Introduction
Osteopontin (OPN) is a secreted extracellular matrix
protein, which can be detected in tissues, serum and urine under
physiological conditions. Due to an increased expression in many
tumor tissues, increased serum levels in cancer patients are
considered as a negative prognostic tumor biomarker. Various
studies have shown that elevated OPN levels are associated with
tumor invasion and metastasis particularly in head and neck tumors,
lung, breast, prostate and colorectal cancer, resulting in a worse
prognosis of the disease (1–3).
Furthermore, increased levels of OPN could be
detected in tumors with reduced oxygen content (tumor hypoxia)
(4–6). In addition, tumor hypoxia is
associated with decreased radiation sensitivity and a poor
prognosis after radiation therapy (7–9).
Furthermore, we and others recently showed, that silencing of OPN
expression caused an increase in radiosensitivity (10–12).
One of the binding partners of OPN is the CD44
(CD44s) receptor and some of its isoforms (13). These are expressed in numerous tumor
types and can be upregulated by OPN itself as shown in stomach,
liver, thyroid and breast cancer tissues (14). Via CD44, signaling pathways are
activated, which regulate cell proliferation, migration and
survival signals and lead to metastasis (12). Recently, it was shown in a mouse
model that a CD44 antagonist delayed the tumor growth of
glioblastoma cells (15). By
interaction of OPN with CD44 and its various splice variants, the
PLC-γ-dependent Akt pathway is activated which contributes to the
motility and survival of tumor cells (16). Although there are many splice
variants of CD44, particularly variant 6 (CD44v6) is highly
expressed along with OPN in many cancers such as breast and gastric
cancer or leukemia and is considered a marker for advanced tumor
disease (17–22).
With this in mind, we analyzed the effect of hypoxia
on the expression of OPN and its receptors CD44s and CD44v6 in 4
different human colorectal carcinoma cell lines (SW480, SW620, HT29
and HCT116). We aimed to ascertain whether hypoxic conditions lead
to an increase in OPN and in parallel to an upregulation of CD44s,
particularly of CD44v6.
Materials and methods
Cell culture
The human colon adenocarcinoma cell lines SW480,
SW620 (this cell line was isolated from a lymph node metastasis of
the same patient from which the SW480 cell line is derived), HT29
and HCT116 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA).
SW480 and SW620 cells were grown in RPMI medium
supplemented with 10% fetal bovine serum (PAA, Cölbe Germany), 2 mM
L-glutamine and penicillin (100 IU/ml)/streptomycin (100 µg/ml) and
10% non-essential amino acids and pyruvat. HT29 and HCT116 cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum, 2 mM L-glutamine and
penicillin (100 IU/ml)/streptomycin (100 µg/ml) in a humidified
atmosphere of 5% CO2 at 37°C.
To compare expression of OPN, CD44s and C44v6 under
normoxic and hypoxic conditions at the mRNA and protein level, the
cells were seeded in standard 75 cm2 tissue culture
flasks (Greiner Bio-One, Frickenhausen, Germany) at a concentration
of 1×106/flask. Afterwards, the cells were allowed to
adhere. While a portion of cells was kept under normoxic conditions
(21% O2, 5% CO2 at 37°C), the other portion
was transferred into a hypoxic glove box in parallel (Coy
Laboratory Products, Grass Lake, MI, USA) and cultured at 0.1%
O2 (5% CO2 at 37°C) for 48 h. Afterwards, the
cells were harvested for preparing RNA or whole-cell lysates.
To examine the effect of additional irradiation on
the expression of OPN and its ligands (CD44 and CD44 v6) under the
conditions already mentioned, a portion of the cells was irradiated
24 h after onset of the cultures at doses of 2 and 8 Gy using a
6-MV linear accelerator (Siemens, Concord, CA, USA) at a dose rate
of 2 Gy/min. After another 24 h of growth under standard or hypoxic
conditions, respectively, the cells were analyzed accordingly. In
the case of the colony forming assay, the cells were treated in the
same way but were irradiated at doses of 2, 3, 5, 7 and 8 Gy or
left untreated.
RNA isolation and real-time
quantitative PCR (RT-qPCR)
Total RNA of the cells was purified with the RNeasy
Mini® kit following the instructions given by the
supplier (Qiagen, Hilden, Germany). The subsequent reverse
transcription of the RNA into cDNA was carried out using First
Strand cDNA Synthesis kit as recommended by the manufacturer
(Fermentas GmbH, St. Leon-Rot, Germany). Afterwards quantitative
real-time PCR was performed to amplify cDNA coding for OPN, CD44s
and the housekeeping gene hypoxanthine phosphoribosyltransferase
(HPRT) using TaqMan® Gene Expression Assays (Applied
Biosystems, Foster City, CA, USA). CAIX RT-qPCR analysis was
performed in the same way to confirm the hypoxic culture
conditions. As the threshold cycle (Ct) the number of cycles
required to cross the threshold was used. The crossing point of the
housekeeping gene HPRT was taken to normalize the amount of cDNA in
each sample. Changes in the expression were detected and quantified
using the comparative Ct method (2−ΔΔCq) (23).
Western blot analyses
Whole-cell lysates were prepared by lysing the cells
in RIPA buffer according to standard protocols. The proteins were
separated by electrophoresis according to their size by running on
4–12% Bis-Tris gradient gels (Invitrogen, Karlsruhe, Germany).
Afterwards they were transferred to polyacrylamide membranes
(Invitrogen) and analyzed by western blotting. Polyclonal rabbit
anti-human-OPN FL-314 and monoclonal mouse anti-human-HPRT
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Heidelberg, Germany). Mouse monoclonal anti-CD44std (BMS 150) and
mouse monoclonal anti-CD44v6 (BMS 125) antibodies were purchased
from eBioscience (Vienna, Austria). The mouse monoclonal antibody
against CAIX is a product of BioScience (Bratislava, Slovakia).
Detection was achieved using species-specific horseradish
peroxidase-coupled secondary antibodies (Dako, Hamburg, Germany)
and Amersham™ ECL™ Select Western Blotting detection reagent (GE
Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
ELISA assays
For detection of OPN, CD44s and CD44v6 protein
levels in the non-concentrated supernatants of the different cell
lines, cultured under normoxic or hypoxic conditions, enzyme-linked
immunosorbent assay (ELISA) kits from IBL (Immuno-Biological
Laboratories Co., Ltd., Gunma, Japan) were used and performed
according to the manufacturer's instructions.
