Introduction
Triple functional domain protein (Trio) is a large
evolutionarily conserved protein that harbors a serine/threonine
kinase domain and two guanine nucleotide exchange factor (GEF)
domains (1). The two GEF domains
activate the GTPases Rac1/RhoG and RhoA, potentially linking
several Rho-GTPase signaling pathways (2). Trio participates in the regulation of
different physiological processes, most notably in neuronal
physiology (3–7). In addition to its main effects on
neuronal development, Trio plays important roles in other aspects
of myogenesis and phagocytosis (8–10).
Since Trio controls a wide range of cellular processes,
dysregulation of its activity is related to the emergence of
diseases. Trio was demonstrated to be highly upregulated in urinary
bladder tumors (11), soft tissue
sarcomas (12), breast cancer
(13), and various other types of
cancer (11–19). Higher Trio levels were closely
related to invasive tumor phenotype, high tumor grade, and rapid
tumor cell proliferation in breast tumors (13). A high expression level of Trio was
also associated with clinicopathological parameters and poor
prognosis of hepatocellular carcinoma patients. Trio was also
expressed at higher levels in glioblastoma compared with low-grade
glioma and was related to poor patient survival (17). Concomitantly, Trio was capable of
serving as a prognostic marker, which helped in determining the
therapeutic modality of colorectal cancer patients (19).
Cervical carcinoma is the second most common cause
of cancer mortality in females and seriously affects the health of
women worldwide (20). Tumor
metastasis results in ~90% of cancer-related deaths (21,22).
For cervical cancer, invasion and migration are important processes
in the progression of cancer metastasis (23). Therefore, studies on molecular
mechanisms underlying tumor invasion and metastasis are very
relevant for understanding tumor occurrence and development.
Despite the major advances in therapeutic approaches, most patients
still succumb to cancer progression (24–27).
Lymph node metastasis acts as an important unfavorable prognostic
factor and a primary cause of cancer-related deaths (28). Previous studies have reported that
Trio was involved in some types of cancer development (11–19).
High expression of Trio was associated with rapid tumor cell
proliferation and invasive tumor growth (18). However, studies on the role of Trio
and its clinical significance in cervical cancer remain
non-existent. In the present study, we identified Trio expression
in cervical cancer and its effects on cell invasion and migration.
Furthermore, we investigated the mechanism of Trio in cervical
cancer migration and invasion.
Materials and methods
Tissue specimens and cell lines
All procedures adhered to the approved medical
ethics practices and the Human Ethics Review Board of the Second
Hospital of Shandong University, China [Approval no.: KYLL-2014
(LW) P-001]. Clinical specimens were collected from patients at the
Second Hospital of Shandong University from October 2014 to
December 2016. Histological classifications and clinical staging
were based on the classification system by the International
Federation of Gynecology and Obstetrics (International Federation
of Gynecology and Obstetrics Cancer Committee; FIGO, 2009)
(29). A tumor size of 4 cm was
chosen as a ‘watershed’ value according to previous studies
(30–32). The human cervical cancer cell lines,
Caski and HeLa, and normal cervical cell, CRL-2614, were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The cells were grown in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS) at 37°C and 5% CO2.
Transfection was performed with Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) following
the manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction analysis
Total RNA was isolated from tissue and cells using
TRIzol reagent (Invitrogen), according to manufacturer's protocols.
Total RNA (1 µg) was reverse-transcribed into cDNA using
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China), and qPCR was conducted using SYBR-Green dye mix
(Invitrogen). Thermocycling conditions for RT-qPCR were as follows:
95°C for 10 min, 40 cycles of 95°C for 20 sec, 55°C for 30 sec, and
72°C for 20 sec. The relative expression level of Trio was
calculated using 2−ΔΔCq, and the expression level of
Trio mRNA was normalized to that of GAPDH. The Trio
sense and antisense primers were designed as follows:
5′-AGATTCTCTGTCGTTAAG-3′, and 5′-TTCTAAGACCAGGATGTA-3′,
respectively. The GAPDH sense and antisense primers were:
5′-CTCAAGATCATCAGCAAT-3′ and 5′-CGATACCAAAGTTGTCAT-3′,
respectively.
CRISPR/Cas9 system targeting human
Trio
The guide RNA sequences targeting the human
Trio gene were designed using an online sgRNA design tool at
https://crispr.mit.edu/. Guide sequences with
high scores for on-target activity and minimal predicted off-target
activity were selected. Table I
displays the three sets of oligonucleotides. Primer
oligonucleotides were annealed under 88°C for 2 min, 65°C for 10
min, 37°C for 10 min, and 25°C for 5 min. The annealing primer was
then purified and cloned into the PX330 vector (Addgene, Cambridge,
MA, USA). The CRISPR/Cas9 backbone and CRISPR/Cas9-gRNA plasmids
were separately transfected into cells using a lipidosome.
 | Table I.The sequences and location of gRNAs
targeting Trio. |
Table I.
The sequences and location of gRNAs
targeting Trio.
| Name | Genomic target | Target
location |
|---|
| g-RNA1 |
TCCGCCGCTGCTGCCGCTCATGG | Exon 1 |
| g-RNA2 |
GCCGCGCTGGCCGCCGCGGCGGG | Exon 1 |
| g-RNA3 |
CACCTCTGAGCCAGGGATACTGG | Exon 24 |
In vitro invasion assay
For the invasion assay, a Transwell chamber placed
into a 24-well plate was coated with 30 µl of Matrigel and
incubated for 40 min at 37°C. In the Transwell assay, the cells
were trypsinized and seeded in chambers at a density of
5×105 cells/well and were cultured in serum-free medium,
while 500 µl of 10% FBS-RPMI-1640 were added to the lower chamber.
