Introduction
Cell lines are usually maintained in defined media
that commonly contain amino acids, vitamins, glucose and inorganic
salts for normal cell metabolism. However, the composition of
medium formulations vary widely in concentrations of glucose and
other metabolic precursors, including amino acids. Thus, many types
of cells showed different responses to culture media in regards to
proliferation and differentiation. Th17 differentiation was induced
more efficiently in Iscove's modified Dulbecco's medium (IMDM) than
in RPMI (1). Bone marrow-derived
dendritic cells in IMDM expressed higher levels of co-stimulatory
and MHC II molecules, compared to the cells generated in RPMI
(2). In addition, hepatocyte
differentiation and propagation and phenotype of corneal
stroma-derived stem cells were modulated by culture media (3,4).
Subsequently, the underlying mechanisms of the medium-induced
changes in cell proliferation and differentiation have been
partially elucidated. Differentiation of Th17 cells was increased
by endogenous aryl hydrocarbon receptor (AhR) agonists derived from
aromatic amino acids such as tryptophan, which is underrepresented
in RPMI compared with IMDM (1).
Mesenchymal stem cells co-cultured with AML12 liver cells were able
to differentiate into hepatocyte-like cells expressing
hepatocyte-specific markers including albumin, α-fetoprotein and
cytokeratin 18 mRNAs, which were expressed at the initial 7-day
culture in DMEM while being expressed during the 7- to 14-day
culture in DMEM/F12 (3). In a study
using MDA-MB-231 breast cancer cells, ~25.6% of genes were
expressed at significantly different levels in cells grown in MEM,
DMEM or RPMI (5). HT-29 cells,
which remain undifferentiated in medium containing glucose, became
differentiated into enterocytes upon removal of glucose (6,7).
HT-29 cells, a human colon carcinoma cell line, can
express, upon exposure to the appropriate inducers (including
sodium butyrate, lack of glucose in culture medium,
12-O-tetradecanoylphorbol-13-acetate and forskolin) distinct
intestinal lineage-specific markers, differentiating along
different lineages that resemble those found in the normal
intestinal epithelium (6,8). Therefore, they are considered
multipotent, similar to the stem cells of the intestinal crypt
(9) and have been extensively used
as an in vitro model for the study of the differentiation
and proliferation of intestinal stem cells.
Various amino acids regulate cell growth and
differentiation either by activating signaling pathways or by their
metabolites. Leucine, arginine and glutamine activate the mammalian
target of rapamycin (mTOR) signaling that controls cell growth and
mRNA translation (10–12). Glutamine is a precursor of
UDP-N-acetylglucosamine, a substrate for cellular
glycosyltransferases, to control T cell self-renewal and malignancy
(13). Metabolisms of threonine and
leucine and isoleucine are required for stem cell self-renewal and
adipocyte differentiation, respectively (14,15).
In addition, tryptophan metabolites such as kynurenine (KN) and
kynurenic acid (KA) are ligands for AhR, a ligand-dependent
transcription factor to regulate cell growth and differentiation
(16,17), and other aromatic amino acids,
including histidine, tyrosine and phenylalanine, could generate
precursors for AhR ligands (18).
DMEM contains higher amounts of aromatic as well as
branched chain amino acids than RPMI. Thus, studies concerning the
growth and differentiation of certain cancer stem cells in
different media containing different amounts of amino acids such as
DMEM and RPMI could elucidate the roles of amino acids in cell
growth and differentiation.
In the present study, we compared the
differentiating ability of HT-29 cells in RPMI-1640 (subsequently
referred to as RPMI) and DMEM which largely differ in regards to
the amounts of many amino acids, including tryptophan
(concentrations of L-histidine, L-tyrosine, L-phenylalanine, and
L-tryptophan, which are 15, 29, 15 and 5 mg/l, respectively, in
RPMI 1640, rise to 42, 104, 66 and 16 mg/l, respectively, in DMEM).
We also investigated which amino acids and metabolites contribute
to this and how they function. When we cultured HT-29 cells in RPMI
and DMEM, it was observed that most of the HT-29 cells
differentiated into goblet cells downregulating the stem cell
marker Lgr5 when cultured in DMEM, but remained undifferentiated in
RPMI. We demonstrated that KN, an indoleamine 2,3 dioxygenase-1
(IDO-1)-mediated tryptophan metabolite, promoted goblet cell
differentiation and reduced β-catenin expression. In addition, the
levels of Notch1 and its active product Notch intracytoplasmic
domain (NICD) were decreased in DMEM when compared to the levels in
RPMI. DMEM reduced expression of transcriptional repressor Hes1
while upregulated expression of Hath1, a transcriptional activator
mediating differentiation of secretory lineages. Finally, AhR
activation moderately induced goblet cell differentiation.
Materials and methods
Cell culture
HT-29 human epithelial cell line was purchased from
the American Type Culture Collection (ATCC; Rockville, MD, USA).
Cells were maintained in RPMI-1640 and DMEM (Gibco; Life
Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v)
heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich, St. Louis,
MO, USA), 100 U/ml penicillin, 100 µg/ml streptomycin and 0.25
µg/ml fungizone (Life Technologies) in a 5% humidified incubator at
37°C. The cell number and viability were assessed by exclusion of
trypan blue dye using a hemacytometer. For experiments, cells were
seeded in a 24-well plate at a density of 1×105
cells/well. Cells were observed under an inverted microscope for
morphologic assessment.
Chemicals and reagents
Indoxyl 3-sulfate (I3S), indole-3-carbinol (I3C),
3,3′-diindolylmethane (DIM), 1-methyl-L-tryptophan and L-kynurenine
were purchased from Sigma-Aldrich. 6-Formylindolo[3,2-b]carbazole
(FICZ) was obtained from Enzo Life Sciences (Farmingdale, NY, USA).
AhR ligands were dissolved in dimethyl sulfoxide (DMSO) and 0.1%
DMSO v/v was used as vector control. Anti-activated Notch1 Ab was
from Abcam (Cambridge, MA, USA) and anti-non-phospho (active)
β-catenin (Ser33/37/Thr41) Ab and anti-β-actin Ab were from Cell
Signaling Technologies (Danvers, MA, USA). Jagged-1 peptide was
obtained from AnaSpec (Fremont, CA, USA).
