Introduction
Osteosarcoma is the most common primary malignant
bone tumor, in which sarcoma cells of malignant proliferation
generate neoplastic osteoid or immature bones directly (1). Its histological characteristic is that
proliferative spindle tumor cells generate osteoid matrix or
immature bones directly (2).
Microscopically, it can be observed that there are many tumor cells
with various sizes and shapes and nuclei, small multinuclear giant
cells, spindle cells, immature chondrocytes and malignant
osteoblasts which are dark with a large nucleus (3). Almost all metastases of osteosarcoma
occur in lungs via blood and the minority of its metastases occurs
in visceral organs such as brain and kidneys and via lymph nodes
(4).
MicroRNAs (miRNAs) are a type of non-coding RNAs
with regulatory function and a length of approximately 20–25
nucleotides, which identify target mRNAs according to the principle
of complementary base pairing and guide silencing complex to
degrade target mRNAs or repress translation of mRNAs (5). Increasing studies found that miRNAs
played an important role in specific cellular processes such as
cell differentiation, morphosis and neoplasia (6). In human cancer, a miRNA can be an
oncogene or a cancer suppressor gene in the process of
tumorigenesis.
Recent research showed that microRNAs (miRNAs)
played an important role in tumorigenesis and tumor development,
which provided a new idea for diagnosis and treatment of tumors
(7). miRNAs are a type of recently
discovered non-coding microRNAs with a length of 22–28 nucleotides,
which widely exist in eucaryon. By complete or partial
complementary pairing with and binding to the target gene mRNA
3′-UTR, miRNAs cause degradation of target miRNA or translational
suppression to regulate the expression of target genes, affecting
biological behavior such as cell proliferation, invasion,
differentiation and apoptosis. Unlike siRNAs, miRNAs have multiple
target genes. As endogenous RNA interference is triggered, miRNAs
regulate cells which are closer to a physiological level instead of
regulation through a simple gene knockout.
The p21-activated kinase 7 (PAK7, also known as
PAK5) is a recently discovered protein involving in cell apoptosis
and a newly found member of the family of p21-activated kinases
(PAKs) (8). p21-activated kinases
are a kind of evolutionarily conserved serine/threonine protein
kinases which participate in regulation of many important
biological activities such as regulation of cytoskeleton, cell
cycle, cell apoptosis, gene transcription and angiogenesis and
especially play an important biological role in tumorigenesis,
tumor invasion and metastases (9).
Materials and methods
Ethics statement, patients, and
samples
Peripheral blood of OS patients and normal
volunteers were collected at The Second Hospital of Jilin
University, (Changchun, China). Serum was collected after 1000 × g
for 10 min and saved at −70°C. The follow-up protocols were
consistent every three months. This study was approved by the
ethics committees of The Second Hospital of Jilin University.
Cell lines and human tumor
samples
The human osteosarcoma cell line F5M2 was acquired
from the American Type Culture Collection (Manassas, VA, USA). F5M2
cells werecultured with RPMI-1640 medium (Gibco Life Technologies,
Gaitherburg, MD, USA) supplemented with 10% fetal bovine serum
(Gibco Life Technologies) and penicillin (100 U/ml)/streptomycin
(100 µg/ml) at 37°C in a humidified atmosphere with 5%
CO2.
Real-time RT-PCR analysis
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies) and reverse-transcribed to cDNA by
the RevertAid First Strand cDNA synthesis kit (Fermentas). The
level of microRNA-95-3p was quantified by RT-qPCR (ABI Prism 7600)
using power SYBR® Green PCR Master Mix (Applied
Biosystems). PCR conditions were predenaturation at 95°C for 5 min,
then 94°C for 1 min, annealing at 58°C for 30 sec and elongation at
72°C for 30 min, for a total of 40 cycles.
MicroRNA reagents
We purchased TGF-β, microRNA-95-3p,
anti-microRNA-95-3p and negative control miRNA mimics from
Guangzhou RioboBio (Guangzhou, Guangdong, China). MicroRNA-95-3p,
anti-microRNA-95-3p and negative control miRNA mimics were
transfected using Lipofectamine 2000 (Gibco Life Technologies).
Cell proliferation and apoptosis
assays
Cells were seeded onto 96-well plates and treated
with methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay
(Sigma-Aldrich, St. Louis, MO, USA) for 4 h. DMSO was added into
cells with shaking for 20 min. The absorbance was determined at 490
nm on a microplate spectrophotometer (SpectraMax, Molecular
Devices, Sunnyvale, CA, USA).
Cells were seeded onto 6-well plates and stained
with Annexin V-FITC/PI Apoptosis Assay (BD Biosciences Pharmingen,
San Diego, CA, USA) for 20 min in the dark. Apoptotic cells were
recorded by applying CellQuest Software (BD Biosciences
Pharmingen).
Western blotting
Total proteins were extracted from cells using RIPA
buffer containing protease inhibitors and phosphatase inhibitors.
Equal amounts (30 µg) of lysate were separated by 8–10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to PVDF membranes (Millipore). Membranes were blocked
with 5% bovine serum albumin (BSA) and incubated with Bax (Cell
Signaling Technology), TGF-β (Cell Signaling Technology), p-Smad2
(Cell Signaling Technology), p21 (Cell Signaling Technology),
cyclin D1 (Cell Signaling Technology) and GAPDH (Cell Signaling
Technology) at 4°C overnight. Then, membranes was washed with TBST
and incubated with HRP secondary antibodies (Cell Signaling
Technology) for 1 h at 37°C. Protein blank was visualized by ECL
Western blotting kit (Millipore).
Statistical analyses
Comparisons between two groups were analyzed using
the Student's t-test. The data were presented as means ± SD. A
P<0.05 was considered statistically significant.
Results
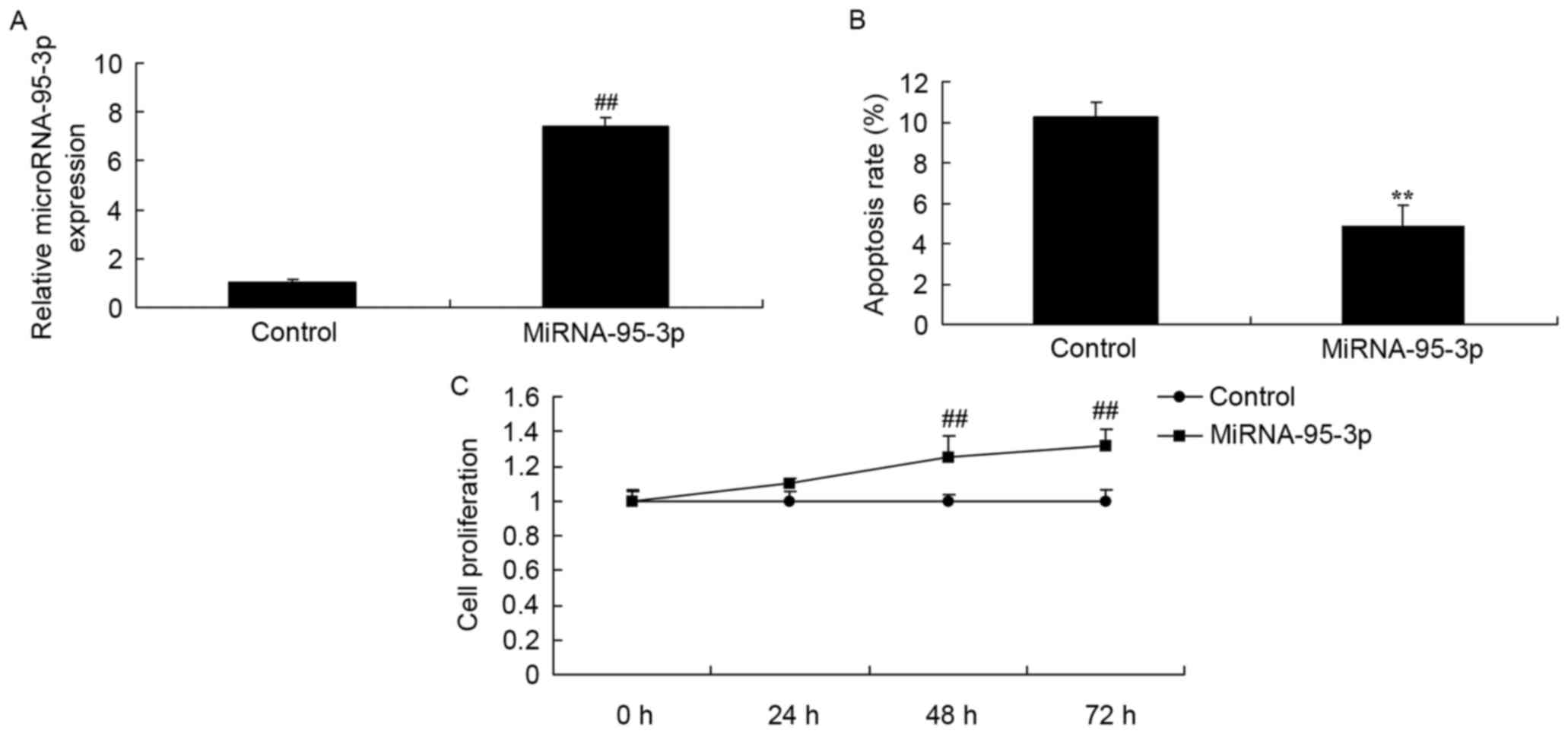
Serum expression of
microRNA-95-3p
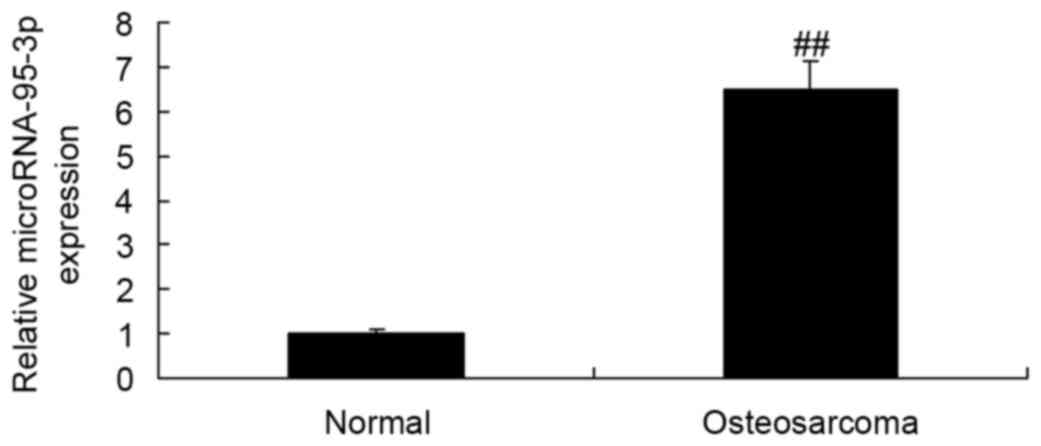
First, we analyzed the serum expression of
microRNA-95-3p using Real-time RT-PCR analysis. As shown in
Fig. 1, compared to healthy
controls, the serum expression of microRNA-95-3p was effectively
upregulated in patients with osteosarcoma.
