Introduction
Hepatocellular carcinoma (HCC) represents
approximately 90% of primary liver cancers and is the second
leading cause of mortality among all human malignancies (1). Unfortunately, patients often present
with advanced HCC that is unresectable, recurrent or metastatic.
Systemic chemotherapy is ineffective in these patients (2). Classical radiation therapy is not a
standard adjuvant for patients with HCC due to intolerance and
resistance of the liver to radiation. In this aspect, developing
effective therapies for liver cancer is one of the most challenging
goals in cancer research. There have been many studies that have
revealed that local expression of interleukin-6 (IL-6) in the liver
was increased in patients with chronic hepatitis. Moreover, signal
transducer and activator of transcription 3 (STAT3) plays a major
role in the pathogenesis of hepatocellular carcinoma (3). IL-6 is a secreted multifunctional
cytokine that has been identified as a master regulator of the
STAT3 signaling pathway (4,5). Furthermore, tumor-associated
macrophages were revealed to activate IL-6/STAT3 signaling in
neighboring HCC stem cells via secretion of IL-6 in the HCC
microenvironment and consequently promote tumor progression.
Numerous studies have demonstrated that STAT3 participates in
regulating gene expression related to cell proliferation (c-myc,
cyclin D1), survival (Mcl-1, Bcl-xL and survivin), invasion (matrix
metalloproteinase-9), and angiogenesis (VEGF) (6). Activation of STAT3 was also associated
with decreased survival rates in HCC (7) and other types of cancer. These
findings indicate that STAT3 has emerged as a therapeutic target
for the development of anticancer agents.
Thus far, the use of anticancer drugs derived from
traditional medicine offers new opportunities to improve existing
standard treatments for cancer and other diseases. Crocus
sativus L. (saffron) has been known as a flavoring agent, food
coloring and traditional herbal medicine. Major components
including safranal, picrocrocin, crocetin and crocin are considered
as the pharmacologically active components of saffron (8). Crocin [digentiobiosyl
all-trans-crocetin (8,8′-di-apocarotene-8,8′-dioic acid) ester] is
a major glycosylated carotenoid found in saffron (9) that has various pharmacological effects
such as antioxidant (10,11), anti-atherosclerotic (12), antidepressant (13) and anti-inflammatory activities
(14,15). Moreover, some studies have shown
anticancer activities of crocin against human leukemia (16), colorectal (17), breast (18) and bladder cancer cell lines
(19). In addition, crocin is a
potential nutraceutical for protecting liver tissue from hepatic
steatosis (20). Based on these
studies, it is expected that crocin could possibly be used to
prevent and treat various cancers in the near future. However, the
direct influence of crocin on the STAT3 signaling pathway has not
been investigated. Thus, we examined whether crocin exerts
therapeutic effects by modulating the IL-6/STAT3 cell signaling
pathways.
In the present study, we revealed that crocin
suppressed the activation of the STAT3 pathway in IL-6-stimulated
hepatocellular carcinoma cells. Crocin suppressed the activation of
non-receptor protein tyrosine kinases (JAK1, JAK2 and Src) and
upregulated SH2-containing tyrosine phosphatase (SHP-1). Crocin
also downregulated STAT3-regulated gene products and promoted
apoptotic progression in liver cancer cells.
Materials and methods
Reagents
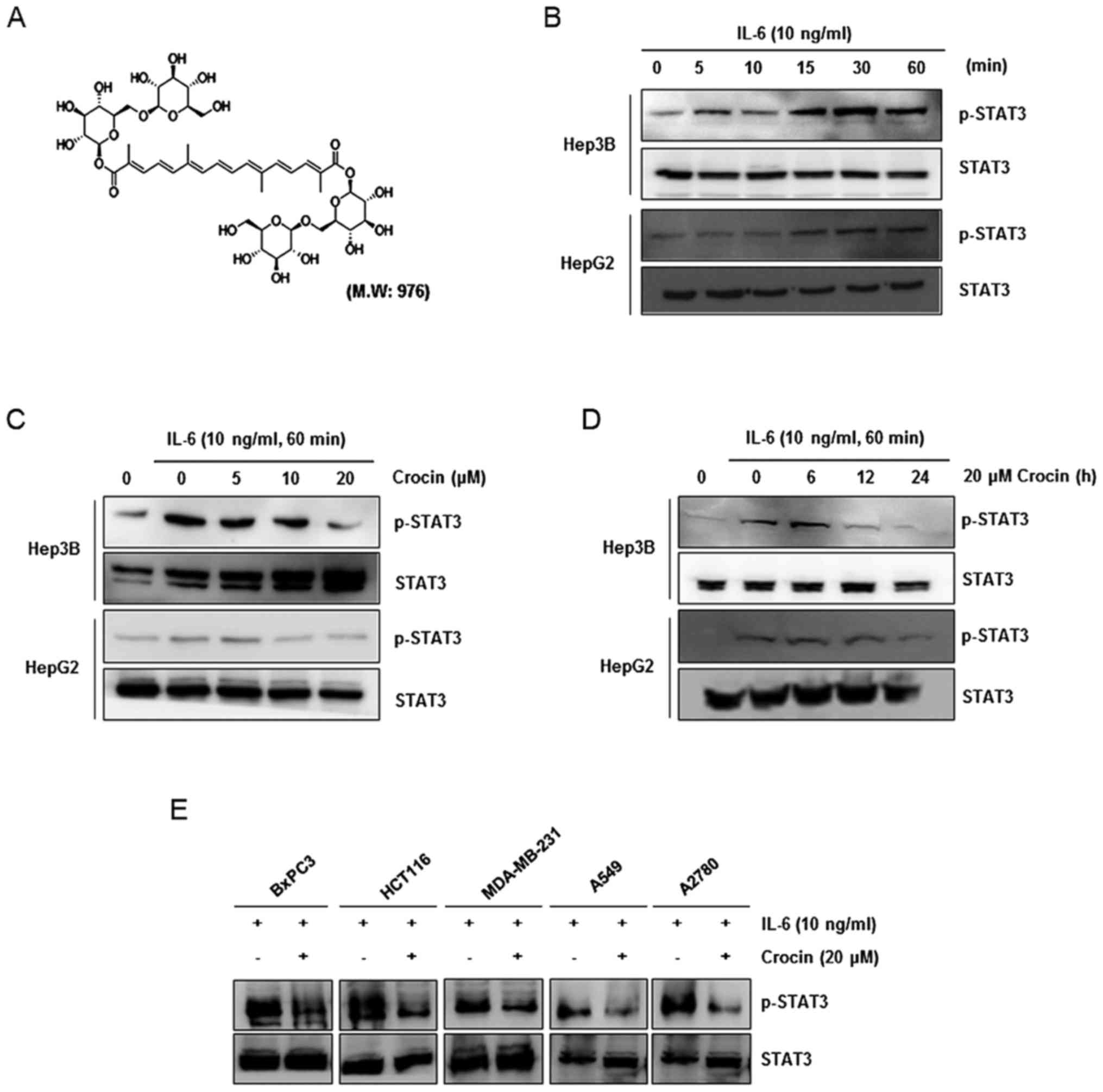
Crocin, with the chemical structure presented in
Fig. 1A, was provided by Dr Ki Yong
Lee (College of Pharmacy, Korea University). A 10-mM solution of
crocin was prepared in dimethyl sulfoxide (DMSO), stored as small
aliquots at −20°C, and then diluted as needed in cell culture
medium. RPMI-1640, fetal bovine serum (FBS), and an
antibiotic-antimycotic mixture were obtained from Gibco-BRL (Grand
Island, NY, USA). The antibodies used were as follows: p-STAT3
(1:1,000; rabbit, monoclonal, cat. no. 9145), STAT3 (1:1,000;
rabbit, monoclonal, cat. no. 12640), p-JAK1 (1:1,000; rabbit,
polyclonal, cat. no. 3331), p-JAK2 (1:1,000; rabbit, monoclonal,
cat. no. 8082), p-Src (1:1,000; rabbit, polyclonal, cat. no. 2101),
Src (1:1,000; rabbit, polyclonal, cat. no. 2108), SHP-1 (1:1,000;
rabbit, monoclonal, cat. no. 3759), cleaved PARP (1:1,000; rabbit,
monoclonal, cat. no. 5625), cleaved caspase-9 (1:1,000; rabbit,
monoclonal, cat. no. 7237), cleaved caspase-3 (1:1,000; rabbit,
polyclonal, cat. no. 9661), β-actin (1:1,000; rabbit, monoclonal,
cat. no. 4970) and anti-rabbit IgG (1:5,000; rabbit, polyclonal,
cat. no. 14708) were obtained from Cell Signaling Technology Inc.
