Introduction
Cervical cancer is the fourth most common cancer in
women, with an estimated 527,600 new cases and 265,700 deaths
worldwide (1). Infection with
high-risk types of human papillomavirus (HPV) leads to nearly all
types of cervical cancers. HPV16 is the predominant HPV, causing
47.7% of cervical cancer cases in sub-Saharan Africa to 69.7% of
cervical cancer cases in Europe/North America (2). In addition to cervical cancer, HPV
infection is also associated with other anogenital and oral cavity
cancers in the penis, vulva/vagina, anus, mouth, and oropharynx
(3). To date, some prophylactic HPV
vaccines have been administered to young women prior to the age of
onset of sexual activity to protect against high-risk HPV
infections (4,5). Although these prophylactic vaccines
have made great success in protecting younger women, individuals
with pre-existing infections have no benefit from prophylactic
vaccines (6). Thus, the research
and development of therapeutic HPV16 vaccines are intensely
required (7).
E6 and E7, two oncogenic HPV16 proteins, are
required for the transformation of infected cells and maintenance
of the transformed state (8,9).
Cervical cancers continuously express E6 and E7 as a result of the
selective integration of the HPV16 genome into transformed cells
(9). Thus, E6 and E7 represent
promising targets for therapeutic HPV16 vaccines. Accordingly,
numerous therapeutic HPV16 vaccines that elicit strong anti-E6/E7
cellular immunity or tumor regression have been reported, including
peptide immunization, DNA immunization, E6/E7 fusion protein
immunization, immunization with recombinant E7-expressing vaccinia
virus, and E7-pulsed dendritic cells (7).
Dendritic cells (DCs) play a central role in the
induction of adaptive immune responses (10). An effective manner of improving
vaccination is targeting the receptor-mediated endocytosis of DCs.
Herein, mannose receptors (MRs), the C-type lectins containing
mannose receptor (MR, CD206), DC-SIGN (CD209), dectin-1 and
langerin, have a high affinity for mannosylated antigens (11,12).
The uptake of mannosylated antigens by these C-type lectins has
great advantages over other approaches (e.g., pinocytosis) in
antigen presentation and T cell stimulation (13–16).
Based on these advantages, antigen mannosylation represents a
promising strategy for therapeutic vaccines in inducing
cell-mediated immune responses (17,18).
As previously reported, the mannosylation of the
HPV16 E7 peptide enhanced the proliferation and activation of
antigen-specific CD8+ T cells and the cytotoxic T cell
response and increased antitumor activity in mice (19,20).
In the present study, we exploited Pichia pastoris to
generate recombinant mannosylated HPV16 E7, which efficiently
stimulated Th1 (type 1 T helper cell) and cell-mediated immune
responses in the presence of monophosphoryl lipid A (MPL). In
addition, mannosylation enhanced the uptake of mE7 by MRs of
peripheral blood mononuclear cells (BMDCs) which then in
vitro stimulated IFN-γ secretion by splenocytes of immunized
mice. Compared with E7, vaccination with mE7 combined with MPL of
C57BL/6 mice increased the production of cytokines (IFN-γ, IL-2 and
TNF-α), induced more E7-specific IFN-γ-secreting CD8+ T
cells in the spleen and PMBCs, promoted E7-specific cytotoxic
CD8+ T cell responses, and improved antitumor effects
against HPV16 E7-expressing tumors. Hence, recombinant mE7 provides
a promising immunotherapy for treating cervical cancer.
Materials and methods
Cells and culture
TC-1 cells, generated by the co-transformation of
C57BL/6 mouse lung epithelial cells with HPV16 E6 and
E7 oncogenes and the human ras oncogene (21), were purchased from the Cancer
Hospital, Chinese Academy of Medical Sciences (ATCC no. CRL-2785).
TC-1 cells were cultured in RPMI-1640 medium (HyClone, Logan, UT,
USA), containing 10% fetal bovine serum (FBS; Gibco, Grand Island,
NY, USA), 100 U/ml penicillin, and 100 U/ml streptomycin (Beyotime,
Shanghai, China). R10 medium, used for culturing BMDCs, contains
standard RPMI-1640 plus 50 nM β-mercaptoethanol (Biosharp, Hefei,
China). All cells were cultured at 37°C in humidified air
supplemented with 5% CO2.
Mice
Specific pathogen-free (SPF) 6- to −8-week-old
female C57BL/6 mice were maintained at the Laboratory Animal Center
of Wuhan Institute of Virology, Chinese Academy of Sciences (CAS).
All mouse studies were performed according to Regulations of the
Administration of Affairs Concerning Experimental Animals in China
(WIVA25201304), and the protocols were reviewed and approved by the
Laboratory Animal Care and Use Committee at the Wuhan Institute of
Virology, CAS. Briefly, all the mice were fed in an independent
ventilated cage (IVC) and the IVCs were kept within an experimental
animal barrier environment. The food was sterilized by
Co60-irradiation and the water was sterilized using an
autoclave. We replenished the food and water twice or three times a
week.
Cloning, expression and purification
of mE7 and E7 in Pichia pastoris KM71
mE7 was generated from the oncogenic E7 gene of
HPV16 (GenBank accession no. AF125673) synthesized at Sangon
Biotech (Shanghai, China) and a linker encoding 15-amino acids
containing two N-X-S/T motifs (GGGGSNGTGGGNASC) with two potential
N-linked glycosylation sites. In contrast to mE7, E7 was
generated from the site-directed mutagenesis of the two potential
N-linked glycosylation sites in the linker region. NGT and
NAS were conservatively mutated to AGT and AAS, respectively.
The mE7 and E7 were cloned into plasmid pPICZαA.
Subsequently, recombinant plasmids pPICZαA-mE7 and pPICZαA-E7 were
linearized using SacI (NEB, Ipswich, MA, USA), purified,
transfected into electrocompetent Pichia pastoris KM71
(pulsed 1.5 kV, 200 Ω, and 25 µF) and subsequently incubated for 2
h in yeast extract peptone dextrose medium (YPD). Recombinant
clones were selected on YPD agar plates, containing 75 µg/ml Zeocin
(Invitrogen, San Diego, CA, USA), and identified using colony PCR.
The expression and purification of recombinant protein in KM71 was
conducted according to Lam et al (15).
