Introduction
According to the 2012 survey conducted by the
International Agency for Research on Cancer (IARC), there are
nearly 1.7 million women suffering from breast cancer and ~520
thousand individuals succuumb to this disease each year worldwide,
with a rising incidence rate affecting younger women (1). For the past decade, molecular-targeted
therapy, targeting a particular gene or protein which can play a
pivotal role in the occurrence and development of malignant tumors,
instead of cytotoxic drugs, has gradually become a ‘hotspot’ in
cancer treatment research (2).
Currently, in this field, studies concerning downstream genes or
proteins of the epidermal growth factor receptor (EGFR) have
emerged in an endless stream, but research focusing on its upstream
genes or proteins is rare (3). A
disintegrin and metalloprotease 17 (ADAM17) is a cell membrane
glycoprotein, originally known for its ability to cleave and
activate tumor necrosis factor-α (TNF-α); thus, it is also named
TNF-α converting enzyme (TACE) (4,5).
However, upon release of TNF-α, ADAM17 also processes various
growth factors and receptors. Thus, it promotes the development of
various diseasess, and particularly participates in the activation
of EGFR, which is closely related to the progression of many
malignant tumors (6,7). ADAM17 is highly expressed in breast
cancer, prostate and colorectal cancer and glioma, playing a
‘signal scissor’ role in the tumor microenvironment (8,9). It
can cut a series of EGFR ligands, such as epidermal growth factor
(EGF), transforming growth factor-α (TGF-α), betacellulin (BTC),
amphiregulin (AREG), neuregulin (NRG), epiregulin (EREG) and
various inflammatory factors, particularly TNF-α (10,11).
EGFR ligand-binding leads to receptor self-dimerization,
autophosphorylation followed by the activation of downstream
MEK/ERK and PI3K/AKT pathways, thereby promoting tumor cell
proliferation, invasion and metastasis (12,13).
In recent years, the action of ADAM17 in breast cancer has
attracted increased attention. In this respect, there has been a
large number of studies on the expression of ADAM17 based on
clinical samples, cells and animal models. Expression of ADAM17 is
weak in normal breast tissues, but is significantly increased in
breast cancer, and becomes higher with an increase in the degree of
malignancy, clinical stage and lymph node metastasis of breast
cancer (14). ADAM17 was found to
exhibit higher expression in highly malignant breast cancers when
compared to that in low malignant cancers, and patients with high
ADAM17 expression were found to have a visibly reduced overall
survival than those with low expression, which implies that ADAM17
may be a favorable breast cancer therapeutic target (15,16).
Our previous study found that the migration and proliferation of
MCF-7 breast cancer cells can be inhibited by the silencing of
ADAM17 via the EGFR/PI3K/AKT signaling pathway in vitro, and
MCF-7 cell xenograft growth was also inhibited by ADAM17-siRNA
in vivo (17). Our present
study found that ADAM17-shRNA suppresses MCF-7 breast cancer cell
growth in vitro and in vivo through the EGFR/PI3K/AKT
and EGFR/MEK/ERK signaling pathways.
Materials and methods
Cell line and cell culture
MCF-7 cell culture was conducted as previously
described (17). The MCF-7 human
breast cancer cell line was obtained from the Institute of Basic
Medical Sciences, Chinese Academy of Medical Sciences (Beijing,
China).
Transfection of MCF-7 cells with
lentivirus-ADAM17-shRNA-GFP
As shown in Table I,
four ADAM17-shRNAs and one non-sense-shNC were designed by
GenePharma (Shanghai, China). Each gene carrier contained an
expression framework of green fluorescent protein (GFP) which could
be expressed in transfected cells. Thus, the transfection
efficiency was evaluated under a Nikon® Eclipse Ti-U
inverted fluorescence microscope (Nikon, Tokyo, Japan). MCF-7 cells
in the ADAM17-shRNA and non-sense-shNC groups were transfected
using a lentivirus following the manufacturer's instructions. An
equal volume of PBS solution was added to the cells of the control
group.
 | Table I.Gene sequences of the ADAM17-shRNAs
and non-sense-shNC. |
Table I.
Gene sequences of the ADAM17-shRNAs
and non-sense-shNC.
| shRNAs | Gene sequences |
|---|
| ADAM17-297 |
5′-GCTCTCAGACTACGATATTCT-3′ |
| ADAM17-1134 |
5′-GCTAGAGCAATTTAGCTTTGA-3′ |
| ADAM17-1219 |
5′-GGAACTCTTGGATTAGCTTAT-3′ |
| ADAM17-1508 |
5′-GCGATCACGAGAACAATAAGA-3′ |
| Non-sense-shNC |
5′-GTTCTCCGAACGTGTCACGT-3′ |
Quantitative real-time polymerase
chain reaction (qRT-PCR)
qRT-PCR experiments were performed as previously
described (17).
Invasion assays in vitro
Invasion of MCF-7 cells was performed using 24-well
Transwell chambers with 8.0-µm pore polycarbonate membranes covered
with Matrigel (BD Biosciences, San Jose, CA, USA). After
trypsinization, cells of the ADAM17-shRNA, non-sense-shNC and
control groups were suspended in DMEM, respectively, with the cell
concentration adjusted to 5×105/ml. The subsequent
experimental procedure, except the cut polycarbonate membrane
stained with hematoxylin, and counting method were identical to
that as previously described (17).
The number of cells that invaded through the Transwell chamber was
an indicator to evaluate invasive ability.
Real-time cell analysis
The effect of ADAM17-shRNA on MCF-7 cell
proliferation was monitored in real-time using the iCELLigence
system (ACEA Biosciences, San Diego, CA, USA). In brief, 150 µl
DMEM containing 10% FBS was dropped in each E-Plate L8 well (ACEA
Biosciences) and then the E-Plates were inserted into the
iCELLigence instrument for background measurement. MCF-7 cells of
the ADAM17-shRNA, non-sense-shNC and control groups were
trypsinized and plated at a concentration of 3×104/well
into the E-Plate L8 in a final volume of 450 µl, respectively, and
were incubated at room temperature for 30 min. Afterwards, E-Plates
were inserted again, and iCELLigence assay was performed. Cell
adhesion, growth, and proliferation process were detected by
measuring electrical impedance and recorded for 120 h. Cell index
(CI) which reflects the number of viable cells was calculated for
each E-plate well and the CI curve was obtained with iCELLigence DA
Software 1.0 (ACEA Biosciences). The experiments were conducted in
triplicate.
Flow cytometry
The effect of ADAM17-shRNA on the cell cycle
distribution of MCF-7 cells was detected by flow cytometric (FCM)
analysis. MCF-7 cells (1×106) during the logarithmic
growth phase in the ADAM17-shRNA, non-sense-shNC, and control
groups were seeded in 25-cm3 culture flasks,
respectively, and maintained for 24 h. Following washing with PBS
and trypsinization, the cells were then collected and fixed with
75% ethanol at 4°C overnight. After centrifugation, the cells were
incubated with PBS containing 50 µg/ml propidium iodide (PI)
(Sigma, St. Louis, MO, USA) and 100 µg/ml RNase A (Invitrogen,
Carlsbad, CA, USA) in darkness at 37°C for 30 min. Finally, the
cells were subjected to flow cytometric analysis using the
FACSCalibur flow cytometer (BD Biosciences). Each experiment was
repeated 3 times.
Effect of ADAM17-shRNA on the growth
of MCF-7 breast cancer cells in vivo
Female (nu/nu) athymic mice with a weight of 15–25 g
were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing,
China). Estrogen (0.2 ml) (0.15 mg/ml) was injected into the
peritoneal cavity of the nude mice every day until sacrifice.
