Introduction
Prostate cancer is the most lethal urogenital system
malignancy in men, accounting for an estimated 161,360 new cases
and 26,730 deaths in the US in 2017 (1). Although great improvement has been
made in the efficacy of therapeutic methods, most prostate cancer
develops into a metastatic pattern, which is a major obstacle for
the treatment of prostate cancer (2). Recent research has reported that
epithelial-to-mesenchymal transition (EMT) is a necessity for the
invasion-metastasis cascade of cancer (3). EMT is a complicated process which
involves the loss of epithelial characteristics and acquisition of
a mesenchymal phenotype, contributing to the initiation of the
metastatic cascade (4). Given that
there are few effective therapeutic methods for the treatment of
cancer metastasis, the development of novel therapeutic agents
against prostate cancer is critically needed.
Thymoquinone (TQ), a major ingredient of black seed
oil (Nigella sativa), has been shown to exhibit broad
pharmacologic effects, such as anti-inflammatory (5) and antioxidant activity (6). Moreover, thymoquinone has been
reported to possess antineoplastic activity against various
cancers, including pancreatic (7),
lung (8) and colon cancer (9) and leukemia (10). Studies have shown that thymoquinone
inhibits the growth and induces cell apoptosis and cycle arrest, as
well as represses metastasis and angiogenesis, which involves Akt
(11), NF-κB signaling (12), mitogen-activated protein kinase
(MAPK) (13) and STAT3 signaling
pathways (14).
In prostate cancer C4-2B and PC3 cells, thymoquinone
was found to suppress proliferation via the accumulation of
reactive oxygen species (ROS) and reduction in the glutathione
(GST) level (15). However, there
is no study concerning the correlation between thymoquinone and EMT
in prostate cancer. In addition, the underlying mechanism by which
thymoquinone suppresses the metastatic phenotype has not yet been
elucidated. The present study aimed to explore the potential
capacity of thymoquinone to reverse EMT in prostate cancer cells
and the related mechanism.
Materials and methods
Reagents and cell culture
Thymoquinone (C10H12O2) was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and dissolved in dimethyl
sulfoxide (DMSO). Stock solutions were stored at −20°C. Antibodies
against transforming growth factor-β (TGF-β), Smad2, Smad3,
E-cadherin, vimentin, Slug and β-actin were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Human prostate cancer cell lines DU145 and PC3 were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). These two cell lines were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (Gibco, Grand
Island, NY, USA), 100 µg/ml streptomycin and 100 U/ml penicillin
(Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified incubator
with 5% CO2.
Cell proliferation assay
A modified MTT assay was used to evaluate cell
proliferation. In brief, DU145 and PC3 cells were seeded at a
density of 1.2×104/well in 96-well plates with 90%
density and subsequently exposed to increasing concentrations of
thymoquinone for 24 h. Then, 20 µl MTT dye solution (5.0 mg/ml) was
mixed with 180 µl medium and added to each well. After incubation
at 37°C for 4 h, the culture medium was wiped out and the cells
were lysed with DMSO to dissolve the formazan crystals. The optical
density (OD) of each well was evaluated at 490 nm wavelength using
a 96-well microplate reader (Bio-Rad, Hercules, CA, USA). The
growth inhibitory rate was calculated as: [(OD 490control cells -
OD 490treated cells)/OD 490control cells] × 100. The experiments
were performed in triplicate.
Wound healing assay
Wound healing assay was performed to detect the
effect of thymoquinone on cell migration. Prostate cancer DU145 or
PC3 cells were seeded onto 6-well plates. When the cell density
reached above 90%, scratch wounds were made across the monolayer
using the tip of a 200-µl pipette. Then, the wounded cultures were
incubated in a serum-free medium with thymoquinone treatment at
different times (0, 24 h), and five random fields (magnification,
×100) were subsequently chosen from each scratch wound and observed
by microscopy to evaluate the migratory capacity. The experiments
were performed in triplicate.
Transwell migration assay
Transwell migration assay is another method to
evaluate cell migratory ability. It was performed using prostate
cancer DU145 and PC3 cells after thymoquinone treatment. Cells
(4×104) with 200 µl serum-free medium were seeded onto
the upper chamber, while 800 µl of medium supplemented with 10%
fetal calf serum was added to the lower chamber. After certain
incubation at 37°C, the adherent cells on the top chambers were
wiped off with a cotton swab. The migrated cells on the lower
surface of the filter were then fixed with 4% paraformaldehyde and
stained with 0.1% crystal violet (Beyotime, Shanghai, China). Cells
were then counted in five randomly chosen visual fields using a
microscope at a magnification of ×100. The experiments were
performed in triplicate.
Matrigel invasion assay
The impact of thymoquinone on the invasion of
prostate cancer cells was evaluated by Matrigel invasion assay
using a Millicell chamber (Millipore, Billerica, MA, USA). The
membrane (polycarbonic membrane, 8 µm pore size) in the top chamber
was pretreated with 50 µl Matrigel (Matrigel, serum-free medium
1:5). After incubation at 37°C for 5 h, the cells
(10×104) in 200 µl serum-free medium were treated with
thymoquinone according to the instructions of the Transwell
migration assay.
