Introduction
PC is one of the most aggressive and fatal cancers
due to rapid metastasis, easy recurrence, early invasion and
resistance to conventional chemotherapy. Survival rates of PC
remained stagnant during the last half century (1). It was confirmatively suggested that
the early detection of PC implied a better prognosis in the
long-term survival (2). It is
essential to search for novel biomarkers for early detection and to
promote the efficiency of chemotherapy.
The TRPV6 channel is a calcium cation channel
protein, which is the major constituent of superfamily of transient
receptor potential (TRP) channels, further in terms of subfamily
vanilloid member 6, which regulates the calcium homeostasis in the
epithelial tissue of human organs (3). TRPV6 is overexpressed in colon cancer,
breast cancer, prostate cancer, parathyroid cancer and thyroid
cancer (3–6), however decreased TRPV6 expression was
observed in esophageal cancer, non-small cell lung cancer and renal
cancer (7–9). Overexpression of TPPV6 in many cancers
indicates the idea of this protein as encoded by a probable
oncogene, but the level of TRPV6 in other cancers showed a tendency
of a tumor-suppressor. Nevertheless, the expression and biological
functions of TRPV6 in PC are less fully elucidated. Therefore, we
analyzed the expression of TRPV6 for clinicopathological
characteristics, predicting survival in the patients, and the
effect of silencing TRPV6 on apoptosis, proliferation, cell cycle,
migration, invasion and chemotherapy sensitivity of PC cells,
respectively, to gemcitabine, oxaliplatin and cisplatin. TRPV6
plays a promising role in the development and progression of PC.
Numb was originally discovered as a determinant of cell fate
(10) responsible for plenty of
signal transduction pathways (Hedgehog, P53), endocytosis, cell
polarity determination, and ubiquitination (11), and to play an important role in
cancers. Numb was recently found as a new interacting partner with
TRPV6 (12). Numb inhibits activity
of TRPV6 via electrostatic interaction in breast cancer cells. Numb
regulates Ca cation influx via TRPV6 (13). Ca cation influx stimulated the
GSK3β, AKT, MAPKinase pathway involved in TRPV6-specific mediating
cell proliferation. Silencing Numb reduced expression of TRPV6 in
prostate cancer cell lines (12).
On the contrary, knockdown of Numb increased the TPRV6 expression
in the breast cancer cells. On the other hand, silencing TRPV6
increased the Numb expression. Numb and TRPV6 regulate the protein
stability and degradation of each other (13). Finally, we observed the interaction
between TRPV6 and Numb in PC cells.
Materials and methods
Pancreatic cancer specimens
Tumor specimens and their corresponding adjacent
noncancerous tissues were selected from patients who were
pathologically confirmative of PC during pancreatic operation in
the Shenyang Medical Center between January 2005 to January 2014.
None received chemotherapy or radiotherapy in the preoperative
period. The pathologic diagnosis and differentiation were confirmed
by three independent pathologists by TNM classification, 7th
edition of the UICC 2010. Fresh specimens were snap-frozen in the
liquid nitrogen rapidly after operation. The study was approved by
the Institutional Ethics Committee of China Medical University and
written informed consent was obtained from the patients.
Pancreatic cancer cell lines
We obtained BxPC-3, AsPC-1, SW1990, PANC-1 cells
from the Cell Bank of the Chinese Academy of Sciences. We purchased
Capan-2 cells from the American Type Culture Collection (ATCC). We
maintained the five cell lines in growth medium supplemented with
10% fetal bovine serum and 100 µg/ml of penicillin and streptomycin
(Hyclone, Logan, UT, USA).
RNA interference
We used small interfering RNA for TRPV6
(TRPV6-siRNA) and counterpart negative control (Neg.Cont)
oligonucleotides (Shanghai GenePharma Co. Ltd.) transfected with
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The target
sequences of oligonucleotides for the TRPV6 siRNA and Neg.Cont were
as follows: TRPV6-Si1, 5′-CCAAGGAGAAAGGGCUAAUTT-3′; TRPV6-Si2,
5′-CCAUAUAUCUGCUGUACAUTT-3′; TRPV6-Si3,
5′-CUGCGUGGGAUAAUCAACATT-3′. Neg.Cont: Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; Antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Immunohistochemistry staining
Immunohistochemistry was performed as previously
described (14). Briefly, tissue
sections (5 µm) were blocked with hydrogen peroxide and then
incubated with mouse polyclonal anti-TRPV6 (1:400, Invitrogen),
overnight at 4°C. As a negative control, normal IgG was used as the
primary antibody at the same dilution. Stained tissue sections were
reviewed and scored according to Masunaga et al (15).
RNA preparation and quantitative
real-time PCR
With TRIzol reagent (Takara), total RNA was
extracted, and then quantitative PCR was performed with a SYBR
Green II (Takara) on a Thermal Cycler Dice Real-time System
(Takara) with the following protocol: 30 sec at 95°C followed by
two-step PCR for 40 cycles of 95°C for 5 sec and 64°C for 30 sec.
Gene expression was normalized relative to its GAPDH mRNA with the
∆∆CT method. The sequences of the PCR primers were as follows:
TRPV6 forward, 5′-ATGGTGATGCGGCTCATCAGTG-3′ and reverse,
5′-GTAGAAGTGGCCTAGCTCCTCG-3′; GAPDH forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′, and reverse
5′-CCCAATACGACCAAATCCGTT-3′.
Western blotting
Pancreatic cancer tissues and pancreatic cancer cell
samples were washed with ice-cold PBS and then lysed by RIPA
(Beyotime, Jiangsu, China). The lysates were separated in SDS-PAGE
(10%). Resolved protein was transferred onto a polyvinylidene
difluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The
membranes were blocked by 5% skim milk solution in TBST buffers,
and were incubated with primary antibodies for TRPV6 (1:500 Abcam,
Cambridge, UK, ab63084), PCNA (1:1000, Abcam, ab29), Bax (1:1000,
Abcam, ab32503), Bcl-2 (1:500, Abcam, ab692), E-cadherin (1:400,
Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc7870) MMP9 (1:400,
Santa Cruz Biotechnology, sc6840), β-catenin (1:1500, ProteinTech
Group, Chicago, IL, USA, 51067–2-AP), cyclin-D1 (1:1000,
ProteinTech Group, 12363-1-AP), Numb (1:1000, Cell Signaling
Technology, Danvers, MA, USA, #8650). overnight at 4°C. PVDF
membranes were washed in TBST and incubated with horseradish
peroxidase-conjugated secondary antibodies (1:10000, ProteinTech
Group, SA00001-15 and SA00001-1) 2 h at 37°C. Antibody against
GAPDH (1:1000, Cell Signaling Technology, #P04406) was used as an
internal control. The protein of interest was visualized using ECL
Western blotting substrate (Pierce, Biotechnology, Rockford, IL,
USA).
Cell Counting Kit-8 assay
When cell proliferation was detected, at an initial
density of 2,000 cells/well, cells were plated in 96-well cultural
dishes, Si-TRPV6 24 h after transfection. Cells were incubated for
0, 24, 48, 72 h. CCK8 (Thiazolyl Blue) solution was added. When
drug susceptibility was detected, cells had reached the density of
5,000 cells/well, 24 h after Si-TRPV6 transfection. Different
concentrations of gemcitabine, oxaliplatin or cisplatin were added,
respectively, and incubated for 48 h.
Cell migration and invasion
assays
After 48 h of transfection, we adjusted the density
of 2×105 cells/ml in every group. The upper Transwell
chamber (Costar; 24-well insert, pore size: 8 µm) was filled with
200 µl cell suspension, and the lower chamber was filled with 500
µl of medium. For the invasion assay, polycarbonate filters coated
with 50 µl Matrigel (1:9, BD Bioscience) were placed in a Transwell
chamber. Cells were incubated for 24 h for the migration assay and
48 h for the invasion assay. Then, the cells on the upper surface
were wiped, and the cells on the lower surface were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet. The
migratory cells were visualized and counted in five random visual
fields per insert under an inverted microscope at ×200
magnification.
Cell cycle and apoptosis
Pancreatic cancer cells were treated with Si-TRPV6
or Neg.Cont oligo. Cells with a density of 500,000/well were
trypsinized, and collected and stained with the Annexin
V-keyFluor555 apoptosis detection kit (KeyGene, Nanjing, China).
Cell cycle analysis was performed after staining with propidium
iodide (Beyotime). The distribution was quantified using a flow
cytometer.
Statistical analysis
Statistical analyses were performed with SPSS 14.0.