Fifteen nude mice were randomly divided into three groups:
ADAM17-shRNA, non-sense-shNC and control group. MCF-7 (0.2 ml)
breast cancer cells (5×107/ml) with three different
treatments were subcutaneously implanted in the right flank of the
nude mice, respectively, after 3 days of estrogen injection. From
the 10th day after implantation, with visable emergence of the
tumor nodule, the tumor diameter was measured with calipers every 4
days and the tumor volume (V) was calculated by the formula: V =
(width)2 × length/2. Mice were sacrificed on day 26
after cell implantation. The mice were euthanized by cervical
dislocation. This study was conducted in accordance with the
ethical standards according to national and international
guidelines, and all experimental procedures were approved by the
Institutional Review Board of North China University of Science and
Technology.
Immunohistochemistry of tumor
tissues
Immunohistochemical staining analysis of ADAM17 and
Ki-67 in the tumor tissues from the different groups was carried
out as previously described (17).
Western blotting
Western blotting was performed as previously
described (17) with the following
modifications. Proteins were separated on 8% SDS-PAGE and two
antibodies: anti-ERK (1:1,000) and anti-phosphorylated ERK
(1:1,000) (both from Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) were used.
Statistical analysis
Results are showed as mean ± SD. Data were
considered statistically significant when the value of P<0.05.
Comparisons among 3 or more groups were made by one-way ANOVA using
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). All experimental
procedures were approved by the Institutional Animal Care and Use
Committee of North China University of Science and Technology.
Results
Transfection efficiency
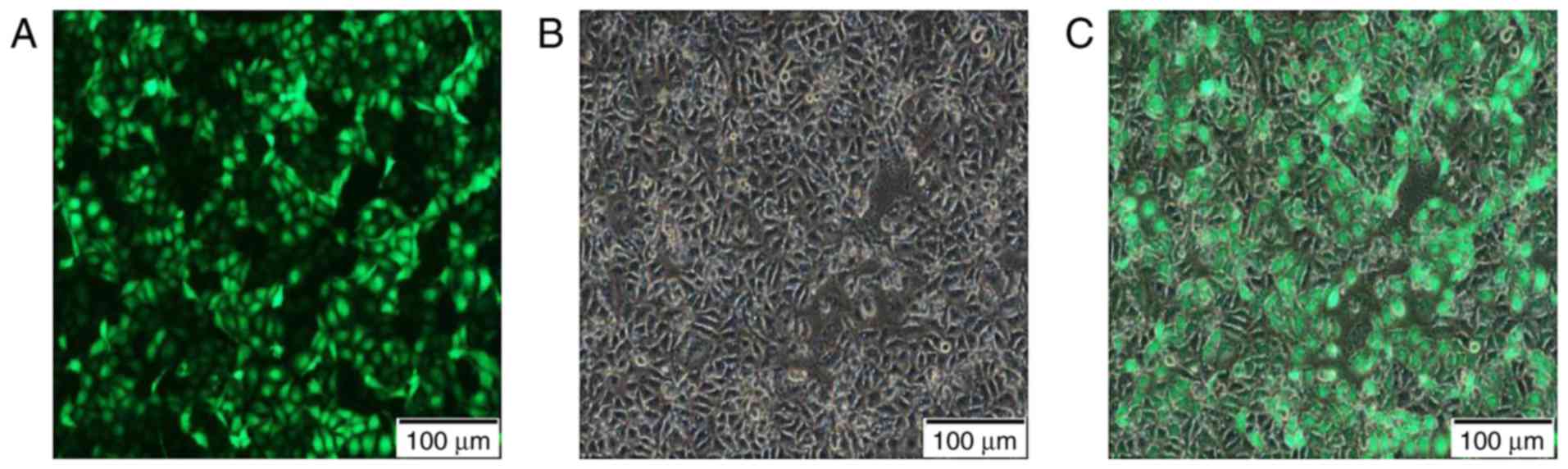
After transfection with a multiplicity of infection
(MOI) set to 100 and continuous culture for 72 h, the cells were
observed under the Nikon® Eclipse Ti-U inverted
fluorescence microscope. According to the expression of the GFP,
the transfection efficiency of this experiment reached >90%.
This verified that the lentivirus transferred ADAM17-shRNA to the
MCF-7 cells efficiently (Fig.
1).
ADAM17 mRNA expression was silenced by
ADAM17-shRNA in MCF-7 cells
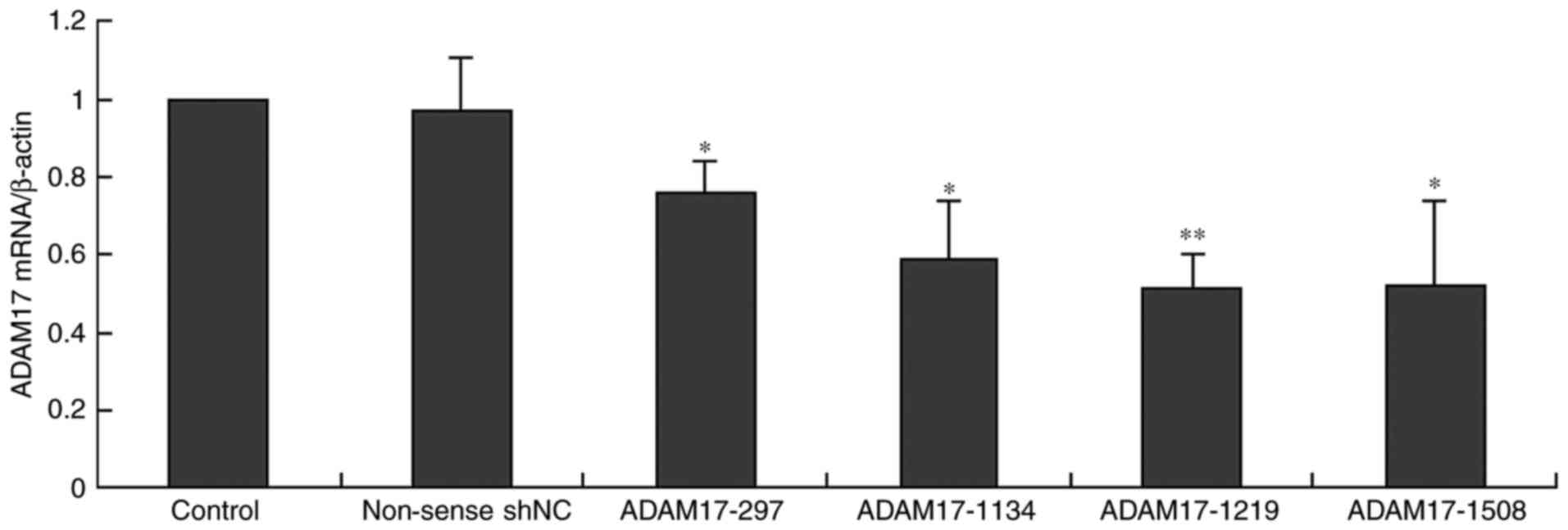
To observe whether ADAM17 expression was inhibited
by ADAM17-shRNA, ADAM17 mRNA levels in MCF-7 cells transfected with
ADAM17-shRNAs and non-sense-shNC were detected by qRT-PCR. The
relative quantity of ADAM17 mRNA in the control group was
considered as 100% after β-actin corrections. Our results revealed
that ADAM17 mRNA was highly expressed in the MCF-7 cells (control),
and non-sense-shNC did not change ADAM17 mRNA expression, while all
ADAM17-shRNAs inhibited the expression (P<0.05, Fig. 2), particularly ADAM17-1219
(P<0.01). Thus, we omitted the other shRNAs in the following
experiments. This data showed that ADAM17 expression was
successfully inhibited by ADAM17-shRNA at the mRNA level.
