Introduction
Pancreatic cancer is a lethal disease. It is the
fourth most common cause of cancer-related death in USA with a
5-year survival rate of <5% (1).
This extremely malignant tumor type exhibits rapid progression
without the presentation of obvious symptoms, and as a consequence
it is advanced in the majority of cases at diagnosis (2,3). At
that time, surgical intervention was not suitable for 80% of the
patients (4,5). Current chemo- and radio-therapy have
also met with limited success. Novel diagnostic and therapeutic
strategies are urgently needed to improve prognosis for pancreatic
cancer patients.
Epigenetic therapeutic agents, such as pan-HDAC
inhibitors and DNA methyltransferase (DNMTs) inhibitors, have
showed efficacy in the treatment of cutaneous T-cell lymphoma
(CTCL) (6), myelodysplastic
syndrome (MDS) (7,8), breast cancers (9), ovarian cancers (10). 5-Aza-CdR is currently one of the
most commonly used demethylation nucleoside analogues (11). It mainly inhibits DNMT expression
under a low concentration. It was approved by the Food and Drug
Administration (FDA) to be mainly used for the treatment of blood
system tumors. Suberoylanilide hydroxamic acid (SAHA) is an HDAC
inhibitor that has the permeability to cross the
blood-brain-barrier and to cause biological responses in the mouse
brain, therefore, making it as a preferred candidate drug for
testing in gliomas (12).
Comprehensive treatment is the main trend for
cancers. Drug combinations are currently an important strategy for
antitumor treatment. The aim of this study was to investigate the
demethylation efficacy of 5-Aza-CdR in combination with SAHA on
pancreatic cancer cells. It was demonstrated that 5-Aza-CdR
combined with SAHA inhibited cell proliferation, migration and
invasion, and induced cell cycle arrest and apoptosis, through
upregulation tumor suppressor genes p16 and p53 and inhibiting
PI3K/AKT/PTEN signaling pathway. This finding might provide a new
strategy for the clinical treatment of pancreatic cancer.
Materials and methods
Cell lines and reagents
Pancreatic cancer cells lines AsPC-1 and SW1990 were
all obtained from the Chinese Academy of Sciences Cell Bank
(Shanghai, China). AsPC-1 were cultured in RPMI-1640 (Gibco)
supplemented with 10% FBS, grown in 5% CO2 saturated
humidity at 37°C. SW1990 cells were cultured in L-15 medium (Gibco)
supplemented with 10% FBS.
5-Aza-CdR and SAHA were purchased from Sigma
(Selleck). RPMI-1640 and Dulbecco's modified Eagle's medium were
obtained from Invitrogen (Carlsbad, CA, USA). The Annexin/PI
apoptosis analysis kit was from BD Biosciences (San Diego, CA,
USA). Total RNA was isolated from the cultured cells by using
miniBEST universal RNA extraction kit and amplificated using SYBR
green RT-PCR mix (Takara Bio, Inc.).
Cell proliferation assay
SRB method was used to test cell proliferation with
the treatment of 5-Aza-CdR and SAHA. Cells were seeded in 96-well
microtiter tissue culture plates and cultured for 24 h. Then we
added indicated concentration of 5-Aza-CdR and SAHA and cultured
for 24, 48 and 72 h, respectively. At the end of the treatment,
cells were fixed with 10% w/v of trichloroacetic acid (100 µl) for
1 h at 4°C. The plates were then washed and air-dried. Samples were
stained with 100 µl of SRB solution (in 0.4% w/v in acetic acid)
for 20 min at room temperature. The plates were then washed with
acetic acid (1%) and air-dried. Tris-base (10 mM, 100 µl, pH 10.0)
was added to each well for solubilization. Optical density (OD)
values were measured at 540 nm with a reference wavelength of 630
nm using microtiter plate reader (VERSMax).
Cell migration and cell invasion
assay
The cell migratory potential was evaluated using
Transwell assay. Briefly, Transwell assay was conducted using
specialized MilliCell chambers (Millipore, Bedford, MA, USA). The
inserts contained an 8-µm pore size polycarbonate membrane.
FBS-containing medium (10%) was placed in the lower chambers to act
as a chemo-attractant. Then, 1×105 AsPC-1, SW1990
harvested from treatment group in a 100-µl volume of serum-free
medium were placed in the upper chambers and incubated at 37°C for
<24 h. Then they were fixed and stained by 0.1% crystal violet
staining solution for 15 min. Cells on the upper surface of
membrane were scraped off with cotton swabs and counted under a
microscope in five randomly selected fields at a magnification of
×200 after dried.
Flow cytometry analysis of cell cycle
and apoptosis
For cell apoptosis analysis, cells treated with
single or two reagents were harvested at 70–80% confluence and
incubated with reagent containing Annexin V-FITC and propidium
iodide (BD Biosciences) for 15 min in darkness at room temperature.
Apoptotic cells were analyzed using FACSCaliber flow cytometer (BD
Biosciences).
Western blot analysis
The cells were treated with 5-Aza-CdR and SAHA,
either alone or in combination for 72 h. The control cells were
treated with 0.1% DMSO only. Total proteins were extracted from the
cells using precooling lysis buffer, and the liquid was collected.