The paired-sample t-test was used for TRPV6 expression in paired
samples of PC and its normal tissue. The TRPV6 expression and
clinicopathological parameters were used for the Chi-squared test.
Differences in survival were assessed with Kaplan-Meier method and
analyzed using the log-rank test. Cox's proportional hazards
regression model was used to analyze independent prognostic
factors. Cell proliferation, apoptosis, cell cycle, invasion and
migration assays were expressed as means ± SE. P<0.05 was
considered to indicate a statistically significant difference.
Results
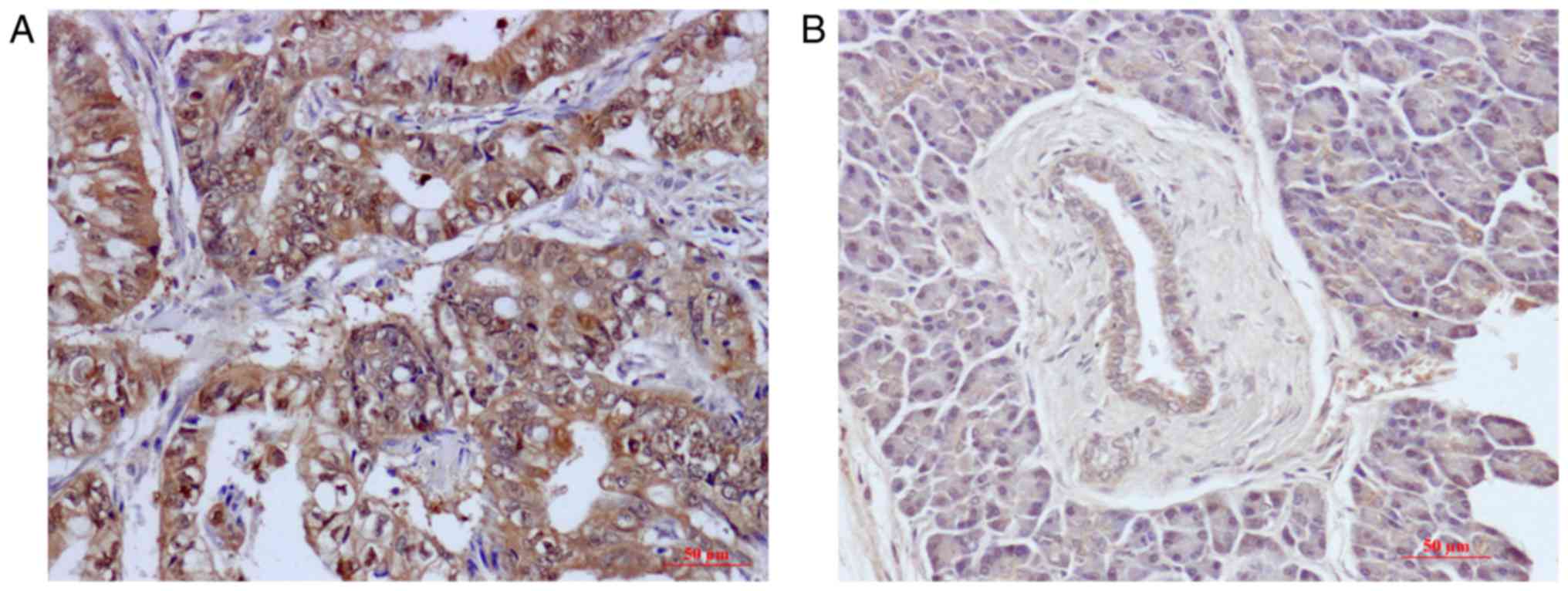
Immunohistochemistry showed that TRPV6 protein was
overexpressed in 57.9% (44/76) in PC tissue and 19.7% (15/76) in
their matched non-tumour adjacent tissues. Specifically, TRPV6
expression was significantly increased in PC tissue in comparison
to their counterpart non-tumor adjacent tissues (t=3.039, P=0.003).
TRPV6 was mostly expressed in the cytoplasm (Fig. 1A and B).
TRPV6 was overexpressed in PC tissues, which
predicted a larger size of tumor (P=0.024) and advanced T
pathological stage (P=0.029) (Table
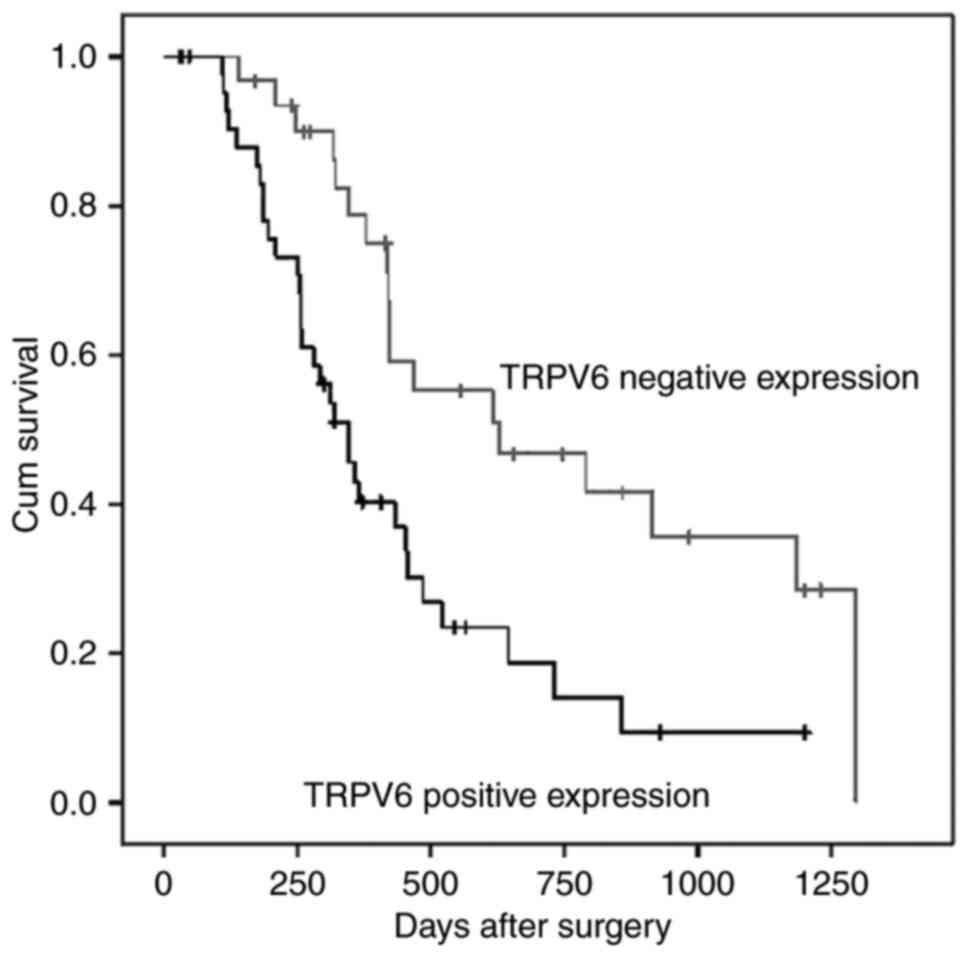
I. Survival rate of the negative TRPV6 expression group was
significantly better than that of the positive TRPV6 expression
group (P=0.003, Fig. 2). Moreover,
univariate analysis showed T stage (P=0.016), and vascular
permeation (P=0.012) influenced patient prognosis (P=0.003,
Fig. 2) as well. Moreover, a
multivariate analysis via the COX proportional hazard model was
conducted to evaluate the independent prognostic value of the TRPV6
expression level. The high level of TRPV6 expression (RR 2.022, 95%
CI 1.075–3.805, P=0.029) and vascular infiltration (RR 1.875, 95%
CI 1.051–3.346, P=0.033) were associated with a poor prognosis,
independent of other clinical covariates (Table II).
 | Table I.TRPV6 expression with
clinicopathological parameters from pancreatic cancer patients. |
Table I.
