Introduction
Bladder cancer is one of the most common
malignancies of the urogenital system worldwide with an estimated
300,00 new cases emerging every year (1). Non-muscle-invasive bladder and
muscle-invasive bladder cancer are the main two patterns of bladder
cancer (2). Most early-stage
bladder cancer is recognized as non-muscle invasive bladder cancer,
which is effectively treated by surgery. However, due to the high
recurrence of bladder cancer after surgical resection, most
advanced bladder cancer develops into a muscle-invasive pattern
resulting in lethal disease without effective treatment (3). Thus, novel potential agents are
urgently needed for the treatment of bladder cancer, and Chinese
medicine has attracted great attention for cancer therapeutics.
Tetrandrine, a bisbenzylisoquinoline alkaloid, is
isolated from the roots of Stephania tetrandra (4). Numerous studies have been reported
that tetrandrine can be broadly used for patients with arthritis,
hypertension, sepsis, inflammation and silicosis (5–9).
Additionally, tetrandrine has been found to inhibit proliferation
and induce apoptosis in various types of cancer cells, such as
gastric and colon cancer, hepatocellular and renal cell carcinoma
(10–13). Moreover, previous research has shown
that tetrandrine induced cell cycle arrest in neuroblastoma cells
(14). In addition, tetrandrine was
reported to have the capacity to reverse multidrug resistance in
cancer cells, indicating the strong anticancer activity of
tetrandrine (15). However, there
is little research concerning the effect of tetrandrine on bladder
cancer. Only one study showed that apoptosis induction by
tetrandrine was mediated via activation of the caspase cascade in
bladder cancer T24 and 5637 cells, but the underlying mechanism is
still undefined (16).
In recent years, autophagy has gained considerable
attention in regards to tumor progression, particularly in cell
apoptosis (17). Autophagy is a
conserved cellular process for the degradation of broken proteins
and aging organelles to maintain intercellular homeostasis
(18). Recent research demonstrated
that autophagy may be a potential antitumor mechanism. It was
revealed that inhibition of autophagy facilitated
epithelial-mesenchymal transition by reactive oxygen species
(ROS)/HO-1 signaling pathway in human ovarian cancer cells
(19).
In our previous study, we confirmed that tetrandrine
at low concentrations reversed epithelial-mesenchymal transition in
bladder cancer cells (20).
Nevertheless, whether tetrandrine could have the ability to induce
autophagy in bladder cancer cells is unknown. In the present study,
we revealed that autophagy induction by tetrandrine enhanced
tetrandrine-induced apoptosis in bladder cancer cells, which was
mediated by the AMPK/mTOR signaling pathway. Our results
demonstrated that tetrandrine may be a potential anticancer agent,
while autophagy may be a crucial mechanism for cancer therapy.
Materials and methods
Reagents and cell culture
Tetrandrine
(C38H42N2O6) was
purchased from Sigma-Aldrich (St. Louis, MO, USA) and stock
solutions with a concentration of 25 mg/ml were stored at −20°C.
Antibodies against microtubule-associated protein 1 light chain 3-β
(LC3-I), LC3-II, p62, p-AMPK (Thr172), AMPK, p-ACC (Ser79), ACC,
p-mTOR, mTOR, p70S6K (Thr389), p70S6K (Ser371) and p-4EBP1
(Thr37/46) were purchased from Cell Signaling Technology (Beverly,
MA, USA). Mouse monoclonal β-actin antibody was purchased from
CWBIO (Beijing, China). Bafilomycin A1 (Baf A1), AICAR, compound C
(Com C) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO,
USA). GFP-RFP-LC3 adenovirus was purchased from Hanbio (Shanghai,
China). The enhanced chemiluminescence (ECL) detection system was
obtained from Amersham Life Science, Inc. (Arlington Heights, IL,
USA).
Human bladder cancer T24 and 5637 cell lines were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and maintained in Dulbecco's modified Eagles medium,
supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA). Cells were
incubated in a humidified atmosphere with 5% CO2 at
37°C.
Transmission electron microscopy
After treatment, cells were washed with
phosphate-buffered saline (PBS) and fixed with glutaraldehyde (pH
7.4). Then, the cells were treated with 1% osmium tetroxide and
dehydrated. Subsequently, the cells were embedded in Ultracut
(Leica Ultracut R; Leica Microsystems, Bensheim, Germany) and cut
into 60-nm sections, followed by uranyl acetate and lead citrate
staining. Ultimately, the ultrathin sections were visualized under
an Hitachi H07650 transmission electron microscope (Hitachi, Ltd.,
Tokyo, Japan).
Confocal fluorescence microscopy
T24 cells were cultured on slides and transfected
with GFP-RFP-LC3 adenovirus. After treatment with tetrandrine,
cells were washed with PBS and fixed with 4% paraformaldehyde.
Then, the slides were blocked with glycerol, and the localization
of LC3 puncta was observed with the confocal fluorescence
microscope (Nikon, Tokyo, Japan).
