Introduction
Human gastric cancer (GC) is the fifth most common
cancer and the second leading cause of cancer-related deaths
worldwide, accounting for approximately 10% of newly diagnosed
cancers, although the incidence and mortality rates have generally
declined during the past few decades (1). The development of optimal strategies
for the treatment of GC is a major focus of current clinical
research. Numerous efforts have been made to identify microRNAs
(miRNAs/miRs) involved in the occurrence and development of GC
(2,3), and evidence to date suggests that
miRNAs involved in the multifaceted and complex mechanisms of GC
are primarily responsible for its metabolism.
miRNAs are a class of endogenous noncoding RNA
molecules of approximately 19–25 nucleotides in length that are
cleaved from 70–100-nucleotide hairpin precursors (pre-miRNAs).
Although miRNAs only account for approximately 1% of all expressed
human genes, accumulating evidence indicates that miRNAs may
regulate the expression of nearly one-third of all human genes, and
may play important roles in cell growth, proliferation,
differentiation and death (4,5).
Recently, evidence has indicated that alterations in miRNA levels,
resulting from mutation or aberrant expression, are associated with
various human types of cancers (6).
Moreover, numerous miRNAs are aberrantly expressed in human types
of cancer, including GC.
miR-133b was initially considered to be a
muscle-specific miRNA and was revealed to be involved in the
development of skeletal muscle (7).
Many studies have now shown that miR-133b is downregulated and
inhibits cell proliferation, migration and invasion while promoting
cell apoptosis in human malignancies, such as osteosarcoma
(8), colorectal (9), lung (10) and bladder cancer (11); however, its molecular mechanism of
action and target genes in tumor development remain to be
elucidated. In the present study we aimed to reveal the novel
regulatory mechanism of miR-133b in the development of GC, with the
hope of providing a new approach for clinical treatment.
In this study, we detected differences in the
expression levels of miR-133b in human gastric tissues compared
with adjacent non-tumor tissues using reverse
transcription-quantitative PCR (RT-qPCR), and confirmed the role of
miR-133b as a tumor suppressor. As expected, the same result was
found in GC cells (MKN-74 and MGC80-3) compared with gastric
epithelial cells (GES-1). miRNA-133b mimics and
miRNA-133b-inhibitors were used to stimulate the overexpression and
knockdown of miR-133b, respectively, and we subsequently identified
that ATP citrate lyase (ACLY) was regulated by miR-133b and
expressed at a higher level in GC tissues and cell lines. Notably,
when ACLY was inhibited by the overexpression of miRNA-133b, MKN-74
cells exhibited decreased proliferative and invasive capacities.
Furthermore, this process was found to be regulated by the
activation of peroxisome proliferator-activated receptor-γ (PPARγ),
a nuclear transcription factor strongly associated with cancer
occurrence and development. These findings contribute to our
understanding of the tumor-suppressive function of miR-133b.
Materials and methods
Cell culture
All cell lines were obtained from the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China). Human GC cell
lines MGC80-3 and MKN-74, gastric epithelial cell line GES-1 were
maintained in RPMI-1640 medium (11875085) and Dulbecco's modified
Eagle's medium (DMEM; 11995065) (both from Gibco, Grand Island, NY,
USA) respectively, and supplemented with 10% (v/v) fetal bovine
serum, 100 U/ml penicillin and 100 mg/ml streptomycin. Cell culture
was conducted at 37°C in a humidified 5 % CO2 incubator.
Cell transfection was performed with a Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) following the
manufacturer's protocol. Briefly, cells were plated at a density of
2×105 cells/well in 6-well plates, cultured overnight,
then transfected with 100 pmol (final 50 nM) of either scramble,
miR-133b-mimic (MC10029, catalog no. 4464066) or miR-133b-inhibitor
(MC10029, cat. no. 4464084; both from Thermo Fisher Scientific,
Inc., Waltham, MA, USA) using the Lipo 2000. Forty-eight hours
later, total protein and RNA samples were prepared; luciferase
assay and cell activity assay were carried out as described below.
Rosiglitazone (S2556) 1 µM and T0070907 (S2871) 1 µM were purchased
from Selleck Chemicals (Shanghai, China) and added to the medium 24
h before harvesting.
Clinical specimens
Human GC tissue and adjacent non-tumor tissue were
obtained from patients diagnosed with colon adenocarcinoma at the
Department of General Surgery, Second Affiliated Hospital of Anhui
Medical University. Stage of disease was reported according to TNM
classification (12). The specimens
were obtained after surgical resection and immediately frozen at
−80°C until use. The study methodologies conformed to the standards
set by the Declaration of Helsinki. Collection and usage of all
specimens were approved by the Ethics Committee of The Second
Affiliated Hospital of Anhui Medical University.