Quantitative real-time PCR assay
DU145 and PC3 cells were treated with different
concentrations of thymoquinone and the total RNA was extracted
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer's protocol. Complementary DNA (cDNA) was
subsequently synthesized using a PrimerScript RT reagent kit
(Takara, Dalian, China). Then, the relative levels of target gene
messenger RNA (mRNA) transcript were measured by quantitative
real-time PCR assay (qRT-PCR) using the SYBR-Green Master Mix. The
sequences of primers for the PCR amplification were forward,
5′-GGCCAGATCCTGTCCAAGC-3′ and reverse, 5′-GTGGGTTTCCACCATTAGCAC-3′
for TGF-β (201 bp); 5′-CGTCCATCTTGCCATTCACG-3′ and reverse,
5′-CTCAAGCTCATCTAATCGTCCTG-3′ for Smad2 (182 bp);
5′-TGGACGCAGGTTCTCCAAAC-3′ and reverse, 5′-CCGGCTCGCAGTAGGTAAC-3′
for Smad3 (90 bp); forward 5′-CGAGAGCTACACGTTCACGG-3′ and reverse,
5′-GGGTGTCGAGGGAAAAATAGG-3′ for E-cadherin (119 bp); forward,
5′-GACGCCATCAACACCGAGTT-3′ and reverse, 5′-CTTTGTCGTTGGTTAGCTGGT-3′
for vimentin (238 bp); forward, 5′-CGAACTGGACACACATACAGTG-3′ and
reverse, 5′-CTGAGGATCTCTGGTTGTGGT-3′ for Slug (87 bp); forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′ for β-actin (250 bp). All the
experiments were performed in triplicate and calculated on the
basis of the ΔΔCt method. The n-fold change in mRNA expression was
evaluated according to the method of 2−ΔΔCt.
Western blotting
Briefly, prostate cancer DU145 and PC3 cells were
collected after thymoquinone treatment, and lysed on ice for 10 min
with a lysis buffer [10 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl,
0.1% sodium dodecyl sulfate (SDS), 1 mmol/l
ethylenediaminetetraacetic acid, 1 mmol/l ethylene glycol
tetraacetic acid, 0.3 mmol/l phenylmethylsulfonyl fluoride, 0.2
mmol/l sodium orthovanadate, 1% NP-40, 10 mg/ml leupeptin and 10
mg/ml aprotinin]. After centrifugation and denaturation, the
clarified protein lysates (~30–60 µg) were subjected to
SDS-polyacrylamide gel electrophoresis (10 or 15%) and transferred
to polyvinylidene membranes (Millipore). Membranes were
