Introduction
Hepatocellular carcinoma (HCC) and hepatoblastoma
(HB) are two common hepatic malignant tumors (HMTs) that typically
occur in adults and children, respectively. HCC originates from
hepatocytes, while the origin of HB is more complex, as it arises
from primary hepatoblasts (1). HCC
is the most common type of HMT, and results in 10,000 deaths
worldwide every year, most of which occur in Asian countries
(2). Additionally, the mortality
rate of HCC ranks second among all primary cancer-related
mortalities, and as many as 90% of these deaths are related to
metastasis (3,4). Viral infection and chronic
inflammatory liver diseases are common risk factors for HCC
(5). Accurate diagnosis and medical
advice in cases of HCC are frequently delayed due to the lack of
obvious symptoms in the early stages (6). At present, the methods of diagnosis
and treatment for HCC are numerous, but the short survival times of
HCC patients indicate the poor prognosis associated with this
disease.
Sigma (σ) receptors were initially described by
Martin et al (7) in 1976 as
subtypes of opioid receptors, and have since been reported to have
high affinity for many antipsychotic drugs. Pharmacological studies
have indicated that there are at least two σ receptor subtypes, of
which only the σ1 receptor (σ1R) has been cloned. The cloned σ1R
has 223 amino acids and shares 30% identity and 67% similarity with
a yeast sterol C8-C7 isomerase (8,9). The
σ1R gene encodes a 25.3-kDa protein with two putative transmembrane
segments, although it remains unclear whether the N and C termini
are cytoplasmic or extracytoplasmic (10,11).
Pharmacological studies have indicated that σ1R
binds to a wide range of compounds, including opiates,
antipsychotics and neurosteroids. Pentazocine and SKF10047 are two
selective agonists for σ1R (12).
However, endogenous ligands for σ receptors have not yet been
defined. The function of σ1R has been explored by studying its
interaction with its ligands. Brent and Pang revealed that certain
σ1R ligands were potent inhibitors of cell proliferation in human
mammary adenocarcinoma, colon carcinoma and melanoma (13). Meanwhile, the σ1R ligand SKF10047
was found to be effective at modulating cell proliferation in the
metastatic breast cancer cell line MDA-MB-231 (14).
Previous studies have found that σ1R is highly
expressed in various cancer tissues, including those of neural and
non-neural origins (15).
Additionally, the upregulation of σ1R was reported to correlate
with the biological behavior of tumors, such as proliferation,
adhesion and cell death (14,16).
Our previous study showed that σ1R was overexpressed in human
esophageal squamous cell carcinoma and that the overexpression of
σ1R was significantly associated with the pathological TNM
classification and lymph node metastasis (18). However, the expression of σ1R and
its biological relevance in HCC have not yet been identified.
In the present study, we first evaluated the
expression status of σ1R in HCC, showing that σ1R expression was
decreased with the progression of HCC and was significantly
correlated with tumor grade. We then increased σ1R expression in
HepG2 cells using a FLAG-SV40-neomycin-plasmid strategy, which
indicated that σ1R upregulation impaired cell proliferation and
induced cell cycle arrest and cell apoptosis in HepG2 cells.
Furthermore, immunoblotting analysis revealed that multiple
migration-associated pathways were regulated by σ1R upregulation,
providing valuable evidence of σ1R-associated mechanisms in HMT
development and progression. Taken together, the present findings
strongly suggest a causal relationship between σ1R expression and
HMT development, indicating that σ1R may serve as a potential
target in research on the pathogenesis and prognosis of HMT.
Materials and methods
Materials and cell lines
HepG2 and SMCC-7721 cell lines, which originate from
primary hepatoblasts (19) and
mature hepatocytes, respectively, were used in the present study.
The human HB cell line HepG2 and the HCC cell line SMCC-7721 were
obtained from GeneChem Co. Ltd. (Shanghai, China) and cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf
serum (FCS), in an atmosphere of 5% CO2 at 37°C.
Polyclonal anti-σ1R (AP2747A; Abgent, San Diego, CA, USA),
anti-NF-κB (8242S; Cell Signaling Technology, Inc., Danvers, MA,
USA), anti-STAT-3 (Ab68153; Abcam, Cambridge, UK), and anti-β-actin
(SAB5500001; Sigma-Aldrich® Co. LLC, St. Louis, MO, USA)
antibodies were used for immunohistochemistry or western blotting.
The peroxidase-conjugated anti-rabbit secondary antibody was
purchased from Santa Cruz Biotechnology, Inc. (sc-2004; Santa Cruz,
CA, USA).
Specimen collection
For the retrospective analysis, archived
formalin-fixed, paraffin-embedded human hepatic tissues from 40
patients were obtained from the Department of Pathology of Jining
First People's Hospital between 2012 and 2014. The 40 samples
included 30 specimens of HCC and 10 specimens of hepatic cavernous
hemangioma (HCH). The HCC patients comprised 26 men and 4 women
(median age, 59 years). Information regarding sex, age, stage of
disease, and histopathological parameters was retrieved from the
medical records, and the patient data are summarized in Table I. All the tumors were confirmed as
HCC by the pathologists at the Clinical Pathology Department of
Jining First People's Hospital. In addition, 26 samples of
cirrhotic liver tissue, from ≥2 cm away from the tumor edge, were
obtained from the 30 HCC patients to represent precancerous
lesions. The evaluation of tumor differentiation was based on
histological criteria of the WHO Pathological Classification of
Tumors guidelines. The study was approved by the Ethics Committee
of the Central Hospital of Jining, the local ethics committee, and
only patients who had provided written informed consent were
included.
 | Table I.Clinical parameters of the HCC
patients. |
Table I.
Clinical parameters of the HCC
patients.
| Clinical
parameters | No. |
|---|
| Age (years) |
|
|
≤59 | 15 |
|
>59 | 14 |
| Sex |
|
|
Male | 25 |
|
Female | 4 |
| Tumor size
(cm) |
|
| ≤3 | 10 |
|
3–5 | 8 |
|
>5 | 11 |
|
Differentiation |
|
| G1 | 10 |
| G2 | 10 |
| G3 | 9 |
| AFP (ng/ml) |
|
|
≤20 | 13 |
|
>20 | 16 |
| Hepatitis B surface
antigen |
|
|
Negative | 7 |
|
Positive | 22 |
| Microvascular
invasion |
|
|
Absent | 17 |
|
Present | 12 |
| Tumor number |
|
|
Single | 27 |
|
Multiple | 2 |
Immunohistochemical staining
The sections were dewaxed in xylene and rehydrated
in a series of graded alcohols. Subsequently, the slides were
submerged for 10 min in a peroxidase quenching solution containing
one part 30% hydrogen peroxide to 9 parts absolute methanol. After
rinsing in PBS, antigen retrieval was carried out by autoclaving
the tissue in 0.01 M sodium citrate buffer (pH 6.0) at 120°C for 3
min. Following autoclaving, the sections were blocked in 10% normal
goat serum for 10 min at room temperature, and then incubated
overnight at 4°C with an anti-σ1R polyclonal antibody (1:400;
Abgent). Subsequently, the sections were subjected to staining with
a 2-Step Plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System
(PV-9000; ZSGB-BIO, Beijing, China) and a Liquid DAB substrate kit
(Invitrogen, Shanghai, China). Samples were rinsed well with
distilled water, then counterstained with Mayer's hematoxylin,
dehydrated and mounted.
