACSCalibur; Becton-Dickinson) using CellQuest
software.
Western blot analysis and measurement
of caspase-3 activity
After transfection for 48 h, cells were lysed using
Radio Immunoprecipitation assay lysis buffer (Beyotime
Biotechnology) and protein concentration was determined by using a
BCA Protein assay kit (Beyotime Biotechnology). Equal amount of
protein was subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE, 6–10%), and transferred onto
polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA,
USA). Western blotting was performed using antibodies against Bax,
p-STAT3, PI3K, p-Akt and GAPDH (Cell Signaling Technology) at 4°C
overnight. Membranes was conducted with a goat anti-rabbit IgG
horseradish peroxidase (HRP) conjugated antibody for 1 h at 37°C
and visualized using enhanced chemiluminescence (Goodbio
Biotechnology). Protein blank was imaged with the Molecular Imager
ChemiDoc™ XRSþ (Bio-Rad) and ImageLab software version 4.1
(Bio-Rad). Equal protein was used to measure caspase-3/9 activities
using caspase-3/9 activities apoptosis kits (Beyotime
Biotechnology). Absorbancy was measured at 405 nm with Microplate
Reader Model 550 (Bio-Rad Laboratories, Japan).
Statistical analysis
Data are presented as the mean ± SD. Statistical
comparisons between two groups were made using one-way ANOVA
followed by least significant difference (LSD) post hoc test.
p<0.05 was considered statistically significant.
Results
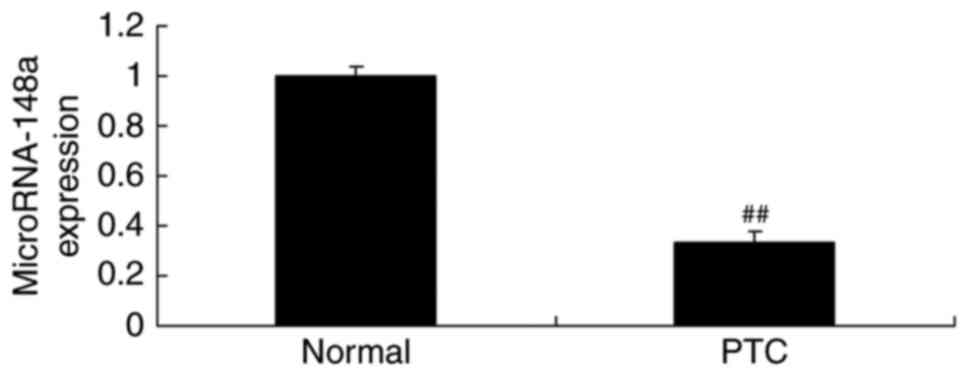
miRNA-148a expression of lymphatic
metastases of papillary thyroid cancer patients
To explore the role of miRNAs in gastric cancer, we
performed qRT-PCR to analyze miRNA-148a expression. As showed in
Fig. 1, miRNA-148a expression of
lymphatic metastases of papillary thyroid cancer patients was
inhibited, compared with normal group.
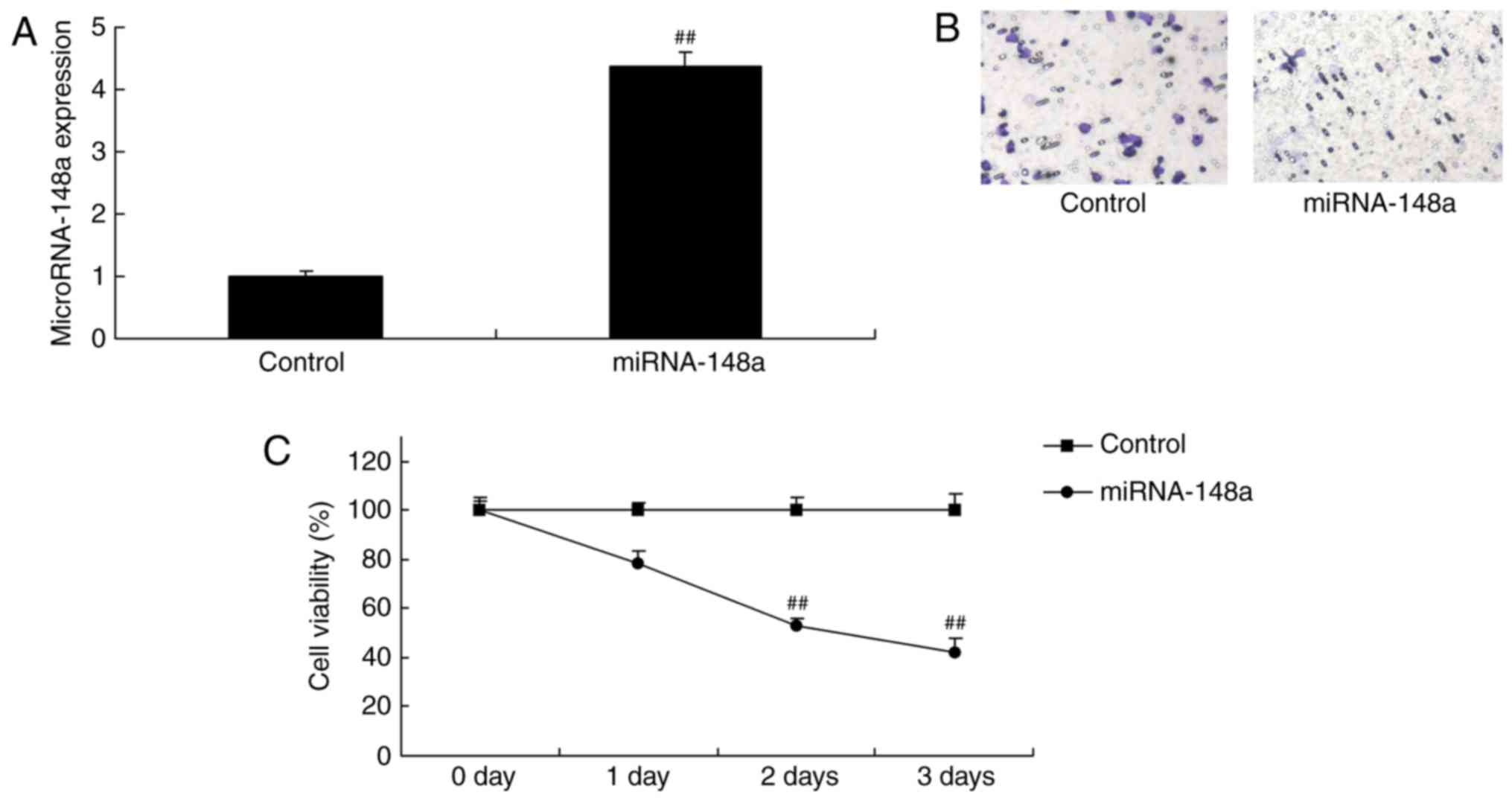
miRNA-148a overexpression reduces cell
cell proliferation and decreased metastases-induced apoptosis of
PTC cells
We validated the function of miRNA-148a on cell
proliferation and metastases of TC cells. Fig. 2 showed that miRNA-148a mimics
increased the expression of miRNA-148a in TPC-1 cells, however,
miRNA-148a overexpression reduced cell-cell proliferation and
decreased metastases induced apoptosis of PTC cells.