Invasion of MCF-7 breast cancer
cells
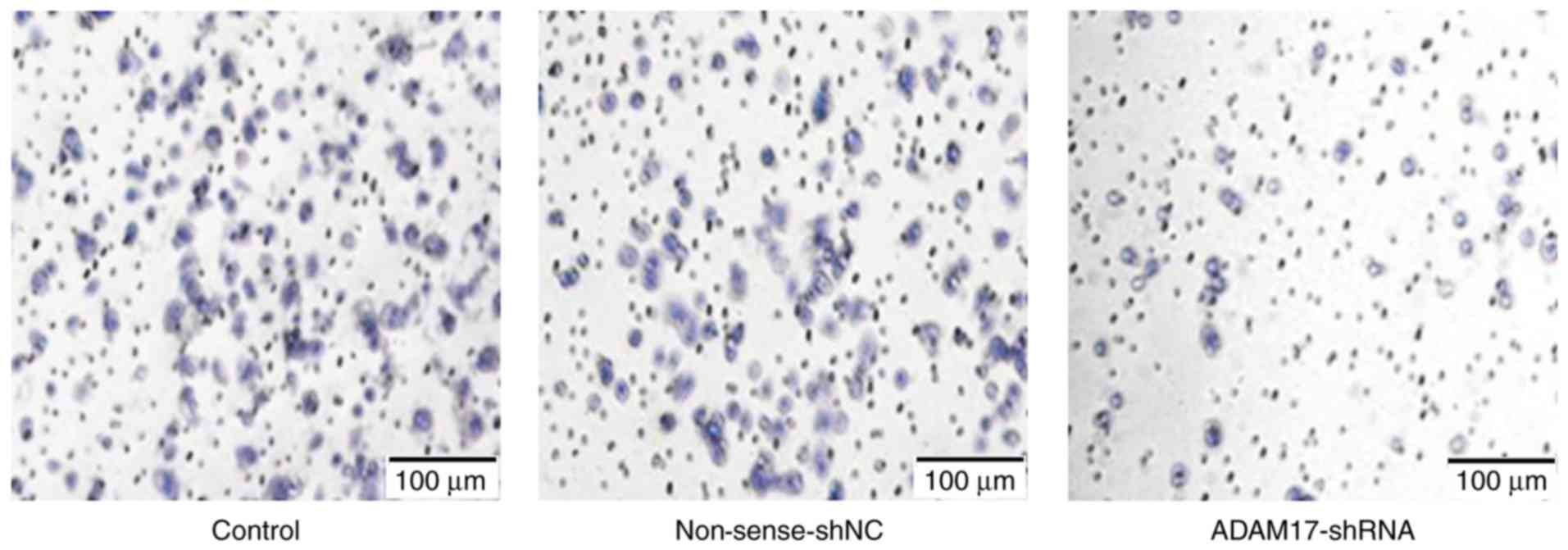
Transwell chamber method was used to test the
invasive ability of the MCF-7 cells. The average number of invaded
cells in each field was 101.75±4.25, 99.13±6.08 and 57.42±3.95 in
the control, non-sense-shNC and ADAM17-shRNA groups, respectively
(Fig. 3). There was no statistical
difference between the control and the non-sense-shNC group
(P>0.05). However, the number was reduced significantly in the
ADAM17-shRNA group (P<0.05). The results revealed that ADAM17
enhanced MCF-7 cell invasion and ADAM17-shRNA successfully
inhibited the invasive ability of the breast cancer cells.
CI curve by iCELLigence system
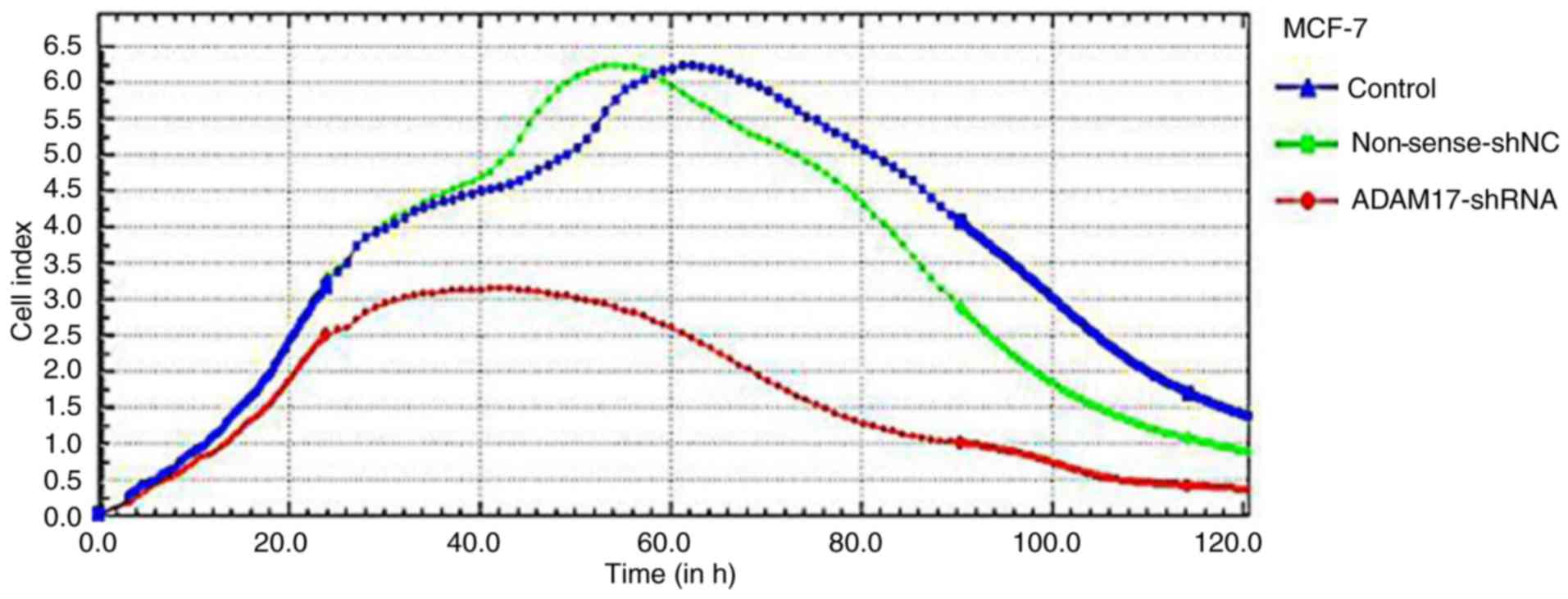
Using the iCELLigence microelectronic biosensor
system, the real-time analysis of MCF-7 cell proliferation was
performed. As shown in Fig. 4,
cells of the control and non-sense-shNC group both displayed high
increases and high levels in CI, but the growth rate and
proliferative activity did not achieve a significant different
(P>0.05). However, the CI of the ADAM17-shRNA group maintained a
shorter increase and significantly lower level than that of the
other two groups, which implies a slowed cell growth rate and
decreased proliferative activity (P<0.05). Thus, the iCELLigence
assay showed that ADAM17-shRNA effectively inhibited the
proliferation of the MCF-7 cells.