After centrifugation at 120,000 × g for 5 min at 4°C, the
supernatant was collected and the protein concentration was
determined using the BCA protein assay kit according to the
manufacturer's instructions. The protein lysates (20 µg/lane) were
separated on 10% SDS polyacrylamide gel and transferred onto a
nitrocellulose membrane. Each membrane was blocked with 5% BSA and
then incubated with the indicated primary antibodies against P16,
TP53, p-AKT, AKT, PTEN and β-actin overnight at 4°C. Subsequently,
the membrane was incubated with the secondary antibodies for 1 h at
room temperature.
Statistical analysis
The results were repeated in at least three separate
experiments. The data are expressed as the means ± SD. Statistical
comparisons were carried out using one-way analysis of variance,
which revealed significant differences between groups. Statistical
analyses were carried out using SPSS version 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
5-Aza-CdR and SAHA inhibits cell
proliferation
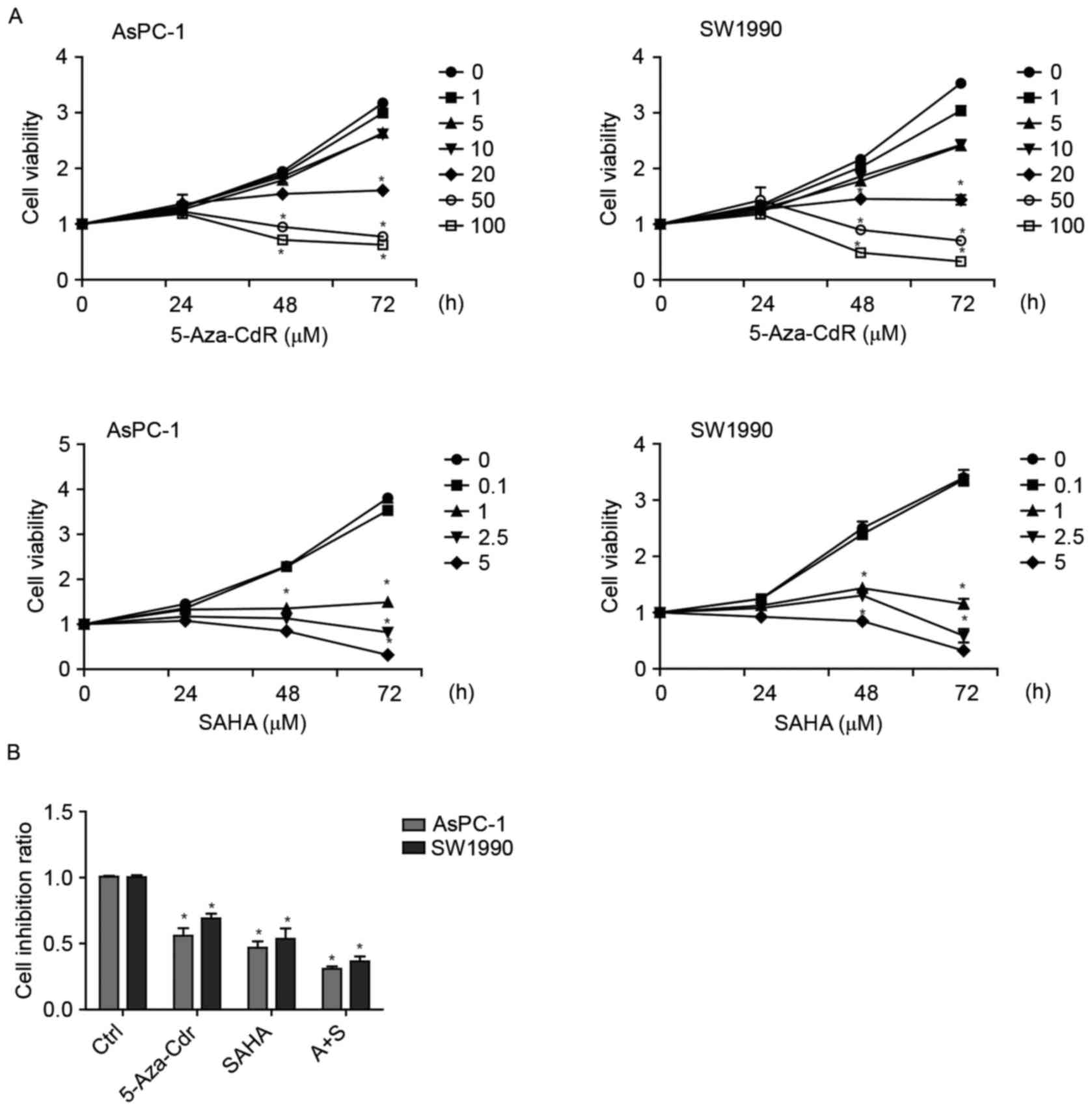
To investigate the effect of 5-Aza-CdR and SAHA on
cell growth, AsPC-1 and SW1990 cells were cultured with 5-Aza-CdR
or/and SAHA for 24, 48 and 72 h. According to SRB assay, 5-Aza-CdR
and SAHA reduced proliferation of the pancreatic cell lines in
either time- or dose-dependent manner (Fig. 1A). Additionally, there was an
enhanced additive effect of 5-Aza-CdR and SAHA on the proliferative
inhibition of pancreatic cancer cells (Fig. 1B).