The immunohistochemical staining results were
assigned a mean score considering both the intensity of staining
and the proportion of tumor cells showing unequivocal positive
reactivity. Each section was independently assessed by two
histopathologists without prior knowledge of the patient data.
Positive reactions were defined as the presence of brown staining
in the cell cytoplasm, nucleus and membrane. For σ1R, a staining
index (values 0–12) was determined by multiplying the score for
staining intensity (0, no staining; 1, weak staining; 2, moderate
staining; and 3, strong staining) by the score for the positive
stained area (1, positive staining in 0–25% of tumor cells; 2,
positive staining in >25-50% of tumor cells; 3, positive
staining in 51–75% of tumor cells; 4, positive staining in
>75-100% of tumor cells). For the purpose of statistical
analyses, scores of 0–4 were considered low expression (−), and
scores of 5–12 were considered high expression (+).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted and purified from the HepG2
and SMCC-7721 cells using TRIzol reagent (Invitrogen) according to
the manufacturer's instructions. RT was performed using a RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) to obtain cDNA. The expression level of σ1R mRNA
was measured by RT-qPCR. Primers used were as follows: σ1R,
5′-ACCATCATCTCTGGCACCTTCC-3′ (forward), and
5′-GCCAAAGAGGTAGGTGGTGAGC-3′ (reverse); β-actin,
5′-CACCCAGCACAATGAAGATCAAGAT-3′ (forward), and
5′-CCAGTTTTTAAATCCTGAGTCAAGC-3′ (reverse). PCR amplification
consisted of 40 cycles (95°C for 15 sec and 60°C for 60 sec), after
an initial denaturation step (95°C for 10 min). The relative
expression of σ1R was normalized to β-actin mRNA level using the
2−ΔΔCq method. All samples were examined in
triplicate.
Construction of expression vectors and
cell transfection
To achieve σ1R overexpression, Lentivector
Expression Systems (GeneChem, Shanghai, China) were obtained to
construct lentiviruses encoding σ1R, which were then transfected
into HepG2 cells. The primers for the σ1R gene were as follows:
forward, 5′-ACGGGCCCTCTAGACTCGAGCGCCACCATGCAGTGGGCCGTGGGCCG-3′; and
reverse, 5′-AGTCACTTAAGCTTGGTACCGAAGGGTCCTGGCCAAAGAGGTAGG-3′. The
designed DNA sequence was inserted into the lentivirus-based GV141
vector (GeneChem) with XhoI/KpnI sites. An empty
vector was used as a negative control (NC). For transfection, HepG2
cells were plated into 6-well plates at 2×105 cells per
well. After 24 h, cells were transfected with the lentivirus
expressing σ1R or the NC lentivirus, and then cultured in a 5%
CO2 incubator at 37°C for another 3 days. Cells were
then harvested and total proteins were extracted to determine the
overexpression efficiency by western blotting.
Protein extraction and immunoblotting
analysis
The total cellular protein was extracted from cells
using 2X RIPA buffer containing protease inhibitors. The protein
concentration was estimated using the Pierce 660nm protein assay
(Thermo Fisher Scientific, Inc.). Equal amounts of tissue lysates
(50 µg) were electrophoresed on 12% polyacrylamide gels at 40 V for
30 min, followed by 60 V for 3 h. The lysates were then transferred
to polyvinylidene fluoride (PVDF) membranes (EMD Millipore,
Bedford, MA, USA). Subsequently, the membranes were blocked in 5%
skim milk in PBS-Tween (0.01 M PBS, 0.05% Tween-20) for 1 h at room
temperature, followed by the addition of the primary antibody
(anti-STAT-3 dilution, 1:1,000; anti-NF-κB dilution, 1:1,000 and
anti-β-actin dilution, 1:2,000, respectively) for 2 h at room
temperature. After that, the membranes were washed and incubated
with peroxidase-conjugated anti-rabbit secondary antibodies
(dilution, 1:2,000) and analyzed using a Pierce™ ECL Western
Blotting Substrate kit (Thermo Fisher Scientific, Inc.). β-actin
was examined as an internal control in this experiment (dilution,
1:2,000).
MTT assay
Cell viability in the σ1R-overexpressing and NC
groups was assessed with
3-(4,5-dimethylthiazol-2-yl)-diphenyltetrazolium bromide (MTT),
which is converted to a colored, soluble formazan product in
metabolically active cells. HepG2 cells (5×103) were
seeded into 96-well plates and incubated for 48 h, after which 10
µl of sterile MTT (5 mg/ml in PBS, pH=7.4) was added to each well
and incubated for 4 h. The medium was then removed from the wells
and replaced with 150 µl dimethyl sulfoxide (DMSO) (Amresco Inc.,
Solon, OH, USA)/well, and the absorbance at 490 nm was measured
within 10 min of DMSO addition. Each experiment was conducted in
triplicate.
Cell migration assay
Cell migration was determined by a Transwell
migration assay. Cells (5×104) were seeded into the top
chambers of 24-well Transwell chambers with 8-µm micropore membrane
filters (Corning Inc., Corning, NY, USA), and the bottom chambers
were filled with 0.5 ml DMEM/F-12 medium with 20% FBS as a
chemoattractant. After 24 h, the non-invaded cells on the upper
surface were carefully removed with a cotton swab, and the
membranes were fixed and stained with crystal violet reagent.
Migration was quantified by counting 3 random fields under a light
microscope (magnification, ×200). Data obtained from 3 separate
chambers were presented as mean values.
Cell cycle analysis
Flow cytometry was used to determine the cell cycle
distribution and to detect apoptosis, and was performed as
previously described (19).
Initially, HepG2 cells (2×105) were transfected with
GV141-σ1R or NC plasmids and incubated at 37°C for 4 days.
Harvested cells were fixed with 75% cold ethanol at 4°C overnight,
washed twice with ice-cold PBS, incubated with 1 mg/ml RNase at
37°C for 40 min, and stained with propidium iodide (PI; 100 µg/ml;
GeneChem). The fluorescence of DNA-bound PI in cells was measured
with a flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA,
USA) and the cell populations in different phases of the cell cycle
were analyzed with ModFit 3.0 software (Verity Software House,
Inc., Topsham, ME, USA). Each experiment was performed in
triplicate.
