Introduction
Gastric cancer is an important cancer type occurring
in the upper digestive tract, accounting for 8% of cancer cases and
10% of deaths globally (1,2). It is estimated that there are
approximately 750,000 new cases diagnosed annually, and 5-year
overall survival rates are <25% (3). Due to its highest morbidity and
mortality, gastric cancer remains a noticeable public health
problem in East Asia (4). Although
great efforts have been made, early diagnosis and current
treatments for this deadly cancer are still not efficacious.
Therefore, it is important to investigate the molecular mechanisms
involved in the transformation and progression of gastric
cancer.
Many factors are associated with the initiation and
progression of gastric cancer: genetic variations, infectious
agents, environmental factors, dietary factors, and pathological
conditions in the stomach (5).
Increasing evidence has showed the positive link between tobacco
smoke and gastric cancer (6–11).
Many compounds found in tobacco smoke are known to induce free
radicals, possess toxic properties and carcinogenic activities,
there by contributing to its potential impact on the transformation
and progression of many cancers (7,12).
Although enormous progress in understanding its molecular
pathogenesisis leading to gastric cancer has been made, the
underlying mechanisms by which tobacco smoke induce gastric cancer
are not fully understood.
Epithelial-mesenchymal transition (EMT) is a cell
biological processes that is very important in various aspects
including embryonic development, cancer progression stem cell
biology, wound healing and fibrosis (13). EMT is characterized by
downregulation of the intercellular tight junctions and acquire
certain properties of mesenchymal cells. Emerging evidence has
revealed that, in addition to facilitating invasion and metastasis,
EMT is also critically involved in the initiation of tumorigenesis.
Exposure of cells to carcinogens, such as tobacco smoke,
benzo(a) pyrenediolepoxide, arsenite and methylnitrosourea,
induced EMT during cell transformation and tumor formation
(14–17). Studies have documented that tobacco
smoke promote the occurrence of EMT process (18–20).
Tobacco smoke triggered EMT has been found to regulate early events
in carcinogenesis: the loss of polarized organization of epithelial
tissue, cell-cell junction breakdown, cell-atrix adhesion
remodeling, and gain properties of mesenchymal cells with invasive
capacity. Nonetheless, the underlying mechanisms by which tobacco
smoke induces EMT and the signaling events that underlie EMT are
poorly understood.
Notch signaling regulates a series of cellular
processes (21). In addition, the
Notch pathway has been reported implicated in a majority of cancers
for promoting the malignant phenotype by inducing cell
proliferation, drug resistance, resistance to apoptosis, invasion
and metastasis (22). Several
studies have revealed that Notch pathway is critically involved in
the process of EMT (21,23,24).
Nonetheless, no studies have been done to examine the role of Notch
pathway in tobacco smoke-induced gastric EMT.
Some studies have illustrated that dietary
phytochemicals with potent anticancer activity are present in
food-based diets. β-carotene is one of the most abundant
carotenoids, abundant in carrot, spinach, kale, pink guava yams,
palm oil, and sweet potato (25).
β-carotene is found to elicit profound effects on the maintenance
of human health and disease prevention (26). Numerous studies have consistently
demonstrated that β-carotene reduce the risk of developing many
diseases such as cardiovascular diseases (27–29),
cataract formation (30,31), age-dependent macula degeneration
(32), and different types of
cancer including stomach, bladder, mouth, pharynx, colon, larynx,
esophagus, rectum, and cervix (33,34).
However, its effect on tobacco smoke triggered gastric EMT has not
been defined.
Herein, we aimed to investigate Notch pathway
regulation of tobacco smoke elicited EMT alterations and the
preventive effects of β-carotene against tobacco smoke induced EMT
in the mouse stomach. We found that Notch regulated tobacco
smoke-mediated gastric EMT and the protective effects of β-carotene
in tobacco smoke-elicited ERK5 activation and EMT in the stomach
tissue by using mouse tobacco smoke exposure models. These findings
indicated that Notch pathway play an important regulatory role and
the chemopreventive effect of β-carotene in tobacco
smoke-associated gastric pathological alterations.
Materials and methods
Reagents and antibodies
Primary antibodies against Notch-1, ZO-1 were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Antibodies against NICD, Hes-1, E-cadherin, Snail-1, N-cadherin,
and vimentin was purchased from Cell Signaling Technology (Danvers,
MA, USA). Anti-CK5 antibody and anti-GAPDH antibody were from
Biogot Technology (Nanjing, China). β-carotene was purchased from
Sigma (St. Louis, MO, USA; purity ≥97%). FLI-06 was purchased from
Tocris Bioscience (Bristol, UK). All the primers were synthesized
by Invitrogen (Carlsbad, CA, USA) according to published sequences.
Sources of other materials are noted in the relevant context.
Experimental animals and
treatment
All experiments were performed in accordance with
the recommendations in the guidelines of the Laboratory Animal
Management Committee of Jiangsu University concerning the care and
treatment of experimental animals. The study was approved by the
Committee on the Ethics of Animal Experiments of Jiangsu
University. A total of 60 male BALB/c mice (6–8 weeks of age)
weighing 18–22 g were used in the current experiments. All mice
were purchased from the Animal Research Center of Jiangsu
University and were fed adaptively for one week.
Six mice were randomly divided into each group. To
serve as a control, the BALB/c mice were exposed to filtered air
and allowed ad libitum access to water until the end of the
experiment. Mice in the tobacco smoke exposure group were exposed
to tobacco smoke 6 h daily for 12 weeks as described in our
previous study. The exposures were monitored and characterized as
follows: carbon monoxide (13.23±2.72 mg/m3), total
particulate matter (TPM) (0 mg/m3) for the control
group; carbon monoxide (157.56±20.12 mg/m3), TPM
(79.73±3.92 mg/m3) for the tobacco smoke exposure
group.
