Introduction
Colon cancer is the most commonly diagnosed cancer
and one of the leading causes of deaths in the United States and
worldwide (1,2). It is the third most common cancer in
men and the second most common cancer in women worldwide (2). Radiation treatment and chemotherapy
are not therapeutically sufficient due to side effects and drug
resistance (3). Several studies
have shown close associations between colon cancer and dietary
factors such as fruit, vegetables, and seaweeds containing a wide
variety of phytochemicals. Some of these phytochemicals have been
shown to protect cells from damage leading to cancer.
Fucoidan is a fucose-rich sulfated polysaccharide
found in various species of brown seaweed (4–7).
Fucoidan has been shown to exert various biological activities,
including antioxidant, anti-inflammatory, anti-angiogenic,
anti-coagulant, anti-bacterial, and anticancer effects (8–18).
Studies have shown anticancer activities of fucoidan in lung,
breast, liver, colon, prostate, and bladder cancer cells (19–23).
Fucoidan has also been shown to induce tumor cell injury leading to
growth arrest and tumor suppression via apoptosis and cell cycle
arrest in various cancer cell types (24–29).
Therefore, fucoidan shows promise as a new therapeutic compound for
cancer treatment. Despite numerous studies showing the
chemopreventive effects of fucoidan in several cancer models, its
mechanism of action has not been fully elucidated.
The insulin-like growth factor (IGF) signaling
system, consisting of ligands (IGF-I and IGF-II), growth factor
receptors (IGF-IR and IGF-IIR), and IGF binding proteins
(IGFBPs-1-6), regulates cell growth, proliferation, transformation,
differentiation, migration and apoptosis (30,31).
IGFs play a vital role in the growth of various cancer cells,
including colon cancer cells (31,32).
IGF-I and IGF-II mRNA levels are highly increased in colon cancer
(33,34). Ligand binding to IGF-IR triggers two
main downstream signaling pathways, the insulin receptor
substrate-1 (IRS-1)/phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT) pathway and the Ras/Raf/extracellular
signal-regulated kinase (ERK) pathway. The IRS-1/PI3K/AKT pathway
is implicated in the transmission of cell survival signals, and the
Ras/Raf/ERK pathway is implicated in receptor-mediated mitogenesis
and transformation (35,36).
In previous studies, fucoidan inhibited HT-29 cell
proliferation by inducing apoptosis (23). In anti-colon cancer-related studies,
the epidermal growth factor (EGF) pathway has been investigated,
but no studies have investigated the IGF-I pathway (37). Therefore, in the present study, we
examined whether fucoidan downregulates IGF-IR signaling in HT-29
cells.
Materials and methods
Preparation of fucoidan
Fucoidan purified from Fucus vesiculosus
(cat. no. sc-255187) was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Fucoidan was dissolved in RPMI-1640 medium
(GenDEPOT, Inc., Barker, TX, USA) at concentrations of 0–1,000
µg/ml.
Cell culture
HT-29 human colon adenocarcinoma cells (cat. no.
30038) were purchased from the Korean Cell Line Bank (Seoul,
Korea). The cells were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS; GenDEPOT, Inc.) containing 50
µg/ml penicillin, 25 µg/ml amphotericin B, and 50 µg/ml
streptomycin, in an incubator with 5% CO2 at 37°C.
Cell proliferation assay
Cell proliferation was estimated using a Cyto X cell
viability assay kit (LPS Solution, Daejeon, Korea). Cells were
seeded in 96-well plates at a density of 4×104
cells/well and allowed to attach for 24 h. Attached cells were
treated with 62.5, 125, 250, 500 or 1,000 µg/ml of fucoidan in
serum-free medium for 24 h. The cell proliferation assay solution
was added and incubated for 1 h, and the absorbance of each well
was measured at a wavelength of 450 nm using a FilterMax F5
microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA).
Immunoprecipitation and western blot
analysis
HT-29 cells were cultured with 0, 62.5, 125, 250, or
500 µg/ml of fucoidan for 24 h. Subsequently, cells were washed
with phosphate-buffered saline (PBS) and lysed with extraction
buffer (1% Nonidet P-40, 1 mM EDTA, 50 mM Tris, pH 7.4, 0.25%
Na-deoxycholate, 150 mM NaCl, 1 mM sodium orthovanadate, 1 µg/ml
aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin A, 1 mM NaF, and 1
mM PMSF). The extracts were centrifuged at 9,750 × g for 10 min,
and the supernatant was used for western blot analysis.
For immunoprecipitation (IP), cells were incubated
for 24 h with 0 or 250 µg/ml of fucoidan, and 10 nM of IGF-I
(recombinant human IGF-I; Invitrogen Life Technologies, Frederick,
MD, USA) was added. At 0, 5, 30 or 60 min after the addition of
IGF-I, the cell lysates were centrifuged at 9,750 × g for 10 min.
Supernatant (0.90 mg protein) were incubated with 3 µl of
anti-IGF-IRβ or IRS-1 antibody overnight at 4°C. Protein A-agarose
beads (GenDEPOT, Inc.) were added to the lysate-antibody mix, which
was then incubated for 4 h at 4°C. The beads were washed 3 times
with extraction buffer. The immunoprecipitates and total protein
(40 µg) were electrophoresed using 8–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). Membranes were blocked with 1% bovine serum
albumin (BSA; GenDEPOT, Inc.) in Tris-buffered saline-Tween-20
(TBS-T; 5 mM Tris-HCl, 20 mM sodium chloride pH 7.4, and 0.1%
Tween-20) incubated with primary antibodies (1:1,000) in 1% BSA in
TBS-T with gentle shaking overnight at 4°C. Membranes were washed
twice for 15 min in TBS-T, and incubated with the corresponding
horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:10,000) for 2 h at room temperature and washed again.