RT-qPCR
Total RNA was extracted from tissues and cultured
cells using TRIzol® reagent (Invitrogen Life
Technologies). DNaseI-treated RNA was used for first strand cDNA
synthesis using M-MLV reverse transcriptase (Promega Corp.,
Madison, WI, USA) and oligo (dT)13 according to the
manufacturer's protocols and 1 µl cDNA samples were used for
conventional PCR amplifications. Real-time quantitative PCR
analysis was performed on a real-time PCR system (StepOne; Applied
Biosystems Life Technologies, Foster City, CA, USA) and the
expression levels of ACLY were normalized to GAPDH determined by a
SYBR Green-based comparative cycle threshold (CT) method. Real-time
PCR primers were: ACLY forward, 5′-GACTTCGGCAGAGGTAGAGC-3′, and
reverse, 5′-TCAGGAGTGACCCGAGCATA-3′; GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′; miR-133b forward,
5′-AAGAAAGATGCCCCCTGCTC-3′, and reverse,
5′-GTAGCTGGTTGAAGGGGACC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Cell lysis and immunoblotting
To obtain total protein lysates, cells were
homogenized and dissolved in RIPA buffer [150 mmol/l NaCl, 1.0%
Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mmol/l Tris
(pH 8.0)] containing proteinase inhibitors and phosphatase
inhibitors. The protein concentration of each lysate was determined
using a BCA protein assay kit (Beyotime, Shanghai, China). The
total cell lysate was applied on 10% SDS-PAGE. After
electrophoresis, the proteins were transferred electrophoretically
from the gel to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The membranes were then blocked for 1 h in
blocking buffer (5% low-fat dried milk in TBS) and probed with the
primary antibodies overnight at 4°C. After washing, horseradish
peroxidase-conjugated secondary antibodies were incubated with the
membranes at room temperature for 1 h, followed by enhanced
chemiluminescence (Amersham). The primary antibodies used were
raised against ACLY (1:1,000 dilution; ab40793), PPARγ (1:1,000
dilution; ab209350) and β-actin (1:3,000 dilution; ab8227), and
were purchased from Abcam (Cambridge, MA, USA).
ACLY activity assay
ACLY activity was assessed via the malate
dehydrogenase-coupled method (13).
RIPA whole-cell lysates were added at a 1:19 ratio to the reaction
mixture containing 100 mM Tris-HCl (pH 8.7), 20 mM potassium
citrate, 10 mM MgCl2, 10 mM DTT, 0.5 U/ml malate
dehydrogenase, 0.33 mM CoASH, 0.14 mM NADH, and 5 mM ATP (all from
Sigma-Aldrich, St. Louis, MO, USA). The change in absorbance at 340
nm was read every 15 sec over 35 min on a SpectraMax 190
spectrophotometer. Change in absorbance in the absence of exogenous
ATP was subtracted from change in the presence of ATP and was
normalized to protein concentration to determine the specific ACLY
activity.
Cell proliferation assay
Cell proliferation was assessed using the MTT assay.
MTT was diluted in phosphate-buffered saline (PBS) to a final
concentration of 5 mg/ml and sterile filtered. Cells were incubated
with a final concentration of 5 µg/ml MTT at 37°C for 4 h. Cell
culture supernatants were carefully removed, and DMSO was added.
The absorbance values were determined using a microplate reader
(MK3, Thermo Fisher Scientific) at a wavelength of 570 nm. The
experiments were performed three times.
Dual-luciferase reporter assay
Luciferase reporter assays were performed in MKN-74
cells. Renilla luciferase (200 ng), luci-ACLY (300 ng), and equal
amounts (200 pmol) of miR-133b-mimic, miR-133b-inhibitor or
scramble control were transfected into cells in 24-well plates with
Lipo 2000. Forty-eight hours after transfection, a luciferase assay
was performed with a Dual-Luciferase Reporter Assay system (E1910;
Promega). The luciferase activity was assessed using a Victor
Luminometer (Perkin Elmer, Inc., Waltham, MA, USA). The firefly
luciferase activity was normalized using co-transfected Renilla
luciferase for transfection efficiency. All experiments were
performed in triplicate.
Statistical analysis
Data were presented as the means ± SE. Data between
groups were analyzed by Student's t-test or one-way ANOVA followed
by Bonferroni-Dunn multiple comparison. P<0.05 was considered as
statistically significant.
