Introduction
Lung cancer is the main cause of cancer-related
mortality worldwide and 80–85% are NSCLC with five-year survival of
only 15% due to delayed diagnosis and ineffective treatment.
Metastasis is the primary cause of death in lung cancer patients
(1). A large number of studies have
shown that EMT is a prerequisite for the invasion and metastasis of
epithelial tumor cells (2–6). The downregulation of epithelial cell
markers, such as E-cadherin and β-catenin, and the upregulation of
interstitial cell markers, such as N-cadherin and vimentin, are the
mainly molecular features of EMT (7). The downregulation or deletion of
E-cadherin is a hallmark of the EMT process and a prerequisite for
tumor invasion and metastasis (8,9).
DAL-1 was first identified as a gene lacking in
NSCLC by using Differential Display PCR (10). The frequent loss of DAL-1 in lung
cancer, breast cancer, gastric cancer, renal clear cell carcinoma
and epithelial ovarian cancers suggests that it could be a tumor
suppressor (11–15). It has been reported that DAL-1 locus
on 18p11.3, and the promoter methylation and loss of heterozygosity
are responsible for the inactivation of DAL-1 (13,16).
We have demonstrated in previous studies that overexpression of
DAL-1 can inhibit the migration and invasion of NSCLC cells by
attenuating EMT and found that the HSPA5 protein was a
DAL-1-related protein which could be directly bound to the DAL-1
protein (17).
HSPA5, also known as immunoglobulin heavy chain
binding protein (Bip) or glucose regulated protein 78 (GRP78),
belongs to the heat shock protein 70 (HSP70) family (18). As a stress-induced endoplasmic
reticulum (ER) chaperone, HSPA5 is overexpressed in many human
cancers including NSCLC. The overexpression of HSPA5 is involved in
the tumor progression, such as regulating EMT, proliferation,
invasion and metastasis (19,20).
HSPA5 has been reported to promote EMT by activating the PI3K/Akt
signaling pathway in NSCLC cells (21).
Herein, the expression and co-localization of DAL-1
protein and HSPA5 protein was identified, and the effect of DAL-1
on the expression and functions of HSPA5 were investigated in NSCLC
cells. We discovered that the expression of DAL-1 was decreased
while the expression of HSPA5 was increased in NSCLC cells. We
revealed that DAL-1 can inhibit EMT, migration, invasion and
proliferation by suppressing HSPA5 expression. We also found that
DAL-1 has a role in inhibiting PI3K/Akt/Mdm2 pathway by depending
on HSPA5.
Materials and methods
Cell lines
The cell lines used in this study (16HBE, A549,
SPCA-1, HA579, H520, H460 and H1299) were purchased from American
Type Culture Collection (ACTT) and incubated in RPMI-1640 medium
containing 10% feral bovine serum (FBS) (Gibco, Carlsbad, CA, USA),
at 37°C, 5% CO2-95% O2 humid incubator.
Lentivirus expression vectors
The lentivirus expression vectors were purchased
from GeneChem Co., Ltd. (Shanghai, China). To obtain stable
infection, cells seeded in 96-well plates were infected with 2
µl/well lentivirus expression vectors (1×108 TU/ml)
using 10 µl/well polybrene (5 µg/ml) reagent and 90 µl/well
RPMI-1640. After 10 h incubation in serum and antibiotic free
condition, the medium with free FBS was replaced by the medium
containing 10% FBS. Cells were incubated with puromycin-containing
medium (2 µg/ml) or selected by means of limited dilution to select
green fluorescent monoclonal cells to select stable cell lines.
Fluorescent staining
The cased slides were washed three times with PBS in
a plate and fixed with 4% paraformaldehyde for 15 min. After
immobilization, the dishes were wash with PBS three times. Normal
goat serum was added to the slide and closed at room temperature
for 30 min. The blocking solution was removed and a sufficient
amount of resistance was added and incubated overnight at 4°C. The
next day, tablets were washed 3 times with PBST, and then
fluorescent secondary antibody was added. Incubated at room
temperature for 1 h and then washed with PBST 3 times, DAPI was
added for 5 min in the dark. The plates were again washed with PBST
4 times and then sealed with a sealant containing a fluorescent
quencher. Finally, images were observed under a laser confocal
microscope.
Western blot analysis
Cells were washed with cold phosphate buffered
saline (PBS) and lysed in RIPA buffer with protease inhibitor
cocktail (CWBIO, Beijing, China) for 20 min. Cell extracts were
separated by 10% SDS-PAGE gel, and transferred to nitrocellulose
membrane (0.45 µm HATF, 10TF of filter; Millipore, Carrigtwohill,
Ireland). After blocking in 0.5% milk for 1 h, the blots were
incubated with rabbit anti-DAL-1 mAb (Abcam, Cambridge, MA, USA),
rabbit anti-HSPA5 mAb (Abcam), rabbit anti-E-cadherin mAb (Cell
Signaling Technology, Danvers, MA, USA), rabbit anti-β-catenin mAb
(Cell Signaling Technology), rabbit anti-N-cadherin mAb (Cell
Signaling Technology), rabbit anti-vimentin mAb (Cell Signaling
Technology), rabbit anti-PI3K mAb (Cell Signaling Technology,
rabbit anti-p-PI3K mAb (Cell Signaling Technology), rabbit anti-Akt
mAb (Cell Signaling Technology), rabbit anti-p-Akt (Ser473) mAb
(Cell Signaling Technology), rabbit anti-Mdm2 mAb (Abcam), rabbit
anti-p-Mdm2 mAb (Abcam), rabbit anti-p53 mAb (Abcam), rabbit
anti-β-actin mAb (Cell Signaling Technology). After being washed
three times, blots were further incubated with 1/1000 anti-rabbit
IgG (Cell Signaling Technology). Chemiluminescence was measured in
a Fusion Solo 2M instrument (Fusion Solo; Vilber Lourmat, Paris,
France).
Quantitative real-time reverse
transcriptase PCR (RT-qPCR)
Total RNA was purified using TRIzol reagent (Takara,
Shiga, Japan) according to the manufacturer's instructions.
Quantification was performed with a NanoDrop 2000 (Thermo Fisher
Scientific, Waltham, MA, USA). RNA (500 ng) was reverse-transcribed
using Prime Script TR Reagent kit (RR037A; Takara). The product was
used in subsequent RT-qPCR using three duplicate PCR reactions
containing 1X SYBR-Green Master Mix (SYBR Premix Ex Taq II; RR820A;
Takara) with the primers in Table
I. Relative expression levels of target genes were determined
by the ∆∆Cq method, using GAPDH gene expression to normalize all
samples.
 | Table I.Sequences of the primers for
quantitative reverse transcriptase real-time polymerase chain
reaction. |
Table I.
Sequences of the primers for
quantitative reverse transcriptase real-time polymerase chain
reaction.
| Genes | Sequences |
|---|
| GAPDH | F:
CGGAGTCAACGGATTTGGTCGTAT |
|
| R:
AGCCTTCTCCATGGTGGTGAAGAC |
| DAL-1 | F:
TGGCCCAAGGTTCTAAAGATTTCA |
|
| R:
CAGCTTAAACCCAATGGTGCTTTC |
| HSPA5 | F:
GGAATTCGATATGATG |
|
| R: GGTAGCTATCA |
| E-cadherin | F:
TCCACCACTAGCCAGTATGATGA |
|
| R:
CACAGTCACACACGCTGACCTCTA |
| β-catenin | F:
GGCAGTGCGTTTAGCTGGT |
|
| R:
TCCACCACTAGCCAGTATGATGA |
| N-cadherin | F:
TTGGTTTGGGGAGGGAGA |
|
| R:
CTGGGGTCAGAGGTGTATCATTT |
| Vimentin | F:
AAGACGGTTGAAACTAGAGATGGAC |
|
| R:
TGCTGGTAATATATTGCTGCACTGA |
Transwell migration and invasion
assay
The Transwell insert was coated with 30 µl (1 µg/ml)
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and
2×105 cells in 200 µl serum-free media were transferred
to the top chamber (6.5 mm; Corning Inc., Corning, NY, USA). The
lower chamber was filled with 700 µl medium containing 5% FBS. The
cells in the lower chamber were fixed by methanol and stained with
Giemsa after culturing for 24 h. For Transwell migration assays,
2×105 cells were plated in the top chamber onto the
non-coated membrane. The invasion and migrating cells were counted
under a microscope from 5 randomly selected fields (magnification,
×200).
Cell proliferation assay
Cells (1×103) per well were seeded in
triplicate into the 96-well plats. After incubation for 24 h, the
CCK-8 reagent (10 µl/well; CCK-8; Dijindo, Kumamoto, Japan) was
added to each well and then incubated for a further 2 h. The
quantity of absorbance on the plat was measured at a wavelength of
450 nm by microplate reader to detect the survival of cells. All
experiments were independently repeated at least three times.