Immunoreactive bands were detected using an enhanced
chemiluminescence substrate (Advansta, Inc., Menlo Park, CA, USA)
and visualized using the GeneSys imaging system (SynGene Synoptics,
Ltd., London, UK). The following primary antibodies from Santa Cruz
Biotechnology, Inc., and Invitrogen were used: anti-p-IGF-IR
(sc-101703, anti-rabbit), anti-IGF-IR (sc-390130, anti-mouse),
anti-phospho-tyrosine (PY99; sc-7020, anti-mouse), anti-p-IRS-1
(sc-17200, anti-goat), anti-IRS-1 (sc-185, anti-mouse), anti-p-AKT
(sc-7985, anti-rabbit), anti-AKT (sc-8312, anti-rabbit),
anti-p-PI3K (PA5-17387, anti-mouse), anti-PI3K (sc-374534,
anti-mouse), anti-Ras (sc-520, anti-rabbit), anti-Raf (sc-227,
anti-rabbit), anti-p-MEK (sc-81503, anti-mouse), anti-MEK
(sc-81504, anti-mouse), anti-p-ERK (sc-7383, anti-mouse), anti-ERK
(sc-292838, anti-rabbit), anti-SOS (sc-259, anti-rabbit), anti-Grb2
(sc-255, anti-rabbit), anti-Shc (sc-967, anti-mouse), anti-PTEN
(sc-7974, anti-mouse), and anti-β-actin (sc-47778, anti-mouse). The
secondary antibodies used were HRP-conjugated anti-mouse IgG
(32430), anti-rabbit IgG (31460), and anti-goat IgG (31400) (all
from Invitrogen Life Technologies).
Statistical analyses
The results are presented as means ± standard
deviation of three independent experiments. The difference in
protein expression levels was determined by quantifying the density
of the bands using ImageJ (NIH). Significant differences among
multiple mean values were assessed using one-way or two-way
analysis of variance followed by Bonferroni's multiple comparison
test using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Fucoidan induces cell death in HT-29
cells
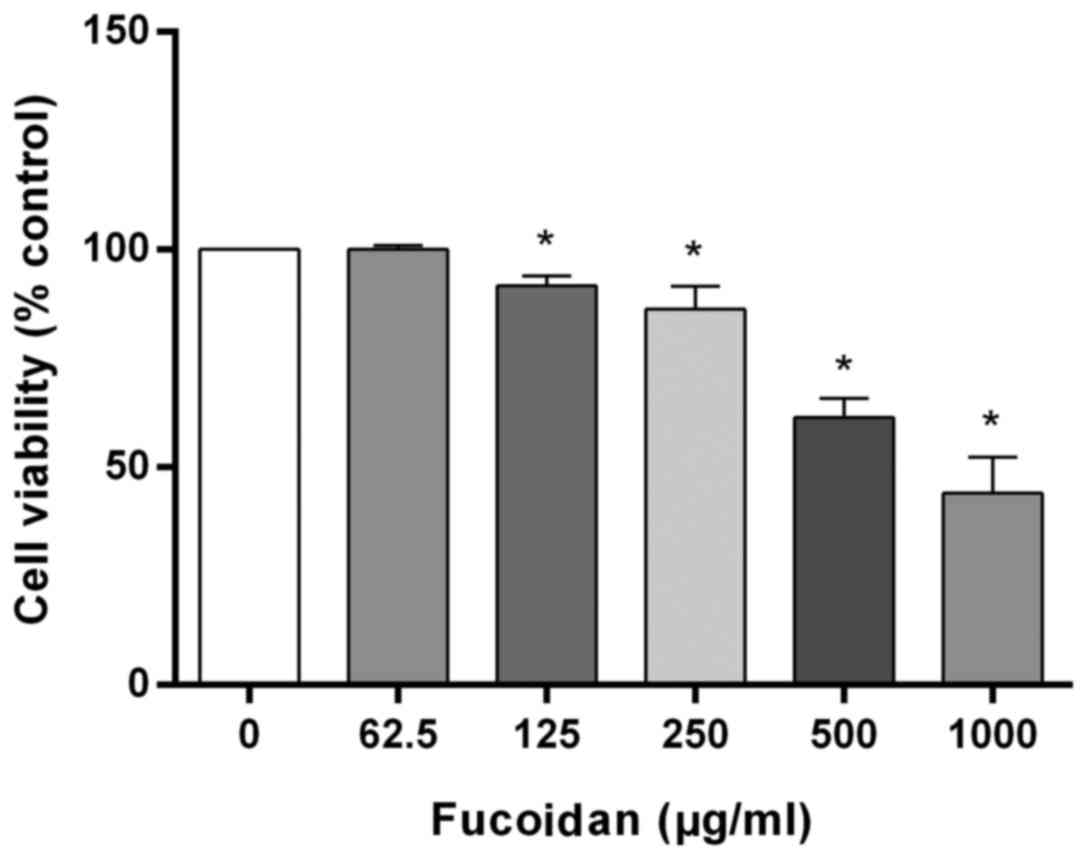
To investigate the effects of fucoidan on cell
viability, HT-29 cells were incubated with various concentrations
(0–1,000 µg/ml) of fucoidan for 24 h. Fucoidan treatment
significantly decreased the viability of HT-29 cells in a
concentration-dependent manner (Fig.
1). After treatment with 125, 250, 500 and 1,000 µg/ml
fucoidan, cell viability was significantly decreased to 91.7±2.3,
86.3±5.3, 61.5±4.4 and 44.1±8.3%, respectively.