(Danvers, MA, USA). Cyclin D1 (1:1,000; rabbit polyclonal, cat. no.
sc-718), VEGF (1:1,000; rabbit, polyclonal, cat. no. sc-152), JAK1
(1:1,000; rabbit, polyclonal, cat. no. sc-277), JAK2 (1:1,000;
rabbit, polyclonal, cat. no. sc-278), Bax (1:1,000; rabbit,
polyclonal, cat. no. sc-493), Bcl-2 (1:1,000; rabbit, polyclonal,
cat. no. sc-492) and goat anti-mouse IgG (1:5,000; mouse,
monoclonal, cat. no. sc-2355) were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). CXCR4 (1:10,000; rabbit,
polyclonal, cat. no. sc-492) was obtained from Abcam (Cambridge,
MA, USA). Recombinant human IL-6 was purchased from Invitrogen
(Groningen, The Netherlands). The siRNA for SHP-1 was obtained from
Ambion (Austin, TX, USA). Control siRNA was obtained from Santa
Cruz Biotechnology (Santa Cruz, CA, USA).
Cell cultures
Human hepatocellular carcinoma cells (Hep3B, HepG2),
human colon cancer cells (HCT116) and human breast cancer cells
(MDA-MB-231) were cultured in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% FBS and 1% antibiotics. Human
pancreatic cancer cells (BxPC3), human lung cancer cells (A549) and
human ovarian cancer cells (A2780) were grown in RPMI-1640 (Gibco
Laboratories, Grand Island, NY, USA) supplemented with 5% FBS and
1% antibiotics. Cells were maintained at 37°C in an atmosphere of
5% CO2-95% air. All cells were passaged at 80%
confluence in 0.25% trypsin-EDTA for 3–5 min.
Western blotting
The whole-cell extracts were lysed with RIPA buffer
(20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 50 mM
β-glycerophosphate, 1% NP-40, 1 mM Na3VO4 and
1× protease inhibitor cocktail). The extracted proteins were then
resolved on sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE). After electrophoresis, the proteins
were electrotransferred to polyvinylidene fluoride (PVDF)
membranes, blocked with 5% non-fat milk. The blots were subjected
to a standard immune detection procedure using specific antibodies
overnight at 4°C. The blot was washed, exposed to HRP-conjugated
secondary antibodies for 1 h, and finally examined by enhanced
chemiluminescence reaction using ECL reagents (Amersham Pharmacia
Biotech, Buckinghamshire, UK).
The primary antibodies used were as follows: p-STAT3
(1:1,000; rabbit, monoclonal; cat. no. 9145), STAT3 (1:1,000;
rabbit, monoclonal; cat. no. 12640), p-JAK1 (1:1,000; rabbit,
polyclonal; cat. no. 3331), p-JAK2 (1:1,000; rabbit, monoclonal;
cat. no. 8082), p-Src (1:1,000; rabbit, polyclonal; cat. no. 2101),
Src (1:1,000; rabbit, polyclonal; cat. no. 2108), SHP-1 (1:1,000;
rabbit, monoclonal; cat. no. 3759), cleaved PARP (1:1,000; rabbit,
monoclonal; cat. no. 5625), cleaved caspase-9 (1:1,000; rabbit,
monoclonal; cat. no. 7237), cleaved caspase-3 (1:1,000; rabbit,
polyclonal; cat. no. 9661), β-actin (1:1,000; rabbit, monoclonal;
cat. no. 4970; all from Cell Signaling Technology, Inc.), cyclin D1
(1:1,000; rabbit, polyclonal; cat. no. sc-718), VEGF (1:1,000;
rabbit, polyclonal; cat. no. sc-152), JAK1 (1:1,000; rabbit,
polyclonal; cat. no. sc-277), JAK2 (1:1,000; rabbit, polyclonal;
cat. no. sc-278), Bax (1:1,000; rabbit, polyclonal; cat. no.
sc-493), Bcl-2 (1:1,000; rabbit, polyclonal; cat. no. sc-492; all
from Santa Cruz Biotechnology), CXCR4 (1:10,000; rabbit,
polyclonal; cat. no. sc-492; Abcam). The secondary antibodies used
were goat anti-mouse IgG (1:5,000; mouse, monoclonal; cat. no.
sc-2355; Santa Cruz Biotechnology) and anti-rabbit IgG (1:5,000;
rabbit, polyclonal; cat. no. 14708; Cell Signaling Technology,
Inc.).
To detect the expression of STAT3-regulated
proteins, Hep3B and HepG2 cells were treated with 20 µM crocin for
the indicated time-points. The cells were then washed and extracted
by incubation for 30 min on ice in RIPA buffer. The lysate was
centrifuged, and the supernatant was collected. Whole-cell protein
extract (20 µg) was resolved on 10–12% SDS-PAGE and then
electro-transferred onto a PVDF membrane. Proteins were detected by
incubation with primary antibodies against CXCR4, SHP-1, p-Src,
Src, p-JAK1, JAK1, p-JAK2, JAK2, VEGF, survivin, Bax, Bcl-2, cyclin
D1, caspase-9, caspase-3, PARP and β-actin, followed by secondary
antibodies conjugated with horseradish peroxidase. Immuno-complexes
were visualized by a chemiluminescence reaction using ECL reagents.
Signal intensity was quantified by an image analyzer (LAS
4000).
Preparation of cytosolic and nuclear
fraction
Hep3B and HepG2 cells were treated with 10 ng/ml
IL-6 for 1 h, and then both cell lines were washed two times and
scraped with PBS. The cell suspension was incubated for 5 min on
ice with buffer A (10 mM HEPES-KOH pH 7.9, 1.5 mm MgCl2,
10 mM KCl, 0.5 mM DTT, 300 mM saccharose, 0.1% NP-40 and 0.5 mM
PMSF) and centrifuged for 5 min at 1,000 × g (4°C). The
supernatants (cytosol fraction) were picked up and the cell pellets
were suspended and incubated for 10 min (4°C) in buffer B (20 mM
HEPES-KOH pH 7.9, 20% glycerol, 100 mM KCl, 100 mM NaCl, 0.2 mM
EDTA, 0.5 mM PMSF and 0.5 mM DTT). These were immediately
centrifuged at 17,000 × g for 5 min (4°C) and the supernatants
(nuclear fraction) were recovered.
