Introduction
Malignant melanoma (MM) is the deadliest skin cancer
due to high aggressiveness and metastasis (1). Regardless of progress in understanding
the initiation and progression of MM, patients with MM experience
gloomy prospects, with a poor prognosis and a low 5-year survival
rate of <15% (2,3). Currently, due to a lack of adequate
treatment, overall survival remains without improvement, requiring
more efforts for searching for effective therapeutic target
(4).
MicroRNAs (miRNAs) have binding sites in the 3′-UTRs
of their target gene and are capable of regulating expression of
the target genes at the transcriptional level by degrading mRNA and
suppressing mRNA translation (5,6).
Accumulating evidence has shown that differentially expressed
miRNAs participate in cancer progression via regulation of cell
proliferation and the cell cycle (7). MicroRNA-106b (miR-106b) is also a
well-accepted participant in the progression of such cancers as
breast cancer, gastric cancer, prostate cancer and hepatocellular
cancer (8–11). According to these studies, miR-106b
upregulation is frequent in tumor progression, and such
upregulation is involved in the development of invasiveness and
metastasis. Moreover, miR-106b has been shown to be heavily
involved in melanoma growth. It has been reported that suppression
of miRNA-106b arrests the cell cycle at the G1 phase and results in
blocked growth of melanoma cells via reactivation of p21/WAF1/Cip1
signaling (12), while
overexpression of miR-106b relates to poor prognosis of cutaneous
melanoma (13). Regretfully, there
is a poor understanding of the effect of miR-106b on its target in
MM progression.
In this study, the status of miR-106b in MM
is identified, and the impact of miR-106b on cell cycle
progression was investigated. Additionally, PTEN
(phosphatase and tensin homolog), which plays important roles in
many cellular processes, for example, cell growth, cell cycle
progression, angiogenesis, migration and invasion (14), was validated as a direct target of
miR-106b. It has been reported that miR-106b
contributes to the development of cancer by targeting PTEN
(15,16). Consistently, the expression of PTEN
was negatively correlated with the expression of miR-106b in
MM cell lines. This study further explored the interaction between
miR-106b and the Akt/ERK pathway downstream of PTEN.
This study reveals the working process of miR-106b in MM
progression and provides a potential molecular therapeutic target
for treatment of MM patients.
Materials and methods
Ethics statement
The present study was conducted under the approval
and guidance of the Medical Ethics Committee of Guizhou Provincial
People's Hospital. All patients included in this study provided
written consent.
Cell culture
SK-MEL-1 and A-375 (Human MM cell lines; American
Type Culture Collection) were incubated with Dulbecco's modified
Eagle's medium (DMEM) (Gibco, USA). Human epidermal melanocytes
(HEM) (Promocell) were cultured in melanocyte growth medium. Both
media contained 10% fetal bovine serum and such cell culture was
conducted at 37°C in 5% CO2 atmosphere.
Transient transfection
Cells were transfected with miR-106b-5p
mimics, mimics control, miR-106b-5p inhibitors and inhibitor
control (GenePharma, Shanghai, China). miR-106b-5p mimics:
5′-UAAAGUGCUGACAGUGCAGAU-3′; miR-106b-5p inhibitor:
5′-AUCUGCACUGUCAGCACUUUA-3′. Cells were cultured for 20 h before
transfection and were transfected at a confluence of 70%.
Oligonucleotides were transfected into MM cells with Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA). The oligonucleotides were
diluted to a final concentration of 100 nM for transfection.
PTEN vector was synthesized (RiboBio, Guangzhou, China).
Tissue specimens
Benign nevi (intradermal or congenital; n=18) or
primary cutaneous melanoma (n=18) was collected from patients
treated with resection at the Guizhou Provincial People's Hospital
between January 2014 and December 2015. All patients were free from
radiotherapy or chemotherapy before resection. All collected
tissues were cultured with RNAlater reagent (Takara, Japan) at
−80°C until use.
Quantitative real-time PCR
(qRT-PCR)
Total-RNA was extracted (Takara PrimeScript™ RT
reagent kit with gDNA Eraser; Takara). For quantification of
miR-106b-5p, TaqMan microRNA assays (Applied Biosystems,
Foster City, CA, USA) were adopted. SYBR Premix Ex Taq II (Takara)
was adopted for qRT-PCR. The following forward and reverse primers
(RiboBio) were used for PTEN: 5′-CACCTATTCCTCAGCCCTTAT-3′
and 5′-AACCCTCATTCAGACCTTCAC-3′; GAPDH:
5′-AATGGGCAGCCGTTAGGAAA-3′ and 5′-TGAAGGGGTCATTGATGGCA-3′;
miR-106b-5p: 5′-CAAGTACCCACAGTGCGGT-3′ and
5′-CTCGCTTCGGCAGCACA-3′); and U6:
5′-CGCTTCGGCAGCACATATACTA-3′ and 5′-CGCTTCACGAATTTGCGTGTCA-3′.
GAPDH and U6 were the internal reference. For
calculation of the relative expression of target genes, the ΔΔCT
method was used (14).
MTT assay, colony formation and flow
cytometry
For the MTT assay, the transfected cells were
cultured (5.0×103 cells/well) in 96-well plate and then
observed for cell proliferation at 24, 48, 72 and 96 h after 4-h
incubation with 20 µl of MTT (5 mg/ml) and the addition of 150 µl
of DMSO. The optical density (OD) at 490 nm was used to determine
the cell proliferation activity. Each well was verified with other
similar 5 wells, and the test on each well was conducted three
times. For colony formation assay, such transfected cells were
incubated for 10 days in 6-well plates, and the formed colonies
were treated with fixation and 5-min staining with 1% crystal
violet (Sigma-Aldrich). Ten different fields were selected for
colony counting, and such counting data was determined to be the
average of the ten fields. For flow cytometry, cells were incubated
(20 min at 37°C) with propidium iodide (PI) and RNase (2 µg/ml,
Sigma, USA). A FACSort flow cytometer was used to identify cell
population at the G1, S, and G2 phases (Becton-Dickinson, CA, USA).