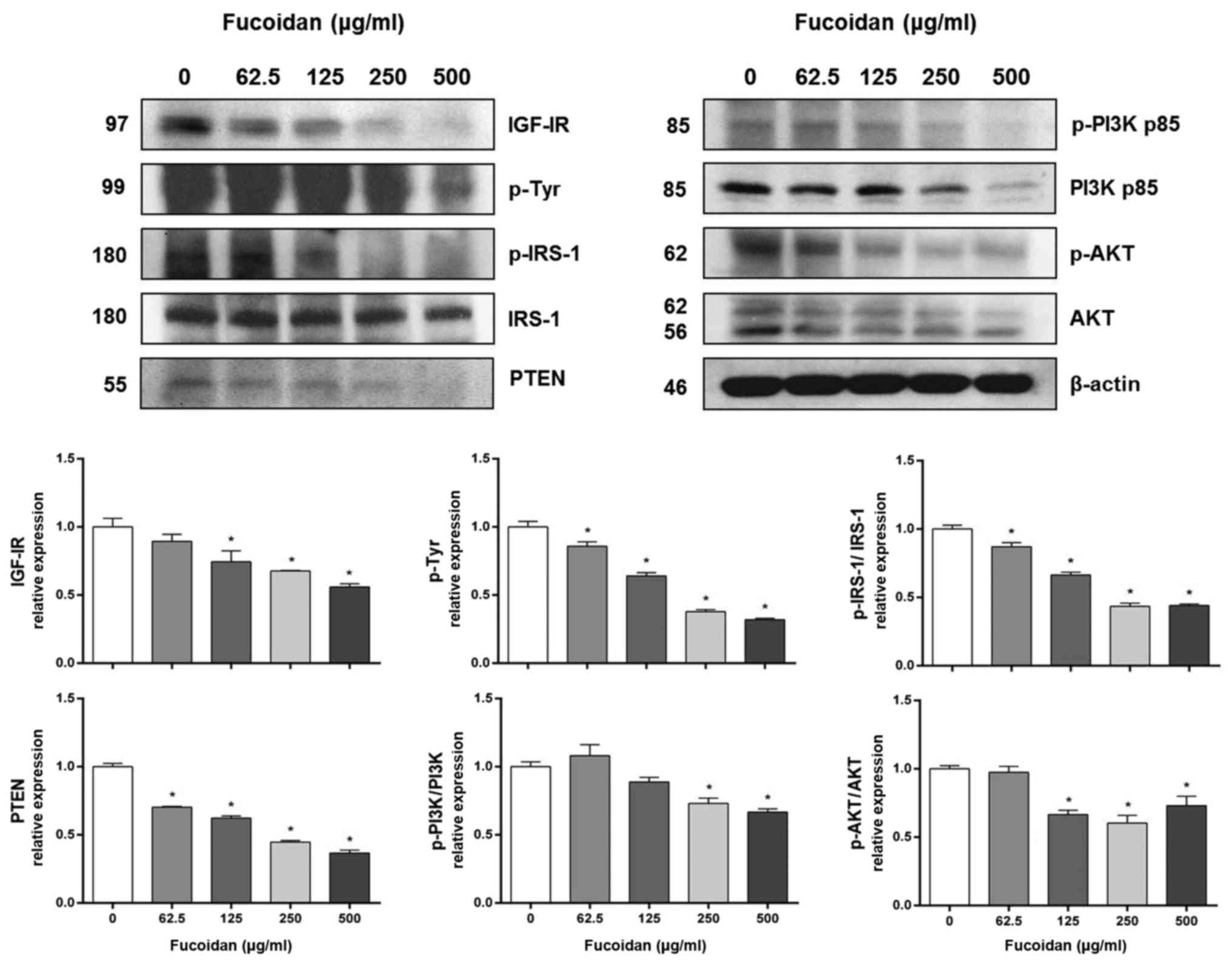
Fucoidan reduces the levels of
IRS-1/PI3K/AKT pathway-related proteins in HT-29 cells
IGFs signaling is known to affect cell survival, and
IGF-I mRNA is increased in colon cancer cells (33,34).
Therefore, we investigated whether the IGF-IR signaling pathway is
involved in fucoidan-induced cell death. First, we investigated
IRS-1/PI3K/AKT pathway-related protein expression levels, one of
the two main downstream IGF-IR signaling pathways. Fucoidan
treatment significantly decreased the expression of IGF-IR,
phospho-tyrosine and PTEN in HT-29 cells in a
concentration-dependent manner (Fig.
2). Fucoidan treatment also significantly decreased the ratios
of p-IRS-1/IRS-1, p-PI3K/PI3K and p-AKT/AKT expression.
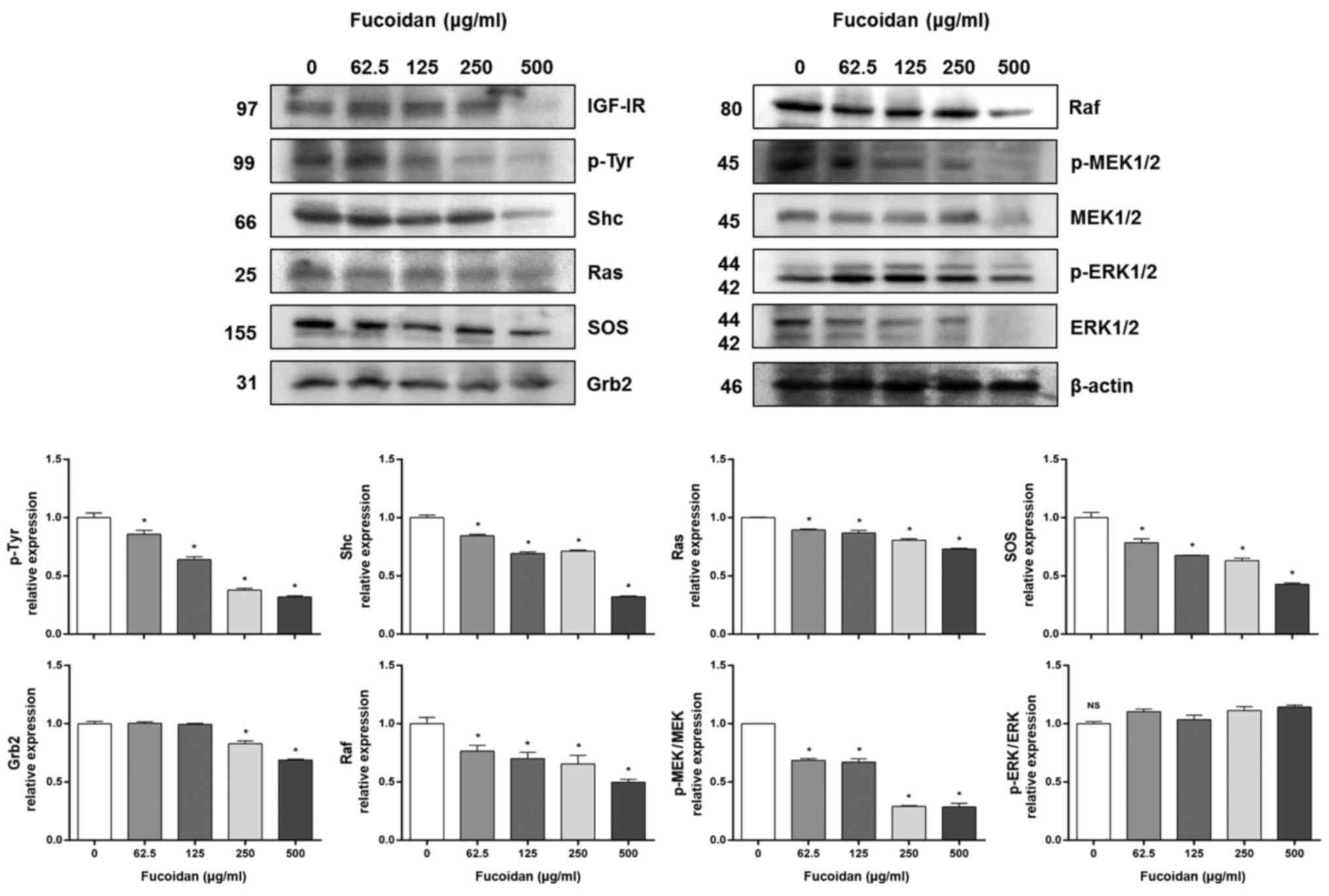
Fucoidan reduces the levels of
Ras/Raf/ERK pathway-related proteins in HT-29 cells
Next, we investigated Ras/Raf/ERK pathway-related
protein expression levels, the other main IGF-IR downstream
signaling pathway. Fucoidan treatment significantly decreased the
expression of IGF-IR, phospho-tyrosine, Shc, Ras, SOS, Grb2, and
Raf in HT-29 cells in a concentration-dependent manner (Fig. 3). Fucoidan treatment also
significantly decreased the expression of p-MEK/MEK, but not
p-ERK/ERK.