Annexin V-FITC apoptosis assay
An Annexin V-FITC apoptosis detection kit (KeyGen
Biotech, Nanjing, China) was used for the labeling of apoptotic
cells, according to the manufacturer's protocol. HepG2 cells
(2×105) were transfected with σ1R-expressing or NC
lentiviruses. After incubation for 4 days, the cells were
harvested, washed with PBS buffer, and resuspended in 200 µl
binding buffer. Then, 5 µl Annexin V-FITC was added into the cell
suspension and incubated at room temperature for 15 min. The cell
cycle was monitored using PI (50 µg/ml; Sigma-Aldrich Co. LLC)
staining of nuclei. Signals were detected with a FACSCalibur flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA). All experiments
were performed in triplicate.
Statistical analysis
Data are expressed as the mean ± standard deviation
of 3 independent experiments. Differences were compared using a
Student's t-test. Associations between σ1R expression and
clinicopathological characteristics were assessed with the
Kendall's τ-b test. All statistical analyses were performed with
SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Each P-value is
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference.
Results
σ1R is decreased in HCC tissues
Immunohistochemical staining was used to examine the
expression patterns of σ1R in tissue samples from 30 HCC patients,
as well as benign liver tissues from 10 HCH patients. From the 30
HCC patients, 29 samples of malignant tumor tissue (after 1 sample
was excluded due to extensive necrosis preventing scoring) as well
as 26 samples of cirrhotic tissue, representing precancerous
lesions, were evaluated. As shown in Fig. 1A, in benign hepatic tissues, the
positive signals were intense and well distributed in the
cytoplasm, whereas weak to moderately positive signals were
observed in precancerous lesions, and weak or negative staining was
observed in the tumor tissues. The rates of σ1R+
expression in benign hepatic tissue, hepatic cirrhosis and HCC
specimens were 90 (9/10), 65.38 (17/26) and 27.59% (8/29),
respectively. The difference was statistically significant between
benign hepatic tissues and HCC (P<0.01) (Fig. 1B). Therefore, we concluded that σ1R
is downregulated in HCC tissues.
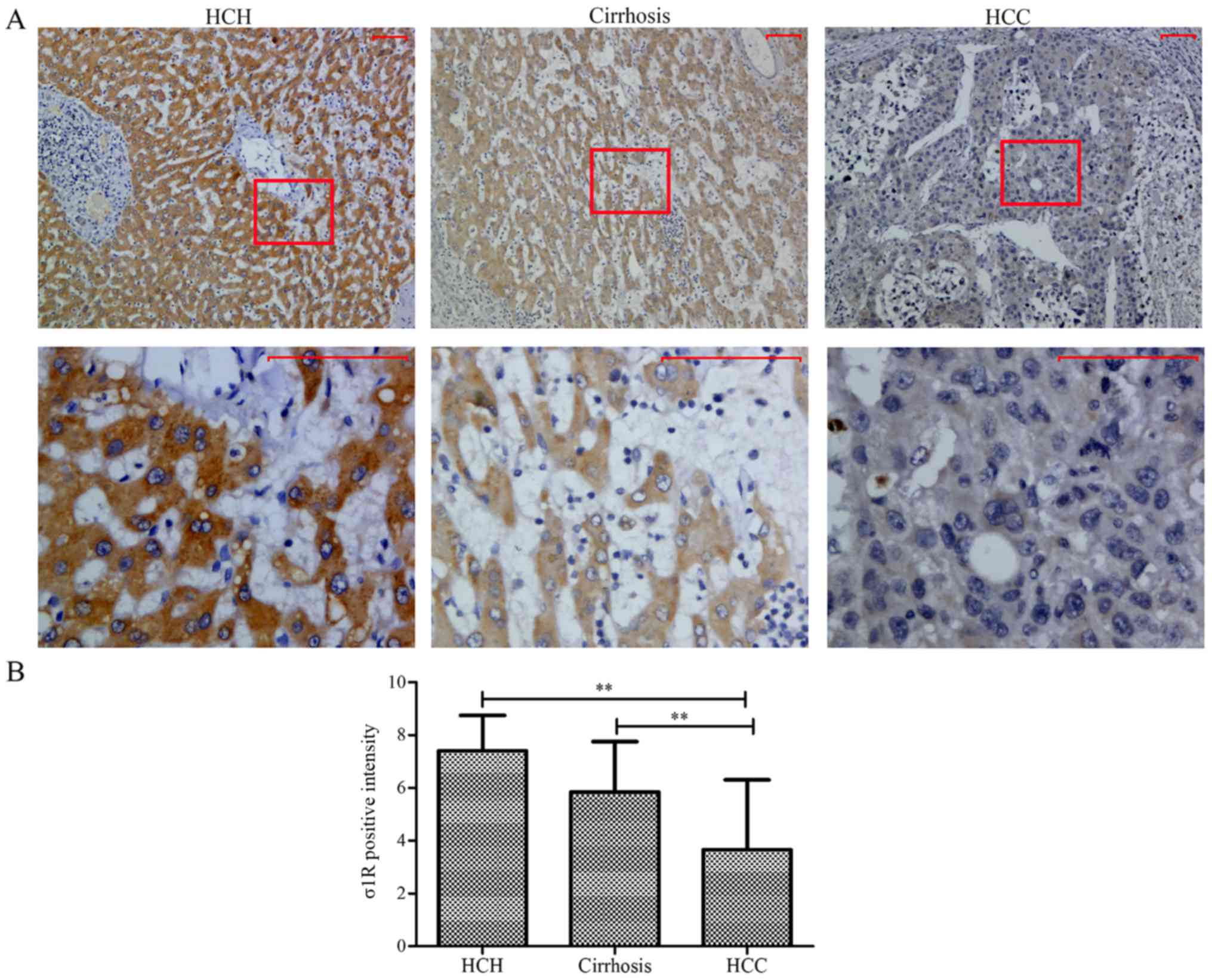 | Figure 1.Immunohistochemical analysis of σ1R in
benign hepatic tissue of HCH, cirrhotic cancer-adjacent liver
tissues, and malignant HCC tissues. (A) σ1R expression in the
progression from benign hepatic tissue to HCC. The staining was
mainly located in the cytoplasm. In HCH tissues, the immunostaining
of σ1R was intense and well-distributed compared with that in
hepatic cirrhosis. Weak or negative staining was observed in HCC.