Electrophoretic mobility shift assay
(EMSA)
Hep3B and HepG2 cells were grown to ~80% confluence
and nuclear protein was prepared. STAT3/DNA-binding activity was
detected by EMSA using the DIG Gel Shift kit (Roche, Mannheim,
Germany) according to the manufacturer's protocol. Briefly, the
nuclear proteins were subjected to hybridization with a
double-stranded, DIG-labelled oligonucleotide probe containing the
consensus binding site for STAT3 (5-CTTCATTTCCCGTAAATCCCTAAAGCT-3
and 5-AGCTTTAGGGATTTACGGGAAATGA-3).
Immuno fluorescence for STAT3
translocation
Crocin-treated cells were plated on a glass slide
and fixed in 3.7% formaldehyde in PBS. The membrane was
permeabilized by treating cells for 5 min with 0.1% Triton X-100 in
PBS. After a brief washing in PBS, the slides were blocked with 5%
bovine serum albumin for 1 h and then incubated with anti-human
STAT3 antibody (1:1,000; rabbit, monoclonal, cat. no. 9145; Cell
Signaling Technology, Inc.) for 1 h at room temperature. After
being washed, the slides were incubated with the secondary antibody
Alexa Flour 488 (1:100; goat anti-rabbit IgG, polyclonal; cat. no.
A11008; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) for 30
min and counterstained for nuclei with Hoechst 33342
(ImmunoChemistry Technologies, LLC, Bloomington, MN, USA) for 10
min. The slides were mounted using ProLong® Gold
Antifade Mountant reagent (Molecular Probes® by Life
Technologies™; Thermo Fisher Scientific, Inc.). Fluorescence
micrographs were acquired with a fluorescence microscope (Nikon
ECLIPSE Ti-U; Nikon Corporation, Tokyo, Japan).
Transfection with SHP-1 siRNA
Human hepatocellular carcinoma cells (Hep3B) were
plated in 6-well plates and allowed to adhere for 24 h. On the day
of transfection, 9 µl of Lipofectamine RNAiMAX transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to 50 nm
SHP-1 siRNA (sense-GGGCAAGAACCGCUACAAGtt,
antisense-CUUGUAGCGGUUCUUGCCCtt) (Ambion) in a final volume of 150
µl of culture medium. After 48 h of transfection, the cells were
treated with crocin for 24 h, and whole-cell extracts were prepared
for SHP-1, STAT3, and phospho-STAT3 analysis by western
blotting.
TUNEL assay
The in situ labeling of apoptotic cells was
performed using a terminal deoxynucleotidyl transferase
(TdT)-mediated deoxyuridine triphosphate-digoxigenin (dUTP)
nick-end labeling (TUNEL) assay with commercial kits (Roche,
Mannheim, Germany).
Statistical analysis
The data are presented as the mean values ± standard
error (SE) of the mean of at least three separate experiments.
Comparisons were made using Student's t-test. For all analysis, a
two-sided P-value <0.05 was considered to indicate statistical
significance.
Results
Crocin inhibits inducible STAT3
activation in IL-6-stimulated cancer cells
Since previous research has shown that STAT3 can be
phosphorylated at tyrosine residue 705 by stimulation with
cytokines (IL-6 and IL-11) and growth factors (EGF and PDGF)
(4,21), we first investigated whether IL-6
induced STAT3 phosphorylation in hepatocellular carcinoma HepG2 and
Hep3B cells. The results revealed that IL-6 induced STAT3
phosphorylation after as few as 15 min and increased its
phosphorylation for up to 60 min (Fig.
1B) in both cell lines. Then, we investigated whether crocin
modulated IL-6-induced STAT3 phosphorylation. HepG2 and Hep3B cells
were incubated with different concentrations of crocin for 24 h and
then treated with IL-6 for 60 min. Whole-cell extracts were
prepared and assessed for STAT3 phosphorylation by western blot
analysis using an antibody that recognizes STAT3 phosphorylated at
tyrosine 705. Crocin inhibited IL-6-induced phosphorylation of
STAT3 in Hep3B and HepG2 cells, with maximum inhibition occurring
at 20 µM (Fig. 1C). When we
examined the incubation time required for crocin to suppress STAT3
activation in both cell lines, inhibition of STAT3 phosphorylation
was revealed to be in a time-dependent manner, with maximum
inhibition occurring at 24 h (Fig.
1D). Persistent or inducible activation of STAT3 has been
frequently found in the majority of human cancers including
pancreatic (22), lung (23), ovarian (24), breast (25) and colorectal (26) cancers. Thus, we investigated whether
crocin inhibited STAT3 activation in IL-6-stimulated various cancer
cells. Our data revealed that crocin suppressed inducible STAT3
activation in HCT116 and MDA-MB-231 cell lines as well as in BxPC3,
A549 and A2780 cells (Fig. 1E), and
these findings revealed that inhibition of STAT3 activation by
crocin was not cell type-specific.
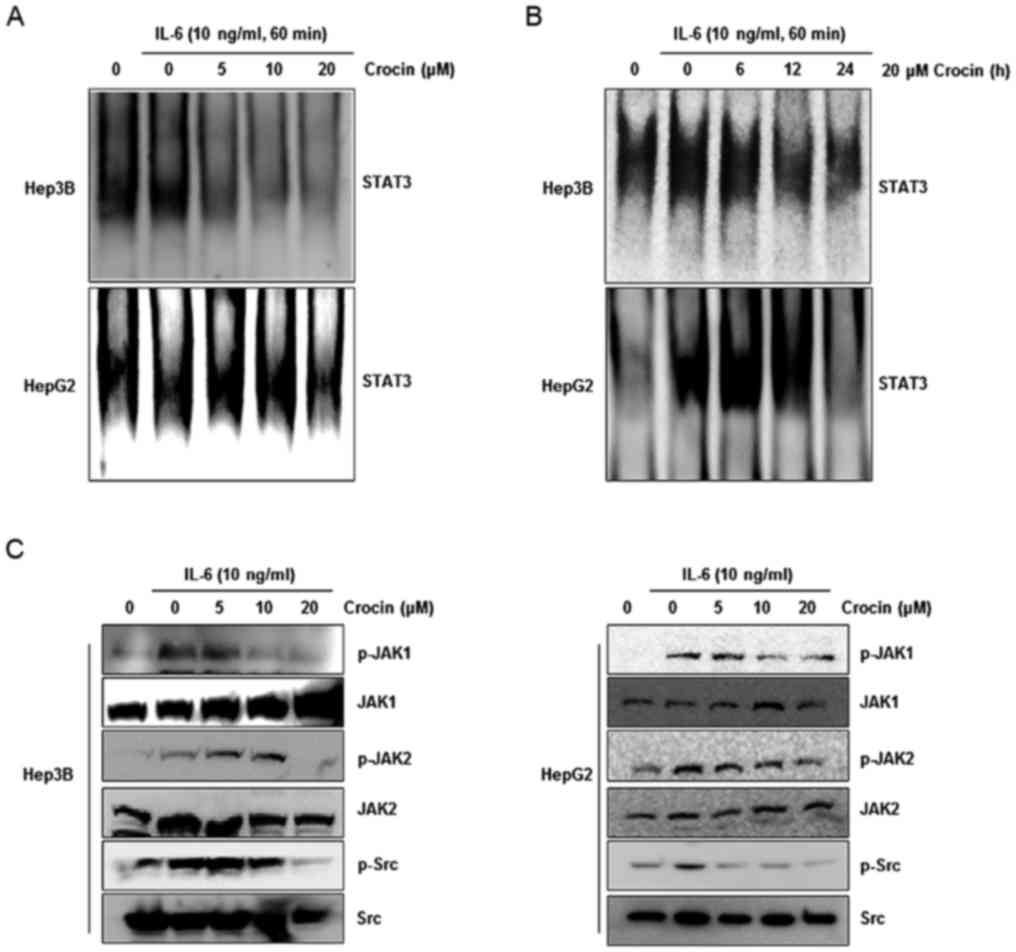
Crocin inhibits the DNA-binding
activity of STAT3 in IL-6-stimulated liver cancer cells
Phosphorylation of STAT3 at Tyr705 leads to
dimerization, nuclear translocation, where it binds to
STAT3-specific DNA-binding elements and regulates gene
transcription (27). We therefore
determined whether crocin suppressed the DNA-binding activity of
STAT3. Cells were treated with different concentrations of crocin
for 24 h or 20 µM crocin at different time-points. Crocin inhibited
STAT3-DNA binding in IL-6-stimulated Hep3B and HepG2 cells in a
dose- and time-dependent manner (Fig.