Generation of BMDCs
BMDCs were generated according to Lutz et al
(22). Briefly, bone marrow cells
from the tibiae and femurs of one 8-week-old C57BL/6 mouse were
cultured in R10 medium supplemented with 200 U/ml rmGM-CSF
(PeproTech, Rocky Hill, NJ, USA). The cells were fed with fresh
medium on days 3, 6, and 8 and matured with fresh R10 medium
containing 100 U/ml rmGM-CSF and 500 U/ml TNF-α (PeproTech) on day
10.
BMDCs uptake of recombinant protein
and T cell polarization
The mature BMDCs were cultured in 6-cm plates and
co-incubated with 10 µg/ml mE7 or E7 in the presence or absence of
10 mg/ml yeast mannans (Sigma, St. Louis, MO, USA) for 45 min.
Subsequently, the cells were collected and washed using PBS. The
collected cells were lysed using RIPA lysis buffer (Beyotime), and
centrifuged at 10,000 × g for 5 min to remove the debris. The
supernatants were precipitated with 4 volumes of acetone, incubated
at −20°C overnight, centrifuged at 13,000 × g for 15 min and
subsequently analyzed by western blotting.
T cell polarization assays were performed following
the method of Satchidanandam et al (23). Briefly, the mE7- or E7-preincubated
BMDCs were cultured for 24 h and then treated with 50 µg/ml
mitomycin C (Sigma) for 1 h followed by extensive washing. The
above BMDCs were co-cultured in a 96-well plate with one million
splenocytes from C57BL/6 mice immunized with mE7, E7 or PBS, at the
BMDC:splenocyte ratio of 1:10 for 72 h. The supernatants were
collected for IFN-γ measurement using capture ELISA (BioLegend, San
Diego, CA, USA), according to the protocol for Mouse IFN-γ ELISA
MAX Standard Sets.
Vaccination of mice with recombinant
proteins
The 6- to 8-week-old C57BL/6 mice were grouped and
subcutaneously (s.c.) vaccinated with 40 µg mE7, 40 µg E7 or 100 µl
PBS in the presence of 5 µg adjuvant MPL (Sigma, L6895) on days 0,
5 and 10. On day 15, the mice were sacrificed by cervical
dislocation under aseptic conditions. Eyeball enucleation was
conducted for blood collection. The sera and splenocytes were
collected and harvested from blood.
Cytokine quantification
Four million splenocytes from each immunized mouse
were cultured for 48 h in 24-well plates in the presence of 10
µg/ml E7 or phorbol-12-myristate-13-acetate (PMA) plus ionomycin
(Dakewe Biotech Co., Shenzhen, China). The supernatants were
collected, and the concentrations of IFN-γ, IL-2 and TNF-α were
measured using capture ELISA (BioLegend), according to the
protocols for Mouse IFN-γ/IL-2/TNF-α ELISA MAX Standard Sets.
Antibody determination
The sera were collected from each immunized mouse at
5 days after the last immunization to determine specific IgG using
ELISA. Polyvinylchloride 96-well plates (Nunc, Rochester, NY, USA)
were coated with E7 at 2 µg/ml in PBS and incubated overnight at
4°C. The plates were subsequently blocked with 10% FBS in PBS,
washed, and incubated with the two-fold diluted serums, followed by
detection with HRP-conjugated Affinipure Goat Anti-Mouse IgG
(Proteintech, Wuhan, China).
FACS staining
One million splenocytes from each immunized mouse
and PBMCs from each group were cultured for 24 h in 96-well plates
in the presence of 10 µg/ml E7 or PMA plus ionomycin as positive
control. For surface staining, cells were incubated with the
following antibodies (BD Biosciences, Franklin Lakes, NJ, USA):
PE-Cy7 anti-CD3e (145-2C11), FITC anti-CD4 (RM4-5), PE anti-CD8a
(53-6.7) and 7-AAD Viability Staining Solution (BioLegend). For
intracellular IFN-γ staining, APC anti-IFN-γ (XMG1.2, BD
Biosciences) was used. All the staining procedures were according
to BD Biosciences protocols. Data were acquired using a BD FACS
flow cytometer and analyzed by FlowJo software (Tree Star, Ashland,
OR, USA).
Cytotoxicity assays
Sixteen million splenocytes from each immunized
mouse were cocultured with one million mitomycin C-pretreated TC-1
cells in RPMI-1640 supplemented with 10% FBS, 50 µg/ml concanavalin
A (Con A; Sigma) and 20 U/ml IL-2 (PeproTech) at 37°C in 5%
CO2. After 5 days, the viable splenocytes were collected
and used as effector cells, and TC-1 cells were used as target
cells. The Pierce LDH Cytotoxicity assay kit (Thermo Fisher
Scientific, Waltham, MA, USA) was used to measure the effector
cells against TC-1 at ratios of 2.5:1 and 5:1 according to the
manufacturer's instructions. Specific lysis was calculated as
percent specific lysis = [(Experimental value - Effector cell
spontaneous control - Target cell spontaneous control)/(Target cell
maximum control - Target cell spontaneous control)] ×100.
Tumor challenge
On day 0, the 6- to 8-week-old C57BL/6 mice were
injected (s.c.) with 2×105 TC-1 cells in the right
flank. Vaccination with mE7, E7 and PBS with MPL was performed on
days 3, 8, and 13. Tumor growth was measured using a caliper, and
tumor size was calculated as volume = (length ×
width2)/2 (24). For
survival analysis, the mice were considered as dead when the tumor
reached 1000 mm3. When the tumor size of the immunized
mice exceeded 1000 mm3, the mice were sacrificed and
dissected. Tumors were separated by ophthalmic scissors and
tweezers, and kept in formalin. Tumor-free mice vaccinated with mE7
were challenged with 2×105 TC-1 cells again on day 31.
The percentage of tumor-free mice was recorded.
Statistical analysis
Statistical analysis was performed using Prism
software version 6.0 (GraphPad, San Diego, CA, USA). All data are
presented as the means ± SD. The cytokine concentration, antibody
titers and FACS results were assessed using an unpaired two-tailed
t-test. Cytotoxicity assays and tumor sizes were assessed using
two-way ANOVA multiple comparisons. Survival percentages were
assessed using the log-rank test. P-values of <0.05 were
considered to indicate statistically significant differences.