RNA preparation, RT-PCR and real-time
PCR
Total cellular RNA was extracted from cells using
the RNAzol method (Tel-Test, Inc., Friendswood, TX, USA). For PCR
analysis, RNA was used after the contaminating DNA was completely
removed by DNase I treatment. RT-PCR analysis was performed using
pairs of oligonucleotide primers. The PCR products were confirmed
to correspond to their original sequence by DNA sequencing.
Gene-specific primers, number of cycles of amplification, annealing
temperature, and expected size of the PCR product are listed in
Table I. Real-time PCR was
performed to quantitate PCR products. Power SYBR-Green PCR Master
Mix and Real-Time PCR System (7300; Applied Biosystems, Foster
City, CA, USA) were used.
 | Table I.Primers used in the RT-PCR. |
Table I.
Primers used in the RT-PCR.
| Name | Nucleotide
sequence | Annealing temp.
(°C) | Cyclesa | Sizeb (bp) |
|---|
| huhgprtfw |
5′-ggccatcacattgtagccct-3′ | 58 | 30 | 408 |
| huhgprtrv |
5′-gtcaagggcatatcctacaac-3′ |
|
|
|
| humuc2fw |
5′-agcacttcgagttcgactgc-3′ | 58 | 30 | 600 |
| humuc2rv |
5′-ggatcttcacgcagctgaag-3′ |
|
|
|
| hualpifw |
5′-atgtgtggaaccgcactgag-3′ | 58 | 35 | 411 |
| hualpirv |
5′-ctttgctgtcctgagccttg-3′ |
|
|
|
| hulyzfw |
5′ggtgtgagttggccagaact-3′ | 58 | 30 | 300 |
| hulyzrv |
5′-cttgtggatcacggacaacc-3′ |
|
|
|
| huahrfw |
5′-ttggctagcctgctgccttt-3′ | 58 | 30 | 350 |
| huahrrv |
5′-gacgctgaaattcagctcgg-3′ |
|
|
|
| hucyp1a1fw |
5′-aggcctgaagaatccaccag-3′ | 58 | 35 | 760 |
| hucyp1a1rv |
5′-gtgctcaatcaggctgtctg-3′ |
|
|
|
| hulgr5fw |
5′-aaacctctccagctgggtag-3′ | 58 | 35 | 590 |
| hulgr5rv |
5′-ttcagcgatcggaggctaag-3′ |
|
|
|
| huhes1fw |
5′-agccagtgtcaacacgacac-3′ | 58 | 35 | 637 |
| huhes1rv |
5′-aagcaaactggccatcggga-3′ |
|
|
|
| huhath1fw |
5′-cactttgcagggcatctgca-3′ | 58 | 35 | 410 |
| huhath1rv |
5′-gccatctgcagggtctcata-3′ |
|
|
|
Western blotting
Cells or tissues were homogenized in lysis buffer
containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA (pH 8.0), 50 µM
sodium vanadate, 20 mM p-nitrophenylphosphate, 50 mM sodium
fluoride, leupeptin (0.5 µg/ml), aprotinin (10 µg/ml) and soybean
trypsin inhibitor (10 µg/ml). Proteins size-fractionated using
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) were transferred to polyvinylidene difluoride (PVDF)
membranes, and the blots were blocked with 3% bovine serum albumin
in TBS buffer (20 mM Tris-HCl pH 7.5/137 mM NaCl). The blots were
sequentially treated with primary and secondary antibodies in
Tris-buffered saline with Tween-20 (TBST) (20 mM Tris-HCl pH
7.5/137 mM NaCl/0.1% Tween-20) with intermittent washing with TBST.
Immunodetection detection was performed with the EzWestLumi Plus
kit (ATTO Corp, Tokyo, Japan). Densitometric analysis was performed
using Image Master 2-D Platinum software (Amersham Biosciences,
Pisactaway, NJ, USA) according to the protocols provided by the
manufacturer.
Statistical analysis