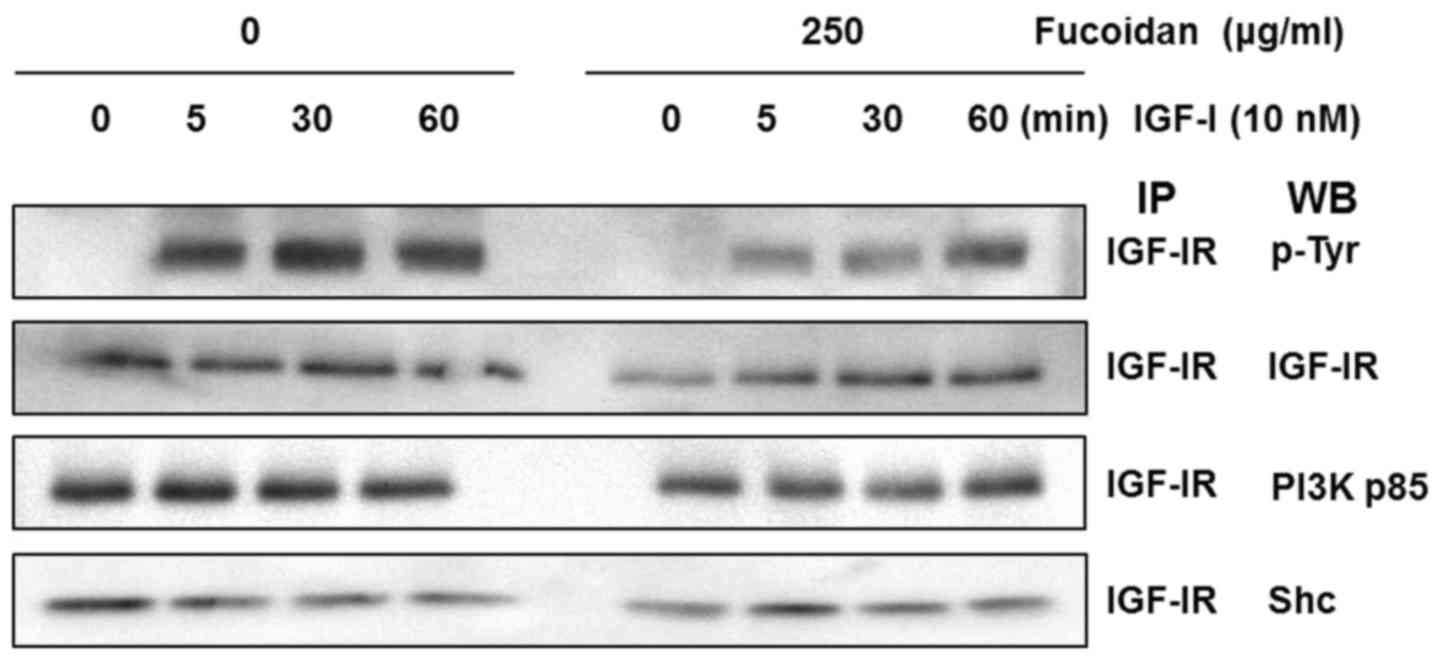
Fucoidan reduces IGF-I-induced IGF-IR
activation of the IGF-IR signaling pathway
We examined whether fucoidan downregulates
IGF-I-induced tyrosine phosphorylation of IGF-IR. HT-29 cells were
treated for 24 h with 0 or 250 µg/ml fucoidan, and IGF-IR was
stimulated with 10 nM IGF-I for 0, 5, 30, or 60 min. Total cell
lysates were prepared and immunoprecipitated using an IGF-IRβ
antibody. The immune complexes were used in western blot analysis
with an anti-phospho-tyrosine antibody (PY99). IGF-I induced
tyrosine phosphorylation of IGF-IR at 5 min, and tyrosine
phosphorylation levels persisted at 60 min in control cells. Cells
treated with fucoidan exhibited significantly inhibited IGF-IRβ
phosphorylation up to 30 min after IGF-I stimulation (Fig. 4).
To investigate the association of the Shc and p85
subunits of PI3K with IGF-IR, we performed IP of cell lysates with
an IGF-IRβ antibody and subsequent western blot analysis with p85
and Shc antibodies. IGF-I stimulated the association of the p85
regulatory subunit of PI3K with IGF-IR. Expression of the p85
regulatory subunit of PI3K in the control group was induced within
5 min, but fucoidan treatment delayed its expression by up to 30
min. IGF-I also stimulated the association of the Shc subunit with
IGF-IR. The expression of Shc in the control cells was induced
within 1 min, but in cells treated with fucoidan, expression was
induced at 5 min.