Results
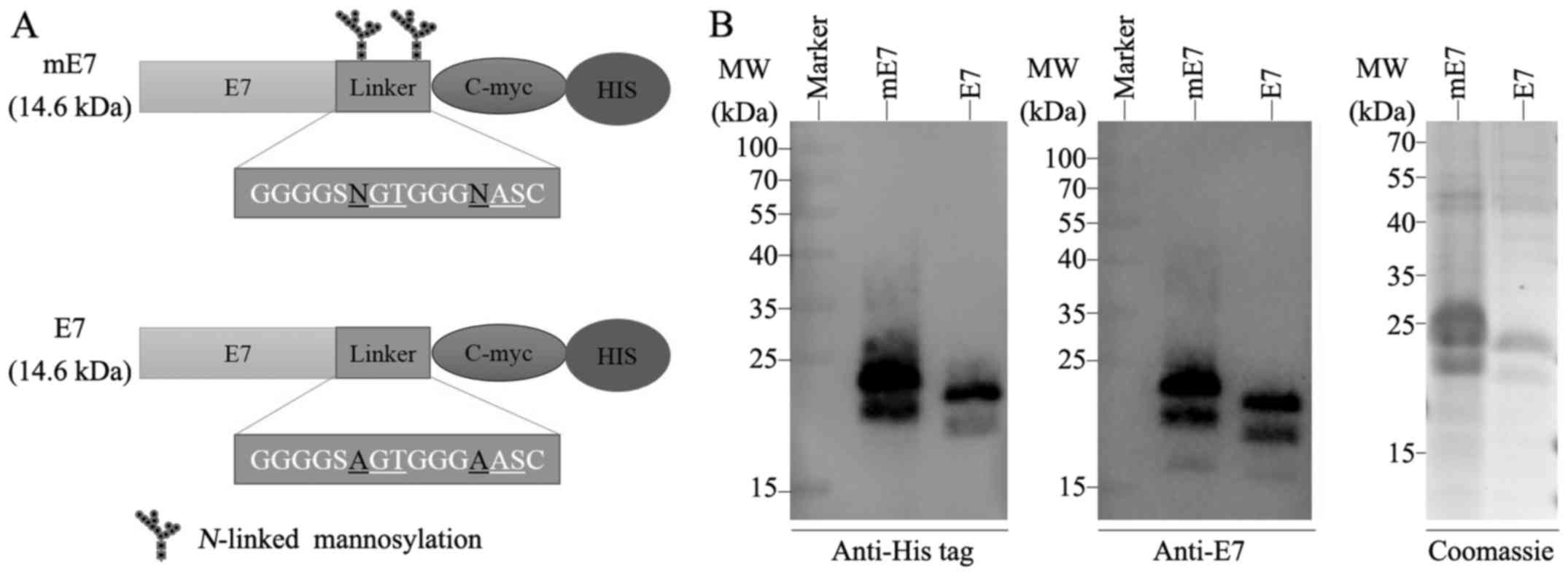
Construction and expression of mE7 and
E7
As previously reported, Pichia pastoris has
been widely used to express mannosylated recombinant proteins
(25). To express a mannosylated
recombinant E7 (defined as mE7), a 15-amino acid linker containing
two N-X-S/T motifs representing potential N-linked
glycosylation sites was fused to the C-terminal of HPV16 E7. As a
control, E7 was subjected to the site-directed mutagenesis of the
two potential N-linked glycosylation sites in the linker
region of mE7. NGT and NAS were conservatively mutated to AGT and
AAS, respectively (Fig. 1A).
The mE7 and E7 recombinant proteins were efficiently
expressed by Pichia pastoris KM71 in secreted forms, and mE7
showed a molecular weight between 22 and 40 kDa, while E7 showed a
molecular weight of ~22 kDa (Fig.
1B), indicating the considerable glycosylation of mE7 in KM71
and the unglycosylation of E7, since the unglycosylated E7
expressed from BL21 (DE3) cells was 22 kDa (data not shown). The
optimum time for mE7 expression (72 h) was longer than that for E7
(36 h).
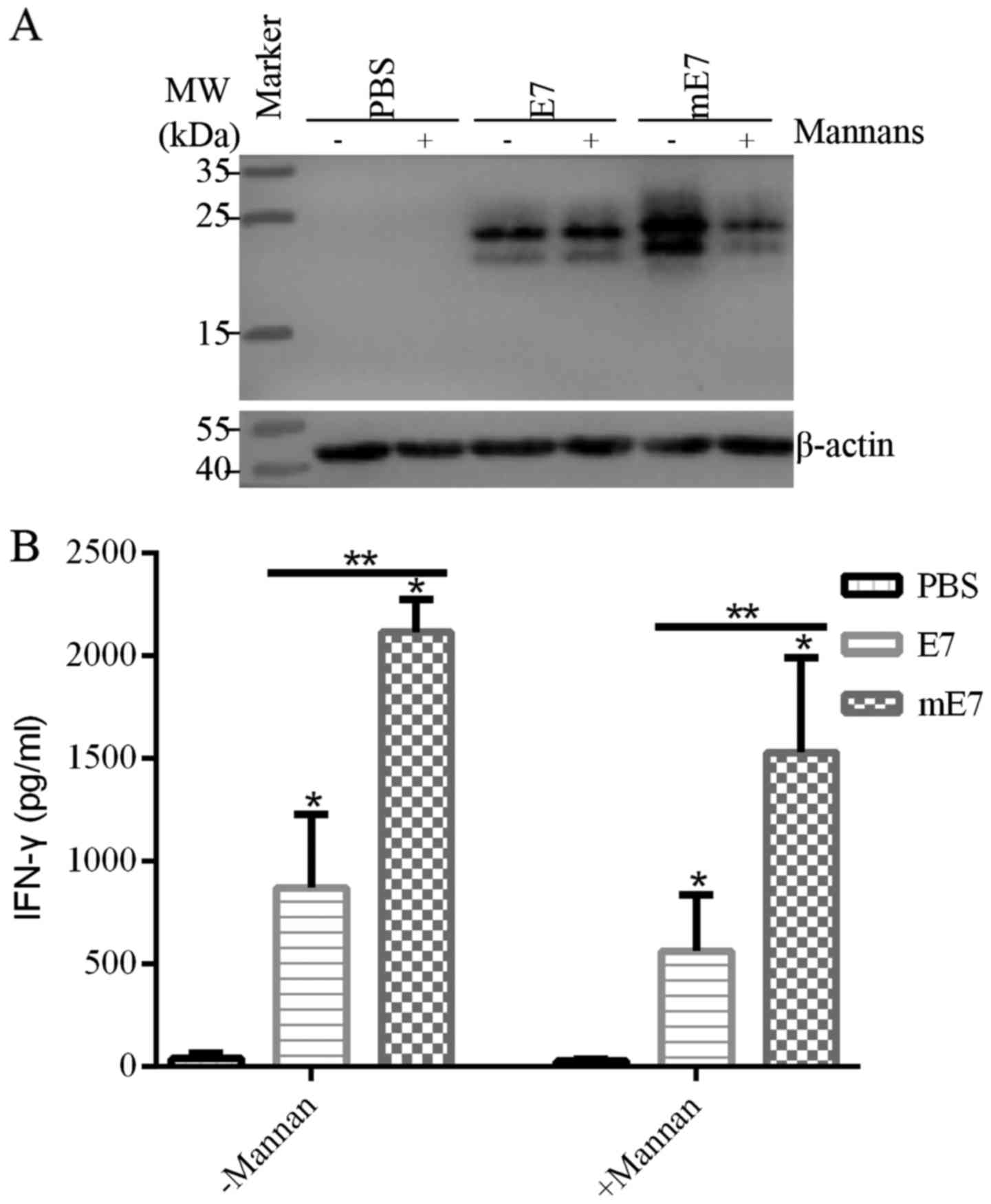
Mannosylation enhances uptake of mE7
by the MRs of BMDCs and T cell polarization
To detect whether mannosylated proteins are more
efficiently taken up by DCs via MR-mediated endocytosis, BMDCs were
incubated with equal mE7 or E7 in the presence or absence of yeast
mannans, which block MR and DC-SIGN (26). BMDCs endocytosed more mE7 than E7,