Scale bars, 50 µm. (B) Rates of positive σ1R expression in HCH,
hepatic cirrhosis and HCC. Statistically significant differences
were observed between HCH and HCC (**P<0.01), and between
hepatic cirrhosis and HCC (**P<0.01), respectively. σ1R, sigma-1
receptor; HCC, hepatocellular carcinoma; NCH, hepatic cavernous
hemangioma. |
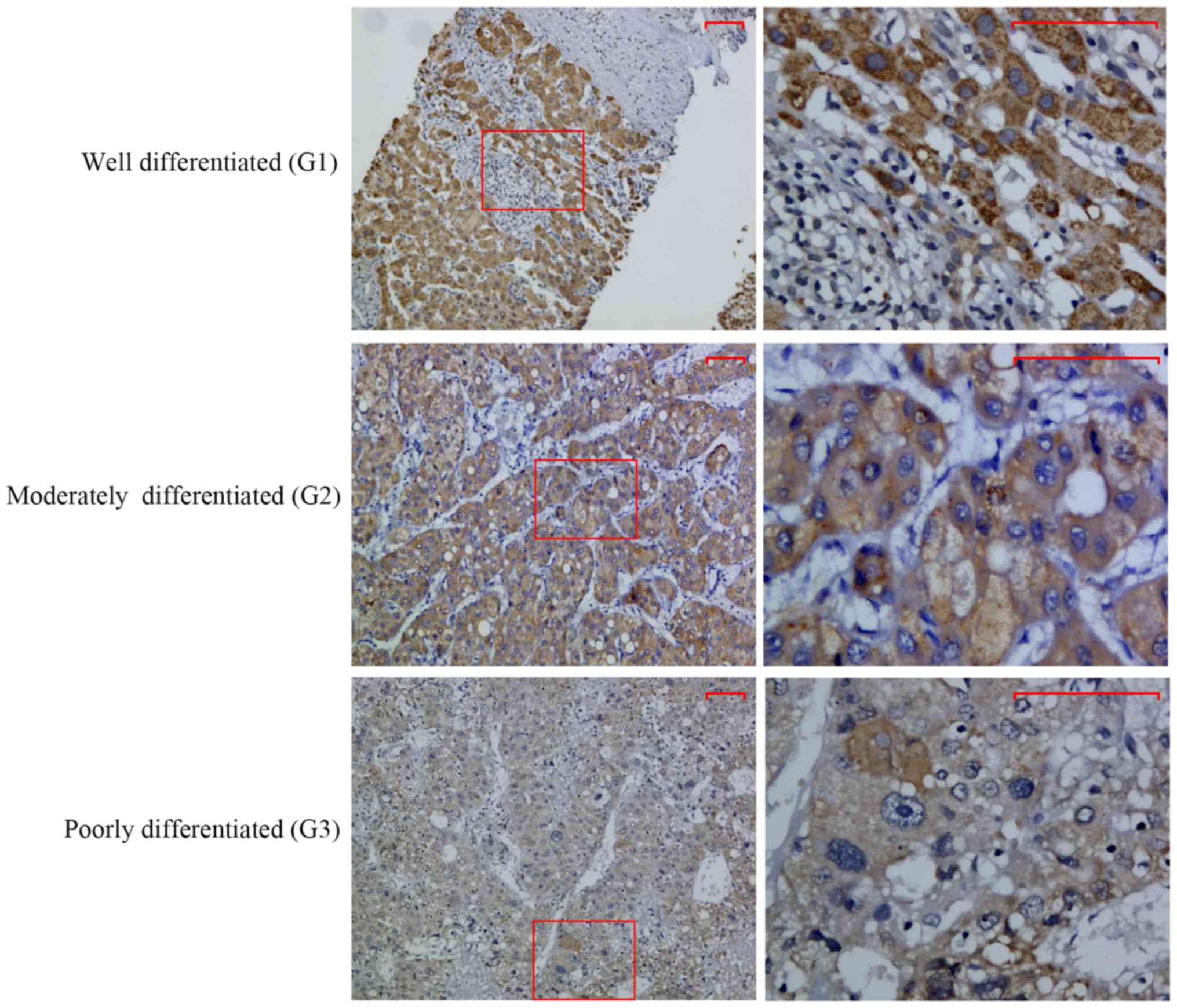
σ1R expression is positively
correlated with HCC grade
Immunohistochemical staining was used to explore the
expression of σ1R in 30 cases of HCC. σ1R immunoreactivity, which
mainly showed a cytoplasmic staining pattern, was analyzed with
respect to various clinicopathological parameters in these cases
(Table II). A significant
correlation was found between σ1R level and the degree of
histological differentiation of the tumors (r=−0.424, P=0.021).
Notably, intense staining was more frequent in well-differentiated
cases of HCC, and σ1R+ expression was observed in 50% of
grade I cases and 15.8% of grade II/III cases (Fig. 2). There were no significant
correlations between σ1R expression and other clinical
parameters.
 | Table II.Association between σ1R expression
and clinical pathological parameters in HCC. |
Table II.
Association between σ1R expression
and clinical pathological parameters in HCC.
|
| σ1R
statusa |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | − | + | rb |
P-valuec |
|---|
| Age (years) |
|
|
|
|
|
≤59 | 12 | 3 | 0.176 | 0.427 |
|
>59 | 9 | 5 |
|
|
| Sex |
|
|
|
|
|
Male | 19 | 6 | 0.201 | 0.552 |
|
Female | 2 | 2 |
|
|
| Tumor size
(cm) |
|
|
|
|
| ≤3 | 7 | 3 | −0.199 | 0.256 |
|
3–5 | 4 | 4 |
|
|
|
>5 | 10 | 1 |