All experiments were performed three times, and a
representative experiment is shown. Data are presented as the mean
± SD and analyzed by the paired Students t-test. A value of
P<0.05 was considered statistically significant.
Results
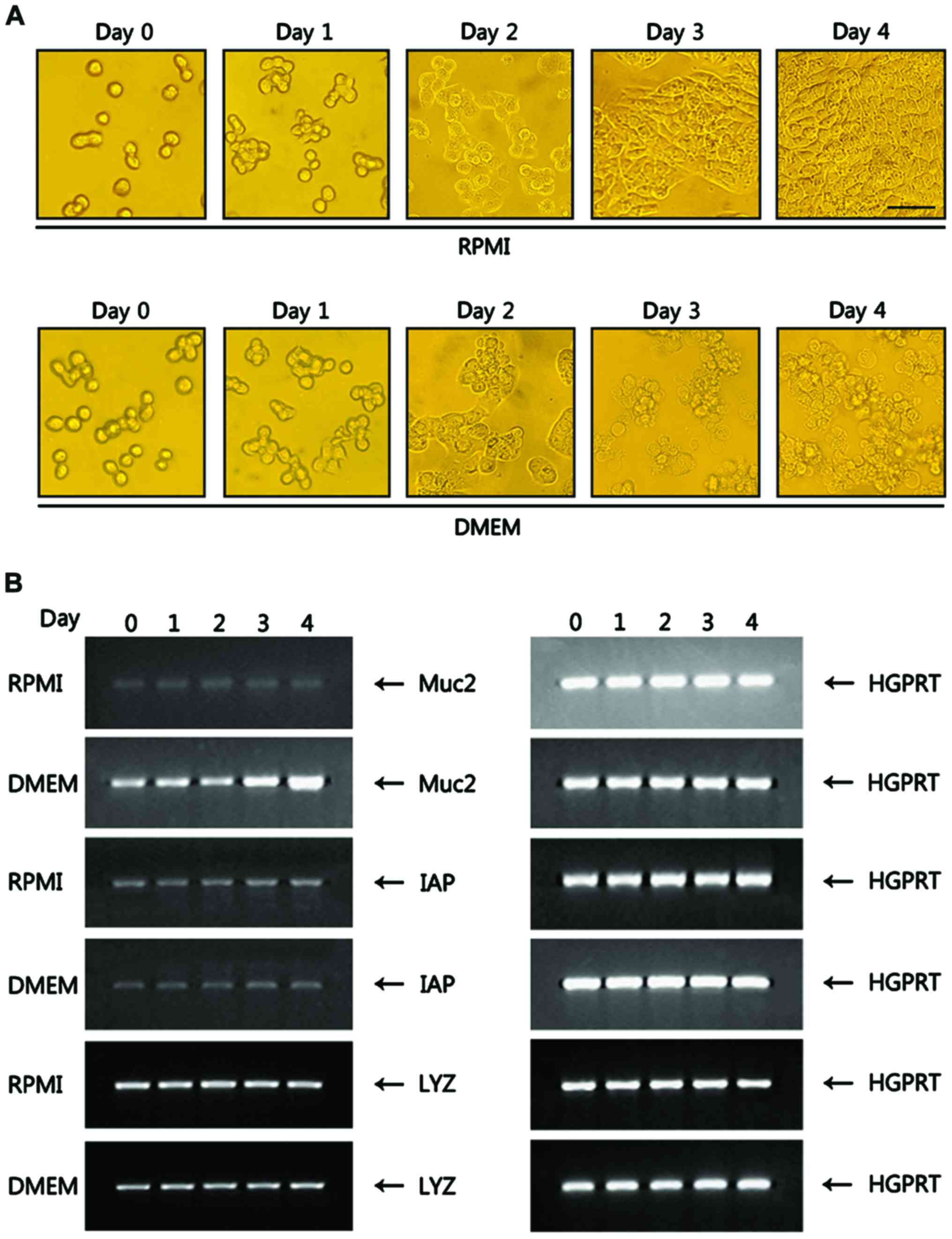
Differentiation of goblet cells from
HT-29 cells is induced in DMEM
HT-29 cells, which are routinely maintained in RPMI
or MEM, grow as monolayers (18,19).
HT-29 cells seeded in a 24-well plate at a density of
1×105 cells/well in RPMI were grown up to 4 days until
achieving >90% confluency in culture dishes. HT-29 cells in RPMI
did not show any morphological changes during the culture period
and remained as monolayers (Fig.
1A). When HT-29 cells were cultured in DMEM, the cells began to
show morphological changes on day 3, including fine blebbings of
secretion material at the cell surface, which is characteristic of
goblet cells. At day 4, most of the HT-29 cells appeared to
differentiate into goblet cells (Fig.
1A). To verify goblet cell differentiation, RNA prepared from
HT-29 cells grown in RPMI and DMEM was analyzed for Muc2, a
molecular marker of goblet cells. Muc2 expression, which was of a
background level in HT-29 cells grown in RPMI, was greatly
increased in HT-29 cells cultured in DMEM, ~5.0-fold at day 4
compared with RPMI (Fig. 1B).
Intestinal alkaline phosphatase (IAP), a marker of enterocytes, was
weakly expressed in both RPMI and DMEM without any change during
the culture period. HT-29 cells are able to differentiate into
Paneth cells (20). The Paneth cell
differentiation marker lysozyme was expressed at a basal level both
in RPMI and DMEM with no changes in expression during the culture
period (Fig. 1B), suggesting that
the DMEM-induced differentiation of goblet cells from HT-29 cells
is lineage-specific. When we did similar experiments with Caco-2
cells, a human colon carcinoma cell line, we observed fine
blebbings of secretion material at the cell surface when cells were
grown in DMEM, but not in RPMI (data not shown).
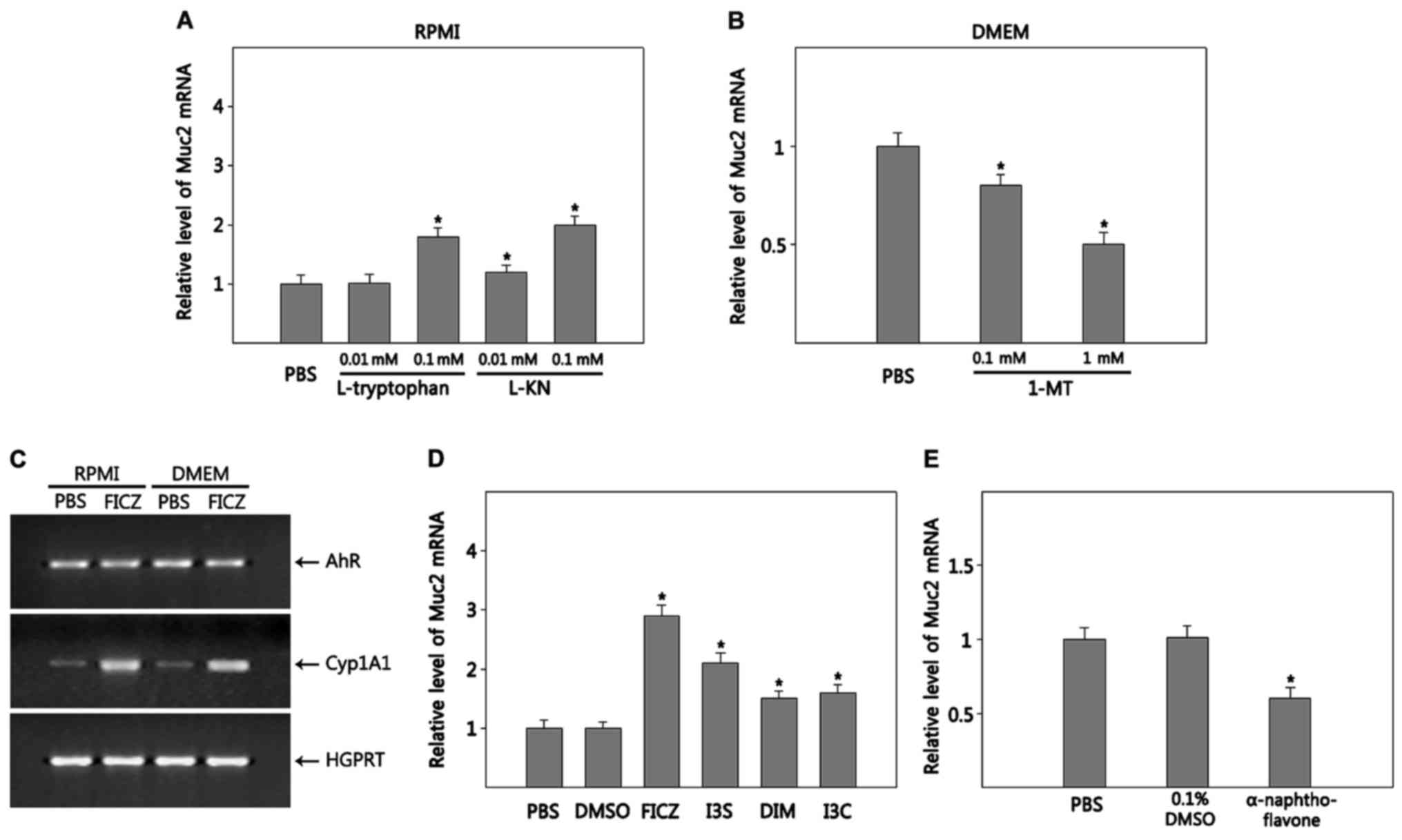
Tryptophan and its metabolite KN
promote the differentiation of goblet cells from HT-29 cells
Tryptophan, which is richer in DMEM compared with
RPMI, is metabolized along 4 pathways converting to many
physiologically important metabolites: i) hydroxylation making
serotonin and melatonin; ii) decaroboxylation producing tryptamine;
iii) transamination producing indolepyruvic acid; and (4) the KN pathway making KN and its
metabolites (21). Thus, we
examined whether tryptophan and its metabolites promote goblet cell
differentiation. HT-29 cells were grown in RPMI supplemented with
L-tryptophan to adjust levels to those in DMEM. To measure the
effects of tryptophan on goblet cell differentiation, we quantified
Muc2 RNA expression. Tryptophan significantly increased Muc2
expression compared with RPMI (~1.8-fold increase at 0.1 mM
