Introduction
Breast cancer is the most prevalent female cancer
worldwide, and most deaths from breast cancer are due to metastasis
(1,2). Triple-negative breast cancer (TNBC)
which is negative for the estrogen receptor (ER), the progesterone
receptor (PR) and the human epidermal growth factor receptor-2
(HER-2), presents the highest risk of recurrence and metastasis
(3,4).
Studies have revealed that epithelial-mesenchymal
transition (EMT) is a critical step in tumor invasion and
metastasis. During the process of EMT, cancer cells lose their
epithelial phenotypes and gain mesenchymal characteristics
(5–7). Breast cancer cells acquire increased
migratory and invasive potential through EMT (8). Our understanding of the molecular
mechanisms of this process may provide effective therapeutic
strategies for reducing breast cancer metastasis.
Livin is the most recently identified member of the
inhibitors of the apoptosis protein (IAP) family. Numerous evidence
has demonstrated that Livin is associated with a high degree of
malignancy and with poor prognosis in cancer patients (9–11).
Livin inhibits cell apoptosis by binding to the regulators of
apoptosis and promotes cell proliferation, migration and invasion
(12). A recent study revealed that
Livin was involved in the regulation of EMT in colorectal cancer
cells (13). However, the role of
Livin in EMT and metastasis of breast cancer and its relevant
mechanisms remain unclear.
In the present study, we investigated the effect of
Livin on the progression and metastasis of breast cancer. Our
findings demonstrated that Livin promoted invasion and metastasis
in breast cancer through the regulation of EMT by activating the
p38/GSK3β pathway, especially in TNBC.
Materials and methods
Tissue samples
The paraffin-embedded specimens of 150 breast cancer
tissues (33 tissues of TNBC cases and 117 of non-TNBC cases) and 30
paracancerous tissues were selected from the First Affiliated
Hospital of China Medical University and the Affiliated Hospital of
Binzhou Medical University. The 16 pairs of primary breast cancer
and the corresponding adjacent non-tumor tissues were acquired from
the First Affiliated Hospital of China Medical University. The
collected fresh samples were immediately stored at −80°C for
protein and RNA extraction. None of the patients had undergone
radiotherapy and chemotherapy prior to the sample collection.
Informed consent was obtained prior to surgery, from all enrolled
patients. The study was approved by the Medical Ethics Committee of
China Medical University and Binzhou Medical University.
Cell culture
Human breast cancer cell lines MCF-7 and MDA-MB-231,
and normal human breast epithelial cell line MCF-10A were obtained
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). MCF-7 and MCF-10A cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) and
MDA-MB-231 cells were grown in Leibovitz's L-15 medium (Gibco).
Both media were supplemented with 10% fetal bovine serum (FBS;
Biological Industries Israel Beit Haemek Ltd., Israel) and 1%
Pen-Strep solution in a 5% CO2 humidified incubator at
37°C.
Quantitative real-time RT-PCR
Total RNA was extracted from the breast tissues and
cells using RNAiso Plus (Takara, Dalian, China) and
reverse-trancribed to synthesize cDNA using the PrimeScript TMRT
reagent kit (Takara). The expression of Livin was determined with
SYBR® Premix Ex Taq™ II (Takara) using the 7900HT Fast
Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
The quantification of Livin was normalized to GAPDH using the ΔΔCt
method. The primers for Livin were
5′-GACAGAGGAGGAAGAGGAGGA-3′/5′-TCAGCGGCCAGTCATAGAAG-3′ and for
GAPDH were 5′-GCACCGTCAAGGCTGAGAAC3′/5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
Total proteins from breast tissues and cells were
extracted in RIPA buffer and quantified using the Bradford method.
Lysates containing 60 µg of total proteins were subjected to 10%
SDS-PAGE and subsequently transferred onto polyvinylidene fluoride
(PVDF) membranes. The membranes were blocked with 5% non-fat milk
powder or 5% bovine serum albumin (BSA) for 2 h at room
temperature. Subsequently, the blots were probed overnight at 4°C
with indicated antibodies against Livin, Snail, Slug, MMP-2 and
MMP-7 (all 1:500 diluted; Santa Cruz Biotechnology, Santa Cruz, CA,
USA), E-cadherin (1:500 diluted; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), vimentin (1:500 diluted; Bioss,
Beijing, China), N-cadherin (1:500 diluted; Bioss), p38, p-p38,
GSK3β, p-GSK3β, p-ATF2 (all 1:1,000 diluted; Cell Signaling
Technology, Beverly, MA, USA), GAPDH (1:2,000 diluted; ZSGB-Bio,
Beijing, China) and incubated with appropriate secondary antibodies
at room temperature for 2 h. Immunolabeled proteins were detected
with ECL (Thermo Fisher Scientific, Waltham, MA, USA). GAPDH was
used as an internal control. To detect the expression of the
relevant protein, the gray level of the indicated protein band was
detected using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Immunohistochemistry
All specimens were made into paraffin sections with
4 µm thickness. Immunohistochemistry staining was performed
according to the Envision method (ZSGB-Bio, Beijing, China),
following the manufacturer's protocol. The primary antibodies were
rabbit polyclonal antibody against Livin (1:200), rabbit polyclonal
antibody against E-cadherin, vimentin and N-cadherin (1:300,
respectively). For the negative controls, the primary antibodies
were replaced by phosphate-buffered saline (PBS). Previously
identified strongly staining breast tissue sections were used as
positive controls. By multiplying the staining intensity and the
percentage of positive cells, we evaluated the expression of Livin.
Livin was mainly located in the cytoplasm. The intensity of
staining was graded as 0–3 (0, none; 1, weak; 2, moderately strong;
3, intense) and positive cell proportion was scored as 0–4 (none,
0; 1–25%, 1; 26–50%, 2; 51–75%, 3; 76–100%, 4). By multiplying
these two factors, positive expression was indicated when the
immunoreactive score was ≥2. E-cadherin mainly localized in the
cytomembrane and ≥50% cells with deep yellow or brown granules was
scored as positive expression. N-cadherin exhibited in the
cytomembrane and (or) cytoplasm and ≥10% cells with deep yellow or
brown granules was scored as positive expression. Vimentin mainly
existing in the cytoplasm and ≥10% cells with deep yellow or brown
granules was scored as positive expression (14,15).
Plasmid construction and
transfection
The pCMV6-myc-Livin plasmid was purchased from Sino
Biological Technology (Beijing, China), and the pCMV6-myc empty
vector was purchased from Origene Technology (Rockville, MD, USA).
Livin-siRNA and NC-siRNA were purchased from Santa Cruz
Biotechnology. Transfection was carried out using the Lipofectamine
3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions.
Immunofluorescence staining
Cells were fixed with 4% paraformaldehyde and
blocked with 5% BSA. The primary antibodies against E-cadherin,
vimentin and N-cadherin (1:100, respectively) were incubated
overnight at 4°C. The following day, the cells were incubated with
FITC-conjugated secondary antibodies in the dark at room
temperature for 2 h. The coverslips were incubated with DAPI for
nuclear counterstaining. The results were observed using a
fluorescence microscope.