The count data were expressed as percentages and analyzed with the
FlowJo 7.6 software.
Luciferase reporter assay
HEK293T cells received 1-day incubation in 24-well
plates (1×105/well) before transfection. pGL3
Dual-Luciferase miRNA Target Expression Vector (Promega, USA) was
synthesized with the predicted mutated (MUT) and wild-type (WT)
miR-106b-5p target on the PTEN 3′-UTR. The vectors
were transfected into cells using polyethylenimine (PEI; Sigma).
After 48 h of transfection, the luciferase activity of the
transfected cells was determined using Dual-Luciferase Reporter
assay system (Promega). Experiments were repeated two additional
times.
Western blot analysis
Total protein extraction was conducted with RIPA
(Thermo Scientific, Rockford, IL, USA). The cells were rinsed with
PBS, followed by solubilization in lysis buffer (1% Nonidet P-40)
and then centrifugation (20,000 × g, 15 min at 4°C). Protein was
measured for the concentration with bicinchoninic acid protein
assay kit (Pierce Biotechnology) and then separated with sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to membranes. Such membranes were first hatched with a
respective primary antibody against phosphorylated rabbit p-Akt,
p-ERK1/2, total AKT, and total ERK1/2, anti-PTEN,
anti-P27Kip1, anti-cyclin D1 or anti-GAPDH (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA or Cell Signaling Technology,
Inc., Danvers, MA, USA) and then continuously incubated with a
horseradish peroxidase-conjugated secondary antibody (Sigma).
Target proteins were quantified using enhanced chemiluminescence
(ECL) assay. The protein expression was normalized to GAPDH
expression.
Statistical analysis
SPSS 22.0 (SPSS Inc., Chicago, IL, USA) was employed
for data analysis, which was based on the mean ± standard
deviations (SD) from repeated experiments. Data comparisons were
made with t-test or one-way ANOVA. P<0.05 indicated statistical
significance.
Results
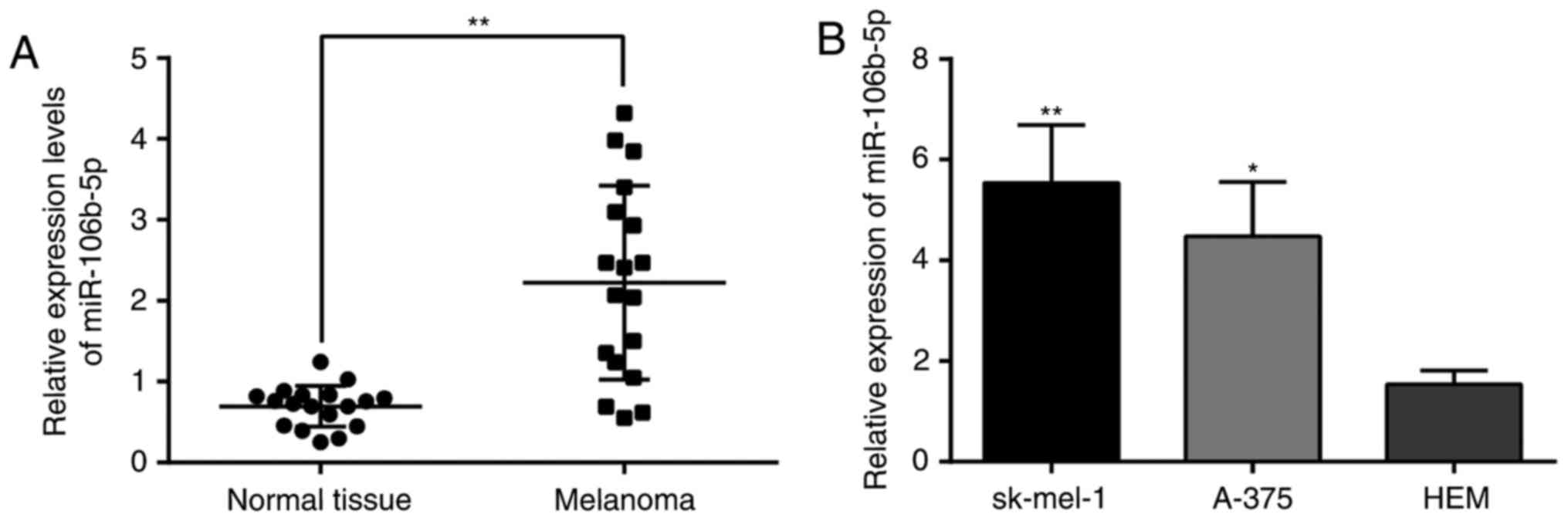
miR-106b-5p is increased in MM tissues
and cells
In light of qRT-PCR, miR-106b-5p greatly
increased in the MM tissues in parallel with that in the nevi
(Fig. 1A), similarly to SK-MEL-1
and A-375 cell lines in parallel with HEMs (Fig. 1B). This finding suggested that
miR-106b-5p may relate to MM progression.