Statistical analysis
All analyses were performed by SPSS software
(version 13.0; SPSS Inc., Chicago, IL, USA). Data were expressed as
mean ± SD from at least three independent experiments. The
difference between different groups was analyzed using a factorial
model one-way analysis of variance and P-value <0.05 was
considered to indicate a statistically significant difference.
Results
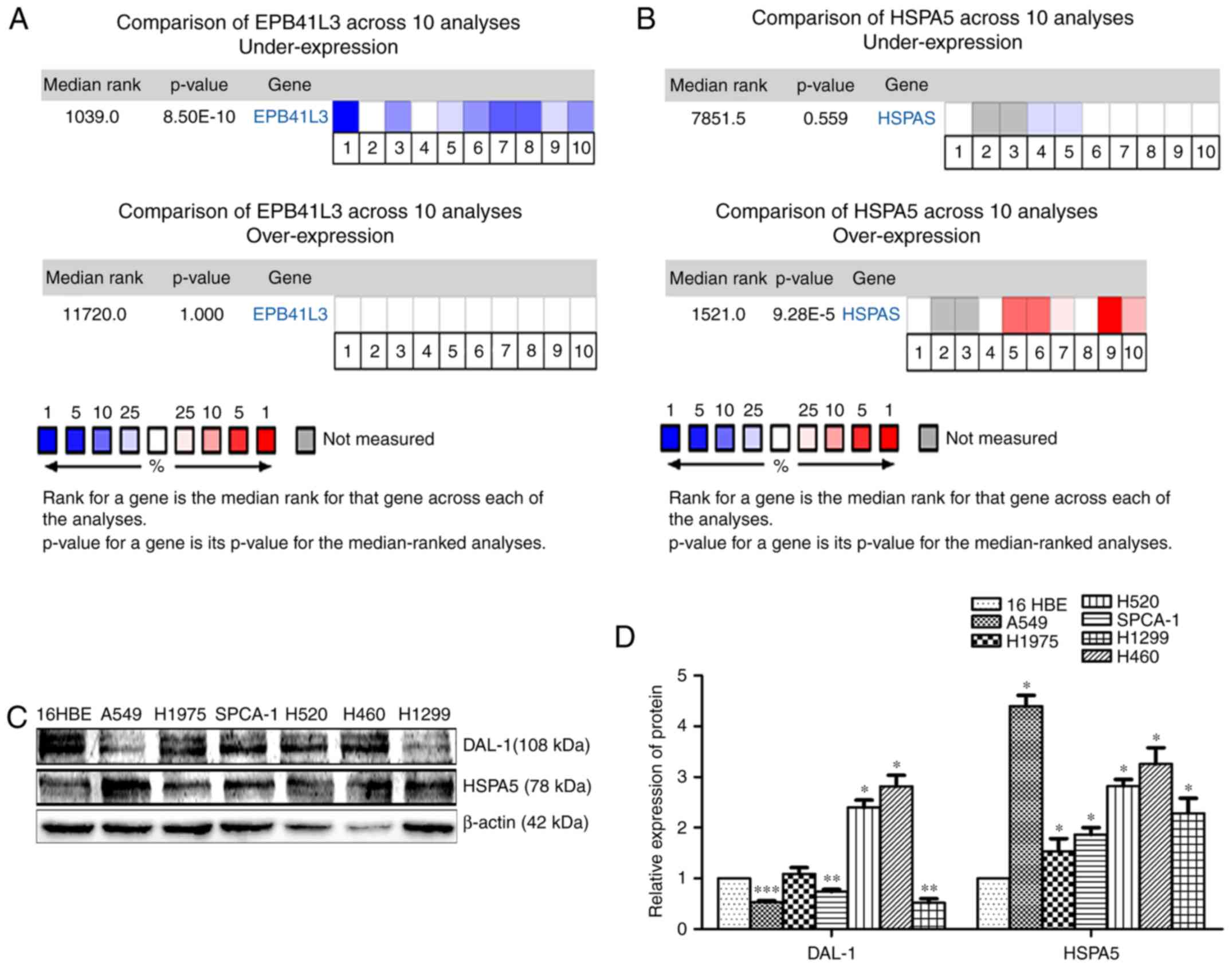
DAL-1 is downregulated while HSPA5 is
upregulated in NSCLC
The Oncomine database (http://www.oncomine.org) was utilized to analyze the
mRNA expression of DAL-1 and HSPA5 in lung cancer patients. Data
from ten databasesdemonstrated that, compared with normal tissues,
the mRNA expression of DAL-1 in lung cancer tissues was
downregulated (P<0.05; Fig. 1A)
while the mRNA expression of HSPA5 was upregulated (P<0.05;
Fig. 1B). The western blot assay
was used to investigate the protein expression level of DAL-1 and
HSPA5 in NSCLC cells (A549, SPCA-1, H1975, H520, H460 and H1299).
As shown in Fig. 1C, compared with
the immortalized bronchial epithelial cells (16HBE), the protein
expression of DAL-1 was lower in A549, SPCA-1 and H1299 cells
(P<0.05) while higher in H520 and H460 cells (P<0.05). The
protein expression of HSPA5 was higher in all the NSCLC cells
(P<0.05). Fig. 1D showed a
statistical analysis of the gray scale of the protein.
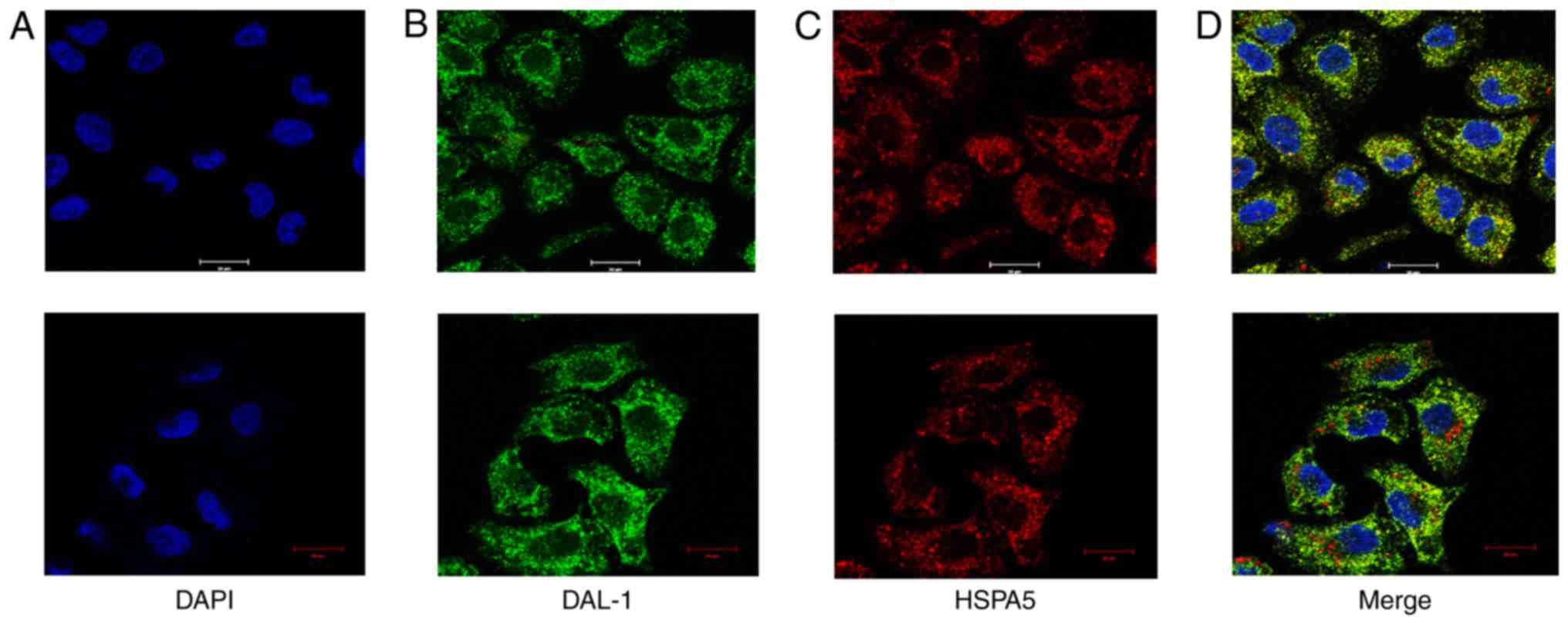
DAL-1 protein and HSPA5 protein are
co-localized in the cytoplasm and nucleus of NSCLC cells
Knowing that DAL-1 protein and HSPA5 protein can be
combined directly in NSCLC cells, we further observed their
co-localization in H460 cells which express the two proteins
simultaneously. The DAL-1 protein and HSPA5 protein were stained by
immunofluorescence and the co-localization of them was observed by
laser confocal scanning microscope. The nucleus labeled by DAPI
showed blue color (Fig. 2A), DAL-1
proteins were marked by green fluorescence (Fig. 2B) and HSPA5 proteins were labeled by
red fluorescence (Fig. 2C). After
merging, we can see these two proteins co-localized in cytoplasm
and nucleus (Fig. 2D).