and yeast mannans inhibited the uptake of mE7, while E7 was not
apparently affected (Fig. 2A). As
shown in Table I, the relative
amount of mE7 ranged from 2.25 (without mannans) to 1.02 (with
mannans) while that of E7 ranged from 1.38 (without mannans) to
1.25 (with mannans). This result indicates that MR or DC-SIGN plays
a dominant role in the uptake of mannosylated proteins by BMDCs,
and mannosylation can promote the uptake of mE7 by BMDCs, which is
crucial to activate downstream immune responses. Subsequently, we
co-cultured antigen-uptake BMDCs with splenocytes of immunized mice
for 72 h. As a results, mE7-uptake BMDCs induced more IFN-γ
secretion by splenocytes than E7, while the levels of IFN-γ were
reduced with mannans, respectively (Fig. 2B). Taken together, mannosylation
enhances the ability of BMDCs to take up mE7 and increases T cell
polarization.
 | Table I.Relative quantification of bands in
the different lanes (Fig. 2A). |
Table I.
Relative quantification of bands in
the different lanes (Fig. 2A).
|
| PBS | PBS+M | E7 | E7+M | mE7 | mE7+M |
|---|
| β-actin | 1.0 | 0.76 | 1.03 | 1.08 | 0.89 | 1.05 |
| Antigen | 0 | 0 | 1.42 | 1.35 | 2.00 | 1.07 |
|
Antigen/β-actin | 0 | 0 | 1.38 | 1.25 | 2.25 | 1.02 |
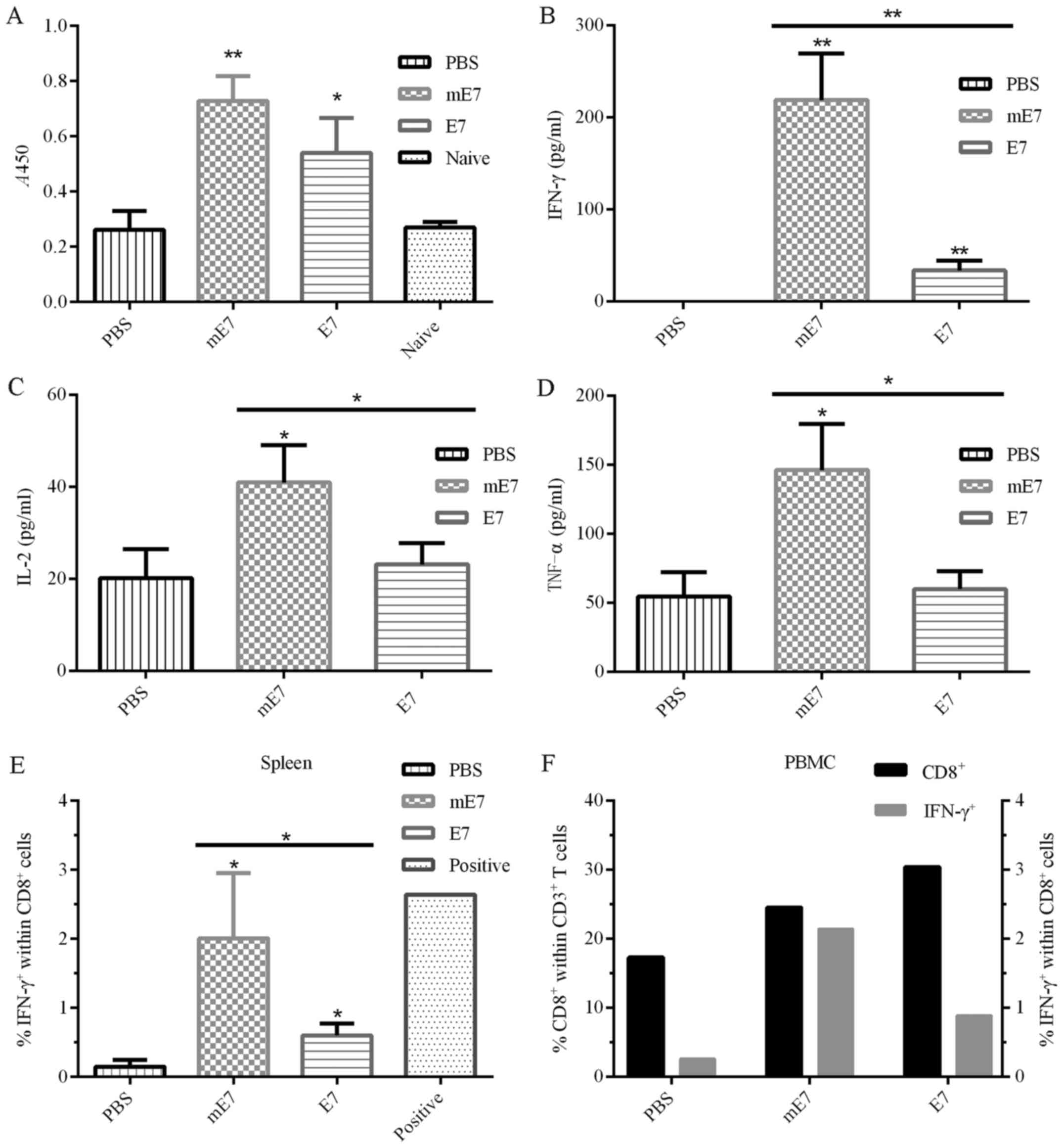
Vaccination with mE7 induces strong
Th1 responses
We measured the E7-specific antibody titers in the
sera of immunized mice using ELISA. The results showed that
vaccination with mE7 developed higher antibody titer than E7
(Fig. 3A).