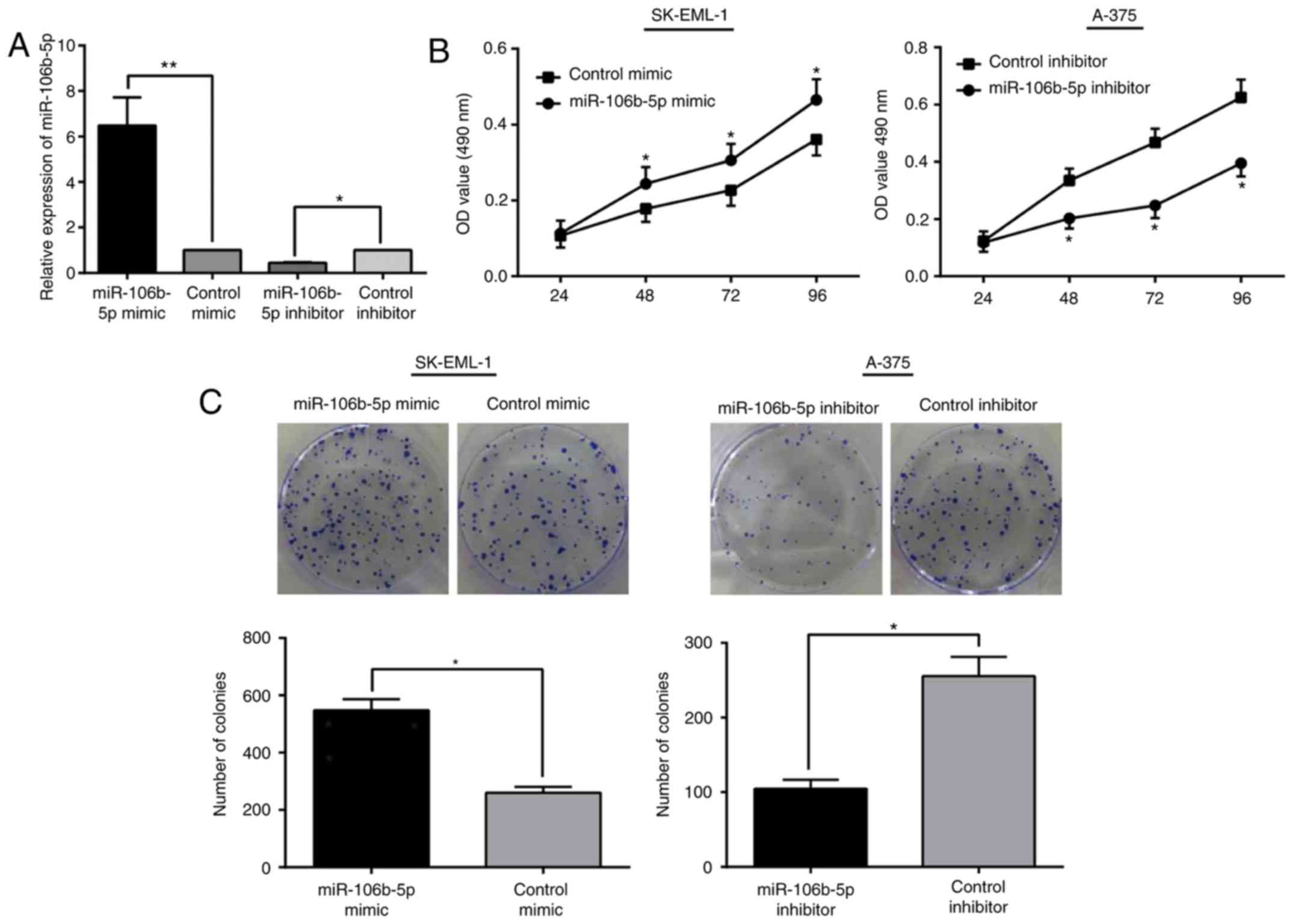
Interaction between miR-106b-5p and MM
cell proliferation in vitro
As demonstrated by qRT-PCR, miR-106b-5p was
obviously increased in SK-EML-1/miR-106b-5p mimic cells (a
100-nm dose), while remarkably downregulated in A-375/miR-106b-5p
inhibitor cells compared with that in the control cells (P<0.05;
Fig. 2A). As observed in the MTT
and colony formation assay, by reference to their control cells,
SK-EML-1/miR-106b-5p mimic cells had higher growth rates and
formed more colonies. In contrast, A-375/miR-106b-5p
inhibitor cells presented decreased growth capacity (Fig. 2B and C). From these results, it is
believed that miR-106b-5p is a promoter for cell
proliferation in MM progression.