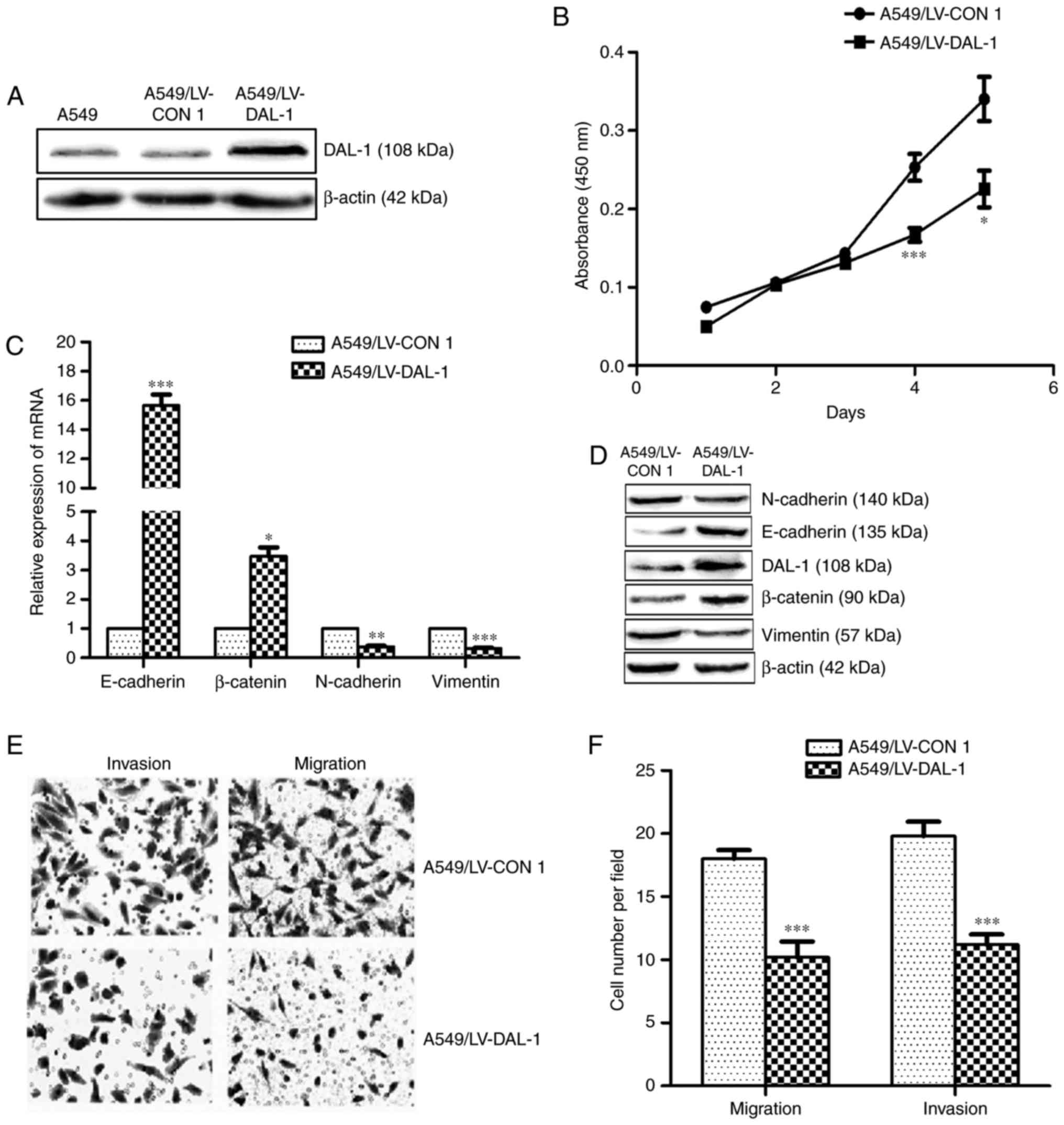
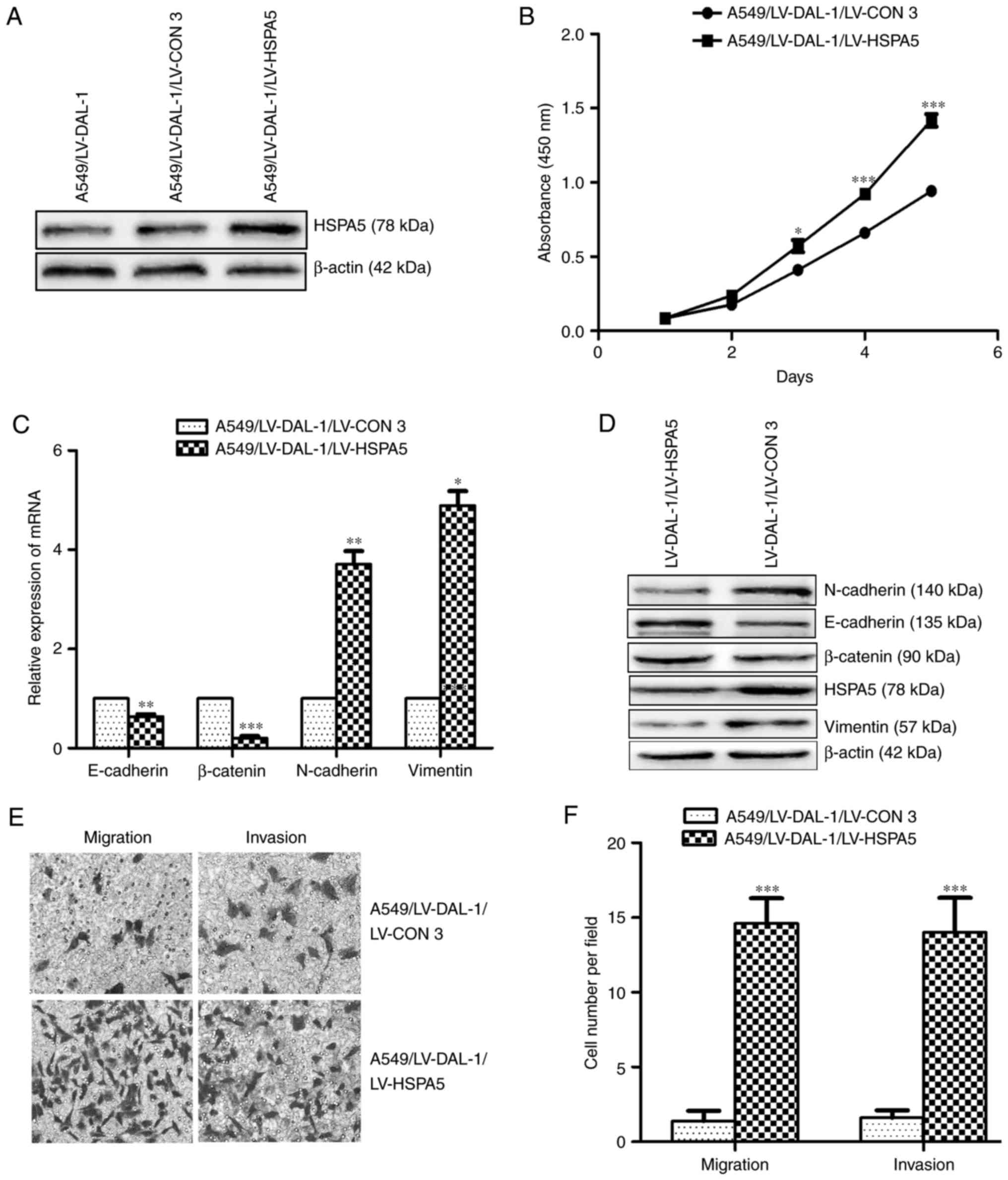
Overexpression of DAL-1 suppresses
EMT, migration, invasion and proliferation of NSCLC cells
In order to investigate the functional role of DAL-1
in NSCLC cells, a lentivirus vector LV-DAL-1 was established to
functionally overexpress DAL-1 in A549 cells. The protein
expression of DAL-1 in stably transfected cells (A549/LV-DAL-1) was
confirmed by western blot assay (Fig.
3A). The mRNA expression of E-cadherin and β-catenin were
increased while the mRNA expression of N-cadherin and vimentin were
decreased in A549/LV-DAL-1 cells compared with control cells
(A549/LV-CON1) (P<0.05; Fig.