tryptophan, P=0.0006) (Fig. 2A),
although its effect was not as dramatic as DMEM (~5-fold increase
in DMEM compared with RPMI, Fig.
1B) in promoting Muc2 expression, suggesting that other factors
in DMEM contribute to goblet cell differentiation. Among the 4
tryptophan degradation pathways, the KN pathway accounts for ~95%
of overall tryptophan degradation (21). IDO-1, which is the first enzyme to
metabolize tryptophan along the KN pathway, is expressed in HT-29
cells (22). Thus, we examined
whether KN stimulates goblet cell differentiation. HT-29 cells
cultured in RPMI supplemented with L-KN showed enhanced expression
of Muc2 dose-dependently; ~1.2- (P=0.016) and 2.0-fold (P=0.0005)
at 0.1 and 1 mM, respectively, compared with RPMI alone (Fig. 2A). KN was as effective as
tryptophane but not as potent as DMEM in driving goblet cell
differentiation. Next, we tested whether inhibition of KN synthesis
suppresses goblet cell differentiation. To do this, HT-29 cells
were grown in DMEM supplemented with 1-MT. Muc2 expression
gradually decreased in DMEM supplemented with 1-MT as the
concentration of 1-MT increased (P=0.017 for 0.1 mM; P=0.002 for 1
mM) (Fig. 2B). Thus, it appears
that KN is involved in the goblet cell differentiation of HT-29
cells.
AhR activation promotes the
differentiation of goblet cells from HT-29 cells
As described earlier in the ‘Introduction’ section,
KN is a ligand of AhR. KN activates AhR in various tumors (23). Thus, it is possible that KN promotes
goblet cell differentiation via activating AhR. To test this, we
first examined whether HT-29 cells express and activate AhR. HT-29
cells grown in both RPMI and DMEM constitutively expressed AhR
(Fig. 2C). Next, we tested whether
AhR is functional in HT-29 cells by examining AhR ligand-induced
expression of Cyp1A1, a target gene of AhR. Expression of Cyp1A1
was induced in HT-29 cells cultured in both RPMI and DMEM by the
addition of FICZ (Fig. 2C). HT-29
cells were cultured in RPMI in the presence of various AhR ligands,
including FICZ, I3S, DIM and I3C (24). Muc2 expression was increased by all
the AhR ligands tested with FICZ being the most effective
(P=0.000025 for FICZ; P=0.0002 for I3S; P=0.008 for DIM; P=0.003
for I3C) (Fig. 2D), although AhR
activation itself was not as effective as DMEM in promoting Muc2
expression (Fig. 1B). In addition,
Muc2 expression in HT-29 cells grown in DMEM was reduced by
α-naphthoflavone (P=0.008), an AhR antagonist, compared with PBS or
vector control (0.1% DMSO), suggesting that DMSO-induced goblet
cell differentiation is partly mediated by AhR (Fig. 2E).
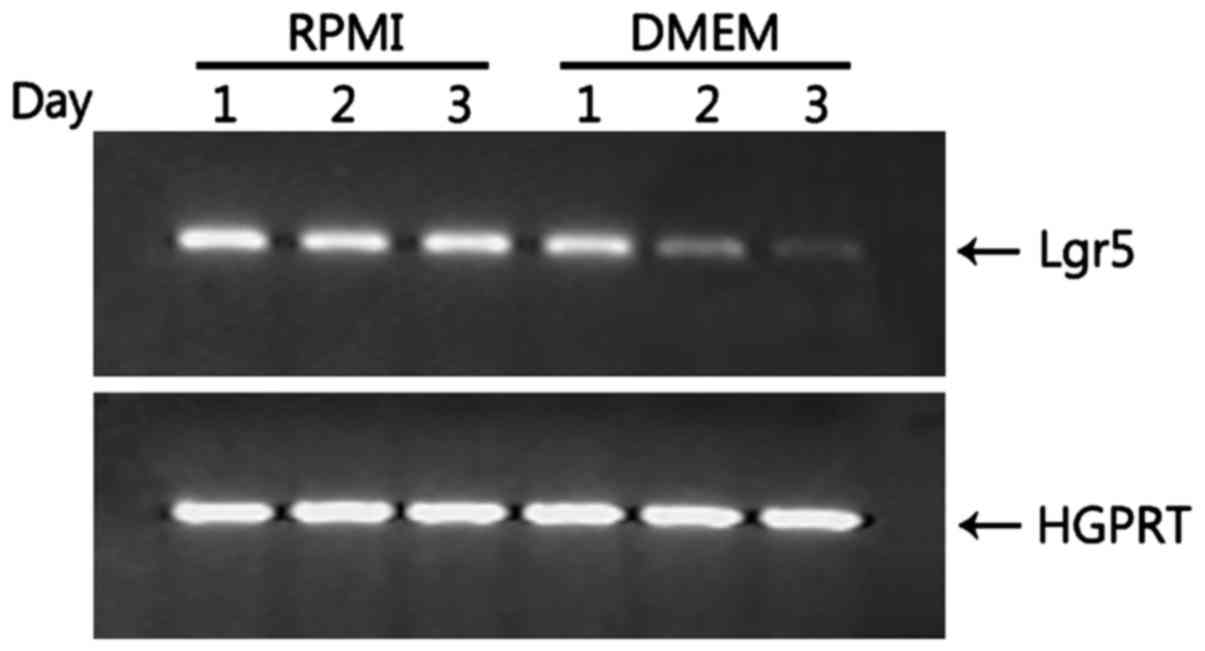
Lgr5 expression is greatly reduced in
HT-29 cells grown in DMEM
Lgr5, a marker for intestinal stem cells, is also
required for the maintenance and proliferation of colorectal cancer
cells via the Wnt3/β-catenin signaling pathway (25,26).