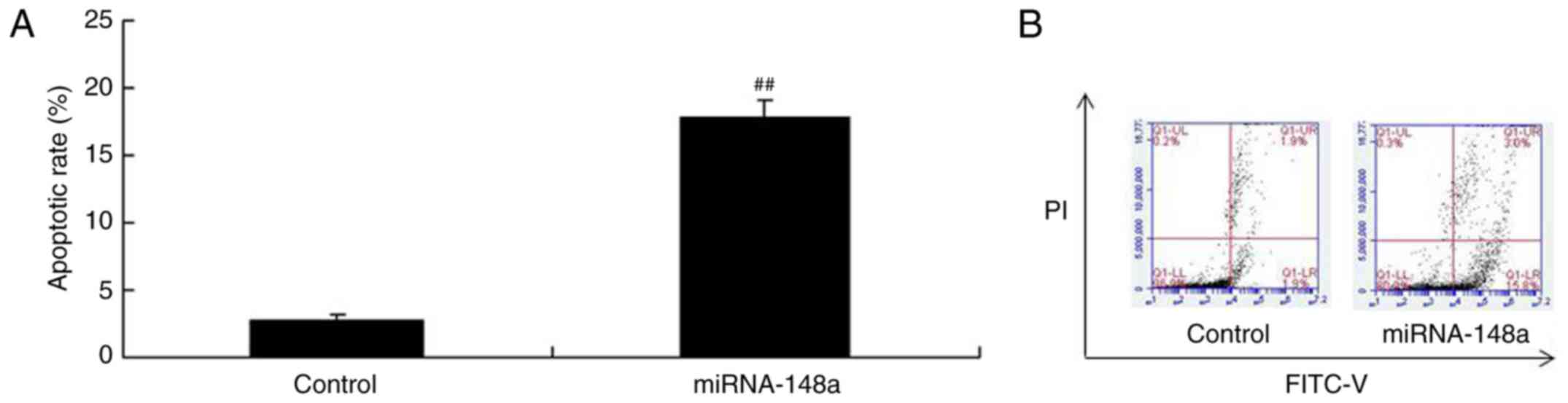
miRNA-148a overexpression induces
apoptosis of PTC cell
To verify our hypothesis, we analyzed apoptosis rate
in TPC-1 cells by miRNA-148a overexpression using flow cytometry.
Conversely, the apoptosis rate of TPC-1 cells transfected with
miRNA-148a mimics were higher than that of control group (Fig. 3). These results indicated that
miRNA-148a adjusts cell growth and death of PTC, however, its
mechanism needs further analysis.
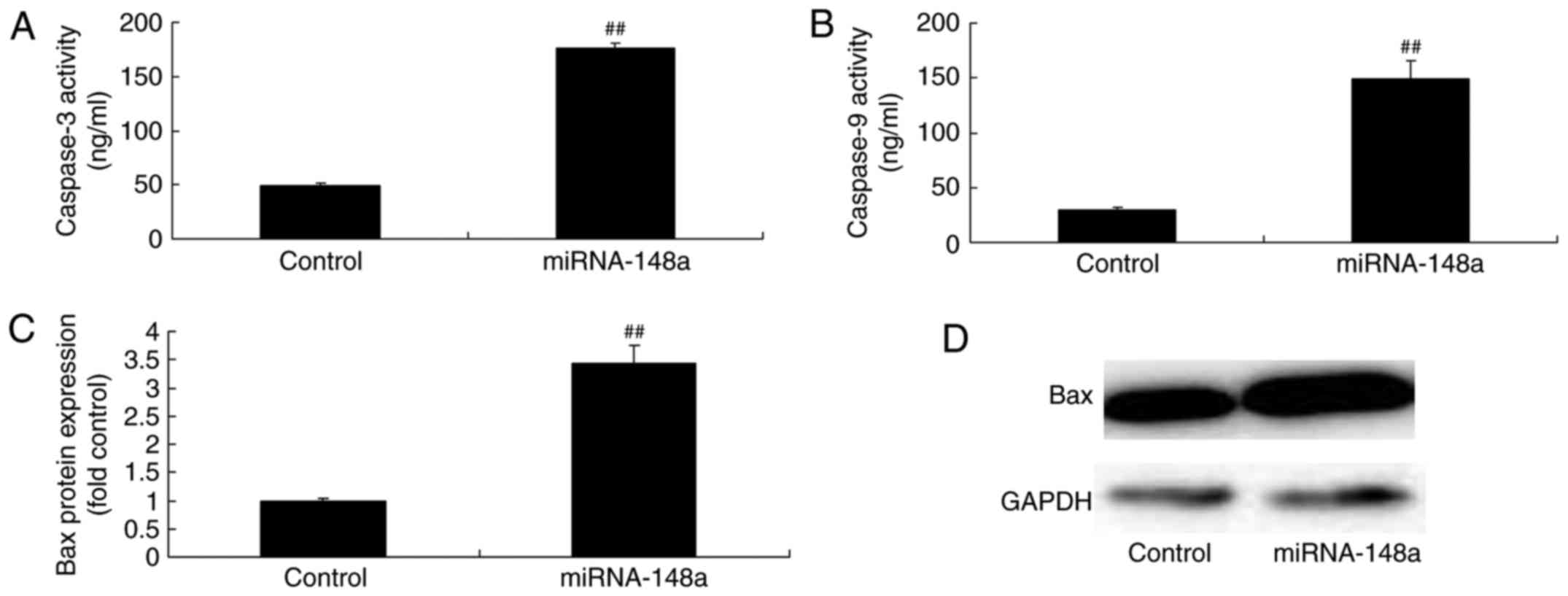
miRNA-148a overexpression induces Bax
protein expression and caspase-3/9 levels of PTC cells
Then, we analyzed Bax protein expression and
caspase-3/9 levels of PTC cells by miRNA-148a overexpression. We
found that miRNA-148a overexpression induced Bax protein expression
and caspase-3/9 levels of PTC cells (Fig. 4).