Western blotting
Briefly, the treated cells were washed with PBS
buffer and lysed with RIPA buffer [10 mmol/l Tris-HCl (pH 7.4), 150
mmol/l NaCl, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/l
ethylenediaminetetraacetic acid, 1 mmol/l ethylene glycol
tetraacetic acid, 0.3 mmol/l phenylmethylsulfonyl fluoride, 0.2
mmol/l sodium orthovanadate, 1% NP-40, 10 mg/ml leupeptin and 10
mg/ml aprotinin], containing proteinase and phosphatase inhibitors
on ice. After centrifugation and denaturation, 30 µg of clarified
cell lysate was separated by SDS-polyacrylamide gel (10 or 15%) and
transferred to polyvinylidene fluoride membranes. Then, the
membranes were blocked with 5% non-fat milk for 1 h at room
temperature and incubated with primary antibody at 4°C overnight.
After being washed with Tris-buffered saline with Tween-20 (TBST)
buffer, the bands were then incubated with horseradish
peroxidase-conjugated secondary antibody for 1 h. Ultimately, the
protein bands were analyzed using an ECL detection system, followed
by exposure to X-ray film.
MTT assay
Briefly, 6×103 cells were seeded onto
96-well plates and treated with different treatments for 24 h.
Then, 20 µl of MTT dye solution was mixed with 180 µl of medium to
each well and incubated at 37°C for 4 h. Subsequently, cells were
lysed with dimethyl sulfoxide was used to dissolve the formazan
crystals. Finally, the optical density (OD) of each well was
detected at a wavelength of 570 nm using a 96-well microplate
reader (Bio-Rad, Hercules, CA, USA).
Flow cytometry
The bladder cancer T24 and 5637 cells were exposed
to a certain treatment for 24 h. The treated cells were washed,
collected and stained with fluorescein isothiocyanate
(FITC)-conjugated Annexin V and propidium iodide (PI) according to
the manufacturer's protocol. The apoptotic cells were subsequently
analyzed by flow cytometry (BD FACScan flow cytometer; BD
Biosciences, San Diego, CA, USA). The experiments were performed in
triplicate.
Statistical analysis
The results are presented as the mean ± SD. All
statistical analyses were performed using GraphPad Prism 5.2
software. Student's t-test (two-sided) was used for comparisons
involving only two groups. P<0.05 was identified as a
significant difference.
Results
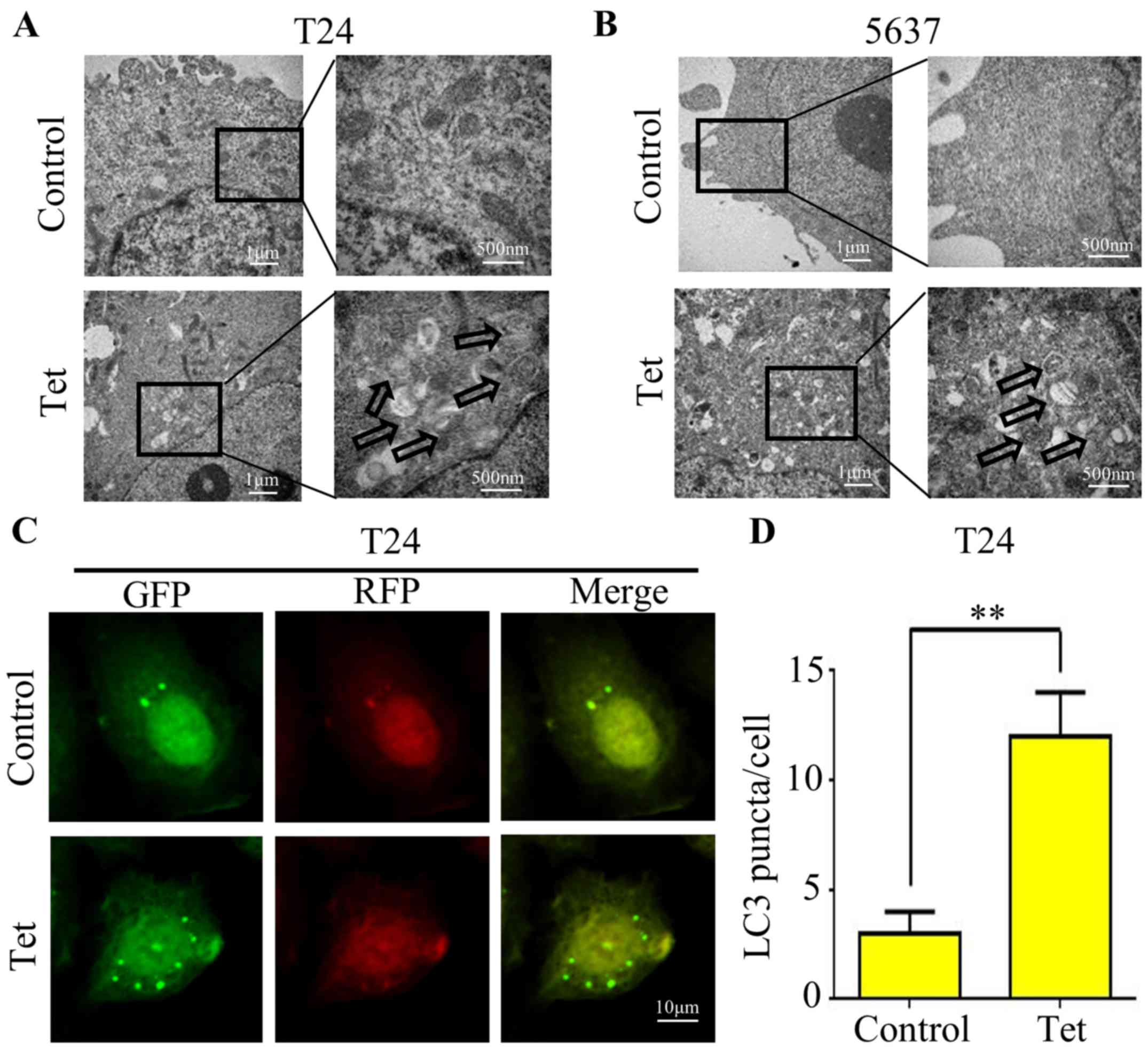
Tetrandrine induces autophagic
vacuoles in bladder cancer T24 and 5637 cell lines
Since there is no study concerning the correlation
between tetrandrine and autophagy in bladder cancer, we firstly
treated T24 (Fig. 1A) and 5637
(Fig. 1B) cell lines with 10 µM
tetrandrine and observed the ultrastructure of the cells by
transmission electron microscopy. A significant increase in
autophagic double-membrane vacuoles was observed in the
tetrandrine-treated cells compared with this number in the control
group.