HT-29 cells grown in RPMI expressed Lgr5 (Fig. 3). Thus, we examined whether Lgr5
expression is modulated by changes in the medium. Once HT-29 cells
were cultured with DMEM, they started to show reduced expression of
Lgr5, barely expressing Lgr5 at day 3 (Fig. 3), suggesting that Lgr5 is required
for proliferation of HT-29 cells so that the reduced expression of
Lgr5 may allow HT-29 cells to differentiate into Lgr5-intestinal
lineages such as goblet cells.
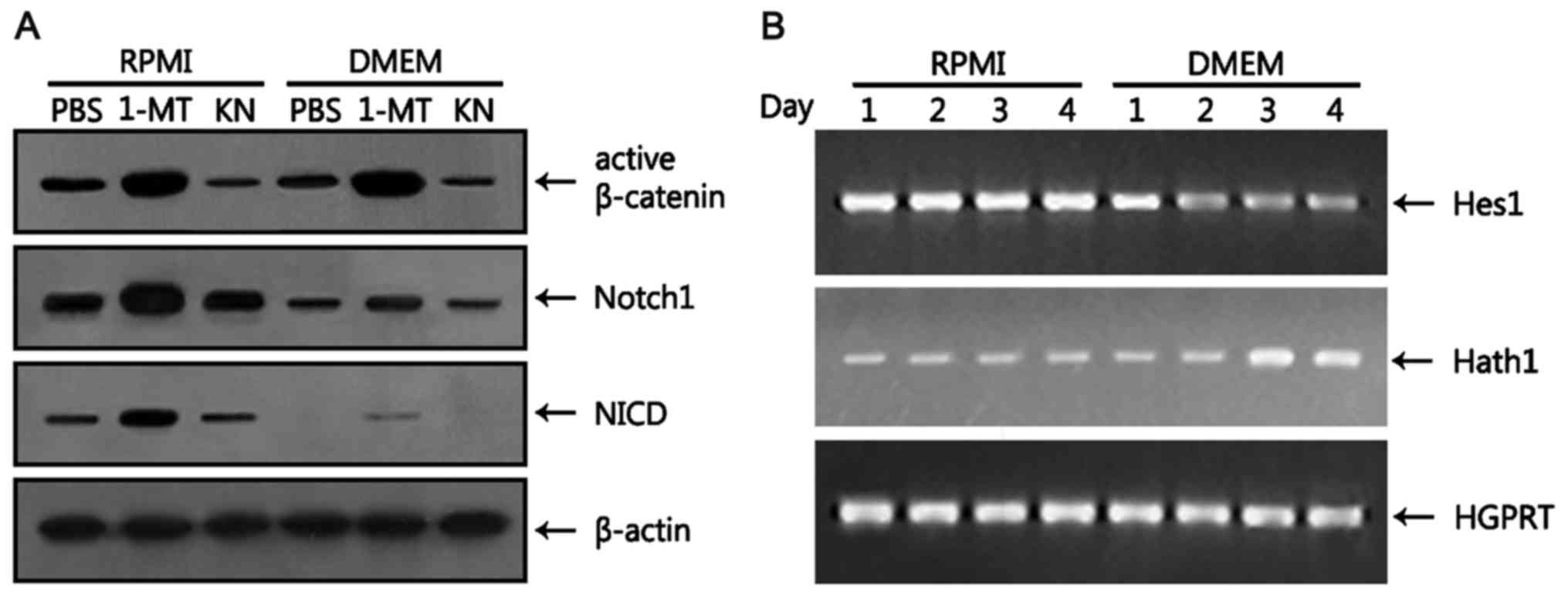
Wnt and Notch signaling pathways are
modulated in HT-29 cells grown in DMEM
The Wnt3/β-catenin signaling pathway, which is
necessary for the proliferation of colorectal cancer cells as
described earlier, also plays a key role in the proliferation of
intestinal stem cells (27,28). Notch is also involved in the
proliferation and differentiation of intestinal stem cells. In the
intestine, Notch has dual functions in the crypt: it directs
proliferation of Lgr5+ stem cells in concert with Wnt
(29) and induces enterocyte
differentiation when Wnt signals are low (30). Inhibition of the Notch pathway
results in a massive increase in goblet cells whereas its
activation results in goblet cell depletion (30). Thus, we examined whether the Wnt and
Notch pathways are modulated by medium changes. Canonical Wnt
signaling leads to stabilization of cytoplasmic β-catenin through
the inhibition of phosphorylation events that otherwise target the
protein for proteosomal degradation (31). In mice, the Wnt signals exhibit a
spatial gradient along the crypt-villus axis, with the highest
activity in proliferating crypt and the lowest activity in
differentiating villus where goblet cells reside (32). To ascertain the effects of DMEM on
Wnt activity, we measured the level of non-phosphorylated (active)
(Ser33/37/Thr41) β-catenin at earlier time points when goblet cell
differentiation was not yet completed. At day 2, there was no
difference between the levels of active β-catenin in HT-29 cells
grown in RPMI and DMEM (Fig. 4A).