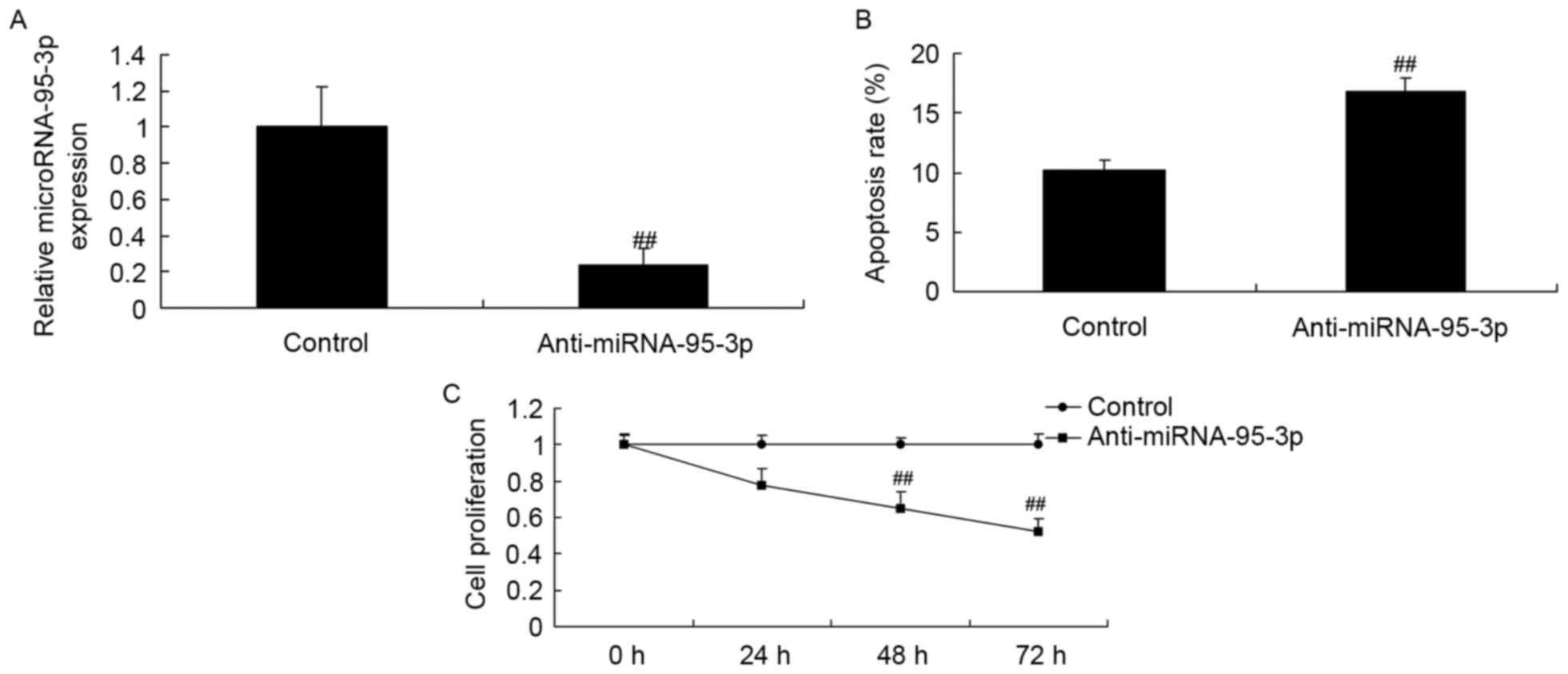
Cell growth and apoptosis of
osteosarcoma cells by microRNA-95-3p downregulation
We determined whether microRNA-95-3p downregulation
affects on cell growth of osteosarcoma in vitro.
MicroRNA-95-3p downregulation significantly inhibited cell
proliferation and induced apoptosis level of osteosarcoma (Fig. 2).