After 24 h, the cells on the upper surface were removed, and the
migrated cells were fixed with 100% methanol for 30 min. The cells
on the bottom surface of the membrane were stained with eosin for
20 min. The cell images were obtained under a phase-contrast
microscope.
Wound healing assays
Cells were seeded in 6-well plates. When the cells
reached 90–100% confluence, the cell monolayers were wounded by
scraping with a micropipette tip. The spreading of wound closure
was observed after 48 h. Images were captured using a
phase-contrast microscope (Olympus, Tokyo, Japan) either
immediately or 48 h after wounding. All experiments were repeated
thrice.
Western blot analysis
Western blot analysis was performed using anti-Trio
(Rabbit, Polyclonal, 1:1,000; Abnova, Taipei, Taiwan), anti-RhoA
(Rabbit, monoclonal, 1:1,000, cat. no. 2117; Cell Signaling
Technology, Beverly, MA, USA), anti-Rock1 (Mouse, monoclonal,
1:800, cat. no. sc-365628), anti-Rock2 (Mouse, monoclonal, 1:800,
cat. no. sc-398519) (both from Santa Cruz Biotechnology, Santa
Cruz, CA, USA), and anti-LIMK1 (Rabbit, Polyclonal, 1:1,000, cat.
no. 3842) antibodies, p-LIMK1 (Thr508) (Rabbit, Polyclonal,
1:1,000, cat. no. 3841) (both from Cell Signaling Technology), with
rabbit anti-β-actin (Mouse, monoclonal, 1:5,000; Bioworld, Nanjing,
China) were used as a loading control. Band intensities of the
western blotting were analyzed using Image Analysis Software v2.0
(Thermo Fisher Scientific Inc.).
RhoA activation assay
RhoA activity was assessed using a Rho Activation
Assay Biochem kit™ (Cytoskeleton, Inc., Denver, CO, USA) according
to the manufacturer's protocol.
Statistical analysis
All values were expressed as the mean ± SD of three
individual experiments. Statistical analyses were performed using
Student's t-test. When appropriate, the Mann-Whitney U-test was
used to compare the two groups. P<0.05 were considered to
indicate a statistically significant result. SPSS software was used
for statistical analyses.
Results
Expression of Trio in cervical cancer
biopsies and cell lines
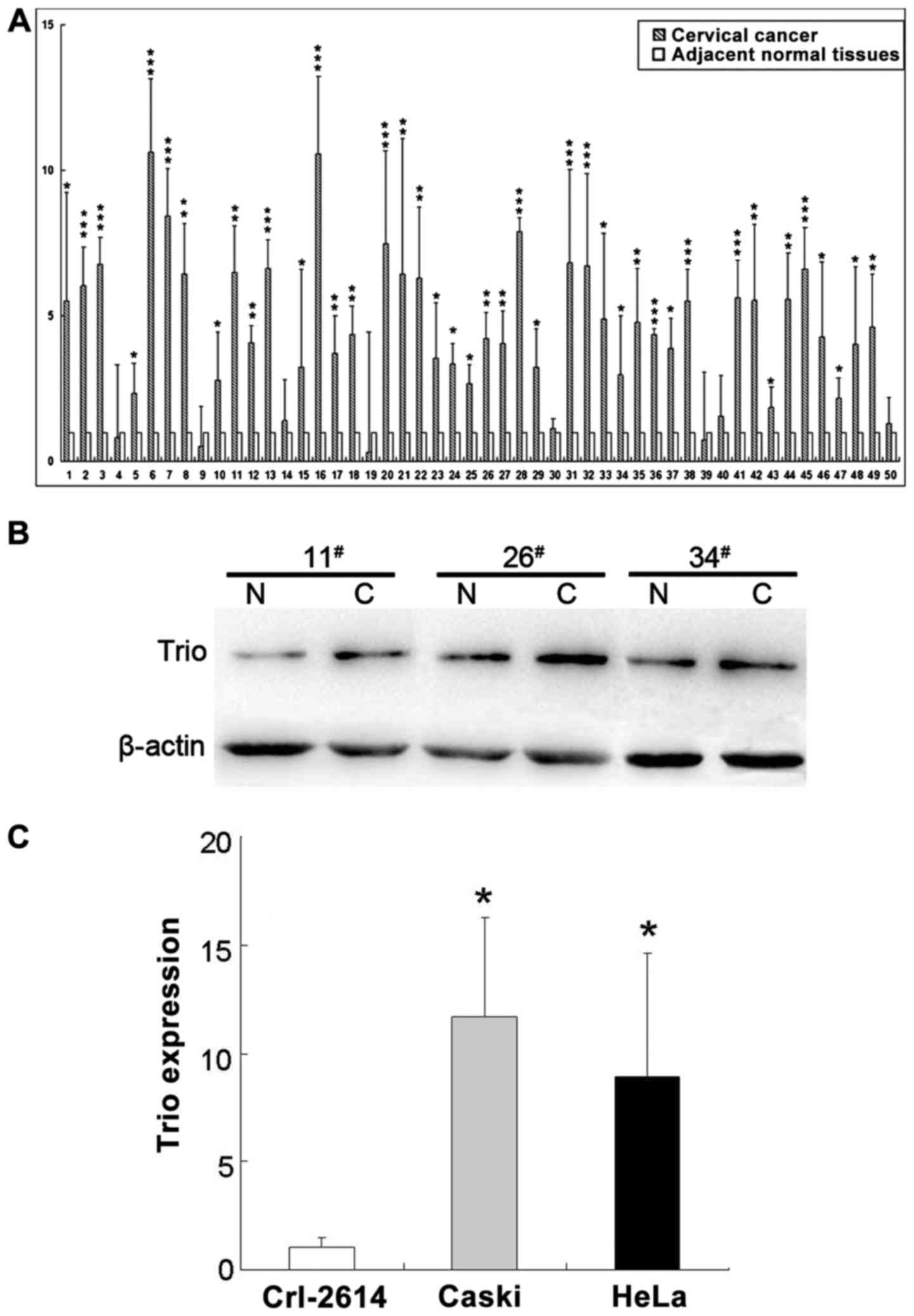
To ascertain Trio expression in cervical
cancer, we performed real-time PCR on 50 clinical specimens from
cervical cancer patients. The expression levels of Trio in
the tumor tissues for all 50 samples were significantly higher than
that in the matching adjacent tissue (Fig. 1A). Furthermore, we determined the
protein level of Trio in randomly selected cancer and adjacent
normal tissues (n=3). The protein level was also increased compared
to the normal tissues (Fig. 1B).