Flow cytometric analysis
To detect OPN in the cells, the cells were fixed in
ice-cold ethanol and permeabilized with 0.5% Triton X for 10 min.
To block unspecific protein-protein interactions, the cells were
incubated with 5% FCS for 1 h at +4°C. Then, an anti-OPN antibody
from Abcam (ab8448; Cambridge, UK) was added overnight (at +4°C).
For the secondary antibody, Alexa Fluor 488 goat anti-rabbit (H+L)
(Invitrogen) was used at a dilution of 1/1,000 for 1 h. The
analyses were performed with a FACScan™ (Becton-Dickinson, Mountain
View, CA, USA). The output data, presented as geometrical means,
were analyzed using the Flowing Software obtained from P. Terho
(Turku Centre for Biotechnology, Turku, Finland).
Clonogenic survival assay
Standard colony formation assays were performed,
analyzing the survival of cells after irradiation by graded single
doses (0–8 Gy). For that, different numbers of cells were seeded in
6-well plates, depending on the radiation dose previously defined,
and were incubated under normoxic (21% O2, 5%
CO2 at 37°C) or hypoxic (0.1% O2, 5%
CO2 at 37°C) conditions for 2 weeks. For each exposure
point, 3 replicates were carried out. Furthermore, the colony
survival assay was carried out 3 times for each cell line. For
visualization of the colony formation (colonies ≥50 cells), cells
were fixed and stained with crystal violet (0.6%). The mean
survival data for each individual cell line were fitted to the
linear quadratic (LQ) model SF = exp(−αX - βX2), where
SF is the survival fraction, X is the irradiation dose, and α and β
are the fitted parameters.
Statistical analysis
All experiments were performed in triplicates and
repeated for at least 2 times. For PCR data, changes in expression
levels were considered as significant when there was a minimum of a
2-fold change. Flow cytometric data were quantified using the
geometric mean. Fittings of experimental curves from the survival
assays were carried out with Origin software (Microcal,
Northampton, MA, USA).
Results
Expression pattern of OPN and CD44s at
the mRNA level under hypoxia
Four different colorectal carcinoma cell lines
(SW480, HT29, HCT116 and SW620) were grown under normoxic (21%
O2) or hypoxic (0.1% O2) conditions. The
effect of these parameters on CD44v6 mRNA expression was not
possible, since TaqMan probes were not available for this splice
variant of CD44. Therefore, CD44v6 expression was examined at the
protein level.
To assess the effects of hypoxia on OPN and its
receptor CD44s at the mRNA level, quantitative RT-PCR (RT-qPCR) was
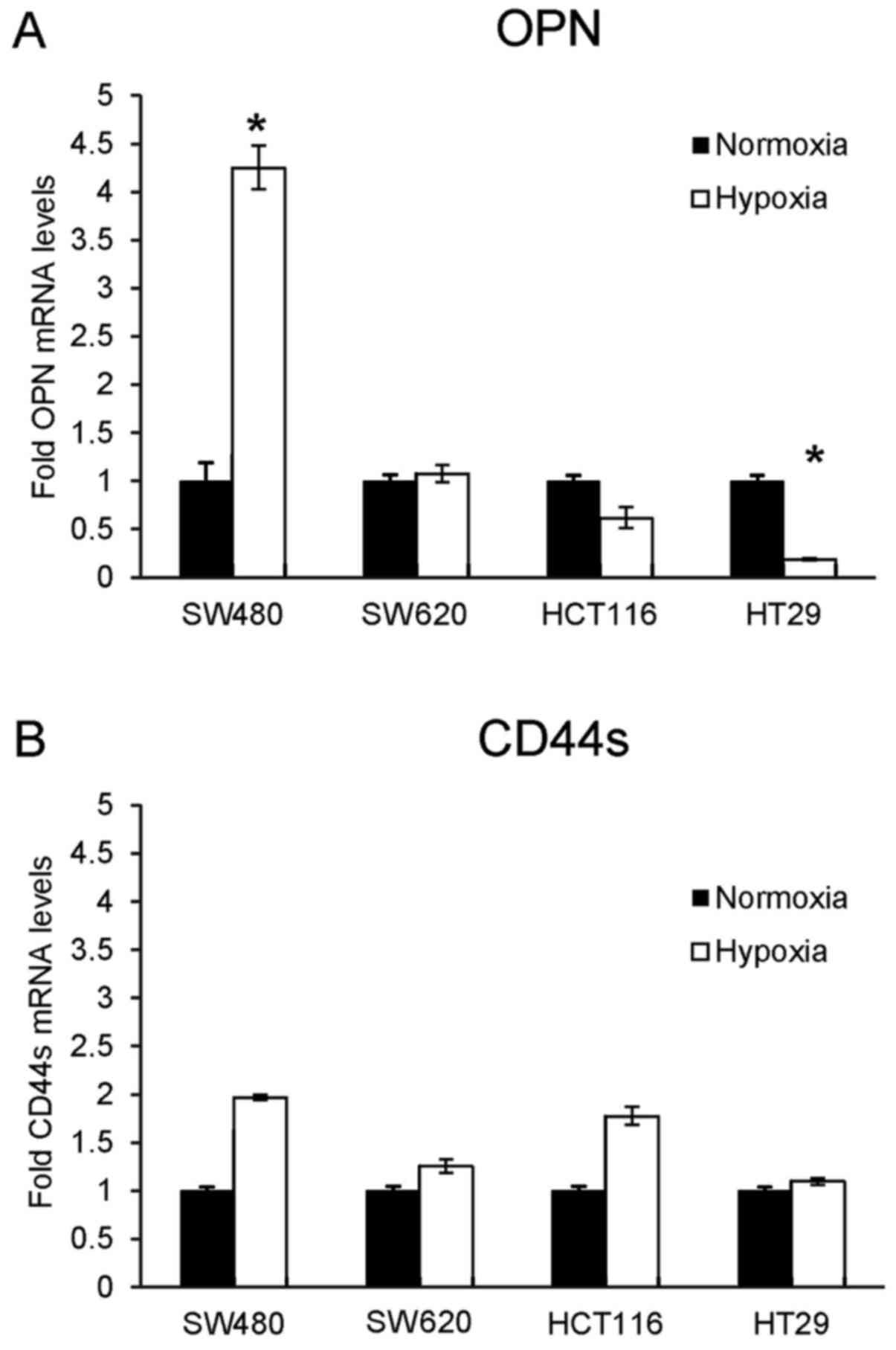
performed. SW480 cells showed a significant increase in OPN mRNA
expression under hypoxic conditions (Fig. 1A). Concordant results were obtained
concerning CD44s mRNA expression, but did not reach significance
(Fig. 1B).