5-Aza-CdR and SAHA inhibit cell
migration
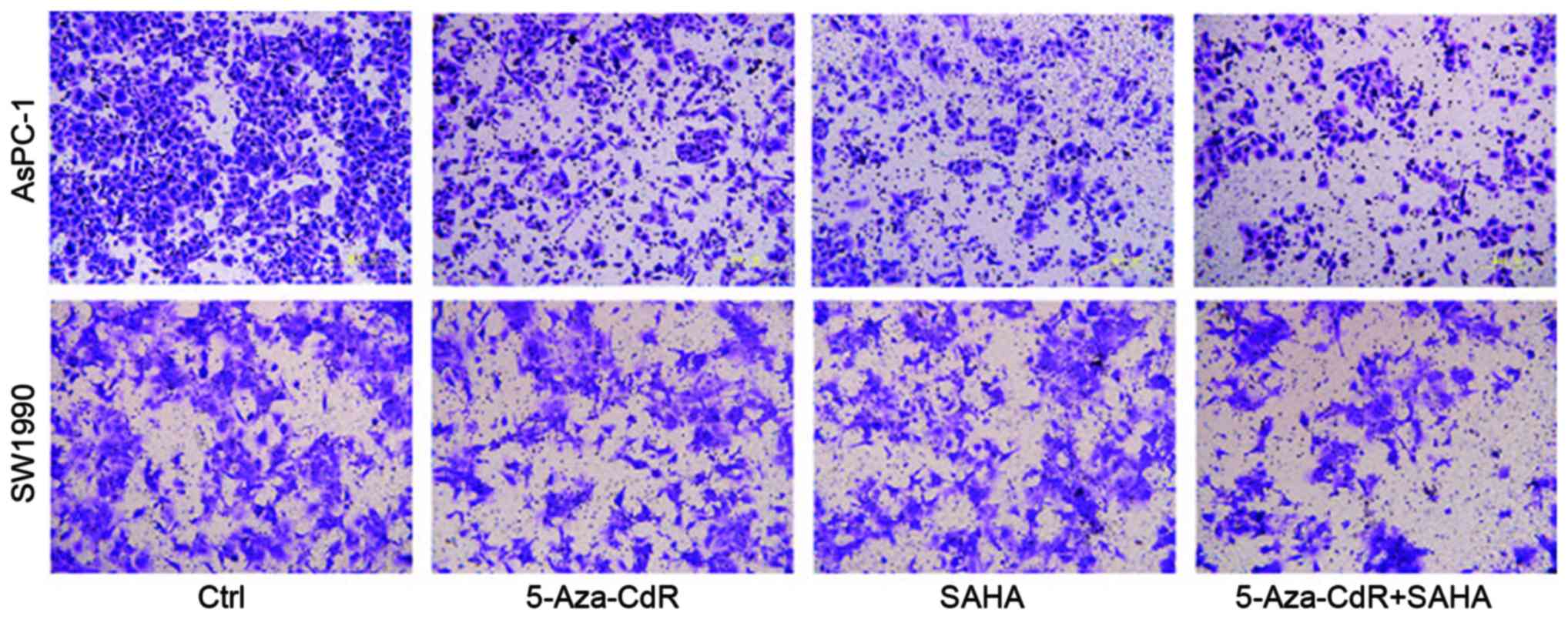
We used a Transwell assay to examine the effects of
5-Aza-CdR and SAHA on pancreatic cancer cell migration. The number
of 5-Aza-CdR or SAHA treated cells were remarkably reduced as
compared to control group, indicating decreased migratory abilities
following the treatment. The combination of both reagents inhibited
cell migrated ablity the most (Fig.
2).