The conversion of LC3-I to LC3-II is a hallmark of
mammalian autophagy. Thus, we transfected the GFP-RFP-LC3
adenovirus containing acid-sensitive GFP and the acid-insensitive
RFP into bladder cancer T24 cells to explore the effect of
tetrandrine on autophagy. Microscopic examination showed a marked
increase in fluorescent puncta of GRP-RFP-LC3, indicating that
tetrandrine induced autophagosome formation and the occurrence of
autophagy (Fig. 1C and D).
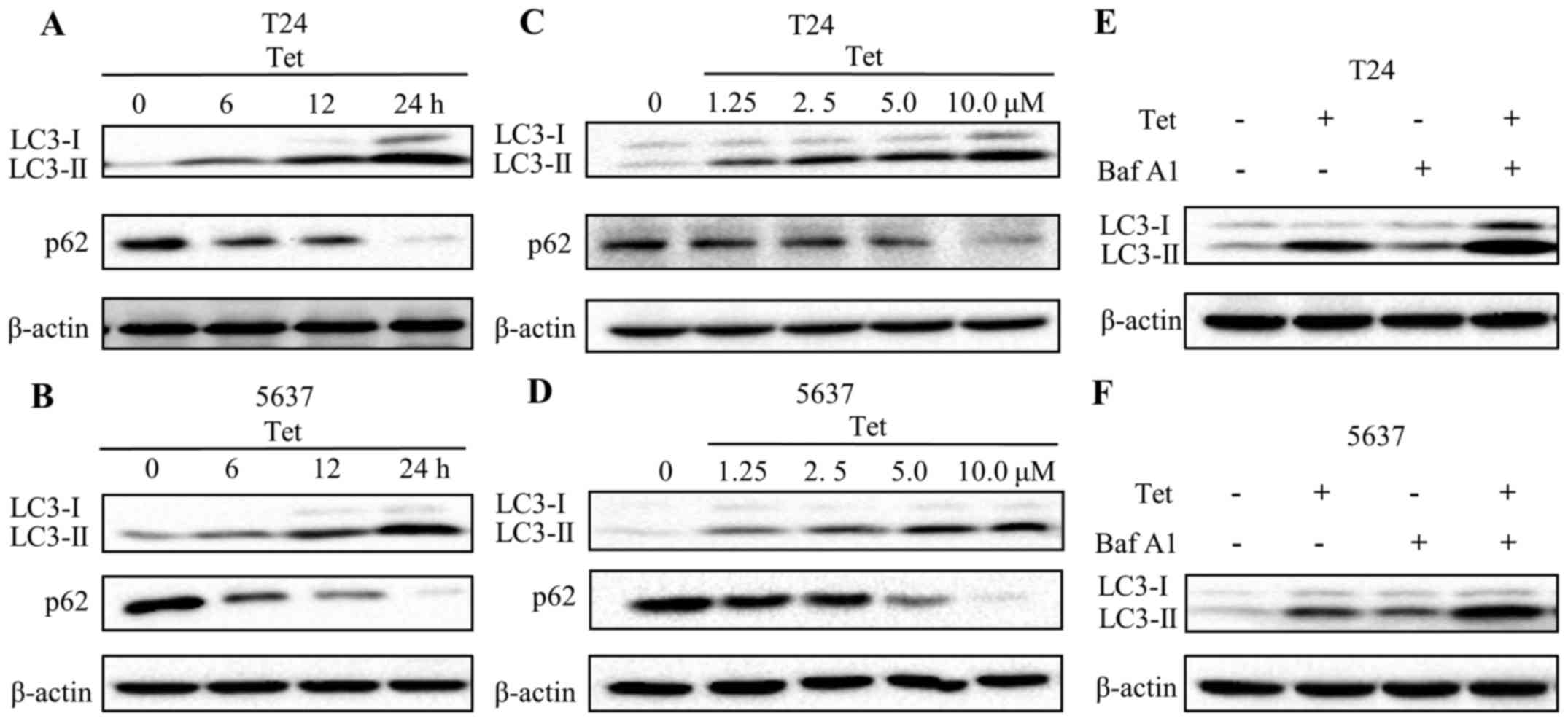
Tetrandrine induces autophagic flux in
bladder cancer T24 and 5637 cells
To further verify whether tetrandrine induces
autophagy in bladder cancer cells, the levels of LC3-II, a membrane
bound form of LC3, were evaluated by western blotting. Results are
presented in Fig. 2A and C.