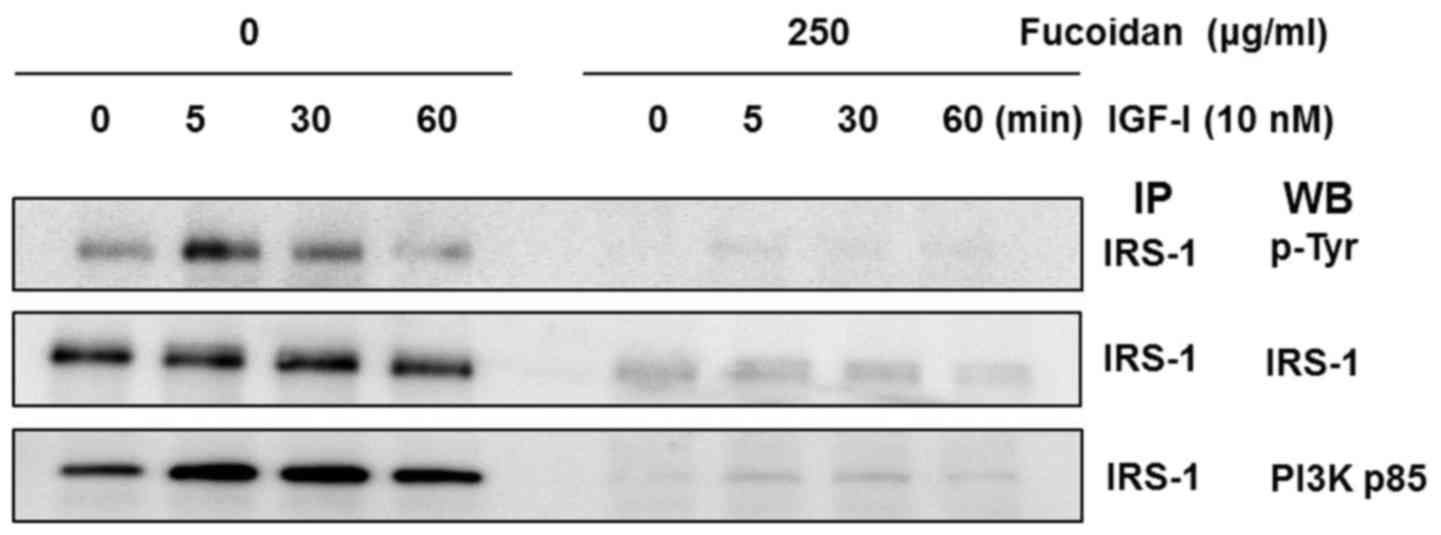
Fucoidan reduces IGF-I-induced IRS-1
activation in the IGF-IR signaling pathway
We examined whether fucoidan downregulates
IGF-I-induced tyrosine phosphorylation of IRS-1. HT-29 cells were
treated for 24 h with 0 or 250 µg/ml fucoidan, and IGF-IR was
stimulated with 10 nM IGF-I for 0, 5, 30, or 60 min. Total cell
lysates were prepared and immunoprecipitated using an IRS-1
antibody. The immune complexes were used in western blot analysis
with anti-phospho-tyrosine antibody. IGF-I induced tyrosine
phosphorylation of IRS-1 at 5 min, and tyrosine phosphorylation
levels persisted for 30 min in control cells. Treatment with
fucoidan significantly inhibited the phosphorylation of IRS-1 for
up to 60 min after IGF-I stimulation (Fig. 5).
To investigate the association of the p85 subunit of
PI3K with IRS-1, we performed IP of cell lysates with an IRS-1
antibody and subsequent western blot analysis with a p85 antibody.
IGF-I stimulated the association of the p85 regulatory subunit of
PI3K and IRS-1 with IRS-1. Expression of the p85 regulatory subunit
of PI3K and IRS-1 in control cells were stimulated within 5 min,
but fucoidan treatment delayed expression for up to 60 min.
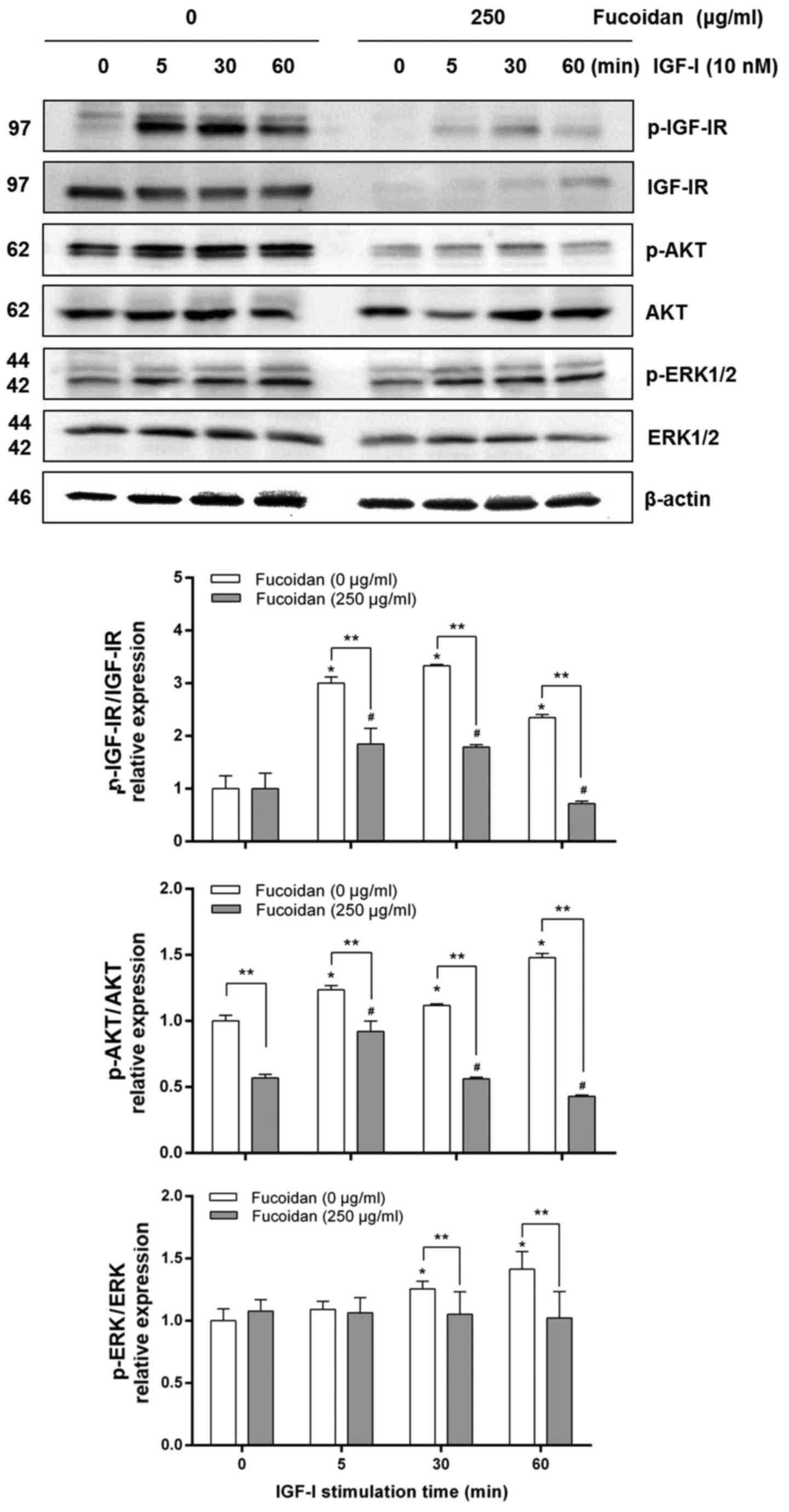
Fucoidan reduces IGF-I-induced
activation of AKT in HT-29 cells
AKT and ERK1/2 are known to play vital roles in cell
survival and are activated by IGF-I (33,37,38).