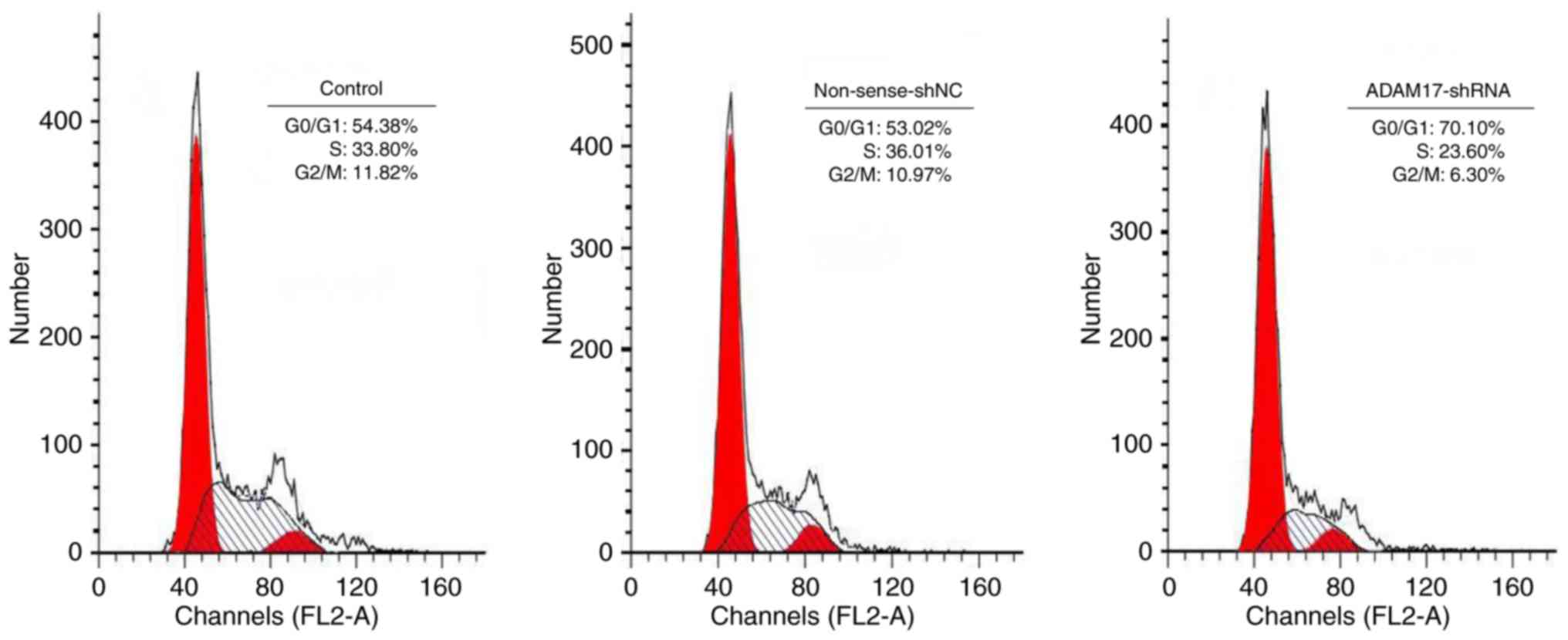
ADAM17-shRNA induces G0/G1 cell cycle
arrest in MCF-7 cells
To further examine the antitumor effect of
ADAM17-shRNA, cell cycle progression in MCF-7 cells was
investigated. The percentage of cells in each phase of the cell
cycle was measured by flow cytometry (Fig. 5). There was no significant
difference in the percentage of G0/G1, S and G2/M phase cells
between the control and non-sense-shNC group (P>0.05). However,
when MCF-7 cells were transfected with ADAM17-shRNA, an
accumulation of the cell population in the G0/G1 phase was
significantly increased, accompanied by a decrease in the
percentage of cells in the S and G2/M phases (P<0.05). These
results indicated that ADAM17-shRNA resulted in G0/G1 phase arrest
of MCF-7 cells, eventually inhibiting the growth of MCF-7
cells.
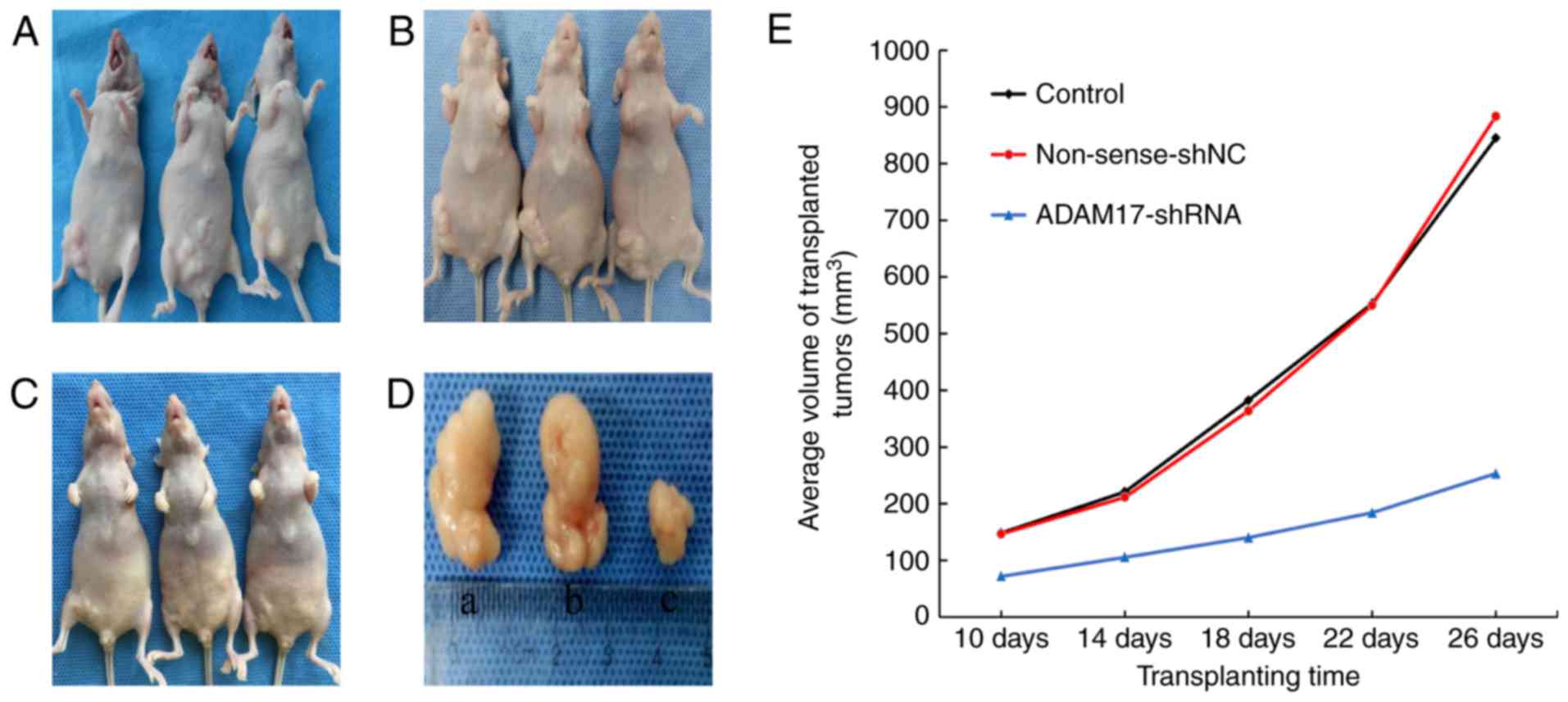
Inhibition of tumor growth by
ADAM17-shRNA
The size of the transplanted tumors was observably
increased (a and b in Fig. 6D) in
the control group (Fig. 6A) and the
non-sense-shNC group (Fig. 6B), but
was significantly smaller (c in Fig.
6D) in the ADAM17-shRNA group (Fig.
6C) than the other two groups (P<0.05). The tumor growth
curve (Fig. 6E), drawn from the
average tumor volumes of the different groups, showed that the
transplanted tumors maintained a sustained and rapid growth trend
in the control and non-sense-shNC groups, but increased slowly in
the ADAM17-shRNA group.