subsequently probed with antibodies against TGF-β, Smad2, Smad3,
E-cadherin, vimentin, Slug and β-actin overnight at 4°C. The
immunoreactive bands were then washed and incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody at room
temperature (25°C). Ultimately, the protein bands were visualized
with ECL substrate and exposed to X-ray film.
Plasmid transfection
TGF-β cDNA was cloned into the pcDNA3.1 vector. When
prostate cancer DU145 or PC3 cells reached 70–80% confluency for
plasmid transfection, the cells were then transfected with
X-tremeGENE HP DNA transfection reagent (Roche, Mannheim, Germany)
for 48 h following the manufacturer's instructions, and prepared
for the subsequent turnover experiments.
Statistical analysis
All statistical analyses were evaluated by GraphPad
Prism (version 5.0) software (GraphPad Software, Inc., La Jolla,
CA, USA), and Student's t-test (two-sided) was used for comparisons
involving only two groups. A value of P<0.05 was identified as a
statistical significant difference.
Results
Thymoquinone inhibits the
proliferation of prostate cancer cells
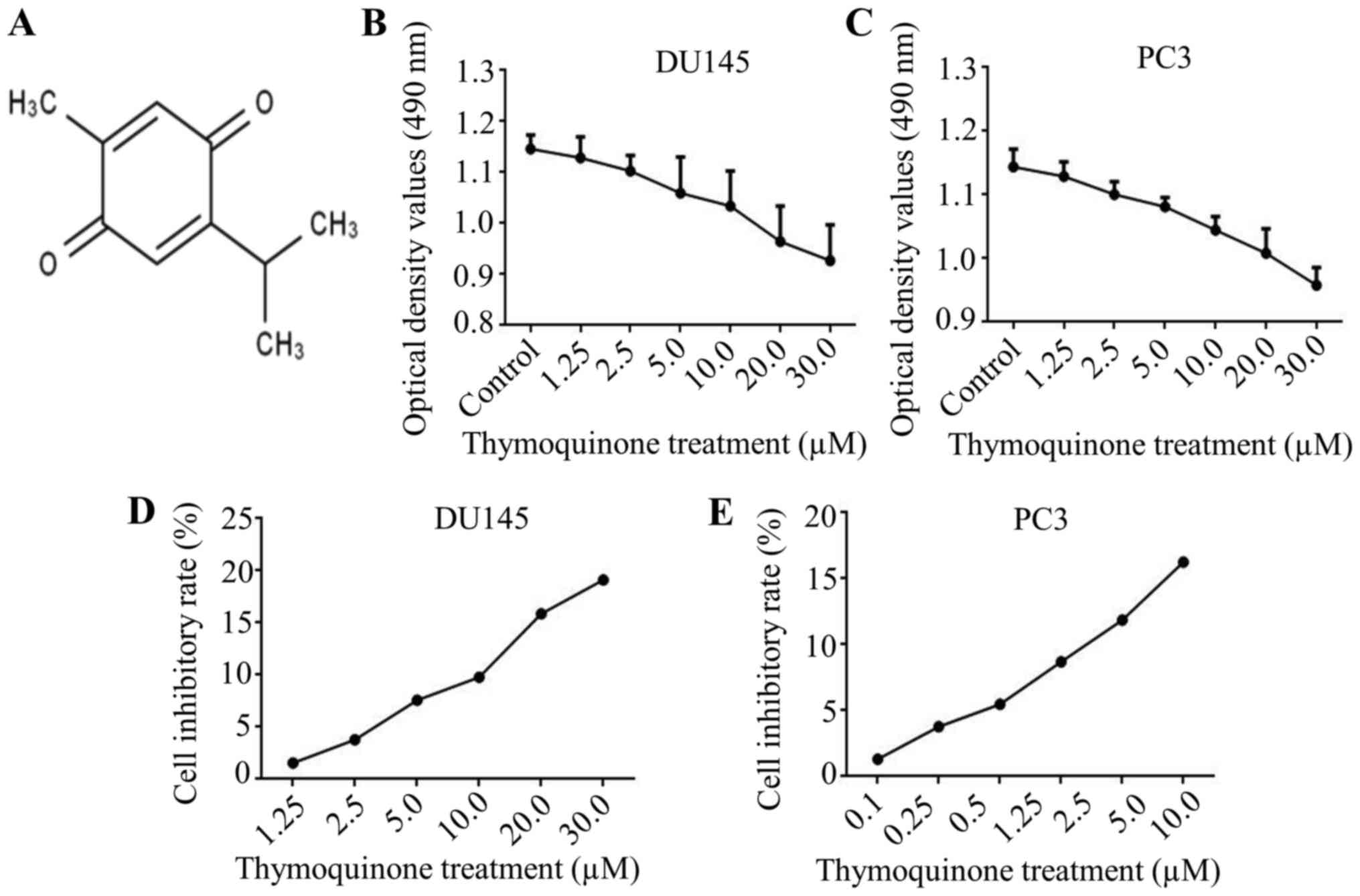
Firstly, the chemical structure of thymoquinone is
presented in Fig. 1A. In view of
the metastatic potential, DU145 and PC3 cells were chosen for the
next research. Subsequently, to exclude the effect of thymoquinone
on growth inhibition in prostate cancer cells, a modified MTT assay
was used. The results demonstrated that the growth of prostate
cancer DU145 and PC3 cells at a density >90% was notably
repressed upon thymoquinone treatment at concentrations ≥10.0 µM
(Fig. 1B-E). Thymoquinone at 10 µM
was used as the adequate concentration in the subsequent research,
to exclude interference from growth inhibition by thymoquinone.
Thymoquinone (TQ) inhibits the
migratory and invasive capacity of prostate cancer DU145 and PC3
cells
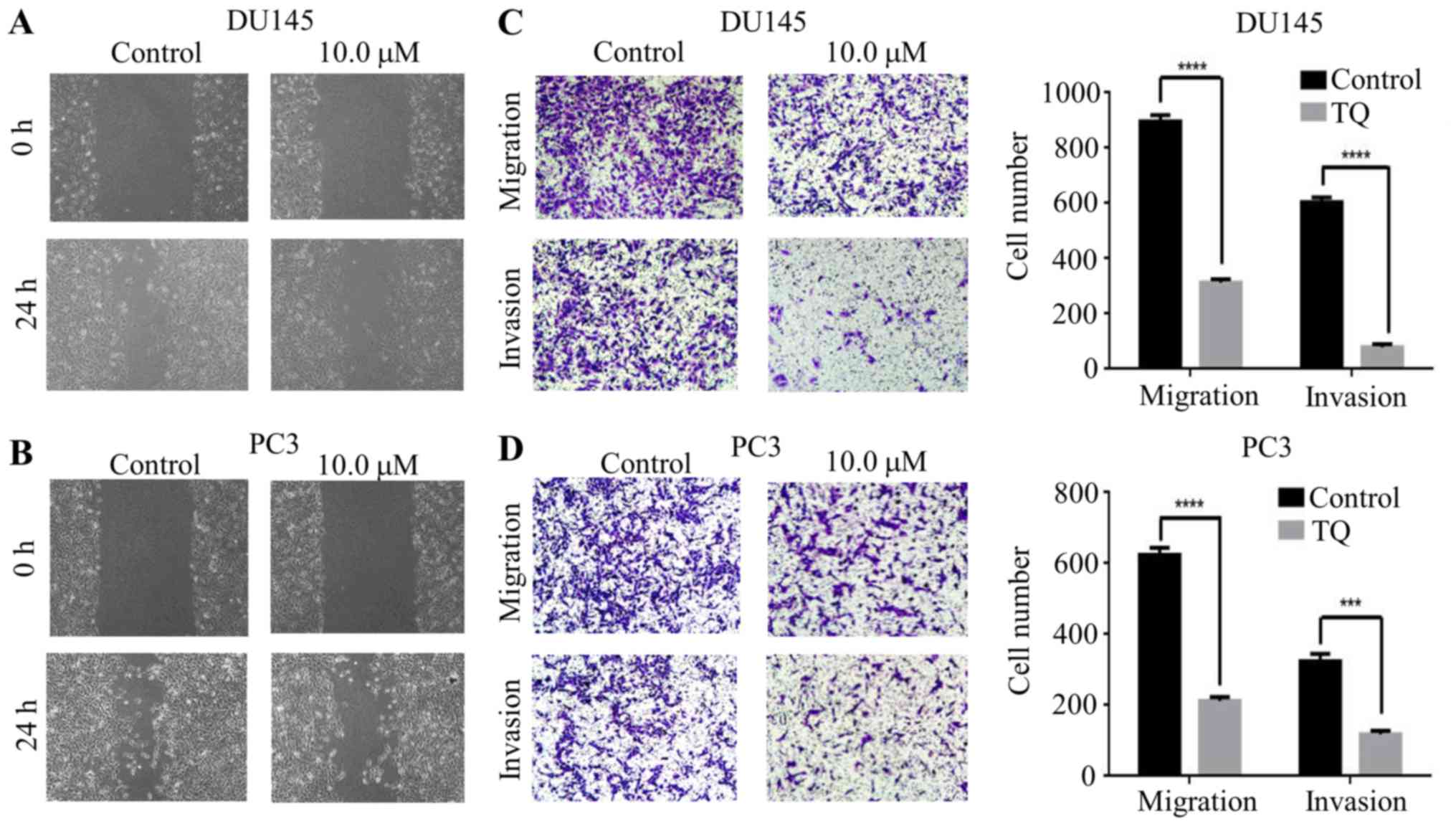
To explore the effect of thymoquinone on migratory
and invasive abilities in prostate cancer cells, a wound healing
assay was used to verify the antimetastatic effect of thymoquinone.