In contrast to SW480 cells, hypoxia caused a
significant decrease in the OPN mRNA level in the HT29 cells and
showed the same trend in the HCT116 cells, but without significance
(Fig. 1A). Additionally, both cell
lines showed contrary results with slightly increasing CD44s mRNA
expression under hypoxic conditions. This observation was more
pronounced in the HCT116 cells than in the HT29 cells (Fig. 1B).
To verify the hypoxic culture conditions, RT-qPCR
was performed for CAIX expression, which is upregulated under
hypoxia, depending on HIF-1α. All cell lines responded to hypoxia
with a clear increase in CAIX expression, which was statistically
significant (data not shown). SW480 cells showed a 73-fold and
HCT116 a 75-fold increase in CAIX levels, respectively. The
increase in CAIX mRNA expression in the HT29 (22-fold) and SW620
cells (27-fold) was not as strong, since both cell lines showed a
background expression of CAIX (see also Fig. 2).
Expression pattern of OPN, CD44s and
CD44v6 at the protein level under hypoxia
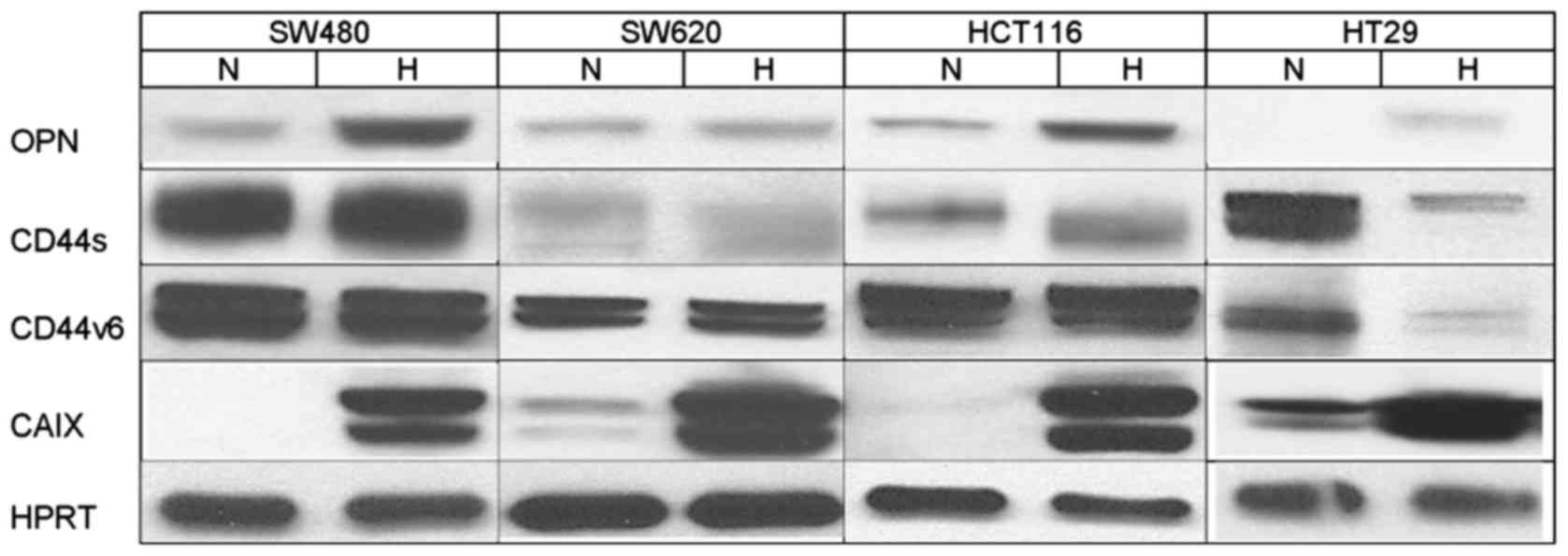
The influence of the different culture conditions on
the expression of OPN, CD44 and CD44v6 at the protein level was
tested by western blot analysis.
In all cell lines cultured under hypoxic conditions,
there was an increase in OPN levels compared with the normoxic
controls (Fig. 2). This increase
was more pronounced in the SW480, HCT116 and HT29 cells than in the
SW620 cells.
Expression of CD44s was slightly enhanced by hypoxia
in the SW480 cells. In the SW620 cells there was nearly no
difference in the CD44s protein levels observed depending on the
culture conditions. HT29 cells responded to hypoxic conditions with
a significant downregulation of CD44s protein, while it was
upregulated in the HCT116 cells (Fig.
2). Protein levels of CD44v6, which is one of the CD44 isoforms
associated with an increase in tumor malignancy, were unaffected by
hypoxia in the cell lines SW480, SW620 and HCT116 (Fig. 2). Only in HT29 cells, hypoxic
conditions led to a clear decrease in the CD44v6 protein level,
comparable to the downregulation of CD44s in the same cell
line.
In all cell lines, a significant upregulation of
CAIX protein expression was observed under hypoxia, which confirmed
cultivation under appropriate conditions. While there was no
expression of CAIX protein detectable under normoxic conditions in
the SW480 and HCT116 cells, HT29 and SW620 cells showed a weak
‘background’ secretion under normoxia as already observed at the
mRNA level. HPRT was used as a loading control, respectively.
