Insulin-like growth factor II m-RNA-binding protein
3 (IMP3) is a member of the IMP family playing an important role in
cell migration in early embryogenesis (1,2). This
‘U3 small nucleolar ribonucleoprotein’ is a component of an RNA
binding protein required for the early cleavage during pre-18s
ribosomal RNA processing. Previously, IMP3 has gained considerable
interest as a cancer-associated protein. IMP3 overexpression has
been reported in a variety of human types of cancer, including lung
cancer (3), germ cell cancer
(4), colon cancer (5), pancreatic cancer (6), gastric cancer (7), liver cancer (8), and kidney cancer (9), and has been linked to advanced disease
stage and adverse clinical outcome in some of these cnacers
(5,7,8,10–12).
Collectively, these studies strongly suggest that IMP3 may
represent a valuable prognostic marker in human cancer. However, as
the number of studies suggesting biological and clinical relevance
of IMP3 is rapidly increasing, there are also a growing number of
reports revealing considerable discrepancies with respect to the
frequency of expression in various types of cancer. For example,
reported frequencies in IMP3 expression ranges from 0 to 83% in
prostate cancer (13–16), from 11 to 86% in papillary thyroid
cancer (17–20), from 11 to 65% in papillary renal
cell cancer (9,12), from 0 to 52% in leiomyoma (21,22),
from 21 to 71% in invasive urinary bladder tumor (23,24),
from 50 to 100% in small cell lung cancer (3,25), and
from 37 to 83% in malignant mesothelioma (26,27).
Such discrepancies may be due to the use of different antibodies,
staining protocols, and scoring criteria in these studies. The
optimal study for assessing the relative importance of a
potentially relevant molecule across tumor types includes the
analysis of as large a number of different normal tissues, cancer
types and subtypes as possible, followed by the evaluation of the
clinical value of IMP3 in selected types of cancer with frequent
IMP3 expression. Moreover, it would be necessary to ensure a
maximal standardization of all these analyses. Tissue microarray
(TMA) technology is a suitable tool for such a study, as a large
number of tissues can be analyzed on few sections that are cut in 1
day and that can be stained in 1 day in a set of reagents under
completely identical staining conditions.
In this study, we utilized a two-step tissue
microarray (TMA) approach to evaluate the clinical utility of IMP3
testing in human normal tissues and cancer. In a first step, we
screened 76 different normal tissue types and samples of 95
different tumor types using a multi-tumor TMA. In a second step,
tumor-type specific TMAs with clinical follow-up data were utilized
to evaluate the clinical significance of IMP3 alterations in five
selected tumor entities. Our approach implicated frequent IMP3
expression in 76 different tumor types and concomitantly
demonstrated the association between IMP3 expression and poor
prognosis in adenocarcinomas of the lung.
Freshly cut TMA sections were analyzed. IMP3
expression was detected with a monoclonal mouse anti-human antibody
(clone 69.1; Dako M3626, Glostrup, Denmark) in a dilution of 1:100
after peroxidase blocking with H2O2 (Dako
S2023) for 10 min. High-temperature pretreatment of slides was
carried out in an autoclave with citrate buffer, pH 9.0 for 5 min.
The Envision system (Dako 5007) was used to visualize the staining.
In normal tissues, a cell type specific distribution of IMP3
expression was recorded, and the staining intensity was estimated
as weak (+), moderate (++), or strong (+++). In tumor tissues,
cytoplasmic staining was evaluated by staining intensity (0, 1+,
2+, and 3+), and the fraction of positive tumor cells was scored
for each tissue spot. A final score was built from these two
parameters according to the following criteria: negative scores had
a staining intensity of 0 and 1+ in ≤10% of tumor cells; weak
scores had a staining intensity of 1+ in >10% and ≤70% of tumor
cells or a staining intensity of 2+ in ≤30% of tumor cells;
moderate scores had a staining intensity of 1+ in >70% of tumor
cells, a staining intensity of 2+ in >30% and ≤70% of tumor
cells or a staining intensity of 3+ in ≤30% of tumor cells; and
strong scores had a staining intensity of 2+ in >70% of tumor
cells or a staining intensity of 3+ in >30% of tumor cells. All
tumors exhibiting at least weak expression were defined as
IMP3-positive.
Positive IMP3 staining was seen in few normal
tissues and in specific cell types only, including amnion (+),
chorion cells (+) syncytiotrophoblast (+++), cytotrophoblast (+++),
decidua (+) and mesenchymal cells (++) of the placenta, lymph
follicles in lymph nodes and tonsils (lymphoblasts (+++),
lymphocytes (+++), thymocytes (+), absorptive cells of the ileum
(+++), crypt cells of rectal mucosa (+), mucus cells (++) of
submandibular and sublingual glands, spermatogonia of the testis
(++), ciliated cells (+) of bronchial mucosa, mucinous acinar cells
of bronchial glands (++), secretory cells of the endocervix (+),
ciliated cells of the fallopian tube (+++), and cells of the
adenohypophysis of the anterior lobe of the pituitary gland
(+).
Normal tissue samples that were also analyzed but
did not exhibit any IMP3 staining included: aorta, heart, striated
muscle, tongue, uterus, appendix, esophagus, stomach, ileum muscle,
colon, kidney, urinary bladder, penis, ovary, fat, skin, lip, oral
cavity, anal canal, ectocervix, spleen, duodenum, gallbladder,
liver, pancreas, parotid gland, bone marrow, prostate, seminal
vesicles, epididymis, nose sinus, lung, breast, adrenal gland,
parathyroid gland, thyroid gland, cerebellum and cerebrum.
Analyzable results could be obtained from 96 of the
99 types of cancer represented in our multi-tumor TMA. At least
weak IMP3 protein expression could be detected in 76 (80%) of the
95 tumor categories with analyzable results, including 64 (67%)
categories where at least one tumor revealed a strong positivity.
The immunohistochemical results are summarized in Table I. IMP3 positivity was most striking
in testicular cancers, where all examined tumors exhibited positive
staining, including 71% (seminomas) and 96% (non-seminomas) with
strong staining. Additional cancers with frequent IMP3 positivity
included Hodgkin's lymphomas (90%), neuroblastomas (88% positive),
squamous cell cancers of various origins, e.g. of the lungs (81%),
oral cavity (73%), esophagus (71%), larynx (67%), penis (59%), and
skin (50%), as well as adenocarcinomas of the esophagus (74%),
pancreas (62%), cervix (58%), stomach (50–55%), and lungs (51%). In
contrast, IMP3 staining was only rarely found in breast cancers of
no special type (7%) and clear cell renal cell cancers (4%).