TRPV6 expression with
clinicopathological parameters from pancreatic cancer patients.
| Parameters | TRPV6 negative | TRPV6 positive | P-value |
|---|
| Age (year) |
|
| 0.466 |
|
<65 | 20 | 31 |
|
|
≥65 | 12 | 13 |
|
| Sex |
|
| 0.450 |
|
Male | 23 | 28 |
|
|
Female | 9 | 16 |
|
| Size (cm) |
|
| 0.024a |
|
<2.5 | 20 | 16 |
|
|
≥2.5 | 12 | 28 |
|
| T Stage |
|
| 0.029a |
|
T1+T2 | 19 | 15 |
|
| T3 | 13 | 29 |
|
| Lymph node |
|
| 0.893 |
| N0 | 23 | 31 |
|
| N1 | 9 | 13 |
|
| Vascular
permeation |
|
| 0.560 |
|
Absent | 19 | 29 |
|
|
Present | 13 | 15 |
|
| CA199 (U/ml) |
|
| 0.518 |
|
<37 | 8 | 14 |
|
|
≥37 | 24 | 30 |
|
| Perineural
invasion |
|
| 0.174 |
|
Absent | 21 | 35 |
|
|
Present | 11 | 9 |
|
| Hepatic
metastasis |
|
| 0.815 |
|
Absent | 21 | 30 |
|
|
Present | 11 | 14 |
|
 | Table II.Univariate and multivariate analysis
of clinicopathological factors for survival in 76 pancreatic cancer
patients. |
Table II.
Univariate and multivariate analysis
of clinicopathological factors for survival in 76 pancreatic cancer
patients.
| Parameters | Median survival
(days) | Univariate analysis
P-value (log-rank) | Multivariate
analysis (95% CI) | P-value |
|---|
| Age (<65/≥65
year) | 365/454 | 0.572 | – |
|
| Sex
(male/female) | 421/345 | 0.897 | – |
|
| Size (<2.5/≥2.5
cm) | 421/418 | 0.151 | – |
|
| T Stage
(T1+T2/T3) | 520/345 | 0.016a | 1.662
(0.899–3.071) | 0.105 |
| Lymph node
(N0/N1) | 454/345 | 0.532 | – |
|
| Perineural invasion
(absent/present) | 454/378 | 0.618 | – |
|
| Vascular permeation
(absent/present) | 615/345 | 0.012a | 1.875
(1.051–3.346) | 0.033a |
| Hepatic metastasis
(absent/present) | 454/378 | 0.361 |
|
|
| TRPV6
(negative/positive) | 629/345 | 0.003 | 2.022
(1.075–3.805) | 0.029a |
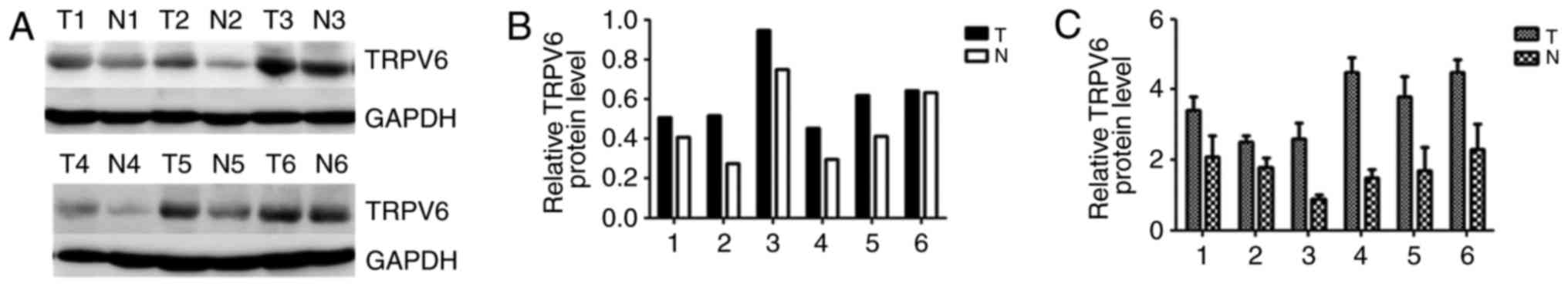
In all six pairs of cases, TRPV6 mRNA and protein
expression were upregulated in PC tissues in comparison to
counterpart normal peritumor tissues (Fig. 3).
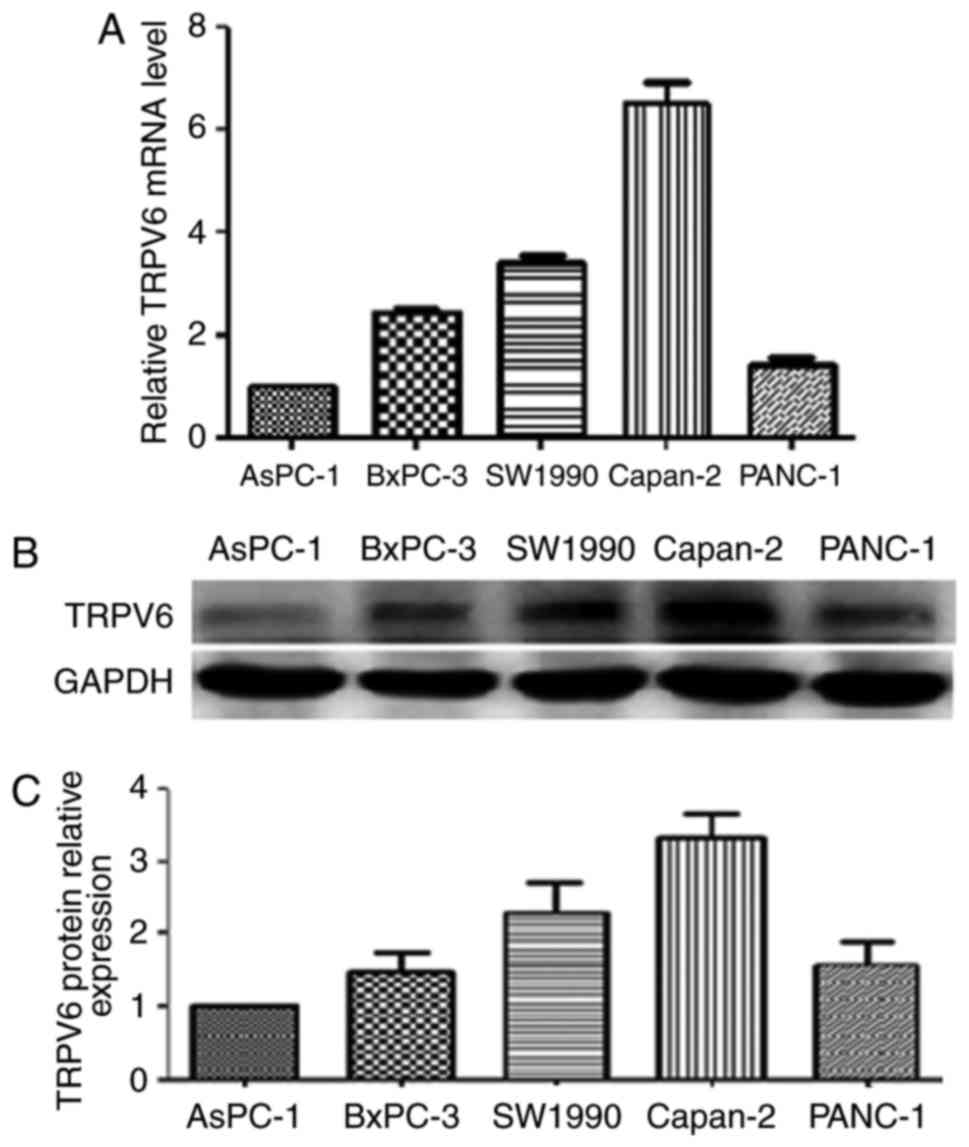
In the TRPV6 mRNA of PC various cell lines including
BxPC-3, AsPC-1, SW1990, Capan-2 and PANC-1, the highest level of
relative mRNA expression detected by qRT-PCR was in Capan-2 cells,
the second highest levels in SW1990 cells and lowest levels in
AsPC-1 cells. In the same five pancreatic cancer cell lines,
consistent with our qRT-PCR analysis, the highest protein levels of
TRPV6 expression was detected by western blot analysis in Capan-2
cells, the second highest levels in SW1990 cells and the lowest
level was in AsPC-1 cells (Fig.
4).