To assess the level of cytokines generated by
vaccination with mE7, the splenocytes from vaccinated mice were
harvested and stimulated with E7 or a positive control PMA plus
ionomycin. Subsequently, the supernatants were collected and
measured using ELISA. Under equal doses of mE7 and E7, vaccination
with mE7 induced more cytokines, including IFN-γ, IL-2 and TNF-α,
than vaccination with E7 (Fig.
3B-D). Compared with E7, mE7 induced almost six times more
IFN-γ (Fig. 3B), which is an
important activator of macrophages, inducer of MHC II expression
and indicator of Th1 responses. Compared with E7, mE7 also induced
approximately twice as much IL-2 (Fig.
3C), which stimulates the differentiation of regulatory T
cells, promotes T cell growth and NK cytolytic activity, and
mediates activation-induced cell death (27). In addition, compared with E7, mE7
induced approximately twice as much TNF-α (Fig. 3D), which induces fever, apoptotic
cell death, cachexia, inflammation and inhibition of tumorigenesis.
Moreover, the E7-specific IFN-γ-secreting CD8+ T cells
in the spleen and PBMCs were analyzed by flow cytometry (Fig. 3E and F). Compared with E7, mE7
induced more E7-specific IFN-γ-secreting CD8+ T cell
within the spleen and PBMCs. These results suggested that
vaccination with mE7 generated stronger Th1 responses which are
crucial for tumor regression and considerable E7-specific antibody
responses.
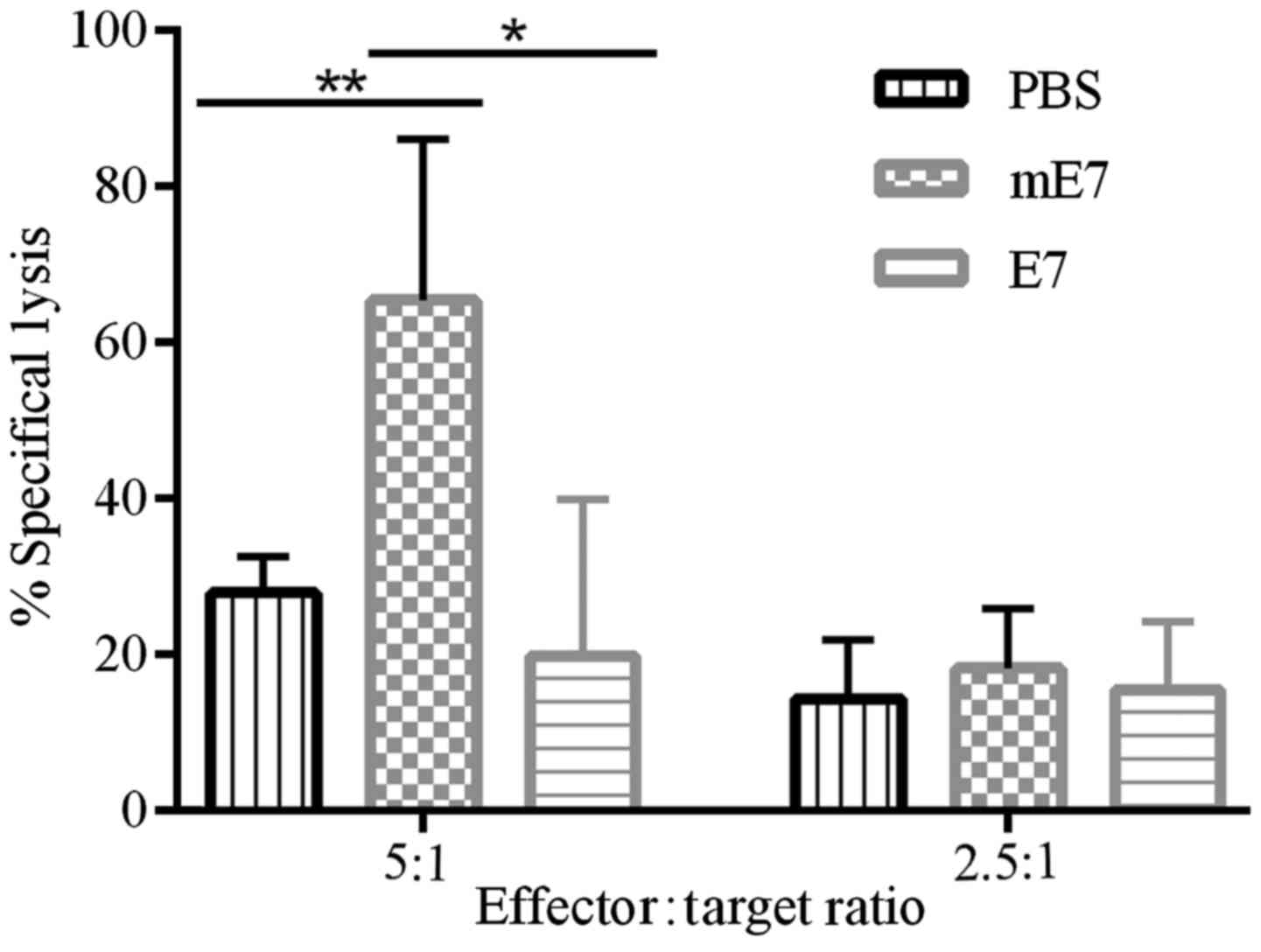
Vaccination with mE7 promotes
E7-specific cytotoxic CD8+ T cell response
The above results that vaccination with mE7 promoted
Th1 responses (Fig. 3B-F)
represents potent E7-specific T cell responses. To examine whether
vaccination with mE7 primes more effective E7-specific cytotoxic
CD8+ T cells than E7, the splenocytes of vaccinated mice
were collected and cocultured with mitomycin C-pretreated TC-1
cells for 5 days. Viable effector cells were measured for cytotoxic
activity against TC-1 cells. As shown in Fig. 4, the effector cells from mice
immunized with mE7 showed stronger cytolytic effects on TC-1 cells
(65.4%) than those from mice immunized with E7 (19.8%) and PBS
(27.9%) when the effector:target ratio was 5:1.