Wound healing assay
When cell confluency reached ~90% after
transfection, wounds were created in the confluent cells using a
200-µl pipette tip. The cells were rinsed with PBS to remove any
free-floating cells and debris. Medium was subsequently added and
culture plates were incubated at 37°C. Wound healing within the
scrape lines was observed at different time-points and
representative scrape lines for each cell line were
photographed.
Cell migration assay
Cells (3×104) were plated in the upper
Transwell chamber (Corning, Lowell, MA, USA) in 100 µl medium with
2% fetal bovine serum (FBS) after they were cultured to the
exponential phase. The lower chamber was filled with 600 µl medium
containing 20% FBS as a chemoattractant. After a 24-h incubation,
the migrated cells were fixed with cold methanol and stained with
hematoxylin. The stained migrated cells were scored and
photographed under microscopic observation.
Matrigel invasion assay
An invasion assay was performed using the Transwell
chamber precoated with diluted Matrigel (BD Biosciences, San Jose,
CA, USA). Cells (1×105) in 100 µl medium containing 2%
FBS were seeded into the upper chamber, whereas the lower chamber
was filled with 600 µl medium containing 20% FBS. The remaining
experimental procedures were in accordance with the cell migration
assay.
Statistical analysis
All statistical analyses were performed using the
SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). The
immunohistochemistry results were analyzed using the Chi-square
test and the Pearson's correlation test. Differences between groups
were assessed by the Student's t-test. Data were processed using
GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Livin is overexpressed in breast
cancer tissues
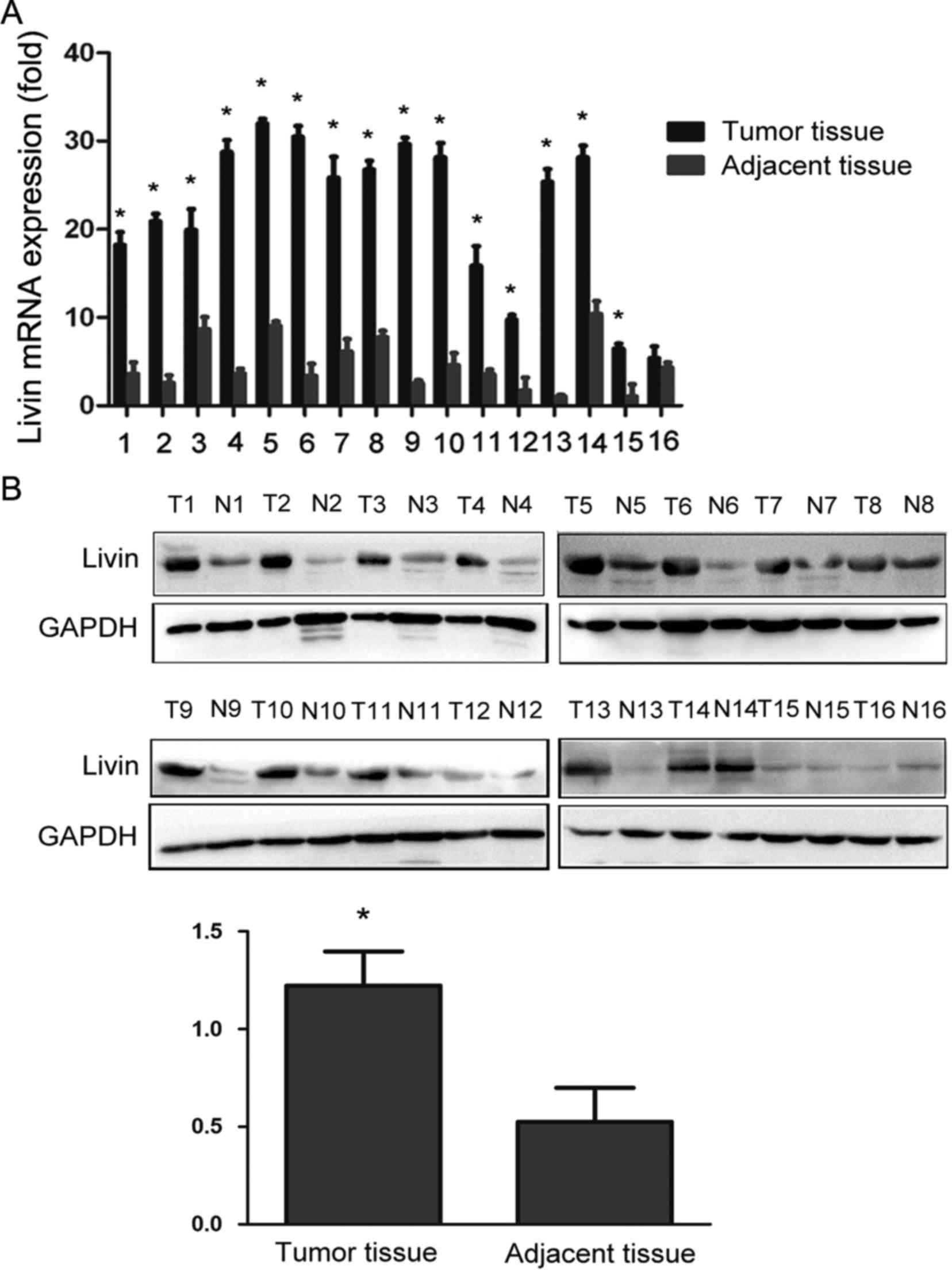
The expression of Livin at transcriptional and
translational levels was assessed in fresh breast tissues by
quantitative real-time RT-PCR (qRT-PCR) and western blot analysis.
As shown in Fig. 1A and B, Livin
mRNA and protein expression were markedly upregulated in breast
cancer tissues compared with that in paired non-tumor tissues.