The results indicated that the scratch width was much wider upon
thymoquinone treatment than that in the control group in the DU145
and PC3 cells, suggesting the effective antimetastatic activity of
thymoquinone on prostate cancer cells (Fig. 2A and B). To further confirm the
effects of thymoquinone on the metastatic phenotype of prostate
cancer cells, Transwell migration and Matrigel invasion assays were
carried out. We found that the migratory ability of DU145 cells was
significantly decreased by thymoquinone treatment (P<0.05)
(Fig. 2C). Similar results were
noted in the prostate cancer PC3 cells treated with thymoquinone
(Fig. 2D). Next, we explored the
effect of thymoquinone on the invasiveness of prostate cancer cells
and our findings indicated that thymoquinone significantly
suppressed the invasive capability of the DU145 and PC3 cells
(P<0.05) (Fig. 2C and D).
Taken together, the results from the wound healing
and Transwell assays revealed that thymoquinone had a strong
antimetastatic effect on human prostate cancer cells.
Thymoquinone represses the expression
of EMT markers in prostate cancer cells
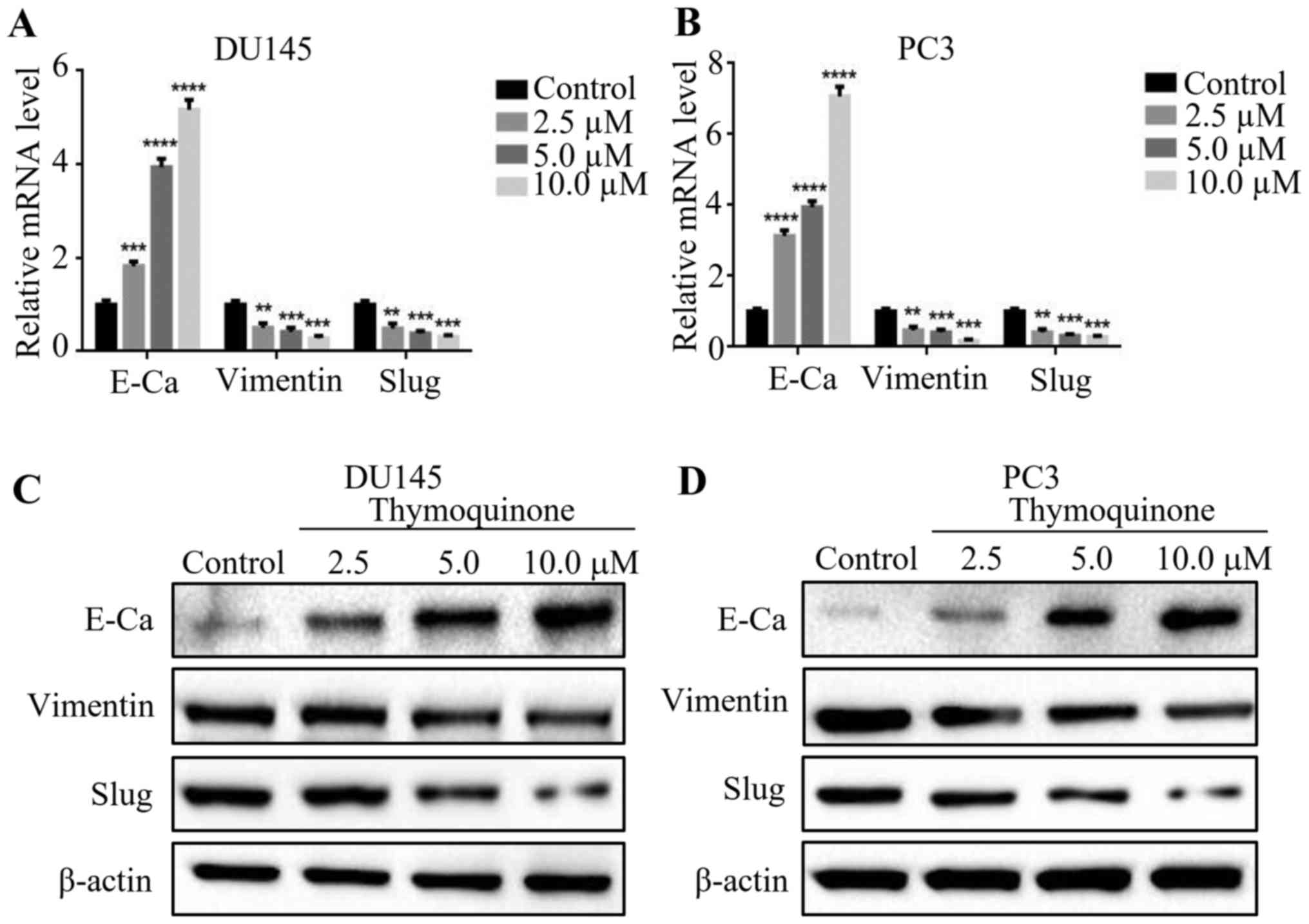
EMT has been widely reported to be associated with
the ability of migration and invasion in various cancers. In view
of that, we first examined the expression levels of EMT markers
including E-cadherin, vimentin and Slug by quantitative real-time
PCR after treatment of DU145 and PC3 cells with 2.5, 5.0 and 10 µM
thymoquinone for 24 h. We found that the mRNA level of E-cadherin
was notably upregulated upon thymoquinone treatment in a
concentration-dependent manner, whereas the expression levels of
vimentin and Slug at mRNA levels were significantly downregulated
(P<0.05)(Fig. 3A and B).