2A and B).
Crocin inhibits JAK1, JAK2, and Src
kinase activation in IL-6-stimulated liver cancer cells
The tyrosine residues in the STAT protein are
phosphorylated by a variety of upstream signaling kinases including
JAKs and Src (28). The effect of
crocin on the activation of JAK1, JAK2 and Src in IL-6-stimulated
Hep3B and HepG2 cells was assessed, since the inhibitory effect of
crocin on STAT3 phosphorylation was due to suppression of the
upstream signaling pathway. The results revealed that JAK1, JAK2
and Src were activated by IL-6, and treatment with crocin inhibited
the phosphorylation of these proteins in a dose-dependent manner
(Fig. 2C).
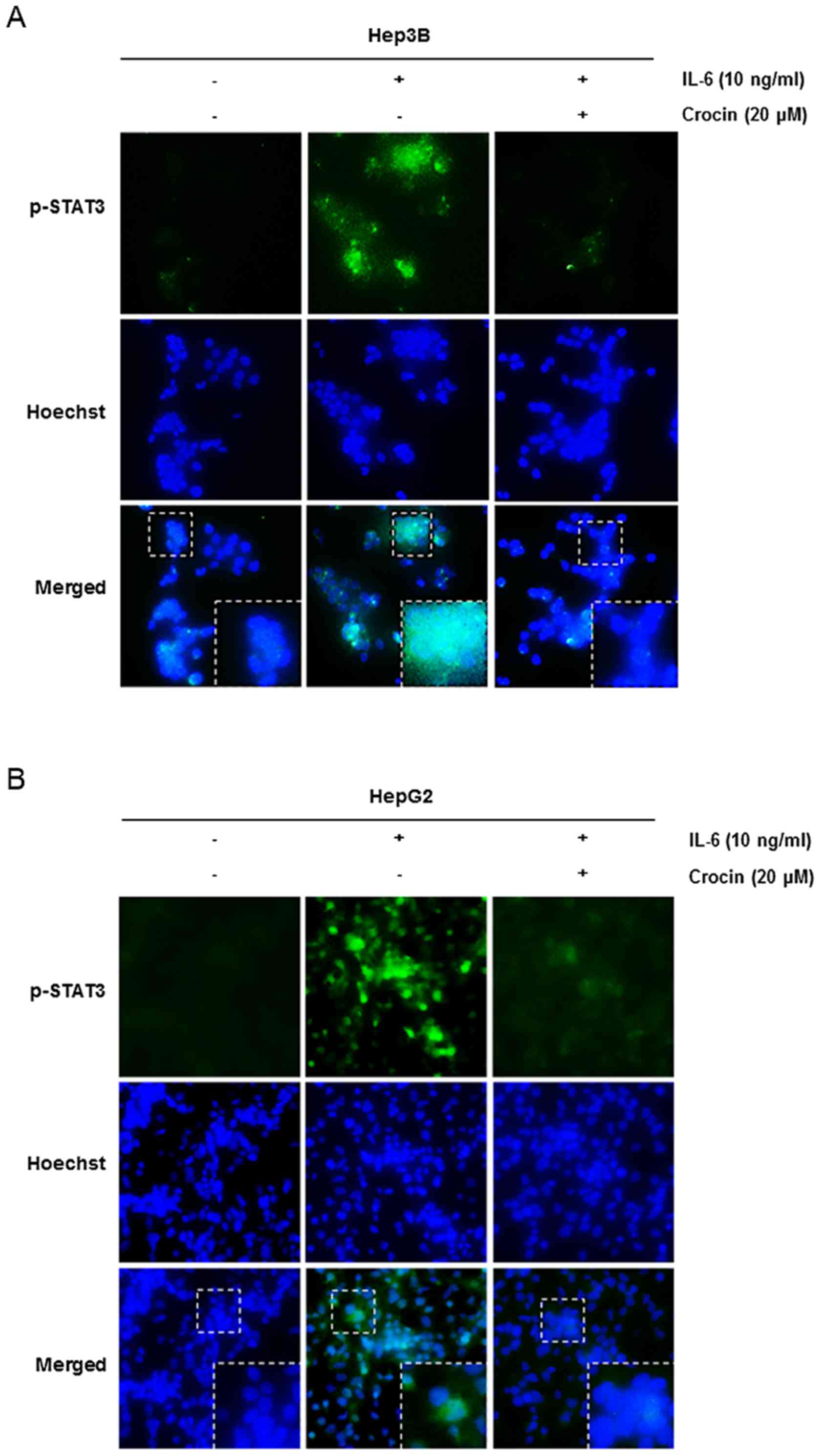
Crocin depletes the nuclear pool of
STAT3 in IL-6-stimulated liver cancer cells
Since it is unclear that phosphorylation is
essential for nuclear transfer and carcinogenic functions of STAT3
(27), we investigated whether
crocin suppresses nuclear translocation of STAT3. As shown in
Fig. 3, crocin inhibited the
translocation of STAT3 to the nucleus in IL-6-stimulated Hep3B
(Fig. 3A) and HepG2 (Fig. 3B) cells. Therefore, these results
indicated that inhibition of STAT3 phosphorylation by crocin
impaired STAT3 transcriptional function by blocking nuclear
translocation.
Pervanadate reverses crocin-mediated
inhibition of STAT3 phosphorylation in IL-6-stimulated Hep3B
cells
Protein tyrosine phosphatases (PTPs) have been
considered to be related to the STAT3 signaling pathway (29). Therefore, we investigated whether
PTPs were involved in blockade of STAT3 signaling by crocin in
Hep3B cells. Sodium pervanadate (a broad-acting tyrosine
phosphatase inhibitor) reversed crocin-mediated inhibition of STAT3
activation induced by IL-6 (Fig.
4A), indicating that the inhibitory effect of crocin was
related to at least one tyrosine phosphatase.
