Introduction
Large areas of surgical resection or high doses of
chemotherapy to treat oral squamous cell carcinoma (OSCC) lead to
serious clinical complications and poor prognosis (1,2). Thus,
to improve the quality of life of OSCC patients, better clinical
treatment strategies with fewer lesions and complications are
warranted.
Ultrasound (US) is already known to be a
chemosensitizer (3–10). US has immediate sonoporation effects
and can achieve the same clinical effect when a lower dose of the
same drug is used (11), resulting
in reduced side-effects. Low-intensity ultrasound (LIUS) can
increase the permeability of the plasma membrane and improve the
effects of anticancer drugs without causing damage to the entire
cell, both in vivo and in vitro (3). LIUS-mediated chemosensitivity is
mainly achieved by increasing the accumulation of intracellular
drugs, although other mechanisms may also be involved (4,9). LIUS
also plays a role in targeted chemotherapy, the direct release of
anticancer drugs, and the induction of apoptosis and necrosis of
cells (12,13).
We previously reported that LIUS (1.1 MHz, 1.0
W/cm2, 10% duty cycle) combined with low doses of
scutellarin or 5-fluorouracil produced enhanced synergistic
antitumor effects in vitro and in vivo, prolonged the
survival time of mice without significant cytotoxicity in normal
cells (3,4). LIUS can limit the side-effects to the
pathological site and minimize damage to surrounding normal
tissues. This treatment modality has great potential in clinical
applications. However, to date, LIUS combined with chemotherapeutic
agents has not yet been used clinically, possibly because the
reported efficacy depends on the drug and tumor model and thus,
further investigation is warranted (6).
Carboplatin (CBP) as the first-line chemotherapeutic
agent for OSCC, is a second-generation platinum drug. Small
inorganic platinum compounds move across cancer cell membranes and
bind to the DNA, forming a variety of inter-strand and intra-strand
cross-links between the platinum compounds and nucleotides, which
trigger a series of intracellular events that ultimately lead to
the cell death (14–16). However, the clinical utility of
these drugs has been limited by their harmful side-effects, which
include nephrotoxicity, myelosuppression, nausea, and vomiting
(14). In addition, the efficacy of
platinum-based drugs is often compromised by the inherent or
acquired resistance of cancer cells (17). The mechanisms underlying platinum
resistance are very complex, and include decreased drug uptake,
increased drug efflux, activation of detoxifying systems,
activation of DNA repair mechanisms, and the evasion of
drug-induced apoptosis (15,17).
Studies have revealed that the reduced accumulation of
intracellular platinum is a prominent feature of platinum
resistance (15). Whether US can
enhance the cytotoxicity of platinum-based drugs remains a subject
of debate. Saad and Hahn (18)
reported that US did not enhance the effects of CDDP in Chinese
hamster ovary cells, however Yu et al (9) demonstrated that US increased
CDDP-induced DNA damage. Therefore, further studies are warranted
to clarify whether this combined treatment modality can serve as a
non-invasive method to improve the local permeability and targeting
of platinum-based drugs in order to increase the clinical treatment
efficacy.
To the best of our knowledge, the development of
pre-clinical drugs and therapeutic modalities mostly rely upon the
subcutaneous inoculation of tumor cells in immunodeficient mice to
establish a tumor xenograft model (19). However, subcutaneous models do not
sufficiently represent the clinical features of tumors,
particularly with respect to metastasis development and response to
treatment (20,21). Chemically-induced orthotopic models
are commonly used for the preclinical and translational studies of
compounds and therapies because they mimic many aspects of human
diseases, provide reproducible results and allow evaluation of the
systemic effects of treatments. Our research team previously
demonstrated that hamster tongue mucosa underwent gradual changes
from hyperplasia, carcinoma in situ to early invasive
carcinoma, when exposed to 7,12-dimethylbenz(alpha)anthracene
(DMBA) (22,23). The pathogenesis of this tongue
cancer model is similar to human tongue carcinoma.
In the present study, DMBA was used to induce an
orthotopic model of tongue cancer, and we used very low acoustic
intensity that complied with current clinical safety regulations
and a current clinical chemotherapeutic drug CBP (14,17) to
investigate whether LIUS combined with low-dose CBP enhances the
anticancer efficacy and decreases the side-effects of CBP.
Materials and methods
Tumor model and chemicals
All animal experimental procedures were approved by
the Laboratory Animal Committee of Harbin Medical University
(Harbin, China). Four-week-old male hamsters (35–55 g) from the
Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China) were housed under specific pathogen-free
conditions. To create an orthotopic tongue cancer model, a 1.5%
DMBA acetone solution (Sigma-Aldrich, St. Louis, MO, USA) was
applied to the left margin of the anterior tongue in a scratched
area, three times a week, and hamsters were fasted for 2 h after
applying DMBA to avoid damage produced by DMBA to the other
alimentary structures other than the tongue according to a
previously published method by Fujita et al (24), until a tumor was formed in the
tongue (~8 weeks). Eight weeks after DMBA application (tumor
diameter, 3~4 mm), the tongue cancer grew rapidly (23,25)
and treatment began. The carboplatin injection was purchased from
Qilu Pharmaceutical Co., Ltd. (Jinan, China) and diluted with 0.9%
sodium chloride solution to the appropriate concentration for
injection. Rabbit polyclonal anti-Chk1 (cat. no. WL01674; IHC,
1:100; WB, 1:500), rabbit polyclonal anti-proliferating cell
nuclear antigen (PCNA; cat. no. WL0341; IHC, 1:50), rabbit
polyclonal anti-Bcl-2 (cat. no. WL01556; IHC, 1:100; WB, 1:500),
rabbit polyclonal anti-cyclin B1 (cat. no. WL01760; IHC, 1:100; WB,
1:500), rabbit polyclonal anti-p53 (cat. no. WL01919; WB, 1:500),
rabbit polyclonal anti-cleaved caspase-3 (cat. no. WL01992; WB,
1:500), rabbit polyclonal anti-phospho-p53 (cat. no. WL02504; WB,
1:500), rabbit polyclonal anti-caspase-8 (cat. no. WL00659; IHC,
1:100; WB, 1:500), rabbit polyclonal anti-Bax (cat. no. WL01637;
IHC, 1:100; WB, 1:500), rabbit polyclonal anti-p21 (cat. no.
WL0362; WB, 1:500), and rabbit polyclonal anti-β-actin (cat. no.
WL01845; WB, 1:1,000) were purchased from Wanleibio Co., Ltd.
(Changchun, China). Rabbit polyclonal anti-Bak (cat. no. PB0506;
IHC, 1:100; WB, 1:500), rabbit polyclonal anti-caspase-3 (cat. no.
BA3968; IHC, 1:100; WB, 1:300) and rabbit polyclonal anti-cyclin D1
(cat. no. BA0770; IHC, 1:100; WB, 1:300) were purchased from Wuhan
Boster Bioengineering Co., Ltd. (Wuhan, China), rabbit polyclonal
anti-MGMT (cat. no. BS1002R; IHC, 1:500; WB, 1:1,000) was purchased
from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China),
Mouse monoclonal anti-γ-H2AX (cat. no. ab26350; IHC, 1:100; WB,
1:1,000) was provided by Abcam (Cambridge, UK) and rabbit
polyclonal anti-Gadd45α (cat. no. E2A6622; IHC, 1:100; WB, 1:300)
was purchased from EnoGene (Nanjing, China).