The expression of Trio was also determined in cervical
cancer cell lines (Caski and HeLa) and in a normal cervical cell
line (Crl-2614) (Fig. 1C), where
the results obtained from the two analyses were in agreement with
the data obtained from real-time PCR, indicating the significant
increase of Trio levels in cervical cancer tissues and cell
lines compared with normal cervical tissue and a cell line.
High expression of Trio in cervical
cancer is correlated with tumor metastasis
We analyzed the expression level of Trio to
determine its clinicopathological significance. Notably,
Trio levels were significantly increased in patients with
lymph node involvement (P=0.005) (Table II). Therefore, Trio
expression associates with lymph node metastasis and is a potential
diagnostic marker for cervical cancer.
 | Table II.The association between Trio
expression with clinicopathological parameters in 50 cervical
cancer patients. |
Table II.
The association between Trio
expression with clinicopathological parameters in 50 cervical
cancer patients.
| Variables | No. | Trio
relative transcript level (mean mRNA/GAPDH ± standard
deviation) | P-value |
|---|
| Age (years) |
|
| 0.156a |
|
≤50 | 18 | 0.0336±0.0309 |
|
|
>50 | 32 | 0.0360±0.0239 |
|
| Tumor size
(cm) |
|
| 0.856a |
| ≤4 | 37 | 0.0340±0.0249 |
|
|
>4 | 13 | 0.0368±0.0376 |
|
| FIGO staging |
|
| 0.983b |
| I | 31 | 0.0363±0.0330 |
|
| II | 10 | 0.0351±0.0235 |
|
|