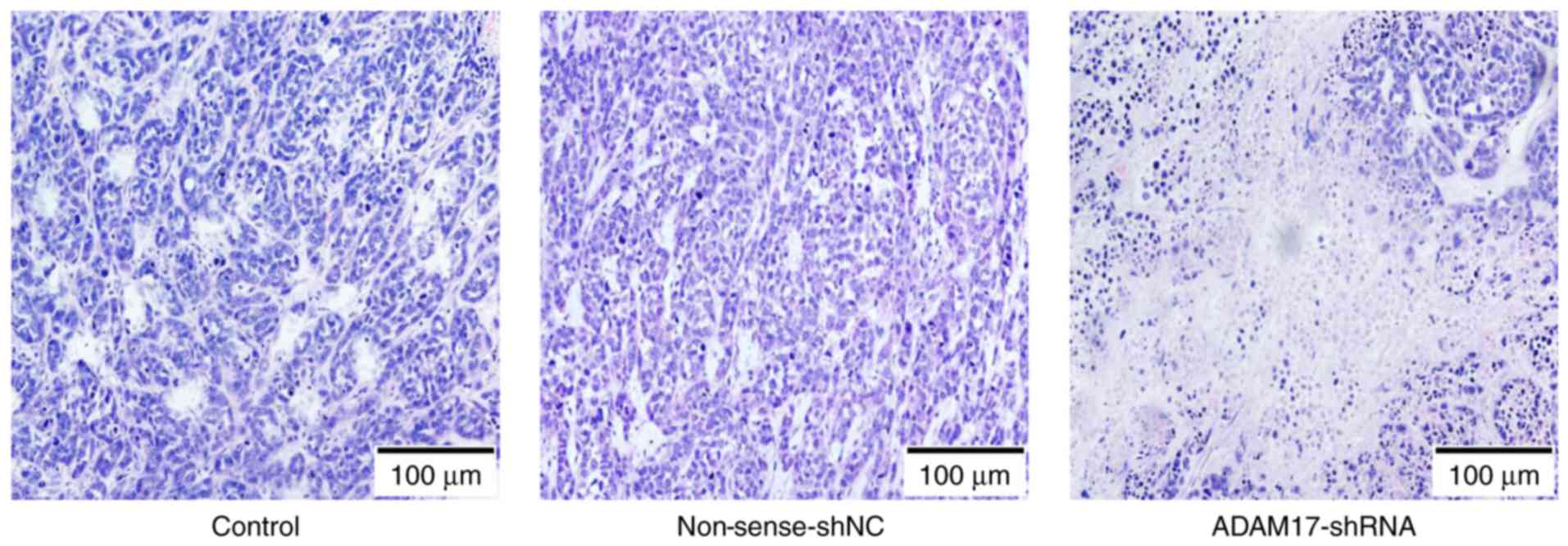
Microscopic features of the
transplanted tumor tissues
Hematoxylin and eosin (H&E) staining displayed
that the transplanted tumors were characterized by breast invasive
ductal carcinoma (cords of breast cancer cells, interstitial
invasion and hemorrhage). There was no obvious difference between
the control and non-sense-shNC group. Compared with the other two
groups, the tumor tissue of the ADAM17-shRNA group developed larger
areas of necrosis, in which the cells were destroyed and cell
structures had disappeared (Fig.
7).
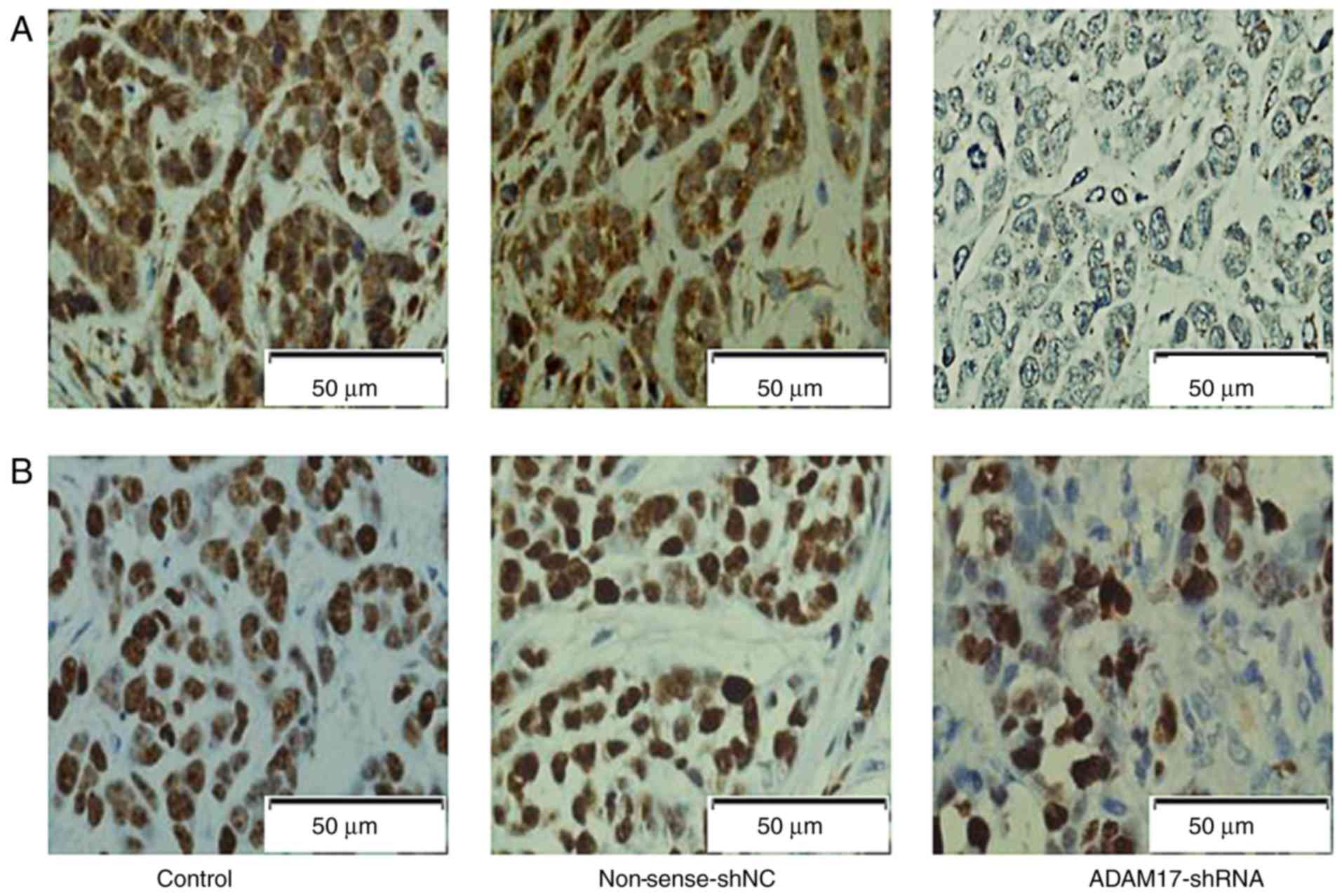
ADAM17 and Ki-67 in tumor tissues as
detected by immunohistochemistry
ADAM17, shown as positive brown staining, was mostly
expressed in the cytoplasm. The staining index score was 7.17±0.27,
7.11±0.21 and 2.65±0.49 in the control, non-sense-shNC and
ADAM17-shRNA group, respectively. The scores indicated a
significant decrease in the ADAM17-shRNA group (P<0.01, Fig. 8A), but had no statistical difference
between the control and non-sense-shNC group (P>0.05). The
results suggested that ADAM17-shRNA inhibited ADAM17 expression in
transplanted tumors.