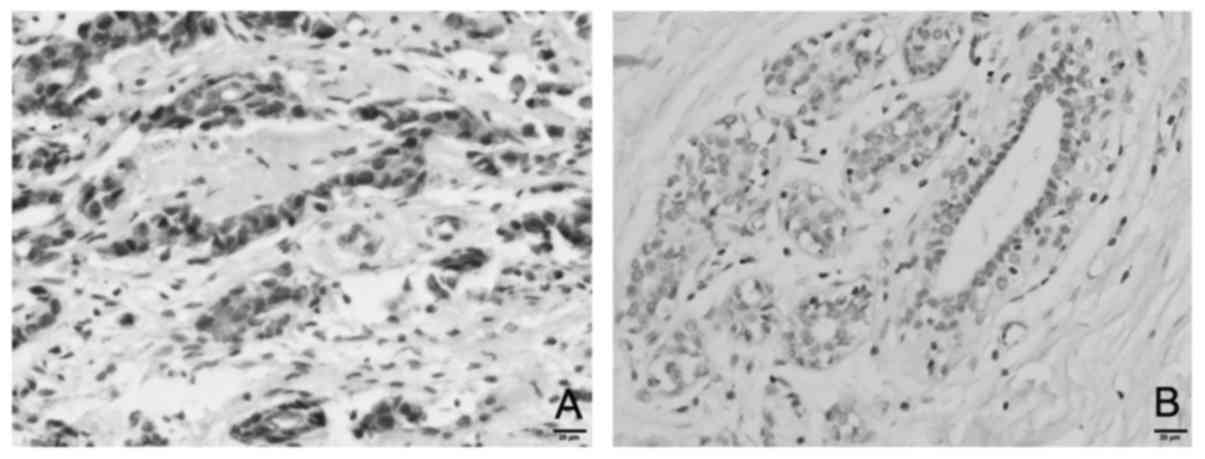
To ascertain this result, an immunohistochemistry
assay was employed to detect the expression of Livin in
paraffin-embedded specimens. The results revealed that Livin was
mainly located in the cytoplasm of cancer cells in breast cancer
tissues (Fig. 2). The rate of
positive expression of Livin in breast cancer tissues (59.3%) was
significantly higher than that in adjacent non-tumor tissues
(23.3%, P<0.001).
Livin is overexpressed in breast
cancer cell lines
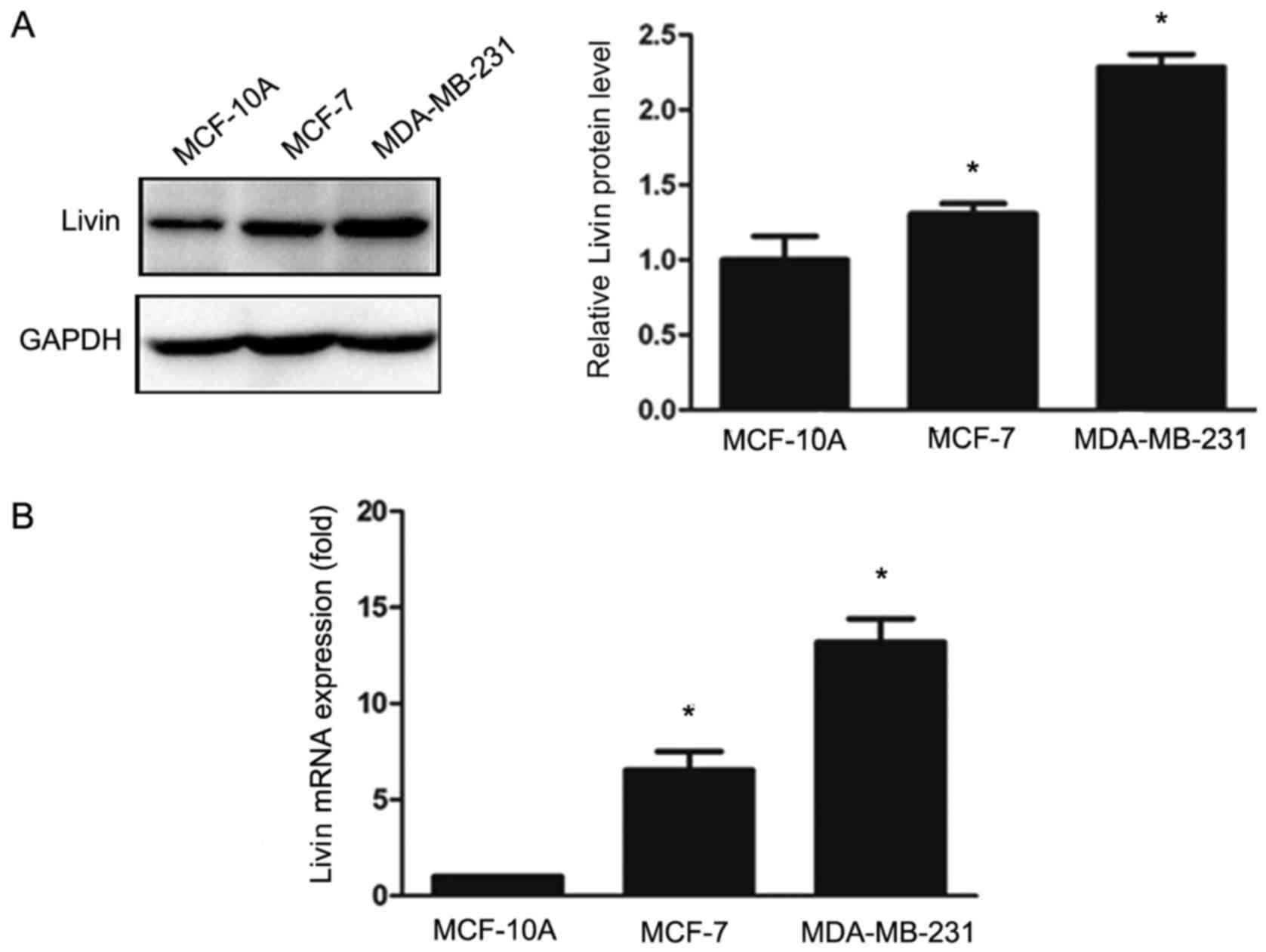
We detected the expression of Livin in the breast
cancer cell lines MCF-7 and MDA-MB-231 and in the normal breast
epithelial cell line MCF-10A using western blot analysis and
qRT-PCR (Fig. 3). The levels of
Livin expression were significantly elevated in the MDA-MB-231 and
MCF-7 cells compared to those in the MCF-10A cells.
Livin expression is correlated with
clinicopathological features of breast cancer
We analyzed the correlation between Livin expression
and the clinicopathological features of the breast cancer patients
(Table I). Livin expression was
significantly positively associated with TNM stage and lymph node
metastasis in the breast cancer cases, especially in TNBC
(P<0.01). In contrast, there was no marked correlation between
Livin expression and patient age, tumor size, or histological
differentiation (P>0.05). Furthermore, the rate of Livin
expression in TNBC tissues was slightly higher than that in
non-TNBC tissues. However, this difference was not statistically
significant. In addition, Livin expression was higher in TNBC
MDA-MB-231 cells than that in non-TNBC MCF-7 cells. These results
suggested that Livin expression is positively associated with the
progression and metastasis of breast cancer and that Livin plays a
more important role in TNBC.
 | Table I.Association of Livin expression with
clinicopathological factors in breast cancer. |
Table I.