III–IV | 9 | 0.0361±0.0257 |
|
| Histological
grade |
|
| 0.996b |
|
Well | 13 | 0.0365±0.0351 |
|
|
Moderately | 10 | 0.0365±0.0321 |
|
|
Poorly | 27 | 0.0370±0.0300 |
|
| Pelvic lymph node
metastasis |
|
| 0.005a |
| No | 34 | 0.0341±0.0348 |
|
|
Yes | 16 | 0.0423±0.0299 |
|
Inhibition of Trio expression by
CRISPR/Cas9
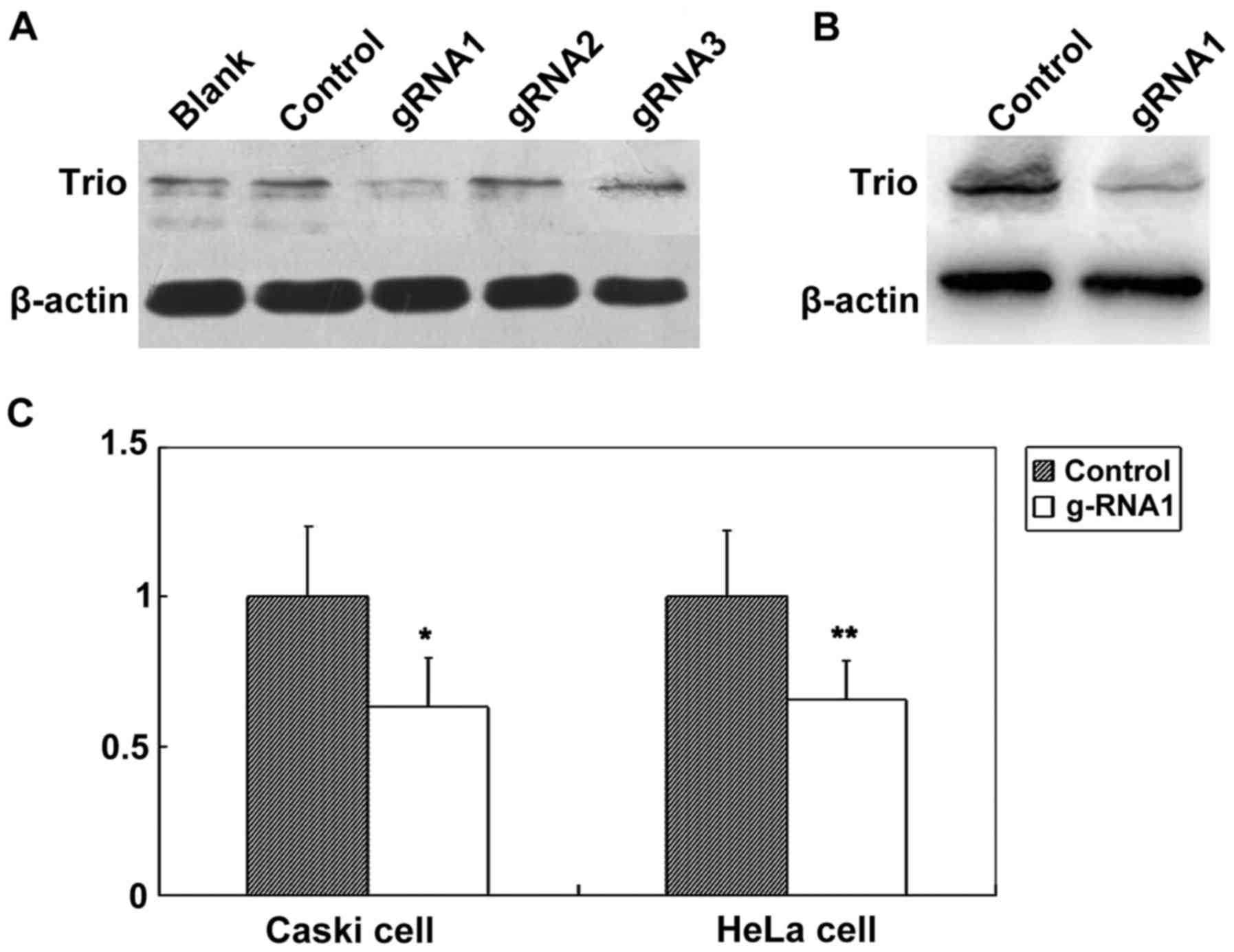
We used Caski cells to examine the effects of
Trio gene knockdown by CRISPR/Cas9. We transfected Caski
cells with the control, CRISPR+Cas9+gRNA empty,
CRISPR+Cas9+Trio-1, CRISPR+Cas9+Trio-2, and
CRISPR+Cas9+Trio-3 and cultured them for two days. Fig. 2A revealed the transfection results
as determined by western blotting, which confirmed the reduced
expression of Trio by CRISPR+Cas9-gRNA1 in comparison with
the control at 48 h. CRISPR+Cas9+Trio-1 exhibited effective
knockdown of Trio gene expression at 48 h (Fig. 2A and C). Significantly decreased
Trio expression was also demonstrated in the HeLa cell line
(Fig. 2B and C) as determined using
western blotting. Therefore, we obtained an effective gRNA for
Trio.
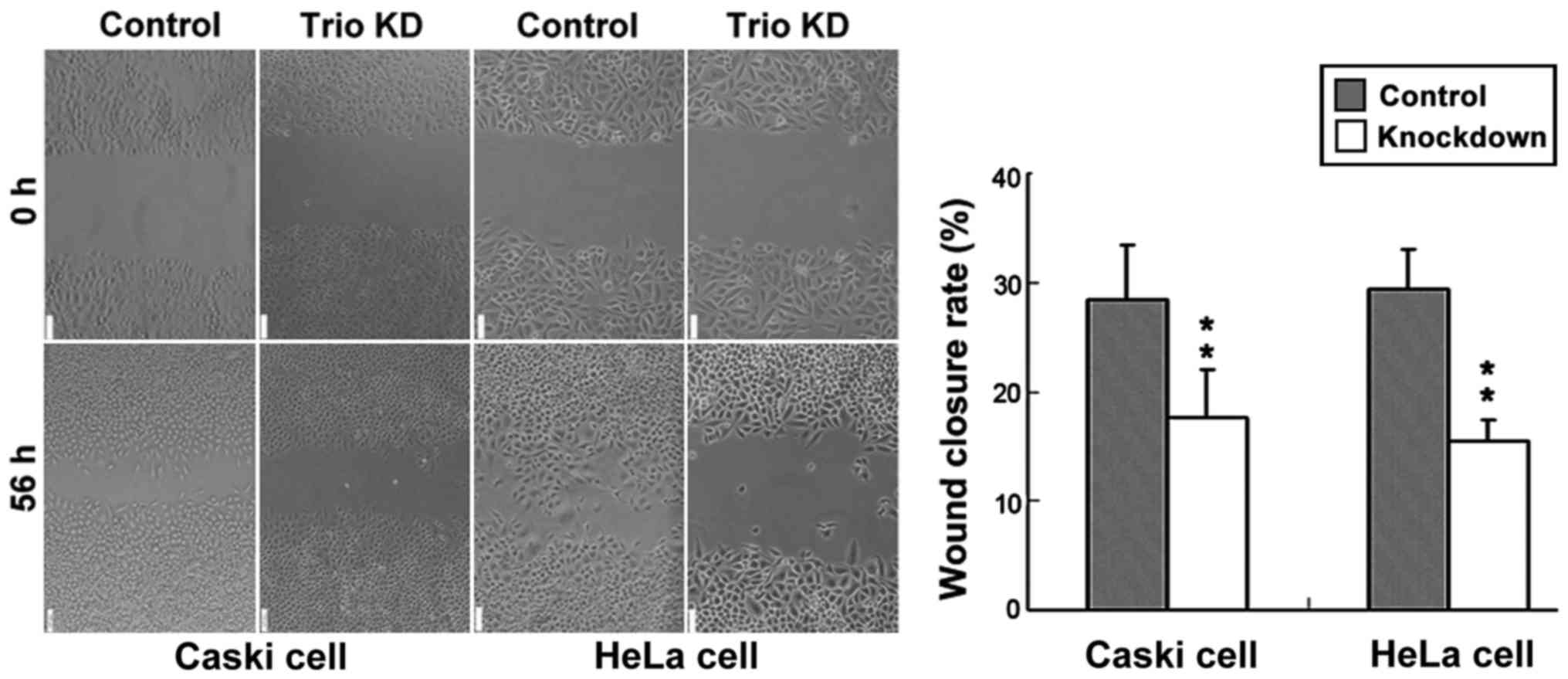
Knockdown of Trio by CRISPR/Cas9
inhibits cervical cancer cell migration and invasion
Migration assay through wound-healing revealed that
the migration ability of Caski and HeLa cells transfected with the
control was significantly higher in cervical cancer cells than that
of cells transfected with knocked down Trio-1 (Fig. 3).