The levels of active β-catenin were increased ~2-fold irrespective
of media when 1-MT was added to the culture while being decreased
~2-fold by the addition of KN (Fig.
4A). These results suggest that the Wnt signal, which is active
in HT-29 cells, is regulated by tryptophan metabolism.
In mouse intestine, Notch1 and Notch2 are
specifically expressed in crypt stem cells (33). The Notch pathway is activated by a
cascade of proteolytic cleavages, resulting in the translocation of
NICD into the nucleus to induce target gene transcription via
interactions with other DNA binding proteins (34). We first examined the levels of
unprocessed Notch1 protein at day 2. The level of Notch1 was
markedly reduced (~2.5-fold) in HT-29 cells grown in DMEM compared
with RPMI (Fig. 4A). The level of
Notch1 was increased by the addition of 1-MT in both culture media,
although the effects of 1-MT were more pronounced in RPMI than in
DMEM. However, addition of KN to both culture media did not change
the level of Notch1. Then, the level of NICD was measured 2 h after
the addition of Jagged-1 peptide. NICD, which was detectable in
HT-29 cells grown in RPMI, was not detected when cells were
cultured in DMEM (Fig. 4A). When
1-MT was added to the culture media, the level of NICD was
increased compared with PBS control in both culture media, although
the level of NICD in HT-29 cells grown in RPMI was significantly
higher than in DMEM. KN showed little effect on the amount of NICD
in HT-29 cells grown in both media treated with Jagged-1 peptide.
Hes1, which is a known Notch target gene (35), is a transcriptional repressor which
represses transcription of the transcription factor Math1 (Hath1 in
humans) (36). Intestinal Math1
expression is required for commitment toward the secretory lineage,
including goblet and Paneth cells (37). Thus, we performed kinetic analyses
of the expression of Hes1 and Hath1 in HT-29 cells. The level of
Hes1, which remained constant in HT-29 cells grown in RPMI, was
reduced 1 day after culturing in DMEM, remaining unchanged
thereafter (Fig. 4B). In contrast,
the level of Hath1, which was constant in HT-29 cells cultured in
RPMI, was increased 2 days after culture in DMEM. These results
suggest that reduced activation of Notch1 resulting from the
decreased expression of Notch1 in HT-29 cells grown in DMEM
promotes the differentiation of goblet cells.
Discussion
Although the Notch pathway plays an important role
in determining lineage decisions between absorptive enterocyte and
secretory cell differentiation (29,30),
there are subtle differences in signaling required for the
differentiation of Paneth, goblet and enteroendocrine cells: Paneth
cells require a high Wnt signal along with Lgr4 while
differentiation of goblet and enteroendocrine cell needs low and no
Wnt activity, respectively (29,38,39).
In the present study, we demonstrated that DMEM induced goblet cell
differentiation from HT-29 colon cancer stem cells in a
lineage-specific manner. Tryptophan metabolism partially mediated
the activity of DMEM via regulating Wnt, Notch and AhR signals.
Tryptophan, which is richer in DMEM than in RPMI,
functions as the precursor to many substances, including KN, KA and
melatonin (21). KN, the first
breakdown product of tryptophan mediated by
indoleamine-2,3-dioxygenases 1 and 2 (IDO1/2) or
tryptophan-2,3-dioxygenase (TDO), activates the aryl hydrocarbon
receptor (AhR), a ligand-activated transcription factor, in various
tumors (16). KN shows contrasting
effects on cell growth depending on cell type; it inhibits growth
of lymphoid cell lines (40) while
promoting proliferation of non-lymphoid tumor cell lines (22). In the present study, we observed
that KN reduced β-catenin expression, which was increased by IDO-1
inhibitor 1-MT. How KN regulates β-catenin expression is not known.
However, as a ligand-activated transcriptional factor, AhR has a
role as a ligand-dependent E3 ubiquitin ligase of certain nuclear
receptors such as the androgen and estrogen receptors (41). It was recently shown that the AhR E3
ubiquitin ligase activated by natural ligands, such as indole
derivatives that are converted from dietary tryptophan by
intestinal microbes, degraded β-catenin, suppressing intestinal
carcinogenesis (42). As presented
earlier in the present study, AhR functions in HT-29 cells
(Fig. 2C). Thus, it is possible
that β-catenin degradation is mediated by activation of AhR by KN.
Alternatively, KN may reduce β-catenin expression via its
metabolites. KA, a metabolite of the KN pathway of tryptophan
degradation, is synthesized in colon-derived normal and cancer
cells (43). KA inhibited
proliferation of several cancer cell lines including colon cancer
cell lines by inhibiting the activation of Akt, ERK1/2 and p38
kinase (43). KA also works as an
AhR ligand (17). Whether KA
mediates goblet cell differentiation remains to be elucidated.
In the present study, Notch1 expression was reduced
in HT-29 cells grown in DMEM compared with RPMI. In addition, 1-MT
increased Notch 1 expression in both media. How DMEM or IDO-1
activity reduces Notch1 expression is not known. AhR promotes
expression of Notch receptors and Notch1 in the testes and NKp46
cells, respectively (44,45), making it unlikely that
transcriptional regulation of Notch1 is mediated by KN. As for
β-catenin degradation by AhR, Notch1 could be degraded by AhR
activation, which remains to be tested. Alternatively, melatonin
could be involved in this event. In the intestine, tryptophan is
metabolized to KN and melatonin (46–48).
Melatonin, which is synthesized from tryptophan by enzymes
N-acetyltransferase and acetylserotonin methyltransferase,
is produced in many tissues, including the gastrointestinal tract
as well as in the pineal gland (47,48).
Melatonin has been shown to inhibit proliferation of many types of
cancer cells such as glioblastoma cells, colon cancer cells, and
breast cancer cells (49–51). Melatonin exerts its
anti-proliferative activity by inactivating FoxO-1 and NF-κB
transcription factors, downregulating myosin light chain kinase
expression through cross-talk with p38 MAPK, or by suppressing
Notch1 signaling (49,52,53).