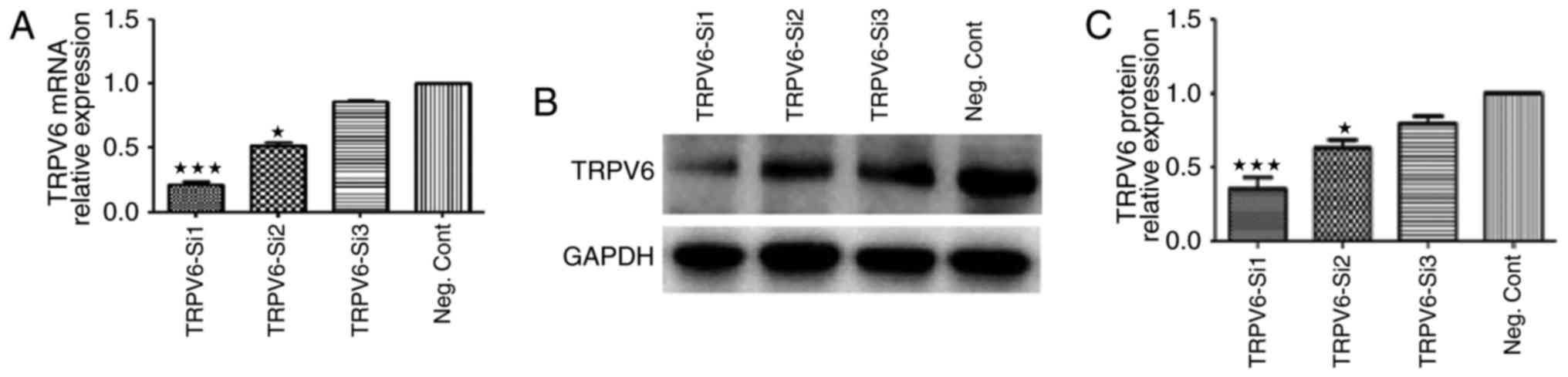
We knocked down TRPV6 expression in Capan-2 cells
via si-RNA transfection, and the efficiency of knockdown of the two
si-RNAs was evaluated. TRPV6 mRNA expression levels of Capan-2
cells by TRPV6-si1 were reduced by 81.5±3.8% (P<0.001), in
comparison to the negative control siRNA groups (Fig. 5A). TRPV6 protein expression levels
of Capan-2 cells by TRPV6-si2 were reduced by 63.4±5.6%
(P<0.001), in comparison to the negative control siRNA groups
(Fig. 5B and C). The most effective
TRPV6-si1 was chosen for the following study.
To further examine whether TRPV6 has a definite role
in PC progression, in vitro functional studies were
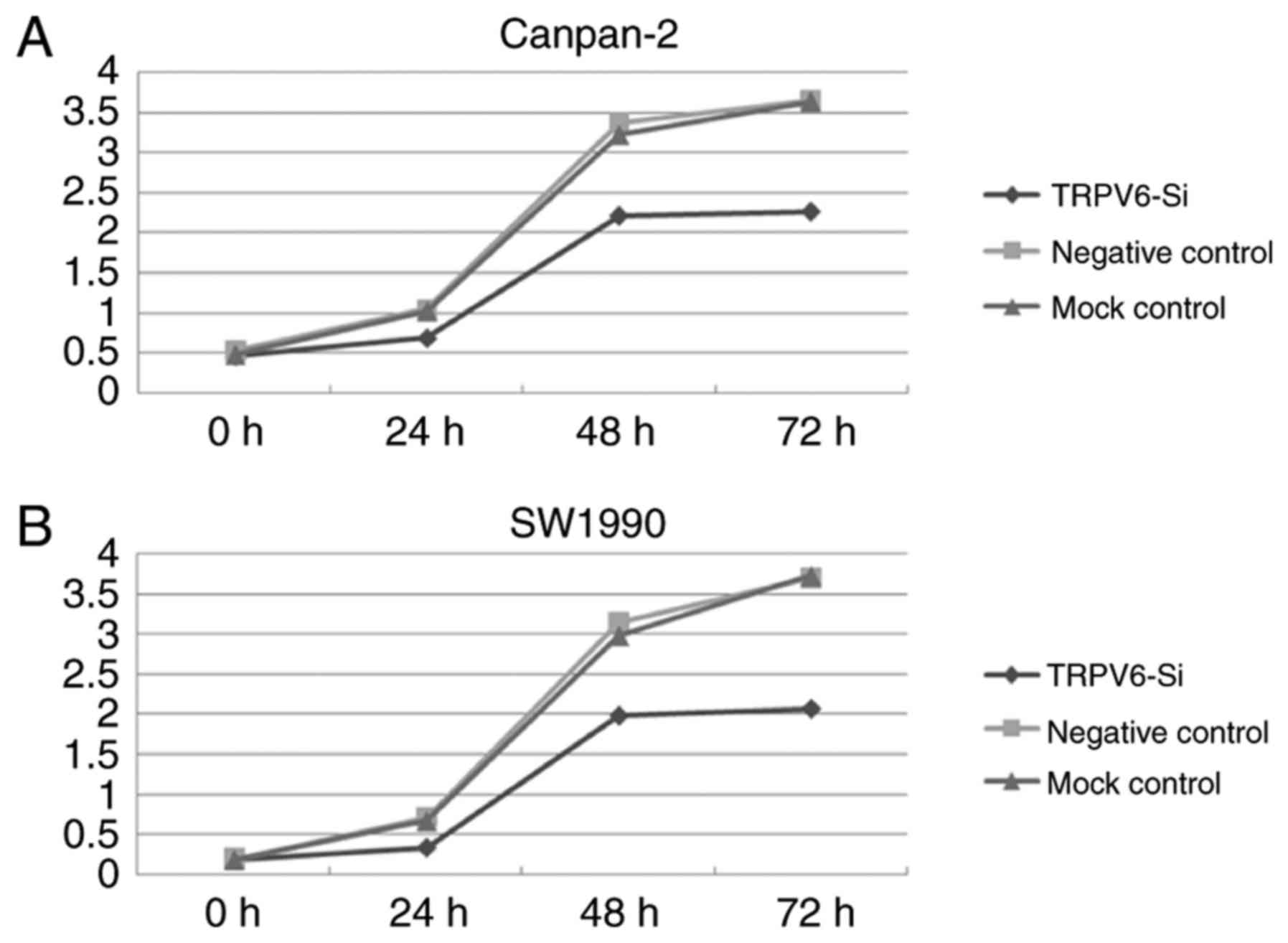
conducted. TRPV6 depletion resulted in decreased proliferation both
in PC cell line Capan-2 and SW1990, as determined by CCK-8 assay.
Downregulation of TRPV6 expression significantly led to 34.6±2.3,
34.4±2.8, 37.9±1.9% decrease in Capan-2 cells (24, 48, 72 h) and
52.1±3.2, 36.4±2.9, 44.2±2.1% decrease in SW1990 cells (24, 48, 72
h) in the proliferation in comparison to the negative control cells
(Fig. 6A and B).