5-Aza-CdR and SAHA induce cell arrest
and promote cell apoptosis
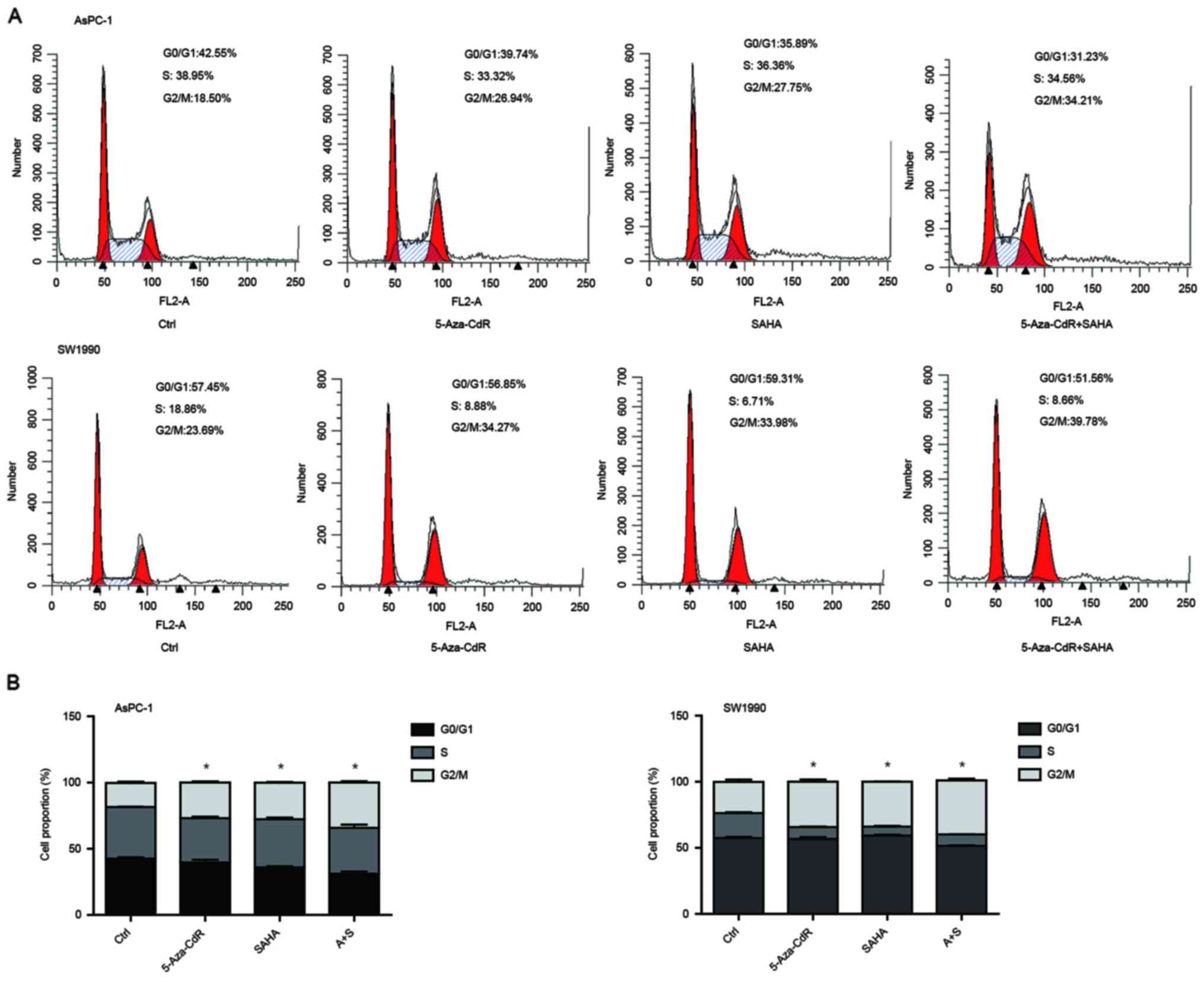
We performed flow cytometric analysis to assess
whether cell cycle and apoptosis were altered. We observed that the
cells were arrested in G2/M phase with the treatment of both
5-Aza-CdR and SAHA, and combination of two reagents induced more
cells in G2 arrest (Fig. 3A). The
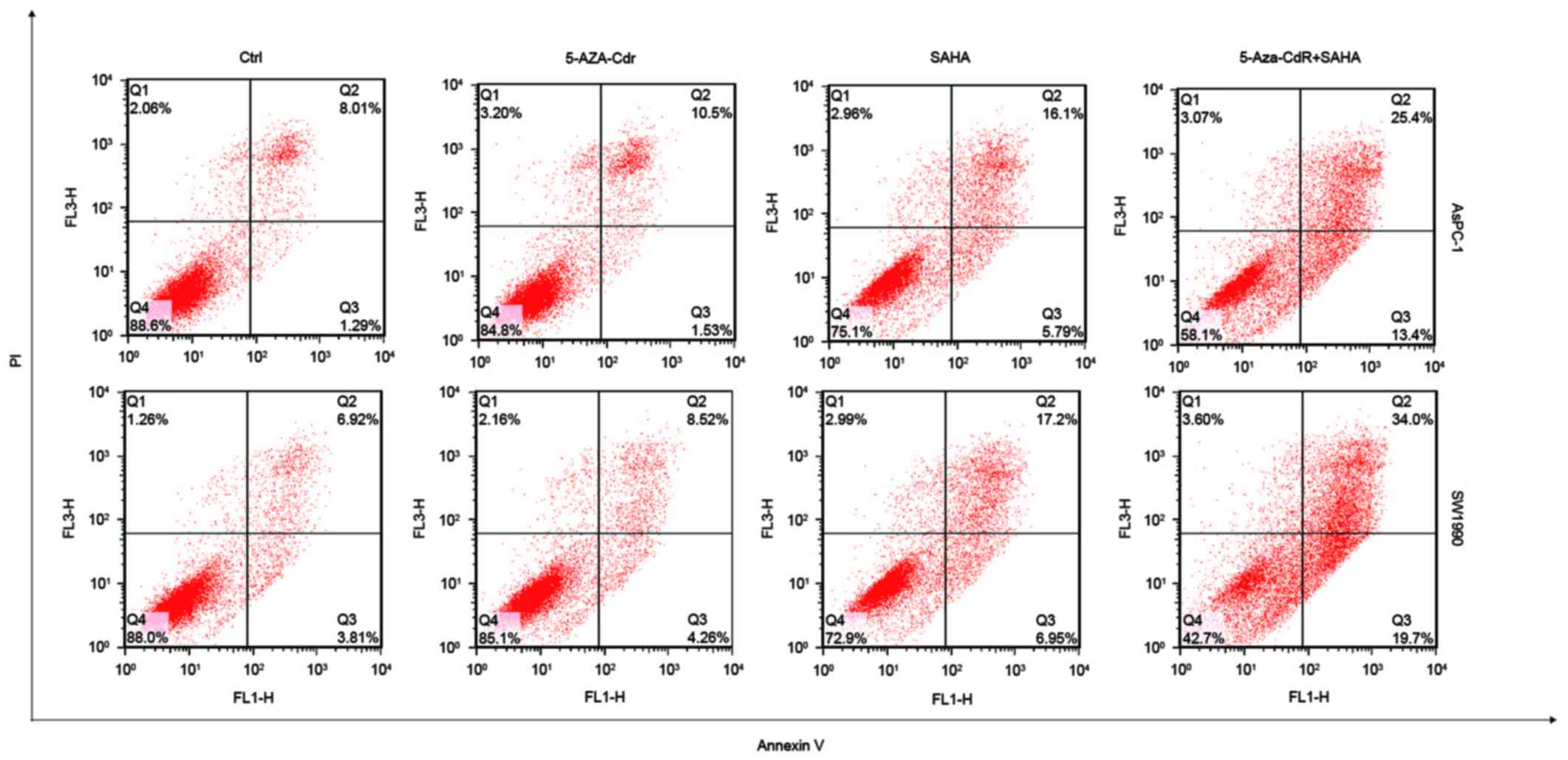
proportion ratio of each period is also shown (Fig. 3B). SAHA alone induced cell
apoptosis, but 5-Aza-CdR alone could not. Interestingly,
combination of 5-Aza-CdR and SAHA remarkably increased apoptosis
(Fig. 4).
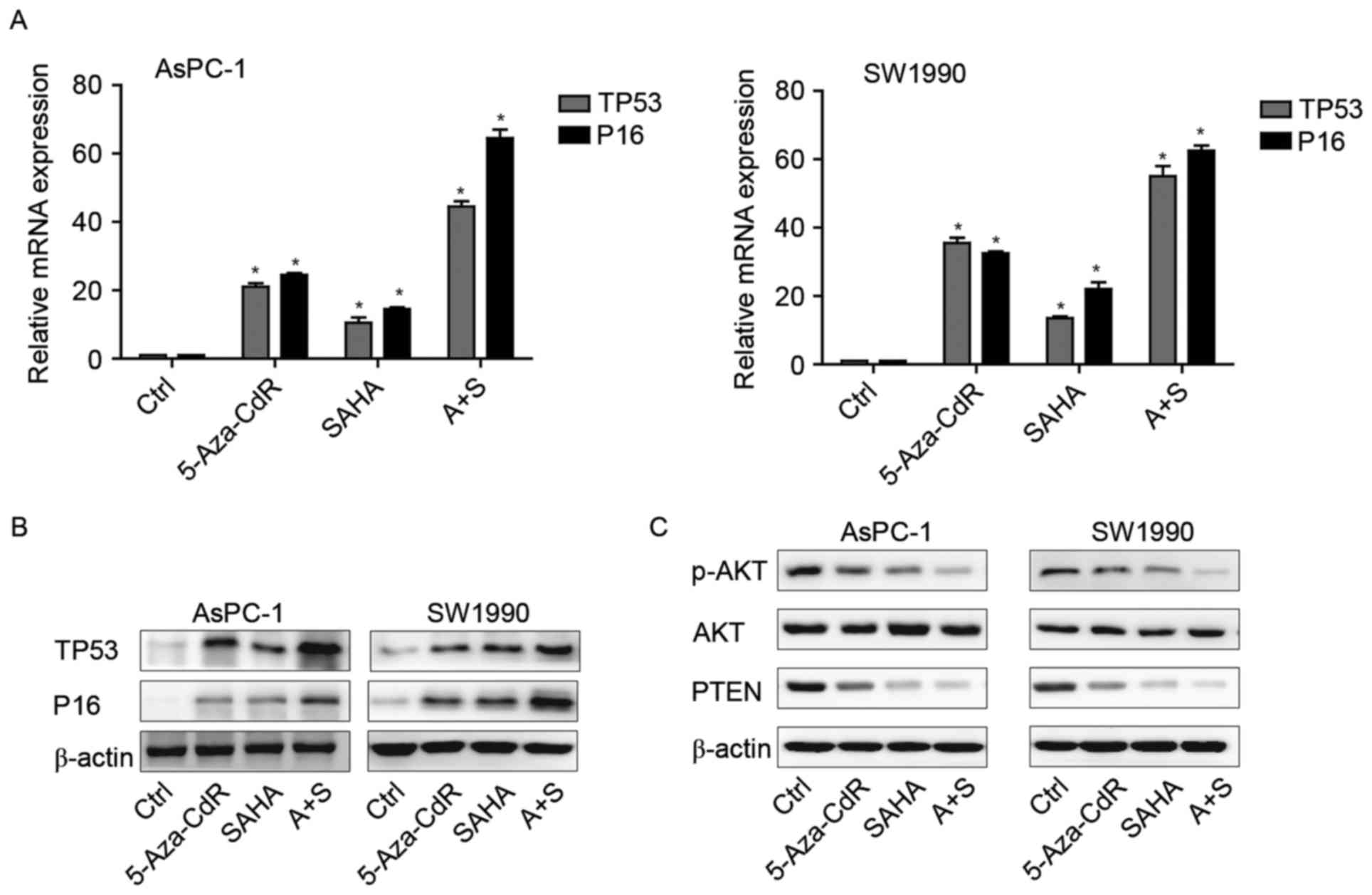
The mRNA and protein expression of
p16, p53 and Rb in pancreatic cancer cells after the exposure to
5-Aza-CdR or/and SAHA
In order to demonstrate whether 5-Aza-CdR and SAHA
have an effect at the transcriptional and protein level, PCR and WB
was used to detect the levels of tumor-suppressor genes P16 and
TP53. After the treatment with 5-Aza-CdR or/and SAHA, the mRNA and
protein expression levels of P16 and TP53 were upregulated in
treated groups compared to those observed in the control. Their
combination was more significantly enhanced when compared with the
levels following treatment with 5-Aza-CdR or SAHA alone (Fig. 5A and B).