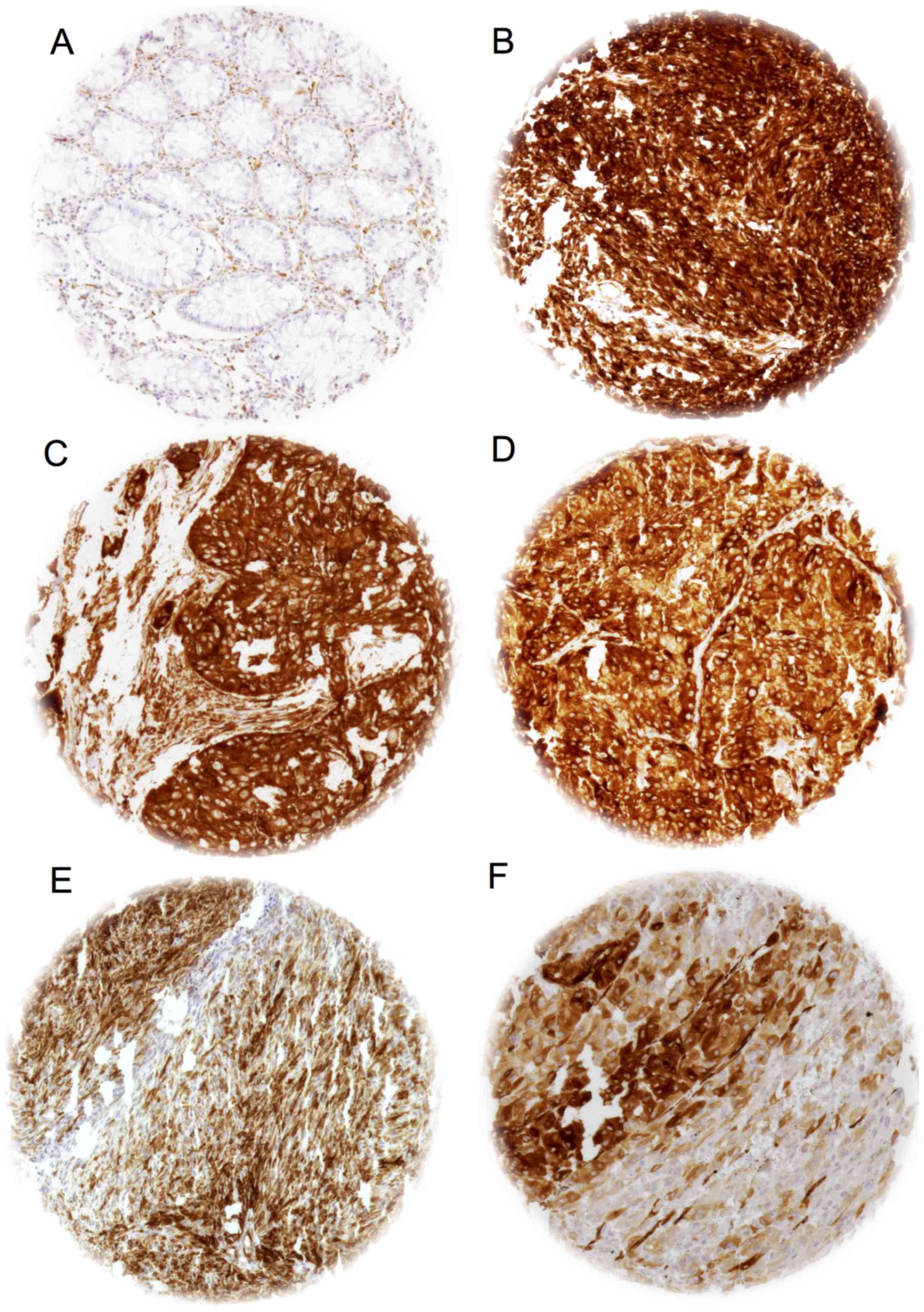
Examples of immunostaining of IMP3 positive and negative tissues
are presented in Fig. 1.
The tumor types where IMP3 protein expression could
never be detected included dermatofibrosarcomas of the skin (0 out
of 4), subtypes of breast cancers (including lobular, mucinous,
tubular cancers and phylloid tumors, 0 out of 11–23), leiomyomas (0
out of 20), stromal sarcomas (0 out of 2), GIST (0 out of 40),
parotid adenomas (0 out of 30), neuroendocrine pancreatic cancers
(0 out of 5), prostate cancers (0 out of 44), pheochromocytomas (0
out of 48), medulloblastomas (0 out of 2), ependymomas (0 out of
1), neurofibromas (0 out of 21), desmoid tumors (0 out of 9),
synovial giant cell carcinomas (0 out of 9) and hemangiopericytomas
(0 out of 5) and. However, the number of examined cases of several
of these tumor types was low. These data therefore do not rule out
that IMP3 expression can sometimes also occur in these tumor
types.
Expression data were available for 639 out of 697
(91.6%) urinary bladder cancers, 1204 out of 1711 (70.4%) colon
cancers, 216 out of 343 (63%) adenocarcinomas of the esophagus, 170
out of 251 (67.7%) squamous cell carcinomas of the esophagus, 641
out of 763 (84%) lung cancers, 280 of 358 (78.2%) pancreas
carcinomas, and 204 out of 230 (88,7%) stomach cancers on the
prognostic TMAs.
IMP3 positivity was found in 21.9% of urinary
bladder cancers (strong: 7.4%, moderate: 8.1%, weak: 6.4%), 63.4%
of colon tumors (strong: 36%, moderate: 16.6%, weak: 10.7%), 74.1%
of esophageal adenocarcinomas (strong: 50.5%, moderate: 11.1%,
weak: 12.5%), 60.6% of esophageal squamous cell cancers (strong:
41.8%, moderate: 8.2%, weak: 10.6%), 63.2% of lung cancers (strong:
47.1%, moderate: 11.4%, weak: 4.7%), 48.9% of pancreatic cancers
(strong: 8.2%, moderate: 10.7%, weak: 30%), and 44.9% of stomach
cancers (strong: 24%, moderate: 10.8%, weak: 20.1%).