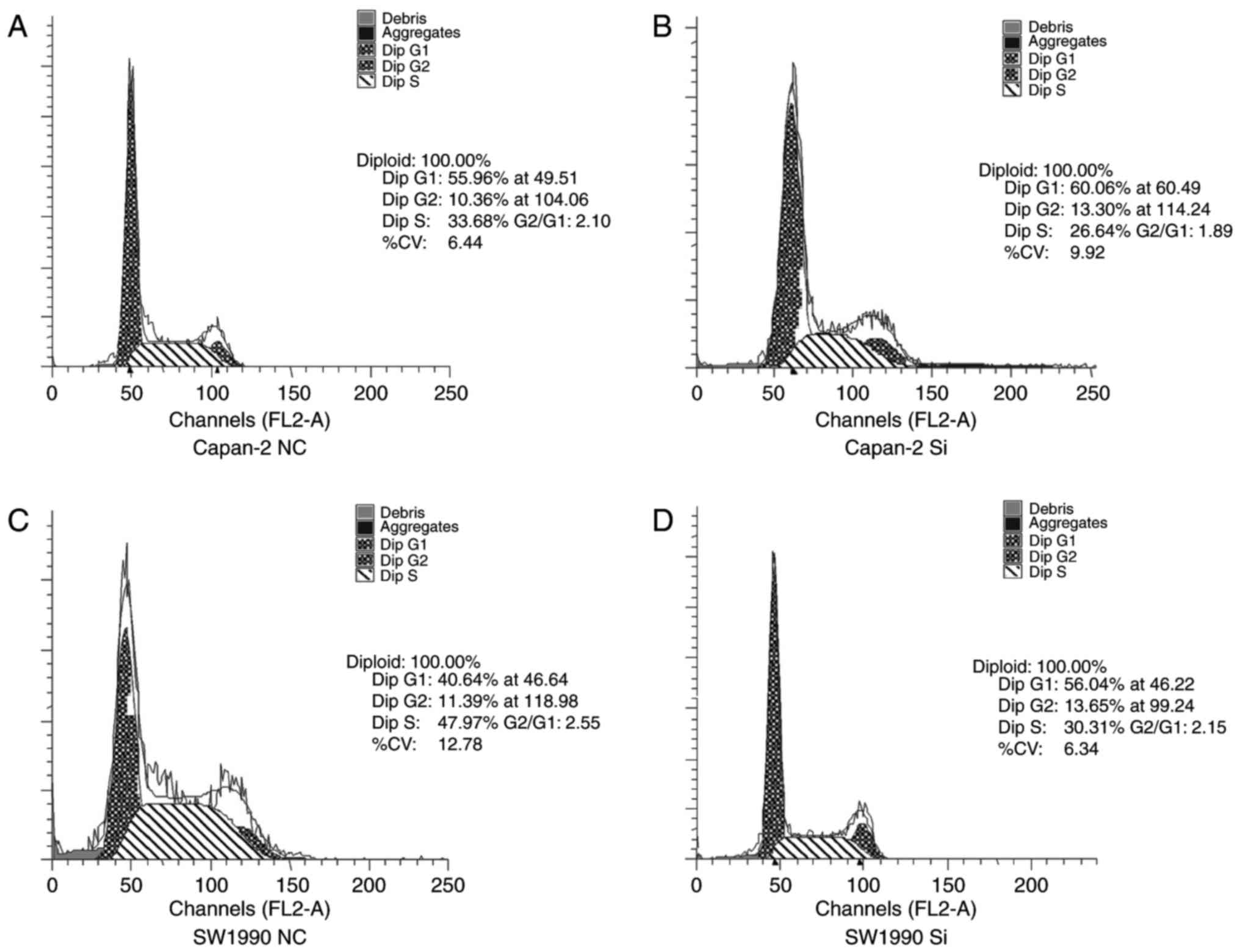
We also performed cell cycle assays after si-RNA
transfection using flow cytometry. The percentage of Capan-2 cells
infected with si-TRPV6 in the G1 phase significantly increased from
53.26±4.52 to 61.36±3.18%, whereas the percentage of cells in the S
phase decreased from 31.48±3.43 to 24.56±3.10% (Fig. 7A and B). The percentage of SW1990
cells infected with Si-TRPV6 in the G1 phase increased from
42.67±5.23 to 55.73±3.21%, whereas the percentage of cells in the S
phase decreased from 46.27±4.62 to 30.26±2.36% (Fig. 7C and D). These results suggest that
suppression of TRPV6 significantly induced G0/G1 phase arrest and
promoted cell cycle progression of PC cells.
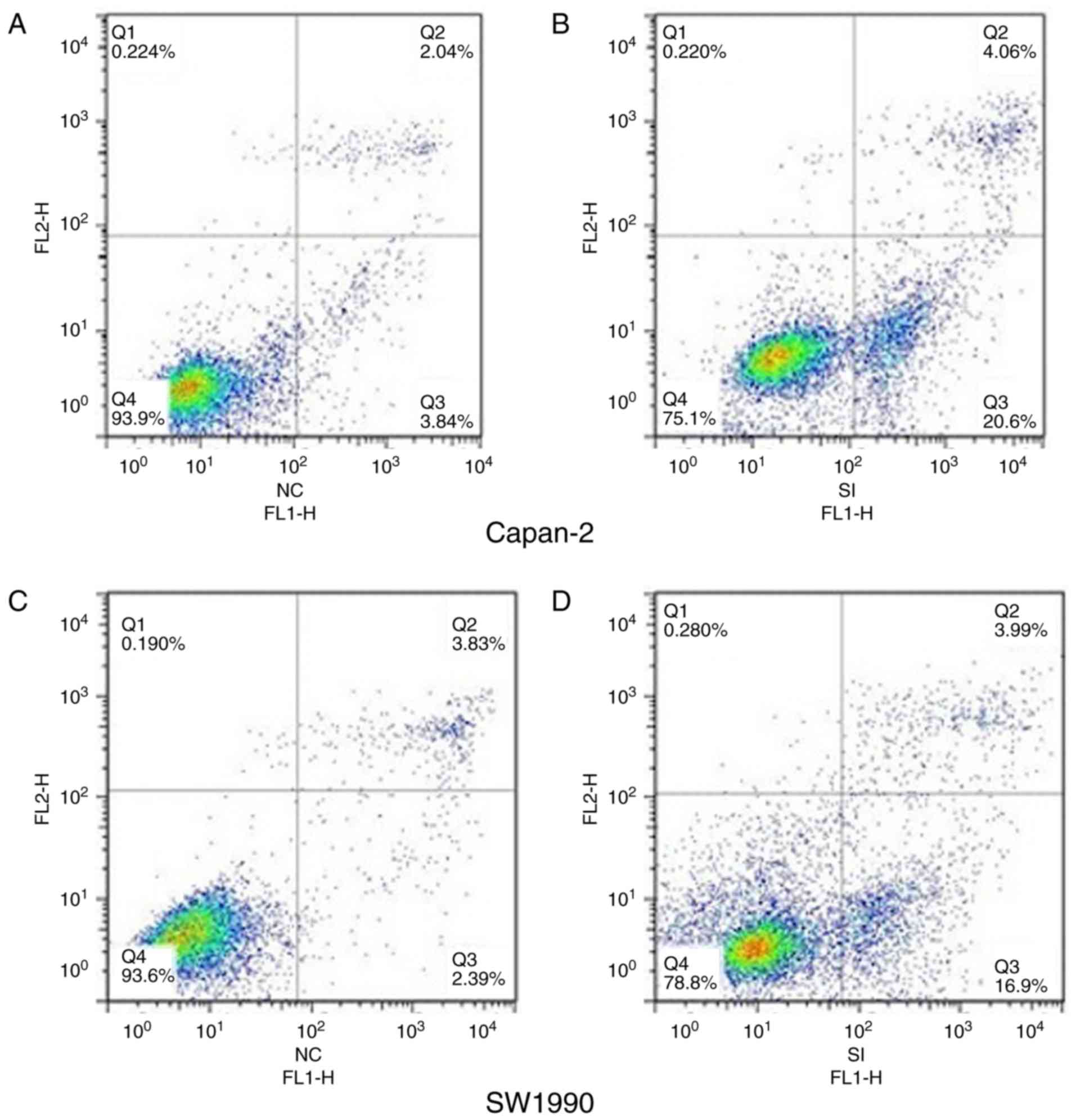
The apoptotic rate of cells infected with
TRPV6-siRNA was significantly increased from 5.23±1.22 to
23.86±2.34% in Capan-2 cells (Fig. 8A
and B) and 6.18±1.87 to 22.49±2.03% in SW1990 cells (Fig. 8C and D), respectively, in comparison
to NC cells. TRPV6-siRNA promoted apoptosis of PC cells, which in
turn makes contribution towards proliferation.