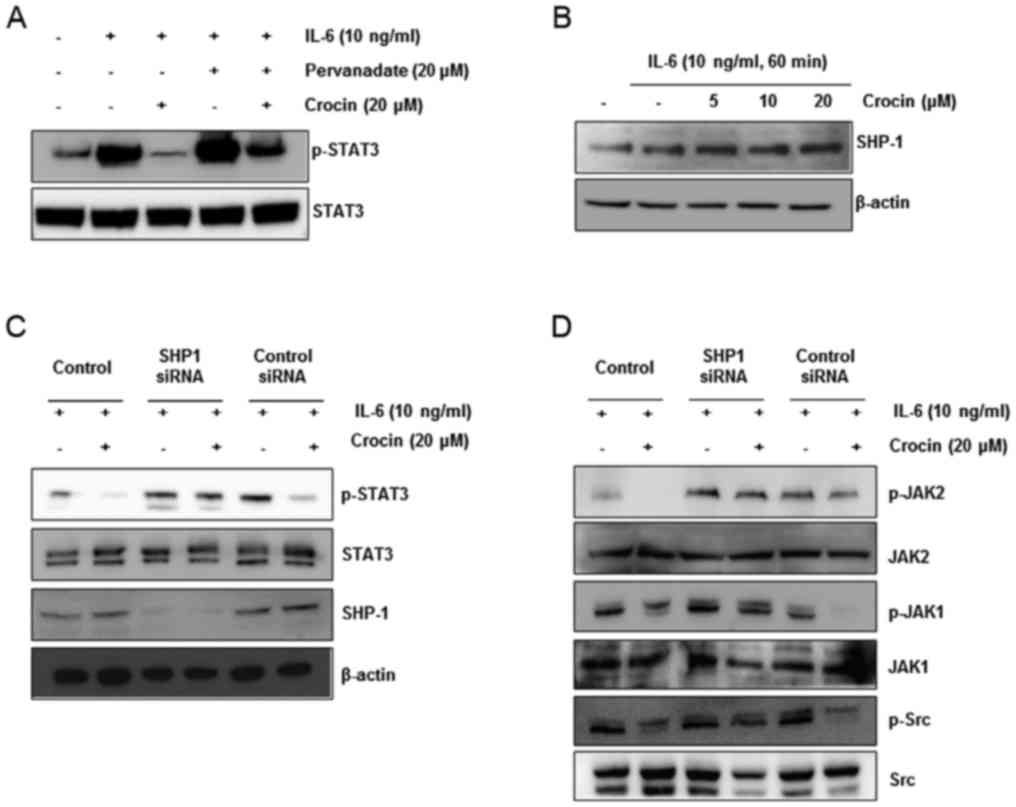 | Figure 4.Crocin induces the expression of
SHP-1 protein in IL-6-stimulated Hep3B cells. (A) Pervanadate
reversed crocin-mediated inhibition of STAT3 phosphorylation in
IL-6-stimulated Hep3B cells. The cells were treated with
pervanadate or crocin for 24 h, and then stimulated with IL-6 for
60 min. The phospho-STAT3 was detected by western blotting, and the
same blots were stripped and reprobed with STAT3 antibodies. (B)
Crocin induced the expression of the SHP-1 protein. Hep3B cells
were treated with 0, 5, 10 and 20 µM crocin for 24 h and then
stimulated with IL-6 (10 ng/ml). After whole-cell extracts were
prepared, SHP-1was detected by western blotting. The same blots
were stripped and reprobed with the β-actin antibody to ascertain
equal protein loading. (C and D) Hep3B cells were transfected with
either SHP-1 siRNA or scrambled siRNA (50 nM). After 24 h, the
cells were treated with or without 20 µM crocin for 24 h and then
stimulated with IL-6 (10 ng/ml) for 60 min. Then, whole-cell
extracts were subjected to western blot analysis for SHP-1,
p-STAT3, p-JAK1, p-JAK2 and p-Src. The same blots were stripped and
reprobed with β-actin, STAT3, JAK1, JAK2 and Src antibodies to
ascertain equal protein loading. |
Since previous studies have shown that various
natural products such as pectolinarigenin (30), capsazepine (31) and ginkgetin (32) dephosphorylate STAT3 through SHP-1
activation, we observed the protein level of SHP-1 in
IL-6-stimulated Hep3B cells after crocin exposure. We determined
that crocin increased SHP-1 expression in a dose-dependent manner
(Fig. 4B). This result revealed
that SHP-1 plays an important role in crocin-mediated inhibition of
STAT3 activity.
Suppression of SHP-1 by a specific
siRNA reverses crocin-mediated inhibition of STAT3
phosphorylation
To confirm that dephosphorylation of STAT3 by crocin
was due to SHP-1, a siRNA against SHP-1 was transfected into
IL-6-stimulated Hep3B cells after treatment with or without crocin.
The results revealed that dephosphorylation of STAT3 by crocin was
confirmed in the control group and the scrambled-siRNA transfected
group. However, STAT3 dephosphorylation was recovered in the
SHP-1-siRNA transfected group (Fig.
4C). These results corroborated our earlier evidence on the
critical role of SHP-1 in suppression of STAT3 phosphorylation by
crocin. SHP-1 gene silencing with siRNA did not suppress activation
of JAK1, JAK2 and Src in IL-6-stimulated Hep3B cells (Fig. 4D). These data indicated that SHP-1
has a critical role in the inhibition of STAT3 signaling by
crocin.
Crocin downregulates gene expression
related to cell proliferation, survival, apoptosis, and invasion in
IL-6-stimulated liver cancer cells
Cyclin D1 is required for cell proliferation and
transition from G1 to S phase of the cell cycle and is regulated by
STAT3. We revealed that crocin treatment suppressed the expression
of cyclin D1 in a time-dependent manner in IL-6-stimulated liver
cancer cells. In addition, there are many studies suggesting that
STAT3 activation is associated with the expression of gene products
involved in cancer metastasis (CXCR4) (33–35)
and angiogenesis (VEGF) (36).
Based on this, we observed that crocin treatment suppressed the
expression of CXCR4 and VEGF in IL-6-stimulated liver cancer cells
in both cell lines. Furthermore, STAT3 activation also regulates
the expression of various gene products related to apoptosis,
including Bcl-2, Bcl-xL, and surviving (6). To identify the role of crocin in
apoptotic cell death, we performed western blot analysis of
anti-apoptotic proteins, Bcl-2 and survivin. The results revealed
that crocin significantly decreased the level of Bcl-2 and survivin
and increased the level of BAX as shown in Fig. 5A. Based on these results, it was
determined that crocin had an effect on antiproliferation,
apoptosis and blockade of invasion in IL-6-stimulated liver cancer
cells.