Influence of hypoxia on extracellular
secretion of OPN, CD44 and CD44v6
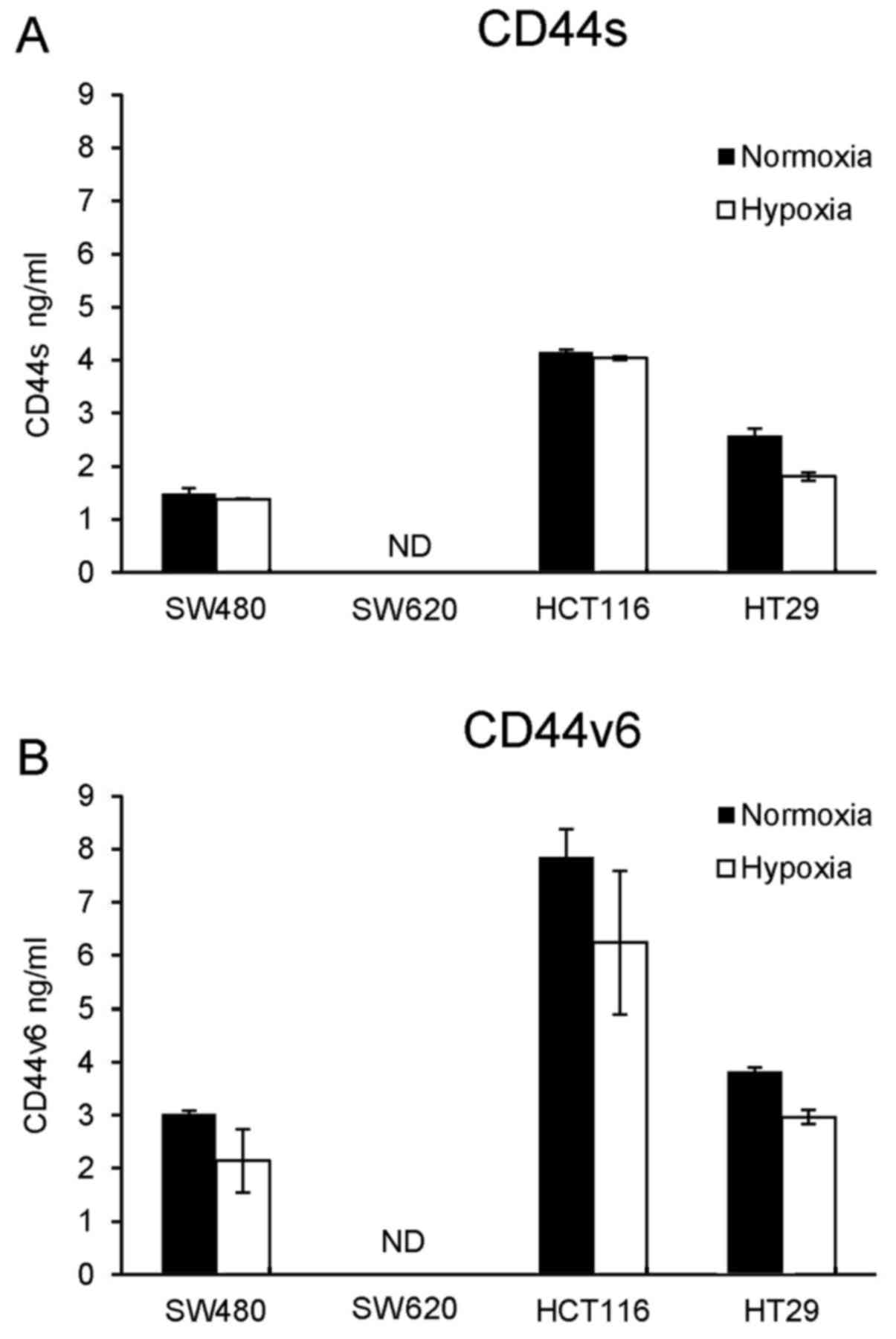
Next, the effect of the different culture conditions
on secretion of OPN, CD44s and CD44 v6 was investigated. Therefore
cell culture supernatants were tested by a commercial ELISA system
without further preparation (e.g. concentration) of the
supernatants. In all cell lines, secretion of OPN was not
detectable. Furthermore, neither CD44s nor CD44v6 was actively
secreted by SW620 cells (Fig. 3A and
B). All other cell lines showed secretion of both OPN receptors
(Fig. 3). In contrast to HT29
cells, which showed reduced CD44s secretion under hypoxic
conditions, SW480 and HCT116 cells showed nearly no reduction in
CD44s secretion under hypoxia (Fig.
3A). In terms of CD44v6, hypoxia caused a decrease in secretion
in all cell lines (Fig. 3B).
Influence of irradiation on expression
of OPN and its ligands CD44 and CD44v6
An effect of irradiation on OPN mRNA expression was
only observed in the SW480 cells grown under standard conditions
(normoxia). While doses of 2 Gy induced only a slight increase,
irradiation with 8 Gy led to a more pronounced OPN mRNA expression
(data not shown). This was not the case for CD44 mRNA expression,
neither in this nor in the other cell lines tested. Furthermore,
there was also no influence of irradiation on protein expression
and secretion of OPN and its ligands CD44 and CD44v6.