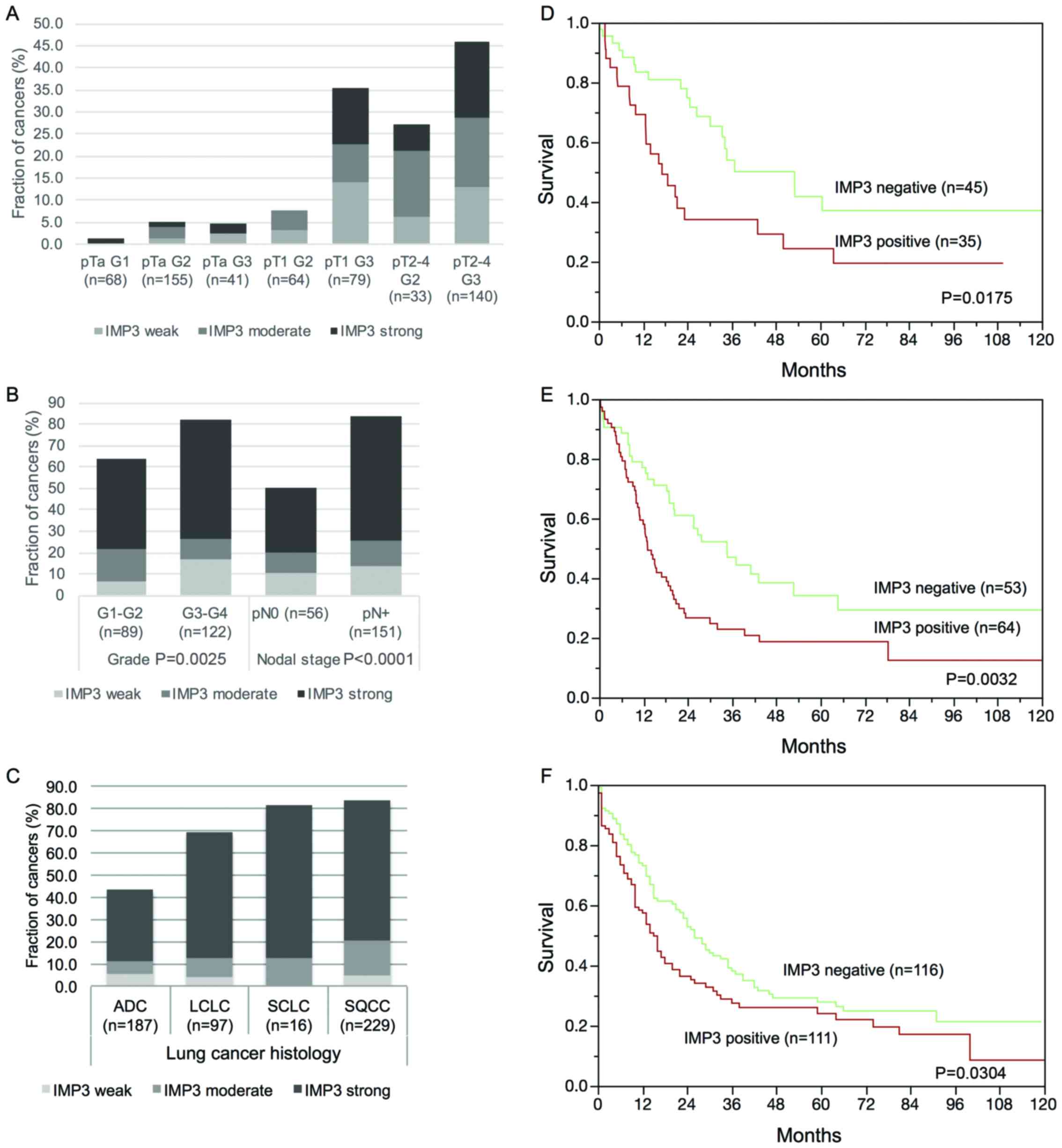
Significant associations were found between IMP3 and
advanced stage and grade in urinary bladder cancers (P<0.0001
each, Fig. 2A), in high grade and
metastatic phenotype in esophageal adenocarcinomas (P≤0.0025,
Fig. 2B), in a squamous cell
phenotype in lung cancers (P<0.0001, Fig. 2C), and in shortened survival in
adenocarcinomas of the lungs (P=0.0175, Fig. 2D), in stomach (P=0.0032, Fig. 2E) and in pancreatic cancers
(P=0.0304, Fig. 2F). No significant
associations between IMP3 expression levels and patient prognosis
were observed in urinary bladder cancers, squamous cell lung
cancers and squamous cell esophageal cancers.
The results of our study provide a comprehensive
catalogue of IMP3 expression across a large variety of human types
of cancer and subtypes. Our findings demonstrated that high levels
of IMP3 protein expression were found in the vast majority of the
analyzed types of cancer and underscored its considerable general
importance in tumor biology. The novelty of this study is that
staining was performed with a single antibody, in 1 laboratory on a
large panel of different tumor entities on a single microarray.
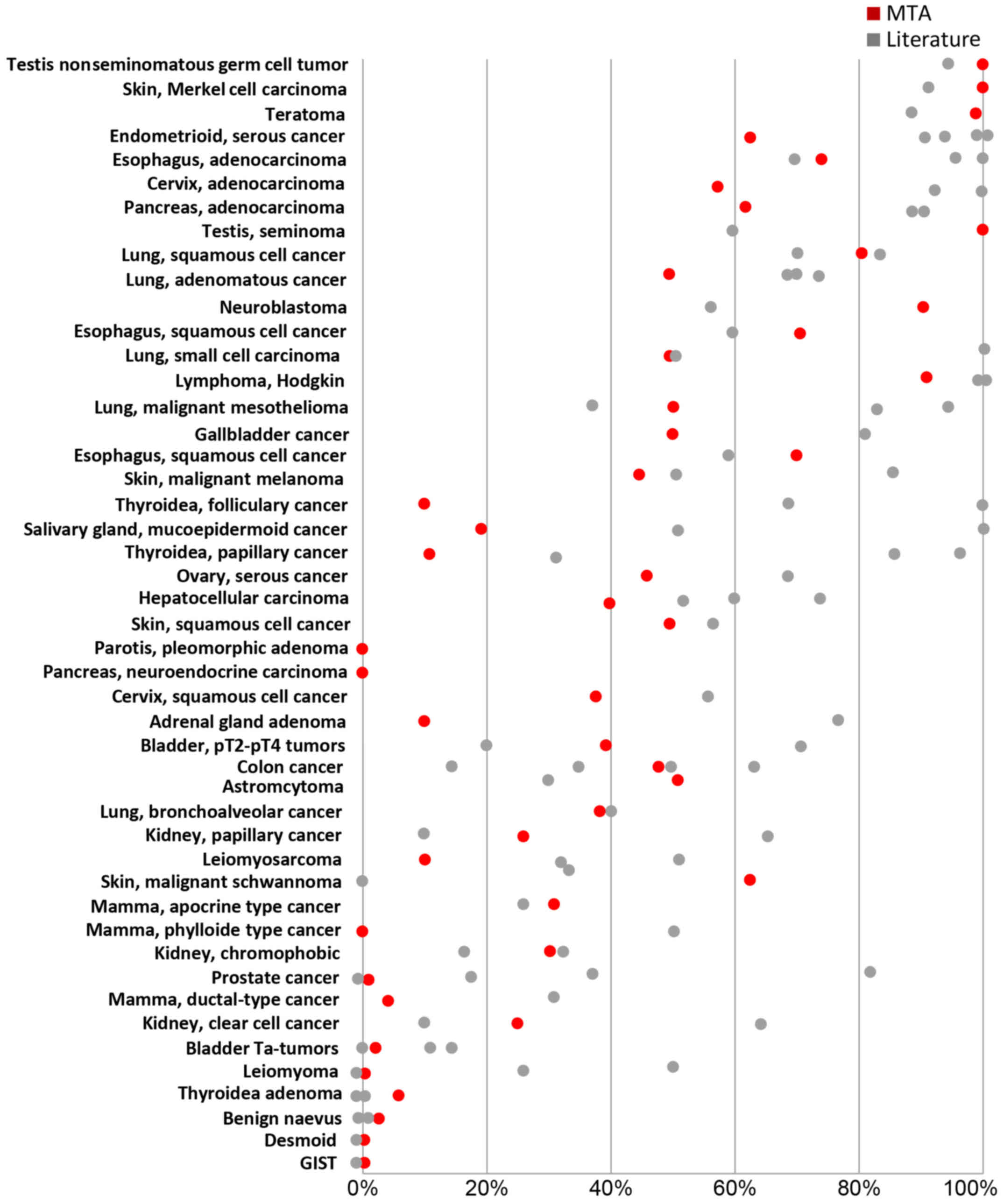
The frequency of IMP3 expression in individual types
of cancer is well in the range of that reported from previous
research (Fig. 3), which
corroborates the validity of our results. For example, virtually
all analyzed testicular cancers; teratomas, Hodgkin's lymphomas and
Merkel cell cancers of the skin were IMP3-positive under the
experimental conditions selected for this study, which fits well
with the 85–99% positivity reported in the literature (4,34–36).