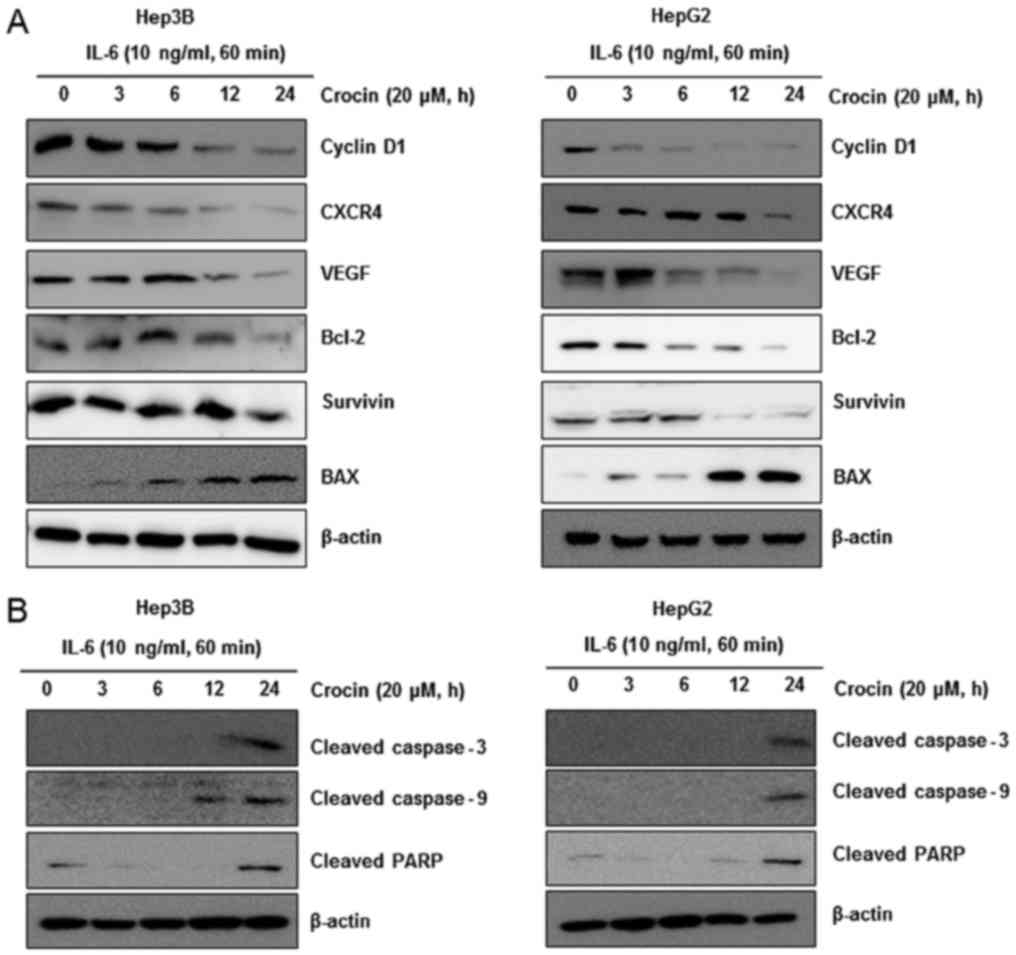 | Figure 5.Crocin downregulates gene expression
related to cell survival, proliferation, apoptosis, and invasion in
IL-6-stimulated liver cancer cells. (A) Crocin suppresses
STAT3-regulated gene products involved in survival, proliferation,
apoptosis and invasion. Hep3B and HepG2 cells (1×106/ml)
were treated with 20 µM crocin for indicated time-points and then
stimulated with IL-6 (10 ng/ml). After whole-cell extracts were
prepared and 30 µg proteins of those extracts were resolved on 12%
SDS-PAGE, the membranes were probed against cyclin D1, CXCR4, VEGF,
Bcl-2, survivin and BAX antibodies. The same blots were stripped
and reprobed with the β-actin antibody to ascertain equal protein
loading. (B) Hep3B (left panel) and HepG2 (right panel) cells
(1×106/ml) were treated with 20 µM crocin for indicated
time-points and then stimulated IL-6 (10 ng/ml). After whole-cell
extracts were prepared and 30 µg proteins of those extracts were
resolved on 8 or 12% SDS-PAGE, the membranes were probed against
cleaved caspase-3, cleaved caspase-9 and cleaved PARP antibodies.
The same blots were stripped and reprobed with the β-actin antibody
to ascertain equal protein loading. |
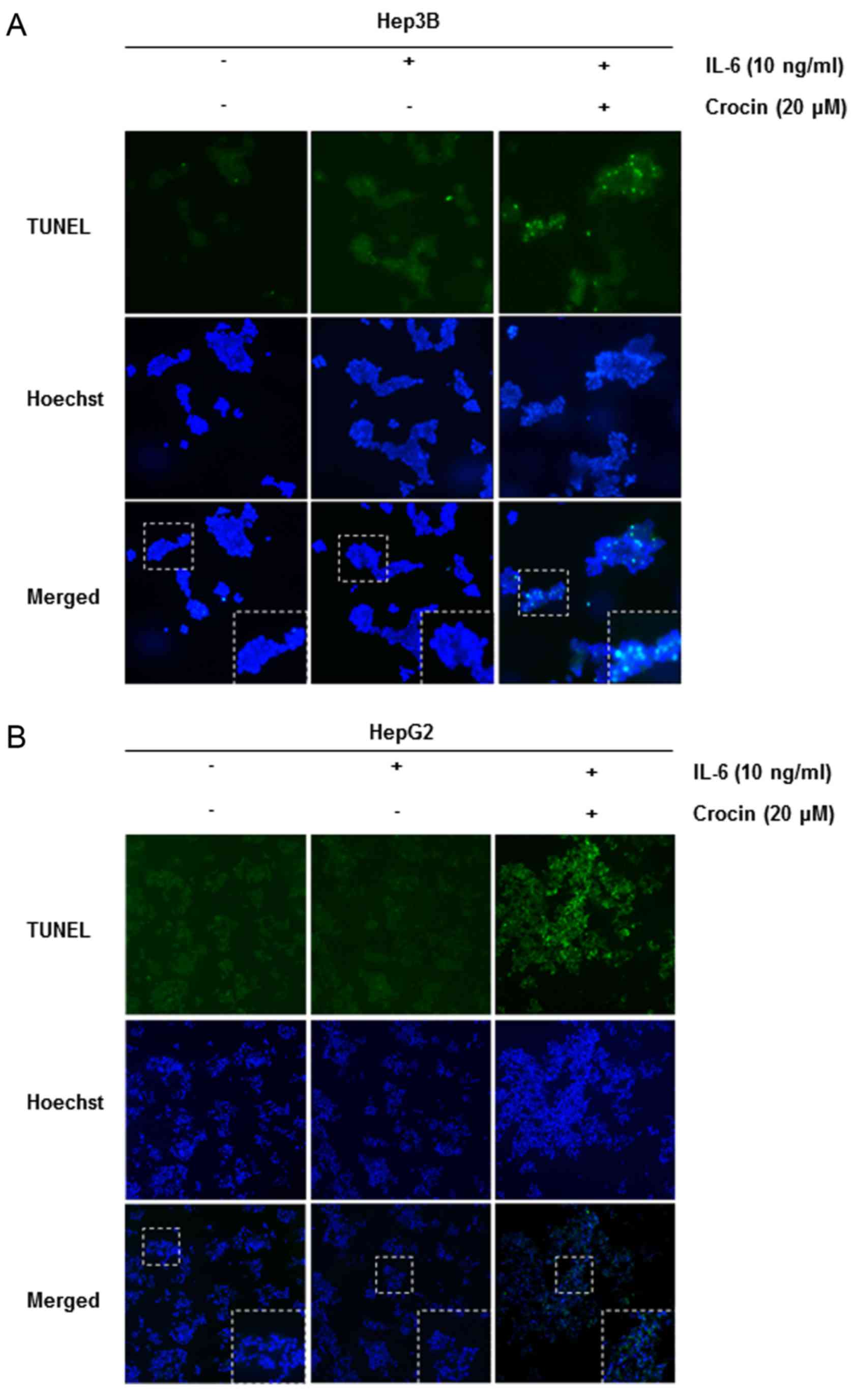
Crocin activates caspase-9, −3 and
PARP cleavage
We also investigated whether suppression of STAT3
activation by crocin led to apoptosis. Cells were treated with 20
µM of crocin for different time-points and then examined for
activation of caspases by western blotting using specific
antibodies. We observed cleavage of caspase-9, caspase-3 and PARP
at 20 µM of crocin for 24 h (Fig.
5B). Finally, we also determined that cells exposed to crocin
were TUNEL-positive, further corroborating with evidence the
initiation of apoptosis in these cells. These results clearly
revealed that crocin induced caspase-dependent apoptosis in
IL-6-stimulated Hep3B (Fig. 6A) and
HepG2 (Fig. 6B).
Discussion
STAT3 activation is closely involved in a wide range
of tumor types such as carcinoma, sarcoma, lymphoma and leukemia
(6,37) and pharmacological inhibition of
STAT3 has shown its potential in cancer prevention and treatment.
In addition, interleukin-6 (IL-6) has been reported to be closely
related to STAT3 activity. The purpose of this study was to examine
whether crocin, derived from Crocus sativus L. (saffron),
exerted its anticancer effects through abrogation of the STAT3
signaling pathway induced by IL-6 in liver cancer cells. We first
investigated whether crocin inhibited STAT3 activation induced by
IL-6 in Hep3B and HepG2 cells. The results revealed that crocin
inhibited STAT3 activation and this was related to inactivation of
upstream kinases and induction of protein tyrosine phosphatase
(PTP). Moreover, blockade of STAT3 activation led to suppression of
gene expression regulated by STAT3. Recent studies have revealed
that STAT3, which plays an important role in carcinogenesis, may
contribute to designing novel targeted therapies (30,31).