Results
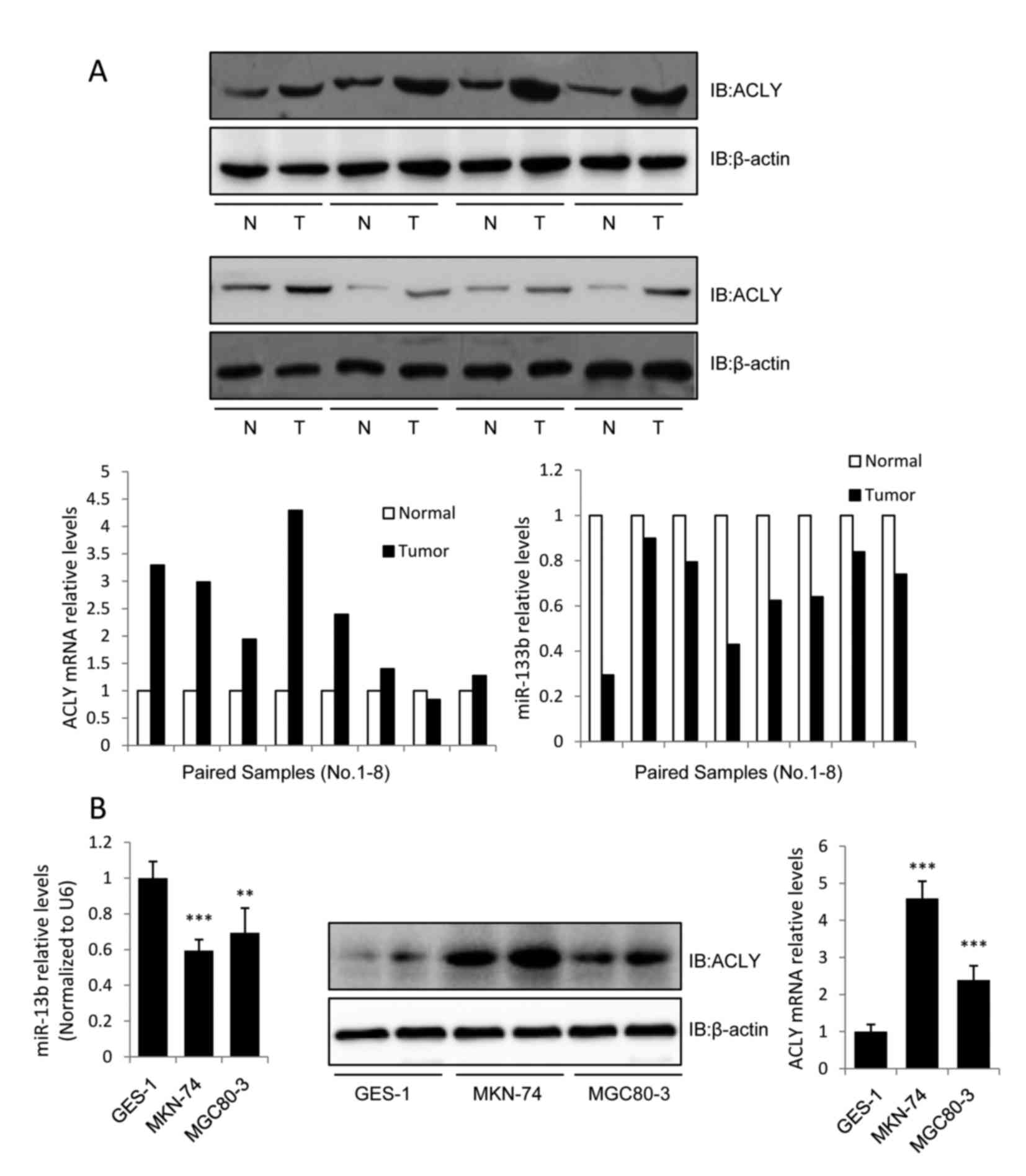
miR-133b is downregulated while ACLY
is upregulated in human GC
To evaluate the association between miR-133b and
ACLY in human GC tissues, we assessed the expression levels of
miR-133b and ACLY in 8 pairs of GC tissue samples using RT-qPCR and
western blot analysis. The 8 GC cases were classified as IV, IIIB,
IIA, IIIA, IIB, IIA, IIIA, and IIIC, respectively, according to the
seventh edition of the AJCC staging system. The results revealed
that miR-133b expression was downregulated while the mRNA and
protein expression levels of ACLY were upregulated in all of the
screened GC tissues (Fig. 1A). We
also investigated the expression levels of miR-133b and ACLY in GC
cell lines. Compared with the normal gastric epithelial cell line
GES-1, miR-133b was downregulated in the GC cell lines MKN-74 and
MGC80-3, while the protein and mRNA levels of ACLY were upregulated
(Fig. 1B).
Previous evaluations of ACLY expression in human
lung, prostate, bladder, breast, liver, stomach, and colon tumors
have identified increased expression levels of this enzyme when
compared with normal tissues (14,15).
Consistent with these previous results, we observed a high-level of
expression of the lipogenic enzyme ACLY in the GC tissues and cell
lines. These data demonstrated that ACLY may be involved in the
tumor-suppressive effect of miR-133b in GC. As MKN-74 cells
expressed higher levels of ACLY than MGC80-3 cells (Fig. 1B), MKN-74 cells were used in
subsequent assays to investigate the correlation between ACLY and
miR-133b.
