Introduction
Glioblastoma, also known as glioblastoma multiforme
(GBM), is the most aggressive primary tumor of the brain (1–3).
Despite advances in surgical technology and adjuvant treatment for
glioblastoma, this tumor remains the most lethal disease worldwide
(2,3). The molecular mechanisms underlying the
invasion and metastasis of glioblastoma are still unknown.
Formerly, Wnts have been divided into two classes:
those that signal through canonical (β-catenin dependent) pathway
and those that signal through non-canonical (β-catenin independent)
pathway (4–6). Wnt signaling participates in the
development of embryo and pathological processes, including
tumorigenesis and metastasis (7). A
homeobox transcription factor, MSX1 was able to inhibit the
Wnt/β-catenin signaling pathway and suppress Wnt/β-catenin-induced
migration and invasion of cultured glioblastoma cells (8). Inhibition of the Wnt/β-catenin pathway
significantly abrogated the invasion effects of irradiation,
indicating a pivotal role of the Wnt/β-catenin pathway in ionizing
radiation-induced invasion of U87 cells (9).
Wnt5a is classified as a non-transforming Wnt family
member that plays complex roles in cancer initiation and metastasis
(10–12). Wnt5a activates small Rho-GTPases and
regulates the cytoskeletal architecture and cellular polarity
during development (13). Wnt5a
signaling is a regulator in the proliferation of human glioma cells
(14). However, very few studies
have reported on the role of Wnt5a in glioblastoma cell invasion
and migration (15,16). In the present study, we propose for
the first time that Wnt5a promotes the invasion of glioblastoma
cells and is upregulated in invasive glioblastoma tissues.
Moreover, the mechanisms whereby the Wnt5a/Daam1/RhoA signaling
pathway regulates glioblastoma cell invasion are described.
Materials and methods
Clinical samples
Tissue samples of nine glioblastoma patients from
the Jinan Fourth People's Hospital from 2015 to 2016 were recruited
in the present study. All patients underwent surgical resection of
the glioblastoma with the intention of maximally resecting the
tumor. Glioblastoma tissues were frozen in liquid nitrogen or fixed
in 10% formalin and embedded in paraffin wax. Slices were stained
with hematoxylin and eosin (H&E) for light microscopy. The
diagnosis of glioblastoma was based on the World Health
Organization (WHO) 2007 and 2016 histopathologic criteria: the
invasive phenotype showing an infiltrative astrocytic neoplasm with
high proliferative activity and microvascular proliferation,
necrosis or both (17,18). The non-invasive glioblastoma was
thus called when the tumor at the anatomical site began to progress
and further yielded a cancerous mass without the invasive phenotype
aforementioned. All glioblastoma tissues with high tumor cell
density were histopathologically confirmed by two pathologists
prior to ELISA and small G-protein activation assay. Ethical
approval of the study (no. LL-20140005) was granted by the Clinical
Research Ethics Committee, Jinan Fourth People's Hospital. Written
informed consent was obtained from each participant.
Cells and small interfering RNA
(siRNA) transfection
U87MG, U251 and T98MG are the most used cell lines
for research on human glioblastoma. The original U87MG cell line
was established in Uppsala University almost 50 years ago (19). However, Allen et al used
short tandem repeat (STR) genotyping to screen out the DNA profile
of U87MG. Different from that of the original cells, this friendly
profile of U87MG is thought to have an unknown origin (20). Thus, two cell lines (U251 and T98MG)
were used in this experiment. Human glioblastoma U251 or T98MG cell
lines were purchased from the Cell Bank of Shanghai (Shanghai,
China) and were grown in Eagle's Minimum Essential Medium (EMEM;
HyClone, Thermo Scientific, Waltham, MA, USA) supplemented with 10%
(v/v) fetal bovine serum (FBS), 2 mmol/l L-glutamine and 100 IU/ml
penicillin, 100 µg streptomycin, 1 mmol/l sodium pyruvate and
non-essential amino acids (HyClone) in a humidified incubator at
37°C with 5% CO2 and 95% humidity. The cells were seeded
in 6-well plates (Costar, Corning, NY, USA) and cultured to 80%
confluence, and then transiently transfected with siRNA against
Daam1 (21) using Lipofectamine
2000 reagent (Invitrogen, Carlsbad, CA, USA) in serum-free Opti-MEM
according to the manufacturer's instructions. The cells were
switched to fresh medium containing 10% FBS 6 h after the
transfection and cultured for 48 h. The cells transfected with
Daam1-siRNA were used for analyzing Rho activation and cell
invasion.
ELISA
The glioblastoma tissues were grinded in liquid
nitrogen. Equal weights of total tissue debris were dissolved in
ice-cold phosphate-buffered saline (PBS) buffer. The experiments
were then performed according to the manufacturer's protocol of the
Wnt5a ELISA kit (CusaBio, Wuhan, China). The concentration of each
glioblastoma tissue was calculated based on the concentration curve
of the Wnt5a standard samples.
Cell invasion assays
Cell invasion was assessed in modified Boyden
chambers (Costar). Two chambers were separated by a polycarbonate
membrane (pore diameter, 8.0 µm). Boyden chamber wells were coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for 30 min
at 37°C. U251 or T98MG cells treated with CCG-1423 (Selleck,
Houston, TX, USA) were added to wells with a membrane placed in the
bottom. Medium containing recombinant Wnt5a (rWnt5a) was added to
the upper and lower compartment of the Boyden chamber. The cells
were allowed to invade for 6 h at 37°C in this assay. Thereafter,
the medium was discarded, stationary cells were removed with a
cotton-tipped applicator, and the membranes were cut out of the
chamber and stained with 0.5% crystal violet. The response was
evaluated on a light microscope by counting the number of cells
that had invaded into the Matrigel and membrane.
Small G-protein activation assay
For RhoA, Cdc42 and Rac1 activation assays, the
glioblastoma tissues were grinded in liquid nitrogen. Equal weights
of total tissue debris were dissolved in ice-cold PBS buffer.
Glioblastoma cells were seeded into 6-well plates and transfected
with Daam1-siRNA or treated with sFRP2 (R&D Systems,
Minneapolis, MN, USA). The experiments were then performed
according to the manufacturer's protocol (Cytoskeleton Inc.,
Denver, CO, USA). The activation of RhoA, Cdc42 and Rac1 was
normalized to the NC control group.