In contrast, and in concordance with previous research, tumor types
largely lacking detectable IMP3 expression included breast cancers
of no special type, gastrointestinal stromal tumors, desmoid
tumors, benign naevi or leiomyomas, which all have been described
as predominantly IMP3-negative before (21,37,38).
More variable findings with respect to published data were made in
tumor types with intermediate IMP3-positivity, such as
hepatocellular carcinomas (53–68% in the literature; 40% in our
study) or colon cancers (15–65% in the literature; 47% in our
study). It is obvious, that different antibodies and
immunohistochemistry protocols, scoring criteria and the
comparatively small number of samples (usually <50 per tumor
category in our study) account for these differences.
There were 14 tumor types that were newly identified
as having occasional IMP3 protein overexpression in this study.
These included many important types of cancer such as squamous cell
cancers of the vagina and vulva, medullary breast and thyroid
cancers, pharyngeal carcinomas, various sarcomas (including
chondro-, angio- and liposarcomas), oncocytomas, as well as some
types of neuronal cancers (e.g. paragangliomas and
oligodendrigliomas). These findings further add to the growing list
of IMP3-expressing types of cancer and emphasize the general role
of IMP3 as a marker for malignant growth of tumors arising from
epithelial, mesenchymal and neuronal tissues.
Since IMP3 is often referred to as a marker for
distinguishing between benign and malignant lesions (39–43),
it was of interest to also find at least occasional expression in a
few individual cells of glandular epithelium, lymphatic tissues and
placenta, which was in accordance to earlier studies demonstrating
that IMP3 was ubiquitously expressed in early developmental stages
of human tissues but also in adult placenta (44,45).
In addition, we also found IMP3 expression in benign lesions such
as Warthin's tumors, schwannomas, colon adenomas and basal cell
adenomas. Such findings are often made in studies including
multiple types of tumors and demonstrate that the specificity of
molecular markers are often overestimated in initial studies
including only a limited number of different samples. For example,
IMP3 has been suggested to aid in the diagnosis of melanoma,
leiomyosarcoma, HCC, papillary thyroid carcinoma, and follicular
thyroid carcinomas, but it is not a sensitive marker for these
tumors as lack of IMP3 expression in these tumors cannot exclude a
malignant phenotype (reviewed in ref. 1).
In addition to analyzing the molecular epidemiology
of IMP3 expression, we took advantage of various pre-existing tumor
type-specific prognostic TMAs for evaluation of the clinical
significance of IMP3 expression in several tumor types. Urinary
bladder, colon, esophageal, lung, pancreatic and stomach cancer
were selected for follow-up studies because IMP3 was frequently
positive in these tumor types and large TMAs were available.
Significant associations between IMP3 expression and tumor
phenotype as well as patient prognosis in our study further added
to the knowledge on the clinical impact of IMP3 in these tumor
types, which have been only studied in comparatively small cohorts
of usually less than 100 cancer samples thus far. For example, the
strong associations between IMP3 expression and advanced stage and
grade in urinary bladder cancers and esophageal adenocarcinomas, or
a squamous cell phenotype in lung cancers are in line with previous
work on 76–384 cancers of the urinary bladder (23,24),
147 esophageal cancers (46) and
89–224 lung cancers (reviewed in refs. 47,48). Furthermore, the
adverse prognostic impact of IMP3 expression in lung
adenocarcinomas, pancreatic cancers and stomach cancers in our
study were supported by previous studies on 40–190 patients in
these types of cancer (10,11,48–50).
In addition a recent meta-analysis revealed a hazard ratio of 2.08
for decreased survival in solid tumors expressing high levels of
IMP3 (51).
Our analysis provides a comprehensive overview on
IMP3 protein expression in neoplastic human tissues. Tissue
microarrays are an ideal tool to massively accelerate
characterization of novel biomarkers. The use of TMAs to jointly
screen many different tumor types for molecular alterations of
interest is an obvious application of this technique. In earlier
studies we had used multi-tumor TMAs for the evaluation of cyclin E
(52), calretinin (53), KIT (54), ERG (55) or copy number changes of 17q23
(56). Others have used comparable
TMAs to evaluate SPANX-B (57), SIL
(58), NOX1 (59), COX2, MMP2, or MMP9 (60).
It is a distinct advantage of the TMA technique that
all tissues are analyzed under maximally standardized conditions.
While automated immunostainers, despite some remaining day-to-day
variability, can provide good standardization of the staining
process, TMAs enable a control of several additional important
parameters affecting immunostaining. For example, TMAs overcome the
issue of slide ageing and decreased immunoreactivity (61) that can become a serious problem in
conventional large studies where tissue sections are often stored
over a longer period of time prior to analysis, because all tissue
samples in a TMA can be easily sectioned and stained within one
day.
In summary, the results of our study show that IMP3
expression is a common feature of most human solid cancer types,
but may also be observed in benign lesions. Strong IMP3 expression
is often linked to adverse tumor features.