5-Aza-CdR or/and SAHA inhibit the
PI3K/AKT/PTEN signaling pathway
PI3K/AKT signaling pathway has been reported to be
activated in pancreatic cancer (13). Here, we investigated whether these
two agents could affect this pathways, leading to pancreatic cancer
cell inhibition. Our findings showed that either alone or combined,
they could downregulate p-AKT and PTEN expression in both cell
types (Fig. 5C).
Discussion
Pancreatic cancer is a world-wide problem that is
hard to conquer. This is because its unique anatomic location and
rapid progress. Gemcitabine has been the standard systemic therapy
for the palliative treatment of pancreatic cancer over the last
decade, although the 1-year survival rate of <20% remains
unsatisfactory (14–17). Many studies have shown epigenetic
modifications associated with cancer (18,19).
Epigenetic modification has been shown as promising antitumor
effect in T cell lymphoma (20),
acute myeloid leukemia (21,22),
breast cancers (9), and some other
malignant tumors (23), however,
with limited efficacy of limited drugs. Therefore, novel therapies
are urgently needed to better inhibit pancreatic cancer cell
proliferation and prolong overall survival time of the patients. At
these circumstances, we consider that some epigenetic inhibitors
might take effect since epigenetic modification is involved in the
pancreatic cancer progression (24). Here, we reported two drugs,
5-Aza-CdR and SAHA, and aimed to define their combination effects
on pancreatic cancer cells.
5-Aza-CdR was first characterized 30 years ago and
it functions as a mechanism-dependent suicide inhibitor of DNA
methyltransferases, with which genes silenced by hypermethylation
can be reactivated (25). SAHA is a
pan-HDAC inhibitor, and the antitumor effects of SAHA have been
reported in chronic myelogenous leukemia (26), lung (27), pancreatic (28), liver (29), cervical (30), head and neck (31), breast (32) and ovarian cancers (33). Our results showed that 5-Aza-CdR or
SAHA could inhibit pancreatic cancer cell proliferation in a dose-
and time-dependent manner. Then we further investigated the
possible mechanism of its inhibition. We detected cell migration,
invasion, the cell cycle and apoptosis. We found that 5-Aza-CdR or
SAHA alone could decrease the migration of pancreatic cancer cells
and induced cell cycle arrest. SAHA increased cell apoptosis,
however, 5-Aza-CdR could not induce cell apoptosis. Combination of
two drugs had stronger inhibition effect than each one alone.
Specially, combination of 5-Aza-CdR and SAHA enhanced apoptosis,
though 5-Aza-CdR had no effects alone. TP53 has been reported with
high methylation and promoter in different cancers, such as mutiple
myeloma (34), esophageal squamous
cell cancer (35), lung cancer
(36), and breast cancer (37). The gene encoding p16 is mutated or
downregulated in several cancer cells (38–40).
However, breast carcinoma progression has been related to
overexpression of p16 (41), and
this has been reported in head and neck squamous carcinoma
(42), in prostate carcinoma
(43). Here, we detected TP53 and
p16 mRNA and protein expression after treatment of 5-Aza-CdR and
SAHA. We found both expressions were upregulated after treatment.
Besides, the two drugs inhibited the PI3K/AKT/PTEN signaling
pathway, leading to inhibition of cell proliferation.