Western blotting
Subconfluent cells were washed twice with PBS, and
then lysed with ice-cold RIPA lysis buffer (Beyotime Biotechnology,
Nantong, China). The lysates were then clarified by centrifugation
at 12,000 × g for 20 min at 4°C. The protein extracts were
separated by 8% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The immunoblotting procedure was
performed as previously described (22), and the following antibodies were
used: anti-GAPDH (Sigma, St. Louis, MO, USA), anti-Daam1 (Santa
Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. Protein bands
were detected by incubation with horseradish peroxidase-conjugated
antibodies and visualized with enhanced chemiluminescence (ECL)
reagent (Thermo Scientific, Rockford, IL, USA).
Pull-down assays
For the detection of active Daam1, GST-RhoA beads
were incubated with 0.1 mmol/l GTPγS (Sigma) at 30°C for 15 min
with constant agitation. Equal volumes of total cellular protein
were incubated with GST-RhoA beads captured on MagneGST Glutathione
Particles (Promega, Madison, WI, USA) at 4°C with constant rotation
for 90 min. The beads were washed three times with washing buffer
(4.2 mmol/l Na2HPO4, 2 mmol/l
KH2PO4, 140 mmol/l NaCl and 10 mmol/l KCl, pH
7.2). At the end of this period, the beads were captured using a
magnet on a magnetic stand. After being washed three times with
ice-cold buffer, the beads were resuspended in Laemmli buffer,
boiled, and subjected to western blot analysis. SDS-PAGE and