Expression of LC3-II in T24 cells was gradually increased in a
time- and concentration-dependent manner following tetrandrine
treatment. Similar results were found in the 5637 cells (Fig. 2B and D). p62, an indicator of
lysosome degradation, has been used for monitoring autophagic flux
(21). Our findings showed a marked
decline in the p62 level upon tetrandrine treatment in a time- and
concentration-dependent pattern (Fig.
2A-D).
To further validate the induction of autophagic flux
by tetrandrine, T24 and 5637 cells were exposed to the combination
treatment of tetrandrine and Baf A1, an inhibitor of lysosomal
acidification. Obviously, the LC3-II levels were increased in both
the control and tetrandrine-treated cells in the presence of Baf
A1. Moreover, the combined group exhibited a higher level of LC3-II
compared with that in the tetrandrine alone or Baf A1 alone treated
groups (Fig. 2E and F). These
results suggested that an increase in production, not a decrease in
degradation, resulted in the accumulation of LC3-II by tetrandrine,
and that tetrandrine significantly induced autophagic flux in the
bladder cancer cells.
Tetrandrine induces autophagy through
the AMPK/mTOR pathway
AMPK is a critical energy sensor that regulates
energy metabolism in eukaryotic cells (22). Recent studies report that autophagy
can be induced by AMP-activated protein kinase (AMPK). To determine
the role of AMPK in tetrandrine-induced autophagy, we evaluated the
protein levels of phosphorylated- and total-AMPK after tetrandrine
treatment. The findings showed that the levels of
phosphorylated-AMPK (Thr172) were markedly increased in a
dose-dependent manner in the T24 and 5637 cells following
tetrandrine treatment, but no significant changes were presented in
total-AMPK levels (Fig. 3A).
Consistent with the above results, the protein level of
phosphorylated-ACC (Ser79), a substrate of phosphorylated-AMPK, is
upregulated by tetrandrine. mTOR, a negative regulator in the
autophagy process, has been verified to be regulated by AMPK. To
examine the role of mTOR signaling in tetrandrine-induced
autophagy, we detected the phosphorylation levels of mTOR, and
subsequently observed a decline in p-mTOR (Ser2448) (Fig. 3A). In accordance with the above
results, the phosphorylation levels of eukaryotic initiation factor