|
|
| Histological
differentiation |
|
|
|
|
| G1 | 5 | 5 | −0.424 | 0.021 |
| G2 | 7 | 3 |
|
|
| G3 | 9 | 0 |
|
|
| AFP (ng/ml) |
|
|
|
|
|
≤20 | 8 | 5 | −0.219 | 0.406 |
|
>20 | 13 | 3 |
|
|
| Hepatitis B surface
antigen |
|
|
|
|
|
Negative | 5 | 2 | 0.012 | 1.000 |
|
Positive | 16 | 6 |
|
|
| Microvascular
invasion |
|
|
|
|
|
Absent | 10 | 7 | 0.362 | 0.093 |
|
Present | 11 | 1 |
|
|
| Tumor number |
|
|
|
|
|
Single | 19 | 8 | −0.168 | 0.586 |
|
Mutiple | 2 | 0 |
|
|
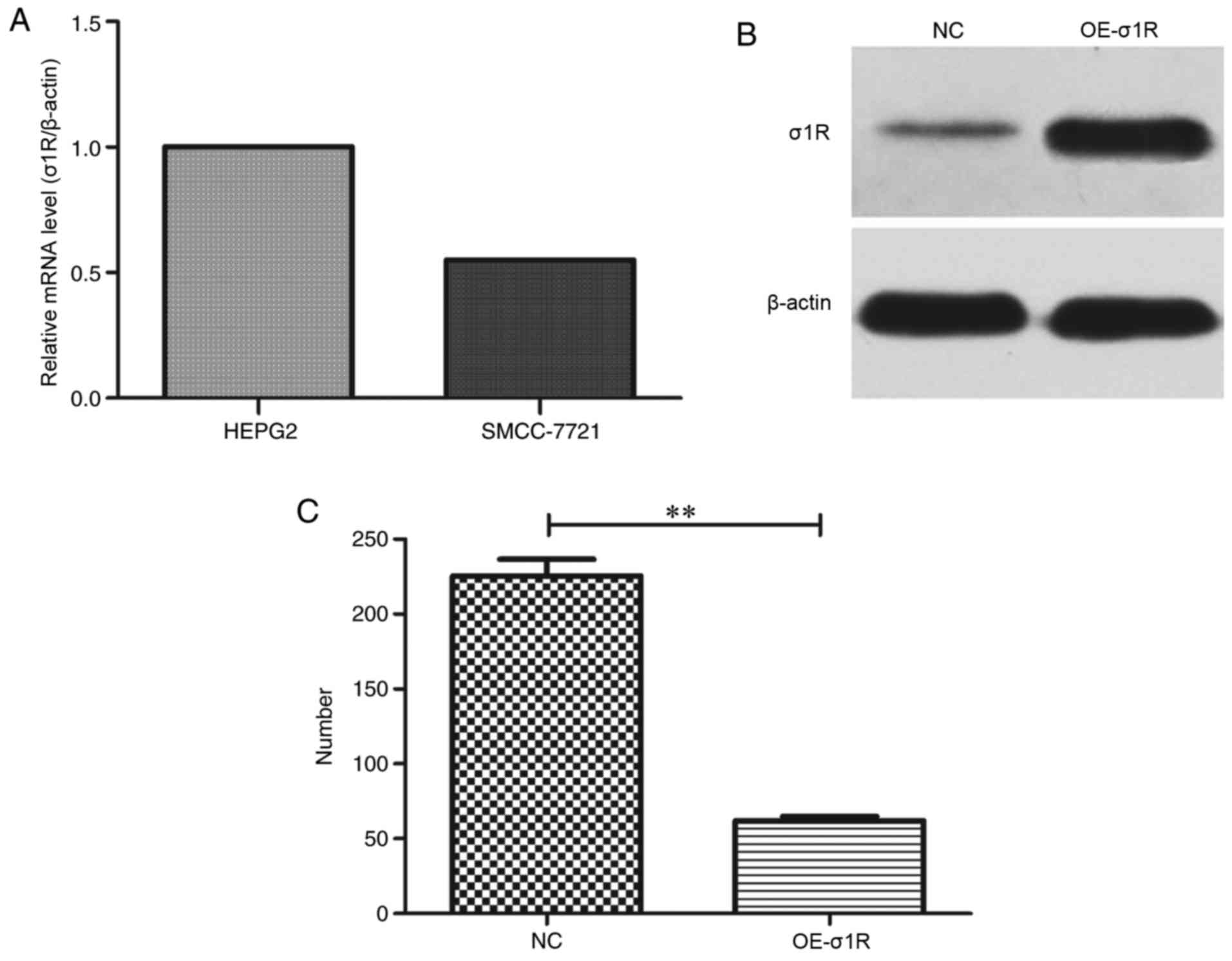
σ1R mRNA expression in HepG2 and
SMCC-7721 cell lines
The mRNA levels of σ1R were assessed in HepG2 and
SMCC-7721 cell lines by RT-qPCR. It was observed that σ1R mRNA was
expressed in the two cell lines, and notably, that the expression
level of σ1R in SMCC-7721 was ~55% of the level in HepG2 cells
(Fig. 3A). As the expression level
in HepG2 was higher than that in SMCC-7721, we selected the HepG2
cell line for subsequent experiments.
σ1R expression is increased
efficiently by lentiviral expression vector transfection in HepG2
cells
To investigate the mechanism by which σ1R
contributes to the malignancy of HMTs, we performed
lentiviral-mediated overexpression of σ1R in HepG2 cells. The
upregulation efficiency of σ1R was evaluated by western blot
analysis. As shown in Fig. 3B and
C, the protein expression level of σ1R was significantly
upregulated compared with that in the NC group.
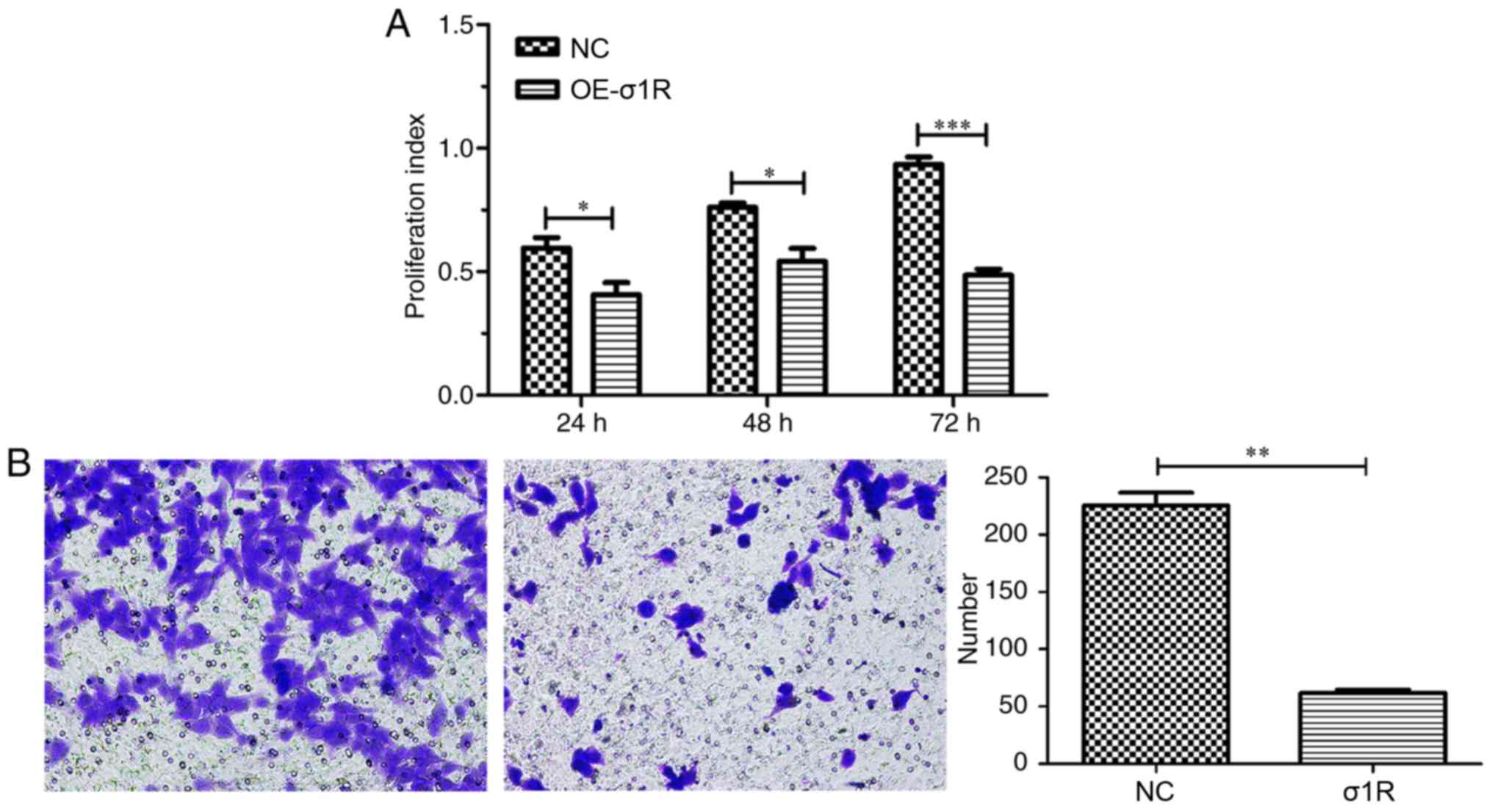
σ1R overexpression inhibits HepG2 cell
growth and migration
The effect of σ1R overexpression on the
proliferative ability of HepG2 cells was determined by MTT assay.