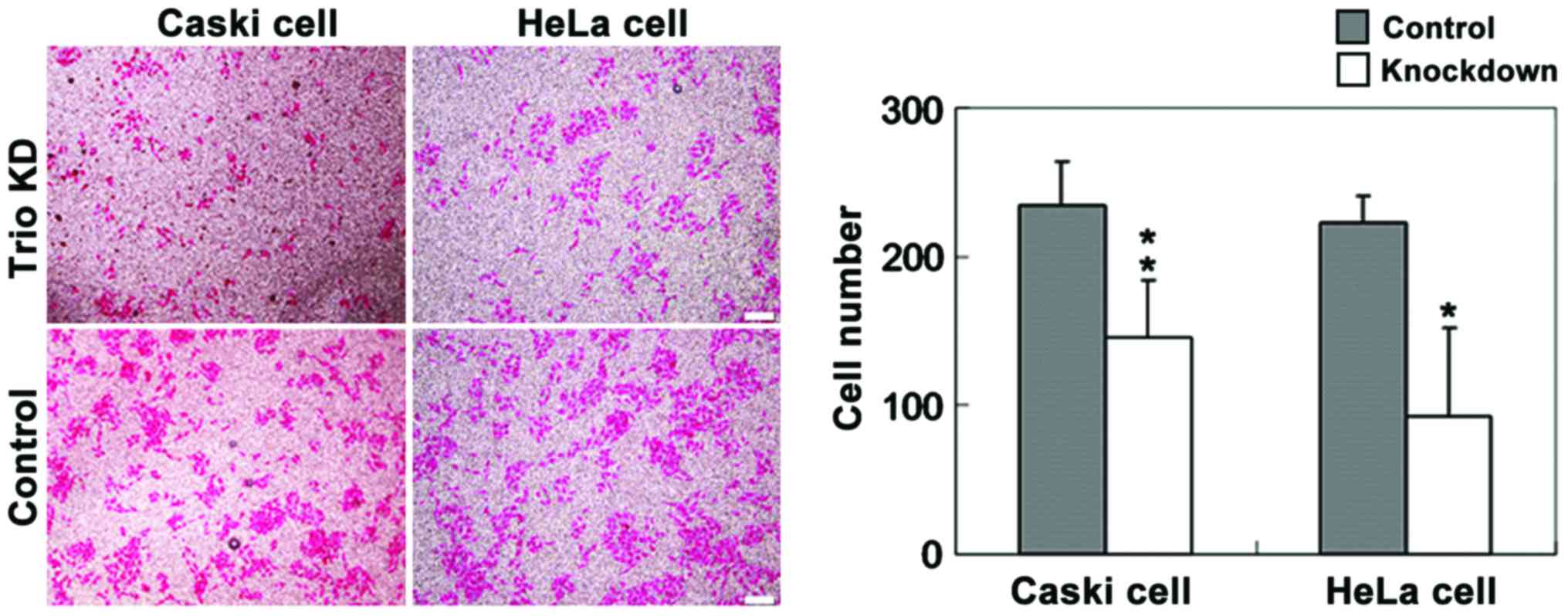
Invasion assay using the Transwell method revealed
that the invasiveness of Caski and HeLa cells transfected with
CRISPR+Cas9+Trio-1 was significantly lower than that of
cells transfected with the control (Fig. 4). Trio silencing inhibited
cervical cancer cell migration and invasion via inactivation of the
RhoA/Rock signaling pathway.