western blotting were performed using standard methods.
Actin cytoskeleton staining and
immunofluorescence
Transfected cells were fixed in 4% paraformaldehyde
in PBS for 20 min, permeabilized in 0.2% Triton X-100 and blocked
in PBS containing 1% BSA for 1 h at room temperature. F-actin was
stained with FITC-labeled phalloidin (5 mg/ml) (Beyotime
Biotechnology) for 40 min at room temperature. After being washed
with PBS, the coverslips were mounted on glass slides with DAPI
Fluoromount-G (Southern Biotech, Birmingham, AL, USA). The images
were acquired with a fluorescence microscope (Zeiss, LSM 710
system; Carl Zeiss, Jena, Germany).
Statistical analysis
The data were analyzed using Student's t-test with
the SPSS statistical software package. All the results were
expressed as the mean ± SD. For all analyses a two-sided P-value of
<0.05 was deemed statistically significant.
Results
Wnt5a and RhoA are upregulated in
invasive glioblastoma tissues
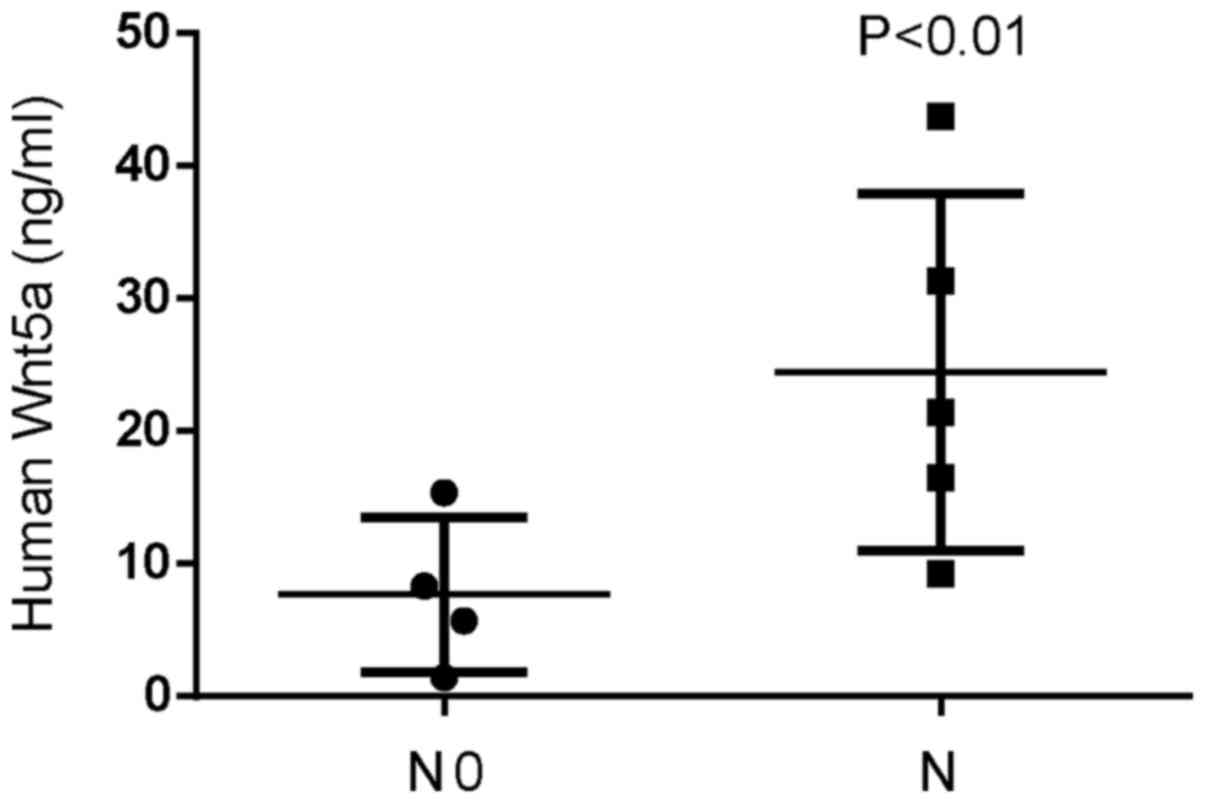
In order to evaluate Wnt5a expression in invasive
glioblastoma tissues, we assessed the expression of Wnt5a using
ELISA assays in nine samples of glioblastoma. Wnt5a expression was
higher in invasive glioblastoma tissues compared to that in
non-invasive glioblastoma tissues, with the highest expression at
43.7 ng/ml (Fig. 1). We also
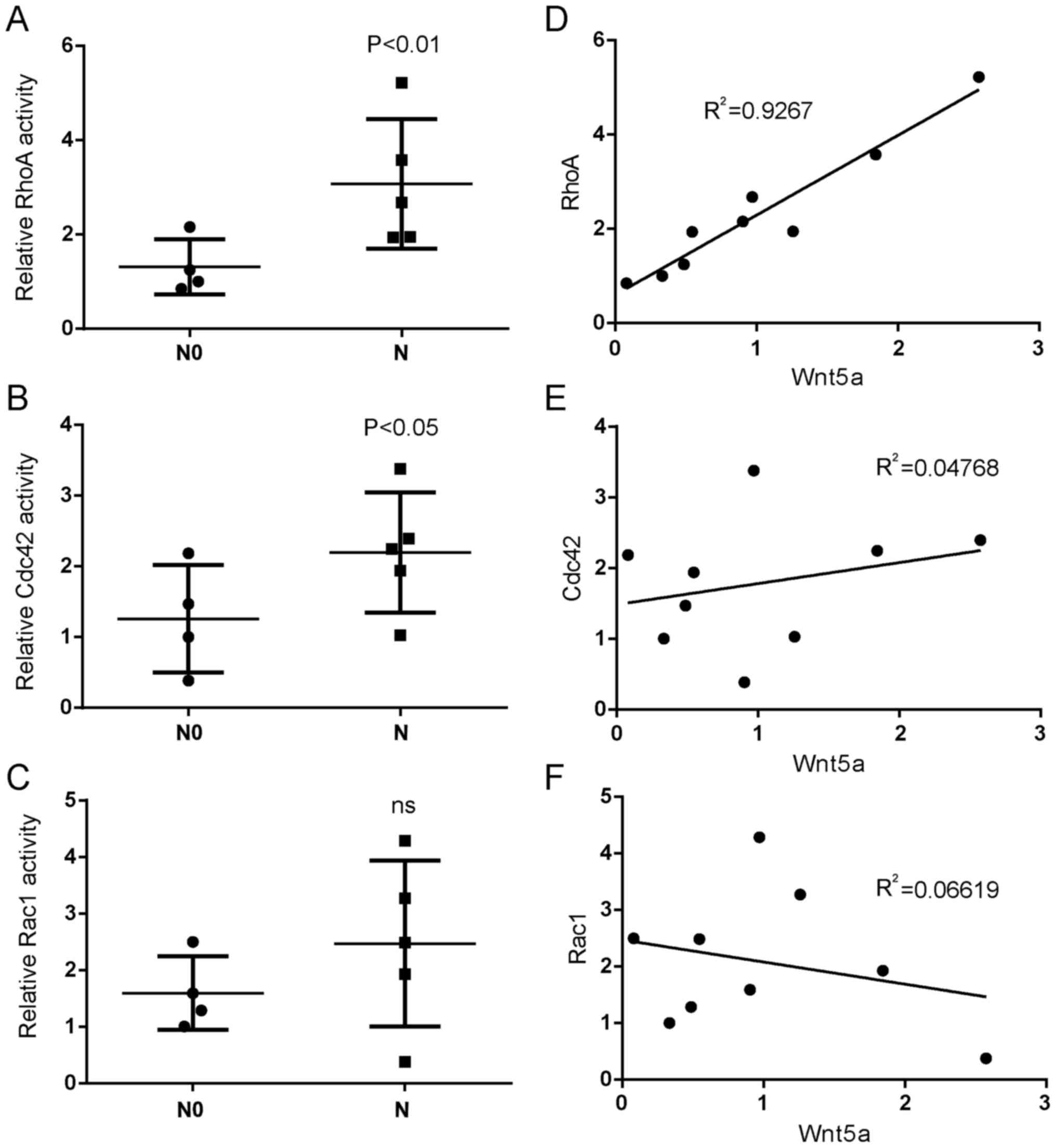
assessed the activations of Rho GTPases in the same glioblastoma