In vivo delivery of specific Notch
inhibitor
Mice were randomly assigned into four groups (n=6
per group): control group, tobacco smoke group, tobacco smoke +
DMSO group and tobacco smoke + FLI-06 group. FLI-06, a specific
Notch inhibitor was reconstituted in sterile DMSO. The mice in
control group and tobacco smoke group were exposed to filtered air
or tobacco smoke alone and mice in tobacco smoke + DMSO group and
tobacco smoke + FLI-06 group were intreperitoneal injected with
FLI-06 (1 mg/kg body weight) or DMSO every other day. After 12
weeks smoke exposure, the mice were sacrificed and stomach tissues
were collected and stored at −80°C until analysis.
β-carotene treatment
In a separate study, mice were divided into four
groups (n=6 per group): control group, tobacco smoke-exposed group,
tobacco smoke + β-carotene 5 mg, tobacco smoke + β-carotene 10 mg.
The mice in control group and tobacco smoke group were exposed to
filtered air or tobacco smoke and received an intragastric
administration of corn oil as a vehicle for 12 weeks. Mice in
tobacco smoke + β-carotene groups were treated with 5 or 10 mg/kg
body weight (BW)/day β-carotene via intragastric administration
dissolved in corn oil and exposed to tobacco smoke throughout the
experimental period. Animals were weighed weekly and the
administration dosages of β-carotene were based on the measurements
of mouse body weight.
Western blot analysis
Frozen stomach tissues were weighed and homogenized
in a lysate buffer. Homogenates were centrifuged (12,000 g, 4°C, 20
min) and supernatants were collected. Protein concentrations were
measured and 60 μg of proteins were fractionated by electrophoresis
through gradient (7.5–10%) sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE). Proteins were then transferred to
polyvinylidene difluoride (PVDF) membranes and blocked with 5% skim
milk. Membranes were subsequently probed with specific primary
antibodies overnight at 4°C, and then incubated with the
appropriate secondary antibodies. For densitometric analyses,
protein bands on the blots were measured by the use of the ImageJ
analysis software.
RNA extraction and quantitative
real-time PCR
Total RNA was extracted from stomach tissues using
TRIzol reagent (Invitrogen) according to the manufacturer's
instructions. Quantitative real-time PCR was performed by using the
Power SYBR Green Master Mix (Takara, Dalian, China) and an Applied
Biosystems 7300 real-time PCR detection system (Applied Biosystems,
Foster City, CA, USA). The primers used were as follows: E-cadherin
forward, 5′-TCGACACCCGATTCAAAGTGG-3′ and reverse,
5′-TTCCAGAAACGGAGGCCTGAT-3′; ZO-1 forward,
5′-GCAGCCACAACCAATTCATAG-3′ and reverse,
5′-GCAGACGATGTTCATAGTTTC-3′; CK5 forward,
5′-CTGGAGAGTAGTCTAGACCAAGCC-3′ and reverse,
5′-GTTAGAACCAAAACAAAATTTGGG-3′; Snail-1 forward,
5′-GACCACTATGCCGCGCTCTT-3′ and reverse,
5′-TCGCTGTAGTTAGGCTTCCGATT-3′; vimentin forward,
5′-CCTTGACATTGAGATTGCCA-3′ and reverse, 5′-GTATCAACCAGAGGGAGTGA-3′;
N-cadherin forward, 5′-ATCAAGTGCCATTAGCCAAG-3′ and reverse,
5′-CTGAGCAGTGAATGTTGTCA-3′; GAPDH forward,
5′-GCTGCCCAACGCACCGAATA-3′ and reverse, 5′-GAGTCAACGGATTTGGTCGT-3′.
Fold changes in gene expression were calculated by a comparative
threshold cycle (Ct) method using the formula
2−ΔΔCt.
Immunohistochemistry
Stomach tissues were fixed in 4% buffered formalin
for 24 h then were paraffin embedded. Sections were cut (5 µm) and
de-waxed in xylene and rehydrated in graded alcohol, after which
endogenous peroxidase activity was quenched by incubating the
sections in 3% (v/v) H2O2 in methanol.
Antigen retrieval was carried out in citrate buffer (pH 6.0) for 20
min in a microwave. Sections were subsequently incubated with a
protein-blocking solution for 30 min, then incubated with the
primary antibody (E-cadherin and vimentin) at 4°C overnight.
Sections were subsequently washed with phosphate-buffered saline
(PBS) before incubation for 1 h with peroxidase-conjugated
secondary antibodies. After 1 h, sections were stained with
3,-3′-diaminobenzidinetetrahydrochloride (DAB) and counterstained
with hematoxylin. Images were collected using a Nikon eclipse Ti-S
microscope (Nikon Corporation, Tokyo, Japan) at a ×200
magnification.
Statistical analysis
Statistical analyses were performed with SPSS 18.0.
All data are expressed as mean ± standard deviation. One-way ANOVA
was used for comparison of statistical differences among multiple
groups, followed by the LSD significant difference test. Unpaired
Student's t-test was also used for the comparison between two
groups. A value of P<0.05 was considered significantly
different.
Results
Tobacco smoke-elicited EMT-like
changes in mice gastric
Our previous study showed that tobacco smoke induced
EMT changes in gastric tissues of mice. In the present study we
also found that 12-week tobacco smoke exposure decreased the
expression of E-cadherin, ZO-1 and CK5, and elevated the mRNA
expression levels of Snial-1, vimentin and N-cadherin (Fig. 1A and B). Immunohistochemical
staining also showed that TS decreased E-cadherin protein
expression and increased vimentin protein expression in the mouse
stomach (Fig. 1C).