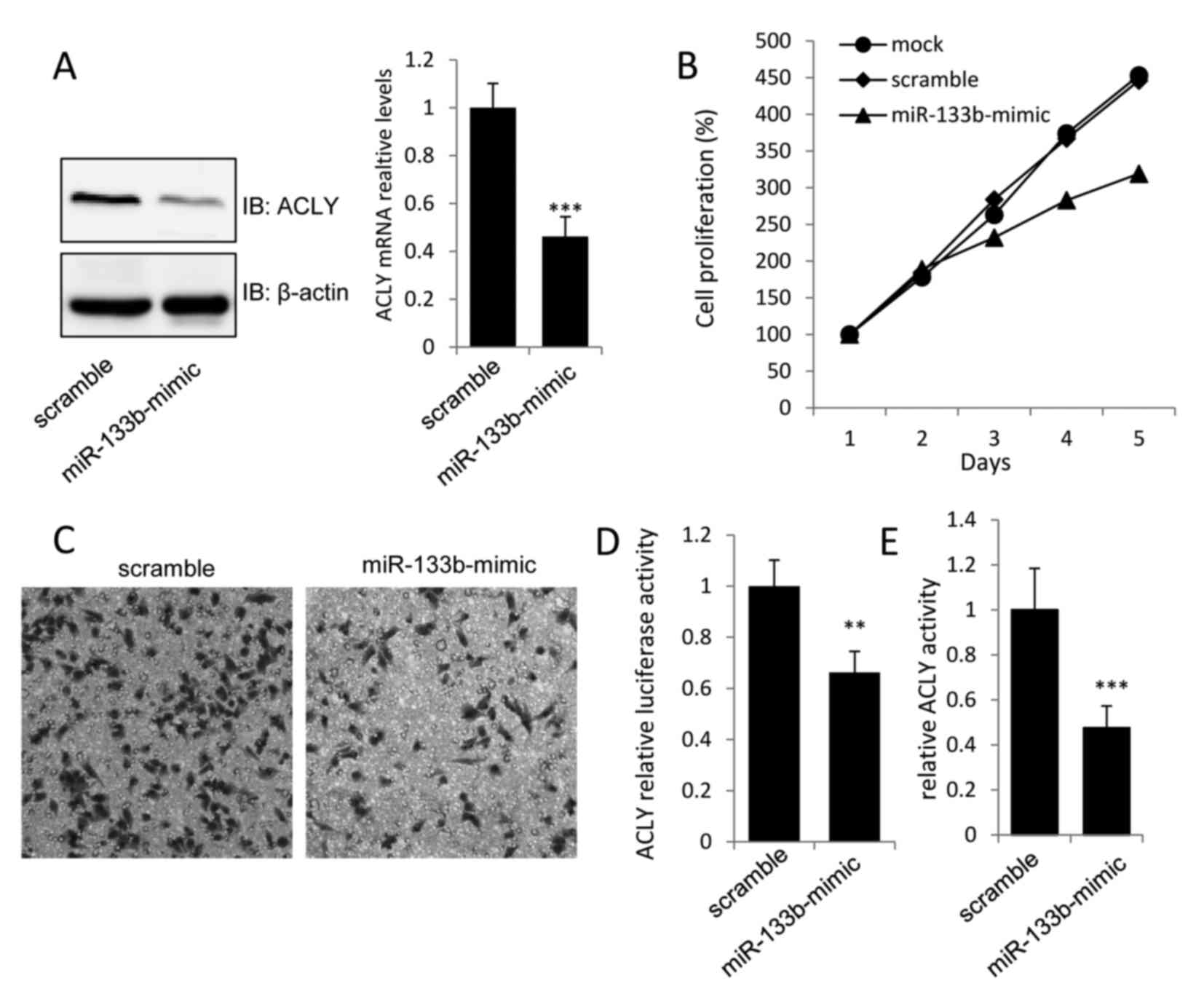
Overexpression of miR-133b suppresses
GC cell proliferation by inhibiting ACLY
To evaluate the biological significance of miR-133b
in GC cell proliferation, miR-133b-mimics were transfected into
MKN-74 cells. The overexpression of miR-133b was detected by
real-time PCR, and was found to induce ~0.6- and 0.45-fold
decreases in the protein and mRNA levels of ACLY, respectively, in
miR-133b-mimic transfected MKN-74 cells when compared with the
vector control (Fig. 2A). To assess
the effects of miR-133b overexpression on the proliferation and
invasion of MKN-74 cells, MTT and Transwell assays were performed
48 h after transfection. As shown in Fig. 2B and C, transfection with
miR-133b-mimic caused a marked decrease in the growth and invasion
of MKN-74 cells. Moreover, the dual-luciferase and ACLY activity
assays revealed that transfection of miR-133b-mimic into MKN-74
cells could inhibit the luciferase activity of the luci-ACLY-3′-UTR
construct, as well as decrease ACLY activity (Fig. 2D and E). These findings provided
evidence that miR-133b could directly regulate the expression and
activation of ACLY to suppress gastric tumor growth.