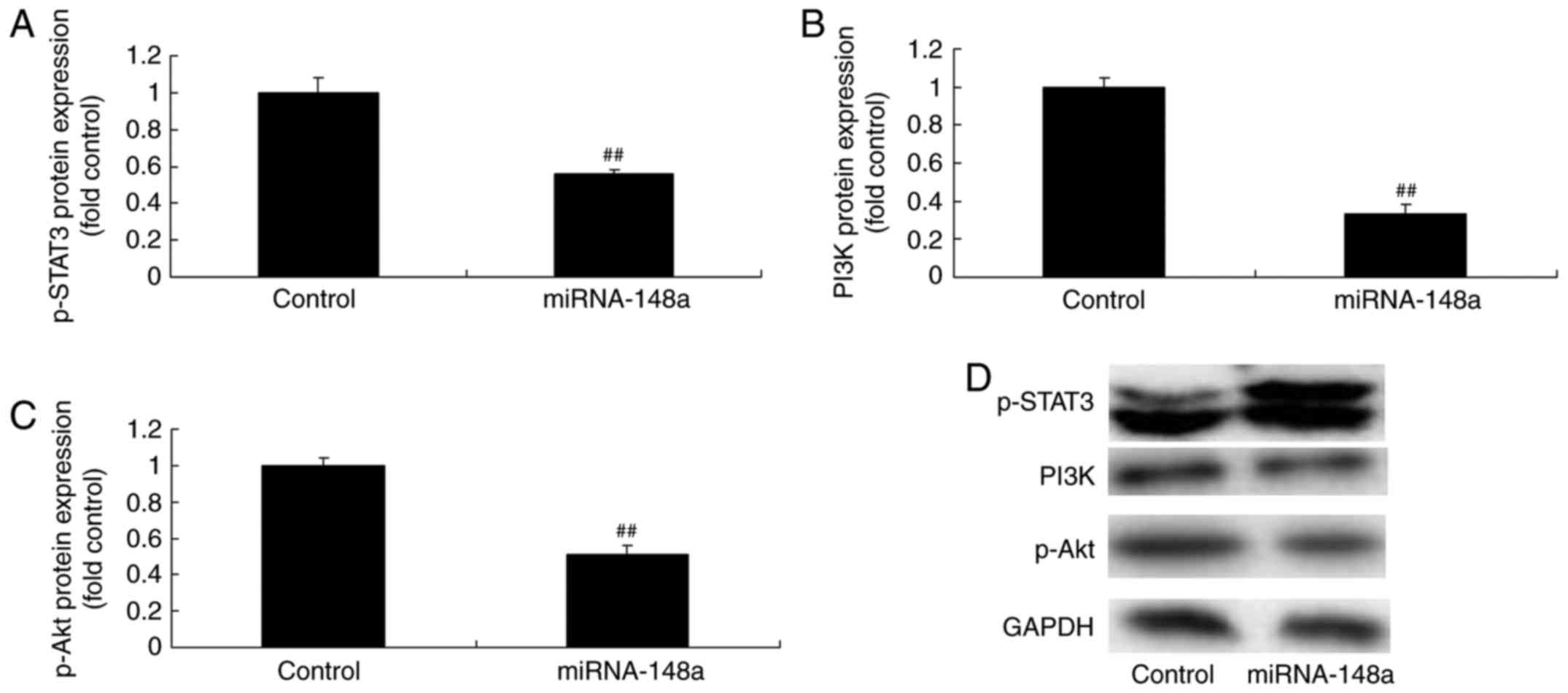
miRNA-148a overexpression suppresses
p-STAT3, PI3K and p-Akt protein expression of PTC cells
Western blot analysis was used to measure p-STAT3,
PI3K and p-Akt protein expression of PTC cells after miRNA-148a
overexpression. As shown in Fig. 5,
miRNA-148a overexpression suppressed p-STAT3, PI3K and p-Akt
protein expression of PTC cells.
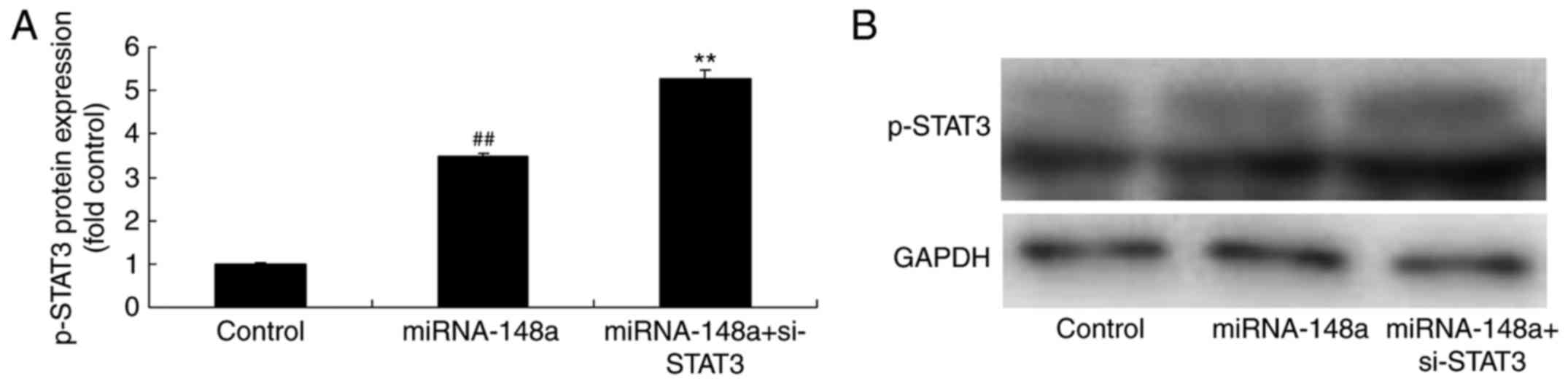
si-STAT3 inhibits p-STAT3 protein
expression, decreased cell proliferation and metastases of PTC
cells after miRNA-148a overexpression
Then, we downregulated p-STAT3 protein expression in
PTC cells following miRNA-148a overexpression. As shown in Fig. 6, si-STAT3 inhibits p-STAT3 protein
expression in PTC cells following miRNA-148a overexpression, and
decreased cell proliferation and metastases of TPC-1 cells,
compared with miRNA-148a overexpression group.