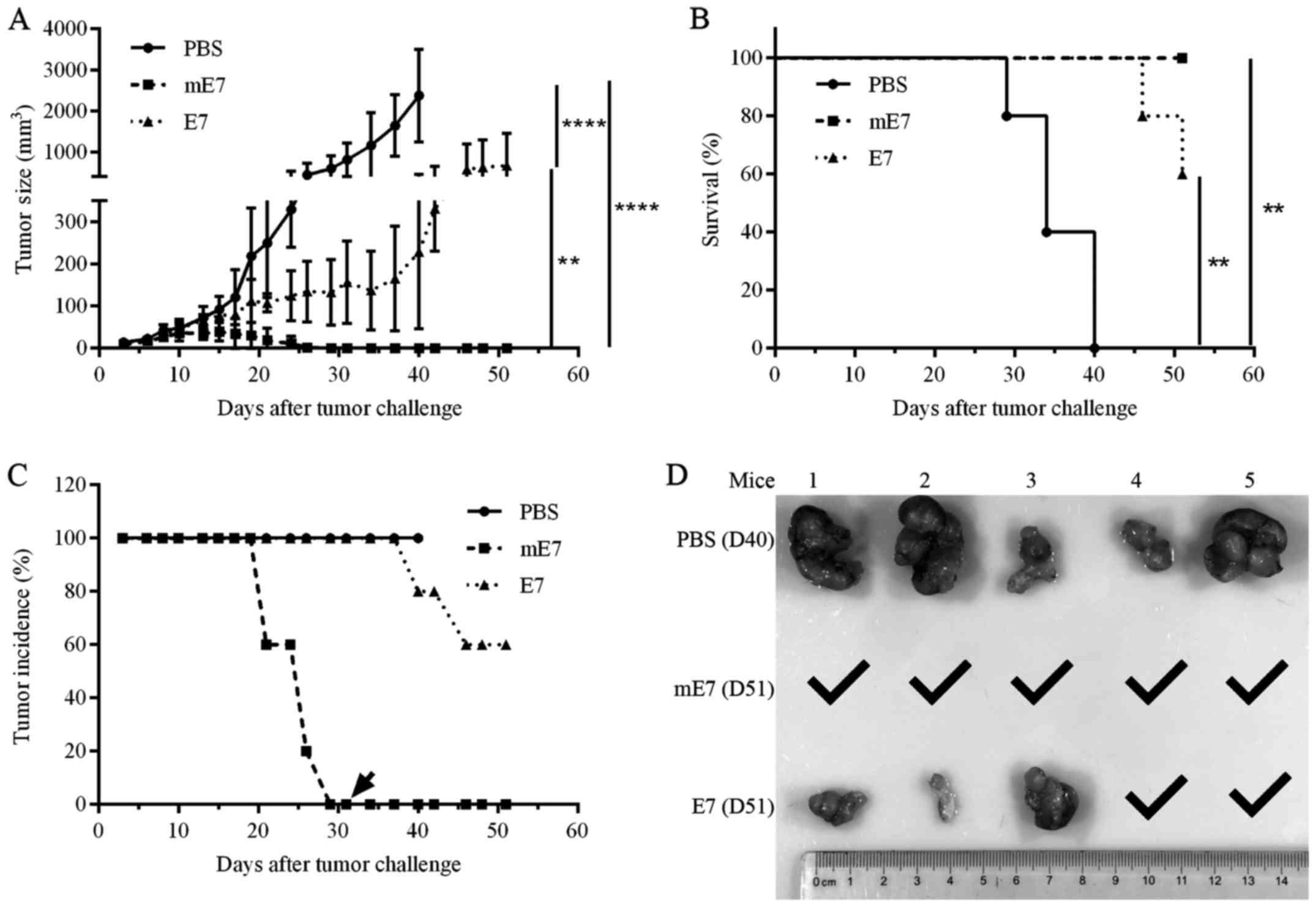
Mannosylation enhances mE7 antitumor
activity against TC-1 tumors
To assess the antitumor activity generated using the
mE7 vaccine, we performed an in vivo tumor challenge assay
with HPV16 E6 and E7-expressing TC-1 cells. C57BL/6 mice were
injected with 2×105 TC-1 cells in the right flank. After
3 days, tumor-challenged mice (5 per group) were vaccinated with
three doses of mE7, E7 or PBS in the presence of MPL every 5 days.
Tumor growth was measured using a caliper every 2 or 3 days. As
shown in Fig. 5A, the mice
vaccinated with mE7 showed lower average tumor sizes than mice
vaccinated with E7 and PBS. All mice vaccinated with mE7 eliminated
tumors at 26 days after tumor challenge, while only 20% of the mice
vaccinated with E7 eliminated tumors at more than 37 days after
tumor challenge. All mice vaccinated with PBS failed to inhibit
tumor growth and were sacrificed after 40 days, when the tumor
sizes reached 1000 mm3. Additionally, all mice
vaccinated with mE7 survived after 51 days. In contrast, 40% of the
mice vaccinated with E7 and 100% of the mice vaccinated with PBS
died after 51 days (Fig. 5B and C).