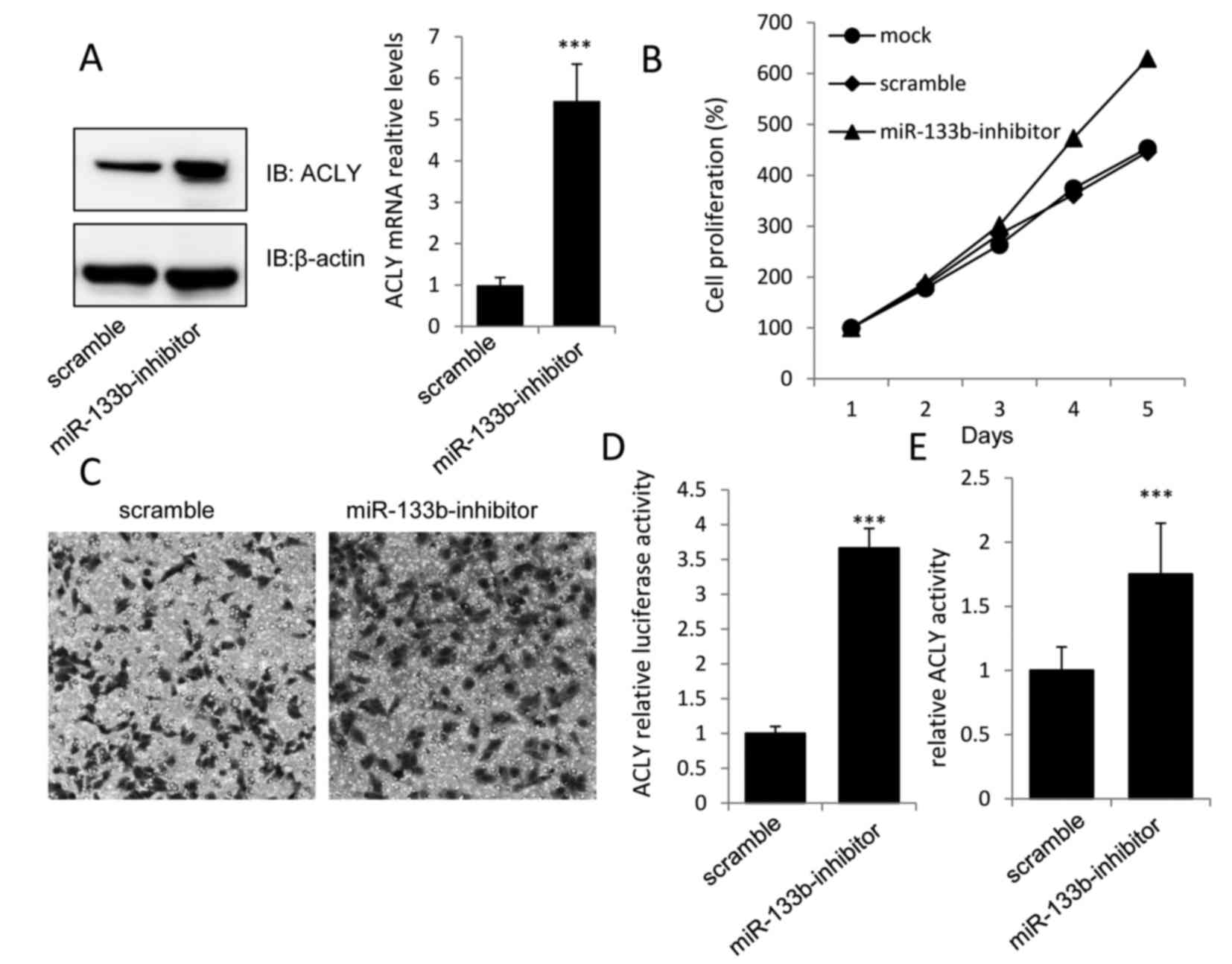
Knockdown of miR-133b promotes GC cell
proliferation by increasing ACLY expression and activation
To further validate whether the increased expression
of miR-133b in MKN-74 cells mediated the downregulation in ACLY,
the functional significance of miR-133b knockdown in MKN-74 cells
was investigated. The results revealed that the miR-133b-inhibitor,
but not the scramble miR, could markedly increase the expression of
ACLY at both the mRNA and protein levels (Fig. 3A).
As predicted, increased levels of cell proliferation
and invasion were observed in MKN-74 cells transfected with the
miR-133b-inhibitor when compared with the scramble group (Fig. 3B and C). Additionally, the
luciferase activity of the ACLY-3′-UTR construct and the relative
ACLY activity were significantly higher in the miR-133b-inhibitor
group than in the scramble group (Fig.
3D and E), which further demonstrated that ACLY may play an
essential role in the tumor-suppressive effect of miR-133b in
MKN-74 cells.