Intracellular immunofluorescence (IF)
staining
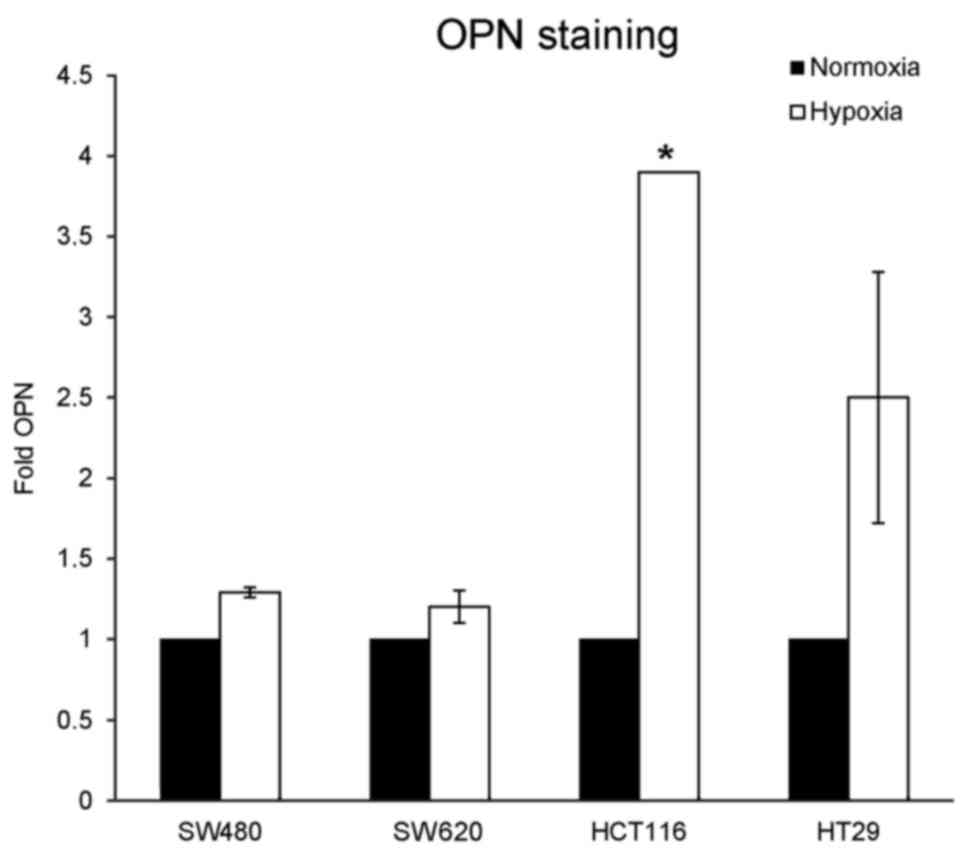
Although the amount of OPN protein increased under
hypoxia, no OPN secretion was detectable in the supernatants of all
cell lines tested by ELISA. Therefore intracellular FACS staining
for OPN was performed to evaluate, whether OPN was stored
intracellularly instead of being secreted into the cell culture
medium. By flow cytometry, positive staining for OPN was observed
in all cell lines, which was more pronounced when cells were
cultured under hypoxia, particularly in HCT116 and HT29 cells
(Fig. 4). These observations are in
line with the results from the western blot analysis.
Effect of hypoxia and irradiation on
radiosensitivity and clonogenic survival
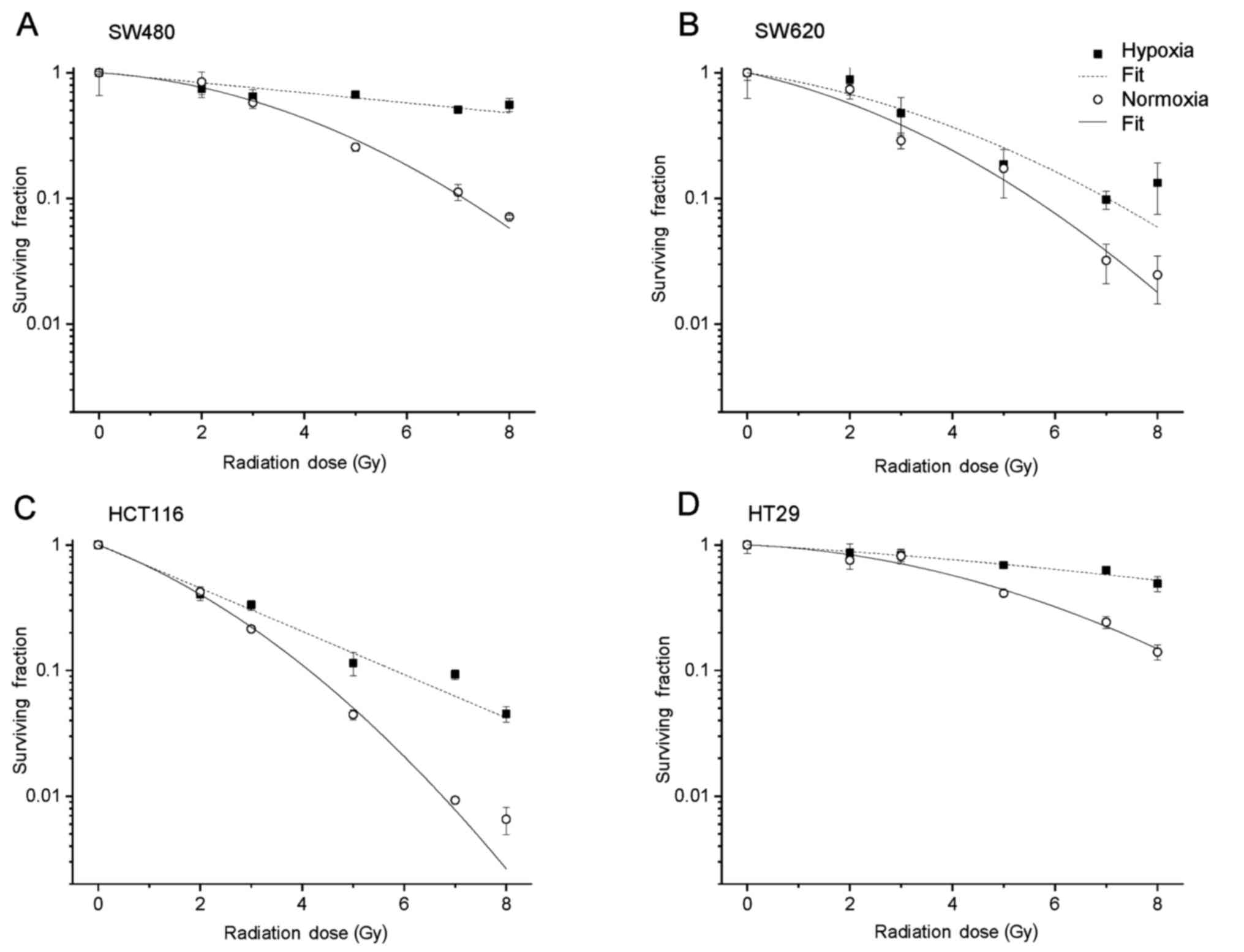
To assess the effects of normoxia and hypoxia on
radiosensitivity and survival, cell lines were cultured under
standard (21% O2) and hypoxic (0.1% O2)
conditions before they were exposed to different increasing
irradiation doses up to 8 Gy. Afterwards, the survival curves of
the colony formation assays were calculated according to the LQ
model. While the plating efficiency in SW480 cells was nearly not
affected by the different culture conditions, all the other cell
lines (SW620, HT29 and HCT116) showed a significant decrease in
this parameter under hypoxia (Table
I). An increase in clonogenic survival (SF2) was observed under
oxygen reduction in all cell lines and ranged from 5% (HCT116), 6%
(HT29) and 7% (SW480) up to 8% in SW620 cells. The radiation dose
yielding 10% survival (D10) showed a significant increase
indicating radioresistance under hypoxia, which was most pronounced
in the SW480 and HT29 cells. In the present study, radiation doses
of 25 and 19.2 Gy were necessary to yield 10% survival,
respectively. The corresponding survival curves are shown in
Fig. 5.
 | Table I.Plating efficiencies and
radiosensitivity parameters of tumor cells cultured under normoxic
and hypoxic conditions, respectively. |
Table I.