3C). Similarly, the protein expression of E-cadherin and
β-catenin were upregulated while the protein of N-cadherin and
vimentin were downregulated in A549/LV-DAL-1 cells (Fig. 3D). Transwell migration and invasion
assays revealed the suppression role of DAL-1 on migration and
invasion abilities in A549 cells (P<0.05; Fig. 3E and F), and CCK-8 cell
proliferation assay indicated the suppression role of DAL-1 on
proliferation (P<0.05; Fig. 3B).
Collectively, these data suggest that DAL-1 plays a role in
suppressing EMT, migration, invasion and proliferation in NSCLC
cells.
Silence of HSPA5 inhibits EMT,
migration, invasion and proliferation ability in NSCLC cells
To investigate the functional role of HSPA5 in NSCLC
cells, the lentivirus vectors LV-HSPA5 RNAi-(1/2/3) were
established to functionally silence HSPA5 expression in A549 cells.
The western blot assay indicated that the protein level of HSPA5 in
A549/LV-HSPA5 RNAi-3 decreased most obviously and was used in
subsequent experiments (Fig. 4A).
The qRT-PCR assay showed that the mRNA expression of E-cadherin and
β-catenin were increased while the mRNA expression of N-cadherin
and vimentin were decreased in silenced-HSPA5 cells (A549/LV-HSPA5
RNAi-3) compared with silenced-control cells (A549/LV-CON2)
(P<0.05; Fig. 4C). The western
blot assay demonstrated that silence of HSPA5 upregulated the
protein expression of E-cadherin and β-catenin, while downregulated
the protein expression of N-cadherin and vimentin of A549 cells
(Fig. 4D). Transwell migration and
invasion assays revealed that HSPA5-silence significantly decreased
the migration and invasion abilities of A549 cells (P<0.05;
Fig. 4E and F). CCK-8 cell
proliferation assay indicated that the proliferation ability was
suppressed by silence of HSPA5 (P<0.05; Fig. 4B). These results demonstrate that
HSPA5 plays a metastasis and growth promoting role in NSCLC
cells.
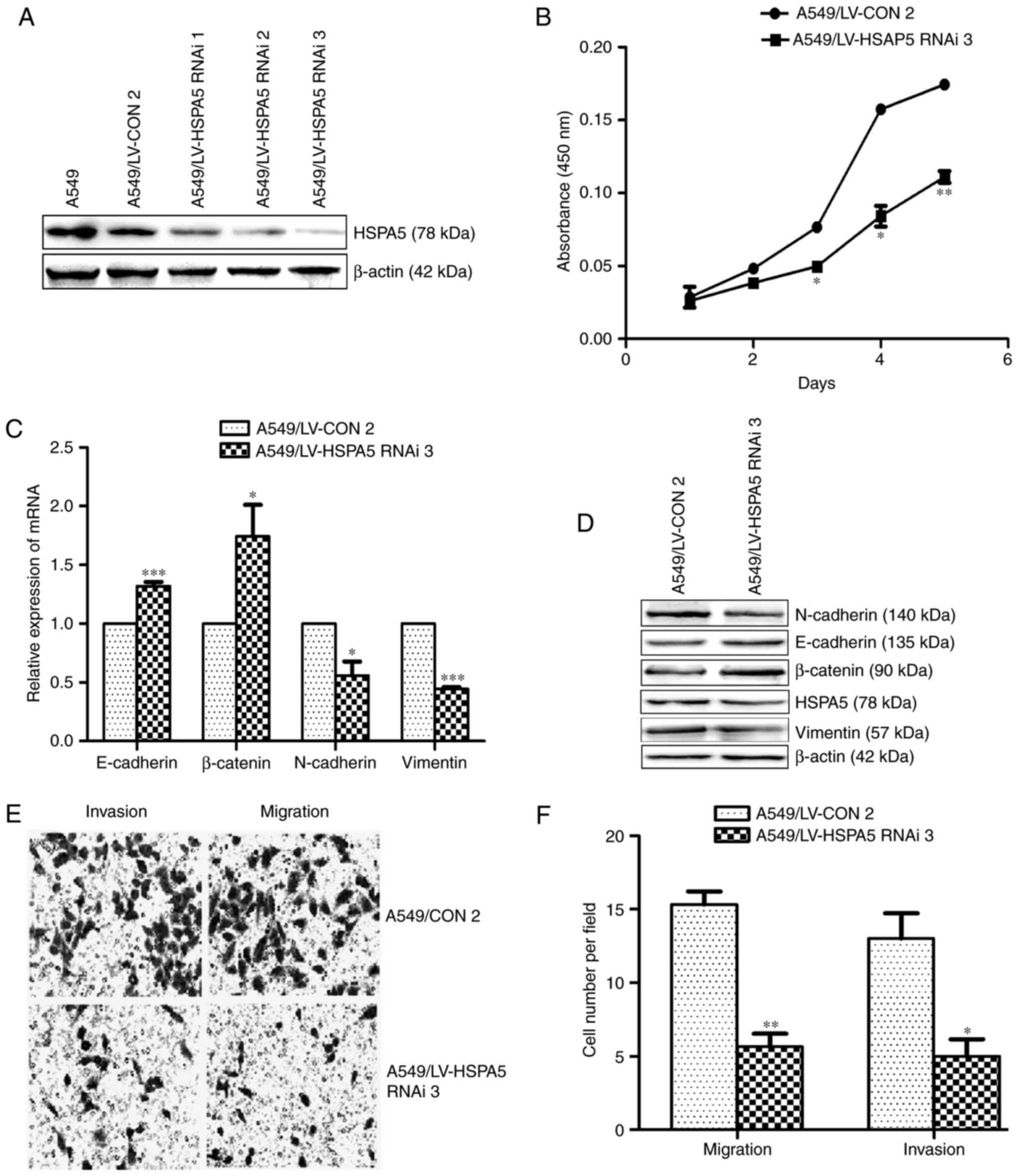 | Figure 4.Silence of HSPA5 attenuates EMT,
migration, invasion and proliferation in NSCLC cells. (A) Western
blot analysis of DAL-1 protein expression in A549 cells after
infecting with LV-HSPA5 RNAi-1, LV-HSPA5 RNAi-2 and LV-HSPA5
RNAi-3. (B) The CCK-8 assay was used to measure the effect of
silencing-HSPA5 on A549 cells proliferation. (C) qRT-PCR of
E-cadherin, β-catenin, N-cadherin and vimentin. (D) Western blot of
E-cadherin, β-catenin, N-cadherin and vimentin. (E) Transwell
migration and invasion assays of the migration and invasion of A549
cells (magnification, ×200). (F) Statistical analysis of Transwell
migration and invasion assays. Data are shown as mean ± SD of three
independent experiments performed in triplicate. *P<0.05,
**P<0.005, ***P<0.001. |
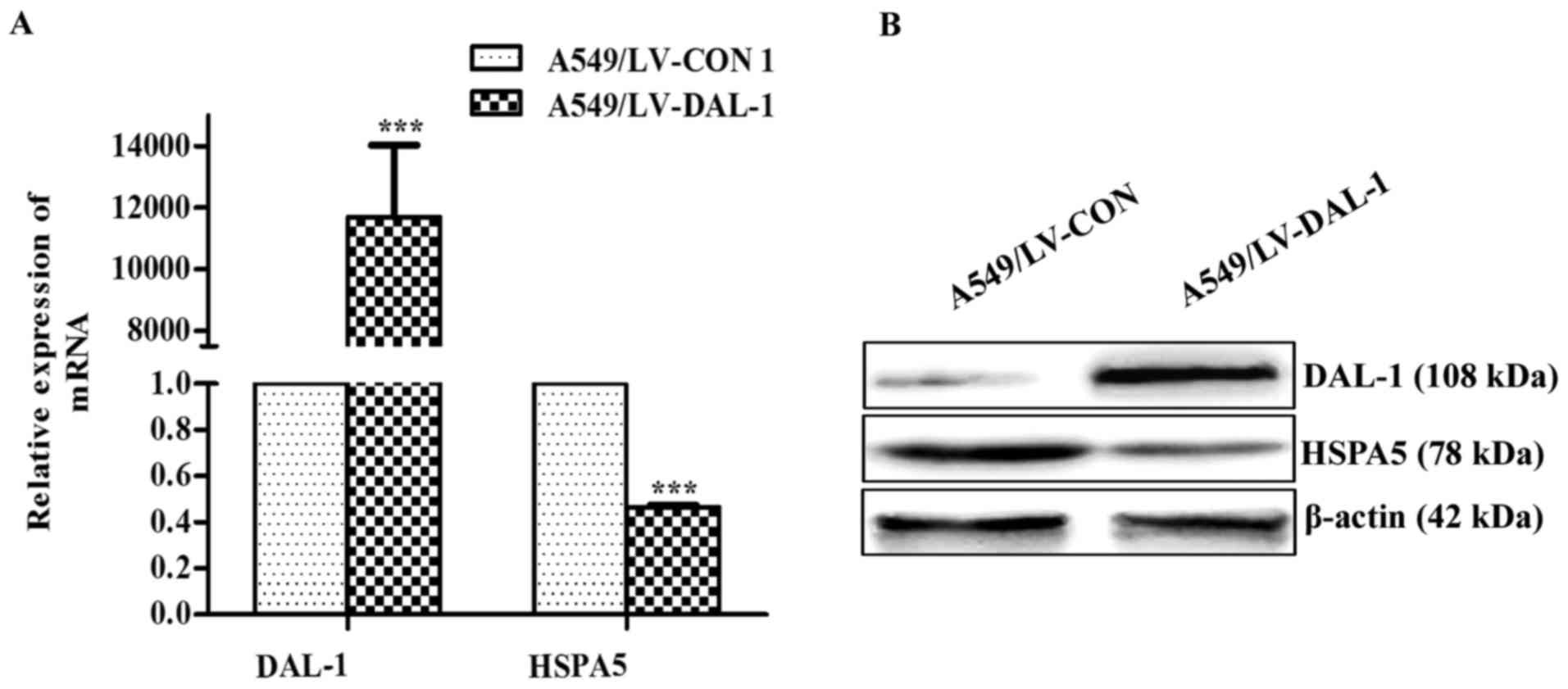
Overexpression of DAL-1 reduces the
expression of HSPA5
To determine whether DAL-1 could influence the
expression of HSPA5, we examined the expression of HSPA5 in
A549/LV-DAL-1 cells and A549/LV-CON1 cells. The result obtained in
the qRT-PCR assay demonstrated that the mRNA expression of HSPA5
was significantly reduced by overexpressing DAL-1 (P<0.001;
Fig. 5A) and western blot assay
indicated that the protein expression of HSPA5 was also suppressed
by DAL-1 (Fig. 5B). The results
suggest that DAL-1 plays a role in suppressing HSPA5
expression.