4E-binding protein (4E-BP1) and p70S6K, two downstream substrates
of mTOR, were also downregulated in the tetrandrine-treated T24 and
5637 cells (Fig. 3A).
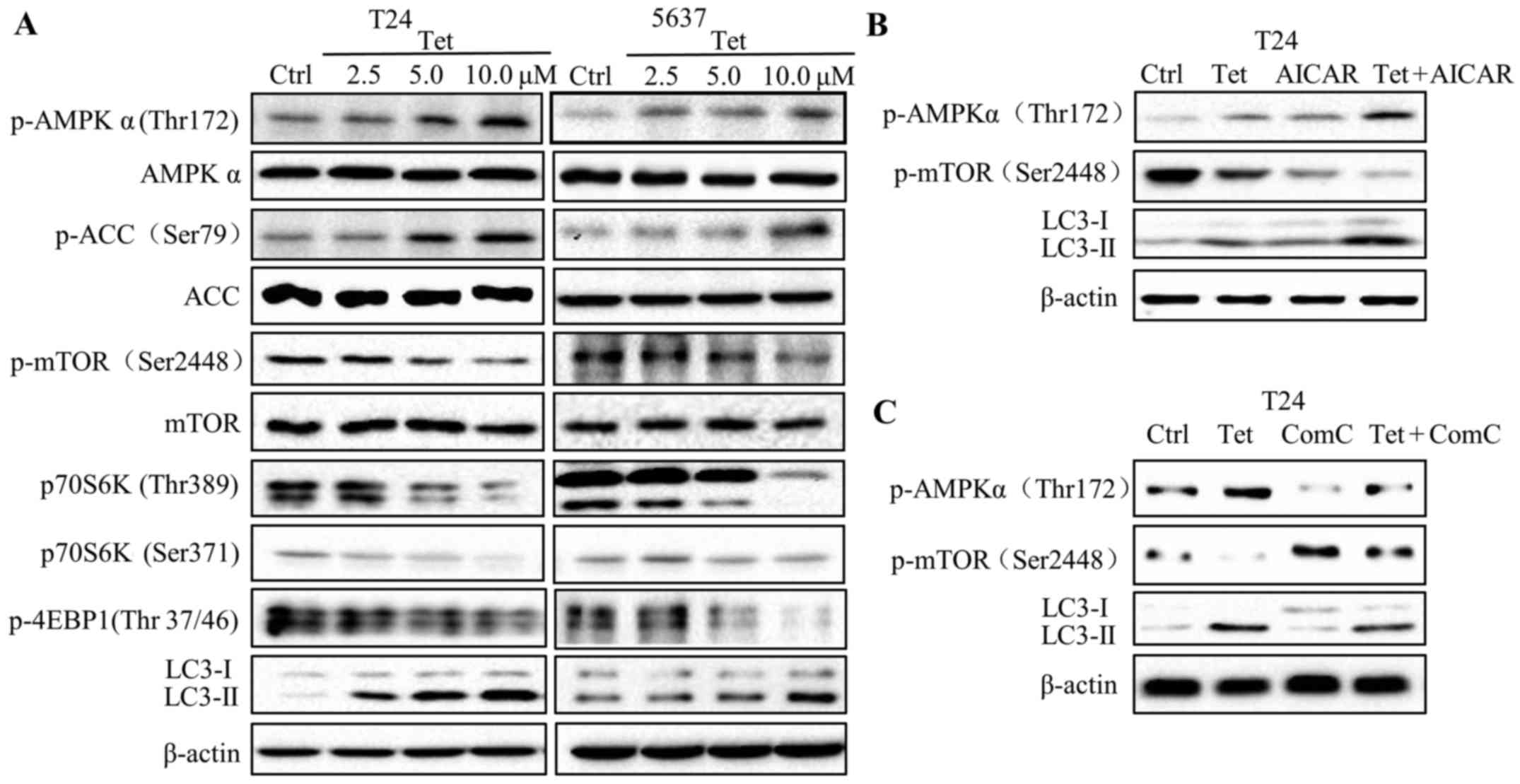 | Figure 3.Tetrandrine induces autophagy through
the AMPK/mTOR pathway. (A) Western blot detection of p-AMPK
(Thr172), AMPK, p-ACC (Ser79), ACC, p-mTOR (Ser2448), mTOR, p70S6K
(Thr389), p70S6K (Ser371), p-4EBP1 (Thr 37/46), LC3-I, LC3-II and
β-actin in T24 and 5637 cells treated with tetrandrine (Tet) for 24
h. Western blot detection of p-AMPK (Thr172), p-mTOR (Ser2448),
LC3-I, LC3-II and β-actin in T24 cells treated with Tet accompanied
by AMPK inhibitor compound C (Com C; 10 µM) (B) and activator AICAR
(1 mM) (C) for 24 h. |
To further confirm the role of AMPK in
tetrandrine-induced autophagy, AICAR (AMPK activator) and compound
C (AMPK inhibitor) were used for combination treatment with
tetrandrine. The results showed that AICAR further increased the
LC3-II levels and decreased the p-mTOR levels compared with
tetrandrine alone in the T24 cells (Fig. 3B). Reversely, compound C (Com C)
inhibited the high expression of LC3-II, while restored the low
expression of p-mTOR, suggesting that tetrandrine induced autophagy
by regulating the AMPK/mTOR signaling pathway (Fig. 3C).
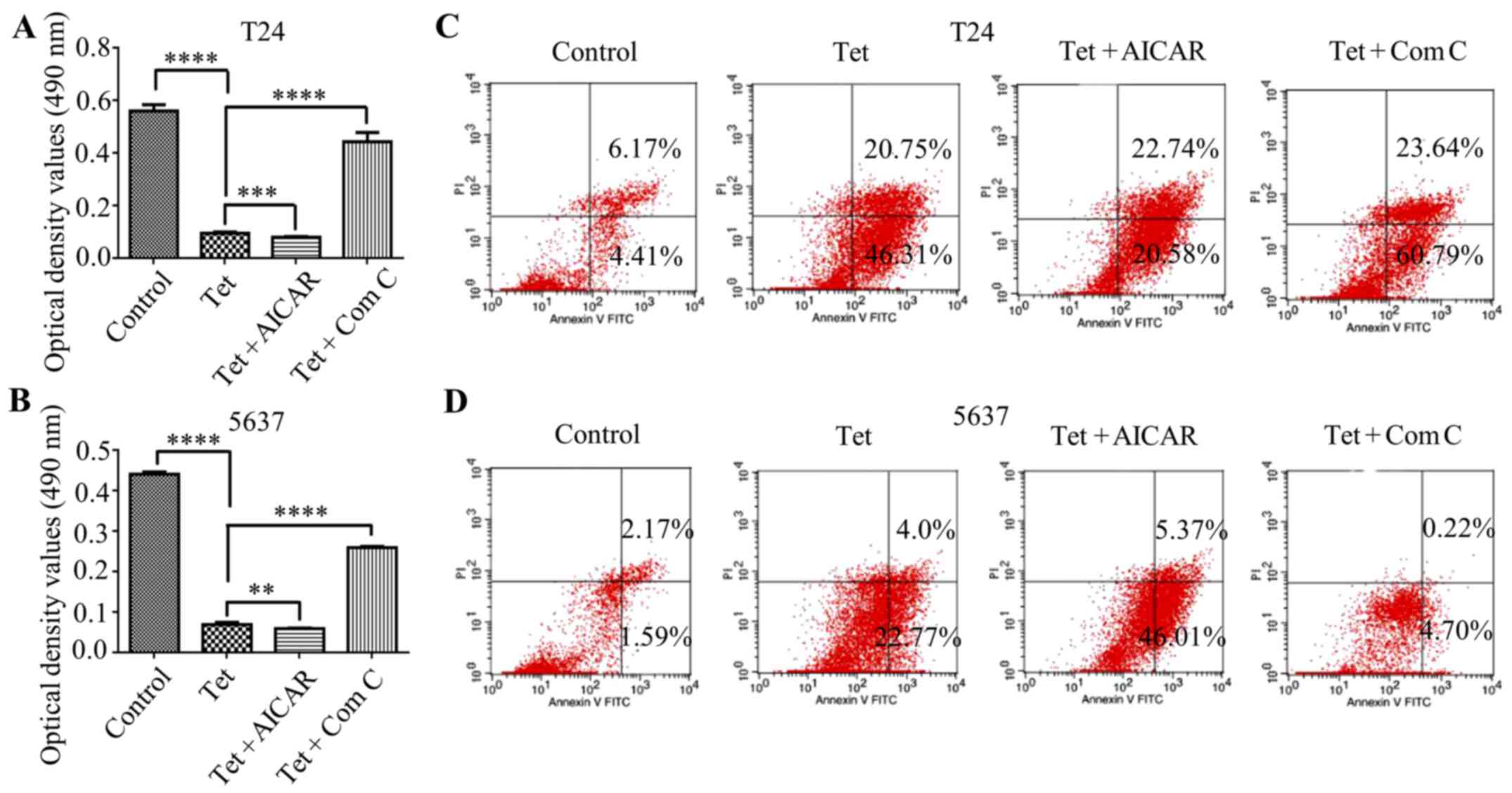
Autophagy induction enhances the
cytotoxic effect of tetrandrine in bladder cancer T24 and 5637
cells
The activation of autophagy has been shown to be
correlated with apoptosis induction. To address whether
AMPK-mediated autophagy contributes to tetrandrine-induced
autophagy, T24 and 5637 cells were treated with tetrandrine and
AICAR to explore the change of tetrandrine on the cytotoxic effect.
As expected, AICAR further enhanced the cytotoxic effect of
tetrandrine in the T24 (Fig. 4A)
and 5637 (Fig. 4B) cells. On the
contrary, compound C partially reversed the growth inhibition of
tetrandrine in these two bladder cancer cell lines (Fig. 4A and B). Consistent with the above
results, the subsequent analysis of flow cytometry showed that
AICAR reinforced the apoptosis induction of tetrandrine in the T24
(Fig. 4C) and 5637 (Fig. 4D) cells, while compound C had the
opposite effect (Fig. 4C and D).
Therefore, these results indicated that AMPK-mediated autophagy
contributed to tetrandrine-induced apoptosis in bladder cancer
cells.
Discussion