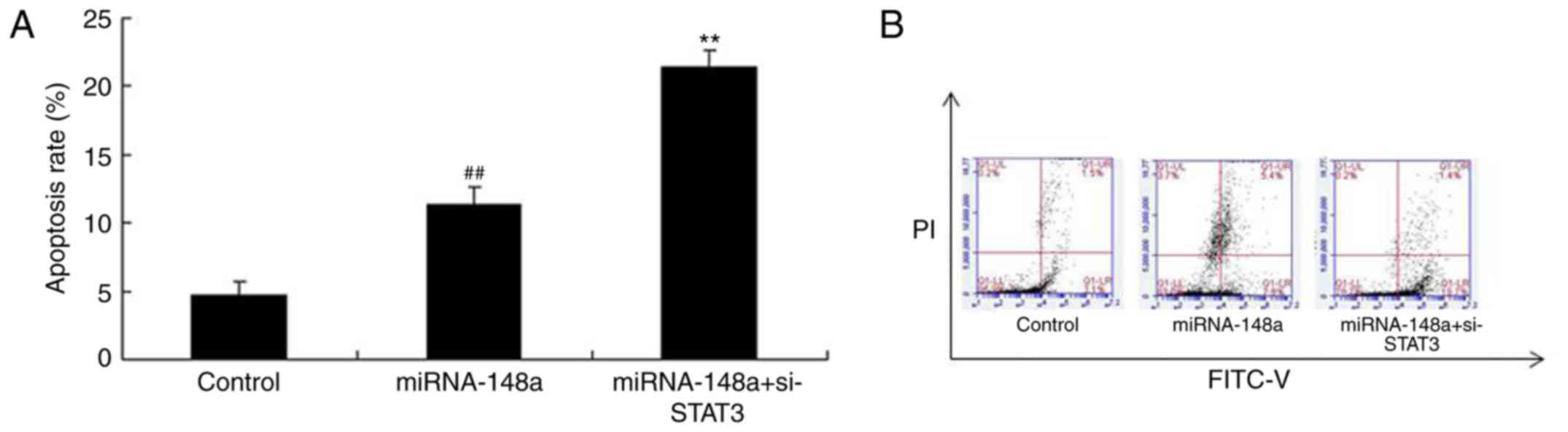
si-STAT3 induces apoptosis of PTC
cells after miRNA-148a overexpression
Then, we found that apoptosis rate of TPC-1 cells
after miRNA-148a overexpression in si-STAT3 were higher than that
of miRNA-148a overexpression group (Fig. 7).
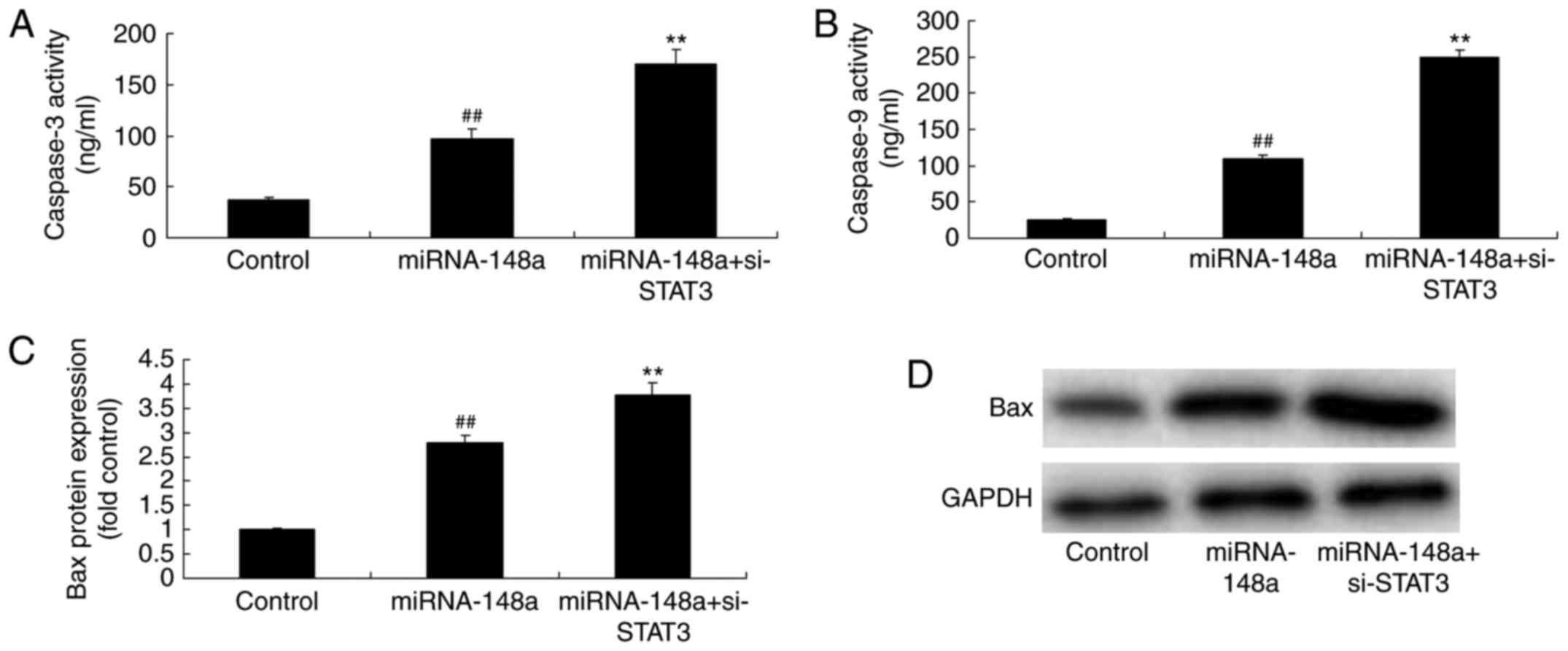
si-STAT3 induces Bax protein
expression and caspase-3/9 levels of PTC cells after miRNA-148a
overexpression
As expected, Bax protein expression and caspase-3/9
levels of PTC cells after miRNA-148a overexpression were markedly
induced by si-STAT3, compared miRNA-148a overexpression group
(Fig. 8). From these results, we
concluded that miR-148a functions as a suppressor in cell growth of
PTC cells through inhibition of STAT3 expression.