HSPA5 is involved in DAL-1-mediated
EMT, migration, invasion and proliferation inhibition
Knowing that DAL-1 can attenuate while HSPA5 can
promote EMT, migration, invasion and proliferation in NSCLC cells,
and overexpression of DAL-1 can reduce the mRNA and protein
expression of HSPA5, we have reason to believe that DAL-1 can
inhibit metastasis and proliferation by suppressing HSPA5
expressing. To elucidate whether HSPA5 is involved in
DAL-1-suppressed EMT, migration, invasion and proliferation,
A549/LV-DAL-1 cells were stably infected with HSPA5-overexpressed
vector LV-HSPA5 (Fig. 6A), and the
role of overexpressed HSPA5 in EMT, migration, invasion and
proliferation was detected. The results of qRT-PCR and western blot
assays in Fig. 6C (P<0.05) and
Fig. 6D revealed that the
overexpression of HSPA5 downregulated the mRNA and protein
expression levels of E-cadherin and β-catenin while upregulated the
mRNA and protein expression levels of N-cadherin and vimentin. The
results of Transwell migration and invasion assays indicated that
the migration and invasion abilities in A549/LV-DAL-1 cells were
obviously increased by overexpressing HSPA5 (P<0.05; Fig. 6C and D). The CCK-8 assay indicated
the proliferation ability of A549/LV-DAL-1 cells could be promoted
by infected LV-HSPA5 (Fig. 6B).
These findings support that HSPA5 is a potential target for DAL-1
and involves in DAL-1-mediated EMT, migration, invasion and
proliferation in NSCLC cells.
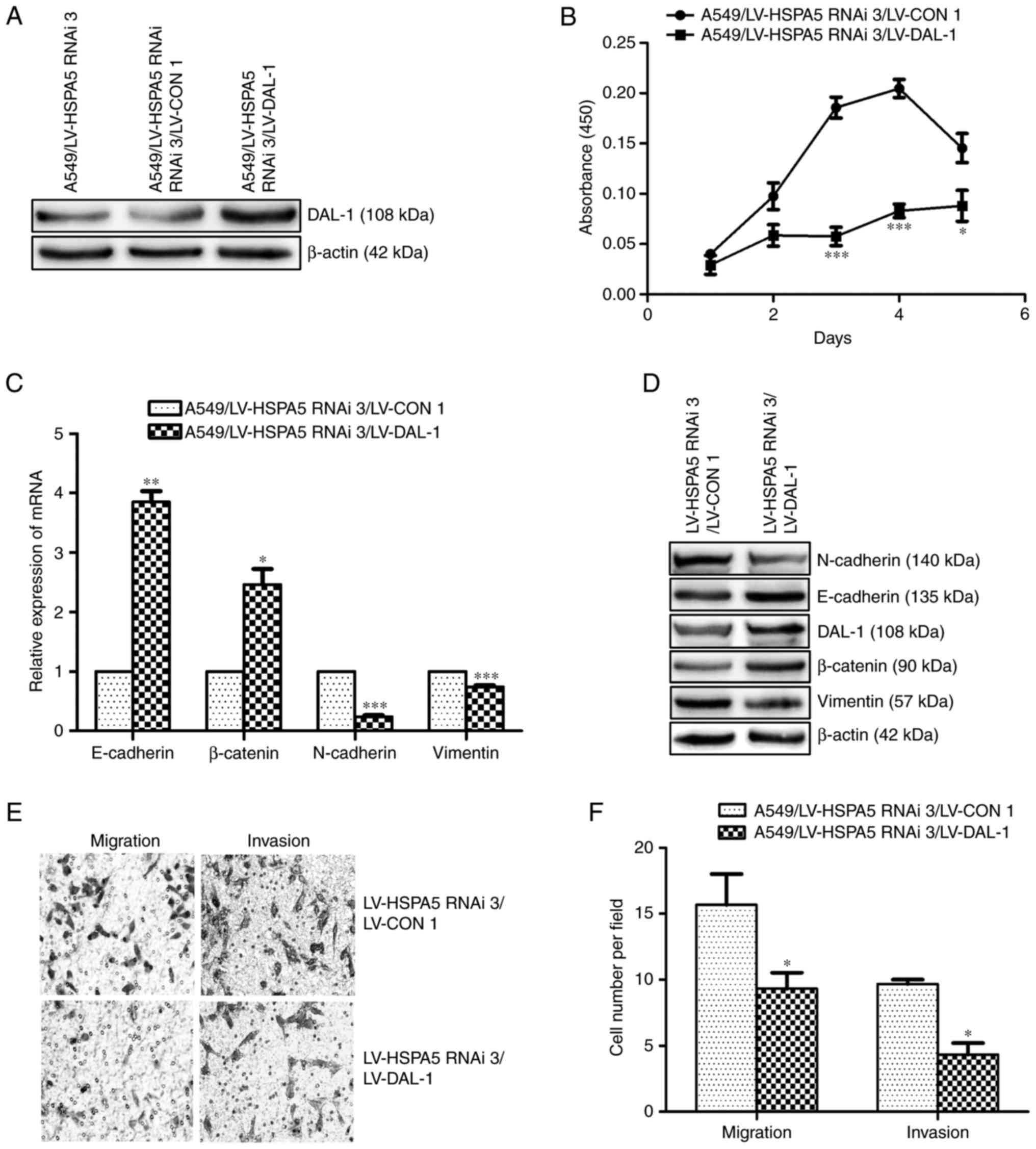
Suppression of HSPA5 is not essential
for DAL-1 to inhibit EMT, migration, invasion and
proliferation
A549/LV-HSPA5 RNAi-3 cells were infected with the
DAL-1 overexpression lentiviral vector LV-DAL-1 (Fig. 7A) to investigate whether the
suppression of HSPA5 is necessary for DAL-1 to inhibit the
metastasis and growth in NSCLC cells. The results of qRT-PCR and
western blot assays shown in Fig.
7C (P<0.05) and Fig. 7D
demonstrated that, after overexpressing DAL-1 in HSPA5-slienced
A549 cells, the mRNA and protein expression of E-cadherin and
β-catenin were increased, while the mRNA and protein expression of
N-cadherin and vimentin were decreased. Transwell migration and
invasion assays shown in Figs. 6E
and 7F (P<0.05), and CCK-8 assay
shown in Fig. 7B (P<0.05)
revealed that overexpression of DAL-1 decreased the migration,
invasion and proliferation ability in A549/LV-HSPA5 RNAi-3 cells.
These data indicate that DAL-1 does not depend on HSPA5 to suppress
EMT, migration, invasion and proliferation.