US device and treatment protocol
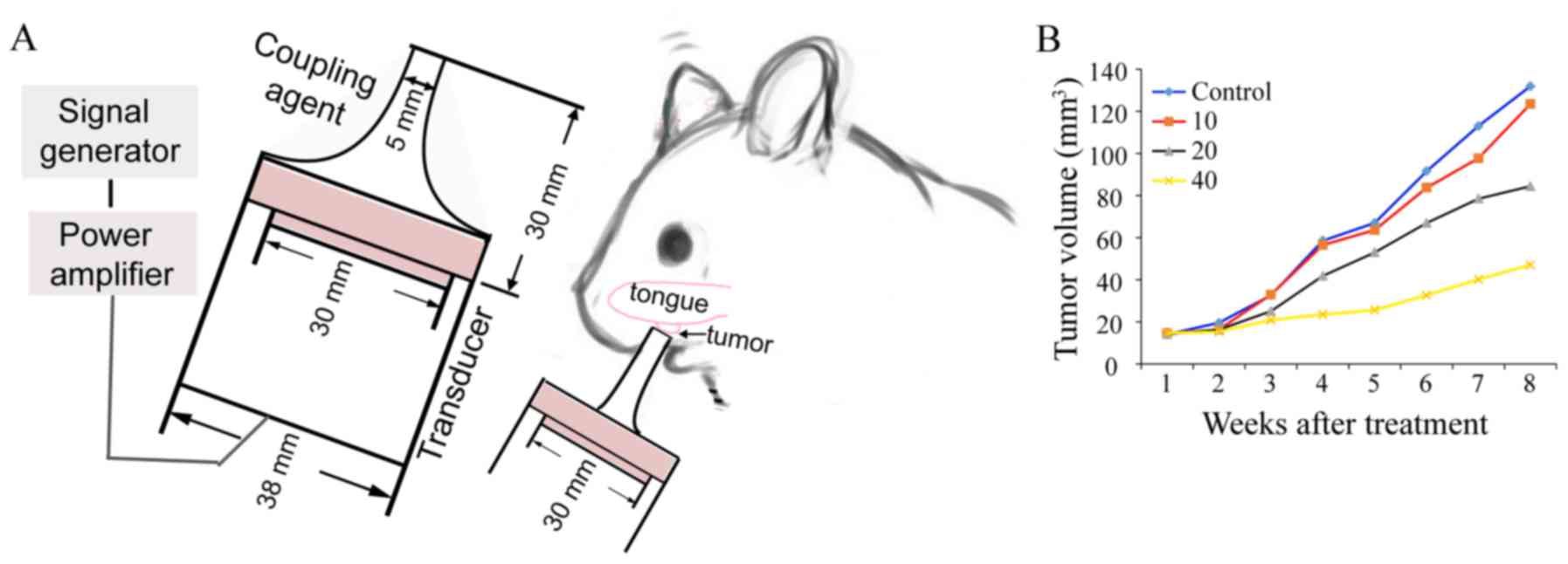
Fig. 1A displays the
US treatment system developed by the Condensed Matter Science and
Technology Institute, Harbin Institute of Technology (Harbin,
China). In the animal treatment experiments, a tone-burst US signal
generated by a 5.0 cm diameter piezoelectric transducer with a
center frequency of 1.0 MHz was applied through a tapered aluminum
buffer head whose front surface (5 mm, diameter) was put directly
in contact with the tongue cancer site using an ultrasonic
couplant. US intensity was measured in degassed water using an
HNC-1000 needle-type hydrophone (0.1 cm active element size, 1~20
MHz bandwidth) (Onda Corporation, Sunnyvale, CA, USA). The sound
pressure level distribution pattern was calculated by finite
element simulations using COMSOL software (26). The US frequency was 1.0 MHz,
provided in tone burst mode with a duty cycle of 20% and a
repetition frequency of 100 Hz; the ultrasonic intensity level was
0.89 W/cm2. We chose 20 mg/kg as a low dose for the
study (Fig. 1B), which can slow the
growth of the hamster tongue cancer but cannot stop the tumor
growth. The hamsters were randomly divided into five groups:
Untreated control (control group, n=10); LIUS treatment (U group,
0.89 W/cm2 intensity, 1.0 MHz frequency, 20% duty
factor, 15 min duration; n=10); intraperitoneal injection of 20
mg/kg CBP (CB20 group, n=10); intraperitoneal injection of 40 mg/kg
CBP (CB40 group, n=10); and LIUS combined with intraperitoneal
injection of CBP (U+CB group, 20 mg/kg CBP administration 1 h
before LIUS treatment; n=10). All animals were treated twice a week
for a total of 8 weeks and then the therapy was terminated. The
tumor volumes and body weights were assessed twice a week. The
tumor volume was calculated using the formula: V = (π/6) × L ×
S2, where L and S are the long and short diameters,
respectively. At the end of the treatment, five hamster tongue
cancer tissues were excised and abdominal aorta blood was drawn
from each group for analysis; the remaining five hamsters were
observed for survival until day 90, and then all hamsters were
sacrificed.
Clinical samples
Archived paraffin-embedded oral tongue squamous cell
carcinoma tissues (OTSCC) and matched adjacent normal tissues were
obtained from 48 patients who had undergone surgical excision at
Harbin Medical University Stomatological Hospital between January
2000 and December 2005 (27). All
of the patients provided informed consent, and the study was
approved by the Research Ethics Committee of Harbin Medical
University. All patients underwent potentially curative surgery
without preoperative therapy. Patient clinical characteristics were
previously described (27).
Immunohistochemistry
Tumors were excised, fixed in 4% paraformaldehyde,
dehydrated with a graded ethanol series, cleared in
dimethylbenzene, and embedded in paraffin. Next, tissue blocks were
cut into 4-µm sections and mounted on glass slides, and then
routinely dewaxed and rehydrated. Antigens were retrieved in 10 mM
citrate buffer (pH 6.0) for 15 min in a pressure cooker. Tissue
sections were treated with endogenous peroxidase at room
temperature. After blocking in 1% bovine serum albumin for 30 min,
the sections were stained with appropriate primary antibodies and
incubated overnight at 4°C. Subsequently, the sections were
incubated with corresponding secondary antibodies (immediate-use
goat anti-rabbit IgG horseradish-peroxidase polymers; cat. no.
PV-6001; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) for 30 min at 37°C. The antibody reaction was visualized
using diaminobenzidine chromogen (cat. no. ZLI-9018; Zhongshan
Golden Bridge Biotechnology). Finally, all the slides were
counterstained with hematoxylin. For protein expression analysis,
10 areas were randomly selected under a microscope at a
magnification of ×200. Image Pro Plus 6.0 (Media Cybernetics, Inc.,
Bethesda, MD, USA) was used to quantify the intensity and extent of
immunopositive expression in cells with integrated optical density
(IOD) values. IOD values were expressed as the mean ± SD per tissue
examined.
Western blotting
The tissue was cut into small pieces on ice, lysed
in cell lysis buffer (cat. no. P0013; Beyotime Institute of
Biotechnology, Nantong, China) in the presence of the Complete Mini
Proteinase Inhibitor Cocktail (Roche Diagnostics, Indianapolis, IN,
USA), resolved on 10% SDS-PAGE gels, and electrotransferred to
nitrocellulose membranes. After blocking in Tris-buffered saline
and Tween-20 (TBS-T) containing 5% non-fat dry milk, the membranes
were incubated overnight at 4°C with primary antibodies against the
target proteins. After washing twice with TBS-T, the membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1 h. The protein levels were detected using a fully
automated chemiluminescence imaging analysis system (Tanon Science
and Technology Co., Ltd., Beijing, China).
Statistical analysis
Three different observers assessed the primary tumor
volume, and two were blind to the research groups. The results were
expressed as the mean ± standard error of the mean (SEM).
Statistical differences were evaluated using one-way analysis of
variance (ANOVA) followed by Dunnett's test. Differences between
any two groups were assessed by the Student Newman-Keuls test, with
P-values <0.05 considered as statistically significant.
Statistical evaluation was performed using SPSS 22.0 (IBM Corp.,
Armonk, NY, USA).
Results
LIUS enhances the anticancer effects
of CBP and prolonged survival
Following corresponding treatment of hamsters in
different groups, tumor images of each group were captured
(Fig. 2A). The results revealed
that the tumor volume in the U+CB group was the smallest among the
5 groups, and the tumor volume changes were 40, 42, 15, 3 and
6-fold for the control, U, CB20, U+CB, and CB40 groups,
respectively (Fig. 2B) at the end
of treatment compared with the tumor volume at the time of
treatment initiation. The results revealed that the growth rate of
the orthotopic tongue carcinoma in the U+CB group was only 1/5th of
that of the group treated with 20 mg/kg CBP alone (CB20 group;
P<0.05).