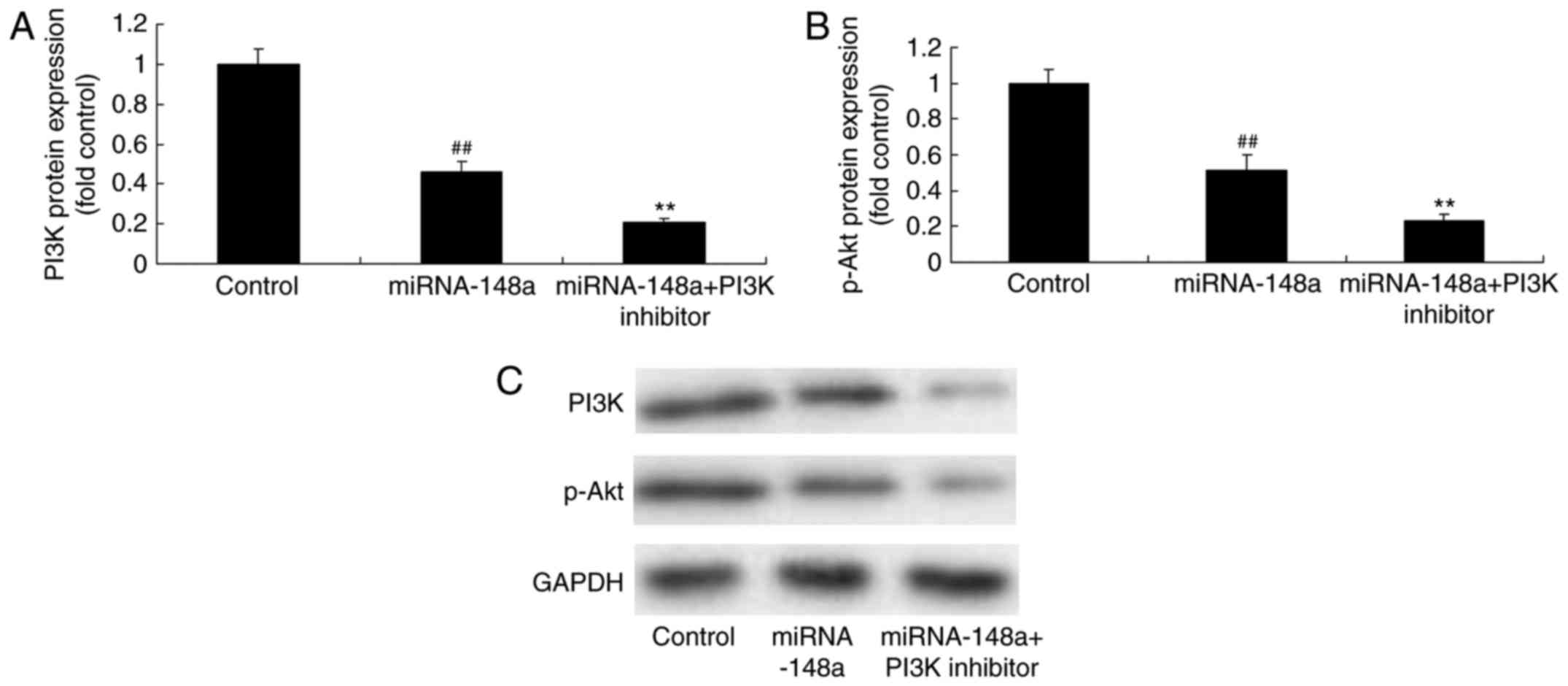
PI3K inhibitor, inhibits PI3K and
p-Akt protein expression of PTC cells after miRNA-148a
overexpression
We next examined whether the inhibition of PI3K
enforced miR-148a expression could suppress tumor growth of PTC.
The results showed that PI3K inhibitor inhibits PI3K and p-Akt
protein expression of PTC cells after miRNA-148a overexpression,
compared with miRNA-148a overexpression group (Fig. 9).
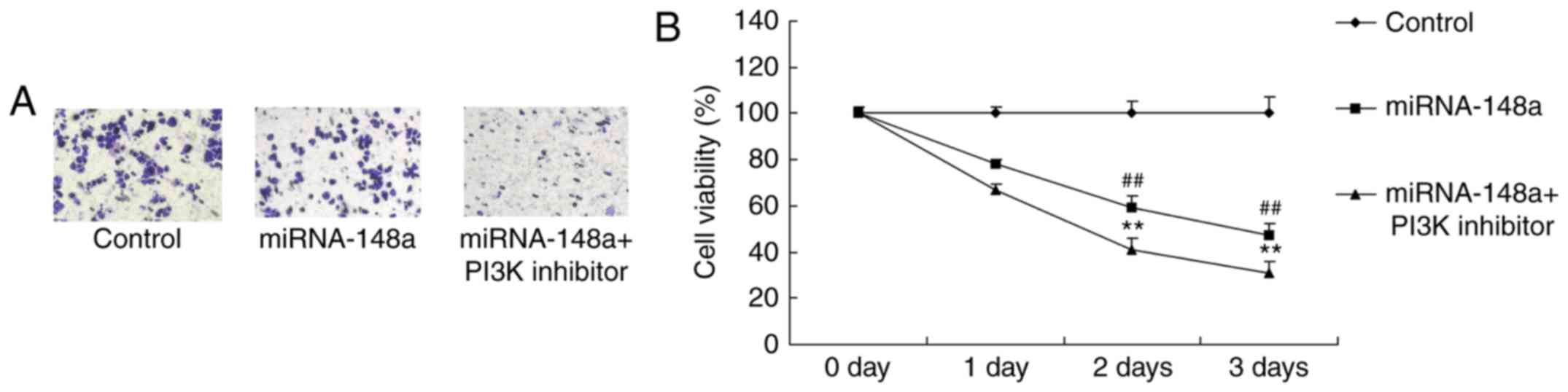
PI3K inhibitor reduces cell
proliferation and decreased metastases of PTC cells after
miRNA-148a overexpression
Then, PI3K inhibitor increased the anticancer
effects of miRNA-148a overexpression on the inhibition of cell
proliferation and metastases of PTC cells, compared with miRNA-148a
overexpression group (Fig.