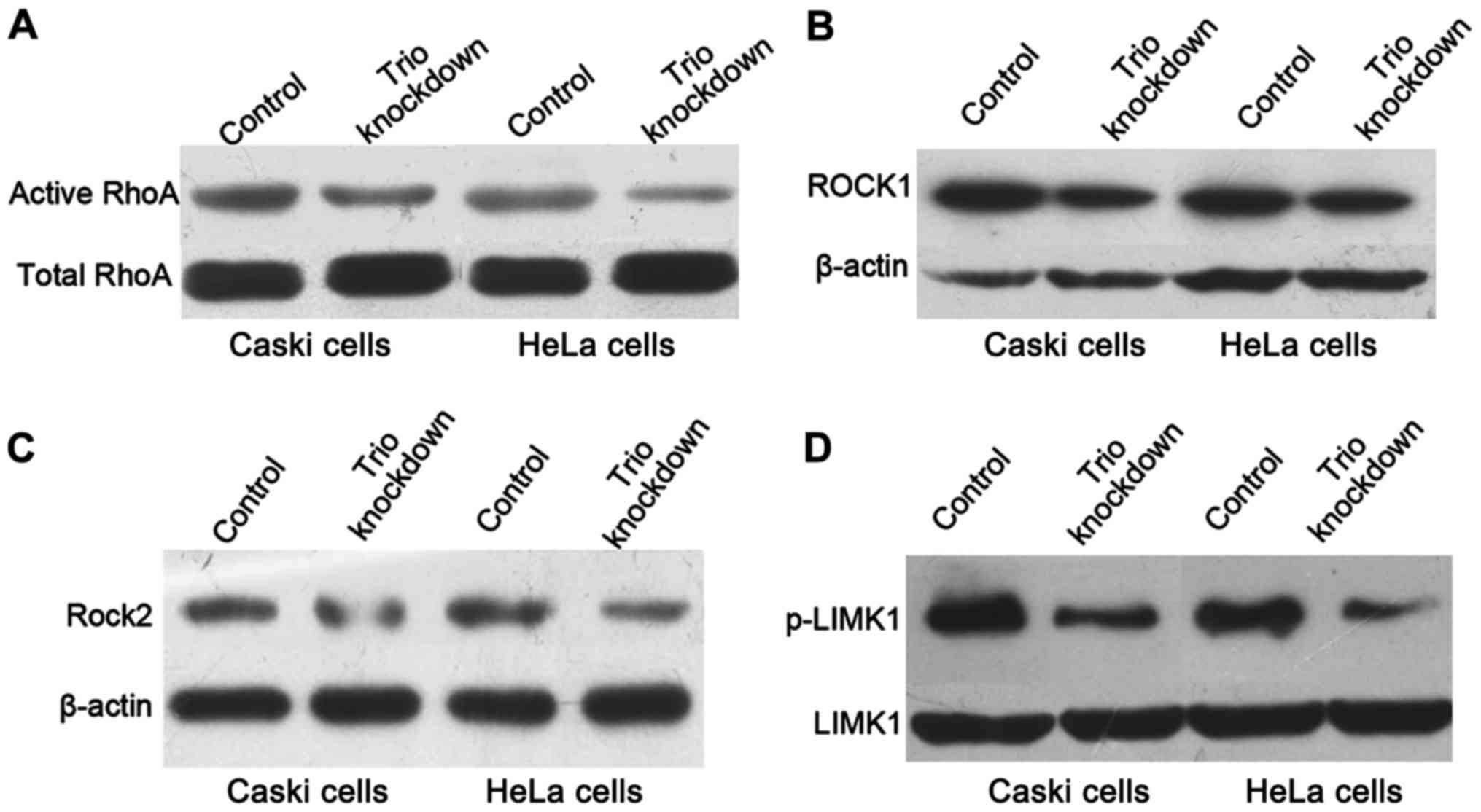
Rho-GTPase proteins participate in cell motility and
actin cytoskeleton reorganization (18). RhoA is a member of the Rho-GTPase
proteins and acts as a switch between the active GTP-bound form and
inactive GDP-bound form. The activated RhoA-GTPase is associated
with invasive cancers and tumor metastasis (30,31).
To determine whether the inactivation of the Rho-GTPase pathway
mediated the inhibitory effect of Trio on cervical cancer cell
migration and invasion, we examined the expression level of the
active RhoA-GTPase under Trio knockdown by western blotting. The
active form of GTP-bound RhoA was downregulated after Trio
decreased. We assessed the protein levels of ROCK1, ROCK2 and
p-LIMK1, a protein directly downstream of ROCK, after transient
transfection of CRISPR+Cas9+gRNA empty and
CRISPR+Cas9+Trio-1 for 48 h. Knockdown of Trio
significantly inhibited the protein expression of activated RhoA,
ROCK1, ROCK2 and p-LIMK1 compared with the control (Fig. 5), indicating that Trio may
mediate the metastatic ability of cervical cancer cells through
inactivation of the RhoA-GTPase pathway.
Discussion
In the present study, we first determined the mRNA
expression levels of Trio in 50 pairs of human cervical
cancer tissues and the matching adjacent tissues by qRT-PCR. Our
data indicated that Trio expression was higher in cervical
cancer tissues than that in adjacent tissues. Consistent with the
results of tissue analysis, the expression of Trio at the
RNA level was also increased in cervical cancer cell lines compared
with the normal cervical cells. Furthermore, the
clinicopathological parameters were evaluated to identify the
correlation between Trio expression and clinical
characteristics. The elevated expression of Trio was
significantly associated with lymph node metastasis in cervical
cancer patients. Our results revealed that the high expression of
Trio was related to lymph node metastasis and serves as a
potential diagnostic marker for cervical cancer.
Cell migration and invasion are key features for
metastatic dissemination of cancer cells and metastatic formation,
which are the leading causes of death in cancer patients (33). In the present study, we performed
scratch wound and Transwell invasion assays to examine the effect
of Trio expression on cervical carcinoma cell migration and
invasion, respectively, and our results revealed that Trio
knockdown significantly inhibited cervical cancer cell migration
and invasion.
Most cancer cells control their migratory and
invasive capabilities by actin cytoskeleton reorganization
(34). Rho-family small GTPases,
which are activated by guanine nucleotide exchange factors (GEFs),
are key regulators of cytoskeleton dynamics (35,36).
Trio is a member of the RhoGEFs family, and has two GEF domains,
one for RAC (GEF1) and the other (GEF2) for RHO (1,2). The
Rho/ROCK signaling pathway participates in tumor growth and
metastasis by regulating actin cytoskeleton reorganization
(37,38). Western blotting demonstrated that
Trio knockdown downregulated the activity of RhoA in Caski and HeLa
cells. Previous studies have demonstrated that ROCK kinase,
activated by RhoA, is a key regulator of intracellular signaling
pathways that contributes to cell migration and invasion. A high
expression of ROCK induces migration and invasion in several types
of tumor (39–42). In our study, the protein level of
ROCK1 and ROCK2 were also decreased during Trio knockdown. We also
examined the phosphorylation of LIM-kinase 1 (LIMK1), which is
activated by the small GTPase Rho and its downstream protein kinase
ROCK (42) and is important for the
regulation of actin cytoskeletal reorganization. In the present
study, the level of the p-LIMK1 expression was also downregulated
during Trio knockdown in human cervical cancer cells.