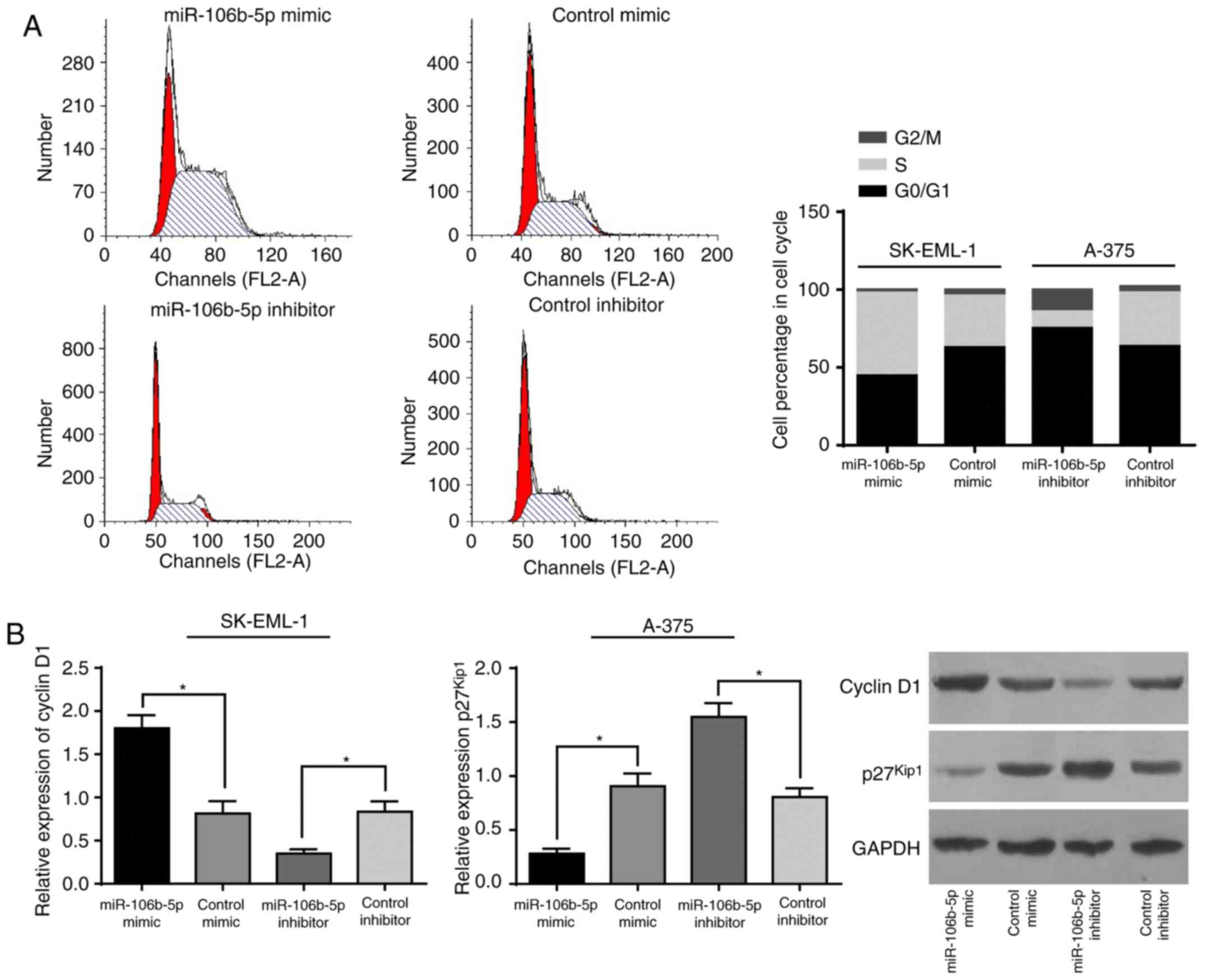
Relation of miR-106b-5p to MM cell
cycle progression in vitro
From the belief that upregulated miR-106b-5p
stimulated proliferation of MM cells, an investigation was carried
out to determine how miR-106b-5p impacts cell cycle
progression. According to flow cytometry, there were fewer SK-MEL-1
cells with upregulated miR-106b-5p at the G1/G0 phase but
more at S phase than the control cells. Conversely, A-375 cells
with downregulation of miR-106b-5p, in comparison with the
control cells, had a larger population at the G1/G0 phase and a
small population at the S phase (Fig.
3A).
It is well accepted that cell cycle progression is
driven forward by bondage between cyclins and CDKs. As detected
with western blot assay (Fig. 3B),
SK-MEL-1 cells with upregulated miR-106b-5p had higher
cyclin D1 expression and lower p27Kip expression, while
A-375 cells with downregulation of miR-106b-5p presented
suppressed cyclin D1 and increased p27Kip1. In
consideration of all these findings, it is reasonable to propose
that miR-106b promotes cell cycle progression when it is
upregulated but arrests cell cycle progression at the G0/G1 phase
via regulation of cyclins and p27Kip.
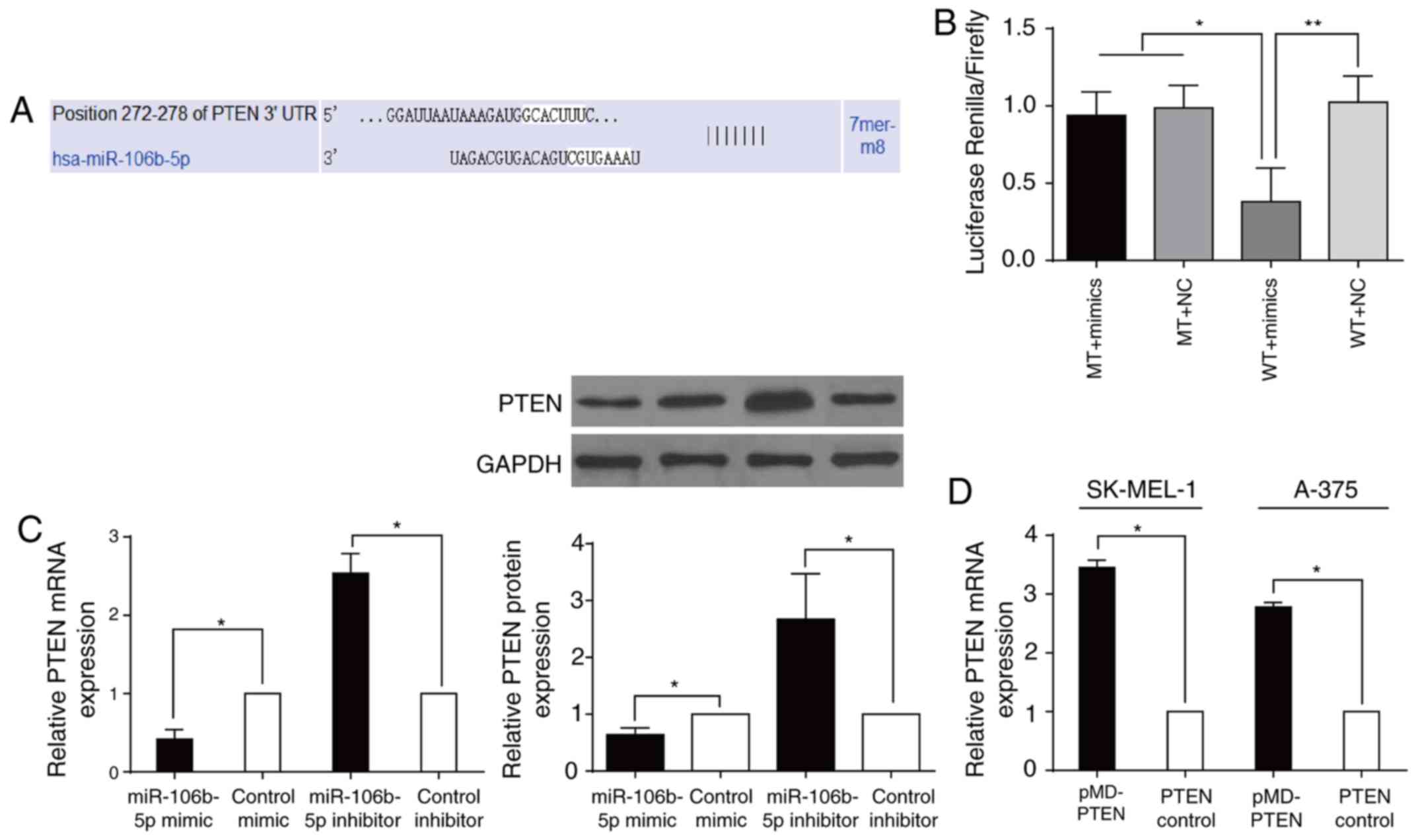
miR-106b-5p directly targeted the PTEN
3′-UTR
It was predicted with TargetScan that miR-106b-5p
can be linked to PTEN at the 3′-UTR (Fig. 4A). This prediction is consistent
with previous studies on other cancers (16,17).