It remains to be tested whether melatonin is involved in goblet
cell differentiation by regulating Notch1 expression.
Notch and Wnt signals interact to regulate target
gene expression (54,55). NICD binds directly to β-catenin and
translocates to the nucleus, where it forms a transcriptional
activator complex with the protein CSL to transcribe target genes
such as Hes1. In the present study, it was observed that Hes1
expression was reduced whereas Hath1 expression was increased in
HT-29 cells grown in DMEM. Notch and Wnt β-catenin signaling can
interact to induce expression of Hes1 by forming a transcriptional
complex of NICD and β-catenin (54). Thus, it is possible that reduced
formation of the transcriptional complex of NICD and β-catenin in
DMEM downregulates the expression of Hes1 which represses Hath1
expression, resulting in the promotion of goblet cell
differentiation.
In the present study, Lgr5 expression gradually
decreased, being almost not detected at day 4 when most of HT-29
cells appeared to be differentiated into goblet cells.
Leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5),
which is a receptor for R-spondins and participates in canonical
Wnt signaling (56), is expressed
in crypt base columnar cells and functions as a stem cell marker
(57). Lgr5 expression is
upregulated by Wnt activity (57).
In addition, Wnt/β-catenin signaling which plays important roles in
the generation of Lgr5+ stem cells is regulated by
nonreceptor tyrosine phosphatase Shp2-mediated MAPK signaling
(20,58). How Lgr5 expression in colon cancer
stem cells is regulated by tryptophan metabolism remains to be
illustrated.
AhR is a ligand-activated transcription factor that
belongs to the basic region-helix-loop-helix (bHLH) superfamily of
DNA binding proteins (59).
However, environmental toxicants, such as
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a number of
structurally diverse low-molecular-weight chemicals, including
indoles and tryptophan metabolites, have been identified as
naturally occurring exogenous and endogenous AhR ligands (60). AhR regulates proliferation and
differentiation of various stem cells. Hematopoietic stem cells
(HSCs) from AhR-knockout mice were hyper-proliferative and had an
altered cell cycle (61). TCDD and
FICZ inhibited proliferation of stem cells derived from the apical
papilla (SCAPs) and HL-60 myeloblastic leukemia cells,
respectively, while increasing proliferation of rodent hepatic stem
cells (62–64). It was previously reported that FICZ
and TCDD inhibit the development of intestinal organoids from
crypts or Lgr5+ stem cells in vitro (65). In the present study, AhR activation
partially induced goblet cell differentiation. How AhR induces
goblet cell differentiation is unknown. It may directly or
indirectly transcriptionally regulate the expression of Notch1,
Hes1 or Hath1. Alternatively, it works by interacting with Notch
and Wnt signals as described earlier.
Although tryptophan and KN induced goblet cell
differentiation, they were not as effective as DMEM, which suggests
that factors other than tryptophan metabolites could contribute to
goblet cell differentiation. FICZ, which can be produced from
tryptophan in light-exposed media, could be partially responsible
for goblet cell differentiation (1). Other amino acids may contribute to
goblet cell differentiation. Self-renewal and differentiation of
mouse embryonic stem cells are critically dependent on the
metabolism of proline, threonine and methionine, which works as
signal molecules (proline) or precursors for donor molecules used
in histone methylation and acetylation (threonine and methionine)
(66). Histidine, tyrosine and
phenylalanine could generate precursors for AhR ligands (18).
In conclusion, the results obtained in the present
study provide evidence that tryptophan metabolites induce the
goblet cell differentiation of colon cancer stem cells by
regulating the Wnt, Notch and AhR signaling pathways. The present
findings may provide clues to understanding goblet cell
differentiation from intestinal crypts. The following questions
remain to be answered. i) Are amino acids other than tryptophan
also involved in goblet cell differentiation? ii) How is Notch1
expression regulated? iii) Does DMEM also regulate the
differentiation of other stem cells?
Acknowledgements
Not applicable.
Funding
The present study was supported by Changwon National
University grants (2017–2018).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
JHP designed research; JML, EJL, DJK and WBH
performed research and JHP, JML, EJL, DJK and WBH analyzed and
interpreted data. JML, EJL, DJK and WBH were involved in drafting
the manuscript and JHP revised it. All authors approved the final
version to be published and agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AhR
|
aryl hydrocarbon receptor
|
|
KN
|
kynurenine
|
|
1-MT
|
1-methyl-L-tryptophan
|
|
IDO-1
|
indoleamine 2,3 dioxygenase-1
|
|
NICD
|
Notch intracytoplasmic domain
|
|
I3S
|
indoxyl 3-sulfate
|
|
I3C
|
indole-3-carbinol
|
|
DIM
|
3,3-diindolylmethane (DIM)
|
|
FICZ
|
6-formylindolo[3,2-b]carbazole.
|
References
|
1
|
Veldhoen M, Hirota K, Christensen J,
O'Garra A and Stockinger B: Natural agonists for aryl hydrocarbon
receptor in culture medium are essential for optimal
differentiation of Th17 T cells. J Exp Med. 206:43–49. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ilchmann A, Krause M, Heilmann M, Burgdorf
S, Vieths S and Toda M: Impact of culture medium on maturation of
bone marrow-derived murine dendritic cells via the aryl hydrocarbon
receptor. Mol Immunol. 51:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu KL, Chang SH, Manousakas I, Huang HH,
Teong B, Chuang CW and Kuo SM: Effects of culturing media on
hepatocytes differentiation using Volvox sphere as co-culturing
vehicle. Biochem Biophys Res Commun. 458:620–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sidney LE, Branch MJ, Dua HS and Hopkinson
A: Effect of culture medium on propagation and phenotype of corneal
stroma-derived stem cells. Cytotherapy. 17:1706–1722. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SW, Kim SJ, Langley RR and Fidler IJ:
Modulation of the cancer cell transcriptome by culture media
formulations and cell density. Int J Oncol. 46:2067–2075. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zweibaum A, Pinto M, Chevalier G, Dussaulx
E, Triadou N, Lacroix B, Haffen K, Brun JL and Rousset M:
Enterocytic differentiation of a subpopulation of the human colon
tumor cell line HT-29 selected for growth in sugar-free medium and
its inhibition by glucose. J Cell Physiol. 122:21–29. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chastre E, Emami S, Rosselin G and Gespach
C: Vasoactive intestinal peptide receptor activity and specificity
during enterocyte-like differentiation and retrodifferentiation of
the human colonic cancerous subclone HT29-18. FEBS Lett.