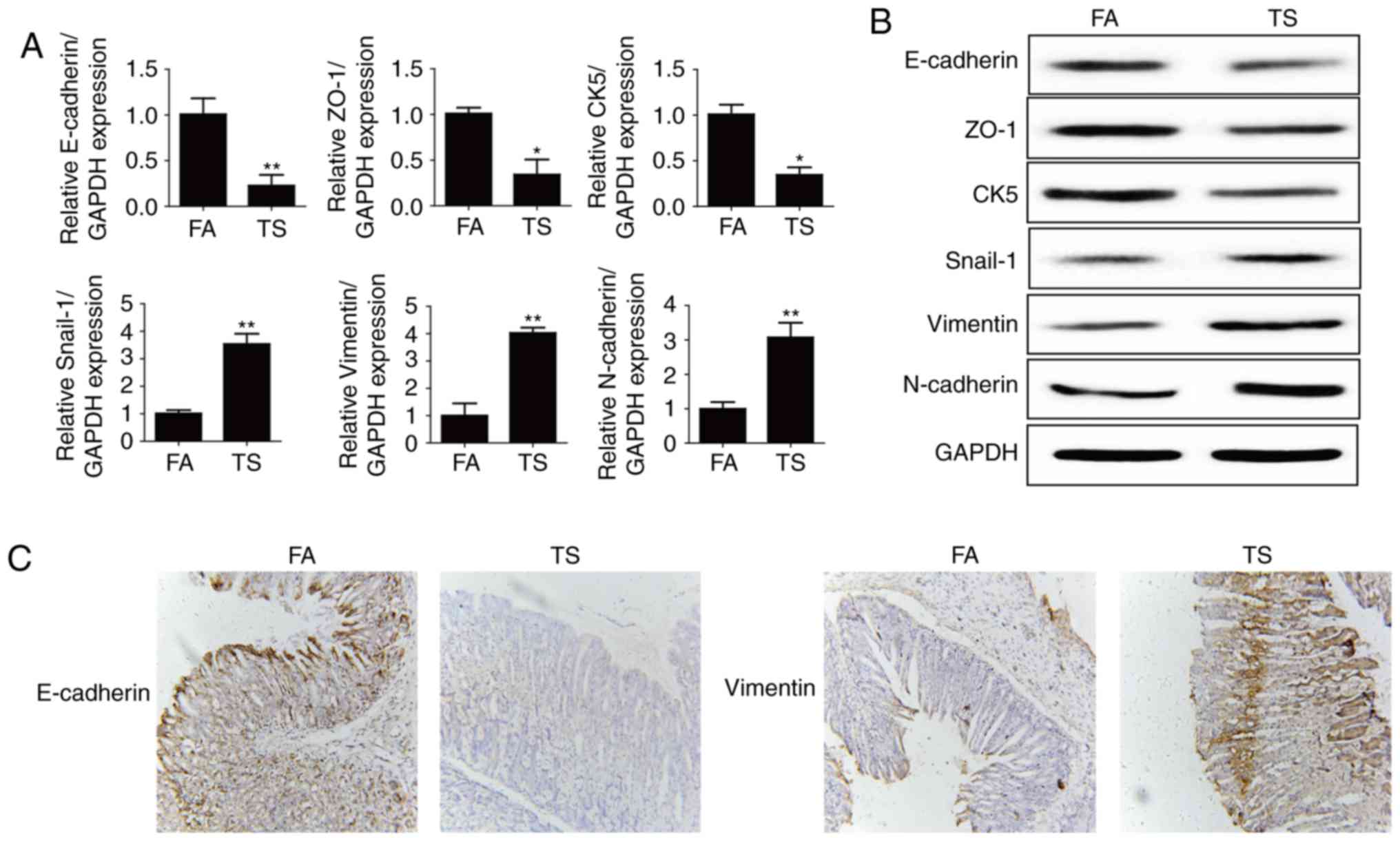 | Figure 1.Tobacco smoke induced alterations in
the expression of EMT markers. (A) Tobacco smoke reduced mRNA
levels of E-cadherin, ZO-1, and CK5, and elevated mRNA levels of
Snial-1, vimentin, and N-cadherin in the stomach of mice. (B)
Tobacco smoke induced alterations in the protein levels of EMT
markers in the stomach of mice. (C) Tobacco smoke decreased
E-cadherin protein expression and increased vimentin protein
expression shown by immunohistochemical staining. *P<0.05,
**P<0.01, compared with FA. EMT, epithelial-mesenchymal
transition; FA, filtered air; TS, tobacco smoke. |
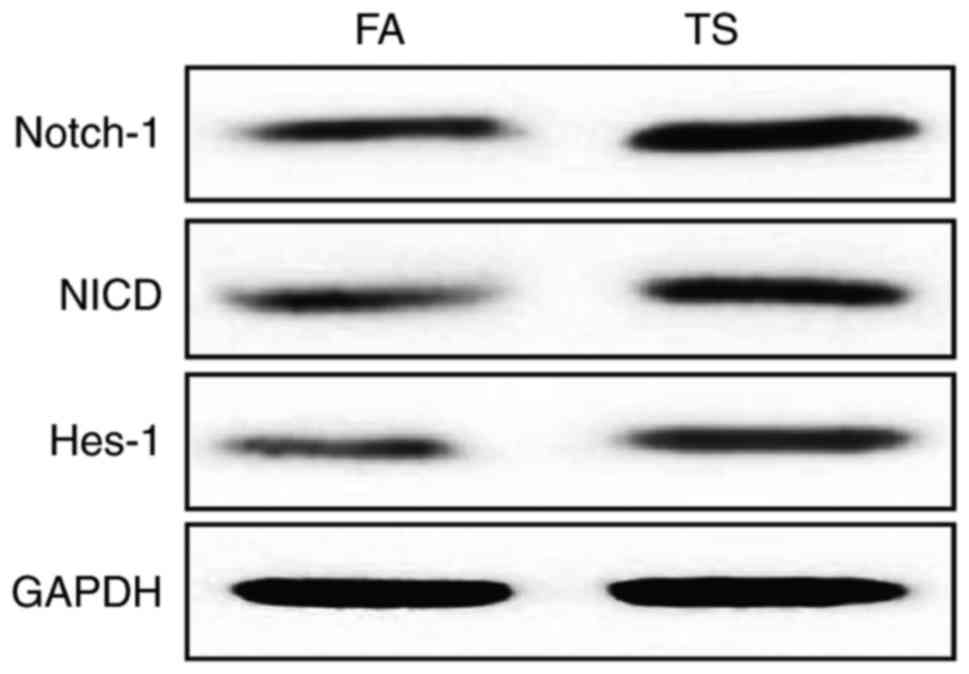
Tobacco smoke-induced gastric EMT is
associated with Notch pathway
Notch pathway has been reported to be implicated in
a majority of cancers for promoting the EMT process. To determine
whether tobacco smoke-triggered gastric EMT is associated with
Notch pathway, the expression level of Notch-1, NICD, and Hes-1
were measured. The results revealed that long-term tobacco smoke
exposure increased the levels of Notch, NICD and Hes-1 in the
gastric tissues of mice (Fig.