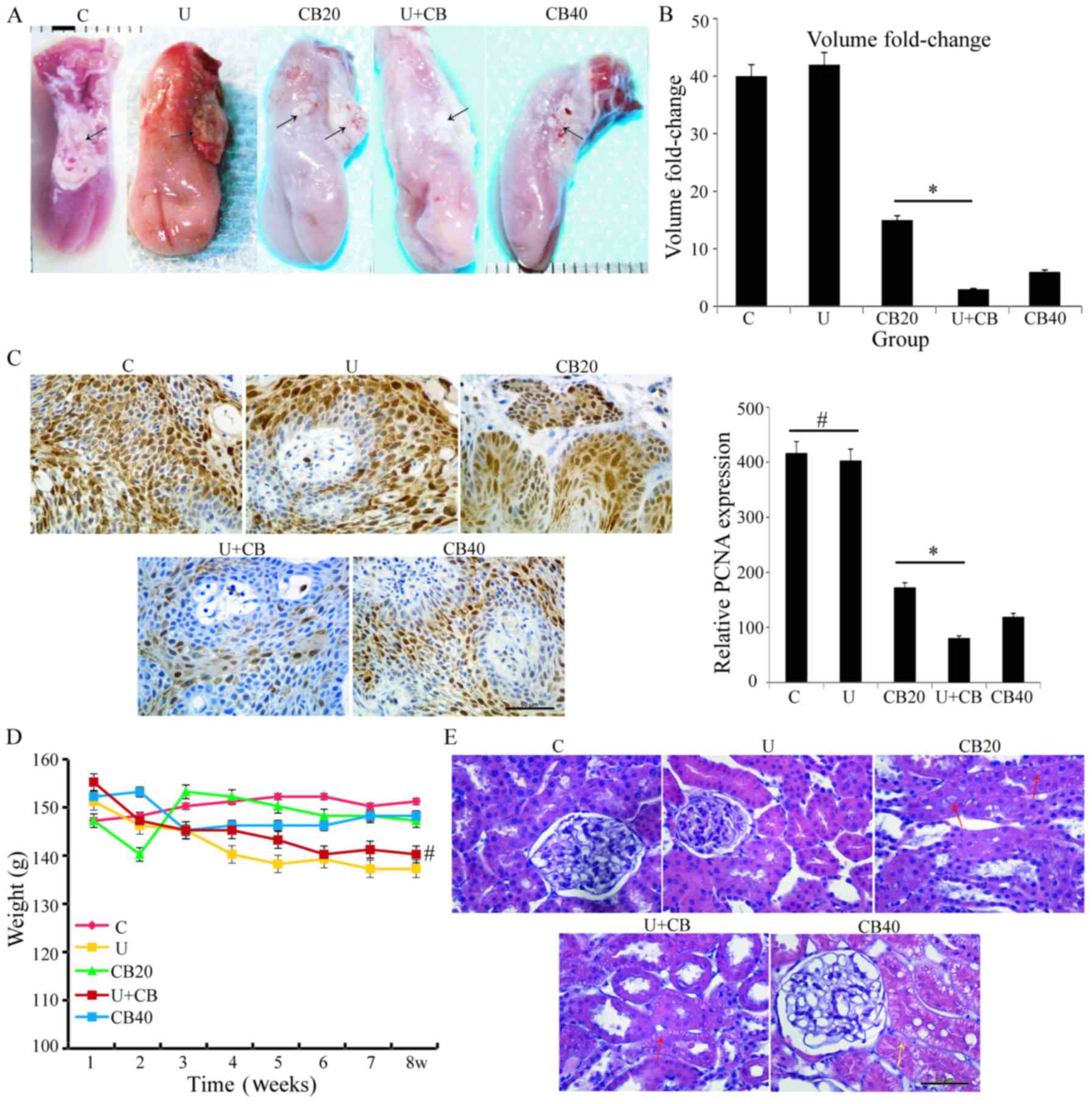 | Figure 2.LIUS enhanced the anticancer effects
of CBP in hamsters with orthotopic tongue carcinoma. (A) Gross view
of a representative orthotopic tongue cancer. The tumor mass
(arrowhead) in the C, U, and CB20 groups was more evident than that
in the U+CB group; Scale bar, 2 mm. (B) Tumor volume changes in the
orthotopic hamster model of tongue cancer (data are expressed as
the mean ± SD; n=5 hamsters/group); CB20 vs. U+CB, *P<0.05. (C)
PCNA expression in the C, U, CB20, CB40, and U+CB groups. Data are
expressed as the mean ± SD. Statistical significance was determined
by one-way ANOVA followed by Dunnett's test. *P<0.05;
#no significance between groups. Scale bar, 50 µm. (D)
Body weight curve in the C, U, CB20, CB40, and U+CB groups.
#No significance between U+CB vs. C. (E) Representative
pathological results of the kidney tissue; renal tubular epithelial
cells exhibiting slight edema (red arrows) and severe edema (yellow
arrow) were observed in the CB40 group. C, control group; U,
low-level ultrasound treatment group; CB20, 20 mg/kg CBP treatment
group; CB40, 40 mg/kg CBP treatment group; U+CB, low-level
ultrasound combined with the 20 mg/kg CBP treatment group. |
To ascertain the cell proliferation status of tumor
cells, we examined the expression of PCNA in the tongue carcinoma
tissues under different treatments. The expression level of PCNA
was high in the LIUS-treated tissue, and there was no significant
difference between the LIUS-treated and the control groups. With
the increase of the CBP dosage, the PCNA expression level gradually
decreased and was further inhibited after combination with LIUS
compared with CBP treatment alone (Fig.
2C). These results indicated that LIUS can enhance the
inhibitory effects of CBP on the proliferation of hamster
orthotopic tongue cancer cells. In our previous study, we
demonstrated that LIUS increased the accumulation of drugs in
cancer cells (4); therefore, we
speculated that LIUS may increase accumulation of intracellular CBP
in hamster orthotopic tongue cancer in this study. There were no
significant differences in body weight between the drug-treated
groups (20 and 40 mg/kg CBP) and the control group; all ultrasonic
treatments (U, U+CB) led to slight but non-significant decreases in
body weight compared to the control group (Fig. 2D; P=0.998). Side-effects were not
observed in any group. Since the ultrasonic treatment needs to be
performed under anesthesia, we hypothesized that the decrease in
body weight was related to the anesthesia. No significant weight
loss occurred in the CB40 group, indicating that this drug dose did
not have significant effects on hamster growth.
To further examine possible side-effects of this
drug, we drew blood from the abdominal aorta of the animals for
blood and renal function tests. There was no significant
differences in erythrocyte (ER), leukocyte (LE), and platelet (PL)
counts between the CB20 and U+CB groups (ER: P=0.999; LE: P=0.014;
PL: P=0.991; Table I). Although CBP
treatment increased serum levels of creatinine (CR), urea nitrogen
(UN), and uric acid (UA) levels compared with the control group,
U+CB treatment did not produce significant differences compared to
the CB20 group (CR: P=0.988; UN: P=0.149; UA: P=0.831; Table I). These findings revealed that
LIUS-enhanced the anticancer effects of CBP in vivo, and did
not worsen the systemic toxicity of the drug.
 | Table I.Systemic toxicity determined by blood
tests. |
Table I.
Systemic toxicity determined by blood
tests.
| Group | ER
(×1012/l) | LE
(×109/l) | PL
(×109/l) | CR (mg/dl) | UN (mg/dl) | UA (µmol/l) |
|---|
| Control | 3.2±0.2 | 6.6±0.2 | 698.4±8.1 | 2.2±0.8 | 25.9±3.8 | 36.4±5.5 |
| US | 3.3±0.1 | 6.3±0.8 | 701.2±10.4 | 2.0±0.7 | 25.4±1.6 | 42±4.6 |
| CBP20 | 3.6±0.1 | 6.0±0.1 | 675.2±5.0 | 2.8±0.8 | 83.0±5.0 | 43.2±3.0 |
| CBP40 | 3.3±0.1 | 5.8±0.1 | 629.6±45.1 | 4.4±1.1 | 117.9±6.3 | 50.8±4.3 |
| CBP+US | 3.6±0.2 | 6.3±0.1 | 670.4±17.3 | 3.0±0.7 | 88.4±1.8 | 45.4±3.5 |
The pathology of the kidney after different
treatments is displayed in Fig. 2E.