Luciferase reporter analysis showed that in HEK293T cells,
miR-106b-5p suppressed the luciferase activity of the
PTEN WT reporter gene but not the MUT type, an indication
that PTEN is subject to the direct regulation of
miR-106b-5p (Fig. 4B). It
was also observed that SK-EML-1 cells with raised
miR-106b-5p had significantly lower PTEN at both the
mRNA and protein levels, while A-375 cells with downregulation of
miR-106b-5p exhibited higher PTEN mRNA and protein
expression (all P<0.05; Fig.
4C). After transfection with pMD-PTEN or PTEN
control in A-375 and SK-MEL-1 cells, cells that were transfected
with pMD-PTEN had significantly upregulated PTEN
(P<0.05; Fig. 4D). These
findings suggested that miR-106b-5p directly targeted
PTEN and affected cell activities via regulation of
PTEN.
miR-106b-5p promotes Akt/ERK1/2
signaling by inhibiting PTEN in MM cells
The AKT/ERK1/2 signaling pathway is a major pathway
in tumor growth (18) and is
subject to dephosphorylation by PTEN (19,20).
Interestingly, p27Kip1 and cyclin D1 are also under the
regulation of PTEN (21,22).
Based on the above facts, it was hypothesized that the Akt/ERK1/2
signaling pathway might play a role in the
miR-106b-5p/PTEN-stimulating MM progression.
Surprisingly, A-375 cells with downregulated miR-106b-5p and
upregulated PTEN had decreased p-AKT expression and p-ERK1/2
expression, while the total-Akt expression and total-ERK1/2
expression in such cells remained unchanged (Fig. 5A and B). Concordantly, SK-EML-1
cells with upregulation of miR-106b-5p increased p-Akt
expression, p-ERK1/2 expression, and total Akt, and total ERK1/2 in
such cells remained in the original expression status (Fig. 5C). After being co-transfected with
pMD-PTEN, SK-EML-1 cells with upregulation of
miR-106b-5p had a reversed trend in Akt and ERK1/2
expression (Fig. 5C). Based on
these findings, it is suggested that MM progression may be
triggered by miR-106b-5p, activating the Akt/ERK signaling
pathways via downregulation of PTEN.
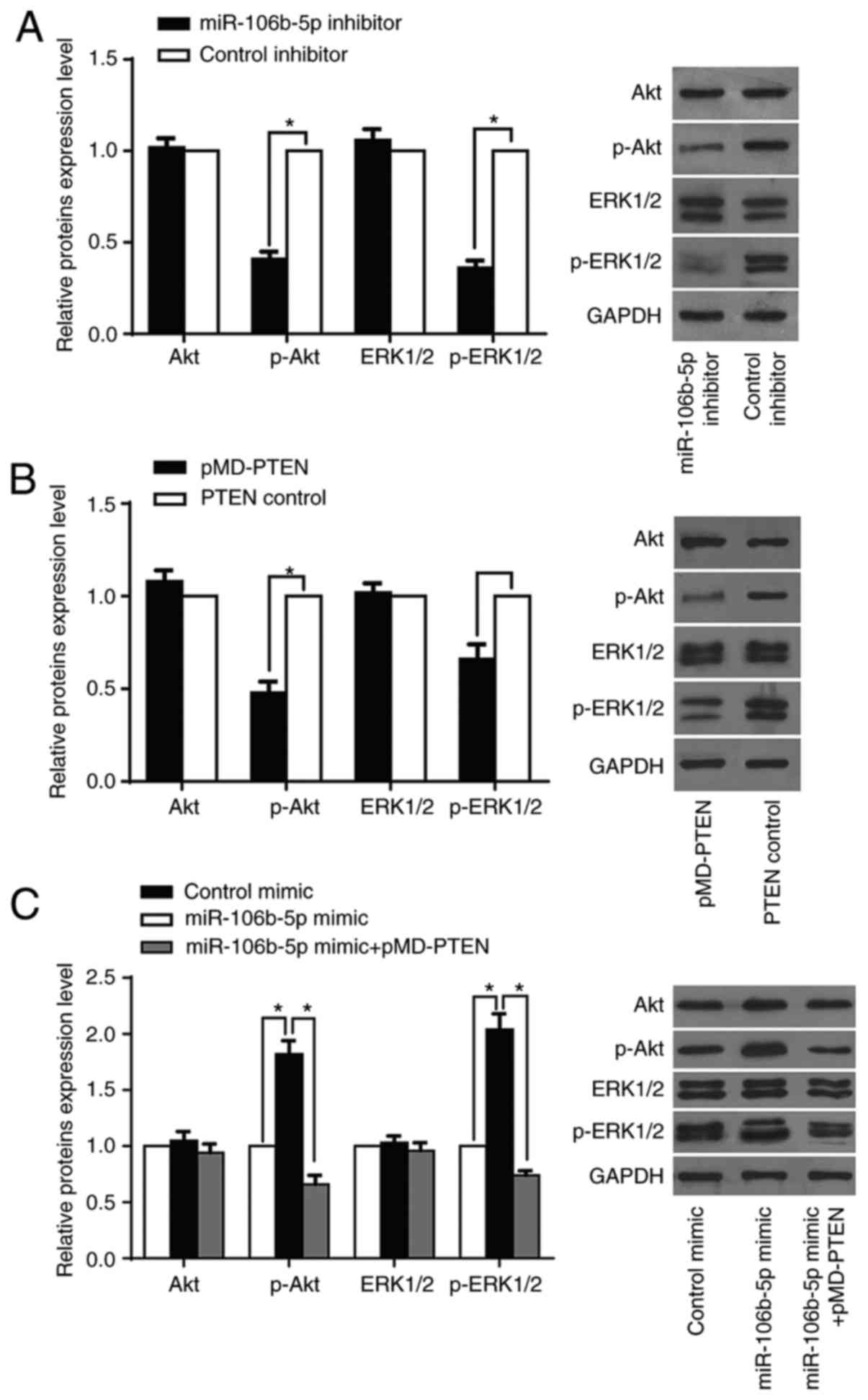 | Figure 5.miR-106b-5p promotes the
Akt/ERK1/2 signaling by inhibiting PTEN in MM cells. (A) The
expression of p-Akt, total Akt, p-ERK1/2, total ERK1/2 was measured
with a western blot assay in A-375/miR-106b-5p inhibitor
cells and A-375/control inhibitor cells. (B) The expression of
p-Akt, total p-Akt, total Akt, p-ERK1/2 and total ERK1/2 proteins
was detected using western blot assay in A-375/PTEN cells
and A-375/control cells. (C) Western blotting was conducted to
detect p-Akt, total Akt, p-ERK1/2, and total ERK1/2 proteins in