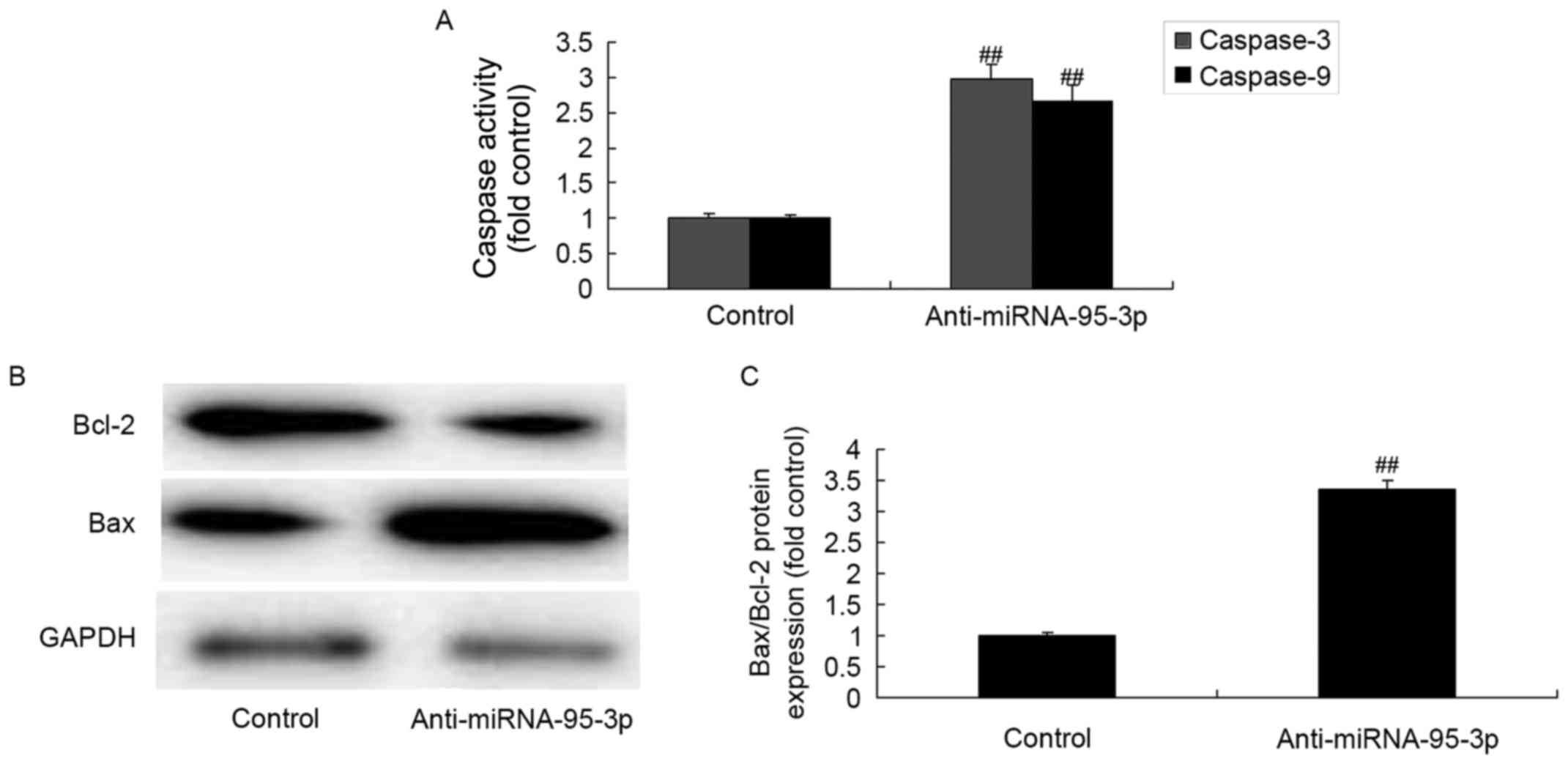
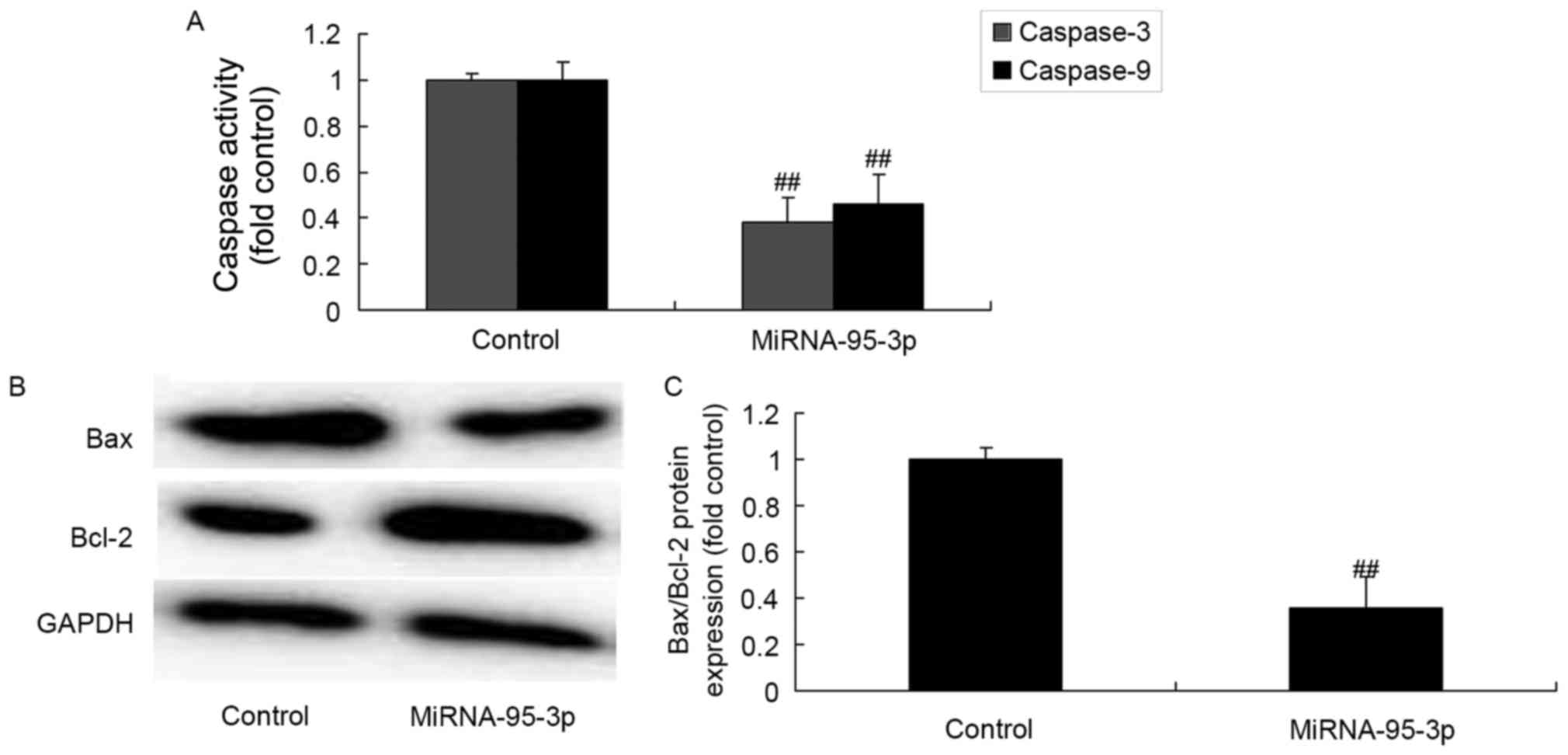
Caspase-3 and caspase-9 activities and
Bax/Bcl-2 protein expression of osteosarcoma cells by
microRNA-95-3p downregulation
Then, we analyzed the mechanism of apoptosis of
microRNA-95-3p on osteosarcoma. However, as compared to control
group, much larger induction of caspase-3 and caspase-9 activity,
and Bax/Bcl-2 protein expression were observed by microRNA-95-3p
downregulation (Fig. 3).
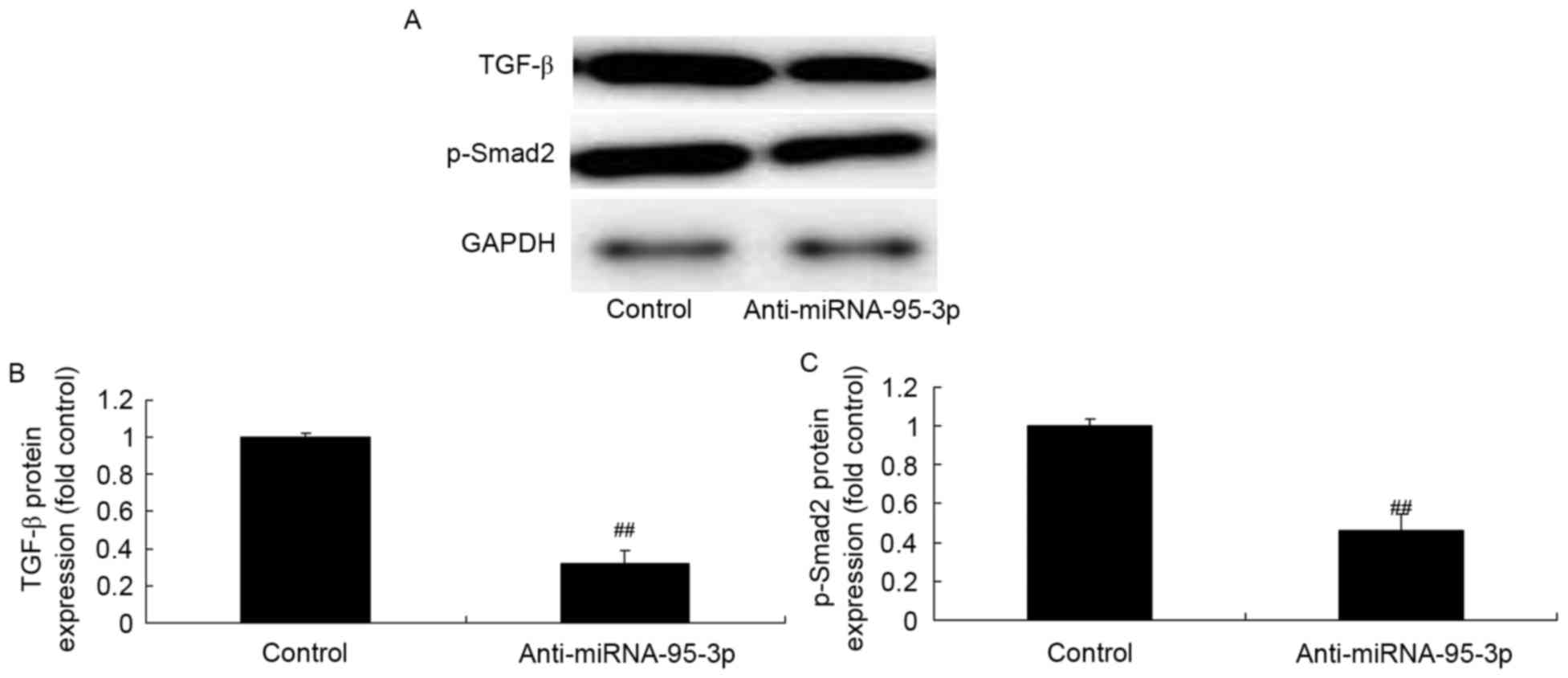
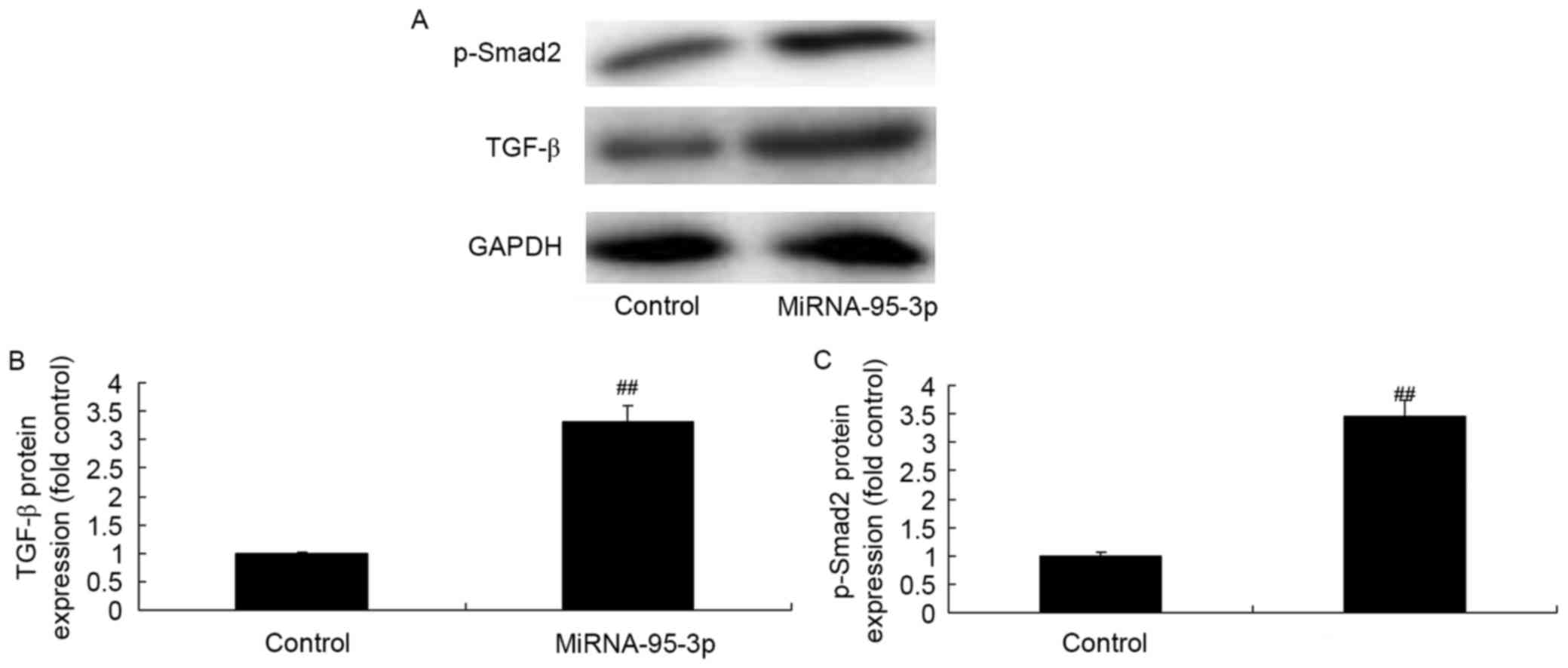
TGF-β and Smad2 protein expression of
osteosarcoma cells by microRNA-95-3p downregulation
To examine the regulative effects of microRNA-95-3p
on apoptosis of osteosarcoma, we conducted western blotting in
TGF-β and Smad2 protein expression. As shown in Fig. 4, TGF-β and Smad2 protein expression
of osteosarcoma was significantly suppressed by microRNA-95-3p
downregulation.
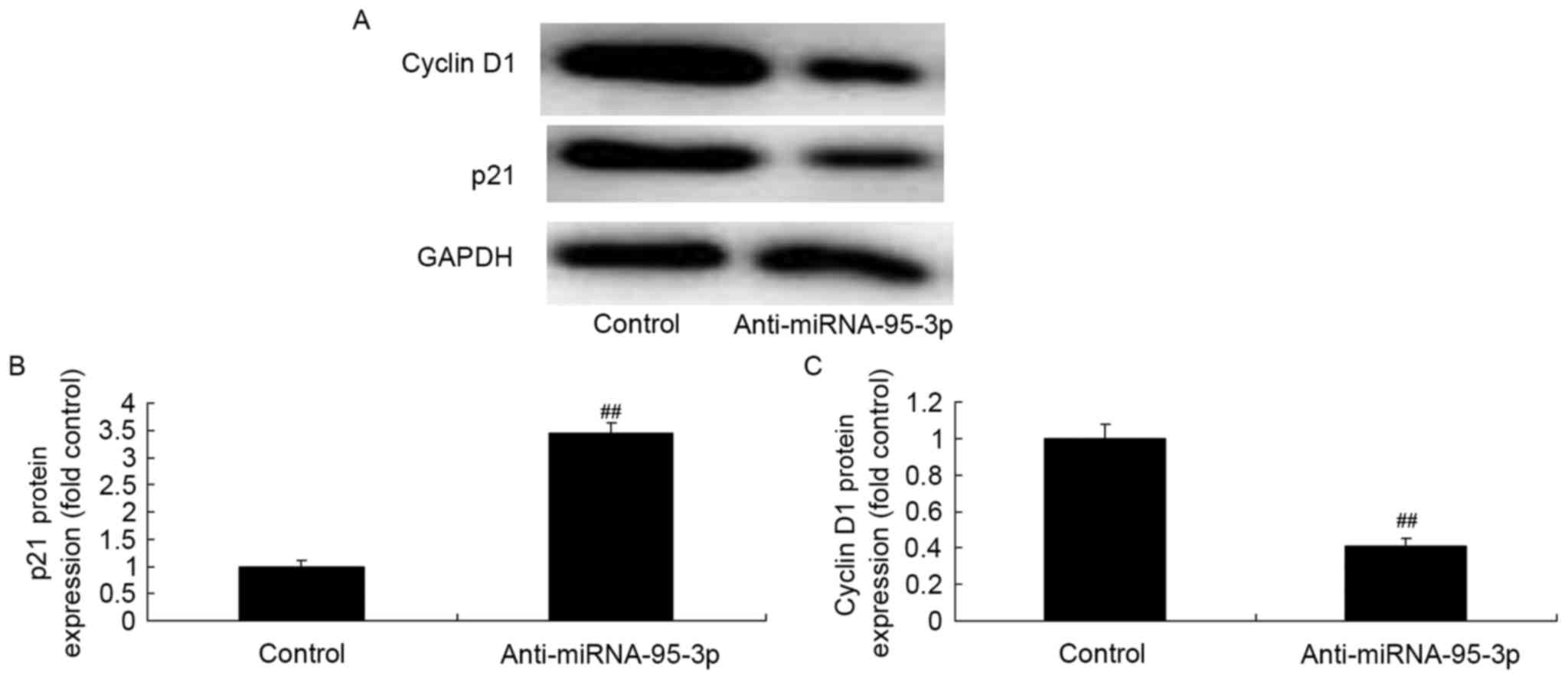
p21 and cyclin D1 protein expression
of osteosarcoma cells by microRNA-95-3p downregulation
Then, we also found that microRNA-95-3p
downregulation significantly reduced p21 and cyclin D1 protein
expression of osteosarcoma in vitro (Fig. 5). Together, these data suggest that
microRNA-95-3p regulates death of osteosarcoma cells by CDKN1A/p21
expression.
Cell growth and apoptosis of
osteosarcoma cells by microRNA-95-3p overexpression
We determined whether the overexpression of
microRNA-95-3p affects cell growth and apoptosis of osteosarcoma
cells in vitro. Compared with control group, cell
proliferation was increased and apoptosis was inhibited in
osteosarcoma cells by microRNA-95-3p overexpression (Fig. 6).
Activity of caspase-3 and caspase-9
and Bax/Bcl-2 protein expression of osteosarcoma cells by
microRNA-95-3p overexpression
To investigate the mechanism for apoptosis of
osteosarcoma cells by microRNA-95-3p overexpression, we examined
the activity of caspase-3 and caspase-9 and Bax/Bcl-2 protein
expression. As showed in Fig. 7,
microRNA-95-3p overexpression suppressed the activity of caspase-3
and caspase-9 and Bax/Bcl-2 protein expression of osteosarcoma
cells in vitro.