Consistent with the above results, western blot analysis showed
that thymoquinone decreased the expression levels of the
mesenchymal markers including vimentin and Slug, while treatment
with thymoquinone increased the expression of E-cadherin (Fig. 3C and D) in the DU145 and PC3 cells.
The results revealed that thymoquinone reversed EMT in prostate
cancer cells.
Thymoquinone reduces the expression of
TGF-β, Smad2 and Smad3 in prostate cancer cells
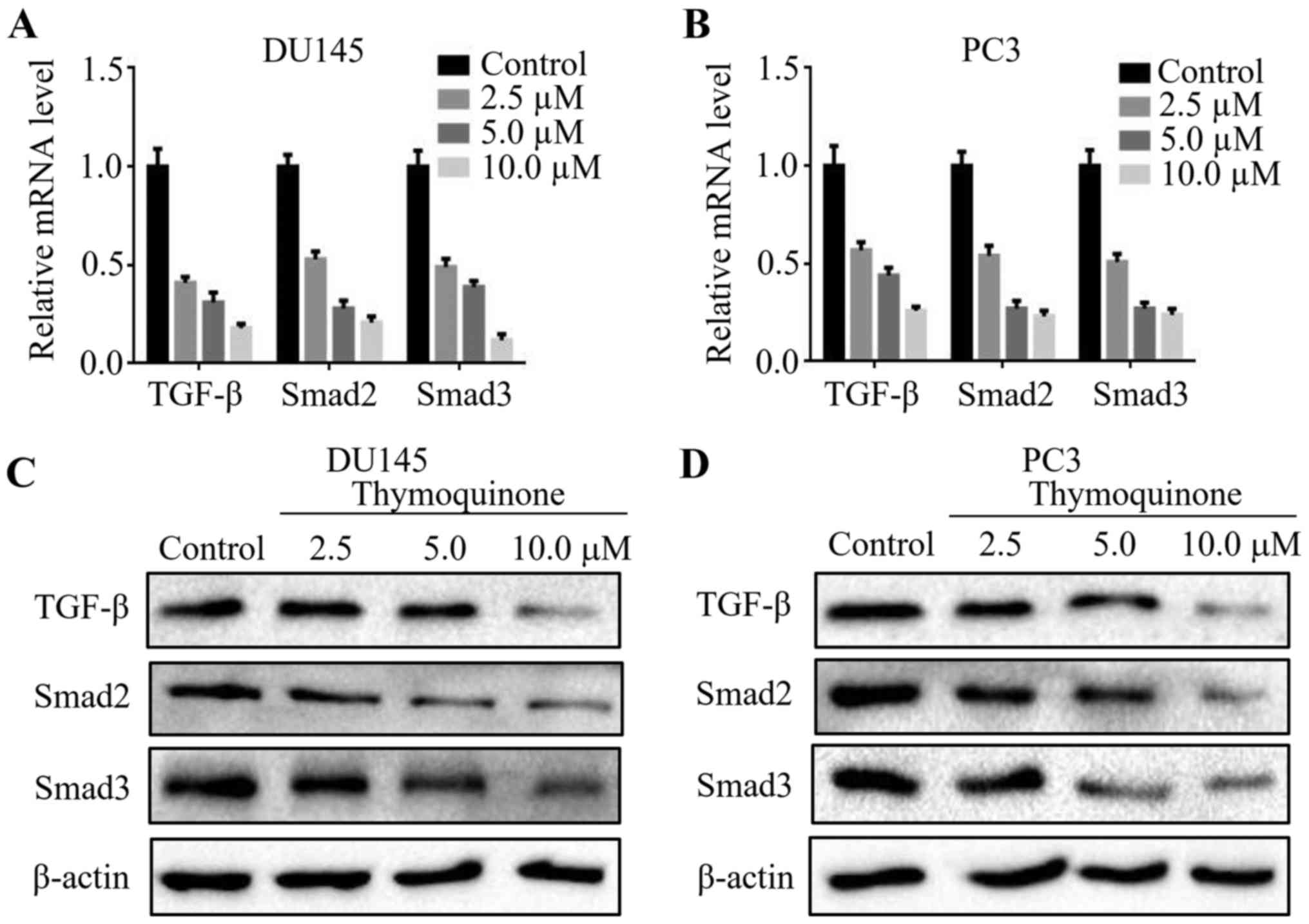
Studies have shown that the TGF-β signaling pathway
is implicated in cancer invasion, metastasis and angiogenesis
(16). To validate the underlying
mechanism involved in the antimetastatic effect of thymoquinone in
prostate cancer cells, we detected the expression levels of TGF-β,
Smad2 and Smad3 in the prostate cancer cells upon thymoquinone
treatment. Notably, thymoquinone downregulated TGF-β, Smad2 and
Smad3 expression at the mRNA level in a concentration-dependent
manner (Fig. 4A and B). In
accordance with the above results, the protein levels of TGF-β,
Smad2 and Smad3 in the DU145 and PC3 cells were significantly
reduced following thymoquinone treatment (P<0.05) (Fig. 4C and D).