LIUS exposure did not cause any histopathological changes in the
kidneys. There was slight edema of renal tubular epithelial cells
in the CB20 and U+CB groups, and edema of renal tubular epithelial
cells was observed in the CB40 group. Histopathological changes in
the kidney resulting from the U+CB treatment were similar to those
observed in the group treated with 20 mg/kg CBP alone.
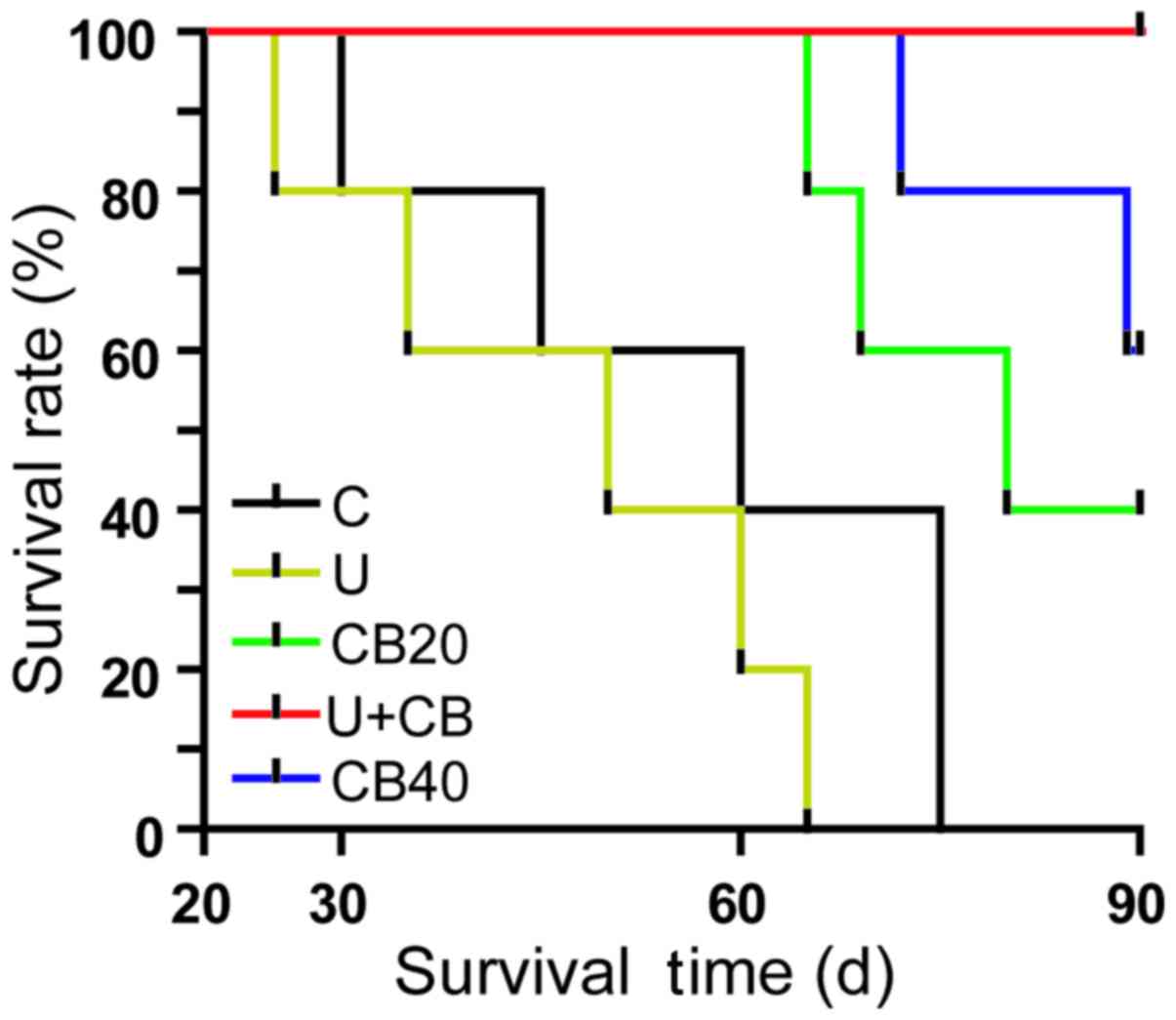
When the tumor grew to ~4 mm in diameter volume,
treatment was initiated and animal survival was monitored daily
until day 90. The results were presented in a Kaplan-Meier graph
(Fig. 3), and the median survival
times for the five groups were 60, 50, 80, 90, and >90 days,
respectively. The survival time was prolonged in the CB20, CB40,
and U+CB groups compared with the control group (P<0.05). These
data clearly indicated that LIUS combined with CBP was more
effective than the treatment with 40 mg/kg CBP alone in prolonging
the animal survival time (P=0.0159), extending the long-term
survival rate presumably due to the more effective CBP delivery
through LIUS-mediated tumor cell targeting. In addition, some
hamsters were in a state of poor health and died during treatment.
In future experiments, systematic investigation will be performed
to determine whether hamsters died of complications or tumor
metastasis.
LIUS treatment alone had no significant effects on
the growth and proliferation of orthotopic tongue cancer cells.
LIUS treatment alone did not significantly reduce the growth in the
xenograft model of human-tongue squamous carcinoma and
hepatocellular carcinoma (3,4).
Carboplatin is not a sensitizer, and the ultrasound-mediated
cavitation effect did not participate in the synergistic effect of
U+CB. Therefore, in subsequent experiments, we did not evaluate
tongue cancer tissue in the LIUS treatment group.
LIUS combined with CBP induces DNA
damage
The antitumor activity of platinum-based
chemotherapy is largely dependent on the DNA repair capacity of
cancer cells, therefore, we examined the expression of DNA damage
repair-related proteins in different treatment groups. First, the
U+CB therapy-induced DNA damage was investigated using IHC to
detect γ-H2AX. As shown in Fig. 4A,
γ-H2AX expression was revealed in the nucleus of the CBP-treated
group, and a higher γ-H2AX expression level was revealed in the
U+CB group compared to the CBP alone group. MGMT was highly
expressed in the control group. Its expression decreased with the
increase of CBP concentration and was the lowest in the U+CB group
(Fig. 4). We observed the
expression of Gadd45α in the mouse model, and determined that
Gadd45α was upregulated in hamster tongue cancer tissues compared
with normal tissues (data not shown). Following treatment with CBP,
the expression of Gadd45α in the cytoplasm and nucleus of the
control group increased, with a higher increase observed in the
nucleus. IHC semi-quantitative and western blot analysis of Gadd45α
protein levels revealed higher Gadd45α expression in the U+CB group
compared to the CB20 group alone (Fig.
4B).