TGF-β and Smad2 protein expression of
osteosarcoma cells by microRNA-95-3p overexpression
To check whether the decrease of microRNA-95-3p
regulates TGF-β and Smad2 protein expression of osteosarcoma cells,
TGF-β and Smad2 protein expression were determined by western
blotting. TGF-β and Smad2 protein expression of osteosarcoma cells
were significantly induced by microRNA-95-3p overexpression
(Fig. 8).
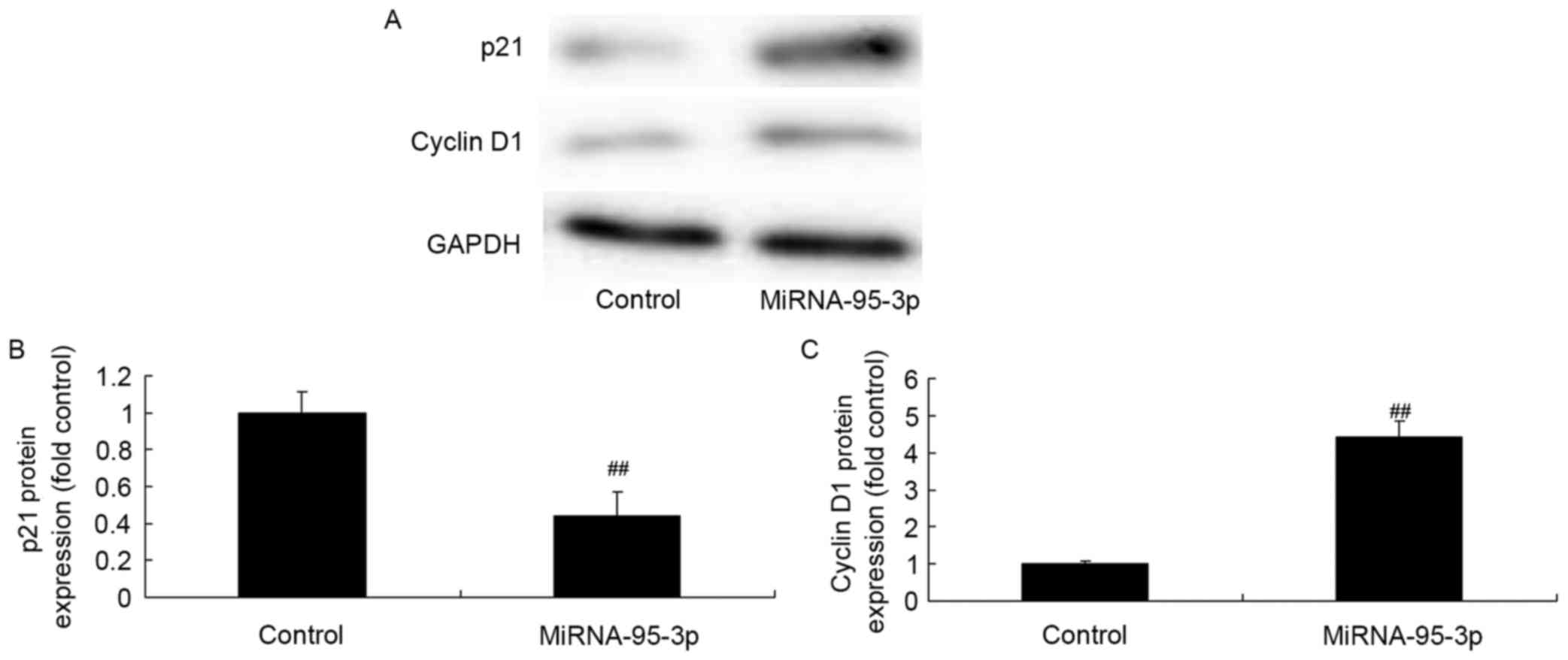
p21 and cyclin D1 protein expression
of osteosarcoma cell by microRNA-95-3p overexpression
To examine whether the observed effects of
microRNA-95-3p are due to regulation of p21 and cyclin D1 protein
expression, anti-microRNA-95-3p mimics were transfected into cells.
p21 protein expression was significantly suppressed and cyclin D1
protein expression was also induced in osteosarcoma cells in
vitro (Fig. 9). Together,
microRNA-95-3p regulates p21 and cyclin D1 protein expression of
osteosarcoma cells.
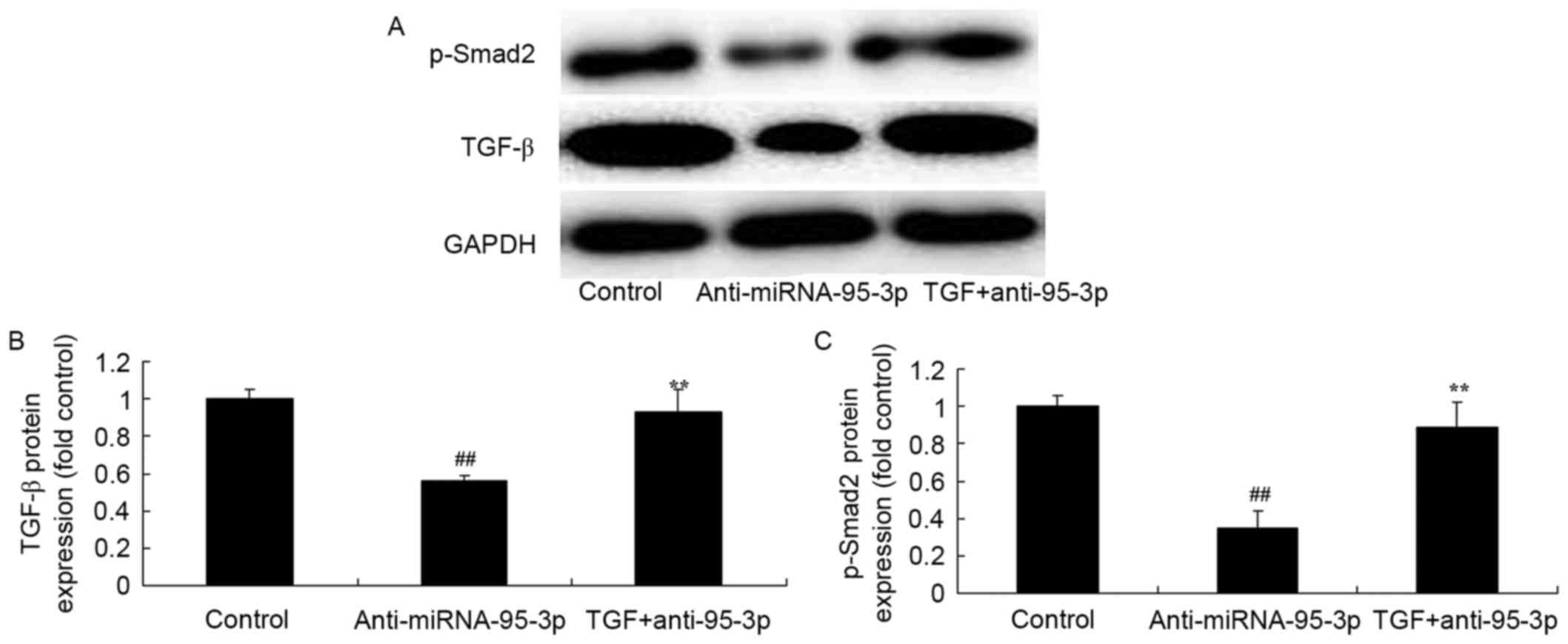
The promotion of TGF-β on TGF-β and
Smad2 protein expression in osteosarcoma cells by microRNA-95-3p
downregulation
To further validate the finding that microRNA-95-3p
regulates TGF-β expression, we examined the protein TGF-β/Smad2
expression. As shown in Fig. 10,
TGF-β expression promotion was significantly induced by TGF-β and
p-Smad2 protein expression in osteosarcoma cells by microRNA-95-3p
downregulation, as compared with microRNA-95-3p downregulation
group.