In conclusion, this study illustrated that treatment
with epigenetic agents decreased cell proliferation and induced
cell death in pancreatic cancer cells, while we also demonstrated
that epigenetic agents were able to upregulate TP53, P16 expression
and inhibited the I3K/AKT/PTEN signaling pathway. These data
suggest that epigenetic therapy has the potential to delay
pancreatic progression, and may have potential application in the
management of pancreatic cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81502017, 81502018,
81572315, 81171887 and 91229117), and in part by National Key
Clinical Discipline-Oncology.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society, . Cancer Facts
and Figures 2008. American Cancer Society; Atlanta, GA: 2008,
https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2008.html
|
|
3
|
American Cancer Society, . Cancer Facts
and Figures 2007. American Cancer Society; Atlanta, GA: 2007,
https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2007.html
|
|
4
|
Von Hoff DD, Evans DB and Hruban RH:
Pancreatic Cancer. 1st. Jones and Bartlett Publishers; Sudbury, MA:
2005
|
|
5
|
Chang DK, Merrett ND and Biankin AV; NSW
Pancreatic Cancer Network, : Improving outcomes for operable
pancreatic cancer: Is access to safer surgery the problem? J
Gastroenterol Hepatol. 23:1036–1045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glaser KB: HDAC inhibitors: Clinical
update and mechanism-based potential. Biochem Pharmacol.
74:659–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gore SD: Combination therapy with DNA
methyltransferase inhibitors in hematologic malignancies. Nat Clin
Pract Oncol. 2 Suppl 1:S30–S35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Issa JP and Byrd JC: Decitabine in chronic
leukemias. Semin Hematol. 42 Suppl 2:S43–S49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Munster PN, Troso-Sandoval T, Rosen N,
Rifkind R, Marks PA and Richon VM: The histone deacetylase
inhibitor suberoylanilide hydroxamic acid induces differentiation
of human breast cancer cells. Cancer Res. 61:8492–8497.
2001.PubMed/NCBI
|
|
10
|
Yang XF, Zhao ZJ, Liu JJ, Yang XH, Gao Y,
Zhao S, Shi S, Huang KQ and Zheng HC: SAHA and/or MG132 reverse the
aggressive phenotypes of glioma cells: An in vitro and vivo study.
Oncotarget. 8:3156–3169. 2017.PubMed/NCBI
|
|
11
|
Brueckner B, Kuck D and Lyko F: DNA
methyltransferase inhibitors for cancer therapy. Cancer J.
13:17–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hockly E, Richon VM, Woodman B, Smith DL,
Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, et
al: Suberoylanilide hydroxamic acid, a histone deacetylase
inhibitor, ameliorates motor deficits in a mouse model of
Huntington's disease. Proc Natl Acad Sci USA. 100:pp. 2041–2046.
2003; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL
and Reddy SA: The PI 3-kinase/Akt signaling pathway is activated
due to aberrant Pten expression and targets transcription factors
NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene.
23:8571–8580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rothenberg ML, Moore MJ, Cripps MC,
Andersen JS, Portenoy RK, Burris HA III, Green MR, Tarassoff PG,
Brown TD, Casper ES, et al: A phase II trial of gemcitabine in
patients with 5-FU-refractory pancreas cancer. Ann Oncol.
7:347–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casper ES, Green MR, Kelsen DP, Heelan RT,
Brown TD, Flombaum CD, Trochanowski B and Tarassoff PG: Phase II
trial of gemcitabine (2,2′-difluorodeoxycytidine) in patients with
adenocarcinoma of the pancreas. Invest New Drugs. 12:29–34. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carmichael J, Fink U, Russell RC, Spittle
MF, Harris AL, Spiessi G and Blatter J: Phase II study of
gemcitabine in patients with advanced pancreatic cancer. Br J
Cancer. 73:101–105. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katsushima K, Natsume A, Ohka F, Shinjo K,
Hatanaka A, Ichimura N, Sato S, Takahashi S, Kimura H, Totoki Y, et
al: Targeting the Notch-regulated non-coding RNA TUG1 for glioma
treatment. Nat Commun. 7:136162016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Y and Teschendorff AE: Epigenetic and
genetic deregulation in cancer target distinct signaling pathway
domains. Nucleic Acids Res. 45:583–596. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batty N, Malouf GG and Issa JP: Histone
deacetylase inhibitors as anti-neoplastic agents. Cancer Lett.
280:192–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flotho C, Claus R, Batz C, Schneider M,
Sandrock I, Ihde S, Plass C, Niemeyer CM and Lübbert M: The DNA
methyltransferase inhibitors azacitidine, decitabine and zebularine
exert differential effects on cancer gene expression in acute
myeloid leukemia cells. Leukemia. 23:1019–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laurenzana A, Petruccelli LA, Pettersson
F, Figueroa ME, Melnick A, Baldwin AS, Paoletti F and Miller WH Jr:
Inhibition of DNA methyltransferase activates tumor necrosis factor
alpha-induced monocytic differentiation in acute myeloid leukemia
cells. Cancer Res. 69:55–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han T, Hu H, Zhuo M, Wang L, Cui JJ, Jiao
F and Wang LW: Long non-coding RNA: An emerging paradigm of
pancreatic cancer. Curr Mol Med. 16:702–709. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe Y and Maekawa M: Methylation of
DNA in cancer. Adv Clin Chem. 52:145–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bu Q, Cui L, Li J, Du X, Zou W, Ding K and
Pan J: SAHA and S116836, a novel tyrosine kinase inhibitor,
synergistically induce apoptosis in imatinib-resistant chronic
myelogenous leukemia cells. Cancer Biol Ther. 15:951–962. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TG, Jeong EH, Kim SY, Kim HR and Kim
CH: The combination of irreversible EGFR TKIs and SAHA induces
apoptosis and autophagy-mediated cell death to overcome acquired
resistance in EGFR T790M-mutated lung cancer. Int J Cancer.