tissues and found that the activation of RhoA and Cdc42 were higher
in invasive glioblastoma tissues compared to that in non-invasive
glioblastoma tissues (Fig. 2A and
B). However, Rac1 activity had an insignificant increase in
invasive glioblastoma tissues compared to non-invasive glioblastoma
tissues (Fig. 2C). Moreover, the
activation of RhoA was positively correlated with the expression of
Wnt5a in glioblastoma tissues (Fig.
2D), while there was a weak correlation between the activation
of Cdc42 or Rac1 and Wnt5a expression (Fig. 2E and F). These results indicated
that Wnt5a and RhoA had tumor-promoting roles in glioblastoma
invasion.
Wnt5a stimulates glioblastoma cell
invasion in vitro
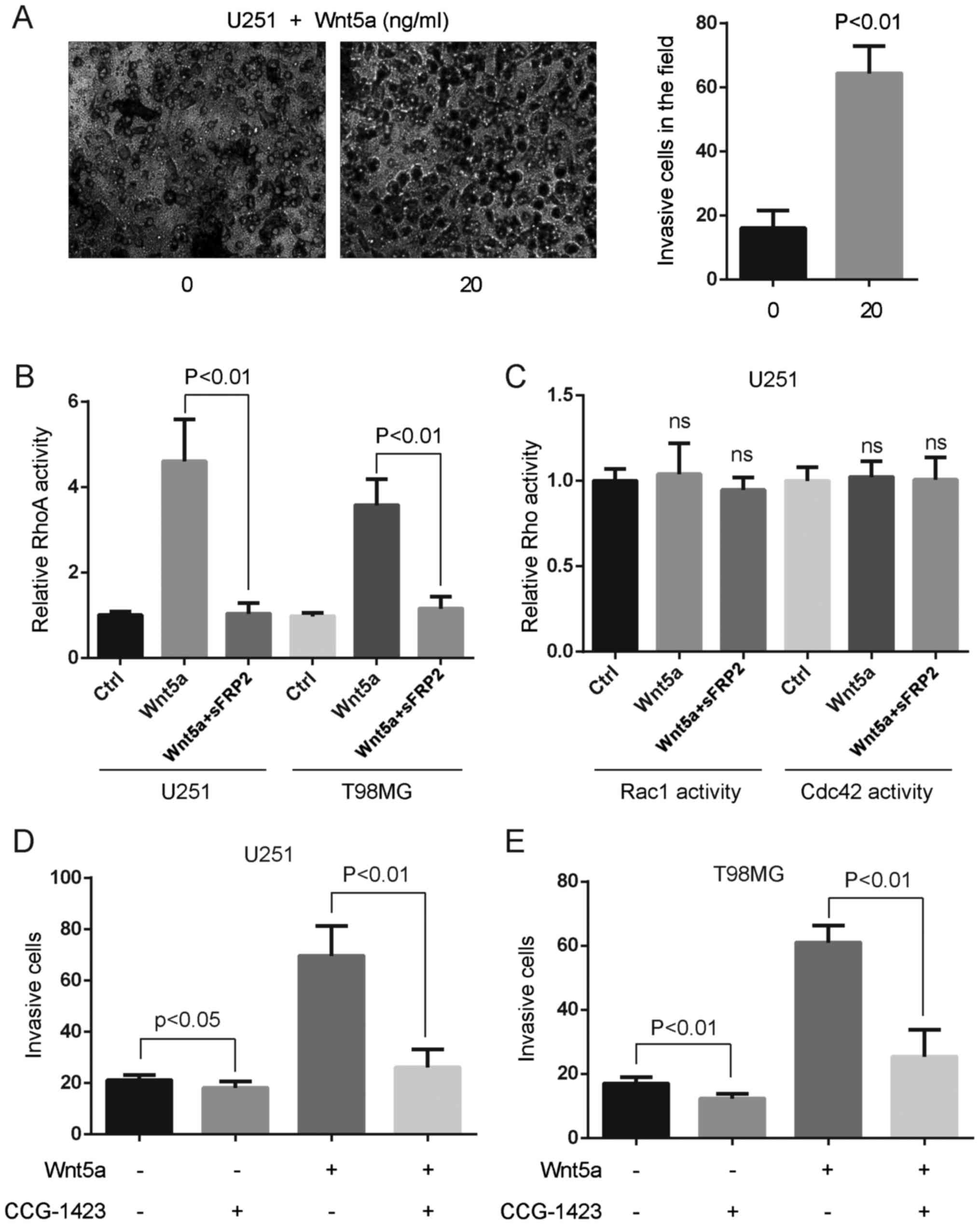
To assess the effect of Wnt5a on glioblastoma cell
invasion, we treated U251 glioblastoma cells with 20 ng/ml rWnt5a,
and assessed the invasion rate using the Boyden chamber assay. An
approximately threefold increase of cell invasion was observed in
U251 cells treated with 20 ng/ml rWnt5a, which indicated that Wnt5a
had a potent stimulatory effect on glioblastoma cell invasion
(Fig. 3A).
RhoA activation participates in
Wnt5a-induced glioblastoma cell invasion
We investigated whether RhoA activation was induced
by Wnt5a in glioblastoma cells. Small G-protein assays revealed a
significant increase of active RhoA after 20 ng/ml rWnt5a treatment
(Fig. 3B). Pre-incubation of