Ki-67, shown as positive brown staining, was mostly
expressed in the nucleus. The staining index score was 9.05±0.34,
8.94±0.42 and 3.76±0.23 in the control, non-sense-shNC and
ADAM17-shRNA group, respectively. The scores indicated a
significant decrease in the ADAM17-shRNA group (P<0.01, Fig. 8B), but no statistical difference was
noted between the control and non-sense-shNC group (P>0.05). The
results indicated that ADAM17-shRNA inhibited Ki-67 expression in
the transplanted tumors.
Exploration of the mechanism
underlying the inhibition of breast cancer growth by
ADAM17-shRNA
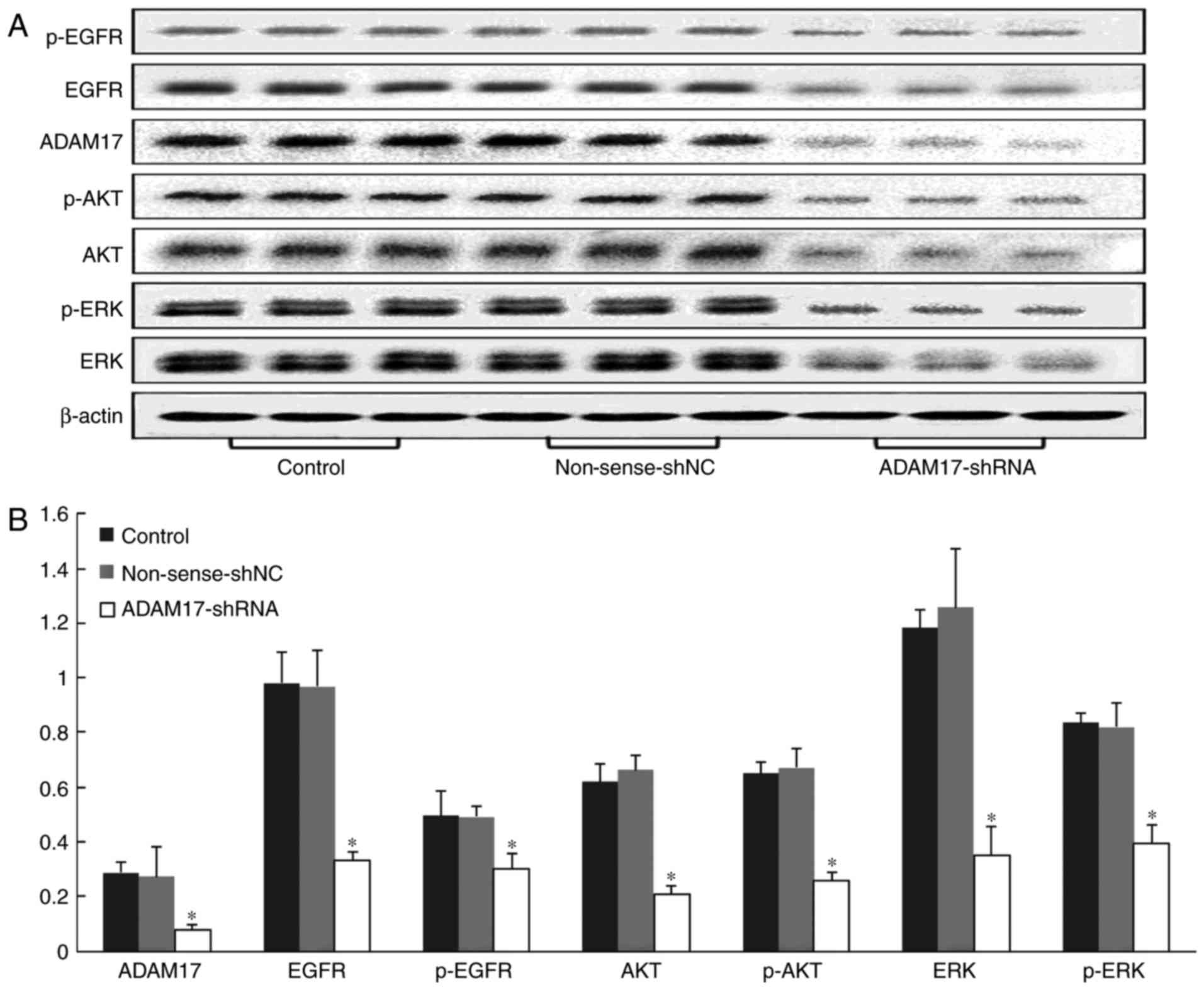
To further reveal the mechanism of ADAM17-shRNA
against MCF-7 breast cancer growth, expression of related proteins
in the transplanted tumors were tested by western blotting. The
results showed that there was high expression of ADAM17, EGFR,
phosphorylated (p)-EGFR, AKT, p-AKT, ERK and p-ERK, both in the
control and non-sense-shNC groups; however, administration of
ADAM17-shRNA significantly reduced these proteins compared with the
other two groups (P<0.01, P<0.01, P=0.01, P<0.01,
P<0.01, P<0.01, P<0.01, respectively, Fig. 9). These data indicated that EGFR,
p-EGFR, AKT, p-AKT, ERK and p-ERK were downstream of ADAM17 and
were involved in the inhibition of MCF-7 breast cancer cells by
ADAM17-shRNA in vivo.
Discussion
ADAM17 is expressed in almost all cells of the human
body, but only obviously when inflammation or cancer occurs
(18). It can cut some
membrane-bound growth factors, growth factor receptors or cytokines
in the extracellular domain, making them activated or released,
thereby modulating a variety of cellular biological behaviors,
which is causally related to neurodevelopment, aging, viral
transmission, immune response and tumorigenesis (8,18).
Our previous study found that migration and
proliferation of MCF-7 breast cancer cells were inhibited by
ADAM17-siRNA via the EGFR/PI3K/AKT signaling pathway in
vitro, and MCF-7 cell xenograft growth was also inhibited by
ADAM17-siRNA in vivo (17).
However, siRNA cannot self-replicate and is easy to be diluted and
degraded in the process of cell differentiation, resulting in a
short duration of silencing action (usually lasting 3–7 days). Once
siRNA disappears in the transfected cells, the function of the
target gene can be restored to the level of pre-transfection. This
limitation makes siRNA only an effective tool for short-term
analysis of gene function (19).
Thus, in our current project, we transfected MCF-7 cells with
ADAM17-shRNA and that siRNA sequence was cloned into the plasmid
vector as ‘short hairpin’. When entering a cell, the hairpin
sequence can be automatically processed into siRNAs, which enable
target gene silencing. Therefore, loading the carrier of the shRNA
can be passed to the progeny cells so that the silencing of genes
can be inherited (19,20). Lentivirus can infect both mitotic
and non-mitotic cells, and the RNA interference sequence that it
carries can be randomly inserted and integrated into the genome of
the host cell for long-term expression (21,22).
Thus, the aim of long-term stable silencing of the ADAM17 gene can
be achieved.
Cell proliferation and invasion are essential to the
development of cancers, which are complicated activities regulated
by many types of factors (23).
Some studies have confirmed that ADAM17 can enhance proliferation
and invasion of breast cancer cells (2,24,25).
In the present study, the MCF-7 cell line was employed for the
research of ADAM17-shRNA against the proliferation and invasion of
breast cancer in vitro. Prior to this, we maintained cell
transfection efficiency above 90% using a lentivirus, in order to
provide full efficacy of ADAM17-shRNA. The qRT-PCR data showed that
our shRNA successfully inhibited ADAM17 expression at the mRNA
level. Real-time cell analysis and Transwell chamber assay showed
that ADAM17-shRNA effectively blocked proliferation and invasion of
MCF-7 cells, and flow cytometric analysis further confirmed that
ADAM17-shRNA resulted in a G0/G1 phase arrest of the MCF-7 cells.
The results suggest that ADAM17-shRNA can inhibit breast cancer
metastasis to other organs.