We would like to thank Christina Koop, Julia
Schumann, Sünje Seekamp, and Inge Brandt for excellent technical
assistance.
|
1
|
Gong Y, Woda BA and Jiang Z: Oncofetal
protein IMP3, a new cancer biomarker. Adv Anat Pathol. 21:191–200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vikesaa J, Hansen TV, Jønson L, Borup R,
Wewer UM, Christiansen J and Nielsen FC: RNA-binding IMPs promote
cell adhesion and invadopodia formation. EMBO J. 25:1456–1468.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu H, Bourne PA, Spaulding BO and Wang HL:
High-grade neuroendocrine carcinomas of the lung express K homology
domain containing protein overexpressed in cancer but carcinoid
tumors do not. Hum Pathol. 38:555–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goodman S, Zhang L, Cheng L and Jiang Z:
Differential expression of IMP3 between male and female mature
teratomas - immunohistochemical evidence of malignant nature.
Histopathology. 65:483–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Yan D, Tang H, Zhou C, Fan J, Li S,
Wang X, Xia J, Huang F, Qiu G, et al: IMP3 is a novel prognostic
marker that correlates with colon cancer progression and
pathogenesis. Ann Surg Oncol. 16:3499–3506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lok T, Chen L, Lin F and Wang HL:
Immunohistochemical distinction between intrahepatic
cholangiocarcinoma and pancreatic ductal adenocarcinoma. Hum
Pathol. 45:394–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Damasceno EA, Carneiro FP, Magalhães AV,
Carneiro MV, Takano GH, Vianna LM, Seidler HB, Castro TM,
Muniz-Junqueira MI, Amorim RF, et al: IMP3 expression in gastric
cancer: Association with clinicopathological features and HER2
status. J Cancer Res Clin Oncol. 140:2163–2168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeng YM, Chang CC, Hu FC, Chou HY, Kao HL,
Wang TH and Hsu HC: RNA-binding protein insulin-like growth factor
II mRNA-binding protein 3 expression promotes tumor invasion and
predicts early recurrence and poor prognosis in hepatocellular
carcinoma. Hepatology. 48:1118–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Z, Lohse CM, Chu PG, Wu CL, Woda BA,
Rock KL and Kwon ED: Oncofetal protein IMP3: A novel molecular
marker that predicts metastasis of papillary and chromophobe renal
cell carcinomas. Cancer. 112:2676–2682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan J, Wei Q, Jian W, Qiu B, Wen J, Liu J,
Fu B, Zhou X and Zhao T: IMP3 predicts invasion and prognosis in
human lung adenocarcinoma. Lung. 194:137–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schaeffer DF, Owen DR, Lim HJ, Buczkowski
AK, Chung SW, Scudamore CH, Huntsman DG, Ng SS and Owen DA:
Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3)
overexpression in pancreatic ductal adenocarcinoma correlates with
poor survival. BMC Cancer. 10:592010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Z, Chu PG, Woda BA, Rock KL, Liu Q,
Hsieh CC, Li C, Chen W, Duan HO, McDougal S, et al: Analysis of
RNA-binding protein IMP3 to predict metastasis and prognosis of
renal-cell carcinoma: A retrospective study. Lancet Oncol.
7:556–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tosun Yildirim H and Sentürk N: Analysis
of IMP3 expression in prostate adenocarcinomas. Turk Patoloji Derg.
28:128–133. 2012.PubMed/NCBI
|
|
14
|
Chromecki TF, Cha EK, Pummer K, Scherr DS,
Tewari AK, Sun M, Fajkovic H, Roehrborn CG, Ashfaq R, Karakiewicz
PI, et al: Prognostic value of insulin-like growth factor II mRNA
binding protein 3 in patients treated with radical prostatectomy.
BJU Int. 110:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikenberg K, Fritzsche FR, Zuerrer-Haerdi
U, Hofmann I, Hermanns T, Seifert H, Müntener M, Provenzano M,
Sulser T, Behnke S, et al: Insulin-like growth factor II mRNA
binding protein 3 (IMP3) is overexpressed in prostate cancer and
correlates with higher Gleason scores. BMC Cancer. 10:3412010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szarvas T, Tschirdewahn S, Niedworok C,
Kramer G, Sevcenco S, Reis H, Shariat SF, Rübben H and vom Dorp F:
Prognostic value of tissue and circulating levels of IMP3 in
prostate cancer. Int J Cancer. 135:1596–1604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulaçoğlu S and Erkılınç G: Imp3
expression in benign and malignant thyroid tumors and hyperplastic
nodules. Balkan Med J. 32:30–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slosar M, Vohra P, Prasad M, Fischer A,
Quinlan R and Khan A: Insulin-like growth factor mRNA binding
protein 3 (IMP3) is differentially expressed in benign and
malignant follicular patterned thyroid tumors. Endocr Pathol.
20:149–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yorukoglu A, Yalcin N, Avci A,
Cakalagaoglu F, Yaylali G, Akin F, Haciyanli M and Ozden A:
Significance of IMP3, nucleophosmin, and Ki-67 expression in
papillary thyroid carcinoma. Int J Surg Pathol. 23:5–12. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin L, Seys AR, Zhang S, Erickson-Johnson
MR, Roth CW, Evers BR, Oliveira AM and Lloyd RV: Diagnostic utility
of IMP3 expression in thyroid neoplasms: A quantitative RT-PCR
study. Diagn Mol Pathol. 19:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto H, Arakaki K, Morimatsu K, Zaitsu
Y, Fujita A, Kohashi K, Hirahashi M, Motoshita J, Oshiro Y and Oda
Y: Insulin-like growth factor II messenger RNA-binding protein 3
expression in gastrointestinal mesenchymal tumors. Hum Pathol.
45:481–487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cornejo K, Shi M and Jiang Z: Oncofetal
protein IMP3: A useful diagnostic biomarker for leiomyosarcoma. Hum
Pathol. 43:1567–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee DJ, Xylinas E, Rieken M, Khani F,
Klatte T, Wood CG, Karam JA, Weizer AZ, Raman JD, Remzi M, et al:
Insulin-like growth factor messenger RNA-binding protein 3
expression helps prognostication in patients with upper tract
urothelial carcinoma. Eur Urol. 66:379–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xylinas E, Cha EK, Khani F, Kluth LA,
Rieken M, Volkmer BG, Hautmann R, Küfer R, Chen YT, Zerbib M, et
al: Association of oncofetal protein expression with clinical
outcomes in patients with urothelial carcinoma of the bladder. J
Urol. 191:830–841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Del Gobbo A, Vaira V, Rocco E Guerini,
Palleschi A, Bulfamante G, Ricca D, Fiori S, Bosari S and Ferrero
S: The oncofetal protein IMP3: A useful marker to predict poor
clinical outcome in neuroendocrine tumors of the lung. J Thorac
Oncol. 9:1656–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang S, Oh MH, Ji SY, Han J, Kim TJ, Eom
M, Kwon KY, Ha SY, Choi YD, Lee CH, et al: Practical utility of
insulin-like growth factor II mRNA-binding protein 3, glucose
transporter 1, and epithelial membrane antigen for distinguishing
malignant mesotheliomas from benign mesothelial proliferations.