We found that crocin suppressed IL-6-induced STAT3 activation and
STAT3-DNA binding in liver cancer cells. Phosphorylation of STAT3
is mediated through activation of non-receptor protein tyrosine
kinases, including Janus-like kinases (JAK)-1, −2, TYK2, and c-Src
kinasec(38). Thus, we examined whether crocin had an inhibitory
effect on these kinases that have demonstrated potential in the
STAT3 activation pathway. Notably, we observed that crocin
inhibited activation of JAK-1, JAK-2 and Src in IL-6-stimulated
Hep3B cells. The SH2 domain-containing phosphatase 1 (SHP-1) is a
non-receptor PTP that has been revealed in hematopoietic cells
(39,40). Specifically, SHP-1 has
tumor-suppression potential due to negative regulation of STAT3
signaling during tumor progression (41,42).
We revealed that crocin treatment significantly increased the
expression of SHP-1 and this was correlated with a decrease in
p-STAT3 expression. We also demonstrated that crocin had no effect
on suppression of p-STAT3 when the cells were transfected with
SHP-1-siRNA. Notably, our present findings revealed that the
tyrosine phosphatase SHP-1, which is upregulated by crocin, plays a
critical role in suppression of the STAT3 signaling pathway in
IL-6-stimulated liver cancer cells.
STAT3 activation, which results in encoding
anti-apoptotic proteins and proliferation-associated proteins and
leads to decreased cell death, is strongly associated with the
development of various types of cancer (43,44).
Apoptosis is the process of programmed cell death characterized by
a series of morphological changes, including plasma and nuclear
membrane blebbing, cell shrinkage, and caspase activation. In the
present study, we detected that crocin significantly promoted the
activation of caspase-3 and caspase-9 along with enhanced cleavage
of PARP, indicating that crocin may exert its anticancer activity
via enabling induction of apoptosis. In addition, STAT3 has been
confirmed to be involved in the regulation of anti-apoptotic
proteins and the inhibitor of apoptosis protein (IAP) family
(45). We also demonstrated that
crocin decreased the expression levels of Bcl-2 and survivin,
suggesting that crocin induces apoptosis in Hep3B and HepG2 cells
stimulated with IL-6. Various natural drugs have been reported to
inhibit cyclin D1, VEGF and CXCR4, which are regulated by STAT3.
For example, quercetin inhibited the IL-6/STAT3 signaling pathway
and inhibited growth and migration of glioblastoma cells (46). Plumbagin downregulated cyclin D1 and
VEGF expression through STAT3 regulation (47), and sinomenine inhibited the invasion
and metastasis through inhibition of the CXCR4-STAT3 pathway
(48). As observed in these
studies, crocin ameliorated the expression of genes related to cell
proliferation and invasion, as evidenced by decreased expression of
cyclin D1, VEGF and CXCR4.
In conclusion, the present study provides evidence
that crocin has the potential for anticancer activity through
inhibition of the IL-6/STAT3 signaling pathway, especially in liver
cancer. However, further research using clinical animal models is
warranted to realize all the potential of this molecule as an
anticancer drug.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (NRF-2016R1A6A1A03011325)
and by the Ministry of Science, ICT & Future Planning
(NRF-2016R1A1A1A05921696).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A, Hernandez-Gea V and Llovet
JM: Medical therapies for hepatocellular carcinoma: A critical view
of the evidence. Nat Rev Gastroenterol Hepatol. 10:34–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao C, Wang W, Yu W, Jou D, Wang Y, Ma H,
Xiao H, Qin H, Zhang C, Lü J, et al: A novel small molecule STAT3
inhibitor, LY5, inhibits cell viability, colony formation, and
migration of colon and liver cancer cells. Oncotarget.
7:12917–12926. 2016.PubMed/NCBI
|
|
4
|
Zhang Z, Mao H, Du X, Zhu J, Xu Y, Wang S,
Xu X, Ji P, Yu Y, Cao B, et al: A novel small molecule agent
displays potent anti-myeloma activity by inhibiting the JAK2-STAT3
signaling pathway. Oncotarget. 7:9296–9308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lanton T, Shriki A, Nechemia-Arbely Y, et
al: IL6-Dependent genomic instability heralds accelerated
carcinogenesis following liver regeneration on a background of
chronic hepatitis. Hepatology. 65:1600–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggarwal BB, Sethi G, Ahn KS, Sandur SK,
Pandey MK, Kunnumakkara AB, Sung B and Ichikawa H: Targeting
signal-transducer-and-activator-of-transcription-3 for prevention
and therapy of cancer: modern target but ancient solution. Ann N Y
Acad Sci. 1091:151–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H,
Wang Y, Yin L, Han Y, Sun L, et al: A novel anti-cancer agent
Icaritin suppresses hepatocellular carcinoma initiation and
malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget.
6:31927–31943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khorasany AR and Hosseinzadeh H:
Therapeutic effects of saffron (Crocus sativus L.) in digestive
disorders: a review. Iran J Basic Med Sci. 19:455–469.
2016.PubMed/NCBI
|
|
9
|
Kim SH, Lee JM, Kim SC, Park CB and Lee
PC: Proposed cytotoxic mechanisms of the saffron carotenoids crocin
and crocetin on cancer cell lines. Biochem Cell Biol. 92:105–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asdaq SM and Inamdar MN: Potential of
Crocus sativus (saffron) and its constituent, crocin, as
hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol.
162:358–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ordoudi SA, Befani CD, Nenadis N, Koliakos
GG and Tsimidou MZ: Further examination of antiradical properties
of Crocus sativus stigmas extract rich in crocins. J Agric Food
Chem. 57:3080–3086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu GL, Yu SQ, Gong ZN and Zhang SQ: Study
of the effect of crocin on rat experimental hyperlipemia and the
underlying mechanisms. Zhongguo Zhong Yao Za Zhi. 30:369–372.
2005.(In Chinese). PubMed/NCBI
|
|
13
|
Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL,
Rahman K and Qin LP: Antidepressant properties of bioactive
fractions from the extract of Crocus sativus L. J Nat Med.
64:24–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nam KN, Park YM, Jung HJ, Lee JY, Min BD,
Park SU, Jung WS, Cho KH, Park JH, Kang I, et al: Anti-inflammatory
effects of crocin and crocetin in rat brain microglial cells. Eur J
Pharmacol. 648:110–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu GL, Li G, Ma HP, Zhong H, Liu F and Ao
GZ: Preventive effect of crocin in inflamed animals and in
LPS-challenged RAW 264.7 cells. J Agric Food Chem. 57:8325–8330.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu HJ, Zhong R, Zhao YX, Li XR, Lu Y, Song
AQ, Pang XY, Yao RY and Sun LR: Proliferative inhibition and
apoptotic induction effects of crocin on human leukemia HL-60 cells
and their mechanisms. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
18:887–892. 2010.(In Chinese). PubMed/NCBI
|
|
17
|
Aung HH, Wang CZ, Ni M, Fishbein A,
Mehendale SR, Xie JT, Shoyama CY and Yuan CS: Crocin from Crocus
sativus possesses significant anti-proliferation effects on human
colorectal cancer cells. Exp Oncol. 29:175–180. 2007.PubMed/NCBI
|
|
18
|
Chryssanthi DG, Lamari FN, Iatrou G,
Pylara A, Karamanos NK and Cordopatis P: Inhibition of breast
cancer cell proliferation by style constituents of different Crocus
species. Anticancer Res. 27:357–362. 2007.PubMed/NCBI
|
|
19
|
Zhao P, Luo CL, Wu XH, Hu HB, Lv CF and Ji
HY: Proliferation apoptotic influence of crocin on human bladder
cancer T24 cell line. Zhongguo Zhong Yao Za Zhi. 33:1869–1873.