Plating efficiencies and
radiosensitivity parameters of tumor cells cultured under normoxic
and hypoxic conditions, respectively.
|
| PE | SF2 | D10 |
|---|
|
|
|
|
|
|---|
| Cell line | Normoxia (%) | Hypoxia (%) | Normoxia | Hypoxia | Normoxia | Hypoxia |
|---|
| SW480 | 55.3 | 54.7 | 0.76 | 0.83 | 7.11 | 25.01 |
| SW620 | 100.0 | 21.2 | 0.41 | 0.49 | 4.04 | 5.09 |
| HCT116 | 40.7 | 7.9 | 0.40 | 0.45 | 4.14 | 5.83 |
| HT29 | 68.0 | 30.3 | 0.83 | 0.89 | 8.87 | 19.18 |
Discussion
The presence of tumor hypoxia is an established
adverse prognostic factor in radiation of different tumors.
Osteopontin (OPN) is a plasma protein that is related with tumor
hypoxia. Furthermore, it is described that OPN expression is
associated with tumors with a poor prognosis. This is also true for
some of its binding partners, such as the cell-surface protein CD44
and its splice variants (particularly CD44v6), expressed in
aggressive cancer types (24,25).
The expression of CD44 has been described in so-called tumor stem
cells and is a proposed marker for tumor-initiating cells in colon
cancer, one of the most common cancers worldwide (26). Recently it was shown that the
downregulation of CD44 expression on stem cells using siRNA or
shRNA suppresses metastasis in breast cancer cells and makes the
stem cells more sensitive to drug treatment (27,28).
In in vitro experiments with stem cell-like brain tumor
cells expressing only large amounts of the isoform CD44v6, cell
growth was inhibited by downregulation of the receptor (knockdown).
Furthermore, it was detected that interaction of OPN and CD44v6
caused an increase in phosphorylated AKT, suggesting the
involvement of the AKT signaling pathway in signal transduction
(29).
In our experiments, we investigated whether, and to
what extent the cultivation of the cells under hypoxia affected the
expression of OPN and its receptors CD44s and particularly CD44v6.
Four different colon cancer cell lines were used: HT29, SW480
(derived from a primary tumor), SW620 (metastasis from the lymph
node of the same patient) and HCT116.
As expected, all tested cell lines had a more or
less strong increase in OPN protein under hypoxic conditions
(Figs. 2 and 4). These results agreed with the enhanced
OPN mRNA expression noted in the SW480 and SW620 cells under
hypoxia (Fig. 1A). In both cell
lines a concordant response to hypoxia with an increase in OPN and
CD44s mRNA was detected (Fig. 1A and
B). This could be a first hint that OPN upregulates its own
receptor under these conditions. However, further functional tests
must be conducted to confirm this hypothesis. The metastatic cell
line SW620 showed a much weaker basal expression of OPN and CD44s
protein than the primary tumor cells (SW480) (Fig. 2). Furthermore, there was nearly no
increase in CD44 expression and only a small increase in OPN
protein under hypoxia. This is consistent with published data
showing CD44 as a metastasis suppressor in some forms of prostate
(30) and in pancreatic cancer
(31). While there was a decrease
in CD44s expression with increasing malignancy in some prostate
cancers, there was a complete loss of CD44s expression in
progressive pancreatic cancer, due to alternative splicing of the
CD44 pre-RNA. Therefore, we assume that the higher tumorigenic and
metastatic potential of SW620 cells (in comparison to SW480) shown
by Hewitt et al is one reason for the different expression
of CD44s and OPN (32).
Our results are also in accordance with recently
published data, showing that colon cancer cells widely differ in
regards to the expression of cancer stem cell markers such as CD44
(33). The authors speculate that
the decreased basal CD44s expression in SW620 cells can be
explained by transition from SW480 to SW620 cells, leading to the
expression of a differentially post-translational modified CD44
protein. This may explain the weak signal for CD44s expression in
comparison to the strong CD44v6 signal detected in the western blot
analysis. Additionally, no CD44s protein could be detected in
supernatants of SW620 cells in contrast to SW480 cells, as shown by
ELISA assay (Fig. 3A). This may
also favor the thesis of post-translationally modified CD44. The
splice-variant CD44v6 was also not detectable in SW620 cell
supernatants, but in supernatants of SW480 cells, where its amount
was reduced under hypoxic conditions (data not shown and Fig. 3A and B).
HT29 cells responded to hypoxia with a distinct
downregulation of CD44s and CD44v6 protein (Fig. 2) accompanied by a significant
decrease in OPN mRNA (Fig. 1A) and
only a weak increase in OPN protein (Fig. 2). In addition, less CD44s and CD44v6
protein was detected in supernatants of the HT29 cells, when cells
were cultured under hypoxic conditions (Fig. 3A and B). Due to the above-cited
results (30), hypoxia may have
caused progressive malignancy in HT29 cells by downregulation of
CD44v6, acting as a metastasis suppressor in this colon cancer cell
line. The development of strong radioresistance under hypoxic
culture conditions could support the speculation of progressive
malignancy in HT29 cells, triggered by hypoxia (Fig. 5). Our speculation of progressive
malignancy in this cell line is supported by Flatmark et al
who showed the aggressive behavior of HT29 cells within a panel of
12 colorectal cancer cell lines which produced lymph node
metastases with a very high frequency (34).
In contrast, HCT116 cells showed an increase in
CD44s at the mRNA and protein level under hypoxia (Figs. 1B and 2). In parallel, expression of its binding
partner OPN was elevated as expected (Fig. 2). However, this was not consistent
with the PCR results, showing a decreased OPN transcription rate
under hypoxic conditions (Fig. 1A).