Thereafter, the antitumor effects of ADAM17-shRNA
were detected in vivo. Xenograft models of MCF-7 breast
cancer were established in nude mice to observe whether
ADAM17-shRNA could inhibit tumor growth. The results showed that
ADAM17-shRNA slowed the growth of transplanted tumors and induced
tumor necrosis. As a valuable biological marker for assessing the
proliferative activity of tumor cells, Ki-67 has demonstrated
positive results in a variety of malignancies, particularly in
breast cancer (26,27). It is often used in clinical work to
evaluate the malignant degree of breast cancer and the prognosis of
patients (27,28). Our immunohistochemistry results
indicated that the expression of ADAM17 and Ki-67 were
significantly decreased in the breast cancer tissues transfected
with ADAM17-shRNA.
EGFR, a tyrosine kinase type receptor, is widely
distributed in all cell surfaces except hematopoietic cells and
plays an important regulatory role in the normal physiological
process of cells (29). However,
overexpression and mutation of EGFR are always associated with
malignant tumors (29,30). A recent study found that EGFR
expression increases with tumor volume, histological grade, lymph
node metastasis and TNM stage of breast cancer, and there are
numerous studies on targeting EGFR in breast cancer treatment
(31–34). EGFR activation relies on binding
ligands (such as EGF, TNF-α) to promote proliferation and invasion
of tumor cells. ADAM17 has been proven to play a pivotal role in
the progression of EGFR-dependent malignancies by hydrolyzing its
ligands (13,16). EGFR ligand-binding may activate the
downstream PI3K/AKT and MEK/ERK signaling pathways, which
contributes to tumor development (2,12,35).
AKT, also known as protein kinase B (PKB), is closely related to
the proliferation, apoptosis, and metabolism of normal cells
(36). The dysregulation of the
PI3K/AKT pathway is associated with diabetes, cardiovascular and
neurological diseases, and malignancies (37). Studies have shown that PI3K/AKT
pathway signals are overexpressed in breast, liver and lung cancer,
soft tissue tumors and other malignant diseases, and are correlated
with tumor size (38–40). Our previous study confirmed that AKT
signaling is induced by ADAM17, which is involved in MCF-7 cell
proliferation and migration (17).
However, in the previous study, the MEK inhibitor (PD0325901) was
found to have no effect on the proliferation and invasion of MCF-7
cells, and thus was discarded in the subsequent experiments,
without further testing the protein expression of the EGFR/MEK/ERK
pathway in different treatments and ascertaining its interaction
with the ERFR/PI3K/ERK pathway. Various studies have shown that
crosstalk exists between the EGFR/PI3K/AKT and EGFR/MEK/ERK
pathways (41–43). Phosphorylated AKT can activate the
mitogen-activated protein kinase (MAPK) pathway (44). Selective MEK inhibitors can cause
the upegulation of PI3K pathway signals, which leads to the drug
resistance to the MEK inhibitor in a complex signal network, while
the combination of MEK inhibitor and PI3K inhibitor can provide a
better therapeutic effect (42,45,46).
Thus, we highly suspect that the inhibitory effect of PD0325901 on
MEK was obscured by the role of the upregulated PI3K signals
through the above mechanism in our last study, so the migration and
proliferation of MCF-7 cells were not reduced after PD0325901
administration. Therefore, we considered that the mechanism of
ADAM17-shRNA in the inhibition of MCF-7 breast cancer needed
further exploration. ERK, an important member of the MAPK family,
is confirmed in a variety of cell models to participate in cell
apoptosis and cell cycle regulation and play a crucial role in cell
proliferation and differentiation (47,48).
Excessive expression of the ERK pathway is common in tumors
(49,50). It has been proven that ERK and p-ERK
expression in breast cancer tissues increases with clinical stage
and lymph node metastasis (51).
Various studies have reported that the EGFR/MEK/ERK pathway can be
activated by ADAM17 (35,52). In the present study, the western
blot results showed that expression of EGFR, AKT, and ERK was
significant lower in the ADAM17-shRNA group than that in the
control and non-sense-shNC groups due to the fact that the growth
of the transplanted tumor was markedly inhibited, and p-EGFR, p-AKT
and p-ERK expression was also suppressed by ADAM17-shRNA.
Our results above indicated that ADAM17-shRNA can
inhibit MCF-7 breast cancer growth in vitro and in
vivo through the EGFR/PI3K/AKT and EGFR/MEK/ERK signaling
pathways. This provides reliable experimental support for the
development of new drugs targeting ADAM17 and opens up a new
approach for the targeted therapy of breast cancer.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Caiazza F, McGowan PM, Mullooly M, Murray
A, Synnott N, O'Donovan N, Flanagan L, Tape CJ, Murphy G, Crown J
and Duffy MJ: Targeting ADAM-17 with an inhibitory monoclonal
antibody has antitumor effects in triple-negative breast cancer
cells. Br J Cancer. 112:1895–1903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ocaña A, Amir E, Seruga B, Martin M and
Pandiella A: The evolving landscape of protein kinases in breast
cancer: Clinical implications. Cancer Treat Rev. 39:68–76. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Black RA, Rauch CT, Kozlosky CJ, Peschon
JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P,
Srinivasan S, et al: A metalloproteinase-disintegrin that releases
tumor-necrosis factor-alpha from cells. Nature. 385:729–733. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moss ML, Jin SL, Milla ME, Bickett DM,
Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, et
al: Cloning of a disintegrin metalloproteinase that processes
precursor tumor-necrosis factor-alpha. Nature. 385:733–736. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baumgart A, Seidl S, Vlachou P, Michel L,
Mitova N, Schatz N, Specht K, Koch I, Schuster T, Grundler R, et
al: ADAM17 regulates epidermal growth factor receptor expression
through the activation of Notch1 in non-small cell lung cancer.
Cancer Res. 70:5368–5378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santiago-Josefat B, Esselens C, Bech-Serra
JJ and Arribas J: Post-transcriptional up-regulation of ADAM17 upon
epidermal growth factor receptor activation and in breast tumors. J
Biol Chem. 282:8325–8331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy G: The ADAMs: Signaling scissors in
the tumor microenvironment. Nat Rev Cancer. 8:929–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng X, Jiang F, Katakowski M, Kalkanis
SN, Hong X, Zhang X, Zhang ZG, Yang H and Chopp M: Inhibition of
ADAM17 reduces hypoxia-induced brain tumor cell invasiveness.
Cancer Sci. 98:674–684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rego SL, Helms RS and Dréau D: Tumor
necrosis factor-alpha-converting enzyme activities and
tumor-associated macrophages in breast cancer. Immunol Res.
58:87–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maretzky T, Zhou W, Huang XY and Blobel
CP: A transforming Src mutant increases the bioavailability of EGFR
ligands via stimulation of the cell-surface metalloproteinase
ADAM17. Oncogene. 30:611–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin CF, Hao K, Tian XD, Xie XH and Yang
YM: Combined effects of EGFR and Hedgehog signaling pathway
inhibition on the proliferation and apoptosis of pancreatic cancer
cells. Oncol Rep. 28:519–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo G, Gong K, Wohlfeld B, Hatanpaa KJ,
Zhao D and Habib AA: Ligand-independent EGFR signaling. Cancer Res.
75:3436–3441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGowan PM, Ryan BM, Hill AD, McDermott E,
O'Higgins N and Duffy MJ: ADAM-17 expression in breast cancer
correlates with variables of tumor progression. Clin Cancer Res.
13:2335–2343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGowan PM, McKiernan E, Bolster F, Ryan
BM, Hill AD, McDermott EW, Evoy D, O'Higgins N, Crown J and Duffy
MJ: ADAM-17 predicts adverse outcome in patients with breast
cancer. Ann Oncol. 19:1075–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kenny PA: TACE: A new target in epidermal
growth factor receptor-dependent tumors. Differentiation.