2).
Notch inhibition reverses tobacco
smoke-mediated gastric EMT
Since the above results revealed that tobacco
smoke-mediated gastric EMT was associated with Notch pathway, we
further determine the role of Notch pathway in tobacco
smoke-induced gastric EMT regulation. Mice were treated with FLI-06
(1 mg/kg body weight), a specific Notch pathway inhibitor. Results
showed that FLI-06 downregulated Notch, NICD and Hes-1 expression
levels (Fig. 3A). Western blot
analyses showed that treated mice with FLI-06 attenuated tobacco
smoke-induced alterations of E-cadherin, ZO-1, CK5, Snial-1,
vimentin and N-cadherin (Fig. 3B).
We also found that the changes in mRNA expression of E-cadherin,
ZO-1, CK5, Snial-1, vimentin and N-cadherin were effectively
reversed by FLI-06 (Fig. 3C).
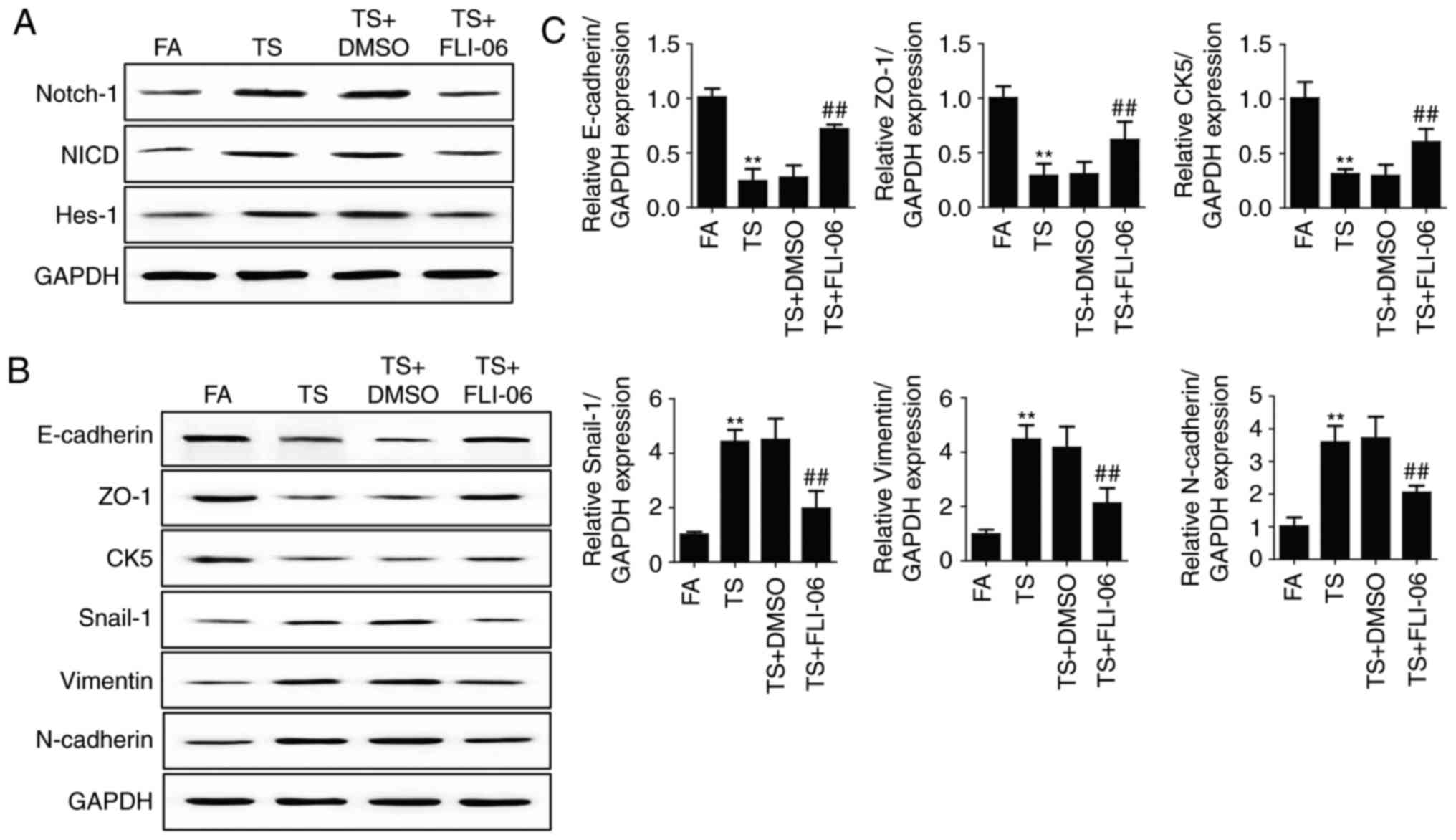 | Figure 3.Notch inhibition reverses tobacco
smoke-mediated gastric EMT in the stomach of mice. (A) Western blot
analyses of Notch, NICD and Hes-1 expression levels after treatment
of mice with FLI-06. (B) Western blot analyses of E-cadherin, ZO-1,
CK5, Snail-1, vimentin, and N-cadherin proteins after treated mice
with FLI-06. (C) qRT-PCR analyses of EMT markers. **P<0.01,
compared with FA; ##P<0.01, compared with TS. EMT,
epithelial-mesenchymal transition; FA, filtered air; TS, tobacco
smoke. |
β-carotene prevented tobacco
smoke-induced EMT in the mouse stomach
To determine the effects of β-carotene on tobacco
smoke-mediated gastric EMT, the BALB/c mice were administered
β-carotene (5 or 10 mg/kg BW/day) and exposed to tobacco smoke for
12 weeks. Our results showed that these alterations in expressions
of the EMT markers induced by tobacco smoke (Fig. 4), including decreases of the
epithelial markers E-cadherin, ZO-1, CK5 and increases of the
mesenchymal markers Snail-1, vimentin, N-cadherin, were
significantly attenuated by β-carotene (10 mg/kg BW/day). These
results indicated that β-carotene prevent tobacco smoke-induced
gastric EMT in the BALB/c mouse model.