secreted frizzled-related protein 2 (sFRP2), an antagonist that
directly binds to Wnt5a (21),
abolished rWnt5a-RhoA activity in U251 and T98MG cells (Fig. 3B). However, Wnt5a and/or sFRP2
treatment did not alter the activation of Rac1 and Cdc42 in U251
cells (Fig. 3C). To assess the
effect of RhoA on glioblastoma cell invasion, we treated
glioblastoma cells with 10 ng/ml RhoA-specific inhibitor CCG-1423
and 20 ng/ml rWnt5a, and assessed the invasion rate using the
Boyden chamber assay. We found that CCG-1423 blocked Wnt5a-induced
glioblastoma cell invasion (Fig. 3D and
E), indicating that RhoA activation was indispensable for
Wnt5a-induced glioblastoma cell invasion.
Wnt5a induces glioblastoma cell
invasion via Daam1 activation
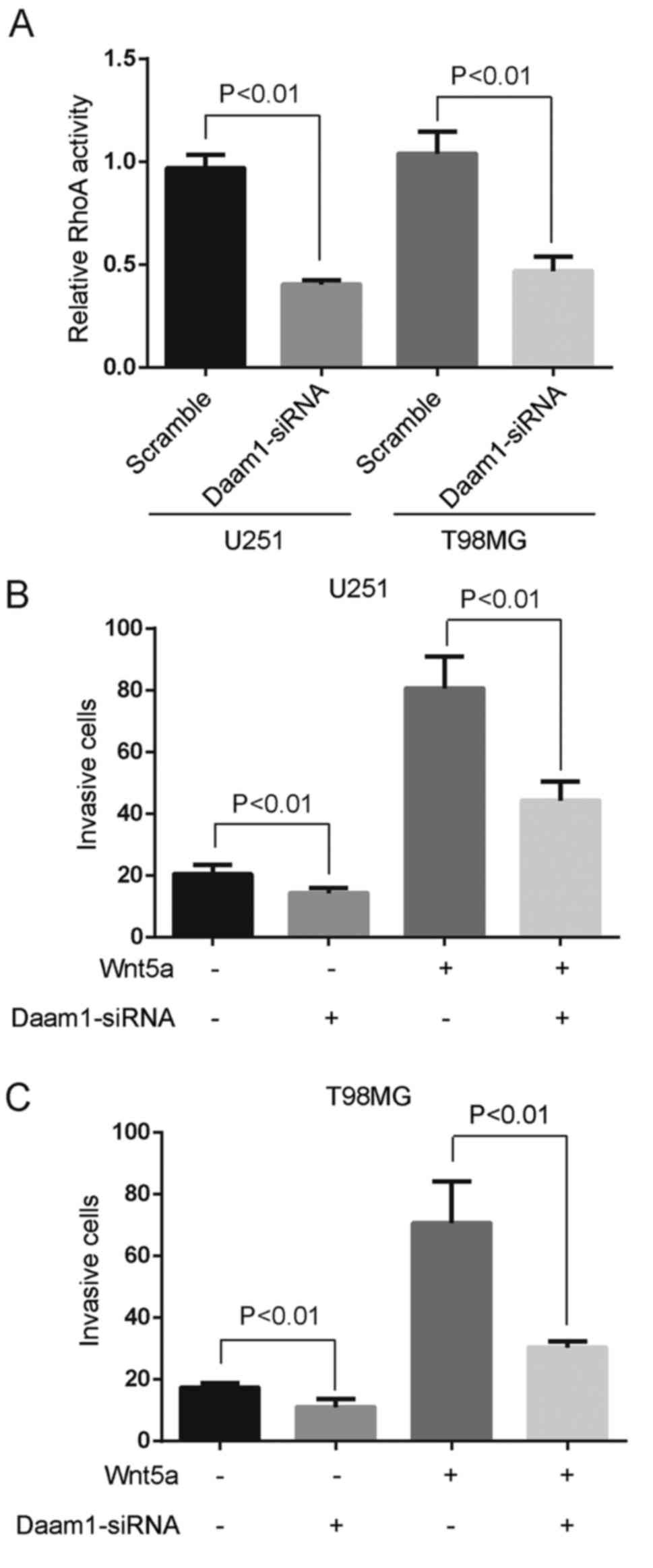
We examined whether Wnt5a-induced RhoA activation
was regulated by Daam1 in glioblastoma cells. Specific siRNA
against Daam1 blocked Wnt5a-induced RhoA activation (Fig. 4A). Moreover, Daam1 siRNA was fully
capable of retarding the invasion of U251 and T98MG cells (Fig. 4B and C). To analyze the role of
Wnt5a on Daam1 activation (a state allowing its interaction with
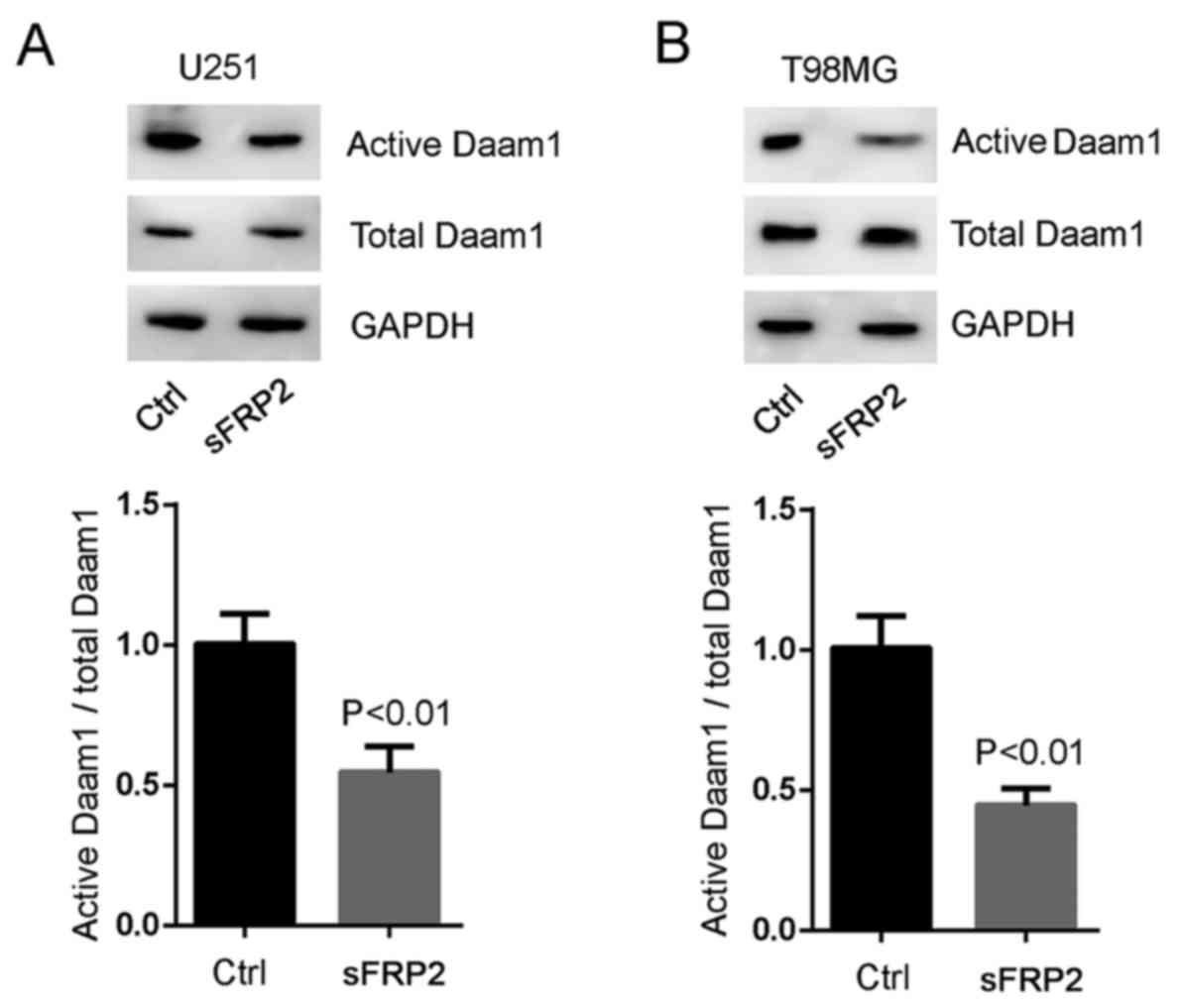
RhoA), we blocked Wnt5a signaling with sFRP2 treatment. SFRP2
significantly inhibited Wnt5a-induced Daam1 activity in U251 and
T98MG cells (Fig. 5A and B). These
experiments demonstrated that RhoA was a downstream target of
Wnt5a/Daam1 signaling in U251 and T98MG cells.