Association of Livin expression with
clinicopathological factors in breast cancer.
|
| Livin (total) | Livin (TNBC) | Livin (non-TNBC) |
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
≤45 | 34 | 42 |
| 5 | 14 |
| 29 | 28 |
|
|
>45 | 27 | 47 | 0.304 | 5 | 9 | 0.561 | 22 | 38 | 0.121 |
| Tumor size
(cm) |
|
|
|
|
|
|
|
|
|
|
<2 | 23 | 27 |
| 3 | 6 |
| 20 | 21 |
|
|
2-5 | 30 | 42 |
| 6 | 10 |
| 24 | 32 |
|
|
>5 | 8 | 20 | 0.314 | 1 | 7 | 0.442 | 7 | 13 | 0.588 |
| Histopathological
grade |
|
|
|
|
|
|
|
|
|
| I | 20 | 15 |
| 4 | 2 |
| 16 | 13 |
|
| II | 28 | 45 |
| 4 | 14 |
| 24 | 31 |
|
|
III | 13 | 29 | 0.057 | 2 | 7 | 0.101 | 11 | 22 | 0.224 |
| TNM stage |
|
|
|
|
|
|
|
|
|
| I | 26 | 16 |
| 5 | 1 |
| 21 | 15 |
|
| II | 26 | 49 |
| 3 | 14 |
| 23 | 35 |
|
|
III | 9 | 24 | 0.003 | 2 | 8 | 0.008 | 7 | 16 | 0.076 |
| Metastatic lymph
node |
|
|
|
|
|
|
|
|
|
|
Negative | 39 | 31 |
| 8 | 6 |
| 31 | 25 |
|
|
Positive | 22 | 58 | 0.000 | 2 | 17 | 0.004 | 20 | 41 | 0.014 |
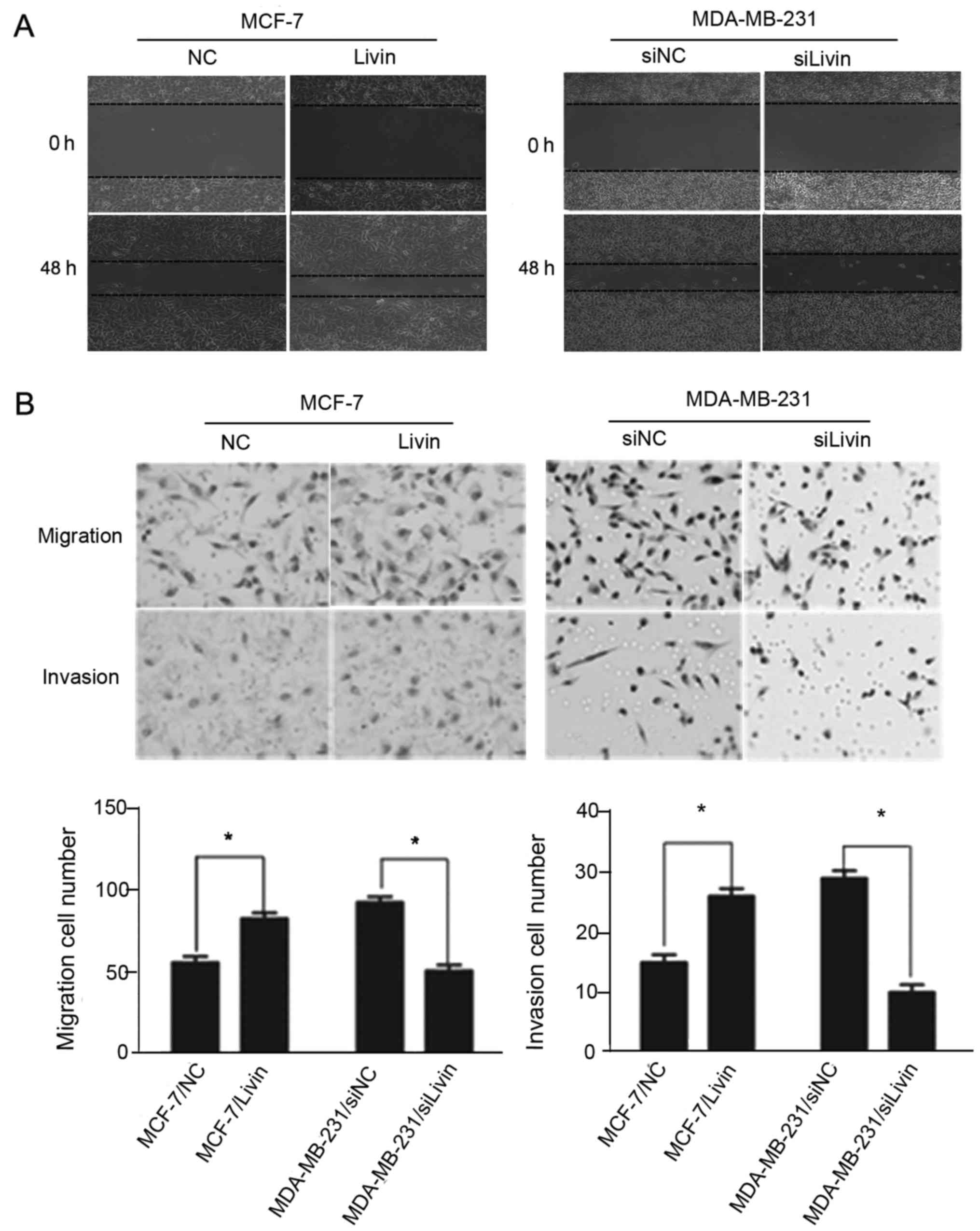
Livin promotes migration and invasion
in breast cancer cells
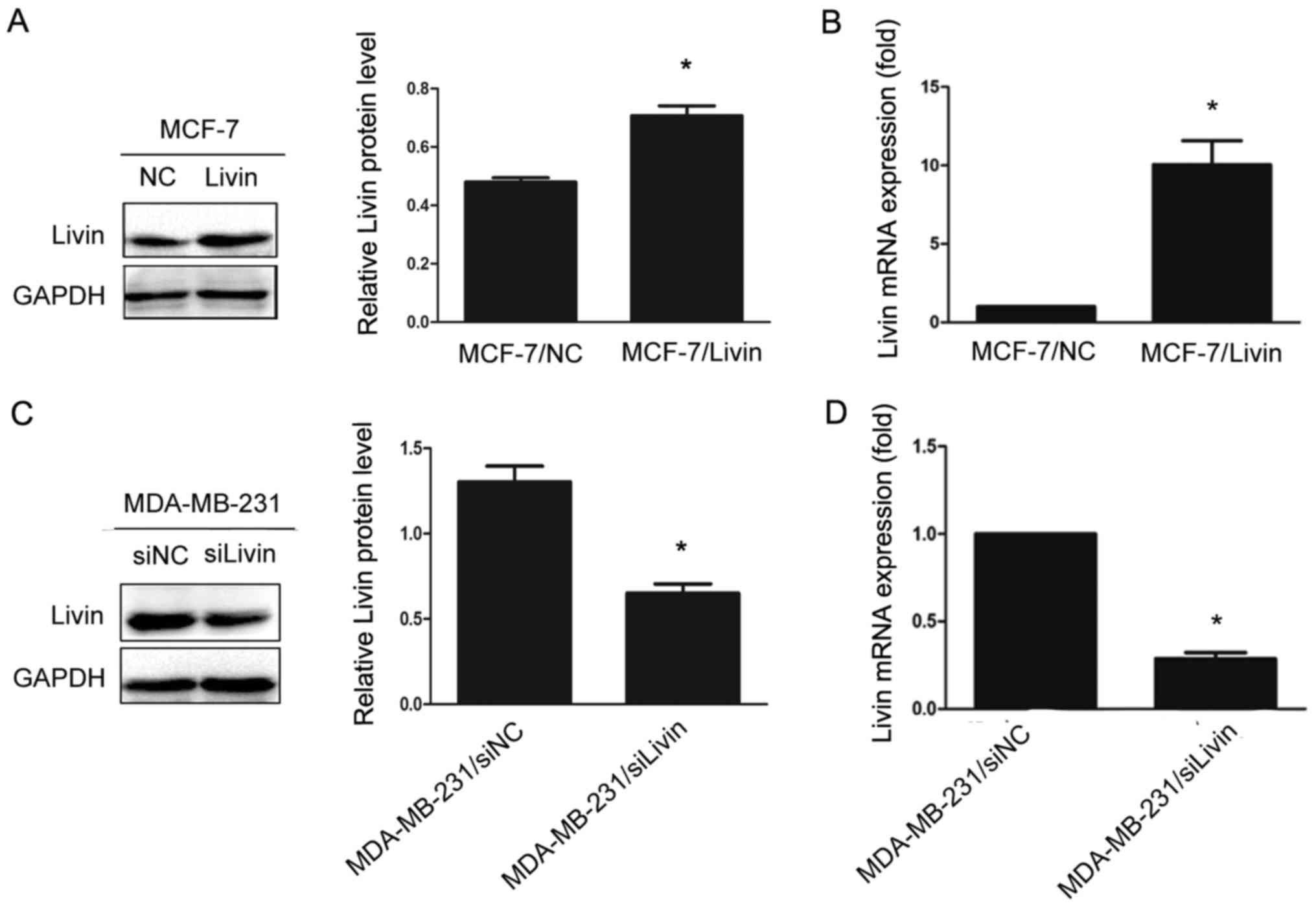
To explore whether Livin affects the migration and
invasion of breast cancer cells, we upregulated Livin expression by
transfection of Livin in the non-TNBC cell line MCF-7, which has
low Livin expression, and knocked down Livin expression by siRNA
treatment in the TNBC cell line MDA-MB-231, which exhibits high