Overexpression of DAL-1 and silence of
HSPA5 inhibit the PI3K/Akt/Mdm2/p53 signaling pathway
Studies have shown that the abnormal activation of
PI3K/Akt/Mdm2/p53 signal pathway was closely related to the
progression of cancer. It has been reported that HSPA5 can promote
lung cancer metastasis by activating PI3K/Akt signaling pathway.
However, there is still no report on the effects of DAL-1 on
PI3K/Akt/Mdm2/p53 signaling pathway. In this study, western blot
assay was utilized to explore the potential roles of DAL-1 and
HSPA5 on PI3K/Akt/Mdm2/p53 signaling pathway in NSCLC cells. The
LV-DAL-1 lentivirus vectors transfected into A549 cells displayed
an inhibition role on expression of PI3K, p-PI3K, Akt, p-Akt, Mdm2
and p-Mdm2 while played a role on upregulating p53 expression
(Fig. 8A). Similarly, the
lentivirus vectors LV-HSPA5 RNAi3 also functioned reducing the
protein expression of PI3K, p-PI3K, Akt, p-Akt, Mdm2 and p-Mdm2
while increased the expression of p53 protein. These results
suggested that DAL-1 could suppress EMT, migration, invasion and
proliferation by inhibiting PI3K/Akt/Mdm2/p53 signaling pathway
through suppressing HSPA5 expression.
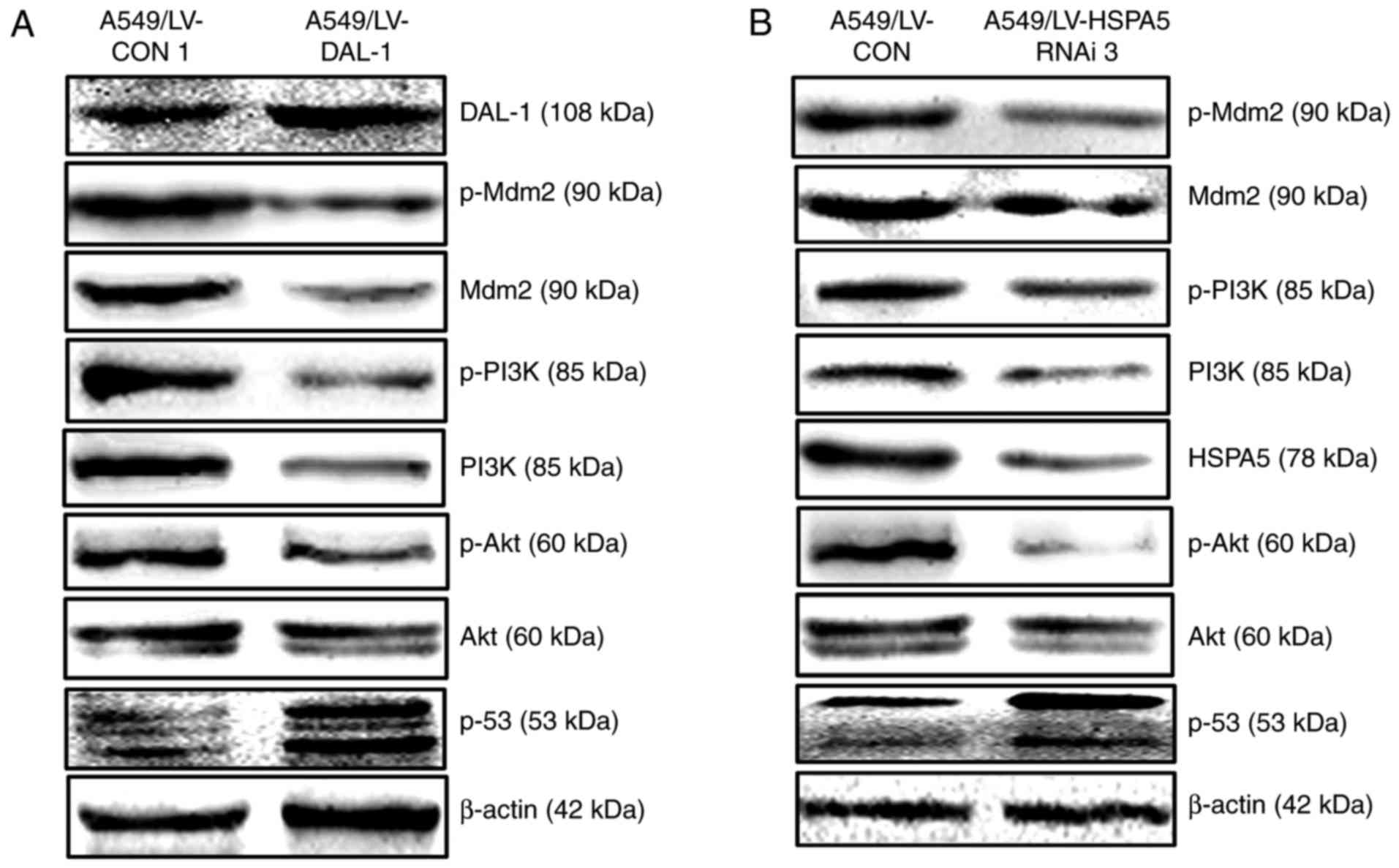 | Figure 8.Overexpression of DAL-1 and silence
of HSPA5 inhibit the PI3K/Akt/Mdm2/p53 signaling pathway. (A)
Western blot assay was used to measure the effect of DAL-1 on the
protein expression of PI3K, p-PI3K, Ake, p-Akt, Mdm2, p-Mdm2 and
p53 in A549 cells. (B) Western blot assay was used to measure the
effect of silencing-HSPA5 on the protein expression of PI3K,
p-PI3K, Ake, p-Akt, Mdm2, p-Mdm2 and p53 in A549 cells. |
Suppression of HSPA5 is essential for
DAL-1 to inhibit PI3K/Akt/Mdm2 signaling pathway
To determine the role of HSPA5 in DAL-1 inhibiting
PI3K/Akt/Mdm2/p53 signaling pathway, the effect of LV-HSPA5 on the
protein expression of PI3K, p-PI3K, Akt, p-Akt, Mdm2, p-Mdm2 and
p53 in A549/LV-DAL-1 cells was investigated. The results shown in
Fig. 9A confirmed that
overexpressing HSPA5 increased the protein expression of PI3K,
p-PI3K, Akt, p-Akt, Mdm2 and p-Mdm2 while decreased the protein
expression of p53 in A549/LV-DAL-1 cells, which told us that HSPA5
is involved in DAL-1-inhibited PI3K/Akt/Mdm2/p53 signaling pathway.
To further study if HSPA5 is necessary for DAL-1 to regulate the
PI3K/Akt/Mdm2 signaling pathway, we examined the protein expression
of PI3K, p-PI3K, Akt, p-Akt, Mdm2, p-Mdm2 and p53 in A549/LV-HSPA5
RNAi-3 cells after infected with LV-DAL-1. The results in Fig. 9B show that, after overexpressing
DAL-1, the protein expression of PI3K, p-PI3K, Mdm2, p-Mdm2, Akt,
p-Akt and p53 in HSPA5-silenced cells were all increased, which
suggested that the suppression of HSPA5 is necessary for DAL-1 to
inhibit PI3K/Akt/Mdm2 signaling pathway.