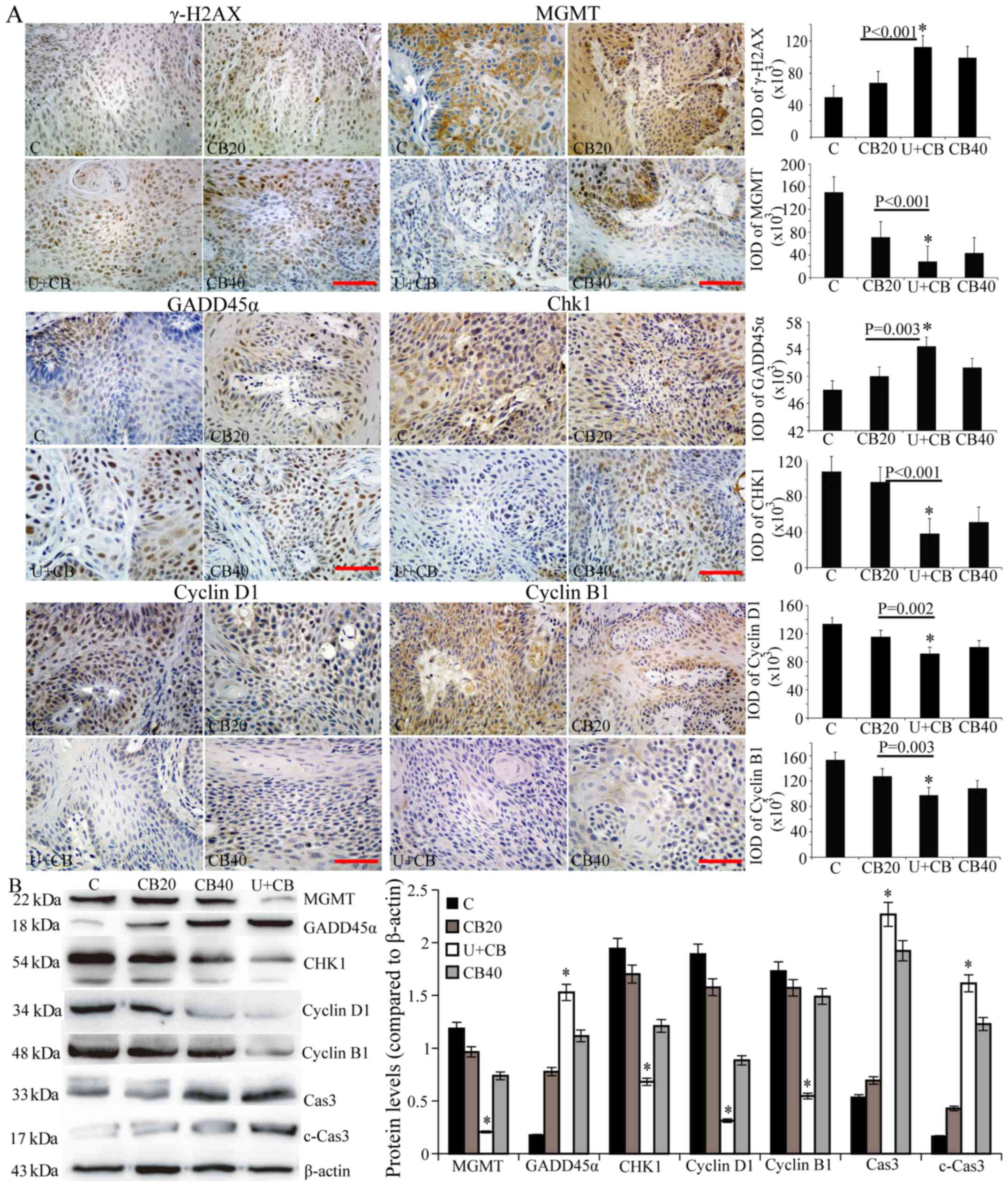 | Figure 4.LIUS and CBP induce DNA damage and
cell cycle arrest. (A) γ-H2AX, MGMT, Gadd45α, Chk1, cyclin D1, and
cyclin B1 expression was evaluated by immunohistochemical staining.
Representative data from three independent experiments are shown.
Scale bar, 50 µm. (B) The expression levels of MGMT, Gadd45α, Chk1,
cyclin D1, cyclin B1, caspase-3, and cleaved caspase-3 protein were
assessed by western blotting; β-actin was used as the internal
control. Data are presented as the mean ± SD (n=3). Statistical
significance was determined by one-way ANOVA followed by Dunnett's
test. *P<0.05 vs. the CB20 group. LIUS, low-intensity
ultrasound; CBP, carboplatin; MGMT,
O6-methylguanine DNA methyltransferase. |
Effect of LIUS combined with CBP on
the expression of Chk1, cyclin D1 and cyclin B1
CBP is a cell cycle non-specific drug that can kill
cells in different phases of the cell cycle. As shown in Fig. 4 LIUS enhanced CBP damage to the
tumor cell DNA. Chk1 is a cell cycle checkpoint kinase that
functions in the repair of damaged DNA by blocking the cell cycle
after DNA damage. To determine its effects on the cell cycle
checkpoints, we examined the expression of Chk1. In the control
group, Chk1 was highly expressed in the cytoplasm of the tumor
cells, with only a small amount of expression in the nucleus
(Fig. 4A). The overall expression
of Chk1 in the treatment group was lower than that in the control
group; its expression decreased in the cytoplasm and increased in
the nucleus with the increase in drug concentration. There were
significant differences in Chk1 expression between the CB20 and
U+CB groups (Fig. 4B; P<0.05),
indicating that LIUS enhanced the inhibitory effects of CBP on
Chk1. Cyclin D1 and cyclin B1 expression was present at lower
levels in the U+CB group than in the other groups (Fig. 4; P<0.05).
Effect of LIUS combined with CBP
treatment on the apoptosis of tongue cancer cells
We revealed that LIUS combined with CBP could
enhance the DNA damage produced by CBP in tumor cells, and inhibit
the expression of Chk1. Then, we determined if these effects
eventually led to apoptosis. The expression levels of caspase-3,
cleaved caspase-3, caspase-8, Bax, and Bak in the U+CB group were
significantly higher than those in the CBP groups, although the
expression of Bcl-2 was inhibited (Figs. 4 and 5). We examined the expression levels of
wild-type p53 and phospho-p53 in the tissues and determined that
although there was no significant difference in total p53 protein
levels among the treated groups, treatment with U+CB significantly
increased the level of phospho-p53 compared with the 20 mg/kg CBP
group (Fig. 5B). In addition, there
was a significant difference in the expression level of p21 between
the U+CB and other treatment groups (Fig. 5B).
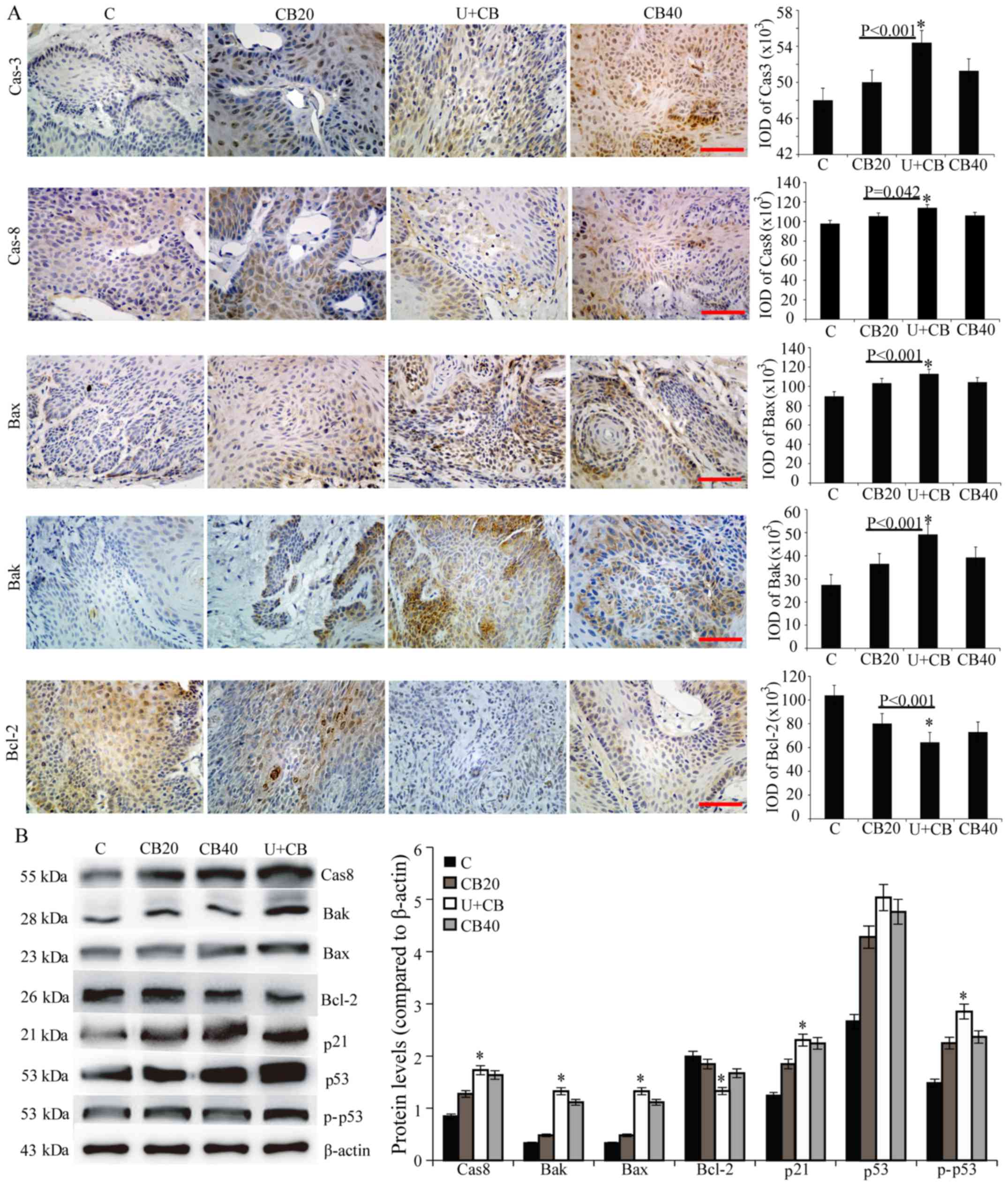 | Figure 5.Effect of U+CB on the expression of
apoptosis proteins in the hamster orthotopic tongue cancer model.