Livin expression. Transfection efficiencies were assessed by
western blot analysis and qRT-PCR, respectively (Fig. 4).
Wound healing, cell migration and Matrigel invasion
assays were used to investigate the effect of Livin on cell
migration and invasion abilities. The upregulation of Livin led to
a significant increase in the migration and invasion of the MCF-7
cells. Conversely, the migratory and invasive abilities of the
MDA-MB-231 cells were suppressed by the Livin-knockdown (Fig. 5).
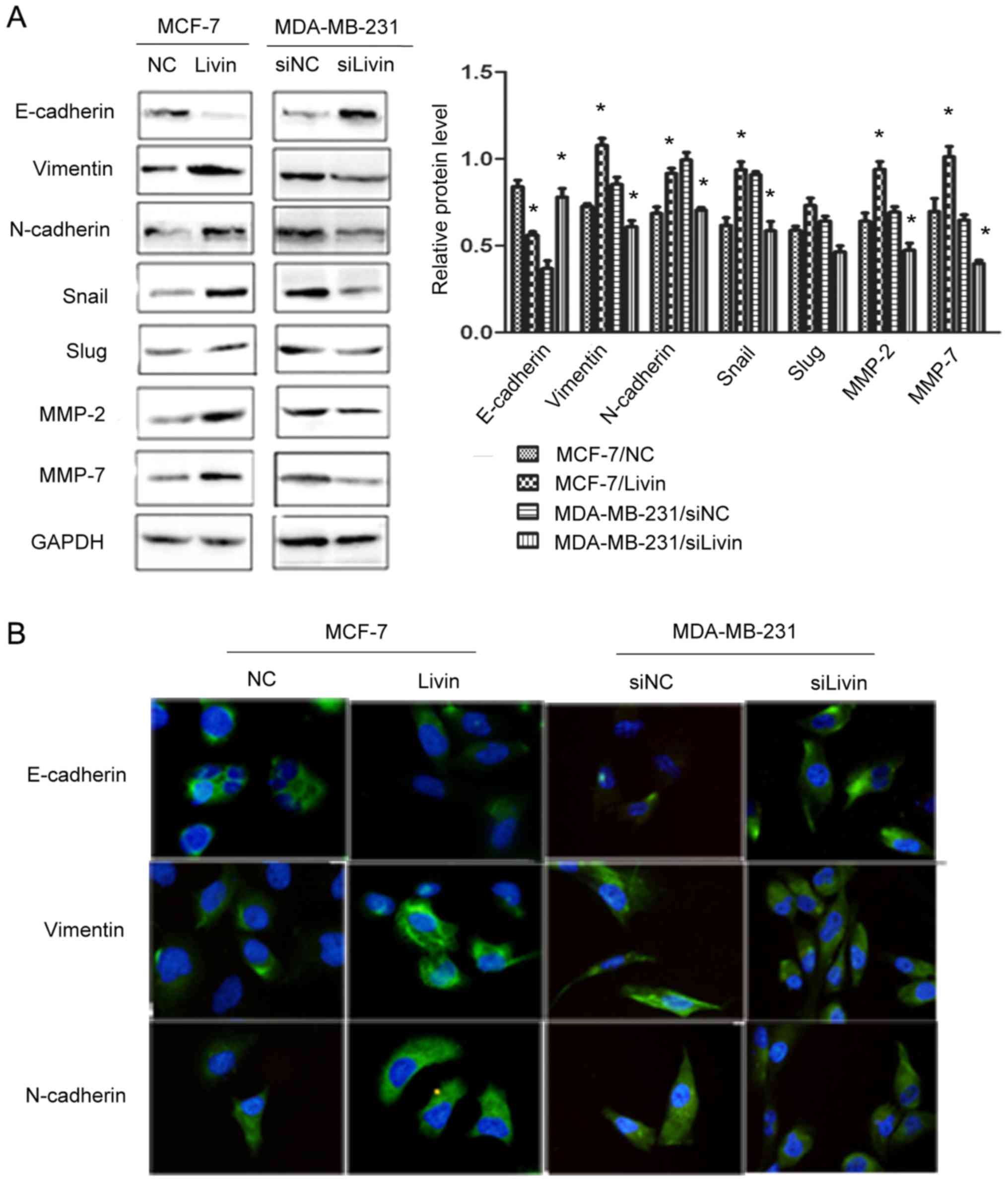
Livin regulates EMT in breast cancer
cells
EMT is highly correlated with tumor invasion and
metastasis (7,8). We therefore observed the influence of
Livin on EMT in breast cancer cells. We examined the levels of
several EMT-related factors using western blot analysis, after
altering the expression of Livin. Livin overexpression caused a
decrease in the expression of the epithelial marker E-cadherin and
increases in the mesenchymal markers vimentin and N-cadherin, as
well as the EMT transcription factor Snail and the
metastasis-associated factors MMP-2 and MMP-7. Depletion of Livin
resulted in an increase in E-cadherin and decreases in vimentin and
N-cadherin as well as Snail, MMP-2 and MMP-7 (Fig. 6A). We further detected the
expression of the EMT marker proteins by immunofluorescence assay
and the results were consistent with those of the western blot
analysis (Fig. 6B).