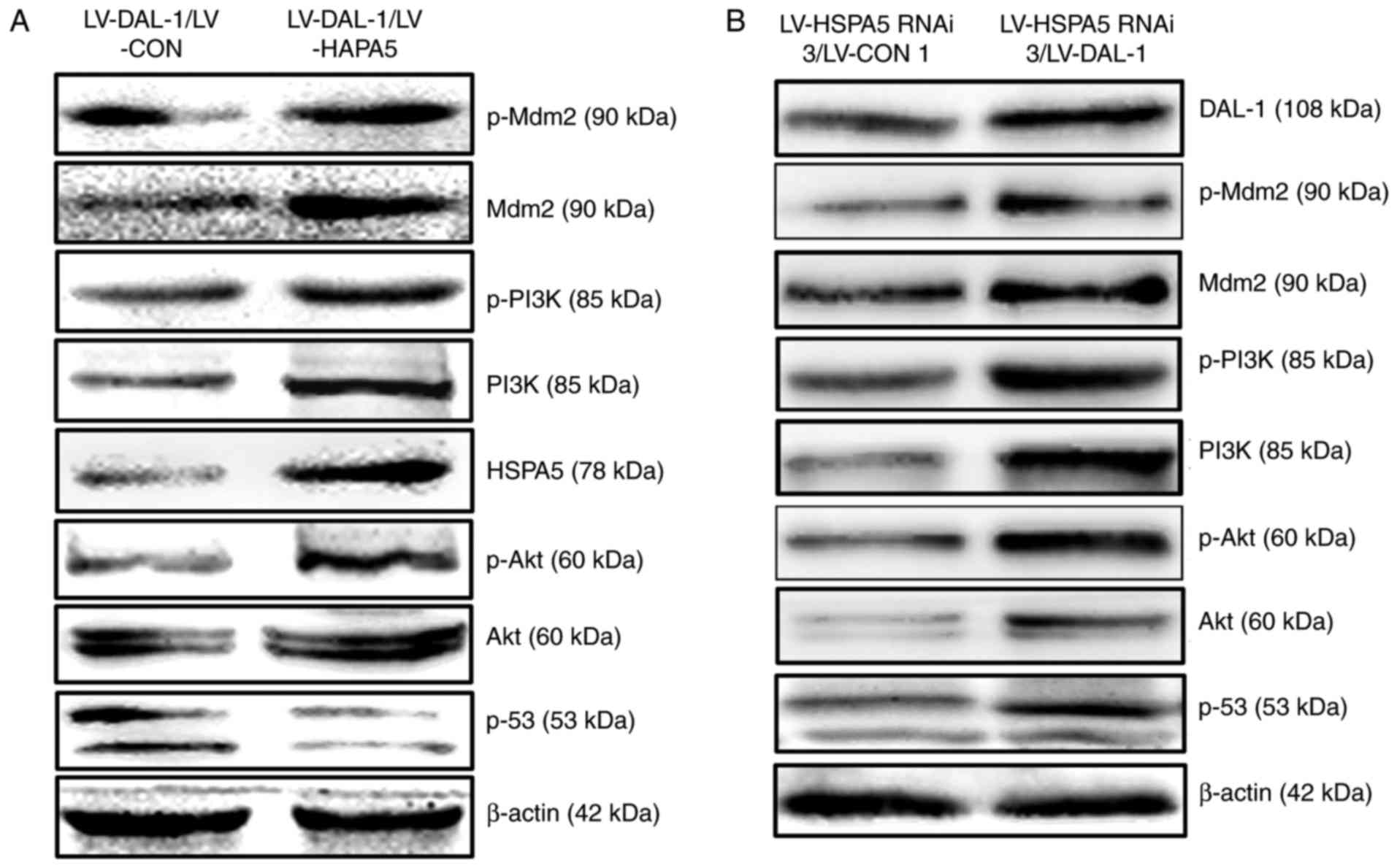 | Figure 9.Suppression of HSPA5 is essential for
DAL-1 to inhibit PI3K/Akt/Mdm2 signaling pathway. (A) Western blot
assay was used to measure the effect of HSPA5 on the protein
expression of PI3K, p-PI3K, Ake, p-Akt, Mdm2, p-Mdm2 and p53 in
A549/LV-DAL-1 cells. (B) Western blot assay was used to measure the
effect of DAL-1 on the protein expression of PI3K, p-PI3K, Ake,
p-Akt, Mdm2, p-Mdm2 and p53 in A549/LV-HSPA5 RNAi-3 cells. |
Discussion
According to previous studies, the occurrence of EMT
is closely related to the metastasis of epithelial malignant tumor.
The invasion and metastasis of tumor cells is based on the
reduction of cell adhesion and the enhancement of cell motility,
and EMT provides conditions for the invasion and metastasis of
epithelial tumor cells (2–6). Some studies also reported that EMT can
stimulate tumor cells to obtain anti-apoptotic ability and enhance
the resistance of tumor cells, but the specific mechanism remains
to be further studied (22,23). We have found that DAL-1 can activate
the E-cadherin promoter to inhibit EMT, thereby inhibiting the
proliferation, invasion and migration abilities in NSCLC cells
(17). The aim of this study was to
further explore the mechanism by which DAL-1 inhibits EMT in NSCLC
cells.
In our previous study, HSPA5 was found to be a
DAL-1-related protein, which can be combined with DAL-1 protein
(17). In this study, we
investigated the co-localization of DAL-1 protein and HSPA5 protein
by laser confocal scanning technique, and found they were
co-located in the nucleus and cytoplasm. At present, there is no
report on the expression relationship between DAL-1 and HSPA5, and
we first studied their expression relationship in NSCLC cells and
found that overexpression of DAL-1 can reduce the mRNA and protein
of HSPA5. We have proved that DAL-1 can function as a transcription
factor to regulate the promoter of E-cadherin (17), which suggested that DAL-1 might
inhibit HSPA5 transcription by suppressing the promoter of HSPA5,
and the reduction of HSPA5 protein might be caused by the
downregulation of mRNA. Double luciferase reporter assay will be
used to validate this conjecture.
To investigate the effects of DAL-1 and HSPA5 on
EMT, proliferation, invasion and migration in NSCLC cells, we
overexpressed DAL-1 or silenced the HSPA5 expression of A549 cells,
which express a low basal level of DAL-1 but a high basal level of
HSPA5. The findings were similar to those of previous studies:
DAL-1 inhibited while HSPA5 promoted the EMT, proliferation,
invasion and migration of NSCLC cells (17,24–26).
We examined the effects of LV-HSPA5 on EMT, migration, invasion and
proliferation of DAL-1-overexpression cells, and found that
overexpression of HSPA5 promoted EMT, migration, invasion and
proliferation. We also determined the effects of LV-DAL-1 on EMT,
migration, invasion and proliferation of HSPA5-silenced cells, and
found that overexpression of DAL-1 decreased EMT, migration,
invasion and proliferation. These findings indicate that DAL-1
could inhibit EMT, migration, invasion and proliferation by
suppressing HSPA5 expression, but the suppression of HSPA5 was not
essential for DAL-1 to inhibit metastasis and growth in NSCLC
cells. Studies have shown that DAL-1 can interact with other
proteins to play physiological function and anti-cancer function.
For example, DAL-1 protein is reported to be involved in
maintaining cell cytoskeleton stability and cell polarity by
binding to CADM1, Spectin, actin, Caspr, MPPs (27–30)
and mediate cell proliferation, inhibit cell growth by binding to
pICIn, CD44 (31,32); by binding to β8-integrin, DAL-1
protein can regulate β8-integrin localization and the downstream
pathway (33).
Emerging evidence indicated that the abnormal
activation of PI3K/Akt/Mdm2/p53 signaling pathway is associated
with the progression of colon cancer (34). In this study, we first studied the
effects of DAL-1 on PI3K/Akt/Mdm2/p53 signaling pathway by western
blot assay. The results reveled that overexpression of DAL-1 and
silence of HSPA5 decreased the expression of PI3K, p-PI3K, Akt,
p-Akt, Mdm2, p-Mdm2 while increased the expression of p53 protein,
which suggested that DAL-1 could attenuate EMT by inhibiting
PI3K/Akt/Mdm2/p53 signaling pathway while HSPA5 could promote EMT
by activating PI3K/Akt/Mdm2/p53 signaling pathway in NSCLC cells.
We further analyzed the correlation of HSPA5 with the suppression
role of DAL-1 in PI3K/Akt/Mdm2/p53 signaling pathway by detecting
the effect of LV-HSPA5 on DAL-1-ovexpression cells and the effect
of LV-DAL-1 on HSPA5-silenced cells. Our findings point toward an
essential role of HSPA5 in DAL-1 inhibiting PI3K/Akt/Mdm2 signaling
pathway by suppressing the protein expression levels of PI3K,
p-PI3K, Akt, p-Akt, Mdm2 and p-Mdm2.
In this study, DAL-1 was found to suppress the mRNA
and protein expression of HSPA5 to inhibit EMT, migration, invasion
and proliferation of NSCLC cells. For the first time, the present
study revealed the relationship between DAL-1 and PI3K/Akt/Mdm2/p53
signaling pathway, indicating the potential role of DAL-1 in the
inhibition of NSCLC development. Furthermore, it underscores the
role of HSPA5 as a potential gene for DAL-1 to inhibit
PI3K/Akt/Mdm2 signaling pathway. In conclusion, this study revealed
that DAL-1 attenuate EMT, metastasis and proliferation by
suppressing HSPA5 expression in NSCLC cells.
Acknowledgements
This work was funded by the National Nature Science
Foundation of China (no. 81401391), the National Nature Science
Foundation of Guangdong Province (no. 2015A030313452), China
Postdoctoral Science Foundation Grant (no. 2015M570696), and
Medical Research Foundation of Guangdong Province (no.
B2014188).