(A) Caspase-3, caspase-8, Bak, Bax, and Bcl-2 expression was
evaluated by immunohistochemical staining. Representative data from
three independent experiments are shown. Scale bar, 50 µm. (B) The
expression levels of caspase-8, Bak, Bax, Bcl-2, p21, p53 and
phospho-p53 protein were assessed by western blotting; β-actin was
used as the internal control. Data are presented as the mean ± SD
(n=3). Statistical significance was determined by one-way ANOVA
followed by Dunnett's test. *P<0.05 vs. CB20 group. CPB,
carboplatin; C, control group; U, low-level ultrasound treatment
group; CB20, 20 mg/kg CBP treatment group; CB40, 40 mg/kg CBP
treatment group; U+CB, low-level ultrasound combined with the 20
mg/kg CBP treatment group. |
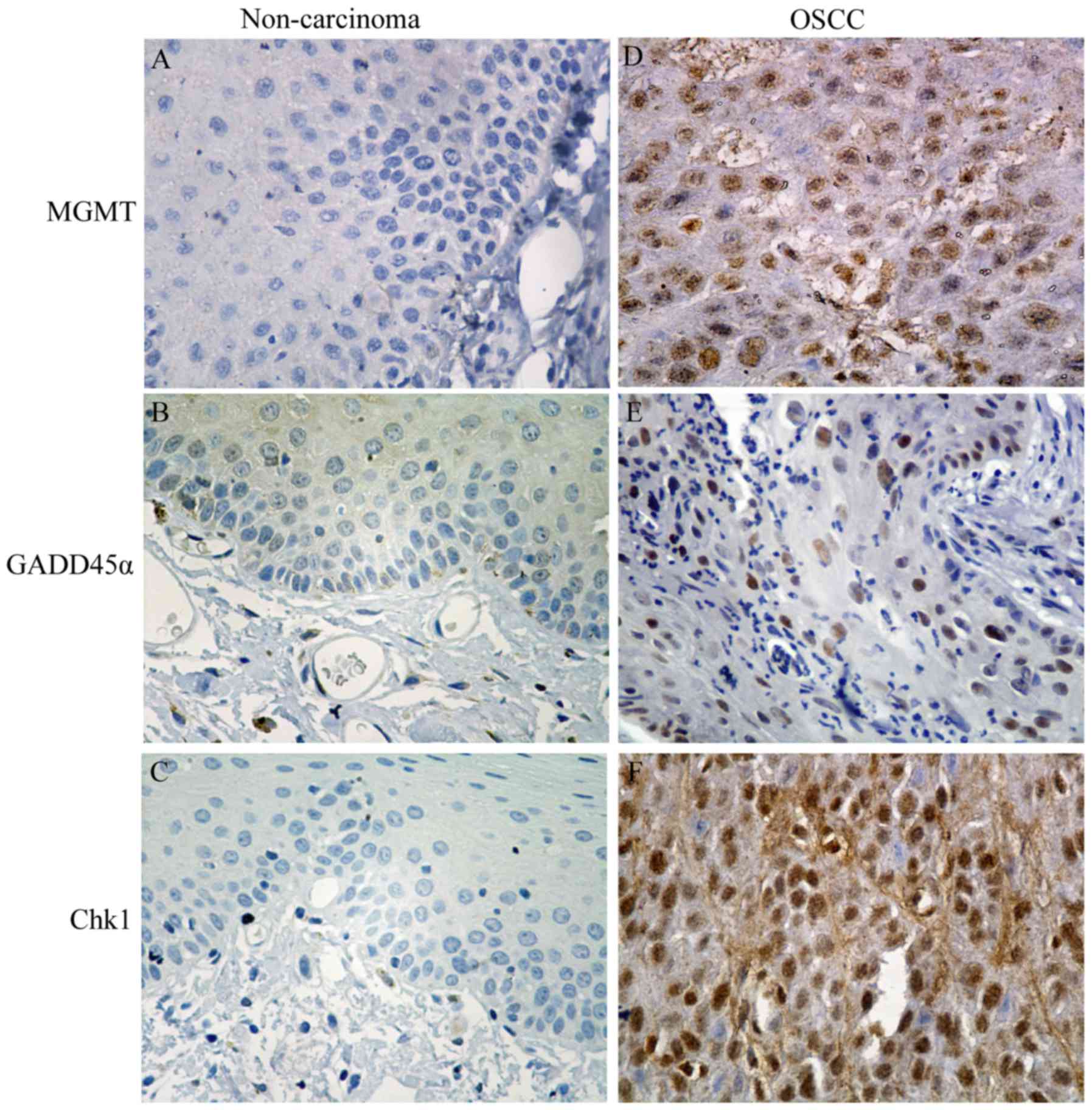
Expression of MGMT, Gadd45α, and Chk1
in non-cancerous and OTSCC tissues
In order to anticipate the potential of chemotherapy
combination with LIUS in OTSCC patients, it was important to detect
whether some tumor markers were overexpressed in human OTSCC
samples. We evaluated the expression of MGMT, Gadd45α, and Chk1 in
48 pairs of OTSCC tissue and adjacent non-cancerous oral tissue
samples. The representative immunostaining profiles of MGMT,
Gadd45α, and Chk1 in OTSCC are displayed in Fig. 6. The immunoreactivities of MGMT,
Gadd45α, and Chk1 were increased in OTSCC compared with matched
adjacent non-cancerous oral tissues. Gadd45α was widely expressed
in OTSCC and adjacent epithelium and was mainly expressed in the
nucleus of the adjacent epithelium with a positive expression rate
of 100%, whereas there was almost no expression of MGMT and Chk1 in
the normal epithelium, and their positive expression rates in OTSCC
tissues were 62.5 and 77.1%, respectively. These results revealed
that the expression of MGMT, Gadd45α, and Chk1 were upregulated in
OTSCC tissues compared with non-cancerous tissues, which may
provide a convincing rationale for considering LIUS combination
with CBP treatment in future clinical studies since our hamster
study revealed that the U+CB treatment can effectively reduce their
expression.
Discussion
LIUS can enhance the sensitivity of drug-resistant
cells to drugs, thereby providing a therapeutic opportunity for
multidrug resistance in tumors (6,10,28).