In addition, to determine whether Livin is
associated with the EMT markers, we further performed
immunohistochemical analysis of the samples from 33 cases of TNBC
and 117 cases of non-TNBC (Table
II). Correlation analysis revealed that Livin expression was
positively correlated with the expression of vimentin and
N-cadherin and negatively correlated with that of E-cadherin in
TNBC. In non-TNBC, enhanced expression of Livin was significantly
associated with enhanced expression of vimentin and reduced
expression of E-cadherin, while Livin expression was not
significantly associated with the expression of N-cadherin. These
findings suggested that Livin induced EMT to promote cell migration
and invasion in breast cancer cells.
 | Table II.Correlation between Livin and EMT
marker proteins in breast cancer. |
Table II.
Correlation between Livin and EMT
marker proteins in breast cancer.
|
| Livin (TNBC) | Livin
(non-TNBC) |
|---|
|
|
|
|
|---|
| EMT markers | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| E-cadherin |
|
|
|
|
|
|
|
Negative | 1 | 13 |
| 9 | 11 |
|
|
Positive | 9 | 10 | 0.013 | 42 | 45 | 0.04 |
| Vimentin |
|
|
|
|
|
|
|
Negative | 10 | 13 |
| 48 | 53 |
|
|
Positive | 0 | 10 | 0.012 | 3 | 13 | 0.03 |
| N-cadherin |
|
|
|
|
|
|
|
Negative | 9 | 11 |
| 43 | 46 |
|
|
Positive | 1 | 12 | 0.023 | 8 | 20 | 0.066 |
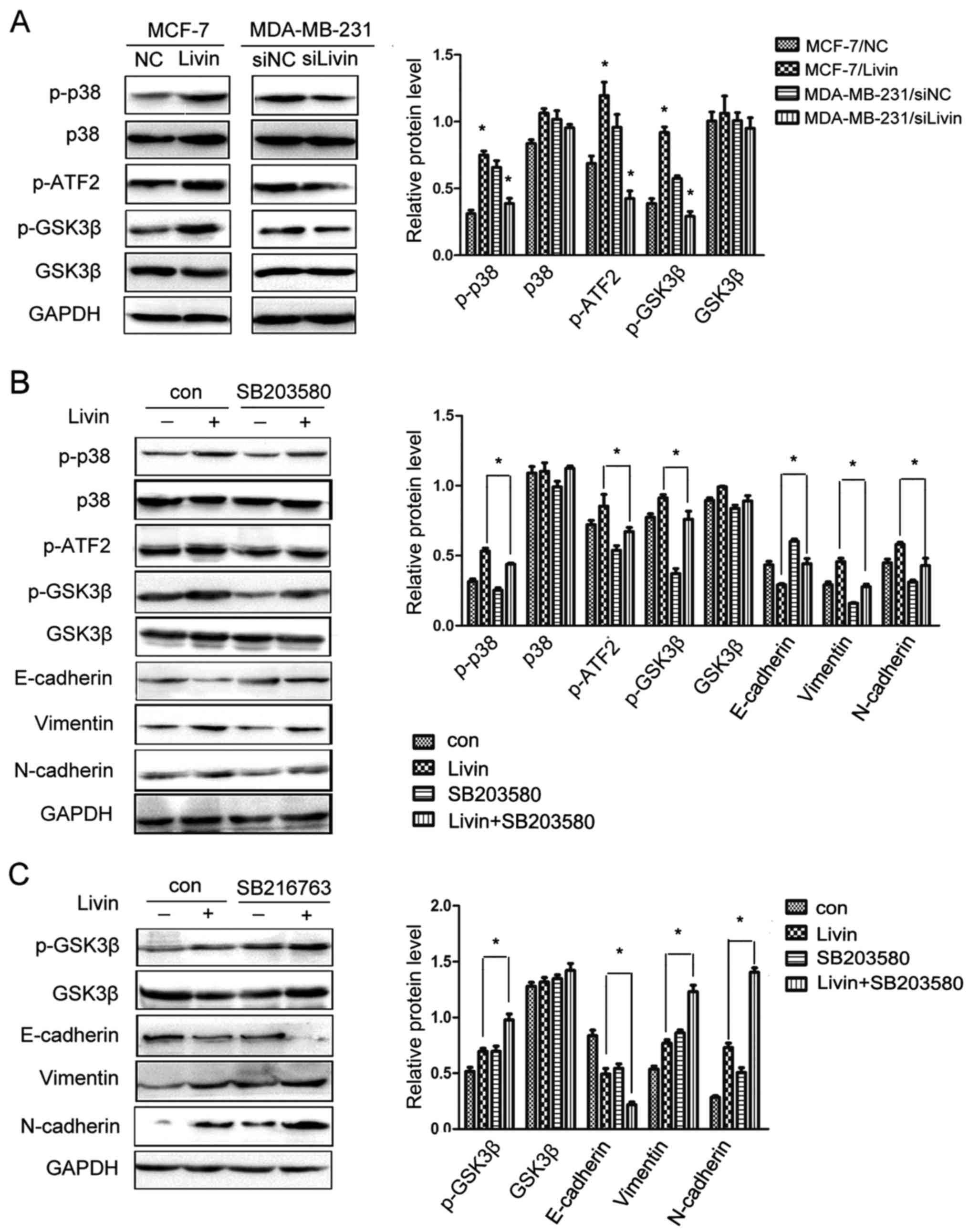
The p38/GSK3β pathway is involved in
the effect of Livin on EMT in breast cancer cells
Recent studies have revealed that the p38 and GSK3β
pathways regulate EMT to promote progression and metastasis in
several types of cancer (16,17),
activating p38 and inhibiting GSK3β to promote EMT. To explore the
possible mechanism of Livin-induced EMT in breast cancer cells, we
examined the expression of p38/GSK3β-associated factors. The
overexpression of Livin in MCF-7 cells markedly enhanced the
phosphorylation of p38, ATF2 and GSK3β. In contrast, the knockdown
of Livin in MDA-MB-231 cells clearly inhibited the phosphorylation
of p38, ATF2 and GSK3β (Fig.
7A).