75:800–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng X, Hu B, Hossain MM, Chen G, Sun Y
and Zhang X: ADAM17-siRNA inhibits MCF-7 breast cancer through
EGFR-PI3K-AKT activation. Int J Oncol. 49:682–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rose-John S: ADAM17, shedding, TACE as
therapeutic targets. Pharmacol Res. 71:19–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rao DD, Vorhies JS, Senzer N and
Nemunaitis J: siRNA vs. shRNA: Similarities and differences. Adv
Drug Deliv Rev. 61:746–759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gvozdeva OV, Prassolov VS, Zenkova MA,
Vlassov VV and Chernolovskaya EL: Silencing of inducible
immunoproteasome subunit expression by chemically modified siRNA
and shRNA. Nucleosides Nucleotides Nucleic Acids. 35:389–403. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stewart SA, Dykxhoorn DM, Palliser D,
Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et
al: Lentivirus-delivered stable gene silencing by RNAi in primary
cells. RNA. 9:493–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klinghoffer RA, Magnus J, Schelter J,
Mehaffey M, Coleman C and Cleary MA: Reduced seed region-based
off-target activity with lentivirus-mediated RNAi. RNA. 16:879–884.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y and Lazo JS: Metastasis-associated
phosphatase PRL-2 regulates tumor cell migration and invasion.
Oncogene. 31:818–827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng X, Jiang F, Katakowski M, Zhang ZG,
Lu QE and Chopp M: ADAM17 promotes breast cancer cell malignant
phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther.
8:1045–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao MQ, Kim BG, Kang S, Choi YP, Yoon JH
and Cho NH: Human breast cancer-associated fibroblasts enhance
cancer cell proliferation through increased TGF-alpha cleavage by
ADAM17. Cancer Lett. 336:240–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshioka T, Hosoda M, Yamamoto M, Taguchi
K, Hatanaka KC, Takakuwa E, Hatanaka Y, Matsuno Y and Yamashita H:
Prognostic significance of pathologic complete response and Ki67
expression after neoadjuvant chemotherapy in breast cancer. Breast
Cancer. 22:185–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao S, He ZX, Yu KD, Yang WT and Shao ZM:
New insights into the prognostic value of Ki-67 labeling index in
patients with triple-negative breast cancer. Oncotarget.
7:24824–24831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang W, Wu J, Zhang P, Fei X, Zong Y, Chen
X, Huang O, He JR, Chen W, Li Y, et al: Prognostic and predictive
value of Ki-67 in triple-negative breast cancer. Oncotarget.
7:31079–31087. 2016.PubMed/NCBI
|
|
29
|
Brand TM, Iida M, Luthar N, Starr MM,
Huppert EJ and Wheeler DL: Nuclear EGFR as a molecular target in
cancer. Radiother Oncol. 108:370–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomas A, Futter CE and Eden ER: EGF
receptor trafficking: Consequences for signaling and cancer. Trends
Cell Biol. 24:26–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Wang Q, Fu L, Liu M and Yu X:
Expression of PTEN, p53, and EGFR in the molecular subtypes of
breast carcinoma and the correlation among them. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 40:973–978. 2015.(In Chinese). PubMed/NCBI
|
|
32
|
Lluch A, Eroles P and Perez-Fidalgo JA:
Emerging EGFR antagonists for breast cancer. Expert Opin Emerg
Drugs. 19:165–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Howe LR and Brown PH: Targeting the
HER/EGFR/ErbB family to prevent breast cancer. Cancer Prev Res.
4:1149–1157. 2011. View Article : Google Scholar
|
|
34
|
Kim S, Lee J, Oh SJ, Nam SJ and Lee JE:
Differential effect of EGFR inhibitors on tamoxifen-resistant
breast cancer cells. Oncol Rep. 34:1613–1619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao LJ, Lin P, Lin F, Liu X, Qin W, Zou
HF, Guo L, Liu W, Wang SJ and Yu XG: ADAM17 targets MMP-2 and MMP-9
via EGFR-MEK-ERK pathway activation to promote prostate cancer cell
invasion. Int J Oncol. 40:1714–1724. 2012.PubMed/NCBI
|
|
36
|
Hers I, Vincent EE and Tavare JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Toker A and Marmiroli S: Signaling
specificity in the Akt pathway in biology and disease. Adv Biol
Regul. 55:28–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dobashi Y, Tsubochi H, Matsubara H, Inoue
J, Inazawa J, Endo S and Ooi A: Diverse involvement of isoforms and
gene aberrations of Akt in human lung carcinomas. Cancer Sci.
106:772–781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dobashi Y, Sato E, Oda Y, Inazawa J and
Ooi A: Significance of Akt activation and AKT gene increases in
soft tissue tumors. Hum Pathol. 45:127–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Won JK, Yang HW, Shin SY, Lee JH, Heo WD
and Cho KH: The crossregulation between ERK and PI3K signaling
pathways determines the tumoricidal efficacy of MEK inhibitor. J
Mol Cell Biol. 4:153–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takai M, Nakagawa T, Tanabe A, Terai Y,
Ohmichi M and Asahi M: Crosstalk between PI3K and Ras pathways via
protein phosphatase 2A in human ovarian clear cell carcinoma.
Cancer Biol Ther. 16:325–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Djukom C, Porro LJ, Mrazek A, Townsend CM
Jr, Hellmich MR and Chao C: Dual inhibition of PI3K and mTOR
signaling pathways decreases human pancreatic neuroendocrine tumor
metastatic progression. Pancreas. 43:88–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tandon M, Chen Z and Pratap J: Role of
Runx2 in crosstalk between Mek/Erk and PI3K/Akt signaling in
MCF-10A cells. J Cell Biochem. 115:2208–2217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoeflich KP, O'Brien C, Boyd Z, Cavet G,
Guerrero S, Jung K, Januario T, Savage H, Punnoose E, Truong T, et
al: In vivo antitumor activity of MEK and phosphatidylinositol
3-kinase inhibitors in basal-like breast cancer models. Clin Cancer
Res. 15:4649–4664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu C, Sun X, Qin S, Wang H, Zheng Z, Xu S,
Luo G, Liu P, Liu J, Du N, et al: Let-7a regulates mammosphere
formation capacity through Ras/NF-κB and Ras/MAPK/ERK pathway in
breast cancer stem cells. Cell Cycle. 14:1686–1697. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hung AC, Tsai CH, Hou MF, Chang WL, Wang
CH, Lee YC, Ko A, Hu SC, Chang FR, Hsieh PW and Yuan SS: The
synthetic β-nitrostyrene derivative CYT-Rx20 induces breast cancer
cell death and autophagy via ROS-mediated MEK/ERK pathway. Cancer
Lett. 371:251–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deschenes-Simard X, Gaumont-Leclerc MF,
Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette
FA, Saba-El-Leil MK, Meloche S, et al: Tumor suppressor activity of
the ERK/MAPK pathway by promoting selective protein degradation.
Genes Dev. 27:900–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang XM, Li BL, Song M and Song JY:
Expression and significance of ERK protein in human breast
carcinoma. Chin J Cancer Res. 16:269–273. 2004. View Article : Google Scholar
|
|
52
|
Beck Gooz M, Maldonado EN, Dang Y, Amria
MY, Higashiyama S, Abboud HE, Lemasters JJ and Bell PD: ADAM17
promotes proliferation of collecting duct kidney epithelial cells
through ERK activation and increased glycolysis in polycystic
kidney disease. Am J Physiol Renal Physiol. 307:F551–F559. 2014.
View Article : Google Scholar : PubMed/NCBI
|