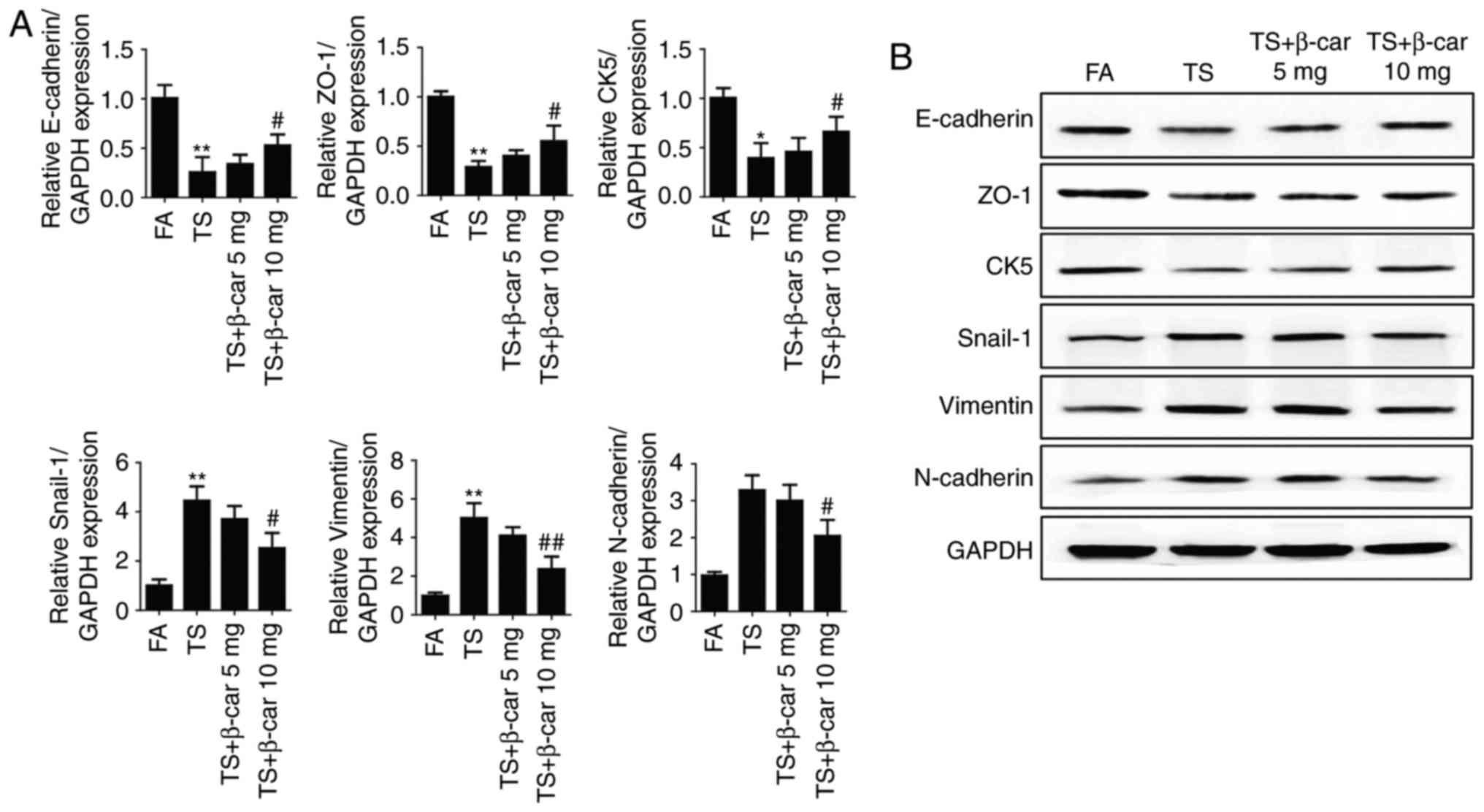 | Figure 4.β-carotene prevents tobacco
smoke-induced EMT in the mouse stomach. (A) qRT-PCR analyses of
E-cadherin, ZO-1, CK5, Snail-1, vimentin, and N-cadherin
mRNAs. (B) Western blot analyses of EMT marker proteins.