As illustrated in Fig. 4A, the
cellular proliferative rate of the σ1R-GV141 group was markedly
reduced from the first day compared with the NC-transfected cells
(P<0.05), and the inhibitory effect on the third day was more
obvious compared with that on the first day of cell incubation
(P<0.001). Furthermore, a Transwell migration assay was
performed to determine the effect of σ1R overexpression on the
invasive ability of HepG2 cells in vitro. The results showed
that σ1R overexpression in HepG2 cells caused a significant
reduction in migration compared with the NC cells (Fig. 4B; P<0.01). Thus, it was
demonstrated that the overexpression of σ1R suppressed the
proliferation and migration of HepG2 cells.
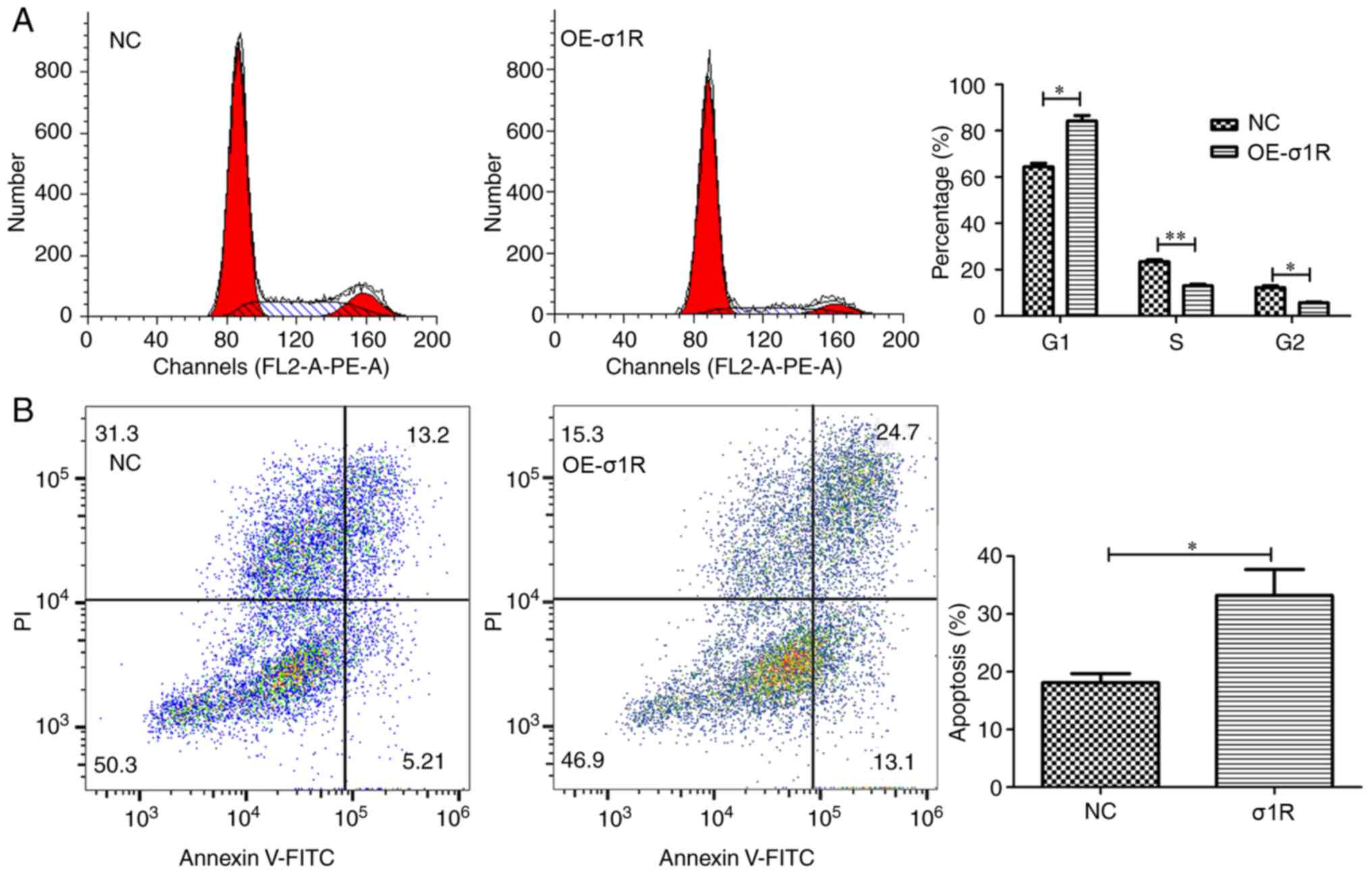
σ1R overexpression causes cell cycle
arrest in the G1 phase and induces apoptosis in HepG2 cells
As changes in cell numbers may result from arrest of
cell cycle progression or induction of apoptosis, both were
analyzed by flow cytometry. As shown in Fig. 5A, for HepG2 cells, the
σ1R-overexpressing group and NC group exhibited the following cell
cycle distributions: G1 phase, 84.17±4.19 vs. 64.42±2.66%,
respectively; S phase, 13.00±1.30 vs. 23.40±1.37%, respectively;
and G2/M phase, 5.59±0.81 vs. 12.17±1.51%, respectively. The
results showed a significant decrease in the proportion of cells in
the S phase (P<0.01) or G2/M phase (P<0.05), and an increase
in the proportion of cells in G1 phase (P<0.05) in the
σ1R-overexpressing group compared with the NC group, suggesting
that σ1R overexpression led to cell cycle arrest in G1 phase. The
ability to resist apoptosis is an important feature of tumor cells
(21). In the present study, the
apoptosis analysis following Annexin V staining showed that the
apoptotic rate of σ1R-overexpressing cells was significantly
increased with respect to the control group (Fig. 5B; 33.20±4.52 vs. 18.13±1.57%;
P<0.05). These data indicated that σ1R may be associated with
apoptosis in HMT.