HT-29 cells were treated for 24 h with 0 or 250 µg/ml fucoidan, and
IGF-IR was stimulated with 10 nM IGF-I for 0, 5, 30, or 60 min. We
investigated whether fucoidan inhibits IGF-I-induced
phosphorylation of IGF-IR, AKT, and ERK, a downstream target of the
IGF-IR signaling pathway. Levels of p-IGF-IR/IGF-IR and p-AKT/AKT
increased in control cells in a time-dependent manner with IGF-I
stimulation (Fig. 6). Fucoidan
treatment delayed p-IGF-IR/IGF-IR and p-AKT/AKT expression for up
to 60 min, lower than the control cells level. In addition, in the
control group, p-ERK/ERK increased with IGF-I stimulation without
changing total ERK levels. There were no differences in
p-ERK/ERK1/2 expression in the fucoidan treatment group.
Discussion
Colon cancer is one of the most common cancers in
both men and women and is prevalent worldwide (1,2).
Globally, the incidence of colon cancer is increasing, seemingly
due to changes in dietary habits and preferences. Dietary habits
can affect the development of colon cancer, and food ingredients
may serve as chemotherapeutic agents (22,24,38–43).
Many studies have investigated potential colon cancer treatments.
Some studies have suggested marine natural products with
pharmacological activities as a method for treating colon cancer
(44). Among these marine natural
products, seaweeds have been reported to contain various useful
compounds such as laminarin, fucoidan, dactylone, and
meroditerpenoids with various effects in cancer cells (37–44).
Numerous studies have investigated the effects of seaweeds on cell
death pathways including apoptosis. The inhibition of apoptosis in
colon cancer cells promotes tumor growth and tumor progression and
imparts tolerance to cytotoxic anticancer drugs (7,22,23,27,40–43).
Studies related to colon cancer have found that
laminarin inhibits cancer cells via Fas and IGF-IR signaling
through the intrinsic apoptotic and ErbB pathways, and induces
Fas-mediated apoptosis by regulating Fas and Fas-associated protein
with death domain (FADD) protein levels (34,45–47).
Fucoidan suppressed growth, decreased metastasis, inhibited
angiogenesis, and induced apoptosis through activated caspases,
resulting in the induction of apoptosis through both death
receptor-mediated and mitochondria-mediated apoptotic pathways
(22,38,40–43).
Dactylone also induced G1-S cell cycle arrest and apoptosis in
tumor cells (44).
In previous studies, fucoidan induced apoptosis
through the apoptotic pathway and cytotoxicity, and inhibited
migration and proliferation in HT-29 colon cancer cells (22,24,28,40,42,43).
Fucoidan exerted an anticancer effect by downregulating the
PI3K-AKT-mTOR signaling pathway (40). In the present study, we investigated
the downregulation of IGF signaling pathways by fucoidan in HT-29
cells.
The present study provided the first evidence that
fucoidan reduces IGF-IR protein expression and IGF-IR-mediated
signaling through the IRS-1/PI3K/AKT pathway. We examined cell
proliferation using a cell viability assay kit with various
concentrations of fucoidan (0–1,000 µg/ml). The results indicated
that fucoidan inhibited cell proliferation in a dose-dependent
manner in HT-29 cells (Fig. 1).
IGF signaling plays a vital role in promoting normal
cell proliferation, tumorigenesis, and cancer cell proliferation.
IGF-I is also known to inhibit cell death and promote growth in
various cancer cells, including colon cancer cells. IGF-IR
expression is increased in colon cancer compared to normal mucosal
tissues (48). In the present
study, we investigated whether the anticancer effects of fucoidan
involve changes in the IGF-IR signaling pathway. The IGF-IR-related
pathway can be divided into two main downstream signaling pathways:
The IRS-1/PI3K/AKT and Ras/Raf/ERK pathways (35,36).
In the IRS-1/PI3K/AKT pathway, the protein expression of pathway
members, including IGF-IR, PTEN, PI3K, and AKT, decreased in a
concentration-dependent manner upon fucoidan treatment (Fig. 2). The protein expression of
Ras/Raf/ERK pathway members, including IGF-IR, Shc, Ras, SOS,
Grab2, Raf, and MEK, also decreased in a concentration-dependent
manner (Fig. 3).
The present study suggests that fucoidan inhibits
phosphorylation of the β-subunit of IGF-IR by downregulating the
α-subunit, directly interfering with the binding of IGF-I to
IGF-IR. In immunoprecipitation assays and western blot analysis,
fucoidan reduced IGF-I-induced tyrosine phosphorylation of IGF-IR
and IRS-1, leading to reduced interaction between PI3K p85 and
IGF-IRβ, and the subsequent activation of PI3K/AKT but not ERK1/2
(Figs. 4–6). AKT plays a vital role in PI3K-mediated
suppression of cell proliferation as a downstream target of the
PI3K/AKT pathway (28,40,49).
We found that fucoidan inhibited IGF-I-induced activation of AKT,
which may have been due to reduced IGF-IR levels and the subsequent
reduction in IGF-IR activation. The moderate reduction in AKT
protein levels may also have contributed to the reduced p-AKT
levels (Fig. 6). However, fucoidan
did not affect IGF-I-induced activation of p-ERK/ERK levels.
Fucoidan was also partially associated with the Ras/Raf pathway in
the Ras/Raf/ERK pathway.
Recent data indicate that fucoidan triggers G1 phase
arrest and apoptosis in HCT116 colon cancer cells through a
p53-independent pathway. In particular, it has been suggested that
fucoidan is able to enhance p21 expression at transcriptional level
in a p53-independent manner (50).
IGF-I signaling is strongly associated with cell growth and has a
significant role in ribosome biogenesis by regulating the
expression of proteins directly involved in the processing of
ribosomal subunits and p53-mediated regulation of cell cycle
progression (51). In particular,
IGF-I activates the murine double minute 2 (MDM2) protein which is
a component crucial regulator of cell proliferation and apoptosis
and acts in association to some ribosomal proteins by inhibiting
the p53 tumor suppressor (51). It
has been demonstrated that fucoidan is able to synergize with
standard anticancer agents as 5-FU and reduce its toxicity
(52,53). Recent data showed that in HCT116
colon cancer cells 5-FU is able to induce perturbation of ribosome
biogenesis led to nucleolar stress (52). This condition activates p53
independent pathway involving ribosomal proteins and MDM2 leading
to p21-mediated cell cycle arrest and apoptosis. In previous study,
fucoidan were induced G1 phase arrest and apoptosis in p53 mutated
colon cancer cell line, HT-29 (23). In present study, we examined whether
HT-29 cells affect the IGF-IR singnaling pathway involved in cell
proliferation during fucoidan. In this result, an interesting
possiblility may be that fucoidan through the IGF-I signlaing could
activate ribosomal protein/MDM2/p21 induced cell death. The
research on this is expected to be further progressed.