Studies have revealed that the use of LIUS combined with low doses
of anticancer drugs can achieve the same anticancer therapeutic
effects as higher drug doses (3,4,11),
however, published studies only used cultured cell levels or nude
mice transplanted with tumors. In the present study, we used DMBA
to induce an orthotopic hamster model of tongue cancer, and for the
first time, used this model to study the effects of LIUS combined
with a marginal dosage of 20 mg/kg CBP on tongue cancer. Our data
revealed that LIUS enhanced the in vivo chemotherapeutic
effects of CBP without increasing its toxicity. It also prolonged
the survival time of the hamsters, indicating that this therapy was
safe in the hamster orthotopic model of tongue cancer (Figs. 2 and 3; Table
I). In the present study, we used very low acoustic energy
combined with a low dose of CBP to treat an orthotopic hamster
model of tongue carcinoma, which is similar to human tongue cancer,
allowing for easy translation from pre-clinical to clinical
studies.
GADD45α was identified as a tumor suppressor of
multiple types of solid tumors, and patients with high expression
of Gadd45α had better prognosis (29). Our results revealed that the
expression of Gadd45α was higher in cancer tissues compared with
adjacent non-cancerous tissues (Fig.
5), which was consistent with a study from Zhang et al
(29). Drug therapy can directly or
indirectly upregulate GADD45α, promote apoptosis, and increase drug
sensitivity (30). We found that
the expression of Gadd45α was increased in hamster tongue cancer
after the U+CB treatment (Fig. 4),
suggesting that LIUS combined with CBP may induce Gadd45α
expression to inhibit cell growth and induce apoptosis (28).
MGMT is a DNA-repair protein, and its expression
level is closely related with the sensitivity of cells to drugs
(31,32). Chen et al observed that MGMT
protected nasopharyngeal carcinoma cells from CDDP-induced DNA
damage by enhancing DNA repair capacity, whereas low expression of
MGMT in these cells made them more sensitive to platinum-based
drugs (33). We found that U+CB led
to a decreased expression of MGMT compared to treatment using CBP
alone, suggesting decreased DNA repair activity. In terms of the
mechanism of LIUS function, further studies are needed in the
future to determine if it promotes drug entry into cells, thereby
increasing intracellular drug levels (4,6,11) or
reduces the threshold of cell destruction (34).
Whether the cell is apoptotic after cell DNA damage
is a critical factor in determining if the damaged DNA can be
repaired (35). The
ATM/ATR-Chk1-p53-p21/Gadd45α pathway can regulate cell cycle
conversion and the repair of DNA damage (28). As a cell cycle checkpoint kinase,
Chk1 undergoes dynamic nuclear-cytoplasmic shuttling under
conditions of both normal growth and DNA damage (36). We observed that Chk1 distribution
underwent ‘cytoplasmic-nuclear’ shuttling in the U+CB group in that
its expression decreased in the cytoplasm (Fig. 4), indicating that there was reduced
ability to regulate the DNA damage checkpoint pathway, leading to
reduced DNA repair (36) and
increased sensitivity to DNA damaging agents (37). The damaged DNA that cannot be
repaired leads to the accumulation of genome mutations in cancer
cells, resulting in higher sensitivity of cancer cells to
chemotherapy than normal cells. Thus, weakening the DNA damage
repair ability can improve the sensitivity to chemotherapy, leading
to better treatment outcomes (38).
The tumor suppressor p53 can inhibit the cancer cell cycle, thereby
affecting DNA damage-induced apoptosis (39). We observed that the expression of
phospho-p53 and p21 increased in orthotopic tongue cancer tissue,
and the expression of cyclin D1 and cyclin B1 decreased in the U+CB
treatment group (Fig. 5). In
addition, the expression of caspase-3, cleaved caspase-3,
caspase-8, Bax, and Bak increased and the expression of Bcl-2
protein decreased (Fig. 5),
suggesting that LIUS plus CBP treatment may activate the downstream
genes p21 and Gadd45α by inducing wild-type p53 expression,
enhancing cell cycle arrest and inducing apoptosis (40,41).
As a cell-cycle non-specific drug, CBP can lead to cell cycle
arrest, and trigger apoptosis. We determined that the expression
levels of cyclin B1 and cyclin D1 were decreased after CBP
treatment. We did not perform further in-depth study on the role of
CBP on the cell cycle since the focus of this paper was mainly on
DNA damage. We will focus on this issue in follow-up
experiments.
The most prominent mode of CBP action is the
induction of the intrinsic apoptotic pathway through the activation
of the DNA damage response (16).
The results of our study revealed that LIUS combined with CBP
treatment enhanced the cytotoxicity of CBP in orthotopic tongue
cancer by enhancing CBP-mediated DNA damage in tongue cancer cells
and inducing apoptosis. To evaluate the clinical relevance of LIUS
combined with chemotherapy for treatment of the orthotopic tongue
cancer animal model, we collected samples of clinical OTSCC tissue
for MGMT, Gadd45α, and Chk1 staining. We found that the expression
of MGMT and Chk1 was significantly higher in the OTSCC tissue than
in the adjacent tissue, and the expression of Gadd45α was very low
in the cytoplasm of the tumor cells (Fig. 6). Wang et al (42) reported that low MGMT expression
resulted in a better prognosis in glioblastoma patients. Gadd45α
significantly increased the chemosensitivity of anticancer drugs
(etoposide, cisplatin, and 5-fluorouracil) in pancreatic cancer
cells due to the induction of abundant apoptosis and cell cycle
arrest (30). The prognosis of OSCC
patients with high Gadd45α expression was better than those with
low expression (29). ATR-Chk1-p53
signal activation was involved in the efficacy of CDDP in OSCC
cells (43). These studies support
our findings, suggesting that the regulation of cell damage-related
factors can enhance the anti-OSCC ability of CBP and inhibit the
malignant progression of OSCC.
Due to hamsters having different sensitivities to
DMBA, leading to differences in tumor development time, tumor
shape, and tumor growth pattern, difficulties in calculating the
tumor size can arise. The LIUS treatment takes time, needs very
skilled people to perform, and can only be performed on a limited
number of animals according to ethical regulations. The number of
surviving animals in the control and LIUS-treated groups are few at
the end of the therapy cycle, which limits our ability to perform
more analysis. In future experiments, we will increase the number
of animals in subsequent experiments to obtain more samples to
detect their molecular changes. Despite various limitations in our
experiments, the achieved significant inhibition of primary tumor
growth was truly encouraging. Compared with CBP treatment alone,
U+CB treatment did not increase side-effects, demonstrating that
this combination therapy is safe, effective and feasible. Thus
combining ultrasound with CBP can be a very promising modality for
clinical treatment of OSCC. In future studies, we plan to refine
the evaluation of tumor growth and increase the number of animals
for survival observations.
In conclusion, LIUS enhanced the ability of low-dose
CBP to damage DNA in orthotopic tongue cancer cells, induced
apoptosis, inhibited tumor growth and progression, and
concomitantly did not increase drug side-effects to normal tissues.
Thus, combining ultrasound with chemotherapy can be a very
promising modality for clinical treatment of tumors.
Acknowledgements
We would like to thank Editage for providing
editorial assistance. This study was supported by grants from the
National Natural Science Foundation of China (no. 81502644), the
Fundamental Research Funds for the Provincial Universities (no.
2017JCZX28).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gharat SA, Momin M and Bhavsar C: Oral
squamous cell carcinoma: Current treatment strategies and
nanotechnology-based approaches for prevention and therapy. Crit
Rev Ther Drug Carrier Syst. 33:363–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Fan H, Wang Z, Zheng J and Cao W:
Potentiation of scutellarin on human tongue carcinoma xenograft by
low-intensity ultrasound. PLoS One. 8:e594732013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Z, Lv G, Li Y, Li E, Li H, Zhou Q, Yang
B and Cao W: Enhancement of anti-tumor effects of 5-fluorouracil on
hepatocellular carcinoma by low-intensity ultrasound. J Exp Clin
Cancer Res. 35:712016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Yu T, Zhang J, Liu S, Huo Y and
Zhang Y: Sonochemotherapy inhibits the adhesion, migration and
invasion of human ovarian cancer cells with highly metastatic
potential. Ultraschall Med. 32 Suppl 1:S14–S20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu T, Li SL, Zhao JZ and Mason TJ:
Ultrasound: A chemotherapy sensitizer. Technol Cancer Res Treat.