After 31 days, the mice vaccinated with mE7 were rechallenged with
2×105 TC-1 cells, and no mice developed tumors until 51
days (Fig. 5C), indicating
immunological memory. At 40 and 51 days, the mice vaccinated with
PBS, mE7 and E7 were sacrificed and dissected, and 5 PBS tumors and
3 E7 tumors were observed (Fig.
5D). Taken together, mE7 represents an effective therapeutic
vaccine against TC-1 tumors.
Discussion
In the present study, we reported that mE7,
expressed by Pichia pastoris, is an effective therapeutic
vaccine candidate against HPV16 E7-expressing tumors. Mannosylation
enhanced the uptake of E7 in BMDCs by MRs and T cell polarization.
As a result, the vaccination of C57BL/6 mice with mE7 induced more
cytokines (IFN-γ, IL-2 and TNF-α), more E7-specific IFN-γ-secreting
CD8+ T cells in the spleen and PBMCs, and more
E7-specific cytotoxic CD8+ T cell responses, suggesting
the activation of E7-specific T cell responses. In tumor challenge
assays, immunization with mE7 significantly inhibited tumor growth
and prolonged the lifespan of tumor-challenged mice.
HPV16 E6 and E7 are often the only viral genes
expressed in cancerous cells. Thus, E6 and E7 represent promising
targets for the immune therapy of HPV-associated lesions or
cancers. Therefore, numerous therapeutic vaccines have focused on
E6 and E7, pursuing E6/E7-specific cellular immunity or tumor
regression. Traditionally, vaccinations with peptide(s), including
minimal or maximal epitopes, have been considered a convenient
method to elicit cellular immunity. However, some limitations
remain, as peptide vaccines may result in T cell tolerance and
become rapidly degraded (28). In
contrast, immunization with deleted (29) or chemically synthesized (30) E7 protein induces T cell immunity in
the presence of Quil A or CpG. In addition, the fusion of E7
proteins with heat shock proteins, virus-like particles, or
listeriolysin, elicits E7-specific CD8+ T cell responses
(7). Moreover, E6/E7-containing DNA
and viral vector vaccines have been exploited to promote T cell
immunity (7). However, the DNA and
viral vector vaccines present risks for the integration of
exogenous E6 or E7 genes into host cells. In contrast, protein
vaccines have some advantages: ⅰ) exogenous proteins have no
expected access to host cells for carcinogenic effects; and ⅱ)
exogenous proteins transiently exist and avoid persistently
transforming host cells. Taken together, these facts indicate the
promising application of E6 or E7 protein-based vaccines. Pichia
pastoris has been used to produce therapeutic glycoproteins
(25). Since glycosylation is of
the high-mannose type, we exploited Pichia pastoris to
produce mE7 to augment the immunogenicity of E7, as mE7 utilizes
the immune recognition of exposed mannoses as pathogen-associated
molecular patterns.
MRs play an important role in the immune system and
connect innate to adaptive immunity (31). Previous studies have shown that MRs
participate in the uptake, processing and presentation of
glycosylated antigens as the immune response to foreign pathogens
(32). DC maturation occurs after
mannosylated antigens bind to MRs and trigger the internalization
of the mannosylated antigens. We observed that Pichia
pastoris mannosylation enhanced the uptake of mE7 by the MRs of
BMDCs and promoted T cell polarization. These data indicate the
involvement of MR and DC-SIGN in the uptake of mE7, as the uptake
activity was inhibited when yeast mannans were added.
Studies have reported that the mannosylation of
antigens enhances MHC I- and MHC II-restricted antigen presentation
and T cell stimulation (13,14,17,33,34).
Furthermore, the immunization of mice with mannosylated vaccines
induced Th1 cytokine production and antigen-specific CTL responses
(17,35,36).
In the present study, vaccination with mE7 improved the production
of Th1 cytokines and the level of E7-specific IFN-γ-secreting
CD8+ T cell in the spleen and PBMCs, such as IFN-γ, IL-2
and TNF-α, and enhanced E7-specific CTL responses.
In conclusion, mannosylated yeast-derived antigens
more effectively induce antigen-specific T cell proliferation than
their unmannosylated counterparts. In the present study, mE7 showed
more potent induction of E7-specific and cytotoxic T cell responses
and antitumor activity than E7, providing a promising immunotherapy
for treating cervical cancer.
Acknowledgements
The authors would like to thank the technicians of
the Laboratory Animal Center of Wuhan Institute of Virology and
Public Technology Service Center of Wuhan Institute of Virology for
providing technical assistance. The present study was supported by
the National Key Research and Development Program of China
(2016YFC1200400).
Glossary
Abbreviations
Abbreviations:
|
HPV
|
human papillomavirus
|
|
BMDCs
|
bone marrow-derived dendritic
cells
|
|
Th1
|
type 1 T helper cells
|
|
MPL
|
monophosphoryl lipid A
|
|
mE7
|
mannosylated HPV16 E7
|
|
DCs
|
dendritic cells
|
|
MRs
|
mannose receptors
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
PMA
|
phorbol-12-myristate-13-acetate
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muñoz N, Bosch FX, Castellsagué X, Díaz M,
de Sanjose S, Hammouda D, Shah KV and Meijer CJ: Against which
human papillomavirus types shall we vaccinate and screen? The
international perspective. Int J Cancer. 111:278–285. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howley P, Schiller J and Lowy D:
PapillomavirusesFields Virology. Knipe DM and Howley PM: 6th.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 1662–1703.
2013
|
|
4
|
Herrero R, González P and Markowitz LE:
Present status of human papillomavirus vaccine development and
implementation. Lancet Oncol. 16:e206–e216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schiller JT and Müller M: Next generation
prophylactic human papillomavirus vaccines. Lancet Oncol.
16:e217–e225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hildesheim A, Herrero R, Wacholder S,
Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin
G, Porras C, et al Costa Rican HPV Vaccine Trial Group, : Effect of
human papillomavirus 16/18 L1 viruslike particle vaccine among
young women with preexisting infection: A randomized trial. JAMA.