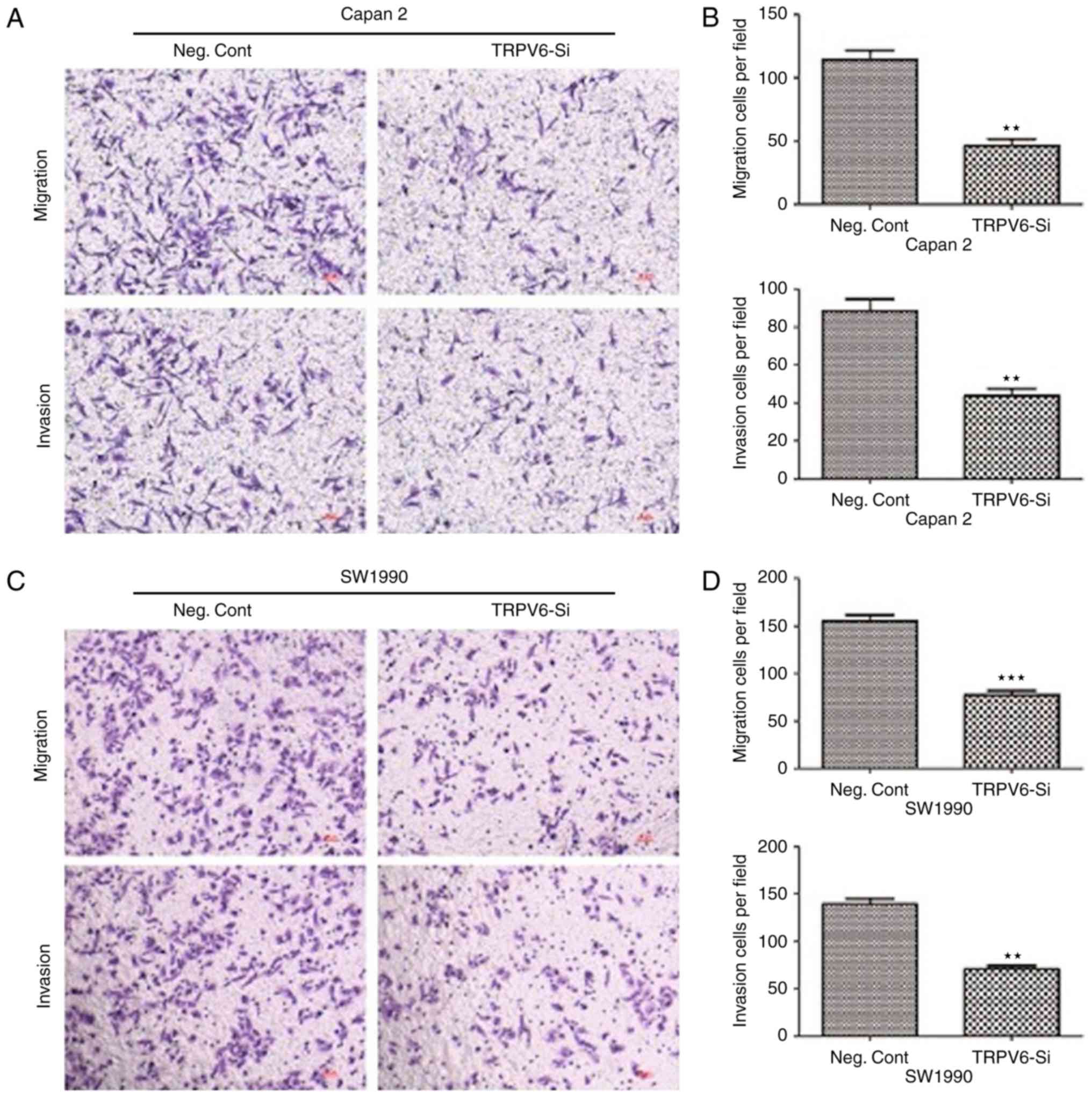
The migratory cells transfected with TRPV6-siRNA
were significantly decreased from 110.4±7.3 to 45±5.4 (Capan-2
cells) and 160±8.6 to 70±4.8 (SW1990 cells), respectively, in
comparison to NC cells. Similarly, the invasive cells transfected
with TRPV6-siRNA were also decreased from 93.8±7.5 to 50±6.8
(Capan-2 cells) and 135.4±4.6 to 73.7±3.9 (SW1990 cells),
respectively, in comparison to NC cells (Fig. 9). TRPV6 plays a promising role in
cell invasion and migration in PC and a mechanism by which
silencing TRPV6 leads to cancer metastasis inhibition in PC.
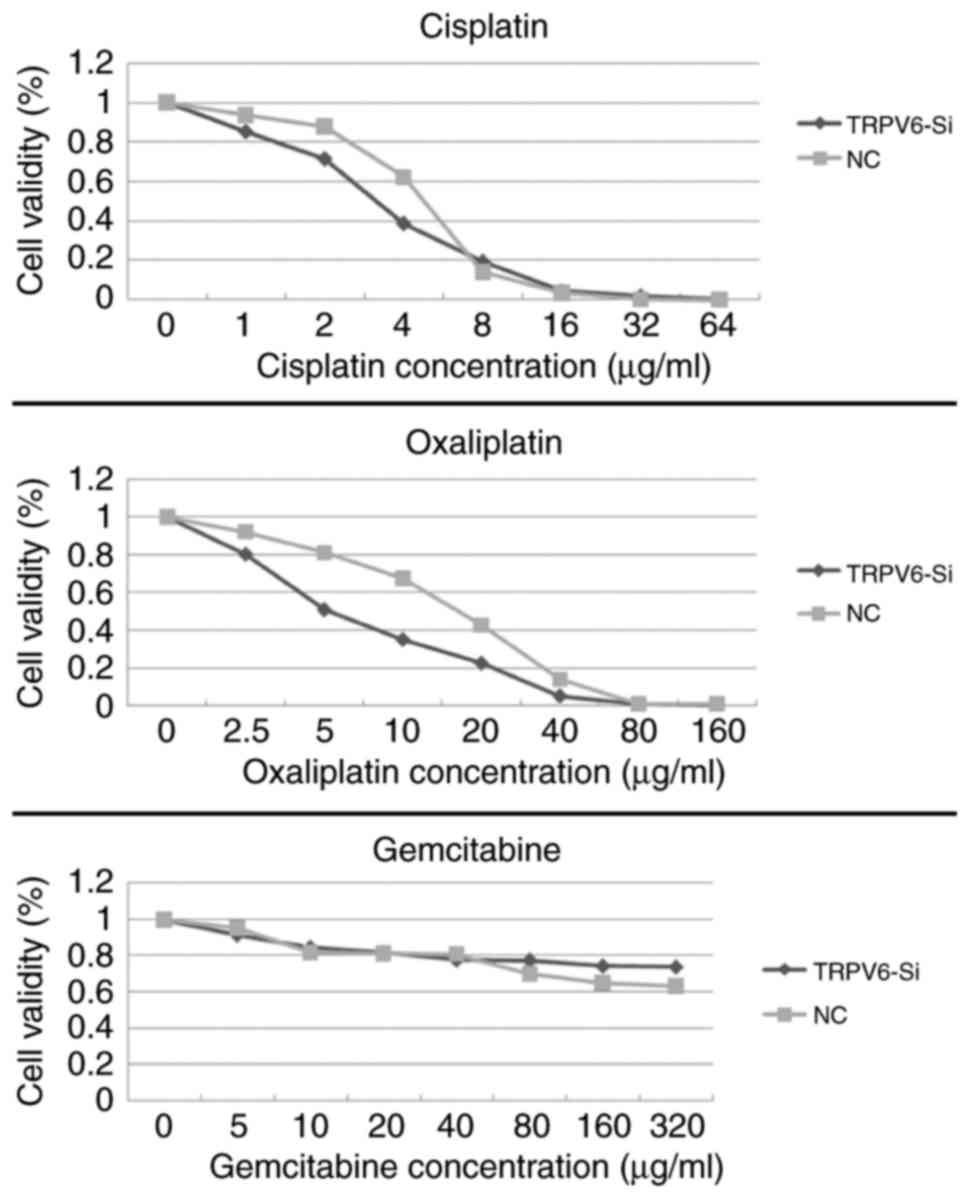
TRPV6-siRNA transfection affected the sensitivity of
PC cells to gemcitabine, oxaliplatin and cisplatin. In comparison
to the NC group, the inhibitory effects of oxaliplatin on cell
proliferation were considerably enhanced in the si-RNA TRPV6 group.
The IC50 of oxaliplatin was reduced from 15.2±1.2 to
4.8±0.7 µg/ml (P<0.05), the IC50 of cisplatin was
reduced from 5.6±1.1 to 3.2±0/9 µg/ml (P>0.05). Gemcitabine had
drug resistance, Si-TRPV6 group and NC group did not reach the
cutoff of IC50 (Fig.
10).