Wnt5a/Daam1/RhoA signaling sustains
the formation of stress fibers
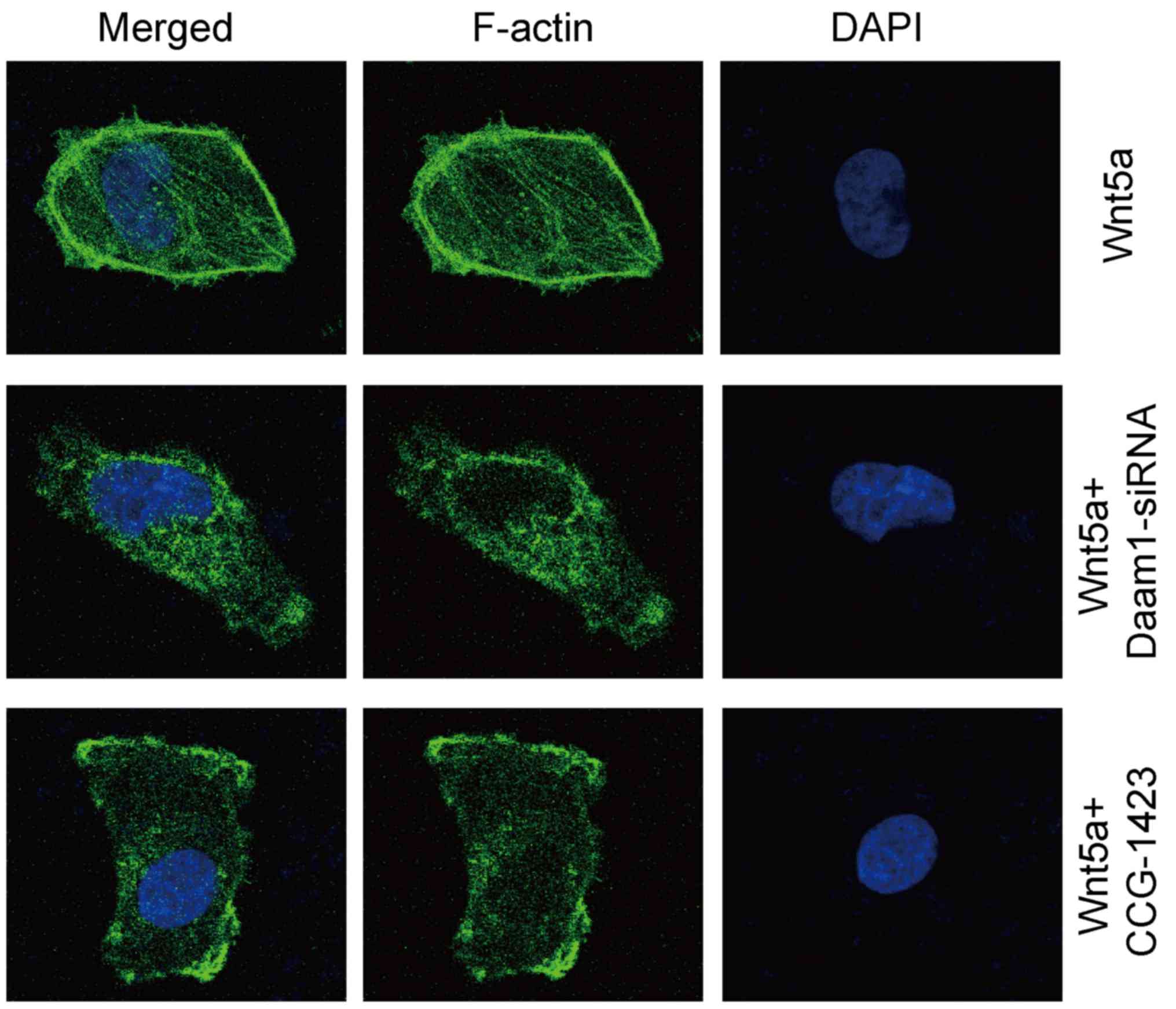
We performed fluorescent phalloidin staining to
investigate the distribution pattern of filamentous actin (F-actin)
in glioblastoma cells. Wnt5a treatment sustained the
formation/maintenance of actin stress fibers in U251 cells
(Fig. 6). In contrast, Daam1-siRNA
or CCG-1423 treatment disrupted the formation of actin stress
fibers in U251 cells (Fig. 6).
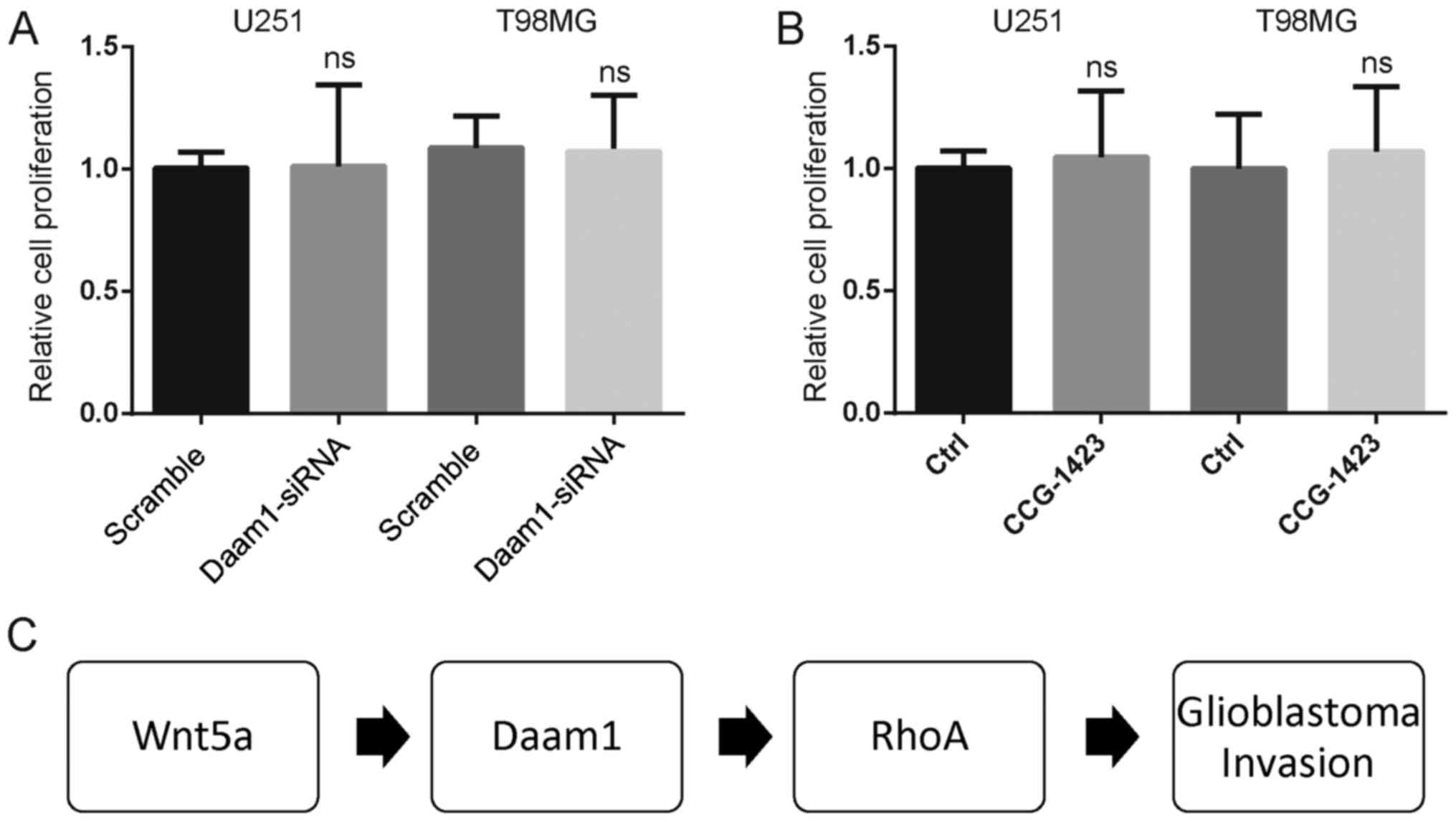
Finally, neither Daam1-siRNA nor CCG-1423 treatment altered the
cell proliferation of U251 and T98MG cells (Fig. 7A and B). Thus, the findings from the
clinical and cellular biological assays indicated that RhoA
activation required Daam1 activity to mediate Wnt5a-induced
glioblastoma cell invasion (Fig.