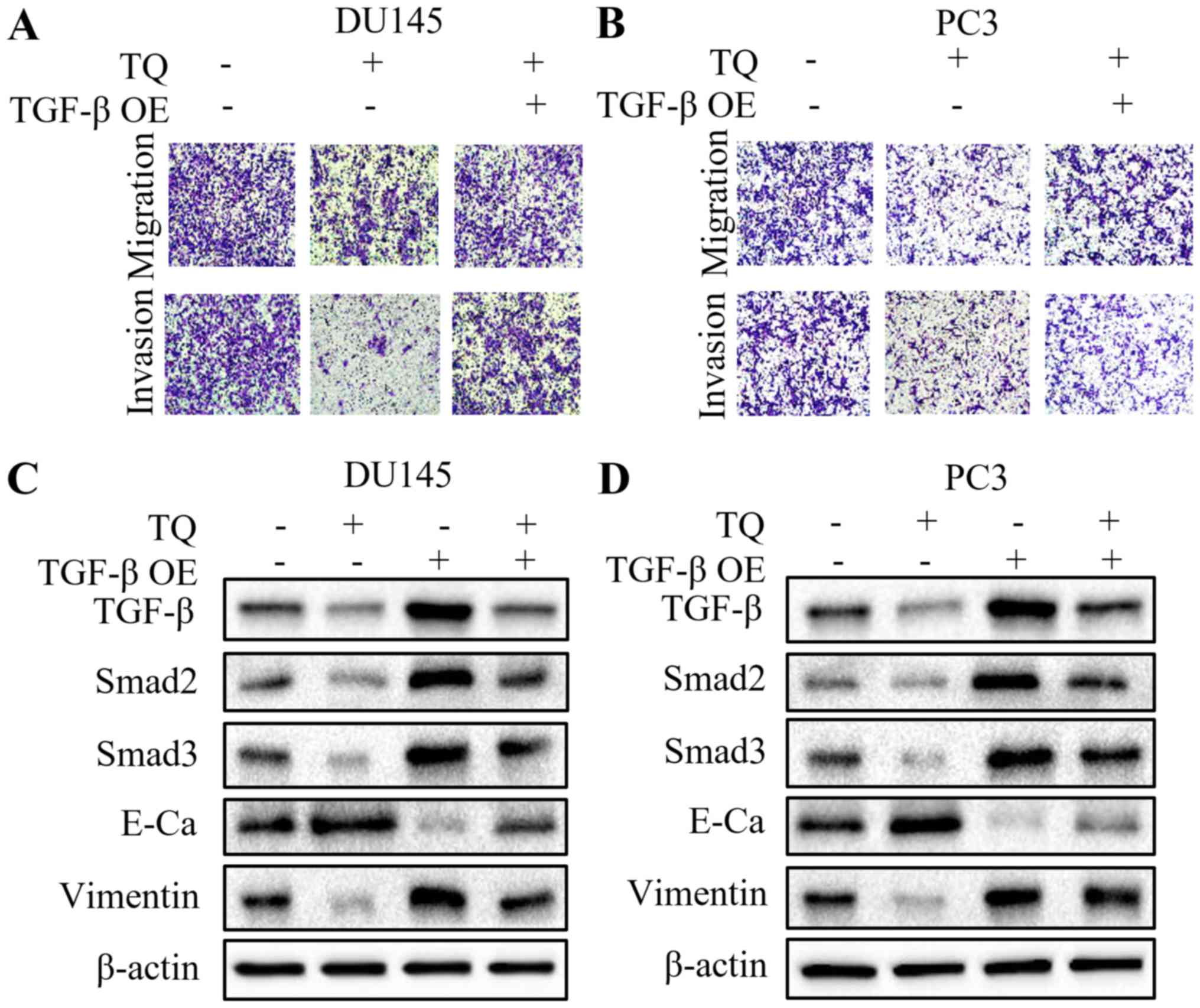
Overexpression of TGF-β attenuates the
antitumor capacity of thymoquinone in prostate cancer cells
To further explore whether the TGF-β signaling
pathway is involved in the antimetastatic effect of thymoquinone on
prostate cancer cells, the TGF-β plasmid was transiently
transfected into DU145 and PC3 cells to overexpress TGF-β.
Intriguingly, overexpression of TGF-β partially restored the
migratory and invasive capacities of the prostate cancer cells,
which was inhibited by thymoquinone (Fig. 5A and B). These findings suggested
that TGF-β may play a crucial role in the antimetastatic activity
of thymoquinone in prostate cancer. Subsequently, we found a marked
reversal of the elevated E-cadherin level, and reduced vimentin,
Smad2 and Smad3 levels following the combined treatment of
thymoquinone and TGF-β overexpression, compared with thymoquinone
treatment alone (Fig. 5C and D).
These results revealed that the antimetastatic capacity of
thymoquinone in prostate cancer may be mediated by the TGF-β
signaling pathway.