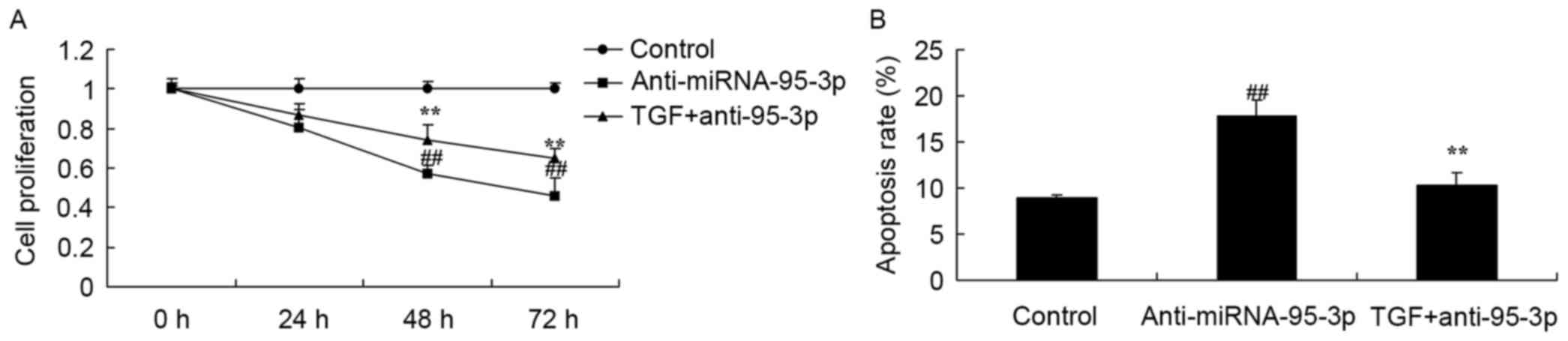
The promotion of TGF-β on cell growth
and apoptosis in osteosarcoma cells by microRNA-95-3p
downregulation
Then, we found significant induction of cell growth
and inhibition of apoptosis in osteosarcoma cells by microRNA-95-3p
downregulation and TGF-β promotion expression (Fig. 11).
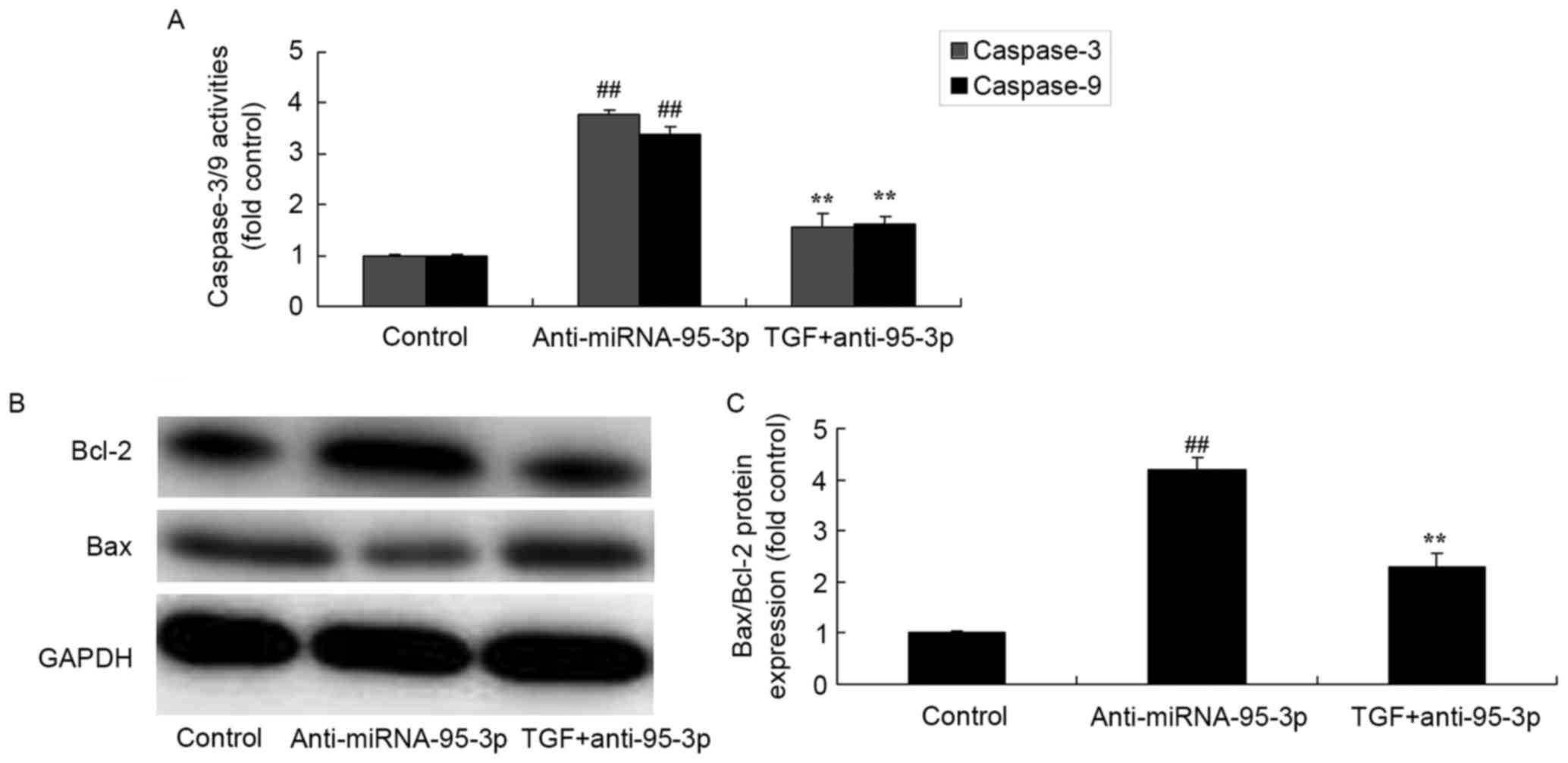
The promotion of TGF-β on caspase-3
and caspase-9 activities and Bax/Bcl-2 protein expression in
osteosarcoma cells by microRNA-95-3p downregulation
To determine the impact of microRNA-95-3p on
osteosarcoma apoptosis mechanism, TGF-β and microRNA-95-3p mimics
was transfected into F5M2 cells. Furthermore, the promotion of
TGF-β led to inhibition of caspase-3 and caspase-9 activity and
Bax/Bcl-2 protein expression in osteosarcoma cell by microRNA-95-3p
downregulation (Fig. 12).
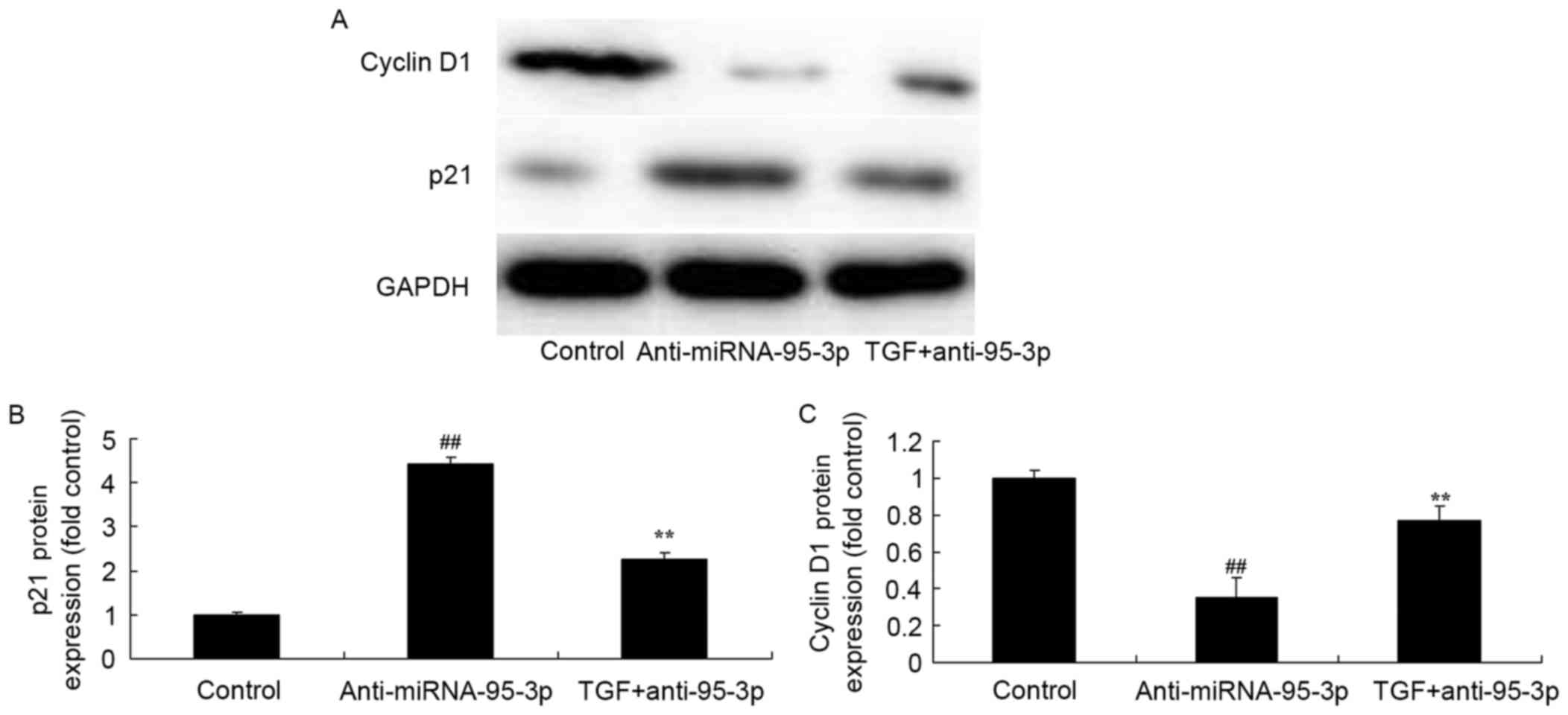
The promotion of TGF-β on p21 and
cyclin D1 protein expression in osteosarcoma cells by
microRNA-95-3p downregulation
The expression level of p21 and cyclin D1 was also
assessed using western blotting. The results of western blotting
showed that the p21 and cyclin D1 protein expression in
osteosarcoma cells by microRNA-95-3p downregulation were
significantly reversed by promotion of TGF-β expression (Fig. 13).