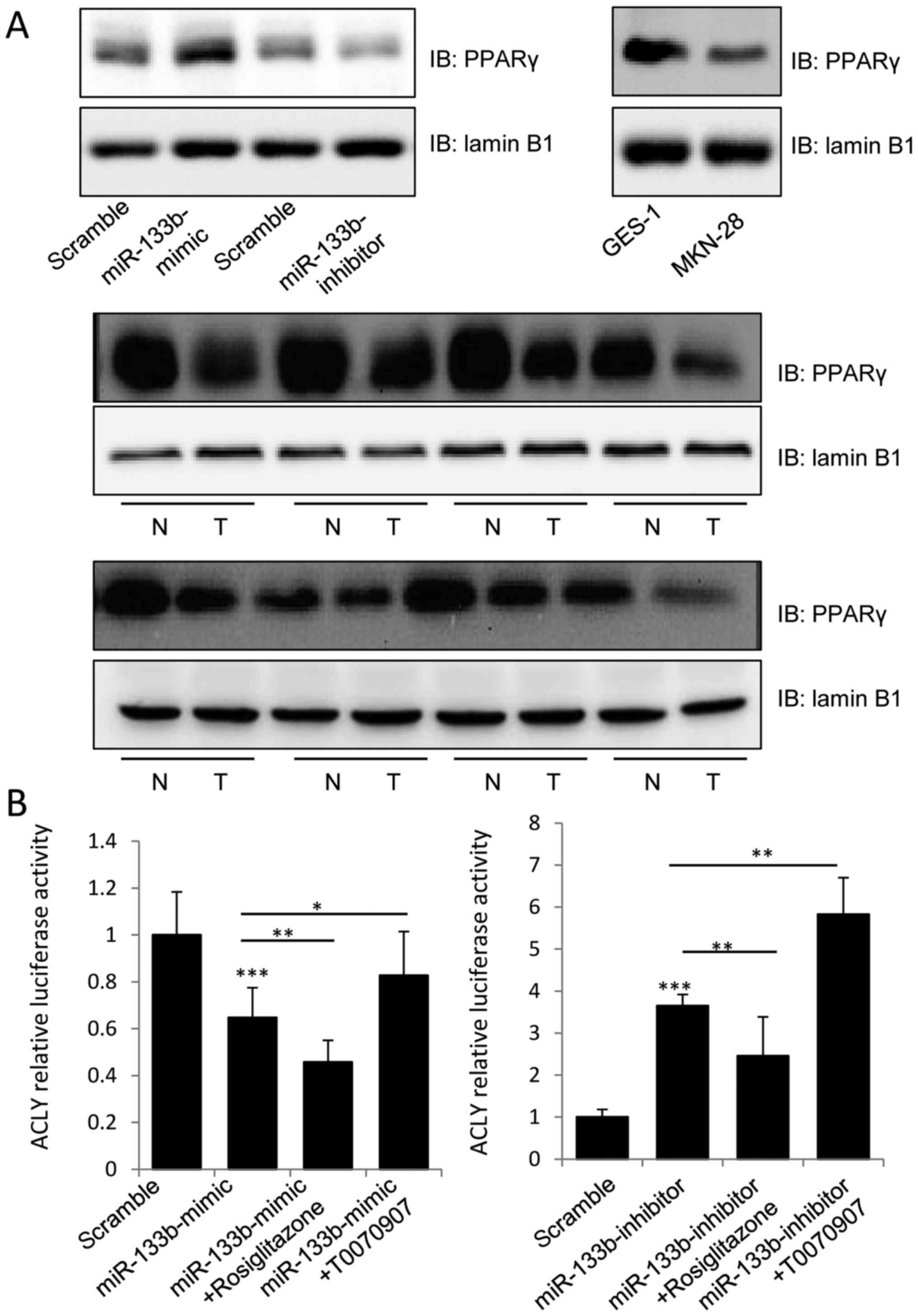
miR-133b regulates the expression of
PPARγ and its transactivation of ACLY
PPARγ is expressed at high levels in primary colon
tumors and colon cancer cell lines, and its agonists have been
reported to decrease the growth and induce the differentiation of
malignant breast epithelial cells (16,17).
Therefore, we aimed to determine whether miR-133b inhibited the
activation of ACLY by regulating PPARγ expression. Notably,
overexpression of miR-133b was found to stimulate the expression of
PPARγ in the nucleus, and knockdown of miR-133b inhibited this
phenomenon (Fig. 4A). We further
evaluated PPARγ expression in the GES-1 and MKN-74 cell lines and
GC tissues, and found lower levels of PPARγ in the nuclear lysates
of MKN-74 cells and tumor tissues samples, which was consistent
with previous research, suggesting that miR-133b may induce PPARγ
expression to inhibit tumor growth.
To further assess whether miR-133b inhibited the
expression of ACLY in a PPARγ-dependent manner, a dual-luciferase
assay was used to detect the transcriptional activity of the
ACLY3′UTR. As shown in Fig. 4B, 1
µM of rosiglitazone (PPARγ agonist) could significantly enhance the
inhibitory effect of miR-133b-mimic on the luciferase activity of
the luci-ACLY 3′UTR construct, while T0070907 (PPARγ inhibitor)
could significantly attenuate the inhibitory effect of the
miR-133b-mimic. By contrast, rosiglitazone suppressed the
stimulatory effect of the miR-133b-inhibitor on the transcriptional
activity of the ACLY-3′UTR, while T0070907 enhanced the stimulatory
effect of miR-133b-inhibitor. Our data clearly indicated that
miR-133b decreased the proliferation of GC cells by targeting the
expression of ACLY in a PPARγ-dependent manner.