7C).
Discussion
Wnt5a acts both as a suppressor and an inducer in
different types of tumors (12,23–26).
The Wnt/planar cell polarity (PCP) pathway triggered by Wnt5a
activates small Rho-GTPases and reassembles the cytoskeletal
architecture and cellular polarity during embryo development and
pathological processes (27–29).
In our previous studies, we found that Wnt5a stimulated the
migration of breast cancer cells via Rho signaling pathways
(21,30). In gastric cancer, Wnt5a also
enhanced the ability of cell mobility via RhoA signaling pathways
(31). In the present study, we
found that Wnt5a induced the invasion of U251 and T98MG
glioblastoma cells. Moreover, Wnt5a was highly expressed in
invasive glioblastoma tissues compared to that in non-invasive
glioblastoma tissues. These results revealed that Wnt5a may
accelerate glioblastoma invasion in vitro and in vivo
and that blocking Wnt5a signaling may prevent glioblastoma
metastasis in clinic. In addition, the overexpression of Wnt5a
increased the proliferation of glioblastoma GBM-05 and U87MG cells,
indicating that Wnt5a is a regulator in the proliferation of human
glioblastoma (14). This evidence
demonstrated that Wnt5a acts as an inducer and promoter in
glioblastoma tumorigenesis and metastasis.
With the help of small G-protein promoting cellular
migration, Wnt5a can remodel the cellular cytoskeleton of melanoma
cells (11). In the present study,
Wnt5a promoted glioblastoma cell invasion by activating the
Daam1/RhoA signaling pathway. In clinical samples, Wnt5a and RhoA
are upregulated in invasive glioblastoma tissues, with a
significant positive correlation between them. Studies with much
larger samples may provide statistical power to validate the role
of Wnt5a and RhoA in glioblastoma. Our previous study revealed that
Rac1 activation was increased by Wnt5a and stimulated the cell
migration of MCF-7 breast cancer cells (30). Unfortunately, Wnt5a did not alter
the activation of Rac1 and Cdc42 in U251 glioblastoma cells in the
present study. These results reveal the specificity of elevated
RhoA activation in glioblastoma invasion.
Containing multiple regulatory domains, Daam1 exists
in an auto-inhibited state through intramolecular interaction in
unstimulated cells (10,32). Our results showed that sFRP2, an
antagonist that directly binds to Wnt5a, markedly blocked the
activity of Wnt5a-induced Daam1 in glioblastoma cells. Knockdown of
Daam1 expression via siRNA transfection inhibited RhoA activation
and the cell invasion stimulated by Wnt5a in U251 and T98MG cells.
Furthermore, we tested the reestablishment of stress fibers in
Daam1-knockdown or RhoA-blocked cells. The decrease of RhoA
activity, formation of stress fibers and cell invasion in
Daam1-knockdown cells indicated that Daam1 may be required for the
activation of RhoA after Wnt5a treatment in glioblastoma. Thus,
these results clearly demonstrated that Daam1/RhoA signaling under
Wnt5a stimulation participated in the invasion of glioblastoma and
that retarding them may prevent glioblastoma metastasis in
clinic.
Soluble frizzled-related proteins (sFRPs) function
as modulators of Wnt signaling through direct interaction with Wnts
(33). We reported in our previous
study that Wnt5a-induced RhoA activation and cell migration could
be abolished by sFRP2 pretreatment in breast cancer cells (21). In the present study, we also found
that sFRP2 blocked the Wnt5a-induced RhoA activation in
glioblastoma cells. These results demonstrated that sFRP2 acted as
an antagonist of Wnt5a mediating the progression of glioblastoma
and breast cancer. A recent study in immunohistochemistry revealed
that the expression level of soluble frizzled-related protein 3
(sFRP3) was decreased in the nucleus in higher grade astrocytoma,
indicating the antagonistic ability of Wnt signaling (34). Further studies are needed to
decipher whether sFRP2 and sFRP3 function in a common pathway or in
parallel pathways to block Wnt5a signaling.
In conclusion, the present study partially clarified
the associations between Wnt5a/RhoA signaling and glioblastoma
progression. It is the first to demonstrate that Wnt5a may regulate
the invasion of glioblastoma cells, at least in part via the
Daam1/RhoA signaling pathway. Therefore, the Wnt5a/Daam1/RhoA
signaling pathway is a candidate accelerator in glioblastoma and
may be a potential clinical classification marker and therapeutic
target for human glioblastoma.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (81472703) to Y.Z., a
sponsorship of Jiangsu Overseas Research and Training Program for
University Prominent Young and Middle-aged Teachers and Presidents
to Y.Z., and a grant from the Joint Research Project of Southeast
University and Nanjing Medical University (2242017K3DN41) to
Y.Z.
References
|
1
|
Agnihotri S, Burrell KE, Wolf A, Jalali S,
Hawkins C, Rutka JT and Zadeh G: Glioblastoma, a brief review of
history, molecular genetics, animal models and novel therapeutic
strategies. Arch Immunol Ther Exp. 61:25–41. 2013. View Article : Google Scholar
|
|
2
|
Rahman M, Abbatematteo J, De Leo EK,
Kubilis PS, Vaziri S, Bova F, Sayour E, Mitchell D and
Quinones-Hinojosa A: The effects of new or worsened postoperative
neurological deficits on survival of patients with glioblastoma. J
Neurosurg. 127:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Konar SK, Bir SC, Maiti TK, Patra DP,
DiPoto Brahmbhatt AC, Jacobsohn JA and Nanda A: Early dural
metastasis from a case of glioblastoma with primitive
neuroectodermal differentiation: A case report and literature
review. J Clin Neurosci. 35:78–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bienz M: Bio-sketch for oncogene review
issue on Wnt signalling. Oncogene. 25:74412006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Widelitz R: Wnt signaling through
canonical and non-canonical pathways: Recent progress. Growth
Factors. 23:111–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao YF, Yong X, Tang B, Qin Y, Zhang JW,
Zhang D, Xie R and Yang SM: Notch and Wnt signaling pathway in
cancer: Crucial role and potential therapeutic targets (Review).