Moreover, we ascertained the above results using a
specific inhibitor of p38, SB203580 (Cell Signaling Technology) and
an effective inhibitor of GSK3β, SB216763 (Cell Signaling
Technology). SB203580 is a specific inhibitor of p38 that inhibits
p38 catalytic activity by binding to the ATP binding pocket, but it
does not inhibit phosphorylation of p38. SB216763 is an effective
inhibitor of the GSK3β activity. Inactivation of GSK3β results in
an increase in phosphorylated GSK3β. GSK3β is a downstream target
of p38 and inhibiting p38 downregulates phosphorylated GSK3β
expression and enhances GSK3β activity. The increased expression of
p-ATF2, p-GSK3β, vimentin and N-cadherin was eliminated and the
expression of E-cadherin was partially restored by SB203580
(Fig. 7B). When SB216763 was added
to the medium after Livin was upregulated, the expression of
p-GSK3β, vimentin and N-cadherin was further increased and
E-cadherin expression was markedly reduced (Fig. 7C). These results indicated that
Livin may activate the p38/GSK3β pathway to promote EMT in breast
cancer cells.
Discussion
Livin has been recently recognized as a vital
molecule that participates in the development and progression of
malignant tumors, such as gastric (18), colorectal (19), prostate (20) and lung cancer (21). In the present study, our results
revealed that Livin expression was increased in breast cancer
tissues and cells. The expression of Livin was positively
correlated with the TNM stage and lymph node metastasis in breast
cancer, particularly in TNBC. Moreover, the overexpression of Livin
enhanced the migratory and invasive abilities of the non-TNBC cell
line MCF-7 and the knockdown of Livin had the opposite effect in
the TNBC cell line MDA-MB-231. These results revealed that Livin
has the potential to facilitate the progression and metastasis of
breast cancer, especially in TNBC, indicating it may be a
therapeutic target for breast cancer treatment.
EMT is highly correlated with tumor development and
progression. Through EMT, cancer cells break down the basement
membrane (BM) and extracellular matrix (ECM), invading the blood or
lymph vessels and spreading to other tissues and organs (22,23).
During the process of EMT, the expression of markers of the
epithelial phenotype, such as E-cadherin, decreases, while the
expression of markers of the mesenchymal phenotype, such as
vimentin and N-cadherin, increases (24–26).
Several transcription factors (such as Snail and Slug) bind to the
promoter regions of genes related to cell-cell adhesion and inhibit
their transcription, which is the critical step in EMT (27–29).
Additionally, EMT also leads to the reorganization of the ECM and
EMT-inducing factors increase the expression of ECM proteins and
proteases (such as MMPs) (30,31).
Therefore, although the potential mechanisms involved in EMT in
breast cancer are not entirely clear, we can assess EMT initiation
through the detection of these factors.
According to a recent study by Ge et al
(13), Livin potentiated the
migration and invasion of colorectal cancer cells by regulating
EMT. Our study revealed that Livin overexpression upregulated
Snail, vimentin, N-cadherin, MMP-2 and MMP-7 and downregulated
E-cadherin. In contrast, Livin knockdown had the opposite effect.
Further immunohistochemistry results also established that Livin
expression was positively associated with EMT in breast cancer.
These results demonstrated that the influence of Livin on breast
cancer aggressiveness was correlated with the induction of EMT.
A wide variety of signaling pathways is implicated
in the EMT process. The p38 pathway has been found to play an
active role in the regulation of EMT (16,32).
Recently, Ou et al (33)
revealed that the knockdown of Livin suppressed the invasion of
gastric cancer cells by inhibiting the phosphorylation of p38.
GSK3β has been reported as a downstream target of p38 (34) and also as a regulator of the EMT
process (17,35). Our results demonstrated that Livin
expression markedly increased the phosphorylation of the p38 and
GSK3β signaling proteins, revealing the activation of the p38/GSK3β
pathway in Livin-overexpressing breast cancer cells. In addition,
the upregulation of vimentin and N-cadherin expression was
eliminated and E-cadherin expression was restored after the
treatment of Livin-transfected cells with the inhibitor of p38.
Similarly, vimentin and N-cadherin were further elevated and
E-cadherin expression was markedly decreased by the inhibitor of
GSK3β. Our findings revealed that Livin overexpression resulted in
the activation of p38, which induced the phosphorylation of GSK3β
and inhibited the GSK3β activity, ultimately contributing to the
initiation of EMT. This observation revealed that Livin promoted
EMT in breast cancer cells, at least partially, through the
activation of the p38/GSK3β pathway. However, the specific
mechanism through which Livin affects this pathway remains unknown
and requires further investigation. The present study is the first
to demonstrate that Livin-induced EMT is, at least partially,
regulated by the p38/GSK3β signaling pathway in breast cancer. Yet,
it is also noteworthy that Livin regulates EMT in breast cancer
through the activation of AKT signaling (36). Whether the AKT and p38/GSK3β
signaling pathways involve crosstalk or whether they are
independent of each other in Livin-induced EMT of breast cancer
requires further investigation. In addition, other Livin-mediated
pathways should be investigated to determine whether there are
other links between Livin and EMT in breast cancer.
Collectively, our results indicated that Livin
promoted the progression and metastasis of breast cancer through
the regulation of EMT by activating the p38/GSK3β pathway. A deeper
understanding of the role of Livin-induced EMT in breast cancer may
provide effective targets for breast cancer therapy, especially in
TNBC.
References
|
1
|
Stebbing J and Ellis P: An overview of
drug development for metastatic breast cancer. Br J Nurs. 21 Sup
4:S18–S22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jia LY, Shanmugam MK, Sethi G and Bishayee
A: Potential role of targeted therapies in the treatment of
triple-negative breast cancer. Anticancer Drugs. 27:147–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saha P and Nanda R: Concepts and targets
in triple-negative breast cancer: Recent results and clinical
implications. Ther Adv Med Oncol. 8:351–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tseng LM, Hsu NC, Chen SC, Lu YS, Lin CH,
Chang DY, Li H, Lin YC, Chang HK, Chao TC, et al: Distant
metastasis in triple-negative breast cancer. Neoplasma. 60:290–294.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar
|
|
6
|
Creighton CJ, Gibbons DL and Kurie JM: The
role of epithelial-mesenchymal transition programming in invasion
and metastasis: A clinical perspective. Cancer Manag Res.
5:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kotiyal S and Bhattacharya S: Epithelial
mesenchymal transition and vascular mimicry in breast cancer stem
cells. Crit Rev Eukaryot Gene Expr. 25:269–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Li H and Ren G:
Epithelial-mesenchymal transition and drug resistance in breast
cancer (Review). Int J Oncol. 47:840–848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kenneth NS and Duckett CS: IAP proteins:
Regulators of cell migration and development. Curr Opin Cell Biol.
24:871–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Argon A, Nart D, Oruç N, Coker A and
Ozütemiz O: The prognostic significance of clinicopathological
features and apoptosis inhibitor proteins in pancreas ductal
adenocarcinoma. Acta Gastroenterol Belg. 77:229–234.