Based on these findings, we conclude that fucoidan
inhibits cell proliferation and induces apoptosis by inhibiting the
IGF-I-induced IGF-IR signaling pathway, including the
IRS-1/P13K/AKT pathway, in HT-29 cells. These results suggest that
downregulation of IGF-IR/IRS-1/PI3K/AKT signaling may be one of the
mechanisms by which fucoidan impacts HT-29 cells. These results
suggest that the anti-proliferative effect of fucoidan may be
mediated through downregulation of the IGF-I/IGF-IR/IRS-1/PI3K/AKT
signaling pathway. Therefore, fucoidan may be useful as a
chemopreventive agent in colon cancer cells.
Acknowledgements
This study was supported by Pukyong National
University (grant no. C-D-2016-0267), Busan, Republic of Korea.
References
|
1
|
Alwarsamy M, Gooneratne R and Ravichandran
R: Effect of fucoidan from Turbinaria conoides on human lung
adenocarcinoma epithelial (A549) cells. Carbohydr Polym.
152:207–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayat MJ, Howlader N, Reichman ME and
Edwards BK: Cancer statistics, trends, and multiple primary cancer
analyses from the Surveillance, Epidemiology, and End Results
(SEER) Program. Oncologist. 12:20–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li B, Lu F, Wei X and Zhao R: Fucoidan:
Structure and bioactivity. Molecules. 13:1671–1695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bilan MI, Grachev AA, Ustuzhanina NE,
Shashkov AS, Nifantiev NE and Usov AI: Structure of a fucoidan from
the brown seaweed Fucus evanescens C. Ag. Carbohydr Res.
337:719–730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen A, Lan Y, Liu J, Zhang F, Zhang L, Li
B and Zhao X: The structure property and endothelial protective
activity of fucoidan from Laminaria japonica. Int J Biol Macromol.
105:1421–1429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moussavou G, Kwak DH, Obiang-Obonou BW,
Maranguy CA, Dinzouna-Boutamba SD, Lee DH, Pissibanganga OG, Ko K,
Seo JI and Choo YK: Anticancer effects of different seaweeds on
human colon and breast cancers. Mar Drugs. 12:4898–4911. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang WN, Chen PW and Huang CY:
Compositional characteristics and in vitro evaluations of
antioxidant and neuroprotective properties of crude extracts of
fucoidan prepared from compressional puffing-pretreated Sargassum
crassifolium. Mar Drugs. 15:E1832017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Palanisamy S, Vinosha M, Marudhupandi T,
Rajasekar P and Prabhu NM: In vitro antioxidant and antibacterial
activity of sulfated polysaccharides isolated from Spatoglossum
asperum. Carbohydr Polym. 170:296–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palanisamy S, Vinosha M, Marudhupandi T,
Rajasekar P and Prabhu NM: Isolation of fucoidan from Sargassum
polycystum brown algae: Structural characterization, in vitro
antioxidant and anticancer activity. Int J Biol Macromol.
102:405–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phull AR, Majid M, Haq IU, Khan MR and Kim
SJ: In vitro and in vivo evaluation of anti-arthritic, antioxidant
efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar.
Int J Biol Macromol. 97:468–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryu MJ and Chung HS: Fucoidan reduces
oxidative stress by regulating the gene expression of HO 1 and SOD
1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol Med
Rep. 14:3255–3260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heeba GH and Morsy MA: Fucoidan
ameliorates steatohepatitis and insulin resistance by suppressing
oxidative stress and inflammatory cytokines in experimental
non-alcoholic fatty liver disease. Environ Toxicol Pharmacol.
40:907–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rui X, Pan HF, Shao SL and Xu XM:
Anti-tumor and anti-angiogenic effects of Fucoidan on prostate
cancer: Possible JAK-STAT3 pathway. BMC Complement Altern Med.
17:3782017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu F, Luo G, Xiao Q and Chen L, Luo X, Lv
J and Chen L: Fucoidan inhibits angiogenesis induced by multiple
myeloma cells. Oncol Rep. 36:1963–1972. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Till S, Jiang C, Knappe S,
Reutterer S, Scheiflinger F, Szabo CM and Dockal M:
Structure-activity relationship of the pro- and anticoagulant
effects of Fucus vesiculosus fucoidan. Thromb Haemost. 111:429–437.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cumashi A, Ushakova NA, Preobrazhenskaya
ME, D'Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE,
Berman AE, Bilan MI, et al Consorzio Interuniversitario Nazionale
per la Bio-Oncologia, Italy, : A comparative study of the
anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive
activities of nine different fucoidans from brown seaweeds.
Glycobiology. 17:541–552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen HY, Li LZ, Xue KC, Hu DD and Gao YJ:
Antitumor activity of fucoidan in anaplastic thyroid cancer via
apoptosis and anti-angiogenesis. Mol Med Rep. 15:2620–2624. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu HY, Lin TY, Lu MK, Leng PJ, Tsao SM
and Wu YC: Fucoidan induces Toll-like receptor 4-regulated reactive
oxygen species and promotes endoplasmic reticulum stress-mediated
apoptosis in lung cancer. Sci Rep. 7:449902017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue M, Ji X, Xue C, Liang H, Ge Y, He X
and Zhang L, Bian K and Zhang L: Caspase-dependent and
caspase-independent induction of apoptosis in breast cancer by
fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro.
Biomed Pharmacother. 94:898–908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho Y, Cho EJ, Lee JH, Yu SJ, Kim YJ, Kim
CY and Yoon JH: Fucoidan-induced ID-1 suppression inhibits the in
vitro and in vivo invasion of hepatocellular carcinoma cells.