Similar, but apparently contradictory results concerning OPN
upregulation at the protein level and downregulation at the mRNA
level were observed in the HT29 cells (Figs. 2 and 1A). Hence, the reduction in
transcriptional activity of OPN mRNA could be an effect of a
possible negative feed-back loop due to intracellular accumulation
of OPN protein, shown by staining (Fig.
4). This fact could also explain the absence of OPN in cell
culture supernatants of all used cell lines, regardless of the
culture conditions; although an increase in OPN protein was
detected by western blot analysis (Fig.
2). These results are in line with the western blotting
outcomes in terms of the amount of OPN protein and recently
published data from our group (35).
We may further examine, whether and to what extent
OPN has an effect on the malignancy of colorectal tumor cell lines
by influencing the expression of HIF-1A and HIF-2A. Both
hypoxia-inducible factors mediate the response of cells to hypoxia
by regulating downstream target genes. However, the functional
roles of the two HIF isoforms are completely different in colon
cancer as shown by Imamura et al, using SW480 cells
(36). In this cell line, induction
of HIF-1A promoted cell growth, while induction of HIF-2A appeared
to restrain it. Furthermore, the loss of HIF-2A expression (but not
of HIF-1A) was found to be associated with advanced tumor stage.
Yang et al showed a significant downregulation of HIF-1A
expression in breast cancer upon the efficient knockdown of OPN,
promoting the radiosensitivity of the cells (37). Furthermore, increased survival of
ovarian cancer cells was promoted by OPN and mediated via induction
of HIF-1A expression through the PI3-K/Akt pathway (38). Jiang et al demonstrated in
bone marrow-derived mesenchymal stem cells (BMSCs) an induction of
adipogenic differentiation under hypoxic conditions regulated by
HIF-1A, while in contrast OPN mRNA was decreased in these
non-malignant cells (39).
Recently, Cheng et al showed for the first time an
association between OPN and HIF-2A expression in chondrocytes. In
this previous study, the expression of HIF-2A at the mRNA level was
inhibited by OPN (40).
In addition, irradiation itself had nearly no
influence on the examined parameters. This is in concordance with
our results obtained in head and neck cancer and glioblastoma cell
lines, where OPN expression was strongly modulated by hypoxia and
only to a minor extent by irradiation (35). An unchanged CD44 expression profile
after irradiation was also observed by another group in Ewing
sarcoma cell lines (41). These
results may be important for the use of radiation therapy in the
clinical setting, showing no risk of upregulation of OPN and its
ligands.
Taken together, the experiments and descriptive
results in this study show that no common statement can be made
concerning the influence of hypoxia on CD44 and CD44v6 expression
in colorectal cancer cell lines. Each cell line behaved
differently, probably according to its state of malignancy, as
discussed. Regarding OPN, all cell lines had in common a more or
less strong increase in its protein expression and the development
of radioresistance under hypoxia. Our planned future projects
should provide more information concerning how hypoxia affects the
cells on a functional level.
References
|
1
|
Wai PY and Kuo PC: Osteopontin: Regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrik D, Lavori PW, Cao H, Zhu Y, Wong P,
Christofferson E, Kaplan MJ, Pinto HA, Sutphin P, Koong AC, et al:
Plasma osteopontin is an independent prognostic marker for head and
neck cancers. J Clin Oncol. 24:5291–5297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mack PC, Redman MW, Chansky K, Williamson
SK, Farneth NC, Lara PN Jr, Franklin WA, Le QT, Crowley JJ and
Gandara DR: SWOG Lower osteopontin plasma levels are associated
with superior outcomes in advanced non-small-cell lung cancer
patients receiving platinum-based chemotherapy: SWOG Study S0003. J
Clin Oncol. 26:4771–4776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Overgaard J, Eriksen JG, Nordsmark M,
Alsner J and Horsman MR; Danish Head and Neck Cancer Study Group, :
Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser
nimorazole in radiotherapy of head and neck cancer: Results from
the DAHANCA 5 randomised double-blind placebo-controlled trial.
Lancet Oncol. 6:757–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bache M, Kappler M, Wichmann H, Rot S,
Hahnel A, Greither T, Said HM, Kotzsch M, Würl P, Taubert H, et al:
Elevated tumor and serum levels of the hypoxia-associated protein
osteopontin are associated with prognosis for soft tissue sarcoma
patients. BMC Cancer. 10:1322010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le QT, Sutphin PD, Raychaudhuri S, Yu SC,
Terris DJ, Lin HS, Lum B, Pinto HA, Koong AC and Giaccia AJ:
Identification of osteopontin as a prognostic plasma marker for
head and neck squamous cell carcinomas. Clin Cancer Res. 9:59–67.
2003.PubMed/NCBI
|
|
7
|
Nordsmark M, Bentzen SM, Rudat V, Brizel
D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, et
al: Prognostic value of tumor oxygenation in 397 head and neck
tumors after primary radiation therapy. An international
multi-center study. Radiother Oncol. 77:18–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hockel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
9
|
Nordsmark M, Alsner J, Keller J, Nielsen
OS, Jensen OM, Horsman MR and Overgaard J: Hypoxia in human soft
tissue sarcomas: Adverse impact on survival and no association with
p53 mutations. Br J Cancer. 84:1070–1075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polat B, Wohlleben G, Katzer A, Djuzenova
CS, Technau A and Flentje M: Influence of osteopontin silencing on
survival and migration of lung cancer cells. Strahlenther Onkol.
189:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hahnel A, Wichmann H, Kappler M, Kotzsch
M, Vordermark D, Taubert H and Bache M: Effects of osteopontin
inhibition on radiosensitivity of MDA-MB-231 breast cancer cells.
Radiat Oncol. 5:822010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hahne JC, Meyer SR, Kranke P, Dietl J,
Guckenberger M, Polat B and Hönig A: Studies on the role of
osteopontin-1 in endometrial cancer cell lines. Strahlenther Onkol.