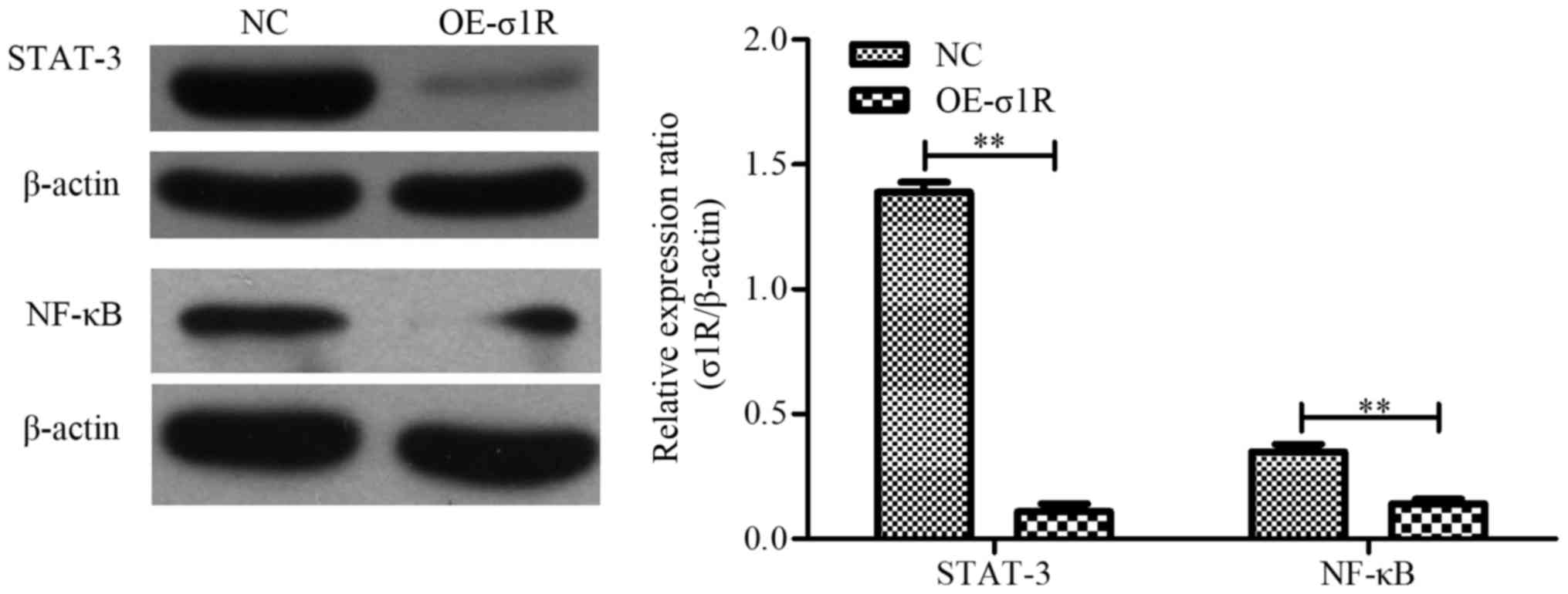
σ1R overexpression inhibits STAT-3 and
NF-κB expression
To explore the molecular mechanisms underlying the
involvement of σ1R in HMT development, we analyzed the effect of
σ1R on oncogenic signaling pathways. Western blotting showed that
STAT-3 and NF-κB were decreased in HepG2 cells overexpressing σ1R
(Fig. 6; P<0.01). These results
suggested that the upregulation of σ1R may be responsible for
STAT-3 and NF-κB downregulation in the context of HepG2 cell
proliferation, apoptosis and migration.
Discussion
HCC is a type of malignant tumor associated with
rapid progression and a poor survival rate (3). Although there have been extensive
studies on HMT, poor prognosis and the limited value of established
prognostic markers have prompted researchers to search for new
biomarkers that are able to predict the prognosis and act as
possible treatment targets in patients with HMT. Sigma-1 receptor
(σ1R) was first discovered in the nervous system by Martin et
al (7), although its expression
has since been identified in other organs, including the liver,
kidneys, lungs and gonads (14,17).
More recently, studies have found that σ1R is highly expressed in
various cancer tissues of neural and non-neural origins (12,15,16,22,23),
and the upregulation of σ1R has been reported to be associated with
biological behavior, such as proliferation, adhesion and cell
death, in tumors (13,24). However, the expression and
biological significance of σ1R in HMT remain unknown.
A novel finding in the present study was that σ1R
was expressed at low levels in HCC, and that there was a
statistically significant difference between its expression levels
in benign hepatic tissue and HCC (P<0.01). The decreased
expression of σ1R in HCC was an interesting phenomenon, which was
inconsistent with former studies in other tumor types (16,18,25).
We considered that this contradiction may indicate a different
molecular biological function in HCC compared with other tumor
types. It has been reported that certain narcotic drugs and oxygen
and glucose deprivation are important factors in inducing σ1R
expression (26,27). The liver is an organ that is central
to metabolism and produces a wide range of chemicals essential to
bodily functioning. External and internal factors may interact with
HCC tumor cells to regulate the expression of σ1R.
Histological differentiation is a significant factor
in estimating the prognosis of cancer patients. Patients with
high-grade HCC often have a poor prognosis. In the present study, a
significant inverse correlation was observed between σ1R expression
and the grade of differentiation of HCC (r=−0.424, P=0.021), and
HCC cases with a high level of σ1R expression were more likely to
have low grade disease. Therefore, we speculated that σ1R may play
a role in the initiation or progression of HCC, and particularly in
histological differentiation.
In the present study, lentivirus-GV141-mediated σ1R
overexpression markedly inhibited HMT cell proliferation and
migration in vitro; the cell proliferation rate and
migratory ability in the σ1R-GV141 group were significantly
decreased or decelerated compared with the NC
lentivirus-transfected group. Furthermore, σ1R overexpression
induced cell cycle arrest in the G1 phase and promoted cell
apoptosis. These findings demonstrate that the downregulation of
σ1R has an important effect on HMT cell proliferation and
migration, and that it may serve as a potential predictive factor
and therapeutic target in the treatment of HMT.
The scientific basis underlying the inhibitory
effect of σ1R overexpression on tumor proliferation is an important
aspect for further research. Ion channels are considered to play a
crucial role in many tumor types (16). Aydar et al found that Kv1.4
and Kv1.5 ion channels were highly sensitive to σ1R ligands in the
presence of σ1R, while the modulation was weak in the absence of
σ1R in Xenopus oocytes, which suggested that σ1R may form a
functional complex with the expressed ion channels (10). In addition, Spruce et al
(28) considered that σ1R ligands
may be involved in the calcium-dependent activation of
phospholipase C and concomitant calcium-independent inhibition of
phosphatidylinositol 3′-kinase pathway signaling in some cancer
cells.
Signal transducer and activator of transcription-3
(STAT-3), a member of the STAT family, is commonly activated in
human epithelial cancers (29,30).
Constitutive activation of STAT3 contributes to oncogenesis and
progression, including increased cell proliferation and survival
(31). Wu et al (32) demonstrated that increased STAT-3
expression was correlated with higher tumor stage and decreased
patient survival in cases of HMT.