Discussion
Numerous studies have shown that thymoquinone is
able to impede tumor progression in a variety of tumors. It is
reported that in prostate cancer PC3 cells, thymoquinone may
suppress tumor angiogenesis and growth by downregulating AKT and
extracellular signal-regulated kinase (ERK) signaling (17). Additionally, thymoquinone notably
suppressed DNA synthesis and proliferation in prostate cancer
LNCaP, C4-2B, DU145 and PC3 cell lines via inactivation of the
androgen receptor (AR) and E2F1, a regulator of cell viability
(18). In addition, it has been
found that thymoquinone could potentiate the cytotoxic and
pro-apoptotic effect of zoledronic acid (ZA) in prostate cancer PC3
and DU145 cells (19). In the
present study, we indeed confirmed the cytotoxic effect of
thymoquinone on prostate cancer DU145 and PC3 cells. The subsequent
wound healing and Transwell assays revealed that thymoquinone
notably suppressed the metastatic phenotype of prostate cancer
cells. Then, we detected the mRNA and protein levels of E-cadherin,
vimentin and Slug, hallmarks of epithelial-to-mesenchymal
transition (EMT). As expected, thymoquinone significantly increased
the expression of E-cadherin, while decreased the expression of
vimentin and Slug at the mRNA and protein levels using quantitative
real-time PCR and western blotting, suggesting that thymoquinone
reversed EMT in prostate cancer DU145 and PC3 cells. Meanwhile,
other studies have demonstrated that thymoquinone decreased the
expression of Twist1 at the transcriptional level, leading to the
reversal of EMT in MDA-MB-435, BT549 and HeLa cells. In addition,
thymoquinone downregulated N-cadherin, Snail and Slug expressions
in bladder cancer T24 cells, which were partially in accordance
with our results (20).
The TGF-β signaling pathway has been confirmed to
regulate malignancy progression and metastasis (21). It is reported that TGF-β may
participate in the regulation of collagen (22), fibronectin (23), laminin (24) and MMP-9 (25), to affect migratory and invasive
capacity. Accumulating evidence shows that Chinese herbal
medicines, such as curcumin and anthocyanidins, reverse EMT and
suppress metastasis via the downregulation of the TGF-β/Smad2/3
signaling pathway (26,27). Our findings revealed a marked
dose-dependent reduction in TGF-β, Smad2 and Smad3 levels upon
thymoquinone treatment in prostate cancer DU145 and PC3 cells,
which was confirmed by quantitative real-time PCR and western
blotting. To further validate that the TGF-β/Smad2/3 signaling
pathway participates in the reversal of EMT by thymoquinone in
prostate cancer, a TGF-β overexpression plasmid was transiently
transfected into DU145 and PC3 cells. The results demonstrated that
overexpression of TGF-β impaired the antimetastatic effect of
thymoquinone and partially abrogated the induction of
mesenchymal-to-epithelial transition (MET) by thymoquinone, as
evidenced by the reversal of the elevated E-cadherin level and
decreased vimentin, Smad2 and Smad3 levels. These results indicated
that the inhibitory effect of thymoquinone on EMT may be mediated
by the TGF-β/Smad2/3 signaling pathway.
In summary, our study confirmed that thymoquinone
suppressed the metastatic phenotype and inhibited EMT in prostate
cancer cells by negatively regulating the TGF-β/Smad2/3 signaling
pathway. In addition, these findings suggest that thymoquinone is a
potential therapeutic agent against prostate cancer which functions
by targeting TGF-β
Acknowledgements
The present study was partially supported by the
National Natural Science Foundation of China (no. 81602562), the
International Science and Technology Cooperative Project of Shaanxi
Province (no. 2017KW-063), the Fundamental Research Funds for the
Central University of Xi'an Jiaotong University (no. 1191329722),
and the Institutional Scientific Development Foundation of the
First Affiliated Hospital of Xi'an Jiaotong University (no.
2015YK17).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leyh-Bannurah SR, Gazdovich S, Budäus L,
Zaffuto E, Briganti A, Abdollah F, Montorsi F, Schiffmann J, Menon
M, Shariat SF, et al: Local therapy improves survival in metastatic
prostate cancer. Eur Urol. 72:118–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mao XY, Li QQ, Gao YF, Zhou HH, Liu ZQ and
Jin WL: Gap junction as an intercellular glue: Emerging roles in
cancer EMT and metastasis. Cancer Lett. 381:133–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cha YH, Yook JI, Kim HS and Kim NH:
Catabolic metabolism during cancer EMT. Arch Pharm Res. 38:313–320.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hossen MJ, Yang WS, Kim D, Aravinthan A,
Kim JH and Cho JY: Thymoquinone: An IRAK1 inhibitor with in vivo
and in vitro anti-inflammatory activities. Sci Rep. 7:429952017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dur A, Kose H, Kocyigit A, Kocaman O,
Ismayilova M and Sonmez FC: The anti-inflammatory and antioxidant
effects of thymoquinone on ceruleine induced acute pancreatitis in
rats. Bratisl Lek Listy. 117:614–618. 2016.PubMed/NCBI
|
|
7
|
Relles D, Chipitsyna GI, Gong Q, Yeo CJ
and Arafat HA: Thymoquinone promotes pancreatic cancer cell death
and reduction of tumor size through combined inhibition of histone
deacetylation and induction of histone acetylation. Adv Prev Med.