2014.PubMed/NCBI
|
|
11
|
Jayakumar J and Anishetty S: Molecular
dynamics simulations of inhibitor of apoptosis proteins and
identification of potential small molecule inhibitors. Bioorg Med
Chem Lett. 24:2098–2104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CJ, Cong Y, Liu XZ, Zhou X, Shi X, Wu
SJ, Zhou GX and Lu M: Research progress on the livin gene and
osteosarcomas. Asian Pac J Cancer Prev. 15:8577–8579. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge Y, Cao X, Wang D, Sun W, Sun H, Han B,
Cui J and Liu B: Overexpression of Livin promotes migration and
invasion of colorectal cancer cells by induction of
epithelial-mesenchymal transition via NF-κB activation. Onco
Targets Ther. 9:1011–1021. 2016.PubMed/NCBI
|
|
14
|
Ryu HS, Chung JH, Lee K, Shin E, Jing J,
Choe G, Kim H, Xu X, Lee HE, Kim DG, et al: Overexpression of
epithelial-mesenchymal transition-related markers according to cell
dedifferentiation: Clinical implications as an independent
predictor of poor prognosis in cholangiocarcinoma. Hum Pathol.
43:2360–2370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryu HS, Park DJ, Kim HH, Kim WH and Lee
HS: Combination of epithelial-mesenchymal transition and cancer
stem cell-like phenotypes has independent prognostic value in
gastric cancer. Hum Pathol. 43:520–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen HH, Zhou XL, Shi YL and Yang J: Roles
of p38 MAPK and JNK in TGF-β1-induced human alveolar epithelial to
mesenchymal transition. Arch Med Res. 44:93–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang B, Yang Y, Shi X, Liao W, Chen M,
Cheng AS, Yan H, Fang C, Zhang S, Xu G, et al: Proton pump
inhibitor pantoprazole abrogates adriamycin-resistant gastric
cancer cell invasiveness via suppression of Akt/GSK-β/β-catenin
signaling and epithelial-mesenchymal transition. Cancer Lett.
356:704–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung CY, Park YL, Kim N, Park HC, Park
HB, Myung DS, Kim JS, Cho SB, Lee WS and Joo YE: Expression and
prognostic significance of Livin in gastric cancer. Oncol Rep.
30:2520–2528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Li Y, Zhou B, Zhang WY, Guan JT,
Wang R, Yang L, Xia QJ, Zhou ZG and Sun XF: Expression of the
apoptosis inhibitor livin in colorectal adenoma-carcinoma sequence:
Correlations with pathology and outcome. Tumour Biol.
35:11791–11798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu J, Ren L, Wang X, Qu C and Zhang Y:
Expression of livin, survivin and caspase-3 in prostatic cancer and
their clinical significance. Int J Clin Exp Pathol. 8:14034–14039.
2015.PubMed/NCBI
|
|
21
|
Li J, Chen P, Li XQ, Bao QL, Dai CH and Ge
LP: Elevated levels of survivin and livin mRNA in bronchial
aspirates as markers to support the diagnosis of lung cancer. Int J
Cancer. 132:1098–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campbell K and Casanova J: A common
framework for EMT and collective cell migration. Development.
143:4291–4300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X and Fan D: The
epithelial-mesenchymal transition and cancer stem cells: Functional
and mechanistic links. Curr Pharm Des. 21:1279–1291. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JY and Kong G: Roles and epigenetic
regulation of epithelial-mesenchymal transition and its
transcription factors in cancer initiation and progression. Cell
Mol Life Sci. 73:4643–4660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawata H, Kamiakito T, Omoto Y, Miyazaki
C, Hozumi Y and Tanaka A: RhoC upregulation is correlated with
reduced E-cadherin in human breast cancer specimens after
chemotherapy and in human breast cancer MCF-7 cells. Horm Cancer.
5:414–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bastos LG, de Marcondes PG,
de-Freitas-Junior JC, Leve F, Mencalha AL, de Souza WF, de Araujo
WM, Tanaka MN, Abdelhay ES and Morgado-Díaz JA: Progeny from
irradiated colorectal cancer cells acquire an EMT-like phenotype
and activate Wnt/β-catenin pathway. J Cell Biochem. 115:2175–2187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muqbil I, Wu J, Aboukameel A, Mohammad RM
and Azmi AS: Snail nuclear transport: The gateways regulating
epithelial-to-mesenchymal transition? Semin Cancer Biol. 27:39–45.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo Q, Ning F, Fang R, Wang HS, Zhang G,
Quan MY, Cai SH and Du J: Endogenous Nodal promotes melanoma
undergoing epithelial-mesenchymal transition via Snail and Slug in
vitro and in vivo. Am J Cancer Res. 5:2098–2112. 2015.PubMed/NCBI
|
|
30
|
Bouris P, Skandalis SS, Piperigkou Z,
Afratis N, Karamanou K, Aletras AJ, Moustakas A, Theocharis AD and
Karamanos NK: Estrogen receptor alpha mediates epithelial to
mesenchymal transition, expression of specific matrix effectors and
functional properties of breast cancer cells. Matrix Biol.
43:42–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao T, Shi Y, Han D, Luan W, Qian J, Zhang
J, Wang Y and You Y: Chinese Glioma Cooperative Group (CGCG): TPM3,
a strong prognosis predictor, is involved in malignant progression
through MMP family members and EMT-like activators in gliomas.
Tumour Biol. 35:9053–9059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang Z, Wu R, Xie W, Geng H, Zhao L, Xie
C, Wu J, Geng S, Li X, Zhu M, et al: Curcumin suppresses MAPK
pathways to reverse tobacco smoke-induced gastric
epithelial-mesenchymal transition in mice. Phytother Res.
29:1665–1671. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ou JM, Ye B, Qiu MK, Dai YX, Dong Q, Shen
J, Dong P, Wang XF, Liu YB, Quan ZW, et al: Knockdown of Livin
inhibits growth and invasion of gastric cancer cells through
blockade of the MAPK pathway in vitro and in vivo.
Int J Oncol. 44:276–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bikkavilli RK, Feigin ME and Malbon CC:
p38 mitogen-activated protein kinase regulates canonical
Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci.
121:3598–3607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Y, Jing Z, Deng H, Li Z, Zhuang Z,
Wang S and Wang Y: Soluble epoxide hydrolase inhibition ameliorates
proteinuria-induced epithelial-mesenchymal transition by regulating
the PI3K-Akt-GSK-3β signaling pathway. Biochem Biophys Res Commun.
463:70–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Yin X, Luo X, Li HY, Su X, Wang XY,
Chen L, Zheng K and Ren GS: Livin promotes progression of breast
cancer through induction of epithelial-mesenchymal transition and
activation of AKT signaling. Cell Signal. 25:1413–1422. 2013.
View Article : Google Scholar : PubMed/NCBI
|