SK-EML-1/miR-106b-5p mimic, SK-EML-1/control mimic cells and
SK-EML-1/miR-106b-5p mimic/pMD-PTEN cells. The
protein expression was measured with GAPDH as an internal
reference. Error bars represent the results of the statistical
analysis. *P<0.05, **P<0.01 and NS, P>0.05. |
Discussion
miRNAs might be potential therapeutic targets for
their roles in regulating many tumor suppressor genes (23,24).
Previous studies reported that miR-106b was differentially
upregulated in several human cancers such as gastric cancer, breast
cancer and glioma (25–27). In agreement with those studies, our
qRT-PCR results also showed that MM tissues and cell lines had
increased miR-106b-5p. Accumulating evidence has shown that
miR-106b-5p is closely correlated with tumor progression
(11,28). However, miR-106b-5p has
varied functions in tumor progression among the studies. One study
defined miR-106b-5p as a promoter of the progression of
esophageal neoplasms by suppressing its two target genes,
p21 and Bim (29).
Another study suggested that it was a tumor-suppressor in type II
invasive endometrial cancer, and its direct interaction with
TWIST could block the development of EMT, thus preventing
tumor invasion and metastasis (30). This study asserts that
miR-106b-5p has different biological functions in different
tumors. Therefore, the present study sought to clarify how
miR-106b-5p works in MM progression.
Control of the cell cycle machinery has a critical
role in regulating cell proliferation and tumor growth of cancer
cells. In our study, which deploys upregulation and knockdown
strategies in the SK-EML-1 and A-375 cell lines, respectively, it
was observed that A-375 cells with suppressed miR-106b-5p
presented lower growth capacity. Additionally, less colony
formation had more cells at the G0/G1 phase and less cells at the S
phase, while SK-EML-1 cells with increased miR-106b-5p had
the reverse trend. Thus, it is suggested that miR-106b-5p is
capable of promoting cell cycle progression. Coincidently, Xiang
et al found that suppression of miR-106b-5p blocked
cell cycle progression at the G0/G1 phase and inhibited cell
proliferation via knockdown of SETD2 (31). By contrast, Ivanovska et al
found that, after being upregulated, miR-106b in cancer
cells sped up cell cycle progression (32). Considering these findings, this
study suggests that miR-106b-5 may accelerate MM progression
and MM cell proliferation by shortening the cell cycle. From this
point, the study progresses to explore the association between
miR-106b-5p and cell cycle-related proteins and
pathways.