2016:14078402016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Acharya BR, Chatterjee A, Ganguli A,
Bhattacharya S and Chakrabarti G: Thymoquinone inhibits microtubule
polymerization by tubulin binding and causes mitotic arrest
following apoptosis in A549 cells. Biochimie. 97:78–91. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohamed AM, Refaat BA, El-Shemi AG,
Kensara OA, Ahmad J and Idris S: Thymoquinone potentiates
chemoprotective effect of Vitamin D3 against colon cancer: A
pre-clinical finding. Am J Transl Res. 9:774–790. 2017.PubMed/NCBI
|
|
10
|
Salim LZ, Othman R, Abdulla MA, Al-Jashamy
K, Ali HM, Hassandarvish P, Dehghan F, Ibrahim MY, Omer FA and
Mohan S: Thymoquinone inhibits murine leukemia WEHI-3 cells in vivo
and in vitro. PLoS One. 9:e1153402014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu D, Ma Y, Zhao B, Li S, Zhang Y, Pan S,
Wu Y, Wang J, Wang D, Pan H, et al: Thymoquinone induces G2/M
arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in
human cholangiocarcinomas both in vitro and in vivo.
Oncol Rep. 31:2063–2070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Bai Y and Yang Y: Thymoquinone
chemosensitizes colon cancer cells through inhibition of NF-κB.
Oncol Lett. 12:2840–2845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woo CC, Hsu A, Kumar AP, Sethi G and Tan
KH: Thymoquinone inhibits tumor growth and induces apoptosis in a
breast cancer xenograft mouse model: The role of p38 MAPK and ROS.
PLoS One. 8:e753562013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu WQ, Wang J, Guo XF, Liu Z and Dong WG:
Thymoquinone inhibits proliferation in gastric cancer via the STAT3
pathway in vivo and in vitro. World J Gastroenterol. 22:4149–4159.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koka PS, Mondal D, Schultz M, Abdel-Mageed
AB and Agrawal KC: Studies on molecular mechanisms of growth
inhibitory effects of thymoquinone against prostate cancer cells:
Role of reactive oxygen species. Exp Biol Med. 235:751–760. 2010.
View Article : Google Scholar
|
|
16
|
Chen W, Zhou S, Mao L, Zhang H, Sun D,
Zhang J, Li J and Tang JH: Crosstalk between TGF-β signaling and
miRNAs in breast cancer metastasis. Tumour Biol. 37:10011–10019.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi T, Cho SG, Yi Z, Pang X, Rodriguez M,
Wang Y, Sethi G, Aggarwal BB and Liu M: Thymoquinone inhibits tumor
angiogenesis and tumor growth through suppressing AKT and
extracellular signal-regulated kinase signaling pathways. Mol
Cancer Ther. 7:1789–1796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaseb AO, Chinnakannu K, Chen D,
Sivanandam A, Tejwani S, Menon M, Dou QP and Reddy GP: Androgen
receptor and E2F-1 targeted thymoquinone therapy for
hormone-refractory prostate cancer. Cancer Res. 67:7782–7788. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dirican A, Erten C, Atmaca H, Bozkurt E,
Kucukzeybek Y, Varol U, Tarhan Oktay M, Karaca B and Uslu R:
Enhanced cytotoxicity and apoptosis by thymoquinone in combination
with zoledronic acid in hormone-and drug-resistant prostate cancer
cell lines. J BUON. 19:1055–1061. 2014.PubMed/NCBI
|
|
20
|
Iskender B, Izgi K, Hizar E, Jauch J,
Arslanhan A, Yuksek EH and Canatan H: Inhibition of
epithelial-mesenchymal transition in bladder cancer cells via
modulation of mTOR signalling. Tumour Biol. 37:8281–8291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Q, Zheng X, Chen L, Xu B, Yang X,
Jiang J and Wu C: Smad2/3/4 pathway contributes to TGF-β-induced
miRNA-181b expression to promote gastric cancer metastasis by
targeting Timp3. Cell Physiol Biochem. 39:453–466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zimmerman KA, Xing D, Pallero MA, Lu A,
Ikawa M, Black L, Hoyt KL, Kabarowski JH, Michalak M and
Murphy-Ullrich JE: Calreticulin regulates neointima formation and
collagen deposition following carotid artery ligation. J Vasc Res.
52:306–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren X, Bo Y, Fan J, Chen M, Xu D, Dong Y,
He H, Ren X, Qu R, Jin Y, et al: Dalbergioidin ameliorates
doxorubicin-induced renal fibrosis by suppressing the TGF-β signal
pathway. Mediators Inflamm. 2016:51475712016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tennant BR, Chen J, Shih AZ, Luciani DS
and Hoffman BG: Myt3 mediates laminin-V/integrin-β1-induced
Islet-cell migration via Tgfbi. Mol Endocrinol.
29:1254–1268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao J, Cheng Q, Ye P, Yang G, Liu S, Ao
Q, Liu Y and Hu Y: Atorvastatin improves pathological changes in
the aged kidney by upregulating peroxisome proliferator-activated
receptor expression and reducing matrix metalloproteinase-9 and
transforming growth factor-β1 levels. Exp Gerontol. 74:37–42. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Cheng X, Gao Y, Zhang C, Bao J,
Guan H, Yu H, Lu R, Xu Q and Sun Y: Curcumin inhibits metastasis in
human papillary thyroid carcinoma BCPAP cells via down-regulation
of the TGF-β/Smad2/3 signaling pathway. Exp Cell Res. 341:157–165.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ouanouki A, Lamy S and Annabi B:
Anthocyanidins inhibit epithelial-mesenchymal transition through a
TGFβ/Smad2 signaling pathway in glioblastoma cells. Mol Carcinog.
56:1088–1099. 2017. View
Article : Google Scholar : PubMed/NCBI
|