2008.(In Chinese). PubMed/NCBI
|
|
20
|
Mashmoul M, Azlan A, Mohtarrudin N, Mohd
Yusof BN, Khaza'ai H, Khoo HE, Farzadnia M and Boroushaki MT:
Protective effects of saffron extract and crocin supplementation on
fatty liver tissue of high-fat diet-induced obese rats. BMC
Complement Altern Med. 16:4012016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng J, Grande F and Neamati N: Small
molecule inhibitors of Stat3 signaling pathway. Curr Cancer Drug
Targets. 7:91–107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu D, Ye T, Xiang Y, Shi Z, Zhang J, Lou
B, Zhang F, Chen B and Zhou M: Quercetin inhibits
epithelial-mesenchymal transition, decreases invasiveness and
metastasis, and reverses IL-6 induced epithelial-mesenchymal
transition, expression of MMP by inhibiting STAT3 signaling in
pancreatic cancer cells. Onco Targets Ther. 10:4719–4729. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Cao L, Wang H, Liu B, Zhang Q,
Meng Z, Wu X, Zhou Q and Xu K: Cancer-associated fibroblasts
enhance metastatic potential of lung cancer cells through
IL-6/STAT3 signaling pathway. Oncotarget. 8:76116–76128.
2017.PubMed/NCBI
|
|
24
|
Zhao X, Huang L, Xu W and Chen X, Shen Y,
Zeng W and Chen X: Physapubescin B inhibits tumorgenesis and
circumvents taxol resistance of ovarian cancer cells through STAT3
signaling. Oncotarget. 8:70130–70141. 2017.PubMed/NCBI
|
|
25
|
Zhang YX, Yan L, Liu GY, Chen WJ, Gong WH
and Yu JM: Inhibition of janus kinase 2 by compound AG490
suppresses the proliferation of MDA-MB-231 cells via up-regulating
SARI (suppressor of AP-1, regulated by IFN). Iran J Basic Med Sci.
18:599–603. 2015.PubMed/NCBI
|
|
26
|
Fan LC, Teng HW, Shiau CW, Tai WT, Hung
MH, Yang SH, Jiang JK and Chen KF: Pharmacological targeting
SHP-1-STAT3 signaling is a promising therapeutic approach for the
treatment of colorectal cancer. Neoplasia. 17:687–696. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu CL, Meyer DJ, Campbell GS, Larner AC,
Carter-Su C, Schwartz J and Jove R: Enhanced DNA-binding activity
of a Stat3-related protein in cells transformed by the Src
oncoprotein. Science. 269:81–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim BH, Won C, Lee YH, Choi JS, Noh KH,
Han S, Lee H, Lee CS, Lee DS, Ye SK and Kim MH: Sophoraflavanone G
induces apoptosis of human cancer cells by targeting upstream
signals of STATs. Biochem Pharmacol. 86:950–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang T, Li S, Li J, Yin F, Hua Y, Wang Z,
Lin B, Wang H, Zou D, Zhou Z, et al: Natural product
pectolinarigenin inhibits osteosarcoma growth and metastasis via
SHP-1-mediated STAT3 signaling inhibition. Cell Death Dis.
7:e24212016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JH, Kim C, Baek SH, Ko JH, Lee SG,
Yang WM, Um JY, Sethi G and Ahn KS: Capsazepine inhibits JAK/STAT3
signaling, tumor growth, and cell survival in prostate cancer.
Oncotarget. 8:17700–17711. 2017.PubMed/NCBI
|
|
32
|
Baek SH, Lee JH, Ko JH, Lee H, Nam D, Lee
SG, Yang WM, Um JY, Lee J, Kim SH, et al: Ginkgetin blocks
constitutive STAT3 activation and induces apoptosis through
induction of SHP-1 and PTEN tyrosine phosphatases. Phytother Res.
30:567–576. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Xiao Q, Bai X, Yu Z, Sun M, Zhao H,
Mi X, Wang E, Yao W, Jin F, et al: Activation of STAT3 is involved
in malignancy mediated by CXCL12-CXCR4 signaling in human breast
cancer. Oncol Rep. 32:2760–2768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pfeiffer M, Hartmann TN, Leick M, Catusse
J, Schmitt-Graeff A and Burger M: Alternative implication of CXCR4
in JAK2/STAT3 activation in small cell lung cancer. Br J Cancer.
100:1949–1956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang Y, Guo Q, Zhi Y, Jin X, Xia B, Guo S,
Tian C and Zhang Y: Role of CXCR4/STAT3 in mesenchymal stromal
cell-mediated drug resistance of acute leukemia cells. Zhonghua Xue
Ye Xue Za Zhi. 37:119–123. 2016.(In Chinese). PubMed/NCBI
|
|
36
|
Yahata Y, Shirakata Y, Tokumaru S,
Yamasaki K, Sayama K, Hanakawa Y, Detmar M and Hashimoto K: Nuclear
translocation of phosphorylated STAT3 is essential for vascular
endothelial growth factor-induced human dermal microvascular
endothelial cell migration and tube formation. J Biol Chem.
278:40026–40031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pandey MK, Sung B and Aggarwal BB:
Betulinic acid suppresses STAT3 activation pathway through
induction of protein tyrosine phosphatase SHP-1 in human multiple
myeloma cells. Int J Cancer. 127:282–292. 2010.PubMed/NCBI
|
|
38
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Banville D, Stocco R and Shen SH: Human
protein tyrosine phosphatase 1C (PTPN6) gene structure: Alternate
promoter usage and exon skipping generate multiple transcripts.
Genomics. 27:165–173. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsui HW, Hasselblatt K, Martin A, Mok SC
and Tsui FW: Molecular mechanisms underlying SHP-1 gene expression.
Eur J Biochem. 269:3057–3064. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu C, Sun M, Liu L and Zhou GW: The
function of the protein tyrosine phosphatase SHP-1 in cancer. Gene.
306:1–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu C, Guan Q, Wang Y, Zhao ZJ and Zhou GW:
SHP-1 suppresses cancer cell growth by promoting degradation of JAK
kinases. J Cell Biochem. 90:1026–1037. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morikawa T, Baba Y, Yamauchi M, Kuchiba A,
Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS and
Ogino S: STAT3 expression, molecular features, inflammation
patterns, and prognosis in a database of 724 colorectal cancers.
Clin Cancer Res. 17:1452–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Michaud-Levesque J, Bousquet-Gagnon N and
Beliveau R: Quercetin abrogates IL-6/STAT3 signaling and inhibits
glioblastoma cell line growth and migration. Exp Cell Res.
318:925–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sandur SK, Pandey MK, Sung B and Aggarwal
BB: 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue,
suppresses STAT3 activation pathway through induction of protein
tyrosine phosphatase, SHP-1: Potential role in chemosensitization.
Mol Cancer Res. 8:107–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xie T, Ren HY, Lin HQ, Mao JP, Zhu T, Wang
SD and Ye ZM: Sinomenine prevents metastasis of human osteosarcoma
cells via S phase arrest and suppression of tumor-related
neovascularization and osteolysis through the CXCR4-STAT3 pathway.
Int J Oncol. 48:2098–2112. 2016. View Article : Google Scholar : PubMed/NCBI
|