*P<0.05, **P<0.01, compared with FA; #P<0.05,
##P<0.01, compared with TS. EMT,
epithelial-mesenchymal transition; FA, filtered air; TS, tobacco
smoke. |
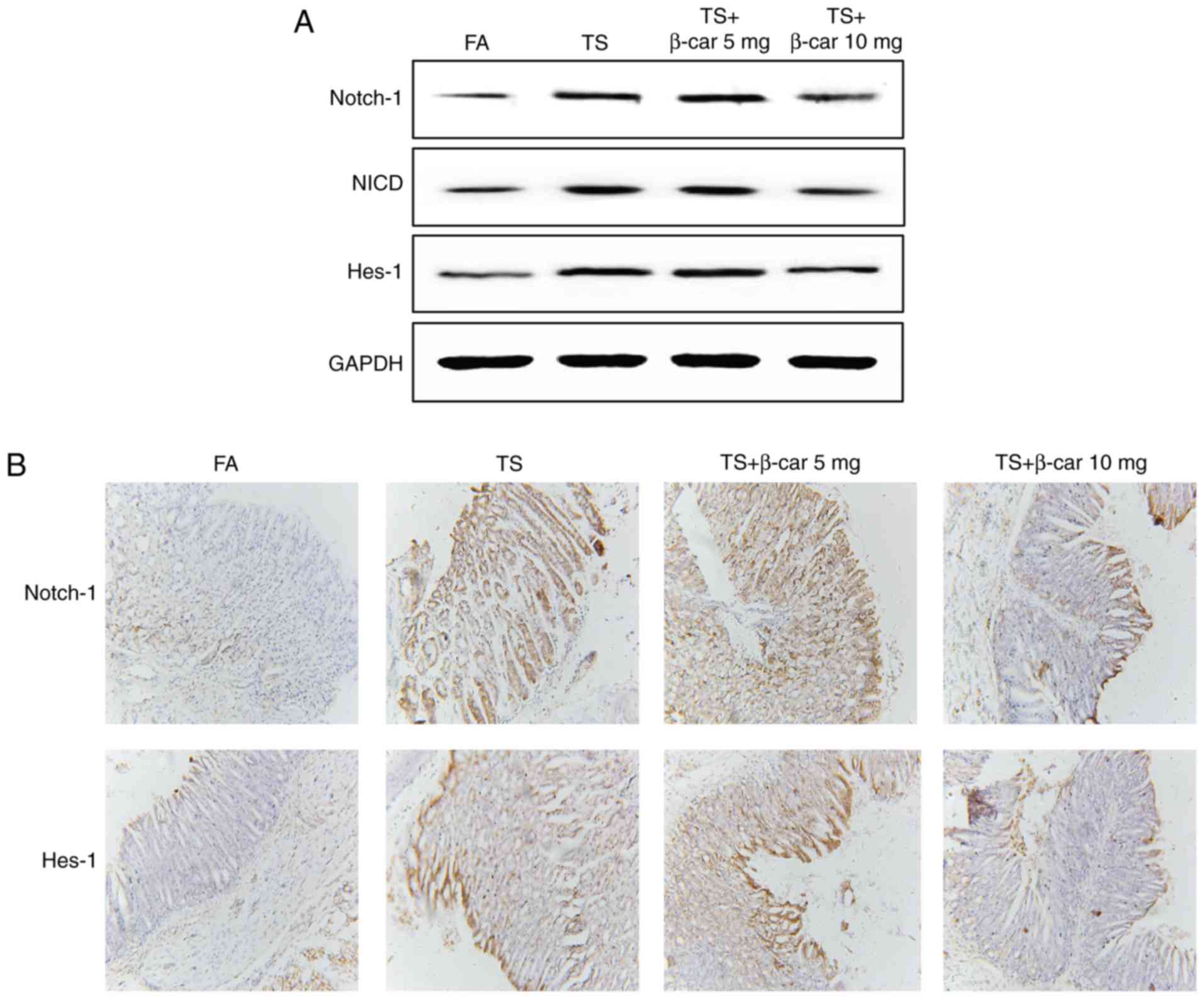
β-carotene attenuated tobacco
smoke-activated Notch pathway
In order to explore the effects of β-carotene on
tobacco smoke-activated Notch pathway, we further examined the
changes of Notch, NICD and Hes-1 expression following β-carotene
treatment in the mouse stomach tissues. Western blot analyses and
immunohistochemical staining showed that 10 mg/kg BW/day β-carotene
obviously inhibited tobacco smoke-induced Notch, NICD and Hes-1
expression levels (Fig. 5).
Discussion
Gastric cancer remains the leading cause of
cancer-related deaths. It is critical to explore the novel
molecular mechanisms and the chemoprevention of gastric cancer.
Tobacco smoke is an important risk factor for gastric cancer, which
promotes the initiation and progression of gastric tumorigenesis.
However, the underlying molecular mechanisms by which tobacco smoke
causes gastric cancer still has not been well defined. The present
study demonstrated for the first time that Notch regulated tobacco
smoke-mediated gastric EMT in BALB/c mice. Moreover, our data
indicated that β-carotene effectively attenuated tobacco
smoke-triggered Notch pathway activation and gastric EMT. These
findings provide new insights into the pathogenesis and the
chemoprevention of tobacco smoke-associated gastric cancer.
Characterized by downregulation of the intercellular
tight junctions, changes in migration and invasion capacity, as
well as the expression of epithelial and mesenchymal markers, EMT
is a crucial process in initiation of tumorigenesis (35,36).
Studies have documented that tobacco smoke promotes the EMT
process, resulting in loss of cellular polarity, downregulation of
epithelial cadherin and the intercellular tight junctions, acquire
certain properties of mesenchymal cells, and increased mobility of
cells (19,20,37).
In the present study, we also found tobacco smoke altered the
expression of EMT markers, including decreased E-cadherin, ZO-1,
and CK5, and increased Snail-1, vimentin, and N-cadherin. These
results revealed that tobacco smoke exposure induced gastric EMT in
BALB/c mice.
Nonetheless, the underlying mechanisms by which
tobacco smoke induces EMT and the signaling events that underlie
EMT are poorly understood. Notch signaling regulates a series of
cellular processes (21). Several
studies have revealed that Notch pathway is critically involved in
the process of EMT (21,23,24).
To examine the role of Notch pathway in tobacco smoke-induced EMT,
we addressed the relationship between Notch and gastric EMT induced
by tobacco smoke. The mice were treated with a specific Notch
inhibitor FLI-06 (1 mg/kg body weight) or DMSO every other day.
Results have shown that FLI-06 downregulated Notch, NICD and Hes-1
expression levels. Furthermore, inhibition of Notch pathway
attenuated tobacco smoke-induced EMT, as indicated by decreased
E-cadherin, ZO-1, and CK5, and increased Snail-1, vimentin, and
N-cadherin. These data indicated that Notch pathway positively
regulates tobacco smoke-induced EMT in the gastric tissues of
mice.