189:1040–1048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marroquin CE, Downey L, Guo H and Kuo PC:
Osteopontin increases CD44 expression and cell adhesion in RAW
264.7 murine leukemia cells. Immunol Lett. 95:109–112. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Y, Stamenkovic I and Yu Q: CD44
attenuates activation of the hippo signaling pathway and is a prime
therapeutic target for glioblastoma. Cancer Res. 70:2455–2464.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denhardt DT, Giachelli CM and Rittling SR:
Role of osteopontin in cellular signaling and toxicant injury. Annu
Rev Pharmacol Toxicol. 41:723–749. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan SA, Cook AC, Kappil M, Günthert U,
Chambers AF, Tuck AB and Denhardt DT: Enhanced cell surface CD44
variant (v6, v9) expression by osteopontin in breast cancer
epithelial cells facilitates tumor cell migration: Novel
post-transcriptional, post-translational regulation. Clin Exp
Metastasis. 22:663–673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christofori G: Changing neighbours,
changing behaviour: Cell adhesion molecule-mediated signalling
during tumour progression. EMBO J. 22:2318–2323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi J, Zhou Z, Di W and Li N: Correlation
of CD44v6 expression with ovarian cancer progression and
recurrence. BMC Cancer. 13:1822013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie JW, Chen PC, Zheng CH, Li P, Wang JB,
Lin JX, Lu J, Chen QY, Cao LL, Lin M, et al: Evaluation of the
prognostic value and functional roles of CD44v6 in gastric cancer.
J Cancer Res Clin Oncol. 141:1809–1817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen P, Huang HF, Lu R, Wu Y and Chen YZ:
Prognostic significance of CD44v6/v7 in acute promyelocytic
leukemia. Asian Pac J Cancer Prev. 13:3791–3794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokota A, Ishii G, Sugaya Y, Nishimura M,
Saito Y and Harigaya K: Expression of exon v6-containing CD44
isoforms is related to poor prognosis of acute myelocytic leukemia.
Hematol Oncol. 16:131–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naor D, Nedvetzki S, Golan I, Melnik L and
Faitelson Y: CD44 in cancer. Crit Rev Clin Lab Sci. 39:527–579.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Günthert U: CD44 in malignant disorders.
Curr Top Microbiol Immunol. 213:271–285. 1996.PubMed/NCBI
|
|
26
|
Huh JW, Kim HR, Kim YJ, Lee JH, Park YS,
Cho SH and Joo JK: Expression of standard CD44 in human colorectal
carcinoma: Association with prognosis. Pathol Int. 59:241–246.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang XJ, Jiang H, Zhao XP and Jiang WM:
The role of a new CD44st in increasing the invasion capability of
the human breast cancer cell line MCF-7. BMC Cancer. 11:2902011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Phuc P, Nhan PL, Nhung TH, Tam NT,
Hoang NM, Tue VG, Thuy DT and Ngoc PK: Downregulation of CD44
reduces doxorubicin resistance of CD44+CD24- breast cancer cells.
Onco Targets Ther. 4:71–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jijiwa M, Demir H, Gupta S, Leung C, Joshi
K, Orozco N, Huang T, Yildiz VO, Shibahara I, de Jesus JA, et al:
CD44v6 regulates growth of brain tumor stem cells partially through
the AKT-mediated pathway. PLoS One. 6:e242172011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao AC, Lou W, Dong JT and Isaacs JT: CD44
is a metastasis suppressor gene for prostatic cancer located on
human chromosome 11p13. Cancer Res. 57:846–849. 1997.PubMed/NCBI
|
|
31
|
Abetamann V, Kern HF and Elsässer HP:
Differential expression of the hyaluronan receptors CD44 and RHAMM
in human pancreatic cancer cells. Clin Cancer Res. 2:1607–1618.
1996.PubMed/NCBI
|
|
32
|
Hewitt RE, McMarlin A, Kleiner D, Wersto
R, Martin P, Tsokos M, Stamp GW and Stetler-Stevenson WG:
Validation of a model of colon cancer progression. J Pathol.
192:446–454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang C, Xie J, Guo J, Manning HC, Gore JC
and Guo N: Evaluation of CD44 and CD133 as cancer stem cell markers
for colorectal cancer. Oncol Rep. 28:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flatmark K, Maelandsmo GM, Martinsen M,
Rasmussen H and Fodstad Ø: Twelve colorectal cancer cell lines
exhibit highly variable growth and metastatic capacities in an
orthotopic model in nude mice. Eur J Cancer. 40:1593–1598. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wohlleben G, Scherzad A, Güttler A,
Vordermark D, Kuger S, Flentje M and Polat B: Influence of hypoxia
and irradiation on osteopontin expression in head and neck cancer
and glioblastoma cell lines. Radiat Oncol. 10:1672015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imamura T, Kikuchi H, Herraiz MT, Park DY,
Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier
RJ, et al: HIF-1alpha and HIF-2alpha have divergent roles in colon
cancer. Int J Cancer. 124:763–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang L, Zhao W, Zuo WS, Wei L, Song XR,
Wang XW, Zheng G and Zheng MZ: Silencing of osteopontin promotes
the radiosensitivity of breast cancer cells by reducing the
expression of hypoxia inducible factor 1 and vascular endothelial
growth factor. Chin Med J. 125:293–299. 2012.PubMed/NCBI
|
|
38
|
Song G, Cai QF, Mao YB, Ming YL, Bao SD
and Ouyang GL: Osteopontin promotes ovarian cancer progression and
cell survival and increases HIF-1alpha expression through the
PI3-K/Akt pathway. Cancer Sci. 99:1901–1907. 2008.PubMed/NCBI
|
|
39
|
Jiang C, Sun J, Dai Y, Cao P, Zhang L,
Peng S, Zhou Y, Li G, Tang J and Xiang J: HIF-1A and C/EBPs
transcriptionally regulate adipogenic differentiation of bone
marrow-derived MSCs in hypoxia. Stem Cell Res Ther. 6:212015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng C, Zhang FJ, Tian J, Tu M, Xiong YL,
Luo W, Li YS, Song BB, Gao SG and Lei GH: Osteopontin inhibits
HIF-2α mRNA expression in osteoarthritic chondrocytes. Exp Ther
Med. 9:2415–2419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Könemann S, Malath J, Bölling T, Kolkmeyer
A, Janke K, Riesenbeck D, Hesselmann S, Nguyen TP, Diallo R,
Vormoor J, et al: Changed adhesion molecule profile of Ewing tumor
cell lines and xenografts under the influence of ionizing
radiation. Anticancer Res. 24:1637–1644. 2004.PubMed/NCBI
|