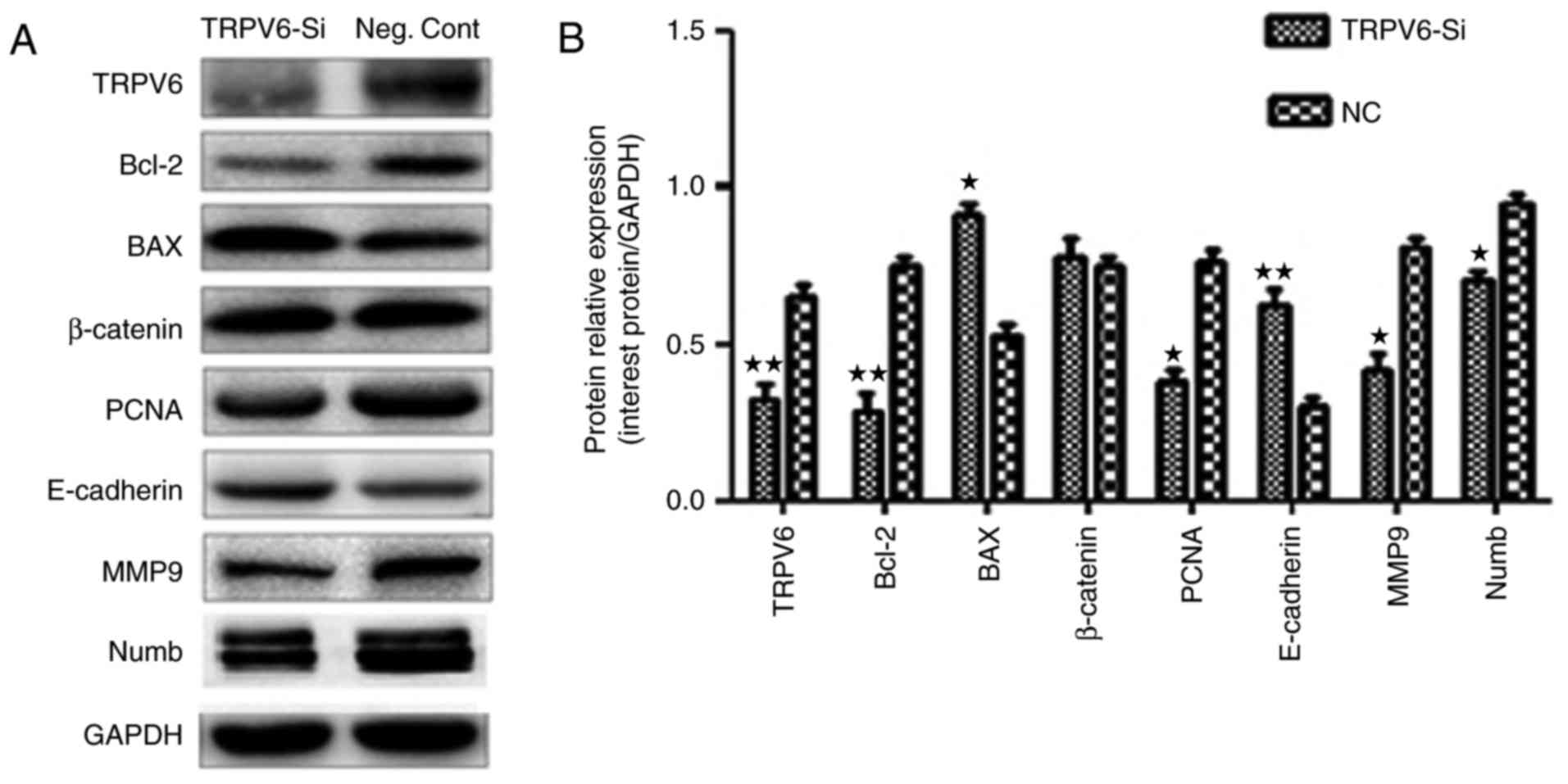
Proteins in the cell cycle pathway and proliferation
were predicted to be regulated by TRPV6 expression including PCNA
in Capan-2 cells (Fig. 11A). Two
core proteins Bax and Bcl-2 in the cell apoptosis pathway were
predicted to be regulated by TRPV6 expression, including in Capan-2
cells (Fig. 11A). E-cadherin and
MMP9 were involved in the invasion pathway were also predicted to
be regulated by TRPV6 (Fig. 11A).
However, expression of β-catenin and other proteins such as
cyclin-D1, cyclin-E1, CDK6, p21 (data not shown) did not change
significantly. TRPV6 may regulate the cell cycle, apoptosis and
invasion processes by regulating these multiple proteins.
Previously, we found the role of Numb-MDM2-P53
interaction in PC cell lines (16).
In the study, Kim et al verified a novel relationship
between Numb-TRPV6 in prostate cancer cells (12). Thus, we asked whether there was a
novel interaction between Numb and TRPV6 in the Capan-2 cells.
TRPV6-siRNA in Capan-2 cells decreased the amount of TRPV6 protein,
consistent with previous results (12,13)
(Fig. 11A). However, knockdown of
Numb expression resulted in no change of TRPV6 expression, in
contrast to these results (12,13)
(data not shown).
Discussion
TRPV6 performed by in situ hybridization
experiments by Wissenbach et al (17) were found in the normal pancreatic
tissue rather than pancreatic carcinoma in only two paired samples.
In this study, it was demonstrated that TRPV6 protein and mRNA
level were generally upregulated in PC tissues compared to adjacent
normal tissue. Positive expression of TRPV6 is significantly
related with the unfavorable survival in the PC patients suggesting
that TRPV6 is an oncogene in PC and might participate in the PC
development and progression. Some reports suggested TRPV6 as
important biomarkers in the development and progression of breast
cancer, colon cancer, prostate cancer, thyroid cancer and
parathyroid cancer (3,5,8,17,18).
However, decreased TRPV6 mRNA and protein level was observed from
esophageal cancer, non-small cell lung cancer and renal cancer. The
results may be related to physiological disorder caused by tumor
development or non-identical modification in tumorigenic cells.
TRPV6 regulated by various upstream genes may result in tumor
suppressive or oncogenic function in different cancers. Some
studies showed that TRPV6 may play a protective role in colon
cancer and gastric cancer (3,19,20).
Curcumin could up-regulate TRPV6 to promote calcium uptake for the
protection against colon cancer (19). The abundance of TRPV6 could
determine its protective role via capsaicin against gastric cancer
(20). Thus, antitumor effect of
TRPV6 regulated by some genes may be associated with the protective
role in some cancers. On the other hand, oncogenic effect of TRPV6
could be regulated by different genes in other cancers. The
pleiotropic functions of TRPV6 and its mechanism should be
clarified according to distinct cellular context. TRVP6 is a highly
selective calcium channel contributing to store-operated calcium
entry (SOCE) activity involving the plasma membrane via the
Orai1/TRPC1-mediated Ca2+/Annexin I/S100 pathway in
prostate cancer cell line (21).
The role of calcium are known to affect proliferation (22), apoptosis (23) and migration (24). Peleg et al found silencing
TRPV6 inhibited proliferation and induced apoptosis in colon
carcinoma cells (25). On the other
hand, Schwarz et al found overexpression of TRPV6 increased
proliferation of HEK-293 cells in a Ca2+ dependent
manner (22). Lehen'kyi et
al regarded that TRPV6-siRNA was directly involved in
proliferation and cell cycle in LNCaP cells (26). In our study, using Capan-2 and
SW1990 PC cell transfected with TRPV6-siRNA, cell proliferation,
migration and invasion was suppressed; the cell cycle was arrested
in the G1 phase; and apoptosis was promoted. Overexpression of
TRPV6 in PC may alter key features of the cells leading to
malignant biological behavior.
At present, gemcitabine is the first line
chemotherapy of pancreatic cancer. There are no randomized data
favoring neoadjuvant (including 5-fluorouracil, irinotecan and
oxaliplatin, gemcitabine with or without abraxane, or docetaxel and
capecitabine) overwhelming adjuvant therapy (27). Docetaxel/oxaliplatin (DocOx)
combination as the second line treatment for advanced pancreatic
cancer is an effective option (28). In our study, silencing TRPV6
expression can significantly increase chemotherapy sensitivity of
PC cells to oxaliplatin, rather than gemcitabine. It could provide
a proper research basis on the drug related to calcium decreasing
the side effect of oxaliplatin for pancreatic cancer.
PCNA has been found to participate in cell cycle
regulation and DNA replication. Our study showed TRPV6-siRNA could
decrease the expression of PCNA, consistent with the study of
Lehen'kyi et al (26).
Although proteins such as cyclin-D1, cyclin-E1, CDK6, p21 did not
change significantly, there would be some genes related to cell
cycle such as cyclin-A, cyclin-B1 to be detected in the future.
Maybe phosphorylation or other modifications occurred in the
protein such as CDK6 instead of alteration of amount of protein.
The further experiments should be validated. Increasing Bax/Bcl-2
ratios by TRPV6-siRNA, apoptosis was promoted suggesting a
mitochondrial apoptotic pathway (29,30).
Functionally, E-cadherin acts as the cancer suppressor gene and
regulates cell polarity, differentiation, migration and invasion
(31). Invasion and metastasis of
tumor involving degradation of ECM (extracellular matrix) and
basement membrane by MMP-9 (matrix metalloproteinase-9) are
critical determinants of cancer morbidity (32). It is probable that upregulation of
E-cadherin and downregulation by TRPV6-siRNA could be involved in
invasion and migration of PC.
We showed that TRPV6 reduced expression of Numb
protein in Capan-2 cells indicating Numb probably acting as a
oncogene. Knockdown of Numb did not alter TRPV6 expression in PC
cell lines. It was very confusing that our previous study showed
Numb as the cancer suppressor gene (33). It is hypothesized that regulatory
factors in TRPV6 toward Numb functions abnormally in PC cells due
to unknown mutations.