Numerous studies have demonstrated that tetrandrine
has promising capacity in cancer therapy, but the effect of
tetrandrine on human bladder cancer remains unknown. It has been
reported that tetrandrine induces apoptosis by triggering the
caspase cascade in bladder cancer T24 and 5637 cells (16). Yet, the underlying mechanism of
apoptosis induction by tetrandrine is still unclear. Accumulating
evidence indicates that autophagy is a dynamic cellular process
that involves the lysosomal degradation of broken proteins and
aging organelles to ensure cellular survival. In addition,
autophagy has been implicated in cancer progression. It was
demonstrated that tetrandrine induced autophagy in human
hepatocellular carcinoma via mitochondrial dysfunction, ROS
accumulation and activation of the extracellular signal-regulated
kinase (ERK) signaling pathway (23). In addition, tetrandrine induced
autophagy by triggering ROS generation and upregulating Notch1
signaling in acute premyelocytic leukemia cells (24). In contrast, tetrandrine was
identified as a potent lysosomal deacidification agent to block
autophagic flux in prostate cancer PC-3 and renal cell carcinoma
786-O cells (25). However, the
correlation between tetrandrine and autophagy in human bladder
cancer cells has not yet been confirmed. In the present study,
tetrandrine induced autophagic vacuoles in bladder cancer cells, as
evidenced by an increase in autophagic double-membrane structure
and fluorescent LC3 puncta. Moreover, analysis of LC3-II and p62
levels, and the subsequent LC3 turnover assay further confirmed
that autophagic flux could be induced by tetrandrine treatment.
Various signaling pathways have been implicated in
the regulation of autophagy. Research has shown that cobalt
chloride induced autophagy and apoptosis in glioma cells by the p53
pathway (26). Another study
revealed that isomahanine-induced autophagy was mediated by p38
mitogen-activated protein kinase (MAPK) signaling pathway in oral
squamous cell carcinoma cells (27). Among the various pathways, the AMPK
pathway has attracted great attention. AMPK is a vital kinase in
autophagy regulation, which senses cellular energy status to
maintain intercellular homeostasis (28). Research supports that AMPK plays a
crucial role in autophagy induction in response to various
stresses, such as starvation (29).