136:2717–2729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumagai T, Wakimoto N, Yin D, Gery S,
Kawamata N, Takai N, Komatsu N, Chumakov A, Imai Y and Koeffler HP:
Histone deacetylase inhibitor, suberoylanilide hydroxamic acid
(Vorinostat, SAHA) profoundly inhibits the growth of human
pancreatic cancer cells. Int J Cancer. 121:656–665. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang BY, Xiong M, Ji GB, Zhang EL, Zhang
ZY, Dong KS, Chen XP and Huang ZY: Synergistic suppressive effect
of PARP-1 inhibitor PJ34 and HDAC inhibitor SAHA on proliferation
of liver cancer cells. J Huazhong Univ Sci Technolog Med Sci.
35:535–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing J, Wang H, Xu S, Han P, Xin M and
Zhou JL: Sensitization of suberoylanilide hydroxamic acid (SAHA) on
chemoradiation for human cervical cancer cells and its mechanism.
Eur J Gynaecol Oncol. 36:117–122. 2015.PubMed/NCBI
|
|
31
|
Kumar B, Yadav A, Lang JC, Teknos TN and
Kumar P: Suberoylanilide hydroxamic acid (SAHA) reverses
chemoresistance in head and neck cancer cells by targeting cancer
stem cells via the downregulation of nanog. Genes Cancer.
6:169–181. 2015.PubMed/NCBI
|
|
32
|
Min A, Im SA, Kim DK, Song SH, Kim HJ, Lee
KH, Kim TY, Han SW, Oh DY, Kim TY, et al: Histone deacetylase
inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances
anti-tumor effects of the poly (ADP-ribose) polymerase (PARP)
inhibitor olaparib in triple-negative breast cancer cells. Breast
Cancer Res. 17:332015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Tong Y, Liu Y, Liu H, Li C, Zhao Y
and Zhang Y: Effects of suberoylanilide hydroxamic acid (SAHA)
combined with paclitaxel (PTX) on paclitaxel-resistant ovarian
cancer cells and insights into the underlying mechanisms. Cancer
Cell Int. 14:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herrero AB, Rojas EA, Misiewicz-Krzeminska
I, Krzeminski P and Gutiérrez NC: Molecular mechanisms of p53
deregulation in cancer: An overview in multiple myeloma. Int J Mol
Sci. 17:172016. View Article : Google Scholar
|
|
35
|
Lv D, Sun R, Yu Q and Zhang X: The long
non-coding RNA maternally expressed gene 3 activates p53 and is
downregulated in esophageal squamous cell cancer. Tumour Biol.
37:16259–16267. 2016. View Article : Google Scholar
|
|
36
|
Blanco J, Lafuente D, Gómez M, García T,
Domingo JL and Sánchez DJ: Polyvinyl pyrrolidone-coated silver
nanoparticles in a human lung cancer cells: Time- and
dose-dependent influence over p53 and caspase-3 protein expression
and epigenetic effects. Arch Toxicol. 91:651–666. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramadoss S, Guo G and Wang CY: Lysine
demethylase KDM3A regulates breast cancer cell invasion and
apoptosis by targeting histone and the non-histone protein p53.
Oncogene. 36:47–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang Z, Ju H, Ling J, Zhuang Z, Li Z,
Wang H, Fleming JB, Freeman JW, Yu D, Huang P, et al: Cooperativity
of oncogenic K-ras and downregulated p16/INK4A in human pancreatic
tumorigenesis. PLoS One. 9:e1014522014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Amano M, Eriksson H, Manning JC, Detjen
KM, André S, Nishimura S, Lehtiö J and Gabius HJ: Tumour suppressor
p16 (INK4a) - anoikis-favouring decrease in N/O-glycan/cell surface
sialylation by downregulation of enzymes in sialic acid
biosynthesis in tandem in a pancreatic carcinoma model. FEBS J.
279:4062–4080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian X, Durkin ME, Wang D, Tripathi BK,
Olson L, Yang XY, Vass WC, Popescu NC and Lowy DR: Inactivation of
the Dlc1 gene cooperates with downregulation of p15INK4b and
p16Ink4a, leading to neoplastic transformation and poor prognosis
in human cancer. Cancer Res. 72:5900–5911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pare R, Shin JS and Lee CS: Increased
expression of senescence markers p14 (ARF) and p16 (INK4a) in
breast cancer is associated with an increased risk of disease
recurrence and poor survival outcome. Histopathology. 69:479–491.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baruah P, Lee M, Wilson PO, Odutoye T,
Williamson P, Hyde N, Kaski JC and Dumitriu IE: Impact of p16
status on pro- and anti-angiogenesis factors in head and neck
cancers. Br J Cancer. 113:653–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Z, Rosen DG, Yao JL, Huang J and Liu
J: Expression of p14ARF, p15INK4b, p16INK4a, and DCR2 increases
during prostate cancer progression. Mod Pathol. 19:1339–1343. 2006.
View Article : Google Scholar : PubMed/NCBI
|