To our knowledge, levels of cyclins and
cyclin-dependent kinase (Cdk) inhibitors are tightly controlled
during normal cell proliferation and are frequently dysregulated in
cancerous cells. Uncontrolled cell division is triggered by
activated cyclins binding to CDKs in the G1 phase, inevitably
driving cells to the S phase. However, the cell cycle exit is
controlled by two major classes of non-enzymatic CDK inhibitors
(CDKIs) that directly interact with cyclin-CDK complexes: the INK4
and the CDK-inhibitory protein (CIP)/kinase-inhibitory protein
(KIP) families (33). The CIP/KIP
family comprises three proteins in mammals:
p21cip1/waf1, p27Kip1, and
p57Kip2. In melanoma, cyclin D1 is highly expressed, and
downregulation of the CDK-inhibitor, p27Kip1, is
associated with a poor prognosis (34). On this basis, this study further
investigated the effect of miR-106b-5p on cyclin D1 and
p27Kip1 during regulation of the cell cycle. Our results
indicate that miR-106b-5p suppressed p27Kip1 and
activated the cell cycle regulator cyclin D1. Such an impact of
miR-106b-5p on p27Kip1 and cyclin D1 can be
mediated by downregulation of miR-106b-5p. Therefore, it can
be concluded that miR-106b-5p promotes cell cycle
progression in MM by regulating cyclins and CDKs. As known,
p27Kip1 and cyclin D1 are subject to transcriptional
regulation by PTEN (35,36),
and PTEN is a well-accepted anti-oncogene in human cancers,
including melanoma (37–39). It is predicated that PTEN can
crosstalk to miR-106b-5p in its 3′-UTR. Using a luciferase
reporter assay, we recognized that PTEN is a target gene of
miR-106b-5p and found that PTEN was negatively
regulated by miR-106b-5p. With upregulation of PTEN,
the growth rate and cell cycle progression of MM cells were
controlled.
It has been reported that the AKT/ERK1/2 signaling
pathway is a crucial pathway in the development of tumor/cancer.
Hydrogen sulfide promotes oral cancer cell proliferation through
activation of the COX2/AKT/ERK1/2 axis (40), and miR-7 inhibits tumor metastasis
and reverses EMT through AKT and ERK1/2 pathway inactivation by
reducing EGFR expression in EOC cell lines. Meanwhile, the
AKT/ERK1/2 signaling pathway is subject to dephosphorylation by
PTEN (19,20). Therefore, miR-106b-5p is
involved in the correlation between PTEN and Akt and ERK1/2
pathway. To address this argument, this study progresses to look
into the relation of miR-106b-5p to Akt/ERK1/2 signaling
pathway, finding that miR-106b-5p positively intensified
phospho-AKT (p-AKT) and phosphor-ERK1/2, while the total Akt
expression and total ERK1/2 expression remained unchanged. Such
intensification is accepted in this study as evidence that MM
progression may be triggered by miR-106b-5p activating
PTEN/Akt/ERK signaling pathways.
There are limitations to this study. First,
miR-106b-5p should have been studied in relation to the
clinicopathological features of MM. Second, miR-106b-5p
promoting cell cycle progression should be further validated in an
in vivo study. Future studies can adequately investigate the
aforementioned limitations. This study summarily argues that
miR-106b-5p is frequently differentially increased in both
MM tissues and MM cell lines and closely relates to cell cycle
progression via targeting PTEN and the Akt/ERK1/2 signaling
pathway. Therefore, miR-106b-5p constitutes an oncogene in
MM progression and a potential target for cellular therapy.
Acknowledgements
The authors would like to acknowledge the helpful
comments on this manuscript that were provided by the
reviewers.
References
|
1
|
Knoll S, Fürst K, Kowtharapu B, Schmitz U,
Marquardt S, Wolkenhauer O, Martin H and Pützer BM: E2F1 induces
miR-224/452 expression to drive EMT through TXNIP downregulation.
EMBO Rep. 15:1315–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al BRIM-3 Study Group, : Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–2516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berrocal A, Cabañas L, Espinosa E,
Fernández-de-Misa R, Martín-Algarra S, Martínez-Cedres JC,
Ríos-Buceta L and Rodríguez-Peralto JL: Melanoma: Diagnosis,
staging, and treatment. Consensus group recommendations. Adv Ther.
31:945–960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Hara SP, Mott JL, Splinter PL, Gores GJ
and LaRusso NF: MicroRNAs: Key modulators of posttranscriptional
gene expression. Gastroenterology. 136:17–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang R, Guo Y, Ma Z, Ma G, Xue Q, Li F
and Liu L: Long non-coding RNA PTENP1 functions as a ceRNA to
modulate PTEN level by decoying miR-106b and miR-93 in gastric
cancer. Oncotarget. 8:26079–26089. 2017.PubMed/NCBI
|
|
9
|
Yen CS, Su ZR, Lee YP, Liu IT and Yen CJ:
miR-106b promotes cancer progression in hepatitis B
virus-associated hepatocellular carcinoma. World J Gastroenterol.
22:5183–5192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi N, Park J, Lee JS, Yoe J, Park GY,
Kim E, Jeon H, Cho YM, Roh TY and Lee Y:
miR-93/miR-106b/miR-375-CIC-CRABP1: A novel regulatory axis in
prostate cancer progression. Oncotarget. 6:23533–23547. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith AL, Iwanaga R, Drasin DJ, Micalizzi
DS, Vartuli RL, Tan AC and Ford HL: The miR-106b-25 cluster targets
Smad7, activates TGF-β signaling, and induces EMT and tumor
initiating cell characteristics downstream of Six1 in human breast
cancer. Oncogene. 31:5162–5171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prasad R and Katiyar SK: Down-regulation
of miRNA-106b inhibits growth of melanoma cells by promoting
G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1
protein. Oncotarget. 5:10636–10649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin N, Zhou Y, Lian X and Tu Y: Expression
of microRNA-106b and its clinical significance in cutaneous
melanoma. Genet Mol Res. 14:16379–16385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin Y and Shen WH: PTEN: A new guardian of
the genome. Oncogene. 27:5443–5453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu T, Liu L, Li J, Yan M, Lin H, Liu Y,
Chu D, Tu H, Gu A and Yao M: MiRNA-10a is upregulated in NSCLC and
may promote cancer by targeting PTEN. Oncotarget. 6:30239–30250.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li KK, Xia T, Ma FM, Zhang R, Mao Y, Wang
Y, Zhou L, Lau KM and Ng HK: miR-106b is overexpressed in
medulloblastomas and interacts directly with PTEN. Neuropathol Appl
Neurobiol. 41:145–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng L, Zhang Y, Liu Y, Zhou M, Lu Y,
Yuan L, Zhang C, Hong M, Wang S and Li X: MiR-106b induces cell
radioresistance via the PTEN/PI3K/AKT pathways and p21 in
colorectal cancer. J Transl Med. 13:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soares AS, Costa VM, Diniz C and Fresco P:
Inosine strongly enhances proliferation of human C32 melanoma cells
through PLC-PKC-MEK1/2-ERK1/2 and PI3K pathways. Basic Clin
Pharmacol Toxicol. 116:25–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouali S, Chrétien AS, Ramacci C, Rouyer
M, Becuwe P and Merlin JL: PTEN expression controls cellular
response to cetuximab by mediating PI3K/AKT and RAS/RAF/MAPK
downstream signaling in KRAS wild-type, hormone refractory prostate
cancer cells. Oncol Rep. 21:731–735. 2009.PubMed/NCBI
|
|
20
|
Chetram MA and Hinton CV: PTEN regulation
of ERK1/2 signaling in cancer. J Recept Signal Transduct Res.