10).
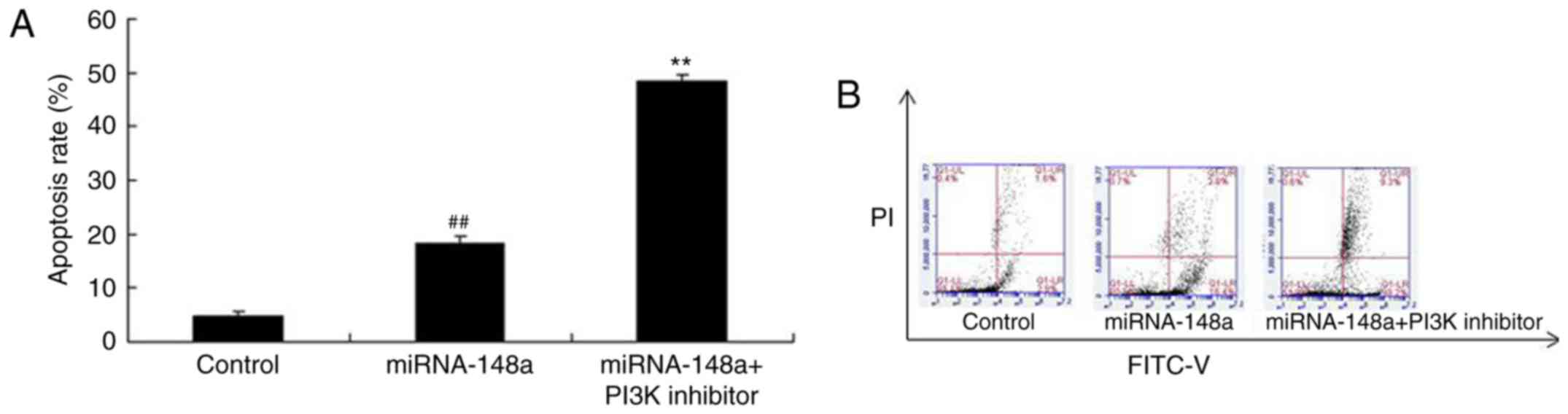
PI3K inhibitor induces apoptosis of
PTC cells after miRNA-148a overexpression
We determined whether PI3K inhibitor affects
apoptosis of PTC cells after miRNA-148a overexpression. As shown in
Fig. 11, PI3K inhibitor induced
apoptosis of PTC cells after miRNA-148a overexpression, compared
with miRNA-148a overexpression group (Fig. 11).
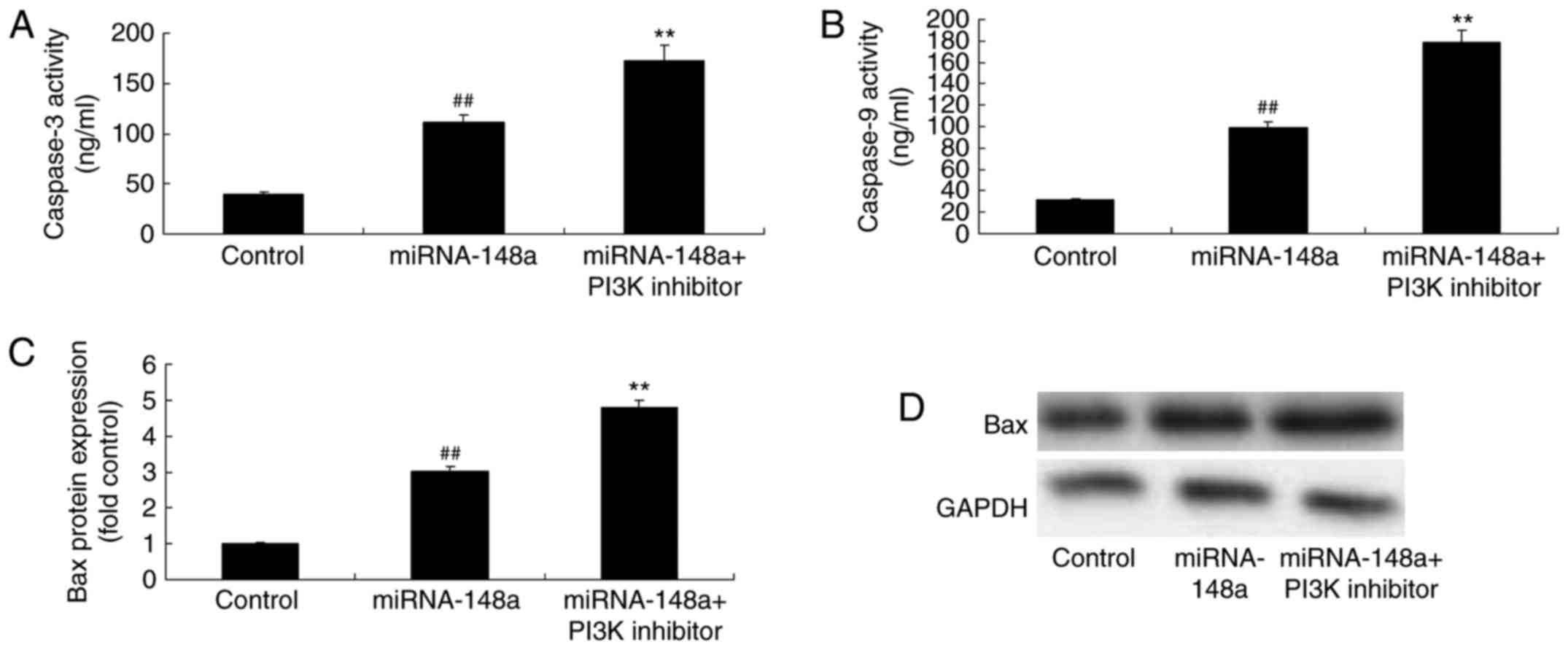
PI3K inhibitor induces Bax protein
expression and caspase-3/9 levels of PTC cells after miRNA-148a
overexpression
Lastly, we found that PI3K inhibitor promoted the
anticancer effects of miRNA-148a overexpression on the induction of
Bax protein expression and caspase-3/9 levels of PTC cells,
compared with miRNA-148a overexpression group (Fig. 12).