298:743–753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su JH, Wu A, Scotney E, Ma B, Monie A,
Hung CF and Wu TC: Immunotherapy for cervical cancer: Research
status and clinical potential. BioDrugs. 24:109–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frazer IH: Prevention of cervical cancer
through papillomavirus vaccination. Nat Rev Immunol. 4:46–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drickamer K: C-type lectin-like domains.
Curr Opin Struct Biol. 9:585–590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGreal EP, Miller JL and Gordon S: Ligand
recognition by antigen-presenting cell C-type lectin receptors.
Curr Opin Immunol. 17:18–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engering AJ, Cella M, Fluitsma D,
Brockhaus M, Hoefsmit EC, Lanzavecchia A and Pieters J: The mannose
receptor functions as a high capacity and broad specificity antigen
receptor in human dendritic cells. Eur J Immunol. 27:2417–2425.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan MC, Mommaas AM, Drijfhout JW, Jordens
R, Onderwater JJ, Verwoerd D, Mulder AA, van der Heiden AN,
Scheidegger D, Oomen LC, et al: Mannose receptor-mediated uptake of
antigens strongly enhances HLA class II-restricted antigen
presentation by cultured dendritic cells. Eur J Immunol.
27:2426–2435. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lam JS, Mansour MK, Specht CA and Levitz
SM: A model vaccine exploiting fungal mannosylation to increase
antigen immunogenicity. J Immunol. 175:7496–7503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levitz SM and Specht CA: The molecular
basis for the immunogenicity of Cryptococcus neoformans
mannoproteins. FEMS Yeast Res. 6:513–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keler T, Ramakrishna V and Fanger MW:
Mannose receptor-targeted vaccines. Expert Opin Biol Ther.
4:1953–1962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irache JM, Salman HH, Gamazo C and
Espuelas S: Mannose-targeted systems for the delivery of
therapeutics. Expert Opin Drug Deliv. 5:703–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moyle PM, Olive C, Ho MF, Pandey M, Dyer
J, Suhrbier A, Fujita Y and Toth I: Toward the development of
prophylactic and therapeutic human papillomavirus type-16
lipopeptide vaccines. J Med Chem. 50:4721–4727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rauen J, Kreer C, Paillard A, van Duikeren
S, Benckhuijsen WE, Camps MG, Valentijn AR, Ossendorp F, Drijfhout
JW, Arens R, et al: Enhanced cross-presentation and improved CD8+ T
cell responses after mannosylation of synthetic long peptides in
mice. PLoS One. 9:e1037552014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin KY, Guarnieri FG, Staveley-O'Carroll
KF, Levitsky HI, August JT, Pardoll DM and Wu TC: Treatment of
established tumors with a novel vaccine that enhances major
histocompatibility class II presentation of tumor antigen. Cancer
Res. 56:21–26. 1996.PubMed/NCBI
|
|
22
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Satchidanandam V, Kumar N, Jumani RS,
Challu V, Elangovan S and Khan NA: The glycosylated Rv1860 protein
of Mycobacterium tuberculosis inhibits dendritic cell mediated TH1
and TH17 polarization of T cells and abrogates protective immunity
conferred by BCG. PLoS Pathog. 10:e10041762014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
25
|
Gerngross TU: Advances in the production
of human therapeutic proteins in yeasts and filamentous fungi. Nat
Biotechnol. 22:1409–1414. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sallusto F, Cella M, Danieli C and
Lanzavecchia A: Dendritic cells use macropinocytosis and the
mannose receptor to concentrate macromolecules in the major
histocompatibility complex class II compartment: Downregulation by
cytokines and bacterial products. J Exp Med. 182:389–400. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: New insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slingluff CL Jr: The present and future of
peptide vaccines for cancer: Single or multiple, long or short,
alone or in combination? Cancer J. 17:343–350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hallez S, Brulet JM, Vandooren C, Maudoux
F, Thomas S, Heinderickx M, Bollen A, Wattiez R and Jacquet A:
Pre-clinical immunogenicity and anti-tumour efficacy of a deleted
recombinant human papillomavirus type 16 E7 protein. Anticancer
Res. 24:2265–2275. 2004.PubMed/NCBI
|
|
30
|
Welters MJ, Filippov DV, van den Eeden SJ,
Franken KL, Nouta J, Valentijn AR, van der Marel GA, Overkleeft HS,
Lipford G, Offringa R, et al: Chemically synthesized protein as
tumour-specific vaccine: Immunogenicity and efficacy of synthetic
HPV16 E7 in the TC-1 mouse tumour model. Vaccine. 23:305–311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weis WI, Taylor ME and Drickamer K: The
C-type lectin superfamily in the immune system. Immunol Rev.
163:19–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Royer PJ, Emara M, Yang C, Al-Ghouleh A,
Tighe P, Jones N, Sewell HF, Shakib F, Martinez-Pomares L and
Ghaemmaghami AM: The mannose receptor mediates the uptake of
diverse native allergens by dendritic cells and determines
allergen-induced T cell polarization through modulation of IDO
activity. J Immunol. 185:1522–1531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hattori Y, Kawakami S, Suzuki S, Yamashita
F and Hashida M: Enhancement of immune responses by DNA vaccination
through targeted gene delivery using mannosylated cationic liposome
formulations following intravenous administration in mice. Biochem
Biophys Res Commun. 317:992–999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arigita C, Bevaart L, Everse LA, Koning
GA, Hennink WE, Crommelin DJ, Van de Winkel JG, van Vugt MJ,
Kersten GF and Jiskoot W: Liposomal meningococcal B vaccination:
Role of dendritic cell targeting in the development of a protective
immune response. Infect Immun. 71:5210–5218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaughan HA, Ho DW, Karanikas V, Sandrin
MS, McKenzie IF and Pietersz GA: The immune response of mice and
cynomolgus monkeys to macaque mucin 1-mannan. Vaccine.
18:3297–3309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toda S, Ishii N, Okada E, Kusakabe KI,
Arai H, Hamajima K, Gorai I, Nishioka K and Okuda K: HIV-1-specific
cell-mediated immune responses induced by DNA vaccination were
enhanced by mannan-coated liposomes and inhibited by
anti-interferon-gamma antibody. Immunology. 92:111–117. 1997.
View Article : Google Scholar : PubMed/NCBI
|