In summary, TRPV6 was significantly overexpressed in
PC tissues and cell lines. This could indicate a promising role of
TRPV6 in PC carcinogenesis. Moreover, TRPV6 had significant
correlation with the cell cycle, apoptosis and metastasis pathways
and regulated expression of related proteins. Inhibiting TRPV6
expression can increase chemotherapy sensitivity of PC cells to the
second line drug oxaliplatin. Our study indicted the feasibility of
TRPV6 as a potential therapeutic for PC.
Acknowledgements
The study was sponsored by Chinese National Science
Foundation (no. 81672835) to M.D.
References
|
1
|
Ansari D, Tingstedt B, Andersson B,
Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M
and Andersson R: Pancreatic cancer: Yesterday, today and tomorrow.
Future Oncol. 12:1929–1946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chari ST, Kelly K, Hollingsworth MA,
Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto
M, Cleeter DF, et al: Early detection of sporadic pancreatic
cancer: Summative review. Pancreas. 44:693–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lehen'kyi V, Raphaël M and Prevarskaya N:
The role of the TRPV6 channel in cancer. J Physiol. 590:1369–1376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wissenbach U, Niemeyer B, Himmerkus N,
Fixemer T, Bonkhoff H and Flockerzi V: TRPV6 and prostate cancer:
Cancer growth beyond the prostate correlates with increased TRPV6
Ca2+ channel expression. Biochem Biophys Res Commun.
322:1359–1363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giusti L, Cetani F, Da Valle Y, Pardi E,
Ciregia F, Donadio E, Gargini C, Piano I, Borsari S, Jaber A, et
al: First evidence of TRPV5 and TRPV6 channels in human parathyroid
glands: Possible involvement in neoplastic transformation. J Cell
Mol Med. 18:1944–1952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ouadid-Ahidouch H, Dhennin-Duthille I,
Gautier M, Sevestre H and Ahidouch A: TRP calcium channel and
breast cancer: Expression, role and correlation with clinical
parameters. Bull Cancer. 99:655–664. 2012.(In French). PubMed/NCBI
|
|
7
|
Zhang SS, Xie X, Wen J, Luo KJ, Liu QW,
Yang H, Hu Y and Fu JH: TRPV6 plays a new role in predicting
survival of patients with esophageal squamous cell carcinoma. Diagn
Pathol. 11:142016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan H, Shen YX and Yuan YF: Expression and
prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer
after curative resection. Asian Pac J Cancer Prev. 15:2559–2563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Miyamoto T, Li K, Nakagomi H, Sawada
N, Kira S, Kobayashi H, Zakohji H, Tsuchida T, Fukazawa M, et al:
Decreased expression of the epithelial Ca2+ channel
TRPV5 and TRPV6 in human renal cell carcinoma associated with
vitamin D receptor. J Urol. 186:2419–2425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uemura T, Shepherd S, Ackerman L, Jan LY
and Jan YN: numb, a gene required in determination of cell fate
during sensory organ formation in Drosophila embryos. Cell.
58:349–360. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gulino A, Di Marcotullio L and Screpanti
I: The multiple functions of Numb. Exp Cell Res. 316:900–906. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SY, Hong C, Wie J, Kim E, Kim BJ, Ha
K, Cho NH, Kim IG, Jeon JH and So I: Reciprocal positive regulation
between TRPV6 and NUMB in PTEN-deficient prostate cancer cells.
Biochem Biophys Res Commun. 447:192–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SY, Yang D, Myeong J, Ha K, Kim SH,
Park EJ, Kim IG, Cho NH, Lee KP, Jeon JH, et al: Regulation of
calcium influx and signaling pathway in cancer cells via
TRPV6-Numb1 interaction. Cell Calcium. 53:102–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamasawa K, Nio Y, Dong M, Yamaguchi K and
Itakura M: Clinicopathological significance of abnormalities in
Gadd45 expression and its relationship to p53 in human pancreatic
cancer. Clin Cancer Res. 8:2563–2569. 2002.PubMed/NCBI
|
|
15
|
Masunaga R, Kohno H, Dhar DK, Ohno S,
Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H and
Nagasue N: Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.PubMed/NCBI
|
|
16
|
Sheng W, Dong M, Zhou J, Li X, Liu Q, Dong
Q and Li F: The relationship and clinicopathological significance
of Numb, MDM2 and p53 expression in human pancreatic cancer.
Zhonghua Wai Ke Za Zhi. 52:675–681. 2014.(In Chinese). PubMed/NCBI
|
|
17
|
Wissenbach U, Niemeyer BA, Fixemer T,
Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H and
Flockerzi V: Expression of CaT-like, a novel calcium-selective
channel, correlates with the malignancy of prostate cancer. J Biol
Chem. 276:19461–19468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dhennin-Duthille I, Gautier M, Faouzi M,
Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H and
Ouadid-Ahidouch H: High expression of transient receptor potential
channels in human breast cancer epithelial cells and tissues:
Correlation with pathological parameters. Cell Physiol Biochem.
28:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartik L, Whitfield GK, Kaczmarska M,
Lowmiller CL, Moffet EW, Furmick JK, Hernandez Z, Haussler CA,
Haussler MR and Jurutka PW: Curcumin: A novel nutritionally derived
ligand of the vitamin D receptor with implications for colon cancer
chemoprevention. J Nutr Biochem. 21:1153–1161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chow J, Norng M, Zhang J and Chai J: TRPV6
mediates capsaicin-induced apoptosis in gastric cancer cells -
Mechanisms behind a possible new ‘hot’ cancer treatment. Biochim
Biophys Acta. 1773:565–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raphaël M, Lehen'kyi V, Vandenberghe M,
Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E,
Bokhobza A, Mihalache A, et al: TRPV6 calcium channel translocates
to the plasma membrane via Orai1-mediated mechanism and controls
cancer cell survival. Proc Natl Acad Sci USA. 111:pp. E3870–E3879.
2014; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwarz EC, Wissenbach U, Niemeyer BA,
Strauss B, Philipp SE, Flockerzi V and Hoth M: TRPV6 potentiates
calcium-dependent cell proliferation. Cell Calcium. 39:163–173.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vanden Abeele F, Roudbaraki M, Shuba Y,
Skryma R and Prevarskaya N: Store-operated Ca2+ current
in prostate cancer epithelial cells. Role of endogenous
Ca2+ transporter type 1. J Biol Chem. 278:15381–15389.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tauzin S, Chaigne-Delalande B, Selva E,
Khadra N, Daburon S, Contin-Bordes C, Blanco P, Le Seyec J, Ducret
T, Counillon L, et al: The naturally processed CD95L elicits a
c-yes/calcium/PI3K-driven cell migration pathway. PLoS Biol.
9:e10010902011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peleg S, Sellin JH, Wang Y, Freeman MR and
Umar S: Suppression of aberrant transient receptor potential cation
channel, subfamily V, member 6 expression in hyperproliferative
colonic crypts by dietary calcium. Am J Physiol Gastrointest Liver
Physiol. 299:G593–G601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lehen'kyi V, Flourakis M, Skryma R and
Prevarskaya N: TRPV6 channel controls prostate cancer cell
proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene.
26:7380–7385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Russo S, Ammori J, Eads J and Dorth J: The
role of neoadjuvant therapy in pancreatic cancer: A review. Future
Oncol. 12:669–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ettrich TJ, Perkhofer L, von Wichert G,
Gress TM, Michl P, Hebart HF, Büchner-Steudel P, Geissler M, Muche
R, Danner B, et al: DocOx (AIO-PK0106): A phase II trial of
docetaxel and oxaliplatin as a second line systemic therapy in
patients with advanced pancreatic ductal adenocarcinoma. BMC
Cancer. 16:212016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rajan S, Choi M, Nguyen QT, Ye H, Liu W,
Toh HT, Kang C, Kamariah N, Li C, Huang H, et al: Structural
transition in Bcl-xL and its potential association with
mitochondrial calcium ion transport. Sci Rep. 5:106092015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luna-Vargas MP and Chipuk JE: The deadly
landscape of pro-apoptotic BCL-2 proteins in the outer
mitochondrial membrane. FEBS J. 283:2676–2689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15:182016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verma S, Kesh K, Gupta A and Swarnakar S:
An overview of matrix metalloproteinase 9 polymorphism and gastric
cancer risk. Asian Pac J Cancer Prev. 16:7393–7400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sheng W, Dong M, Zhou J, Li X, Liu Q, Dong
Q and Li F: Cooperation among Numb, MDM2 and p53 in the development
and progression of pancreatic cancer. Cell Tissue Res. 354:521–532.
2013. View Article : Google Scholar : PubMed/NCBI
|