Invasion and migration are not the only signs of
tumor progression, but are also the major reasons behind failures
in clinical treatment and patient deaths. Our study revealed the
importance of Trio in the migration and invasion of cervical
cancer. Since all active forms of proteins were reduced in
Trio-deficient cells, the aberrant cell migration and invasion upon
Trio knockdown may be results of lower RhoA activity, and impaired
migration and invasion possibly arose from the drop in Rock and
p-LIMK1 activity. The Trio/RhoA/ROCK pathway regulates the
progression of cervical cancer metastasis, and blocking the Trio
signaling pathway could be a feasible treatment strategy in
inhibiting tumor invasion and migration. In conclusion, the present
study provided evidence that Trio expression was increased in
cervical cancer tissue samples, and was closely correlated with
lymph node metastasis. Our findings indicate that Trio is a
promising diagnostic marker for the identification of cervical
cancer individuals who are at high risk of lymph node
metastasis.
Acknowledgements
The present study was supported by grants from the
Seed Foundation of the Second Hospital of Shandong University
(grant no. 26010275618012), the Shandong Provincial Science and
Technology Key Program (2016GSF201158), and the Shandong Provincial
Natural Science Foundation of China (ZR2015PC020).
References
|
1
|
Schmidt S and Debant A: Function and
regulation of the Rho guanine nucleotide exchange factor Trio.
Small GTPases. 5:e297692014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellanger JM, Lazaro JB, Diriong S,
Fernandez A, Lamb N and Debant A: The two guanine nucleotide
exchange factor domains of Trio link the Rac1 and the RhoA pathways
in vivo. Oncogene. 16:147–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steven R, Kubiseski TJ, Zheng H, Kulkarni
S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T and Culotti J:
UNC-73 activates the Rac GTPase and is required for cell and growth
cone migrations in C. elegans. Cell. 92:785–795. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awasaki T, Saito M, Sone M, Suzuki E,
Sakai R, Ito K and Hama C: The Drosophila trio plays an essential
role in patterning of axons by regulating their directional
extension. Neuron. 26:119–131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bateman J, Shu H and Van Vactor D: The
guanine nucleotide exchange factor trio mediates axonal development
in the Drosophila embryo. Neuron. 26:93–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liebl EC, Forsthoefel DJ, Franco LS,
Sample SH, Hess JE, Cowger JA, Chandler MP, Shupert AM and Seeger
MA: Dosage-sensitive, reciprocal genetic interactions between the
Abl tyrosine kinase and the putative GEF trio reveal trio's role in
axon pathfinding. Neuron. 26:107–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newsome TP, Schmidt S, Dietzl G, Keleman
K, Asling B, Debant A and Dickson BJ: Trio combines with dock to
regulate Pak activity during photoreceptor axon pathfinding in
Drosophila. Cell. 101:283–294. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Brien SP, Seipel K, Medley QG, Bronson
R, Segal R and Streuli M: Skeletal muscle deformity and neuronal
disorder in Trio exchange factor-deficient mouse embryos. Proc Natl
Acad Sci USA. 97:pp. 12074–12078. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charrasse S, Comunale F, Fortier M,
Portales-Casamar E, Debant A and Gauthier-Rouvière C: M-cadherin
activates Rac1 GTPase through the Rho-GEF trio during myoblast
fusion. Mol Biol Cell. 18:1734–1743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
deBakker CD, Haney LB, Kinchen JM,
Grimsley C, Lu M, Klingele D, Hsu PK, Chou BK, Cheng LC, Blangy A,
et al: Phagocytosis of apoptotic cells is regulated by a
UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of
CED-12/ELMO. Curr Biol. 14:2208–2216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng M, Simon R, Mirlacher M, Maurer R,
Gasser T, Forster T, Diener PA, Mihatsch MJ, Sauter G and Schraml
P: TRIO amplification and abundant mRNA expression is associated
with invasive tumor growth and rapid tumor cell proliferation in
urinary bladder cancer. Am J Pathol. 165:63–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adamowicz M, Radlwimmer B, Rieker RJ,
Mertens D, Schwarzbach M, Schraml P, Benner A, Lichter P,
Mechtersheimer G and Joos S: Frequent amplifications and abundant
expression of Trio, NKD2, and IRX2 in soft tissue sarcomas. Genes
Chromosomes Cancer. 45:829–838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lane J, Martin TA, Mansel RE and Jiang WG:
The expression and prognostic value of the guanine nucleotide
exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast
cancer. Int Semin Surg Oncol. 5:232008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baldwin C, Garnis C, Zhang L, Rosin MP and
Lam WL: Multiple microalterations detected at high frequency in
oral cancer. Cancer Res. 65:7561–7567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chattopadhyay I, Singh A, Phukan R,
Purkayastha J, Kataki A, Mahanta J, Saxena S and Kapur S:
Genome-wide analysis of chromosomal alterations in patients with
esophageal squamous cell carcinoma exposed to tobacco and betel
quid from high-risk area in India. Mutat Res. 696:130–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coe BP, Henderson LJ, Garnis C, Tsao MS,
Gazdar AF, Minna J, Lam S, Macaulay C and Lam WL: High-resolution
chromosome arm 5p array CGH analysis of small cell lung carcinoma
cell lines. Genes Chromosomes Cancer. 42:308–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salhia B, Tran NL, Chan A, Wolf A, Nakada
M, Rutka F, Ennis M, McDonough WS, Berens ME, Symons M, et al: The
guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate
the invasive behavior of glioblastoma. Am J Pathol. 173:1828–1838.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang B, Fang J, Qu L, Cao Z, Zhou J and
Deng B: Upregulated TRIO expression correlates with a malignant
phenotype in human hepatocellular carcinoma. Tumour Biol.