It has been reported that approximately one third of
cancers can be prevented by controlling diet and regular physical
activities. Dietary phytochemicals have been shown to be a very
promising approach to the prevention of cancer development.
β-carotene is one of carotenoids, abundant in carrot, spinach,
kale, pink guava yams, palm oil, and sweet potato (25). Studies have demonstrated the safety
of β-carotene as well as its anticancer activities in different
types of cancer (33). The doses of
β-carotene used in our present study were 5 or 10 mg/kg BW per day.
After treatment with β-carotene and tobacco smoke for 12 weeks, the
effect of β-carotene on tobacco smoke-induced alterations in the
expression of the EMT markers were examined. As shown in Fig. 4 tobacco smoke-triggered EMT were
significantly attenuated by 10 mg/kg BW/day dose of β-carotene. In
order to explore the effects of β-carotene on tobacco
smoke-activated Notch pathway, we further examine the changes of
Notch pathway following β-carotene treatment. Tobacco
smoke-elevated expression levels of Notch, NICD and Hes-1 were
obviously inhibited following the 12-week β-carotene treatment
(Fig. 5).
The present study demonstrated for the first time
that Notch pathway positively regulates tobacco smoke-induced
gastric EMT and the protective effects of β-carotene in tobacco
smoke-induced Notch pathway activation and EMT in vivo.
These findings provide new insights into the mechanisms and the
chemoprevention of tobacco smoke-associated gastric
tumorigenesis.
Acknowledgements
We are thankful to the Laboratory Animal Management
Committee of Jiangsu University for providing necessary facilities
related to our study. This study was supported by the National
Natural Science Foundation of China (no. 81602883), China
Postdoctoral Science Foundation Funded Project (no. 2016M591792),
and Science and Technology Project of Zhenjiang (SH2014065).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu L, Chen J, Tang H, Bai L, Lu C, Wang K,
Li M, Yan Y, Tang L, Wu R, et al: EGCG Suppresses ERK5 activation
to reverse tobacco smoke-triggered gastric epithelial-mesenchymal
transition in BALB/c Mice. Nutrients. 8(pii): E3802016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu M, Chen L, Xu T, Xu B, Jiang J and Wu
C: Prognostic values of tissue factor and its alternatively splice
transcripts in human gastric cancer tissues. Oncotarget.
8:53137–53145. 2017.PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li F, Guo Y, Liu J and Zhang R: The
significance of elevated plasma expression of microRNA 106b~25
clusters in gastric cancer. PLoS One. 12:e01784272017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang Z, Wu R, Xie W, Geng H, Zhao L, Xie
C, Wu J, Geng S, Li X, Zhu M, et al: Curcumin suppresses MAPK
pathways to reverse tobacco smoke-induced gastric
epithelial-mesenchymal transition in mice. Phytother Res.
29:1665–1671. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malakar M, Devi KR, Phukan RK, Kaur T,
Deka M, Puia L, Baruah D, Mahanta J and Narain K: CYP2E1 genetic
polymorphism with dietary, tobacco, alcohol habits, H. Pylori
infection status and susceptibility to stomach cancer in Mizoram,
India. Asian Pac J Cancer Prev. 15:8815–8822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li LF, Chan RL, Lu L, Shen J, Zhang L, Wu
WK, Wang L, Hu T, Li MX and Cho CH: Cigarette smoking and
gastrointestinal diseases: The causal relationship and underlying
molecular mechanisms (review). Int J Mol Med. 34:372–380. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin VY, Jin H, Ng EK, Cheng AS, Chong WW,
Wong CY, Leung WK, Sung JJ and Chu KM: NF-κB targets miR-16 and
miR-21 in gastric cancer: Involvement of prostaglandin E receptors.
Carcinogenesis. 32:240–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phukan RK, Zomawia E, Narain K, Hazarika
NC and Mahanta J: Tobacco use and stomach cancer in Mizoram, India.
Cancer Epidemiol Biomarkers Prev. 14:1892–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Babhadiashar N, Sotoudeh M, Azizi E,
Bashiri J, Didevar R, Malekzadeh R and Ghahremani MH: Correlation
between cigarette smoking and urine cotinine level in gastric
cancer patients. Iran J Pharm Res. 13:313–318. 2014.PubMed/NCBI
|
|
11
|
Sjödahl K, Lu Y, Nilsen TI, Ye W, Hveem K,
Vatten L and Lagergren J: Smoking and alcohol drinking in relation
to risk of gastric cancer: A population-based, prospective cohort
study. Int J Cancer. 120:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Ren JW, Wong CC, Wu WK, Ren SX,
Shen J, Chan RL and Cho CH: Effects of cigarette smoke and its
active components on ulcer formation and healing in the
gastrointestinal mucosa. Curr Med Chem. 19:63–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prieto-García E, Díaz-García CV,
García-Ruiz I and Agulló-Ortuño MT: Epithelial-to-mesenchymal
transition in tumor progression. Med Oncol. 34:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun JL, Chen DL, Hu ZQ, Xu YZ, Fang HS,
Wang XY, Kan L and Wang SY: Arsenite promotes intestinal tumor cell
proliferation and invasion by stimulating epithelial-to-mesenchymal
transition. Cancer Biol Ther. 15:1312–1319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, Lu
X, Zhao Y, Luo F, Wang B, et al: MicroRNA-191, by promoting the EMT
and increasing CSC-like properties, is involved in neoplastic and
metastatic properties of transformed human bronchial epithelial
cells. Mol Carcinog. 54 Suppl 1:E148–E161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tellez CS, Juri DE, Do K, Bernauer AM,
Thomas CL, Damiani LA, Tessema M, Leng S and Belinsky SA: EMT and
stem cell-like properties associated with miR-205 and miR-200
epigenetic silencing are early manifestations during
carcinogen-induced transformation of human lung epithelial cells.