Discussion
miRNAs are endogenous non-coding RNAs that interact
with the 3′UTRs of target mRNAs to induce mRNA cleavage, which
enables miRNAs to regulate protein expression at the
transcriptional level (18). In
recent years, it has been reported that miRNAs can alter the
expression of tumor-suppressor genes and oncogenes, thus
implicating them in the regulation of tumor development and
progression. Therefore, the identification of cancer-related miRNAs
and their targets is essential to understand their roles in
tumorigenesis, and may be important for diagnosis and targeted
therapeutic treatment.
miR-133b is located on chromosome 18 in the same
bicistronic unit as miR-133 (19).
miR-133b has long been recognized as a muscle-specific miRNA that
may regulate myoblast differentiation and participate in many
myogenic diseases and in myocardial infarction (20,21).
Recently, researchers found that miR-133b was significantly
downregulated in a number of types of cancer, such as colorectal
cancer, non-small-lung cancer, and GC (5,22–24),
but its target genes remain unclear. Therefore, we focused on the
downstream target genes of miR-133b and aimed to identify the
molecular mechanism underlying its tumor-suppressive effect on GC,
since it is the fifth most common malignancy worldwide and the
second most common cancer in China (25).
PPARγ is a ligand-activated transcription factor
that belongs to the nuclear hormone receptor superfamily (26). Activation of PPARγ results in the
inhibition of tumor growth in various types of cancer, and
activating ligands of PPARγ have been shown to induce
differentiation and inhibit tumor growth in multiple tumor types
(27). The miRNA miR-122 can
regulate the PPARc/RXRa complex through interactions with
corepressors and H3K9 histone methyltransferase in hepatocellular
carcinoma cells (28). In the
present study we investigated whether miR-133b could regulate the
expression of PPARγ to inhibit tumor growth in GC and, notably, we
demonstrated that PPARγ expression was increased by miR-133b
overexpression in GC cells.
As a cytosolic enzyme that catalyzes the generation
of acetyl CoA from citrate, ACLY has been found to be involved in
cancer progression, potentially via its regulatory role in cancer
cell metabolism (15). It has been
reported that ACLY expression and activity are markedly increased
in lung, prostate, bladder, breast, liver, stomach, and colon
tumors (14,29). Moreover, ACLY inhibition by siRNAs
or the selective inhibitor SB-204990 has been found to suppress the
growth and survival of tumor cells in vitro and in
vivo (30).
In the present study we detected increased
expression of ACLY in the GC cell lines MKN-74 and MGC80-3 at both
the mRNA and protein levels when compared with the gastric
epithelial cell line GES-1. Notably, this upregulation was
concomitant with decreased expression levels of miR-133b. The in
vivo findings in GC tissues were consistent with the in
vitro data. Our subsequent observations indicated that the
exogenous expression of miR-133b in MKN-74 cells could inhibit cell
proliferation and invasion, which was accompanied by decreased
expression of ACLY at both the mRNA and protein levels.
Furthermore, the essential role of ACLY in miR-133b-induced tumor
suppression was implicated by the findings that knockdown of
miR-133b could increase ACLY activity and the growth and invasive
capacities of MKN-74 cells in vitro. These findings revealed
that ACLY could be a critical downstream target of the tumor
suppressor activity of miR-133b in GC.
To further elucidate the molecular mechanism of
miR-133b with regard to its inhibition of ACLY in GC, the
transcription of PPARγ in the nucleus was analyzed by western
blotting, which indicated that miR-133b could induce PPARγ
expression to inhibit tumor growth. A luciferase assay also
revealed that transfection with miR-133b could decrease the
transcriptional activity of the ACLY 3′UTR in a PPARγ-dependent
manner within MKN-74 cells, suggesting that the apparent
tumor-suppressive effect of miR-133b may be mediated by PPARγ, thus
enabling it to regulate the expression of ACLY in GC cells.
Our present study provides evidence that
manipulation of the miR-133b/ACLY axis may be a novel strategy for
the treatment of GC. However, the extent to which the miR-133b/ACLY
axis functionally aids in tumor suppression in GC requires further
investigation.
Acknowledgements
This study was supported by the Provincial Teaching
Research Project of Anhui Province (2014jyxm704) and the Natural
Science Foundation of Anhui Province China (1708085MH213).