In the present study, tetrandrine markedly increased p-AMPK and
p-ACC in the T24 and 5637 cells. mTOR, a downstream substrate of
AMPK, negatively regulates autophagy. Research as also demonstrated
that hypoxia promotes autophagy in nucleus pulposus cells
independent of mTOR signaling (30). In the present study, the
phosphorylated levels of mTOR, 4E-BP1 and p70S6K were downregulated
upon tetrandrine treatment. Then, the combined treatment of
tetrandrine and AICAR or compound C in the subsequent assay
suggested that tetrandrine-induced autophagy was mediated by the
AMPK/mTOR signaling pathway.
It has been widely reported that autophagy is a
double-edged sword in tumor development and suppression (31). In response to adverse stress, such
as nutrient starvation, autophagy may be triggered for the
degradation of unnecessary molecules, serving as a potential
survival mechanism to maintain intercellular homeostasis, regulate
the immune response and remodel development (32,33).
Studies suggest that autophagy induction is a mechanism of
chemoresistance (34,35). On the contrary, numerous anticancer
agents, such as resveratrol, induce autophagic cell death,
indicating that autophagy may be a vital mechanism for anticancer
therapy (36). Results showed that
3-methyladenine (3-MA) and bafilomycin A1 partially restored the
antiproliferative effect of tetrandrine on human oral cancer cells
(37). In the present study, the
AMPK activator AICAR reinforced the growth inhibition and apoptosis
induction of tetrandrine in T24 and 5637 cells, while compound C
protected tetrandrine-treated bladder cancer cells against a
decrease in cell viability, indicating that AMPK-mediated autophagy
contributed to tetrandrine-induced apoptosis in human bladder
cancer cells.
In conclusion, our findings indicate that activation
of AMPK signaling is critical for tetrandrine-induced autophagy in
bladder cancer cells, which enhances the apoptosis induction of
tetrandrine. Tetrandrine may be an alternative anticancer candidate
for the treatment of bladder cancer, and autophagy may be a
possible mechanism for cancer therapy.
Acknowledgements
The present study was partially supported by the
National Natural Science Foundation of China (no. 81602562), the
Fundamental Research Funds for the Central University of Xi'an
Jiaotong University (no. 1191329722), and the Institutional
Scientific Development Foundation of the First Affiliated Hospital
of Xi'an Jiaotong University (no. 2015YK17).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panebianco V, De Berardinis E, Barchetti
G, Simone G, Leonardo C, Grompone MD, Del Monte M, Carano D,
Gallucci M, Catto J, et al: An evaluation of morphological and
functional multi-parametric MRI sequences in classifying non-muscle
and muscle invasive bladder cancer. Eur Radiol. 27:3759–3766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A;
European Association of Urology, : EAU guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2013
guidelines. Eur Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan X, Tong B, Dou Y, Wu X, Wei Z and Dai
Y: Tetrandrine ameliorates collagen-induced arthritis in mice by
restoring the balance between Th17 and Treg cells via the aryl
hydrocarbon receptor. Biochem Pharmacol. 101:87–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Yu B, Zhang XQ, Sheng ZF, Li SJ,
Wang ZJ, Cui XY, Cui SY and Zhang YH: Tetrandrine, an
antihypertensive alkaloid, improves the sleep state of
spontaneously hypertensive rats (SHRs). J Ethnopharmacol.
151:729–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye Z, Van Dyke K and Rossan RN: Effective
treatment with a tetrandrine/chloroquine combination for
chloroquine-resistant falciparum malaria in Aotus monkeys. Malar J.
12:1172013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang OH, An HJ, Kim SB, Mun SH, Seo YS,
Joung DK, Choi JG, Shin DW and Kwon DY: Tetrandrine suppresses
pro-inflammatory mediators in PMA plus A23187-induced HMC-1 cells.
Int J Mol Med. 33:1335–1340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu NF, Luo CH, Wu JC, Zheng YY, Gan YJ,
Ling JA, Liang HQ, Liang DY, Xie J, Chen XQ, et al: Clearance of
free silica in rat lungs by spraying with chinese herbal kombucha.