Biomed Pharmacother. 83:607–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Somasundaram SN, Shanmugam S, Subramanian
B and Jaganathan R: Cytotoxic effect of fucoidan extracted from
Sargassum cinereum on colon cancer cell line HCT-15. Int J Biol
Macromol. 91:1215–1223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim IH, Kwon MJ and Nam TJ: Differences in
cell death and cell cycle following fucoidan treatment in
high-density HT-29 colon cancer cells. Mol Med Rep. 15:4116–4122.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han MH, Lee DS, Jeong JW, Hong SH, Choi
IW, Cha HJ, Kim S, Kim HS, Park C, Kim GY, et al: Fucoidan induces
ROS-dependent apoptosis in 5637 human bladder cancer cells by
downregulating telomerase activity via inactivation of the PI3K/Akt
signaling pathway. Drug Dev Res. 78:37–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue M, Ge Y, Zhang J, Liu Y, Wang Q, Hou L
and Zheng Z: Fucoidan inhibited 4T1 mouse breast cancer cell growth
in vivo and in vitro via downregulation of Wnt/β-catenin signaling.
Nutr Cancer. 65:460–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HY, Kim GY, Moon SK, Kim WJ, Yoo YH
and Choi YH: Fucoidan inhibits the proliferation of human urinary
bladder cancer T24 cells by blocking cell cycle progression and
inducing apoptosis. Molecules. 19:5981–5998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han YS, Lee JH and Lee SH: Antitumor
effects of fucoidan on human colon cancer cells via activation of
Akt signaling. Biomol Ther (Seoul). 23:225–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang G, Zhang Q, Kong Y, Xie B, Gao M, Tao
Y, Xu H, Zhan F, Dai B, Shi J, et al: Antitumor activity of
fucoidan against diffuse large B cell lymphoma in vitro and in
vivo. Acta Biochim Biophys Sin (Shanghai). 47:925–931. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atashrazm F, Lowenthal RM, Woods GM,
Holloway AF, Karpiniec SS and Dickinson JL: Fucoidan suppresses the
growth of human acute promyelocytic leukemia cells in vitro and in
vivo. J Cell Physiol. 231:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scagliotti GV and Novello S: The role of
the insulin-like growth factor signaling pathway in non-small cell
lung cancer and other solid tumors. Cancer Treat Rev. 38:292–302.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu L, Zhou R, Yuan L, Wang S, Li X, Ma H,
Zhou M, Pan C, Zhang J, Huang N, et al: IGF1/IGF1R/STAT3
signaling-inducible IFITM2 promotes gastric cancer growth and
metastasis. Cancer Lett. 393:76–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frasca F, Pandini G, Sciacca L, Pezzino V,
Squatrito S, Belfiore A and Vigneri R: The role of insulin
receptors and IGF-I receptors in cancer and other diseases. Arch
Physiol Biochem. 114:23–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis by laminarin, regulating the insulin-like
growth factor-IR signaling pathways in HT-29 human colon cells. Int
J Mol Med. 30:734–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HS, Cho HJ, Kwon GT and Park JH:
Kaempferol downregulates insulin-like growth factor-I receptor and
ErbB3 signaling in HT-29 human colon cancer cells. J Cancer Prev.
19:161–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fidler MJ, Shersher DD, Borgia JA and
Bonomi P: Targeting the insulin-like growth factor receptor pathway
in lung cancer: Problems and pitfalls. Ther Adv Med Oncol. 4:51–60.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vishchuk OS, Sun H, Wang Z, Ermakova SP,
Xiao J, Lu T, Xue P, Zvyagintseva TN, Xiong H, Shao C, et al:
PDZ-binding kinase/T-LAK cell-originated protein kinase is a target
of the fucoidan from brown alga Fucus evanescens in the prevention
of EGF-induced neoplastic cell transformation and colon cancer
growth. Oncotarget. 7:18763–18773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL and
Park JH: Conjugated linoleic acid downregulates insulin-like growth
factor-I receptor levels in HT-29 human colon cancer cells. J Nutr.
133:2675–2681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pollak MN, Schernhammer ES and Hankinson
SE: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han YS, Lee JH and Lee SH: Fucoidan
inhibits the migration and proliferation of HT-29 human colon
cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic
target of rapamycin pathways. Mol Med Rep. 12:3446–3452. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thinh PD, Menshova RV, Ermakova SP,
Anastyuk SD, Ly BM and Zvyagintseva TN: Structural characteristics
and anticancer activity of fucoidan from the brown alga Sargassum
mcclurei. Mar Drugs. 11:1456–1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim EJ, Park SY, Lee JY and Park JH:
Fucoidan present in brown algae induces apoptosis of human colon
cancer cells. BMC Gastroenterol. 10:962010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hyun JH, Kim SC, Kang JI, Kim MK, Boo HJ,
Kwon JM, Koh YS, Hyun JW, Park DB, Yoo ES, et al: Apoptosis
inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol
Pharm Bull. 32:1760–1764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fedorov SN, Shubina LK, Bode AM, Stonik VA
and Dong Z: Dactylone inhibits epidermal growth factor-induced
transformation and phenotype expression of human cancer cells and
induces G1-S arrest and apoptosis. Cancer Res. 67:5914–5920. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park HK, Kim IH, Kim J and Nam TJ:
Induction of apoptosis and the regulation of ErbB signaling by
laminarin in HT-29 human colon cancer cells. Int J Mol Med.
32:291–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ji CF and Ji YB: Laminarin-induced
apoptosis in human colon cancer LoVo cells. Oncol Lett.
7:1728–1732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ji YB, Ji CF and Zhang H: Laminarin
induces apoptosis of human colon cancer LOVO cells through a
mitochondrial pathway. Molecules. 17:9947–9960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo YS, Narayan S, Yallampalli C and Singh
P: Characterization of insulinlike growth factor I receptors in
human colon cancer. Gastroenterology. 102:1101–1108. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
Downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park HY, Park SH, Jeong JW, Yoon D, Han
MH, Lee DS, Choi G, Yim MJ, Lee JM, Kim DH, et al: Induction of
p53-independent apoptosis and G1 cell cycle arrest by fucoidan in
HCT116 human colorectal carcinoma cells. Mar Drugs. 15:E1542017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Worrall C, Suleymanova N, Crudden C,
Trocoli Drakensjö I, Candrea E, Nedelcu D, Takahashi SI, Girnita L
and Girnita A: Unbalancing p53/Mdm2/IGF-1R axis by Mdm2 activation
restrains the IGF-1-dependent invasive phenotype of skin melanoma.
Oncogene. 36:3274–3286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pagliara V, Saide A, Mitidieri E,
d'Emmanuele di Villa Bianca R, Sorrentino R, Russo G and Russo A:
5-FU targets rpL3 to induce mitochondrial apoptosis via
cystathionine-β-synthase in colon cancer cells lacking p53.
Oncotarget. 7:50333–50348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: rpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NFκB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar : PubMed/NCBI
|