Int J Oncol. 48:437–449. 2016.PubMed/NCBI
|
|
8
|
Tao H, Guo L, Chen L, Qiao G, Meng X, Xu B
and Ye W: MSX1 inhibits cell migration and invasion through
regulating the Wnt/β-catenin pathway in glioblastoma. Tumour Biol.
37:1097–1104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Z, Zhou L, Han N, Zhang M and Lyu X:
Wnt/β-catenin pathway involvement in ionizing radiation-induced
invasion of U87 glioblastoma cells. Strahlenther Onkol.
191:672–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Habas R, Kato Y and He X: Wnt/Frizzled
activation of Rho regulates vertebrate gastrulation and requires a
novel Formin homology protein Daam1. Cell. 107:843–854. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witze ES, Litman ES, Argast GM, Moon RT
and Ahn NG: Wnt5a control of cell polarity and directional movement
by polarized redistribution of adhesion receptors. Science.
320:365–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu C, Wang X, Zhu H, Feng J, Ni S and
Huang J: Over-expression of ROR2 and Wnt5a cooperatively correlates
with unfavorable prognosis in patients with non-small cell lung
cancer. Oncotarget. 6:24912–24921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos A, Bakker AD, de Blieck-Hogervorst
JM and Klein-Nulend J: WNT5A induces osteogenic differentiation of
human adipose stem cells via rho-associated kinase ROCK.
Cytotherapy. 12:924–932. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu JM, Jun ES and Jung JS, Suh SY, Han JY,
Kim JY, Kim KW and Jung JS: Role of Wnt5a in the proliferation of
human glioblastoma cells. Cancer Lett. 257:172–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu B, Wang Q, Wang YA, Hua S, Sauvé CG,
Ong D, Lan ZD, Chang Q, Ho YW, Monasterio MM, et al: Epigenetic
activation of WNT5A drives glioblastoma stem cell differentiation
and invasive growth. Cell. 167:1281–1295.e18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Binda E, Visioli A, Giani F, Trivieri N,
Palumbo O, Restelli S, Dezi F, Mazza T, Fusilli C, Legnani F, et
al: Wnt5a drives an invasive phenotype in human glioblastoma
stem-like cells. Cancer Res. 77:996–1007. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pontén J and Macintyre EH: Long term
culture of normal and neoplastic human glia. Acta Pathol Microbiol
Scand. 74:465–486. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J
and Gu L: Dvl2-dependent activation of Daam1 and RhoA regulates
Wnt5a-induced breast cancer cell migration. PLoS One. 7:e378232012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu M, Wang T, He M, Cheng W, Yan T, Huang
Z, Zhang L, Zhang H, Zhu W, Zhu Y, et al: Tumor suppressor role of
miR-3622b-5p in ERBB2-positive cancer. Oncotarget. 8:23008–23019.
2017.PubMed/NCBI
|
|
23
|
Zoico E, Darra E, Rizzatti V, Budui S,
Franceschetti G, Mazzali G, Rossi AP, Fantin F, Menegazzi M, Cinti
S, et al: Adipocytes WNT5a mediated dedifferentiation: A possible
target in pancreatic cancer microenvironment. Oncotarget.
7:20223–20235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bitler BG, Nicodemus JP, Li H, Cai Q, Wu
H, Hua X, Li T, Birrer MJ, Godwin AK, Cairns P, et al: Wnt5a
suppresses epithelial ovarian cancer by promoting cellular
senescence. Cancer Res. 71:6184–6194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang CL, Liu D, Nakano J, Ishikawa S,
Kontani K, Yokomise H and Ueno M: Wnt5a expression is associated
with the tumor proliferation and the stromal vascular endothelial
growth factor - an expression in non-small-cell lung cancer. J Clin
Oncol. 23:8765–8773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee GT, Kang DI, Ha YS, Jung YS, Chung J,
Min K, Kim TH, Moon KH, Chung JM, Lee DH, et al: Prostate cancer
bone metastases acquire resistance to androgen deprivation via
WNT5A-mediated BMP-6 induction. Br J Cancer. 110:1634–1644. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J and Mlodzik M: Wnt/PCP instructions
for cilia in left-right asymmetry. Dev Cell. 40:423–424. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Y, Guo X, Yang Z, Chen S, Lei Y, Lin
M, Wang L, Feng C and Ke Z: AEG-1 activates Wnt/PCP signaling to
promote metastasis in tongue squamous cell carcinoma. Oncotarget.
7:2093–2104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
López-Escobar B, Cano DA, Rojas A, de
Felipe B, Palma F, Sánchez-Alcázar JA, Henderson D and
Ybot-González P: The effect of maternal diabetes on the Wnt-PCP
pathway during embryogenesis as reflected in the developing mouse
eye. Dis Model Mech. 8:157–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu Y, Shen T, Liu J, Zheng J, Zhang Y, Xu
R, Sun C, Du J, Chen Y and Gu L: Rab35 is required for
Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7
breast cancer cells. Cell Signal. 25:1075–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Zhang Y, Xu R, Du J, Hu Z, Yang L,
Chen Y, Zhu Y and Gu L: PI3K/Akt-dependent phosphorylation of GSK3β
and activation of RhoA regulate Wnt5a-induced gastric cancer cell
migration. Cell Signal. 25:447–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu W, Sato A, Khadka D, Bharti R, Diaz H,
Runnels LW and Habas R: Mechanism of activation of the Formin
protein Daam1. Proc Natl Acad Sci USA. 105:pp. 210–215. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones SE and Jomary C: Secreted
Frizzled-related proteins: Searching for relationships and
patterns. BioEssays. 24:811–820. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pećina-Šlaus N, Kafka A, Varošanec AM,
Marković L, Krsnik Ž, Njirić N and Mrak G: Expression patterns of
Wnt signaling component, secreted frizzled-related protein 3 in
astrocytoma and glioblastoma. Mol Med Rep. 13:4245–4251. 2016.
View Article : Google Scholar : PubMed/NCBI
|