5:51–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trendowski M, Yu G, Wong V, Acquafondata
C, Christen T and Fondy TP: The real deal: Using cytochalasin B in
sonodynamic therapy to preferentially damage leukemia cells.
Anticancer Res. 34:2195–2202. 2014.PubMed/NCBI
|
|
8
|
Trendowski M: The promise of sonodynamic
therapy. Cancer Metastasis Rev. 33:143–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu T, Yang Y, Zhang J, He H and Ren X:
Circumvention of cisplatin resistance in ovarian cancer by
combination of cyclosporin A and low-intensity ultrasound. Eur J
Pharm Biopharm. 91:103–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Xu K, Bi Y, Yu G, Wang S, Qi X
and Zhong H: Low intensity ultrasound promotes the sensitivity of
rat brain glioma to Doxorubicin by down-regulating the expressions
of p-glucoprotein and multidrug resistance protein 1 in vitro and
in vivo. PLoS One. 8:e706852013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kotopoulis S, Delalande A, Popa M, Mamaeva
V, Dimcevski G, Gilja OH, Postema M, Gjertsen BT and McCormack E:
Sonoporation-enhanced chemotherapy significantly reduces primary
tumour burden in an orthotopic pancreatic cancer xenograft. Mol
Imaging Biol. 16:53–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu FT, Chen X, Wang J, Qin B and
Villanueva FS: Low intensity ultrasound mediated liposomal
doxorubicin delivery using polymer microbubbles. Mol Pharm.
13:55–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rizzitelli S, Giustetto P, Faletto D,
Delli Castelli D, Aime S and Terreno E: The release of Doxorubicin
from liposomes monitored by MRI and triggered by a combination of
US stimuli led to a complete tumor regression in a breast cancer
mouse model. J Control Release. 230:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lorusso D, Petrelli F, Coinu A,
Raspagliesi F and Barni S: A systematic review comparing cisplatin
and carboplatin plus paclitaxel-based chemotherapy for recurrent or
metastatic cervical cancer. Gynecol Oncol. 133:117–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saad AH and Hahn GM: Ultrasound enhanced
drug toxicity of Chinese hamster ovary cells in vitro. Cancer Res.
49:5931–5934. 1989.PubMed/NCBI
|
|
19
|
Sausville EA and Burger AM: Contributions
of human tumor xenografts to anticancer drug development. Cancer
Res. 66:3351–3354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teicher BA: Tumor models for efficacy
determination. Mol Cancer Ther. 5:2435–2443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan H, Jiang W, Li H, Fang M, Xu Y and
Zheng J: MMP-1/2 and TIMP-1/2 expression levels, and the levels of
collagenous and elastic fibers correlate with disease progression
in a hamster model of tongue cancer. Oncol Lett. 11:63–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng J, Xie L, Teng H, Liu S, Yoshimura
K, Kageyama I and Kobayashi K: Morphological changes in the lingual
papillae and their connective tissue cores on the
7,12-dimethylbenz[alpha]anthracene (DMBA) stimulated rat
experimental model. Okajimas Folia Anat Jpn. 85:129–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujita K, Kaku T, Sasaki M and Onoé T:
Experimental production of lingual carcinomas in hamsters: Tumor
characteristics and site of formation. J Dent Res. 52:1176–1185.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng J, Wang Q, Li H, et al: Comparative
study on the tongue carcinoma model induced by local application
both of 7,12-dimethylbenz[a]anthracene (DMBA) and injury. Anatomy
Res. 29:405–409. 2007.
|
|
26
|
Lv Y, Zheng J, Zhou Q, Jia L, Wang C, Liu
N, Zhao H, Ji H, Li B and Cao W: Antiproliferative and
apoptosis-inducing effect of exo-protoporphyrin IX based
sonodynamic therapy on human oral squamous cell carcinoma. Sci Rep.
7:409672017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan HX, Li HX, Chen D, Gao ZX and Zheng
JH: Changes in the expression of MMP2, MMP9, and ColIV in stromal
cells in oral squamous tongue cell carcinoma: Relationships and
prognostic implications. J Exp Clin Cancer Res. 31:902012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Chen J, Chen L, Yang X, Zhong H,
Qi X, Bi Y and Xu K: Low frequency and intensity ultrasound induces
apoptosis of brain glioma in rats mediated by caspase-3, Bcl-2, and
survivin. Brain Res. 1473:25–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XY, Xun-Qu, Wang CQ, Liu GX, Zhou CJ
and Wang ZG: Expression of growth arrest and DNA damage inducible
45a in human oral squamous cell carcinoma is associated with tumor
progression and clinical outcome. J Cancer Res Ther. 10
Suppl:C108–C113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Qian H, Li X, Wang H, Yu J, Liu Y,
Zhang X, Liang X, Fu M, Zhan Q and Lin C: Adenoviral-mediated gene
transfer of Gadd45a results in suppression by inducing apoptosis
and cell cycle arrest in pancreatic cancer cell. J Gene Med.
11:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pegg AE: Multifaceted roles of
alkyltransferase and related proteins in DNA repair, DNA damage,
resistance to chemotherapy, and research tools. Chem Res Toxicol.
24:618–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christmann M, Verbeek B, Roos WP and Kaina
B: O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal
tissues and tumors: Enzyme activity, promoter methylation and
immunohistochemistry. Biochim Biophys Acta. 1816:179–190.
2011.PubMed/NCBI
|
|
33
|
Chen SH, Kuo CC, Li CF, Cheung CH, Tsou
TC, Chiang HC, Yang YN, Chang SL, Lin LC, Pan HY, et al:
O6-methylguanine DNA methyltransferase repairs platinum-DNA adducts
following cisplatin treatment and predicts prognoses of
nasopharyngeal carcinoma. Int J Cancer. 137:1291–1305. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu T, Xiong Z, Chen S and Tu G: The use of
models in ‘target theory to evaluate the survival curves of human
ovarian carcinoma cell line exposure to adriamycin combined with
ultrasound. Ultrason Sonochem. 12:345–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Gao S, Jiang W, Luo C, Xu M,
Bohlin L, Rosendahl M and Huang W: Antioxidative dietary compounds
modulate gene expression associated with apoptosis, DNA repair,
inhibition of cell proliferation and migration. Int J Mol Sci.
15:16226–16245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Han X, Feng X, Wang Z and Zhang Y:
Coupling cellular localization and function of checkpoint kinase 1
(Chk1) in checkpoints and cell viability. J Biol Chem.
287:25501–25509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y and Hunter T: Roles of Chk1 in
cell biology and cancer therapy. Int J Cancer. 134:1013–1023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jackson SP and Helleday T: DNA REPAIR.
Drugging DNA repair. Science. 352:1178–1179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Simpson ER and Brown KA: p53:
Protection against tumor growth beyond effects on cell cycle and
apoptosis. Cancer Res. 75:5001–5007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng SY, Seo J, Huang BT, Napolitano T
and Champeil E: Mitomycin C and decarbamoyl mitomycin C induce
p53-independent p21WAF1/CIP1 activation. Int J Oncol.
49:1815–1824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Canne IG, Merrick KA, Morandell S, Zhu CQ,
Braun CJ, Grant RA, Cameron ER, Tsao MS, Hemann MT and Yaffe MB: A
pleiotropic RNA-binding protein controls distinct cell cycle
checkpoints to drive resistance of p53-defective tumors to
chemotherapy. Cancer Cell. 28:8312015. View Article : Google Scholar
|
|
42
|
Wang W, Zhang L, Wang Z, Yang F, Wang H,
Liang T, Wu F, Lan Q, Wang J and Zhao J: A three-gene signature for
prognosis in patients with MGMT promoter-methylated glioblastoma.
Oncotarget. 7:69991–69999. 2016.PubMed/NCBI
|
|
43
|
Hung CC, Chien CY, Chiang WF, Lin CS, Hour
TC, Chen HR, Wang LF, Ko JY, Chang CH and Chen JY: p22phox confers
resistance to cisplatin, by blocking its entry into the nucleus.
Oncotarget. 6:4110–4125. 2015. View Article : Google Scholar : PubMed/NCBI
|