Pathol Int. 64:607–612. 2014.PubMed/NCBI
|
|
27
|
Üçer Ö, Dağli AF, Kiliçarslan A and Artaş
G: Value of Glut-1 and Koc markers in the differential diagnosis of
reactive mesothelial hyperplasia, malignant mesothelioma and
pulmonary adenocarcinoma. Turk Patoloji Derg. 29:94–100.
2013.PubMed/NCBI
|
|
28
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steurer S, Singer JM, Rink M, Chun F,
Dahlem R, Simon R, Burandt E, Stahl P, Terracciano L, Schlomm T, et
al: MALDI imaging-based identification of prognostically relevant
signals in bladder cancer using large-scale tissue microarrays.
Urol Oncol. 32:1225–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
von Loga K, Kohlhaussen J, Burkhardt L,
Simon R, Steurer S, Burdak-Rothkamm S, Jacobsen F, Sauter G and
Krech T: FGFR1 amplification is often homogeneous and strongly
linked to the squamous cell carcinoma subtype in esophageal
carcinoma. PLoS One. 10:e01418672015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grob TJ, Kannengiesser I, Tsourlakis MC,
Atanackovic D, Koenig AM, Vashist YK, Klose H, Marx AH, Koops S,
Simon R, et al: Heterogeneity of ERBB2 amplification in
adenocarcinoma, squamous cell carcinoma and large cell
undifferentiated carcinoma of the lung. Mod Pathol. 25:1566–1573.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gebauer F, Tachezy M, Effenberger K, von
Loga K, Zander H, Marx A, Kaifi JT, Sauter G, Izbicki JR and
Bockhorn M: Prognostic impact of CXCR4 and CXCR7 expression in
pancreatic adenocarcinoma. J Surg Oncol. 104:140–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mina S, Bohn BA, Simon R, Krohn A, Reeh M,
Arnold D, Bokemeyer C, Sauter G, Izbicki JR, Marx A, et al: PTEN
deletion is rare but often homogeneous in gastric cancer. J Clin
Pathol. 65:693–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammer NA, Hansen T, Byskov AG, Rajpert-De
Meyts E, Grøndahl ML, Bredkjaer HE, Wewer UM, Christiansen J and
Nielsen FC: Expression of IGF-II mRNA-binding proteins (IMPs) in
gonads and testicular cancer. Reproduction. 130:203–212. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang H, Wei Q, Ge J, Jian W, Liu J, Zhong
L, Fu B and Zhao T: IMP3 as a supplemental diagnostic marker for
Hodgkin lymphoma. Hum Pathol. 44:2167–2172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pryor JG, Simon RA, Bourne PA, Spaulding
BO, Scott GA and Xu H: Merkel cell carcinoma expresses K homology
domain-containing protein overexpressed in cancer similar to other
high-grade neuroendocrine carcinomas. Hum Pathol. 40:238–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vranic S, Gurjeva O, Frkovic-Grazio S,
Palazzo J, Tawfik O and Gatalica Z: IMP3, a proposed novel basal
phenotype marker, is commonly overexpressed in adenoid cystic
carcinomas but not in apocrine carcinomas of the breast. Appl
Immunohistochem Mol Morphol. 19:413–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chokoeva AA, Ananiev J, Wollina U, Tana C,
Lotti T, Cardoso JC and Tchernev G: Imp-3 expression in benign
melanocytic nevi, dysplastic nevi and malignant melanoma:
preliminary findings in Bulgarian patients. J Biol Regul Homeost
Agents. 29:695–699. 2015.PubMed/NCBI
|
|
39
|
Shooshtarizadeh T, Nazeri A, Zare-Mirzaie
A and Movahedinia S: Expression of insulin-like growth factor II
mRNA binding protein 3 (IMP3) in enchondroma and chondrosarcoma.
Pathol Res Pract. 212:335–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee AF, Gown AM and Churg A: IMP3 and
GLUT-1 immunohistochemistry for distinguishing benign from
malignant mesothelial proliferations. Am J Surg Pathol. 37:421–426.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ikeda K, Tate G, Suzuki T, Kitamura T and
Mitsuya T: IMP3/L523S, a novel immunocytochemical marker that
distinguishes benign and malignant cells: The expression profiles
of IMP3/L523S in effusion cytology. Hum Pathol. 41:745–750. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pryor JG, Bourne PA, Yang Q, Spaulding BO,
Scott GA and Xu H: IMP-3 is a novel progression marker in malignant
melanoma. Mod Pathol. 21:431–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yantiss RK, Woda BA, Fanger GR, Kalos M,
Whalen GF, Tada H, Andersen DK, Rock KL and Dresser K: KOC (K
homology domain containing protein overexpressed in cancer): A
novel molecular marker that distinguishes between benign and
malignant lesions of the pancreas. Am J Surg Pathol. 29:188–195.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nielsen J, Christiansen J, Lykke-Andersen
J, Johnsen AH, Wewer UM and Nielsen FC: A family of insulin-like
growth factor II mRNA-binding proteins represses translation in
late development. Mol Cell Biol. 19:1262–1270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mueller-Pillasch F, Pohl B, Wilda M,
Lacher U, Beil M, Wallrapp C, Hameister H, Knöchel W, Adler G and
Gress TM: Expression of the highly conserved RNA binding protein
KOC in embryogenesis. Mech Dev. 88:95–99. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu D, Vohra P, Chu PG, Woda B, Rock KL and
Jiang Z: An oncofetal protein IMP3: A new molecular marker for the
detection of esophageal adenocarcinoma and high-grade dysplasia. Am
J Surg Pathol. 33:521–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szarvas T, vom Dorp F, Niedworok C,
Melchior-Becker A, Fischer JW, Singer BB, Reis H, Bánkfalvi Á,
Schmid KW, Romics I, et al: High insulin-like growth factor
mRNA-binding protein 3 (IMP3) protein expression is associated with
poor survival in muscle-invasive bladder cancer. BJU Int.
110:E308–E317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Findeis-Hosey JJ and Xu H: Insulin-like
growth factor II-messenger RNA-binding protein-3 and lung cancer.
Biotech Histochem. 87:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Li HG, Xia ZS, Lü J and Peng TS:
IMP3 is a novel biomarker to predict metastasis and prognosis of
gastric adenocarcinoma: A retrospective study. Chin Med J (Engl).