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
DAL-1
|
differentially expressed in
adenocarcinoma of the lung
|
|
HSPA5
|
heat shock protein 5
|
|
Bip
|
heavy chain binding protein
|
|
GRP78
|
glucose regulated protein 78
|
|
HSP70
|
heat shock protein 70
|
|
ER
|
endoplasmic reticulum
|
References
|
1
|
Al Mohammad B, Brennan PC and Mello-Thoms
C: A review of lung cancer screening and the role of computer-aided
detection. Clin Radiol. 72:433–442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu B, Qi L, Liu S, Liu W, Ou Z, Chen M,
Liu L, Zu X, Wang J and Li Y: CLASP2 is involved in the EMT and
early progression after transurethral resection of the bladder
tumor. BMC Cancer. 17:1052017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He YX, Song XH, Zhao ZY and Zhao H: HOXA13
upregulation in gastric cancer is associated with enhanced cancer
cell invasion and epithelial-to-mesenchymal transition. Eur Rev Med
Pharmacol Sci. 21:258–265. 2017.PubMed/NCBI
|
|
4
|
Chen J, Zhang H, Chen Y, Qiao G, Jiang W,
Ni P, Liu X and Ma L: miR-598 inhibits metastasis in colorectal
cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell
Res. 352:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng H, Lu JJ, Wang Y, Pei L and Chen X:
Osthole inhibited TGF β-induced epithelial-mesenchymal transition
(EMT) by suppressing NF-κB mediated Snail activation in lung cancer
A549 cells. Cell Adhes Migr. Feb 1–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
6
|
Xu J, Zhang X, Wang H, Ge S, Gao T, Song
L, Wang X, Li H, Qin Y and Zhang Z: HCRP1 downregulation promotes
hepatocellular carcinoma cell migration and invasion through the
induction of EGFR activation and epithelial-mesenchymal transition.
Biomed Pharmacother. 88:421–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Otto W, Breyer J, Herdegen S, Eder F,
Bertz S, May M, Mayr R, Lausenmeyer EM, Denzinger S, van Rhijn BW,
et al: WHO 1973 grade 3 and infiltrative growth pattern proved,
aberrant E-cadherin expression tends to be of predictive value for
progression in a series of stage T1 high-grade bladder cancer after
organ-sparing approach. Int Urol Nephrol. 49:431–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Yin S, Zhang L, Liu W and Chen B:
Prognostic value of reduced E-cadherin expression in breast cancer:
A meta-analysis. Oncotarget. 8:16445–16455. 2017.PubMed/NCBI
|
|
10
|
Tran YK, Bögler O, Gorse KM, Wieland I,
Green MR and Newsham IF: A novel member of the NF2/ERM/4.1
superfamily with growth suppressing properties in lung cancer.
Cancer Res. 59:35–43. 1999.PubMed/NCBI
|
|
11
|
Li L, Li S, Cai T, Wang H, Xie X, Liu Z
and Zhang Y: The targeted inhibitory effects of human amniotic
fluid stem cells carrying CXCR4 promoter and DAL-1 on non-small
cell lung carcinoma growth. Gene Ther. 23:214–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi Y, Iwai M, Kawai T, Arakawa A,
Ito T, Sakurai-Yageta M, Ito A, Goto A, Saito M, Kasumi F, et al:
Aberrant expression of tumor suppressors CADM1 and 4.1B in invasive
lesions of primary breast cancer. Breast Cancer. 19:242–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Xu M, Cui X, Liu Y, Zhang Y, Sui
Y, Wang D, Peng L, Wang D and Yu J: Aberrant expression of the
candidate tumor suppressor gene DAL-1 due to hypermethylation in
gastric cancer. Sci Rep. 6:217552016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagata M, Sakurai-Yageta M, Yamada D, Goto
A, Ito A, Fukuhara H, Kume H, Morikawa T, Fukayama M, Homma Y, et
al: Aberrations of a cell adhesion molecule CADM4 in renal clear
cell carcinoma. Int J Cancer. 130:1329–1337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dafou D, Grun B, Sinclair J, Lawrenson K,
Benjamin EC, Hogdall E, Kruger-Kjaer S, Christensen L, Sowter HM,
Al-Attar A, et al: Microcell-mediated chromosome transfer
identifies EPB41L3 as a functional suppressor of epithelial ovarian
cancers. Neoplasia. 12:579–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kittiniyom K, Mastronardi M, Roemer M,
Wells WA, Greenberg ER, Titus-Ernstoff L and Newsham IF:
Allele-specific loss of heterozygosity at the DAL-1/4.1B (EPB41L3)
tumor-suppressor gene locus in the absence of mutation. Genes
Chromosomes Cancer. 40:190–203. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Guan X, Zhang H, Xie X, Wang H,
Long J, Cai T, Li S, Liu Z and Zhang Y: DAL-1 attenuates
epithelial-to mesenchymal transition in lung cancer. J Exp Clin
Cancer Res. 34:32015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moreno JA and Tiffany-Castiglioni E: The
chaperone Grp78 in protein folding disorders of the nervous system.
Neurochem Res. 40:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu T, Guo Z, Fan H, Song J, Liu Y, Gao Z
and Wang Q: Cancer-associated fibroblasts promote non-small cell
lung cancer cell invasion by upregulation of glucose-regulated
protein 78 (GRP78) expression in an integrated bionic microfluidic
device. Oncotarget. 7:25593–25603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen HA, Chang YW, Tseng CF, Chiu CF, Hong
CC, Wang W, Wang MY, Hsiao M, Ma JT, Chen CH, et al: E1A-mediated
inhibition of HSPA5 suppresses cell migration and invasion in
triple-negative breast cancer. Ann Surg Oncol. 22:889–898. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra
SK, Krasnoperov V, Dong D, Liu S, Li D, et al: Monoclonal antibody
against cell surface GRP78 as a novel agent in suppressing PI3K/AKT
signaling, tumor growth, and metastasis. Clin Cancer Res.
19:6802–6811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dekervel J, Bulle A, Windmolders P,
Lambrechts D, Van Cutsem E, Verslype C and van Pelt J: Acriflavine
inhibits acquired drug resistance by blocking the
epithelial-to-mesenchymal transition and the unfolded protein
response. Transl Oncol. 10:59–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li
J and and Zhang Q: Resveratrol reverses Doxorubicin resistance by
inhibiting epithelial-mesenchymal transition (EMT) through
modulating PTEN/Akt signaling pathway in gastric cancer. J Exp Clin
Cancer Res. 36:192017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Xu R, Li G, Xie X, Long J and
Wang H: Loss of expression of the differentially expressed in
adenocarcinoma of the lung (DAL-1) protein is associated with
metastasis of non-small cell lung carcinoma cells. Tumour Biol.
33:1915–1925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Q, Hua J, Wang Q, Xu W, Zhang J, Zhang
J, Kang J and Li M: Expressions of GRP78 and Bax associate with
differentiation, metastasis, and apoptosis in non-small cell lung
cancer. Mol Biol Rep. 39:6753–6761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HM, Jiang ZF, Fan XY, Wang T, Ke Xu,
Yan XB, Ma Y, Xiao WH and Liu RY: Reversed expression of GRIM-1 and
GRP78 in human non-small cell lung cancer. Hum Pathol.
45:1936–1943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Busam RD, Thorsell AG, Flores A,
Hammarström M, Persson C, Öbrink B and Hallberg BM: Structural
basis of tumor suppressor in lung cancer 1 (TSLC1) binding to
differentially expressed in adenocarcinoma of the lung
(DAL-1/4.1B). J Biol Chem. 286:4511–4516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh V, Miranda TB, Jiang W, Frankel A,
Roemer ME, Robb VA, Gutmann DH, Herschman HR, Clarke S and Newsham
IF: DAL-1/4.1B tumor suppressor interacts with protein arginine
N-methyltransferase 3 (PRMT3) and inhibits its ability to methylate
substrates in vitro and in vivo. Oncogene. 23:7761–7771. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horresh I, Bar V, Kissil JL and Peles E:
Organization of myelinated axons by Caspr and Caspr2 requires the
cytoskeletal adapter protein 4.1B. J Neurosci. 30:2480–2489. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamijo A, Saitoh Y, Ohno N, Ohno S and
Terada N: Immunohistochemical study of the membrane skeletal
protein, membrane protein palmitoylated 6 (MPP6), in the mouse
small intestine. Histochem Cell Biol. 145:81–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang W, Roemer ME and Newsham IF: The
tumor suppressor DAL-1/4.1B modulates protein arginine
N-methyltransferase 5 activity in a substrate-specific manner.
Biochem Biophys Res Commun. 329:522–530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robb VA, Gerber MA, Hart-Mahon EK and
Gutmann DH: Membrane localization of the U2 domain of Protein 4.1B
is necessary and sufficient for meningioma growth suppression.
Oncogene. 24:1946–1957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCarty JH, Cook AA and Hynes RO: An
interaction between {alpha}v{beta}8 integrin and Band 4.1B via a
highly conserved region of the Band 4.1 C-terminal domain. Proc
Natl Acad Sci USA. 102:pp. 13479–13483. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Zhu S, Shi X and Sha W: The
silence of p66(Shc) in HCT8 cells inhibits the viability via
PI3K/AKT/Mdm-2/p53 signaling pathway. Int J Clin Exp Pathol.
8:9097–9104. 2015.PubMed/NCBI
|