Evid Based Complement Alternat Med. 2013:7907922013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Lu X, Xu H, Zhu Z, Yin H, Qian X, Li
R, Jiang X and Liu B: Paclitaxel/tetrandrine coloaded nanoparticles
effectively promote the apoptosis of gastric cancer cells based on
‘oxidation therapy’. Mol Pharm. 9:222–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu JM, Chen Y, Chen JC, Lin TY and Tseng
SH: Tetrandrine induces apoptosis and growth suppression of colon
cancer cells in mice. Cancer Lett. 287:187–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Liu W, Wang K, Fan Y, Chen J, Ma
J, Wang X, He D, Zeng J and Li L: Tetrandrine inhibits migration
and invasion of human renal cell carcinoma by regulating
Akt/NF-κB/MMP-9 signaling. PLoS One. 12:e01737252017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Q, Kang C, Soh Y, Sohn NW, Lee J, Cho
YH, Baik HH and Kang I: Tetrandrine cytotoxicity and its dual
effect on oxidative stress-induced apoptosis through modulating
cellular redox states in Neuro 2a mouse neuroblastoma cells. Life
Sci. 71:2053–2066. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Li F, Xu T and Sun J: Tetrandrine
prevents multidrug resistance in the osteosarcoma cell line, U-2OS,
by preventing Pgp overexpression through the inhibition of NF-κB
signaling. Int J Mol Med. 39:993–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–e51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ravegnini G, Sammarini G, Nannini M,
Pantaleo MA, Biasco G, Hrelia P and Angelini S: Gastrointestinal
stromal tumors (GIST): Facing cell death between autophagy and
apoptosis. Autophagy. 13:452–463. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galluzzi L, Pietrocola F, Levine B and
Kroemer G: Metabolic control of autophagy. Cell. 159:1263–1276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Z, Zhao J, Xue J, Zhao X and Liu P:
Autophagy inhibition promotes epithelial-mesenchymal transition
through ROS/HO-1 pathway in ovarian cancer cells. Am J Cancer Res.
6:2162–2177. 2016.PubMed/NCBI
|
|
20
|
Zhang Y, Liu W, He W, Zhang Y, Deng X, Ma
Y, Zeng J and Kou B: Tetrandrine reverses epithelial-mesenchymal
transition in bladder cancer by downregulating Gli-1. Int J Oncol.
48:2035–2042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hewitt G, Carroll B, Sarallah R,
Correia-Melo C, Ogrodnik M, Nelson G, Otten EG, Manni D, Antrobus
R, Morgan BA, et al: SQSTM1/p62 mediates crosstalk between
autophagy and the UPS in DNA repair. Autophagy. 12:1917–1930. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carling D: AMPK signalling in health and
disease. Curr Opin Cell Biol. 45:31–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong K, Chen C, Zhan Y, Chen Y, Huang Z
and Li W: Autophagy-related gene 7 (ATG7) and reactive oxygen
species/extracellular signal-regulated kinase regulate
tetrandrine-induced autophagy in human hepatocellular carcinoma. J
Biol Chem. 287:35576–35588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Men Q, Wu G, Yu C, Huang Z, Liu X
and Li W: Tetrandrine induces autophagy and differentiation by
activating ROS and Notch1 signaling in leukemia cells. Oncotarget.
6:7992–8006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu W, Su M, Xie F, Ai J, Ren Y, Zhang J,
Guan R, He W, Gong Y and Guo Y: Tetrandrine blocks autophagic flux
and induces apoptosis via energetic impairment in cancer cells.
Cell Death Dis. 5:e11232014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng BC, Chen JT, Yang ST, Chio CC, Liu
SH and Chen RM: Cobalt chloride treatment induces autophagic
apoptosis in human glioma cells via a p53-dependent pathway. Int J
Oncol. 50:964–974. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Utaipan T, Athipornchai A, Suksamrarn A,
Chunsrivirot S and Chunglok W: Isomahanine induces endoplasmic
reticulum stress and simultaneously triggers p38 MAPK-mediated
apoptosis and autophagy in multidrug-resistant human oral squamous
cell carcinoma cells. Oncol Rep. 37:1243–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hardie DG: AMP-activated/SNF1 protein
kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vingtdeux V, Giliberto L, Zhao H,
Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies
P, et al: AMP-activated protein kinase signaling activation by
resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem.
285:9100–9113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi H, Merceron C, Mangiavini L, Seifert
EL, Schipani E, Shapiro IM and Risbud MV: Hypoxia promotes
noncanonical autophagy in nucleus pulposus cells independent of
MTOR and HIF1A signaling. Autophagy. 12:1631–1646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
White E, Mehnert JM and Chan CS:
Autophagy, metabolism, and cancer. Clin Cancer Res. 21:5037–5046.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng Y, Ren X, Hait WN and Yang JM:
Therapeutic targeting of autophagy in disease: Biology and
pharmacology. Pharmacol Rev. 65:1162–1197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Höhn A and Grune T: Lipofuscin: Formation,
effects and role of macroautophagy. Redox Biol. 1:140–144. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim M, Jung JY, Choi S, Lee H, Morales LD,
Koh JT, Kim SH, Choi YD, Choi C, Slaga TJ, et al: GFRA1 promotes
cisplatin-induced chemoresistance in osteosarcoma by inducing
autophagy. Autophagy. 13:149–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang
C, Wang F, Zhang CY, Zen K and Li L: MiR-26 enhances
chemosensitivity and promotes apoptosis of hepatocellular carcinoma
cells through inhibiting autophagy. Cell Death Dis. 8:e25402017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang AC, Lien JC, Lin MW, Yang JS, Wu PP,
Chang SJ and Lai TY: Tetrandrine induces cell death in SAS human
oral cancer cells through caspase activation-dependent apoptosis
and LC3-I and LC3-II activation-dependent autophagy. Int J Oncol.
43:485–494. 2013. View Article : Google Scholar : PubMed/NCBI
|