123:3554–3558. 2010.PubMed/NCBI
|
|
50
|
Morimatsu K, Aishima S, Yamamoto H,
Hayashi A, Nakata K and Oda Y, Shindo K, Fujino M, Tanaka M and Oda
Y: Insulin-like growth factor II messenger RNA-binding protein-3 is
a valuable diagnostic and prognostic marker of intraductal
papillary mucinous neoplasm. Hum Pathol. 44:1714–1721. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen L, Xie Y, Li X, Gu L, Gao Y, Tang L,
Chen J and Zhang X: Prognostic value of high IMP3 expression in
solid tumors: A meta-analysis. Onco Targets Ther. 10:2849–2863.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schraml P, Bucher C, Bissig H, Nocito A,
Haas P, Wilber K, Seelig S, Kononen J, Mihatsch MJ, Dirnhofer S, et
al: Cyclin E overexpression and amplification in human tumours. J
Pathol. 200:375–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lugli A, Forster Y, Haas P, Nocito A,
Bucher C, Bissig H, Mirlacher M, Storz M, Mihatsch MJ and Sauter G:
Calretinin expression in human normal and neoplastic tissues: A
tissue microarray analysis on 5233 tissue samples. Hum Pathol.
34:994–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Went PT, Dirnhofer S, Bundi M, Mirlacher
M, Schraml P, Mangialaio S, Dimitrijevic S, Kononen J, Lugli A,
Simon R, et al: Prevalence of KIT expression in human tumors. J
Clin Oncol. 22:4514–4522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Minner S, Luebke AM, Kluth M, Bokemeyer C,
Jänicke F, Izbicki J, Schlomm T, Sauter G and Wilczak W: High level
of Ets-related gene expression has high specificity for prostate
cancer: A tissue microarray study of 11 483 cancers.
Histopathology. 61:445–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Andersen CL, Monni O, Wagner U, Kononen J,
Bärlund M, Bucher C, Haas P, Nocito A, Bissig H, Sauter G, et al:
High-throughput copy number analysis of 17q23 in 3520 tissue
specimens by fluorescence in situ hybridization to tissue
microarrays. Am J Pathol. 161:73–79. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Almanzar G, Olkhanud PB, Bodogai M,
Dell'agnola C, Baatar D, Hewitt SM, Ghimenton C, Tummala MK,
Weeraratna AT, Hoek KS, et al: Sperm-derived SPANX-B is a
clinically relevant tumor antigen that is expressed in human tumors
and readily recognized by human CD4+ and CD8+ T cells. Clin Cancer
Res. 15:1954–1963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Erez A, Perelman M, Hewitt SM, Cojacaru G,
Goldberg I, Shahar I, Yaron P, Muler I, Campaner S, Amariglio N, et
al: Sil overexpression in lung cancer characterizes tumors with
increased mitotic activity. Oncogene. 23:5371–5377. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Geiszt M, Lekstrom K, Brenner S, Hewitt
SM, Dana R, Malech HL and Leto TL: NAD(P)H oxidase 1, a product of
differentiated colon epithelial cells, can partially replace
glycoprotein 91phox in the regulated production of superoxide by
phagocytes. J Immunol. 171:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dicken BJ, Graham K, Hamilton SM, Andrews
S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S and
Cass C: Lymphovascular invasion is associated with poor survival in
gastric cancer: An application of gene-expression and tissue array
techniques. Ann Surg. 243:64–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mirlacher M, Kasper M, Storz M, Knecht Y,
Dürmüller U, Simon R, Mihatsch MJ and Sauter G: Influence of slide
aging on results of translational research studies using
immunohistochemistry. Mod Pathol. 17:1414–1420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mhawech-Fauceglia P, Herrmann FR, Rai H,
Tchabo N, Lele S, Izevbaye I, Odunsi K and Cheney RT: IMP3
distinguishes uterine serous carcinoma from endometrial
endometrioid adenocarcinoma. Am J Clin Pathol. 133:899–908. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zheng W, Yi X, Fadare O, Liang SX, Martel
M, Schwartz PE and Jiang Z: The oncofetal protein IMP3: A novel
biomarker for endometrial serous carcinoma. Am J Surg Pathol.
32:304–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yi X and Zheng W: Endometrial glandular
dysplasia and endometrial intraepithelial neoplasia. Curr Opin
Obstet Gynecol. 20:20–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li C, Zota V, Woda BA, Rock KL, Fraire AE,
Jiang Z, Lu D, Xu B, Dresser K, Lutman CV, et al: Expression of a
novel oncofetal mRNA-binding protein IMP3 in endometrial
carcinomas: Diagnostic significance and clinicopathologic
correlations. Mod Pathol. 20:1263–1268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kazeminezhad B, Mirafsharieh SA, Dinyari
K, Azizi D and Ebrahimi A: Usefulness of insulin-like growth factor
II mRNA-binding protein 3 (IMP3) as a new marker for the diagnosis
of esophageal adenocarcinoma in challenging cases. Turk J
Gastroenterol. 25:253–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Feng W, Zhou Z, Peters JH, Khoury T, Zhai
Q, Wei Q, Truong CD, Song SW and Tan D: Expression of insulin-like
growth factor II mRNA-binding protein 3 in human esophageal
adenocarcinoma and its precursor lesions. Arch Pathol Lab Med.
135:1024–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
He Y, Li L, Jiang W, Wang DQ, Xu L, Huang
Q, Zhang Y and Yang KX: Expression of the insulin-like growth
factor-II mRNA-binding protein 3 (IMP3) and carcinoembryonic
antigen (CEA) in mucinous minimal deviation adenocarcinoma. Pathol
Res Pract. 207:295–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wachter DL, Schlabrakowski A, Hoegel J,
Kristiansen G, Hartmann A and Riener MO: Diagnostic value of
immunohistochemical IMP3 expression in core needle biopsies of
pancreatic ductal adenocarcinoma. Am J Surg Pathol. 35:873–877.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lin L, Zhang J, Wang Y, Zheng L, Lin Z and
Cai Y: Expression of insulin-like growth factor 2 mRNA-binding
protein 3 expression and analysis of prognosis in the patients with
lung squamous cell carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
29:694–697. 2013.(In Chinese). PubMed/NCBI
|
|
71
|
Perak R Beljan, Durdov MG, Capkun V,
Ivcevic V, Pavlovic A, Soljic V and Peric M: IMP3 can predict
aggressive behaviour of lung adenocarcinoma. Diagn Pathol.
7:1652012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Findeis-Hosey JJ, Yang Q, Spaulding BO,
Wang HL and Xu H: IMP3 expression is correlated with histologic
grade of lung adenocarcinoma. Hum Pathol. 41:477–484. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen ST, Jeng YM, Chang CC, Chang HH,
Huang MC, Juan HF, Hsu CH, Lee H, Liao YF, Lee YL, et al:
Insulin-like growth factor II mRNA-binding protein 3 expression
predicts unfavorable prognosis in patients with neuroblastoma.