Discussion
PTC is the most commonly seen malignant endocrine
system tumor, which severely impair female health (16). The morbidity of PTC in China shows a
significantly increasing trend in recent years, which is even
higher in costal cities, exceeding the growth rate of other
malignant tumors (17). Searching
for new methods for the diagnosis and treatment of PTC is of
particular importance since cancer has caused tremendous burdens on
the society, families and individuals (18). PTC is a common human adenocarcinoma,
which is dominated by papillary carcinoma, accounting for ~70% of
all PTCs (17). The growth rate of
such cancer is relatively slow, with relatively low malignancy
grade. However, it is adjacent to human neck and is prone to
develop cervical lymph node metastasis (1). Our results showed that, for the first
time, miRNA-148a expression of lymphatic metastases of papillary
thyroid cancer patients was inhibited, compared with normal group.
Feng et al showed that microRNA-148a suppressed cell
proliferation and migration of pancreatic cancer cells (6).
So far, >700 miRNAs in human genome have been
identified (19). These microRNAs
are the non-coding sequences in the genome with the length of
~21–25 nucleotides. They are highly conserved in evolution and can
regulate gene expression at translation level (19), they participle in multiple
physiological metabolic processes, including development, cell
differentiation, apoptosis, and energy metabolism. With the
advancement in research on molecular biology and molecular
genetics, increasing number of studies have demonstrated that
miRNAs play important roles in tumor development, metastasis and
prognosis. For instance, miRNAs play certain roles in granulocytic
leukemia, non-small cell lung cancer, gastric cancer, pancreatic
cancer, colon cancer and liver cancer (20). Our results suggested that miRNA-148a
overexpression effectively reduced cell-cell proliferation and
metastases, and induced apoptosis of papillary thyroid cancer in
vitro. Yu et al showed that miR-148a suppressed human
gastric cancer cell growth by inactivating STAT3 and Akt (21). Li et al reported that
silencing of miRNA-148a activates nasopharyngeal carcinoma, and can
act as as a biomarker as well as a therapeutic target for
nasopharyngeal carcinoma (22).
These results were in keeping with our study.
Caspase family includes important proteins for
executing apoptosis discovered in research on the molecular
mechanism of cell apoptosis in recent years. Among them,
caspase-3/9 is one of the pro-apoptotic factors locating in the
upstream of caspase cascade reaction. Moreover, it is also one of
the most critical components in the caspase system that induces
cell apoptosis (23,24). We also confirmed that overexpression
of miRNA-148a significantly induced Bax protein expression and
caspase-3/9 levels, and suppressed phosphorylation of STAT3
(p-STAT3), PI3K and p-Akt protein expression of papillary thyroid
cancer in vitro.
STAT3 phosphorylation can result from multiple
factors that promote the genesis and development of cancer. The
activated STAT3 can inhibit cell apoptosis, promotes cell
proliferation and differentiation, and results in carcinogenesis.
Therefore, it is defined as an oncogene (25). Abnormally active STAT3 expression in
PTC cells has been reported in relevant literature. Its expression
difference is positively correlated with the clinical pathological
type and stage of PTC (11). We
showed that overexpression of miRNA-148a significantly suppressed
p-STAT3 protein expression of papillary thyroid cancer in
vitro. si-STAT3, could inhibit p-STAT3 protein expression,
reduced cell cell proliferation and metastases, and induced
apoptosis of papillary thyroid cancer following miRNA-148a
overexpression. He et al showed that microRNA-148a
suppressed cell proliferation and invasion potential through
regulation of STAT3 in non-small cell lung carcinomas (7).
The growth factor receptor, PI3K, Akt and eIF-4E in
the PI3K/Akt/mTOR signaling pathway are all proteins encoded by
oncogenes (26). It has been
discovered currently that numerous tumors are accompanied with
abnormal regulation of the mTOR signaling pathway. In addition,
cell proliferation, cell cycle regulation, and cell migration,
which are closely associated with tumor genesis, are all regulated
by mTOR (13). It has been
indicated in research that, the PI3K/Akt/mTOR signaling pathway is
excessively activated in the genesis and development of PTC
(15). The PI3K/Akt/mTOR signaling
pathway is activated in PTC, especially in patients with lymph node
metastases (27). It is proposed
that the PI3K/Akt/mTOR signaling pathway supports the malignant
characteristics of PTC cell model, and the effects of mTOR targeted
therapy on advanced PTC have been approved (27). In our study, we determined that
overexpression of miRNA-148a significantly suppressed PI3K and
p-Akt of papillary thyroid cancer in vitro. PI3K inhibitor,
could inhibit PI3K and p-Akt protein expression, reduced cell cell
proliferation and metastases, and induced apoptosis of papillary
thyroid cancer following miRNA-148a overexpression. Zhang et
al reported that microRNA-148a promotes cancer cell growth by
targeting PI3K signaling pathway in osteosarcoma (7).
In conclusion, our results indicate that miRNA-148a
inhibits cell growth and metastases of papillary thyroid cancer
through STAT3 and PI3K/AKT signaling pathways. To our knowledge,
this is the first study to describe a potential role for miRNA-148a
as potential targets in future studies on the prevention and
treatment of papillary thyroid cancer.
References
|
1
|
Sawka AM, Straus S, Rodin G, Thorpe KE,
Ezzat S, Gafni A and Goldstein DP: Decision aid on radioactive
iodine treatment for early stage papillary thyroid cancer: Update
to study protocol with follow-up extension. Trials. 16:3022015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan Y, Fang M, Wu CY and Ling EA:
Scutellarin as a potential therapeutic agent for microglia-mediated
neuroinflammation in cerebral ischemia. Neuromolecular Med.
18:264–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Titov SE, Ivanov MK, Karpinskaya EV,
Tsivlikova EV, Shevchenko SP, Veryaskina YA, Akhmerova LG, Poloz
TL, Klimova OA, Gulyaeva LF, et al: miRNA profiling, detection of
BRAF V600E mutation and RET-PTC1 translocation in patients from
Novosibirsk oblast (Russia) with different types of thyroid tumors.
BMC Cancer. 16:2012016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoruker EE, Terzioglu D, Teksoz S, Uslu
FE, Gezer U and Dalay N: MicroRNA expression profiles in papillary
thyroid carcinoma, benign thyroid nodules and healthy controls. J
Cancer. 7:803–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mutalib NS, Yusof AM, Mokhtar NM, Harun R,
Muhammad R and Jamal R: MicroRNAs and lymph node metastasis in
papillary thyroid cancers. Asian Pac J Cancer Prev. 17:25–35. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng H, Wang Y, Su J, Liang H, Zhang CY,
Chen X and Yao W: MicroRNA-148a suppresses the proliferation and
migration of pancreatic cancer cells by downregulating ErbB3.