32:190–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao J, Li S, Palmqvist L, Fogelstrand L,
Wei SY, Busayavalasa K, Liu K and Liu VM: p27(KIP1) and PTEN
cooperate in myeloproliferative neoplasm tumor suppression in mice.
Exp Hematol Oncol. 5:172016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Macdonald FH, Yao D, Quinn JA and
Greenhalgh DA: PTEN ablation in Ras(Ha)/Fos skin carcinogenesis
invokes p53-dependent p21 to delay conversion while p53-independent
p21 limits progression via cyclin D1/E2 inhibition. Oncogene.
33:4132–4143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beg MS, Brenner AJ, Sachdev J, Borad M,
Kang YK, Stoudemire J, Smith S, Bader AG, Kim S and Hong DS: Phase
I study of MRX34, a liposomal miR-34a mimic, administered twice
weekly in patients with advanced solid tumors. Invest New Drugs.
35:180–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen MM: Getting miRNA Therapeutics into
the target cells for neurodegenerative diseases: A mini-review.
Front Mol Neurosci. 9:1292016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng R, Pan L, Gao J, Ye X, Chen L, Zhang
X, Tang W and Zheng W: Prognostic value of miR-106b expression in
breast cancer patients. J Surg Res. 195:158–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang A, Hao J, Wang K, Huang Q, Yu K,
Kang C, Wang G, Jia Z, Han L and Pu P: Down-regulation of miR-106b
suppresses the growth of human glioma cells. J Neurooncol.
112:179–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Tan W, Neo TW, Aung MO, Wasser S,
Lim SG and Tan TM: Role of the miR-106b-25 microRNA cluster in
hepatocellular carcinoma. Cancer Sci. 100:1234–1242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kan T, Sato F, Ito T, Matsumura N, David
S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, et al: The
miR-106b-25 polycistron, activated by genomic amplification,
functions as an oncogene by suppressing p21 and Bim.
Gastroenterology. 136:1689–1700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong P, Kaneuchi M, Watari H, Sudo S and
Sakuragi N: MicroRNA-106b modulates epithelial-mesenchymal
transition by targeting TWIST1 in invasive endometrial cancer cell
lines. Mol Carcinog. 53:349–359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiang W, He J, Huang C, Chen L, Tao D, Wu
X, Wang M, Luo G, Xiao X, Zeng F, et al: miR-106b-5p targets tumor
suppressor gene SETD2 to inactive its function in clear cell renal
cell carcinoma. Oncotarget. 6:4066–4079. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ivanovska I, Ball AS, Diaz RL, Magnus JF,
Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson
AL, et al: MicroRNAs in the miR-106b family regulate p21/CDKN1A and
promote cell cycle progression. Mol Cell Biol. 28:2167–2174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhatt KV, Spofford LS, Aram G, McMullen M,
Pumiglia K and Aplin AE: Adhesion control of cyclin D1 and p27Kip1
levels is deregulated in melanoma cells through BRAF-MEK-ERK
signaling. Oncogene. 24:3459–3471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jonason JH, Gavrilova N, Wu M, Zhang H and
Sun H: Regulation of SCF(SKP2) ubiquitin E3 ligase assembly and
p27(KIP1) proteolysis by the PTEN pathway and cyclin D1. Cell
Cycle. 6:951–961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao L, Pan TJ, Wu GJ, Shen GQ, Yang JR,
Wen HD, Xie S and Qian WH: Effects of adenovirus-mediated PTEN on
the proliferation of prostate cancer PC-3 cells and expressions of
cyclin D1 and p21. Zhonghua Nan Ke Xue. 20:207–212. 2014.(In
Chinese). PubMed/NCBI
|
|
37
|
Jian B, Li Z, Xiao D, He G, Bai L and Yang
Q: Downregulation of microRNA-193-3p inhibits tumor proliferation
migration and chemoresistance in human gastric cancer by regulating
PTEN gene. Tumour Biol. 37:8941–8949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong Y, Richards JA, Gupta R, Aung PP,
Emley A, Kluger Y, Dogra SK, Mahalingam M and Wajapeyee N: PTEN
functions as a melanoma tumor suppressor by promoting host immune
response. Oncogene. 33:4632–4642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yari K, Payandeh M and Rahimi Z:
Association of the hypermethylation status of PTEN tumor suppressor
gene with the risk of breast cancer among Kurdish population from
Western Iran. Tumour Biol. 37:8145–8152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang S, Bian H, Li X, Wu H, Bi Q, Yan Y
and Wang Y: Hydrogen sulfide promotes cell proliferation of oral
cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep.
35:2825–2832. 2016. View Article : Google Scholar : PubMed/NCBI
|