Cancer Sci. 102:2191–2198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Takata A, Takiguchi S, Okada K, Takahashi
T, Kurokawa Y, Yamasaki M, Miyata H, Nakajima K, Mori M and Doki Y:
Expression of insulin-like growth factor-II mRNA-binding protein-3
as a marker for predicting clinical outcome in patients with
esophageal squamous cell carcinoma. Oncol Lett. 8:2027–2031.
2014.PubMed/NCBI
|
|
75
|
King RL, Pasha T, Roullet MR, Zhang PJ and
Bagg A: IMP-3 is differentially expressed in normal and neoplastic
lymphoid tissue. Hum Pathol. 40:1699–1705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gaete S: Consciousness as meaning. Actas
Luso Esp Neurol Psiquiatr. 28:306–330. 1969.(In Spanish).
PubMed/NCBI
|
|
77
|
Minato H, Kurose N, Fukushima M, Nojima T,
Usuda K, Sagawa M, Sakuma T, Ooi A, Matsumoto I, Oda M, et al:
Comparative immunohistochemical analysis of IMP3, GLUT1, EMA,
CD146, and desmin for distinguishing malignant mesothelioma from
reactive mesothelial cells. Am J Clin Pathol. 141:85–93. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shi J, Liu H, Wang HL, Prichard JW and Lin
F: Diagnostic utility of von Hippel-Lindau gene product, maspin,
IMP3, and S100P in adenocarcinoma of the gallbladder. Hum Pathol.
44:503–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yu L, Xu H, Wasco MJ, Bourne PA and Ma L:
IMP-3 expression in melanocytic lesions. J Cutan Pathol.
37:316–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Elshafey MR, Ahmed RA, Mourad MI and
Gaballah ET: The oncofetal protein IMP3 is an indicator of early
recurrence and poor outcome in mucoepidermoid carcinoma of salivary
glands. Cancer Biol Med. 13:286–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ismerim AB, Ferreira SV, Lessa AM, Júnior
AS Pereira, Gurgel CA, Coutinho-Camillo CM, Soares FA, Vilas-Bôas
DS, Vidal MT and Santos JN: Insulin-like growth factor ii messenger
RNA-binding protein 3 in salivary gland tumors. Appl
Immunohistochem Mol Morphol. 24:422–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chisté M, Alexis J and Recine M: IMP3
expression in serous tumors of the ovary. Appl Immunohistochem Mol
Morphol. 22:658–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hu S, Wu X, Zhou B, Xu Z, Qin J, Lu H, Lv
L, Gao Y, Deng L, Yin J, et al: IMP3 combined with CD44s, a novel
predictor for prognosis of patients with hepatocellular carcinoma.
J Cancer Res Clin Oncol. 140:883–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wachter DL, Kristiansen G, Soll C,
Hellerbrand C, Breuhahn K, Fritzsche F, Agaimy A, Hartmann A and
Riener MO: Insulin-like growth factor II mRNA-binding protein 3
(IMP3) expression in hepatocellular carcinoma. A
clinicopathological analysis with emphasis on diagnostic value.
Histopathology. 60:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Soddu S, Di Felice E, Cabras S,
Castellanos ME, Atzori L, Faa G and Pilloni L: IMP-3 expression in
keratoacanthomas and squamous cell carcinomas of the skin: An
immunohistochemical study. Eur J Histochem. 57:e62013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Denby KS, Briones AJ, Bourne PA, Spaulding
BO, Lu D, Fischer-Colbrie R, Qu Z, Wang HL and Xu H: IMP3, NESP55,
TTF-1 and CDX2 serve as an immunohistochemical panel in the
distinction among small-cell carcinoma, gastrointestinal carcinoid,
and pancreatic endocrine tumor metastasized to the liver. Appl
Immunohistochem Mol Morphol. 20:573–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li C, Rock KL, Woda BA, Jiang Z, Fraire AE
and Dresser K: IMP3 is a novel biomarker for adenocarcinoma in situ
of the uterine cervix: An immunohistochemical study in comparison
with p16(INK4a) expression. Mod Pathol. 20:242–247. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kumara H Shantha, Kirchoff D, Caballero
OL, Su T, Ahmed A, Herath SA, Njoh L, Cekic V, Simpson AJ,
Cordon-Cardo C, et al: Expression of the cancer testis antigen
IGF2BP3 in colorectal cancers; IGF2BP3 holds promise as a specific
immunotherapy target. Oncoscience. 2:607–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lochhead P, Imamura Y, Morikawa T, Kuchiba
A, Yamauchi M, Liao X, Qian ZR, Nishihara R, Wu K, Meyerhardt JA,
et al: Insulin-like growth factor 2 messenger RNA binding protein 3
(IGF2BP3) is a marker of unfavourable prognosis in colorectal
cancer. Eur J Cancer. 48:3405–3413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu W, Wang P, Li Z, Xu W, Dai L, Wang K
and Zhang J: Evaluation of tumour-associated antigen (TAA)
miniarray in immunodiagnosis of colon cancer. Scand J Immunol.
69:57–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Barton VN, Donson AM, Birks DK,
Kleinschmidt-DeMasters BK, Handler MH, Foreman NK and Rush SZ:
Insulin-like growth factor 2 mRNA binding protein 3 expression is
an independent prognostic factor in pediatric pilocytic and
pilomyxoid astrocytoma. J Neuropathol Exp Neurol. 72:442–449. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bellezza G, Prosperi E, Del Sordo R,
Colella R, Rulli A and Sidoni A: IMP3 is strongly expressed in
malignant phyllodes tumors of the breast: An immunohistochemical
study. Int J Surg Pathol. 24:37–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Walter O, Prasad M, Lu S, Quinlan RM,
Edmiston KL and Khan A: IMP3 is a novel biomarker for triple
negative invasive mammary carcinoma associated with a more
aggressive phenotype. Hum Pathol. 40:1528–1533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hoffmann NE, Sheinin Y, Lohse CM, Parker
AS, Leibovich BC, Jiang Z and Kwon ED: External validation of IMP3
expression as an independent prognostic marker for metastatic
progression and death for patients with clear cell renal cell
carcinoma. Cancer. 112:1471–1479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sitnikova L, Mendese G, Liu Q, Woda BA, Lu
D, Dresser K, Mohanty S, Rock KL and Jiang Z: IMP3 predicts
aggressive superficial urothelial carcinoma of the bladder. Clin
Cancer Res. 14:1701–1706. 2008. View Article : Google Scholar : PubMed/NCBI
|