Pancreas. 45:1263–1271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Wang Y, Xu T, Li C, Wu J, He Q,
Wang G, Ding C, Liu K, Tang H, et al: Increased expression of
microRNA-148a in osteosarcoma promotes cancer cell growth by
targeting PTEN. Oncol Lett. 12:3208–3214. 2016.PubMed/NCBI
|
|
8
|
Zhang J, Gill A, Atmore B, Johns A,
Delbridge L, Lai R and McMullen T: Upregulation of the signal
transducers and activators of transcription 3 (STAT3) pathway in
lymphatic metastases of papillary thyroid cancer. Int J Clin Exp
Pathol. 4:356–362. 2011.PubMed/NCBI
|
|
9
|
Kim YR, Byun HS, Won M, Park KA, Kim JM,
Choi BL, Lee H, Hong JH, Park J, Seok JH, et al: Modulatory role of
phospholipase D in the activation of signal transducer and
activator of transcription (STAT)-3 by thyroid oncogenic kinase
RET/PTC. BMC Cancer. 8:1442008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim DW, Chung HK, Park KC, Hwang JH, Jo
YS, Chung J, Kalvakolanu DV, Resta N and Shong M: Tumor suppressor
LKB1 inhibits activation of signal transducer and activator of
transcription 3 (STAT3) by thyroid oncogenic tyrosine kinase
rearranged in transformation (RET)/papillary thyroid carcinoma
(PTC). Mol Endocrinol. 21:3039–3049. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Couto JP, Daly L, Almeida A, Knauf JA,
Fagin JA, Sobrinho-Simões M, Lima J, Máximo V, Soares P, Lyden D,
et al: STAT3 negatively regulates thyroid tumorigenesis. Proc Natl
Acad Sci USA. 109:pp. E2361–E2370. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trovato M, Grosso M, Vitarelli E, Ruggeri
RM, Alesci S, Trimarchi F, Barresi G and Benvenga S: Distinctive
expression of STAT3 in papillary thyroid carcinomas and a subset of
follicular adenomas. Histol Histopathol. 18:393–399.
2003.PubMed/NCBI
|
|
13
|
Liu Q, Guan JZ, Sun Y, Le Z, Zhang P, Yu D
and Liu Y: Insulin-like growth factor 1 receptor-mediated cell
survival in hypoxia depends on the promotion of autophagy via
suppression of the PI3K/Akt/mTOR signaling pathway. Mol Med Rep.
15:2136–2142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao Y, Xi L, Li Q, Cai Z, Lai Y, Zhang X
and Yu C: Regulation of cell apoptosis and proliferation in
pancreatic cancer through PI3K/Akt pathway via Polo-like kinase 1.
Oncol Rep. 36:49–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petrulea MS, Plantinga TS, Smit JW,
Georgescu CE and Netea-Maier RT: PI3K/Akt/mTOR: A promising
therapeutic target for non-medullary thyroid carcinoma. Cancer
Treat Rev. 41:707–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gweon HM, Son EJ, Youk JH, Kim JA and Park
CS: Preoperative assessment of extrathyroidal extension of
papillary thyroid carcinoma: Comparison of 2- and 3-dimensional
sonography. J Ultrasound Med. 33:819–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei S, Wang D, Ge J, Liu H, Zhao D, Li G
and Ding Z: Single-center study of familial papillary thyroid
cancer in China: Surgical considerations. World J Surg Oncol.
13:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Proczko M, Stefaniak T, Sworczak K,
Kobiela J, Lachiński AJ, Stepaniak P and Sledziński Z: Completion
thyroidectomy of well-differentiated thyroid cancer - a
prospective, randomised study. Endokrynol Pol. 64:335–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen CT, Qiu ZL, Song HJ, Wei WJ and Luo
QY: miRNA-106a directly targeting RARB associates with the
expression of Na(+)/I(−) symporter in thyroid cancer by regulating
MAPK signaling pathway. J Exp Clin Cancer Res. 35:1012016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geraldo MV, Nakaya HI and Kimura ET:
Downregulation of 14q32-encoded miRNAs and tumor suppressor role
for miR-654-3p in papillary thyroid cancer. Oncotarget.
8:9597–9607. 2017.PubMed/NCBI
|
|
21
|
Yu B, Lv X, Su L, Li J, Yu Y, Gu Q, Yan M,
Zhu Z and Liu B: MiR-148a functions as a tumor suppressor by
targeting CCK-BR via inactivating STAT3 and Akt in human gastric
cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HP, Huang HY, Lai YR, Huang JX, Chang
KP, Hsueh C and Chang YS: Silencing of miRNA-148a by
hypermethylation activates the integrin-mediated signaling pathway
in nasopharyngeal carcinoma. Oncotarget. 5:7610–7624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin SM, Jang HW, Sohn SY, Kim NK, Joung
JY, Cho YY, Kim SW and Chung JH: Role of autophagy in the
resistance to tumour necrosis factor-related apoptosis-inducing
ligand-induced apoptosis in papillary and anaplastic thyroid cancer
cells. Endocrine. 45:256–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saffar H, Sanii S, Emami B, Heshmat R,
Panah VH, Azimi S and Tavangar SM: Evaluation of MMP2 and Caspase-3
expression in 107 cases of papillary thyroid carcinoma and its
association with prognostic factors. Pathol Res Pract. 209:195–199.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong W, Cui J, Tian X, He L, Wang Z, Zhang
P and Zhang H: Aberrant sonic hedgehog signaling pathway and STAT3
activation in papillary thyroid cancer. Int J Clin Exp Med.
7:1786–1793. 2014.PubMed/NCBI
|
|
26
|
Zhao P, Guan HT, Dai ZJ, Ma YG, Liu XX and
Wang XJ: Knockdown of SPOCK1 inhibits the proliferation and
invasion in colorectal cancer cells by suppressing the PI3K/Akt
pathway. Oncol Res. 24:437–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonçalves AP, Videira A, Soares P and
Máximo V: Orthovanadate-induced cell death in RET/PTC1-harboring
cancer cells involves the activation of caspases and altered
signaling through PI3K/Akt/mTOR. Life Sci. 89:371–377. 2011.
View Article : Google Scholar : PubMed/NCBI
|