References
|
1
|
Murray CJ and Lopez AD: Alternative
projections of mortality and disability by cause 1990–2020: Global
Burden of Disease Study. Lancet. 349:1498–1504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo J, Miao Y, Xiao B, Huan R, Jiang Z,
Meng D and Wang Y: Differential expression of microRNA species in
human gastric cancer versus non-tumorous tissues. J Gastroenterol
Hepatol. 24:652–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:pp. 2257–2261.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bandrés E, Cubedo E, Agirre X, Malumbres
R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M, et
al: Identification by Real-time PCR of 13 mature microRNAs
differentially expressed in colorectal cancer and non-tumoral
tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong X, Coukos G and Zhang L: miRNAs in
human cancerNext-Generation MicroRNA Expression Profiling
Technology: Methods and Protocols, Methods in Molecular Biology.
Fan JB: 822. Humana Press; New York: pp. 295–306. 2012, View Article : Google Scholar
|
|
7
|
Chiba Y, Tanabe M, Goto K, Sakai H and
Misawa M: Down-regulation of miR-133a contributes to up-regulation
of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care
Med. 180:713–719. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated in human osteosarcoma and
inhibits osteosarcoma cells proliferation, migration and invasion,
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu G, Chen D, Li X, Yang K, Wang H and Wu
W: miR-133b regulates the MET proto-oncogene and inhibits the
growth of colorectal cancer cells in vitro and in vivo. Cancer Biol
Ther. 10:190–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crawford M, Batte K, Yu L, Wu X, Nuovo GJ,
Marsh CB, Otterson GA and Nana-Sinkam SP: MicroRNA 133B targets
pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem
Biophys Res Commun. 388:483–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han Y, Chen J, Zhao X, Liang C, Wang Y,
Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al: MicroRNA expression
signatures of bladder cancer revealed by deep sequencing. PLoS One.
6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Hoboken, NJ: 2009
|
|
13
|
Srere PA: The citrate cleavage enzyme. I.
Distribution and purification. J Biol Chem. 234:2544–2547.
1959.PubMed/NCBI
|
|
14
|
Migita T, Narita T, Nomura K, Miyagi E,
Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y,
et al: ATP citrate lyase: Activation and therapeutic implications
in non-small cell lung cancer. Cancer Res. 68:8547–8554. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaidi N, Swinnen JV and Smans K:
ATP-citrate lyase: A key player in cancer metabolism. Cancer Res.
72:3709–3714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mueller E, Sarraf P, Tontonoz P, Evans RM,
Martin KJ, Zhang M, Fletcher C, Singer S and Spiegelman BM:
Terminal differentiation of human breast cancer through PPARγ. Mol
Cell. 1:465–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DuBois RN, Gupta R, Brockman J, Reddy BS,
Krakow SL and Lazar MA: The nuclear eicosanoid receptor, PPARγ, is
aberrantly expressed in colonic cancers. Carcinogenesis. 19:49–53.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kano M, Seki N, Kikkawa N, Fujimura L,
Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M and
Matsubara H: miR-145, miR-133a and miR-133b: Tumor-suppressive
miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J
Cancer. 127:2804–2814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams AH, Liu N, van Rooij E and Olson
EN: MicroRNA control of muscle development and disease. Curr Opin
Cell Biol. 21:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boštjančič E, Zidar N, Štajer D and Glavač
D: MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated
in human myocardial infarction. Cardiology. 115:163–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiang KM and Li XR: MiR-133b acts as a
tumor suppressor and negatively regulates TBPL1 in colorectal
cancer cells. Asian Pac J Cancer Prev. 15:3767–3772. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Shao X, Gao W, Zhang Z, Liu P, Wang
R, Huang P, Yin Y and Shu Y: MicroRNA-133b inhibits the growth of
non-small-cell lung cancer by targeting the epidermal growth factor
receptor. FEBS J. 279:3800–3812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen D, Li S, Ji F, Cao H, Jiang W, Zhu J
and Fang X: miR-133b acts as a tumor suppressor and negatively
regulates FGFR1 in gastric cancer. Tumour Biol. 34:793–803. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stewart B and Wild CP: World cancer report
2014. International Agency for Research on Cancer (IARC); Lyon,
France: 2015
|
|
26
|
Lemberger T, Desvergne B and Wahli W:
Peroxisome proliferator-activated receptors: A nuclear receptor
signaling pathway in lipid physiology. Annu Rev Cell Dev Biol.
12:335–363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang C, Ting AT and Seed B: PPAR-γ
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song K, Han C, Zhang J, Lu D, Dash S,
Feitelson M, Lim K and Wu T: Epigenetic regulation of MicroRNA-122
by peroxisome proliferator activated receptor-gamma and hepatitis b
virus X protein in hepatocellular carcinoma cells. Hepatology.
58:1681–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hatzivassiliou G, Zhao F, Bauer DE,
Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA and
Thompson CB: ATP citrate lyase inhibition can suppress tumor cell
growth. Cancer Cell. 8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bauer DE, Hatzivassiliou G, Zhao F,
Andreadis C and Thompson CB: ATP citrate lyase is an important
component of cell growth and transformation. Oncogene.
24:6314–6322. 2005. View Article : Google Scholar : PubMed/NCBI
|