Discussion
Osteosarcoma is the most common malignant osteogenic
tumor with easy metastases, characterized by generation of osteoid
matrix from tumor cells and constituted by sarcoma osteoblasts,
osteoid tissue directly generated from osteoid tissue and new bones
(10). In osteosarcoma,
osteogenesis exists with osteoclasia at the same time, so it is
classified into an osteoblastic type and an osteolytic type
according to proportions of osteogenesis and osteoclasia (11). Osteosarcoma is highly malignant with
extremely poor prognosis (12).
Even when a therapeutic schedule combining surgery with
chemotherapy is adopted, the five-year survival rate of patients is
only 55–68% (12). The main cause
of the poor prognosis is that metastases occur in its early stage.
As a result, the current anti-metastasis treatment cannot achieve a
satisfactory effect (13). In
addition, the large side-effect of systemic chemotherapy will
damage various organs of human body to a certain degree and it is
often hard for patients to accept severe impacts on their limb
functions caused by surgical treatment (13). Hence, treatment of osteosarcoma has
become a problem for researchers to solve urgently (3). We found that the serum expression of
microRNA-95-3p was effectively upregulated in patients with
osteosarcoma. Conversely, miR-95-3p expression in patients with
osteosarcoma had significant association with clinical stage, which
showed microRNA-95-3p may regulate osteosarcoma development and
progression. Fan et al reported that the microRNA-95-3p
downregulation inhibits proliferation and invasion promoting
apoptosis of glioma cells (14).
miRNAs are a type of recently discovered non-coding
microRNAs with a length of 22–28 nucleotides, which widely exist in
eucaryon (5). By complete or
partial complementary pairing with and binding to the target gene
mRNA 3′-UTR, miRNAs cause degradation of target miRNA or
translational suppression to regulate the expression of target
genes, affecting biological behavior such as cell proliferation,
invasion, differentiation and apoptosis (15). Unlike siRNAs, miRNAs have multiple
target genes (15). As endogenous
RNA interference is triggered, miRNAs regulate cells which are
closer to a physiological level instead of regulation through a
simple gene knockout (16). We
found that downregulation of microRNA-95-3p expression suppresses
cell growth, induced apoptosis, increased caspase-3 and caspase-9
activities and Bax protein expression in osteosarcoma cells;
overexpression of microRNA-95-3p increased cell growth, and
inhibited apoptosis of osteosarcoma cells, suggesting that
microRNA-95-3p has a potential benefit in osteosarcoma.
p21-activated kinase (PAK) proteins are
evolutionarily conserved serine/threonine protein kinases which
participate in regulation of biological functions such as
cytoskeleton rearrangement, cell survival, cell apoptosis,
angiogenesis and mitosis (15). p21
is a currently known as cell cyclin inhibition protein with the
most extensive kinase inhibitory activity (17). Some scholars reported that positive
expression rates of p21 in colon cancer and gastric cancer were
50.5 and 39%, respectively, and research showed that the expression
of p21 protein was related to tumorigenesis, tumor differentiation,
invasion and metastases and clinical stages of tumors (18). In our results, downregulation of
microRNA-95-3p expression induced p21 protein expression and
overexpression of microRNA-95-3p suppressed p21 protein expression
in osteosarcoma cells. Ye et al demonstrated that
upregulation of miR-95-3p promotes tumorigenesis of hepatocellular
cells via p21 expression (19).
TGF-β/Smad promotes angiogenesis and strengthens
interactions between tumor cells and extracellular matrix to
enhance invasion and metastases of tumors mainly through
immunosuppression/immune escape (20). New vessels of tumor cells are
necessary for invasion and metastases of tumors and these vessels
are immature vascular structures formed by endothelial cells
falling off from mature vessels around the tumor with high
permeability. Tumor cells permeate in the blood system, causing
tumor metastases (21). TGF-β/Smad
can induce expression levels of angiogenic factors, such as VGEF
and connective tissue growth factors (CTGFs), in epithelial cells
and fibroblasts and expression, secretion and activation of matrix
metalloproteinases, MMP-2 and MMP-9, in endothelial cells and tumor
cells, leading to endothelial cell detachment from basement
membranes (22). Our results
demonstrate that downregulation of microRNA-95-3p expression
suppressed TGF-β and p-Smad2 protein expression, and overexpression
of microRNA-95-3p induced TGF-β and p-Smad2 protein expression in
osteosarcoma cells. Taken together, our results demonstrate that
microRNA-95-3p regulates TGF-β/Smad2 expression in
osteosarcoma.
Gene p21waf1/cip1 is located on human chromosome
6p21.2 and Mr of the protein that it codes is 21×103.
P21 has an extensive inhibiting effect on CDKs (cyclin-dependent
kinases), especially on CDK2 and CDK4 (23). The Cyclin-CDK complex is a positive
regulatory factor in the cell cycle, called the engine of cell
cycles, and can help cells quickly pass checkpoints of the cell
cycles (23). Its overexpression
can reduce cell mass and promote proliferation and transformation.
P21waf1/cip1 plays a role of checkpoint control in inhibition of
Cyclin-CDK (24). Cyclin D1 is cell
cycle protein synthesized from early G1 phase and reaching its peak
during middle G1 phase (7,25). It promotes cells to enter S phase
from G1 phase and overexpression of cyclin D1 can shorten G1 phase
and make cells step into S phase from G1 phase in advance,
resulting in uncontrolled growth, transformation and cancerization
of cells (26). In our experiments,
TGF-β promotion reversed the anticancer effects of microRNA-95-3p
on osteosarcoma cells through p21/cyclin D1 expression. Hwang et
al reported that microRNA-95-3p overexpression reduced brain
metastasis of lung adenocarcinoma via cyclin D1 expression
(27).
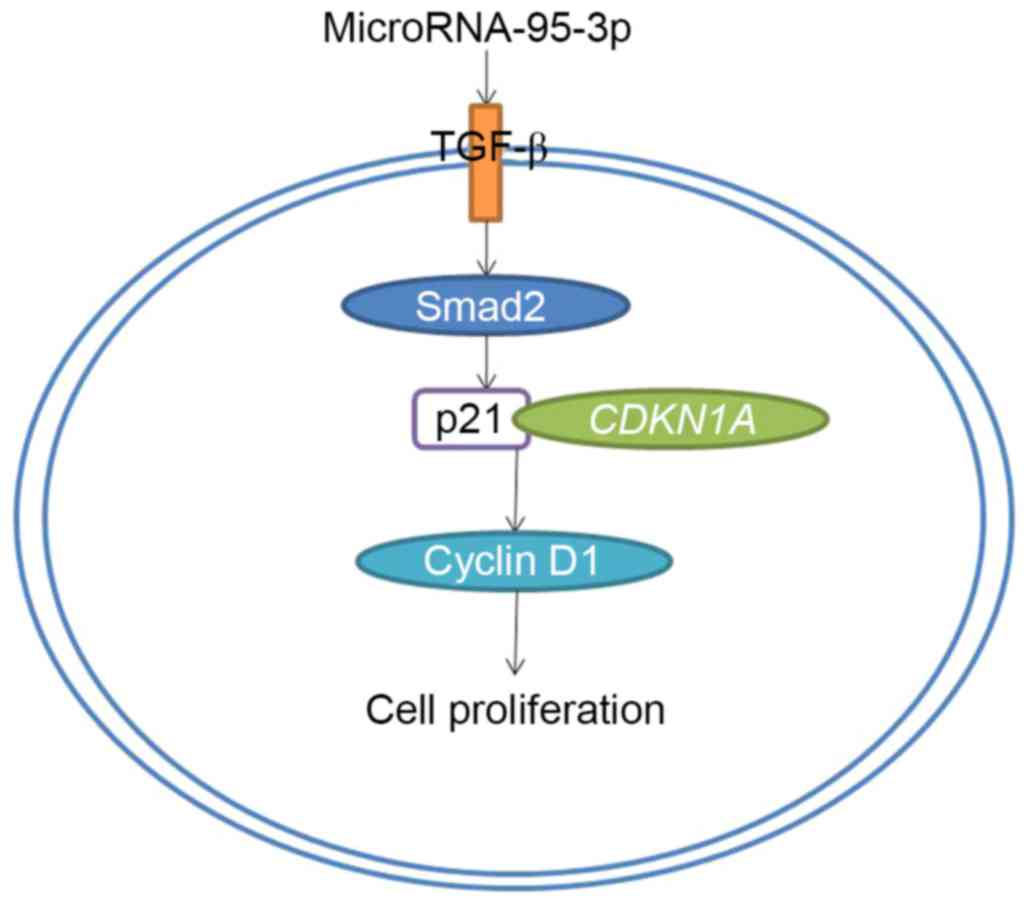
In summary, we have found that the serum expression
of microRNA-95-3p was effectively upregulated in patients with
osteosarcoma. Downregulation of microRNA-95-3p expression
suppresses cell growth, induced apoptosis, increased caspase-3 and
caspase-9 activities and Bax/Bcl-2 protein expression in
osteosarcoma cell through TGF-β/CDKN1A/p21/cyclin D1 expression
(Fig. 14). This study established
microRNA-95-3p as a potential biomarker for diagnosis of
osteosarcoma through TGF-β/CDKN1A/p21/cyclin D1 expression and as a
new therapeutic target for prevention and treatment of
osteosarcoma.