Cancer Res. 71:3087–3097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Luo F, Xu Y, Wang B, Zhao Y, Xu W,
Shi L, Lu X and Liu Q: Epithelial-mesenchymal transition and cancer
stem cells, mediated by a long non-coding RNA, HOTAIR, are involved
in cell malignant transformation induced by cigarette smoke
extract. Toxicol Appl Pharmacol. 282:9–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Z, Xie W, Wu R, Geng H, Zhao L, Xie
C, Li X, Zhu M, Zhu W, Zhu J, et al: Inhibition of tobacco
smoke-induced bladder MAPK activation and epithelial-mesenchymal
transition in mice by curcumin. Int J Clin Exp Pathol. 8:4503–4513.
2015.PubMed/NCBI
|
|
19
|
Zhang L, Gallup M, Zlock L, Basbaum C,
Finkbeiner WE and McNamara NA: Cigarette smoke disrupts the
integrity of airway adherens junctions through the aberrant
interaction of p120-catenin with the cytoplasmic tail of MUC1. J
Pathol. 229:74–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Liu H, Borok Z, Davies KJ, Ursini
F and Forman HJ: Cigarette smoke extract stimulates
epithelial-mesenchymal transition through Src activation. Free
Radic Biol Med. 52:1437–1442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Guo W, Wang L, Yu L, Mei H, Fang
S, Chen A, Liu Y, Xia K and Liu G: Notch signaling is important for
epithelial-mesenchymal transition induced by low concentrations of
doxorubicin in osteosarcoma cell lines. Oncol Lett. 13:2260–2268.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zang MD, Hu L, Fan ZY, Wang HX, Zhu ZL,
Cao S, Wu XY, Li JF, Su LP, Li C, et al: Luteolin suppresses
gastric cancer progression by reversing epithelial-mesenchymal
transition via suppression of the Notch signaling pathway. J Transl
Med. 15:522017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang G, Zhao Z, Zhang X, Wu A, Huang Y,
Miao Y and Yang M: Effect of berberine on the renal tubular
epithelial-to-mesenchymal transition by inhibition of the
Notch/snail pathway in diabetic nephropathy model KKAy mice. Drug
Des Devel Ther. 11:1065–1079. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Sha J, Yang G, Huang X, Bo J and
Huang Y: Activation of Notch pathway is linked with
epithelial-mesenchymal transition in prostate cancer cells. Cell
Cycle. 16:999–1007. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thakur D, Jain A, Ghoshal G, Shivhare US
and Katare OP: Microencapsulation of β-carotene based on
casein/guar gum blend using zeta potential-yield stress phenomenon:
An approach to enhance photo-stability and retention of
functionality. AAPS Pharm Sci Tech. 18:1447–1459. 2017. View Article : Google Scholar
|
|
26
|
Sowmya Shree G, Yogendra Prasad K, Arpitha
HS, Deepika UR, Nawneet Kumar K, Mondal P and Ganesan P: β-carotene
at physiologically attainable concentration induces apoptosis and
down-regulates cell survival and antioxidant markers in human
breast cancer (MCF-7) cells. Mol Cell Biochem. 436:1–12. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaziano JM, Manson JE, Branch LG, Colditz
GA, Willett WC and Buring JE: A prospective study of consumption of
carotenoids in fruits and vegetables and decreased cardiovascular
mortality in the elderly. Ann Epidemiol. 5:255–260. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gey KF, Moser UK, Jordan P, Stähelin HB,
Eichholzer M and Lüdin E: Increased risk of cardiovascular disease
at suboptimal plasma concentrations of essential antioxidants: An
epidemiological update with special attention to carotene and
vitamin C. Am J Clin Nutr. 57 5 Suppl:S787–S797. 1993. View Article : Google Scholar
|
|
29
|
Ito Y, Kurata M, Suzuki K, Hamajima N,
Hishida H and Aoki K: Cardiovascular disease mortality and serum
carotenoid levels: A Japanese population-based follow-up study. J
Epidemiol. 16:154–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gale CR, Hall NF, Phillips DI and Martyn
CN: Plasma antioxidant vitamins and carotenoids and age-related
cataract. Ophthalmology. 108:1992–1998. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacques PF and Chylack LT Jr:
Epidemiologic evidence of a role for the antioxidant vitamins and
carotenoids in cataract prevention. Am J Clin Nutr. 53 Suppl
1:S352–S355. 1991. View Article : Google Scholar
|
|
32
|
Seddon JM, Ajani UA, Sperduto RD, Hiller
R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller
DT, et al: Dietary carotenoids, vitamins A, C, and E, and advanced
age-related macular degeneration. Eye Disease Case-Control Study
Group. JAMA. 272:1413–1420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haider C, Ferk F, Bojaxhi E, Martano G,
Stutz H, Bresgen N, Knasmüller S, Alija A and Eckl PM: Effects of
β-carotene and its cleavage products in primary pneumocyte type II
cells. Antioxidants (Basel). 6(pii): E372017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heinen MM, Verhage BA, Goldbohm RA and van
den Brandt PA: Intake of vegetables, fruits, carotenoids and
vitamins C and E and pancreatic cancer risk in The Netherlands
Cohort Study. Int J Cancer. 130:147–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao J, Dong D and Sun L, Zhang G and Sun
L: Prognostic significance of the epithelial-to-mesenchymal
transition markers e-cadherin, vimentin and twist in bladder
cancer. Int Braz J Urol. 40:179–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Hu G, Chen D, Gong AY, Soori GS,
Dobleman TJ and Chen XM: Suppression of SCARA5 by Snail1 is
essential for EMT-associated cell migration of A549 cells.
Oncogenesis. 2:e732013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin VY, Jin HC, Ng EK, Sung JJ, Chu KM
and Cho CH: Activation of 5-lipoxygenase is required for nicotine
mediated epithelial-mesenchymal transition and tumor cell growth.
Cancer Lett. 292:237–245. 2010. View Article : Google Scholar : PubMed/NCBI
|