36:6901–6908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sonoshita M, Itatani Y, Kakizaki F,
Sakimura K, Terashima T, Katsuyama Y, Sakai Y and Taketo MM:
Promotion of colorectal cancer invasion and metastasis through
activation of NOTCH-DAB1-ABL-RHOGEF protein TRIO. Cancer Discov.
5:198–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang PP, Sun BC, Zhao JH, Chen KX, Lou YL
and Hao X: Changes in incidence rates of cervical cancer in a
geographically defined Chinese population and their implications in
screening and education programs. Ann Epidemiol. 15:630–631. 2005.
View Article : Google Scholar
|
|
22
|
Bray F, Loos AH, McCarron P, Weiderpass E,
Arbyn M, Møller H, Hakama M and Parkin DM: Trends in cervical
squamous cell carcinoma incidence in 13 European countries:
Changing risk and the effects of screening. Cancer Epidemiol
Biomarkers Prev. 14:677–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ward KK, Shah NR, Saenz CC, McHale MT,
Alvarez EA and Plaxe SC: Changing demographics of cervical cancer
in the United States (1973Y2008). Gynecol Oncol. 126:330–333. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu JT, Abdullah NA, Chou HH, Lin CT, Jung
SM, Wang CC, Chen MY, Huang KG, Chang TC and Lai CH: Outcomes and
prognosis of patients with recurrent cervical cancer after radical
hysterectomy. Gynecol Oncol. 127:472–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tong YQ, Liu B, Zheng HY, He YJ, Gu J, Li
F and Li Y: Overexpression of BMI-1 is associated with poor
prognosis in cervical cancer. Asia Pac J Clin Oncol. 8:e55–e62.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng X, Wu Z, Yu L, Li J, Xu W, Chan HC,
Zhang Y and Hu L: Overexpression of cystic fibrosis transmembrane
conductance regulator (CFTR) is associated with human cervical
cancer malignancy, progression and prognosis. Gynecol Oncol.
125:470–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cibula D, Abu-Rustum NR, Dusek L, Slama J,
Zikán M, Zaal A, Sevcik L, Kenter G, Querleu D, Jach R, et al:
Bilateral ultrastaging of sentinel lymph node in cervical cancer:
Lowering the false-negative rate and improving the detection of
micrometastasis. Gynecol Oncol. 127:462–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi EJ, Kim MS, Yoo NJ and Lee SH: TRIO
gene encoding Trio Rho guanine nucleotide exchange factor harbors
frameshift mutations of in gastric and colorectal cancers. Pathol
Oncol Res. Feb 21–2017.(Epub ahead of print). View Article : Google Scholar
|
|
29
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma DM, Xu YP and Zhu L: Expression of
vascular endothelial growth factor C correlates with a poor
prognosis based on analysis of prognostic factors in patients with
cervical carcinomas. J Obstet Gynaecol Res. 37:1519–1524. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen MX and Ding JB: Expression levels and
roles of EMC-6, Beclin1, and Rab5a in the cervical cancer. Eur Rev
Med Pharmacol Sci. 21:3038–3046. 2017.PubMed/NCBI
|
|
32
|
Song Z, Zhang X, Ye X, Feng C, Yang G, Lu
Y, Lin Y and Dong C: High expression of stromal cell-derived factor
1 (SDF-1) and NF-κB predicts poor prognosis in cervical cancer. Med
Sci Monit. 23:151–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
36
|
Ridley AJ: Rho family proteins:
Coordinating cell responses. Trends Cell Biol. 11:471–477. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsui T, Amano M, Yamamoto T, Chihara K,
Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A and Kaibuchi K:
Rho-associated kinase, a novel serine/threonine kinase, as a
putative target for small GTP binding protein Rho. EMBO J.
15:2208–2216. 1996.PubMed/NCBI
|
|
38
|
Kosako H, Yoshida T, Matsumura F, Ishizaki
T, Narumiya S and Inagaki M: Rho-kinase/ROCK is involved in
cytokinesis through the phosphorylation of myosin light chain and
not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene.
19:6059–6064. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bourguignon LY, Zhu H, Shao L, Zhu D and
Chen YW: Rho-kinase (ROK) promotes CD44v (3,8–10)-ankyrin
interaction and tumor cell migration in metastatic breast cancer
cells. Cell Motil Cytoskeleton. 43:269–287. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Itoh K, Yoshioka K, Akedo H, Uehata M,
Ishizaki T and Narumiya S: An essential part for Rho-associated
kinase in the transcellular invasion of tumor cells. Nat Med.
5:221–225. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li B, Zhao WD, Tan ZM, Fang WG, Zhu L and
Chen YH: Involvement of Rho/ROCK signalling in small cell lung
cancer migration through human brain microvascular endothelial
cells. FEBS Lett. 580:4252–4260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maekawa M, Ishizaki T, Boku S, Watanabe N,
Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K and Narumiya S:
Signaling from Rho to the actin cytoskeleton through protein
kinases ROCK and LIM-kinase. Science. 285:895–898. 1999. View Article : Google Scholar : PubMed/NCBI
|