References
|
1
|
Ebb D, Meyers P, Grier H, Bernstein M,
Gorlick R, Lipshultz SE, Krailo M, Devidas M, Barkauskas DA, Siegal
GP, et al: Phase II trial of trastuzumab in combination with
cytotoxic chemotherapy for treatment of metastatic osteosarcoma
with human epidermal growth factor receptor 2 overexpression: A
report from the children's oncology group. J Clin Oncol.
30:2545–2551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Guo Z, Wang Z, Fan H and Fu J: Does
microwave ablation of the tumor edge allow for joint-sparing
surgery in patients with osteosarcoma of the proximal tibia? Clin
Orthop Relat Res. 473:3204–3211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bajpai J, Gamanagatti S, Sharma MC, Kumar
R, Vishnubhatla S, Khan SA, Rastogi S, Malhotra A and Bakhshi S:
Noninvasive imaging surrogate of angiogenesis in osteosarcoma.
Pediatr Blood Cancer. 54:526–531. 2010.PubMed/NCBI
|
|
4
|
Bacci G, Ferrari S, Longhi A, Picci P,
Mercuri M, Alvegard TA, Saeter G, Donati D, Manfrini M, Lari S, et
al Italian Sarcoma Group/Scandinavian Sarcoma Group, : High dose
ifosfamide in combination with high dose methotrexate, adriamycin
and cisplatin in the neoadjuvant treatment of extremity
osteosarcoma: Preliminary results of an Italian Sarcoma
Group/Scandinavian Sarcoma Group pilot study. J Chemother.
14:198–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Ning Y, Yang L, Liu H, Wu C, Wang
S and Guo X: Diagnostic value of circulating microRNAs for
osteosarcoma in Asian populations: A meta-analysis. Clin Exp Med.
17:175–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen R, Li X, He B and Hu W: MicroRNA-410
regulates autophagy-related gene ATG16L1 expression and enhances
chemosensitivity via autophagy inhibition in osteosarcoma. Mol Med
Rep. 15:1326–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang Y, Wang LQ, Guo QY and Shi TL:
MicroRNA-196a overexpression promotes cell proliferation and
inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int J Clin
Exp Pathol. 8:2461–2472. 2015.PubMed/NCBI
|
|
8
|
Han K, Zhou Y, Gan ZH, Qi WX, Zhang JJ,
Fen T, Meng W, Jiang L, Shen Z and Min DL: p21-activated kinase 7
is an oncogene in human osteosarcoma. Cell Biol Int. 38:1394–1402.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Ma W, Cui J, Yao H, Zhou H, Ge Y,
Xiao L, Hu X, Liu BH, Yang J, et al: Regulation of p21 by TWIST2
contributes to its tumor-suppressor function in human acute myeloid
leukemia. Oncogene. 34:3000–3010. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pappo AS, Vassal G, Crowley JJ, Bolejack
V, Hogendoorn PC, Chugh R, Ladanyi M, Grippo JF, Dall G, Staddon
AP, et al: A phase 2 trial of R1507, a monoclonal antibody to the
insulin-like growth factor-1 receptor (IGF-1R), in patients with
recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial
sarcoma, and other soft tissue sarcomas: Results of a Sarcoma
Alliance for Research Through Collaboration study. Cancer.
120:2448–2456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudawara I, Aoki Y, Ueda T, Araki N, Naka
N, Nakanishi H, Matsumine A, Ieguchi M, Mori S, Myoui A, et al:
Neoadjuvant and adjuvant chemotherapy with high-dose ifosfamide,
doxorubicin, cisplatin and high-dose methotrexate in non-metastatic
osteosarcoma of the extremities: A phase II trial in Japan. J
Chemother. 25:41–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al Italian Sarcoma Group, : Sorafenib
and everolimus for patients with unresectable high-grade
osteosarcoma progressing after standard treatment: A non-randomised
phase 2 clinical trial. Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
den Hengst WA, Hendriks JM, Balduyck B,
Rodrigus I, Vermorken JB, Lardon F, Versteegh MI, Braun J,
Gelderblom H, Schramel FM, et al: Phase II multicenter clinical
trial of pulmonary metastasectomy and isolated lung perfusion with
melphalan in patients with resectable lung metastases. J Thorac
Oncol. 9:1547–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan B, Jiao BH, Fan FS, Lu SK, Song J, Guo
CY, Yang JK and Yang L: Downregulation of miR-95-3p inhibits
proliferation, and invasion promoting apoptosis of glioma cells by
targeting CELF2. Int J Oncol. 47:1025–1033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Z, He R, Xia H, Wei YU and Wu S:
MicroRNA-101 has a suppressive role in osteosarcoma cells through
the targeting of c-FOS. Exp Ther Med. 11:1293–1299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu M, Xie Y, Sheng W, Miao J and Yang J:
Adenovirus-mediated ING4 gene transfer in osteosarcoma suppresses
tumor growth via induction of apoptosis and inhibition of tumor
angiogenesis. Technol Cancer Res Treat. 14:369–378. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yong ST and Wang XF: A novel,
non-apoptotic role for Scythe/BAT3: A functional switch between the
pro- and anti-proliferative roles of p21 during the cell cycle.
PLoS One. 7:e380852012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye J, Yao Y, Song Q, Li S, Hu Z, Yu Y, Hu
C, Da X, Li H, Chen Q, et al: Up-regulation of miR-95-3p in
hepatocellular carcinoma promotes tumorigenesis by targeting p21
expression. Sci Rep. 6:340342016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim BG, Lee JH, Yasuda J, Ryoo HM and Cho
JY: Phospho-Smad1 modulation by nedd4 E3 ligase in BMP/TGF-β
signaling. J Bone Miner Res. 26:1411–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Navid F, Letterio JJ, Yeung CL, Pegtel M
and Helman LJ: Autocrine transforming growth factor-beta growth
pathway in murine osteosarcoma cell lines associated with inability
to affect phosphorylation of retinoblastoma protein. Sarcoma.
4:93–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li F, Li S and Cheng T: TGF-β1 promotes
osteosarcoma cell migration and invasion through the
miR-143-versican pathway. Cell Physiol Biochem. 34:2169–2179. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chow LT: Giant cell rich osteosarcoma
revisited-diagnostic criteria and histopathologic patterns, Ki67,
CDK4, and MDM2 expression, changes in response to bisphosphonate
and denosumab treatment. Virchows Arch. 468:741–755. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benassi MS, Molendini L, Gamberi G,
Ragazzini P, Sollazzo MR, Merli M, Asp J, Magagnoli G, Balladelli
A, Bertoni F, et al: Alteration of pRb/p16/cdk4 regulation in human
osteosarcoma. Int J Cancer. 84:489–493. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Zhao Y and Zeng B: Enhanced
chemosensitivity by simultaneously inhibiting cell cycle
progression and promoting apoptosis of drug-resistant osteosarcoma
MG63/DXR cells by targeting cyclin D1 and Bcl-2. Cancer Biomark.
12:155–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang J, Ni J, Yi S, Song D and Ding M:
Protein inhibitor of activated STAT xα depresses cyclin D and
cyclin D kinase, and contributes to the inhibition of osteosarcoma
cell progression. Mol Med Rep. 13:1645–1652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang SJ, Lee HW, Kim HR, Song HJ, Lee DH,
Lee H, Shin CH, Joung JG, Kim DH, Joo KM, et al: Overexpression of
microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma
through downregulation of cyclin D1. Oncotarget. 6:20434–20448.
2015. View Article : Google Scholar : PubMed/NCBI
|