Nuclear factor-κB (NF-κB) is a well-known nuclear
transcription factor that regulates the expression of a variety of
genes critical for the regulation of apoptosis (33). The activation of NF-κB induces
antiapoptotic gene expression to promote cell survival. In the
present study, we found that the expression levels of STAT-3 and
NF-κB were decreased in σ1R-overexpressing HepG2 cells relative to
the NC group. Our findings preliminarily indicate that σ1R
overexpression may suppress HepG2 cell proliferation and migration
through inactivation of STAT-3 and NF-κB.
In summary, the present findings indicate that σ1R
is decreased in HCC and is closely correlated with histological
differentiation. The overexpression of σ1R suppressed cell
proliferation, inhibited cell migration and induced cell cycle
arrest and cell apoptosis in HepG2 hepatoblastoma cells. Therefore,
we hypothesized that σ1R has an important role in promoting HCC
cell differentiation. Elucidation of the functions and detailed
mechanisms of σ1R in regulating HMT tumorigenesis and progression
are the subjects of our ongoing research.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Shandong Province (grant no.
ZR2014HP014).
References
|
1
|
Bell D, Ranganathan S, Tao J and Monga SP:
Novel advances in understanding of molecular pathogenesis of
hepatoblastoma: A Wnt/β-catenin perspective. Gene Expr. 17:141–154.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J,
Wang J, Zhang D, Cheng S and Liu S: Expression of intercellular
adhesion molecule 1 by hepatocellular carcinoma stem cells and
circulating tumor cells. Gastroenterology. 144:1031–1041. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
agency for research on cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stravitz RT, Heuman DM, Chand N, Sterling
RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles
HC, et al: Surveillance for hepatocellular carcinoma in patients
with cirrhosis improves outcome. Am J Med. 121:119–126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin WR, Eades CG, Thompson JA, Huppler
RE and Gilbert PE: The effects of morphine- and nalorphine-like
drugs in the nondependent and morphine-dependent chronic spinal
dog. J Pharmacol Exp Ther. 197:517–532. 1976.PubMed/NCBI
|
|
8
|
Walker JM, Bowen WD, Walker FO, Matsumoto
RR, de Costa B and Rice KC: Sigma receptors: Biology and function.
Pharm Rev. 42:355–402. 1990.PubMed/NCBI
|
|
9
|
Hanner M, Moebius FF, Flandorfer A, Knaus
HG, Striessnig J, Kempner E and Glossmann H: Purification,
molecular cloning, and the expression of the mammalian
sigma1-binding site. Proc Natl Acad Sci USA. 93:pp.
8072–8077. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aydar E, Palmer CP, Klyachko VA and
Jackson MB: The sigma receptor as a ligand-regulated auxiliary
potassium channel subunit. Neuron. 34:399–410. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayashi T and Su TP: Sigma-1 receptor
chaperones at the ER-mitochondrion interface regulate
Ca2+ signaling and cell survival. Cell. 131:596–610.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crawford KW and Bowen WD: Sigma-2 receptor
agonists activate a novel apoptotic pathway and potentiate
antineoplastic drugs in breast tumor cell lines. Cancer Res.
62:313–322. 2002.PubMed/NCBI
|
|
13
|
Brent PJ and Pang GT: Sigma binding site
ligands inhibit cell proliferation in mammary and colon carcinoma
cell lines and melanoma cells in culture. Eur J Pharmacol.
278:151–160. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hellewell SB, Bruce A, Feinstein G,
Orringer J, Williams W and Bowen WD: Rat liver and kidney contain
high densities of sigma 1 and sigma 2 receptors: Characterization
by ligand binding and photoaffinity labeling. Eur J Pharmacol.
268:9–18. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vilner BJ, John CS and Bowen WD: Sigma-1
and sigma-2 receptors are expressed in a wide variety of human and
rodent tumor cell lines. Cancer Res. 55:408–413. 1995.PubMed/NCBI
|
|
16
|
Aydar E, Palmer CP and Djamgoz MB: Sigma
receptors and cancer: Possible involvement of ion channels. Cancer
Res. 64:5029–5035. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolfe SA Jr, Culp SG and De Souza EB:
Sigma-receptors in endocrine organs: Identification,
characterization, and autoradiographic localization in rat
pituitary, adrenal, testis, and ovary. Endocrinology.
124:1160–1172. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu QX, Li EM, Zhang YF, Liao LD, Xu XE, Wu
ZY, Shen JH and Xu LY: Overexpression of sigma1 receptor and its
positive associations with pathologic TNM classification in
esophageal squamous cell carcinoma. J Histochem Cytochem.
60:457–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
20
|
Milner AE, Levens JM and Gregory CD: Flow
cytometric methods of analyzing apoptotic cells. Methods Mol Biol.
80:347–354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas GE, Szücs M, Mamone JY, Bem WT,
Rush MD, Johnson FE and Coscia CJ: Sigma and opioid receptors in
human brain tumors. Life Sci. 46:1279–1286. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bem WT, Thomas GE, Mamone JY, Homan SM,
Levy BK, Johnson FE and Coscia CJ: Overexpression of sigma
receptors in nonneural human tumors. Cancer Res. 51:6558–6562.
1991.PubMed/NCBI
|
|
24
|
Aydar E, Onganer P, Perrett R, Djamgoz MB
and Palmer CP: The expression and functional characterization of
sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett.
242:245–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crottès D, Guizouarn H, Martin P, Borgese
F and Soriani O: The sigma-1 receptor: A regulator of cancer cell
electrical plasticity? Front Physiol. 4:1752013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guitart X, Codony X and Monroy X: Sigma
receptors: Biology and therapeutic potential. Psychopharmacology.
174:301–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruscher K, Shamloo M, Rickhag M, Ladunga
I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg
D, Urfer R, et al: The sigma-1 receptor enhances brain plasticity
and functional recovery after experimental stroke. Brain.
134:732–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spruce BA, Campbell LA, McTavish N, Cooper
MA, Appleyard MV, O'Neill M, Howie J, Samson J, Watt S, Murray K,
et al: Small molecule antagonists of the sigma-1 receptor cause
selective release of the death program in tumor and self-reliant
cells and inhibit tumor growth in vitro and in vivo. Cancer Res.
64:4875–4886. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng JY, Sun D, Liu XY, Pan Y and Liang H:
STAT-3 correlates with lymph node metastasis and cell survival in
gastric cancer. World J Gastroenterol. 16:5380–5387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HS, Park YH, Lee J, Ahn JS, Kim J,
Shim YM, Kim JH, Park K, Han J and Ahn MJ: Clinical impact of
phosphorylated signal transducer and activator of transcription 3,
epidermal growth factor receptor, p53, and vascular endothelial
growth factor receptor 1 expression in resected adenocarcinoma of
lung using tissue microarray. Cancer. 116:676–685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu WY, Li J, Wu ZS, Zhang CL, Meng XL and
Lobie PE: Prognostic significance of phosphorylated signal
transducer and activator of transcription 3 and suppressor of
cytokine signaling 3 expression in hepatocellular carcinoma. Exp
Ther Med. 2:647–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|