188:197–204. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Velcich A, Palumbo L, Jarry A, Laboisse C,
Racevskis J and Augenlicht L: Patterns of expression of
lineage-specific markers during the in vitro-induced
differentiation of HT29 colon carcinoma cells. Cell Growth Differ.
6:749–757. 1995.PubMed/NCBI
|
|
9
|
Huet C, Sahuquillo-Merino C, Coudrier E
and Louvard D: Absorptive and mucus-secreting subclones isolated
from a multipotent intestinal cell line (HT-29) provide new models
for cell polarity and terminal differentiation. J Cell Biol.
105:345–357. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boultwood J, Yip BH, Vuppusetty C,
Pellagatti A and Wainscoat JS: Activation of the mTOR pathway by
the amino acid L-leucine in the 5q-syndrome and other
ribosomopathies. Adv Biol Regul. 53:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma X, Han M, Li D, Hu S, Gilbreath KR,
Bazer FW and Wu G: L-Arginine promotes protein synthesis and cell
growth in brown adipocyte precursor cells via the mTOR signal
pathway. Amino Acids. 49:957–964. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai Y, Sun Z, Zhang J, Kang K, Chen J and
Zhang W: Activation of the TOR signalling pathway by glutamine
regulates insect fecundity. Sci Rep. 5:106942015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swamy M, Pathak S, Grzes KM, Damerow S,
Sinclair LV, van Aalten DM and Cantrell DA: Glucose and glutamine
fuel protein O-GlcNAcylation to control T cell self-renewal and
malignancy. Nat Immunol. 17:712–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen G and Wang J: Threonine metabolism
and embryonic stem cell self-renewal. Curr Opin Clin Nutr Metab
Care. 17:80–85. 2014.PubMed/NCBI
|
|
15
|
Green CR, Wallace M, Divakaruni AS,
Phillips SA, Murphy AN, Ciaraldi TP and Metallo CM: Branched-chain
amino acid catabolism fuels adipocyte differentiation and
lipogenesis. Nat Chem Biol. 12:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rannug U, Rannug A, Sjöberg U, Li H,
Westerholm R and Bergman J: Structure elucidation of two
tryptophan-derived, high affinity Ah receptor ligands. Chem Biol.
2:841–845. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DiNatale BC, Murray IA, Schroeder JC,
Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ and Perdew GH:
Kynurenic acid is a potent endogenous aryl hydrocarbon receptor
ligand that synergistically induces interleukin-6 in the presence
of inflammatory signaling. Toxicol Sci. 115:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paine AJ and Francis JE: The induction of
benzo[a]pyrene-3-mono-oxygenase by singlet oxygen in liver cell
culture is mediated by oxidation products of histidine. Chem Biol
Interact. 30:343–353. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi HJ, Kim J, Park SH, Do KH, Yang H and
Moon Y: Pro-inflammatory NF-κB and early growth response gene 1
regulate epithelial barrier disruption by food additive carrageenan
in human intestinal epithelial cells. Toxicol Lett. 211:289–295.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heuberger J, Kosel F, Qi J, Grossmann KS,
Rajewsky K and Birchmeier W: Shp2/MAPK signaling controls
goblet/paneth cell fate decisions in the intestine. Proc Natl Acad
Sci USA. 111:pp. 3472–3477. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Badawy AA: Tryptophan metabolism in
alcoholism. Nutr Res Rev. 15:123–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thaker AI, Rao MS, Bishnupuri KS, Kerr TA,
Foster L, Marinshaw JM, Newberry RD, Stenson WF and Ciorba MA: IDO1
metabolites activate β-catenin signaling to promote cancer cell
proliferation and colon tumorigenesis in mice. Gastroenterology.
145:416–25.e1, 4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Opitz CA, Litzenburger UM, Sahm F, Ott M,
Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller
M, et al: An endogenous tumour-promoting ligand of the human aryl
hydrocarbon receptor. Nature. 478:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen LP and Bradfield CA: The search for
endogenous activators of the aryl hydrocarbon receptor. Chem Res
Toxicol. 21:102–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Wei B, Han X, Zheng Z, Huang J,
Liu J, Huang Y and Wei H: LGR5 is required for the maintenance of
spheroid-derived colon cancer stem cells. Int J Mol Med. 34:35–42.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin YU, Wu T, Yao Q, Zi S, Cui L, Yang M
and Li J: LGR5 promotes the proliferation of colorectal cancer
cells via the Wnt/β-catenin signaling pathway. Oncol Lett.
9:2859–2863. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pećina-Slaus N: Wnt signal transduction
pathway and apoptosis: A review. Cancer Cell Int. 10:222010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fre S, Huyghe M, Mourikis P, Robine S,
Louvard D and Artavanis-Tsakonas S: Notch signals control the fate
of immature progenitor cells in the intestine. Nature. 435:964–968.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Es JH, van Gijn ME, Riccio O, van den
Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ,
Radtke F, et al: Notch/gamma-secretase inhibition turns
proliferative cells in intestinal crypts and adenomas into goblet
cells. Nature. 435:959–963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vries RG, Huch M and Clevers H: Stem cells
and cancer of the stomach and intestine. Mol Oncol. 4:373–384.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fre S, Hannezo E, Sale S, Huyghe M, Lafkas
D, Kissel H, Louvi A, Greve J, Louvard D and Artavanis-Tsakonas S:
Notch lineages and activity in intestinal stem cells determined by
a new set of knock-in mice. PLoS One. 6:e257852011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohtsuka T, Ishibashi M, Gradwohl G,
Nakanishi S, Guillemot F and Kageyama R: Hes1 and Hes5 as Notch
effectors in mammalian neuronal differentiation. EMBO J.
18:2196–2207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jensen J, Pedersen EE, Galante P, Hald J,
Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P and Madsen
OD: Control of endodermal endocrine development by Hes-1. Nat
Genet. 24:36–44. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Q, Bermingham NA, Finegold MJ and
Zoghbi HY: Requirement of Math1 for secretory cell lineage
commitment in the mouse intestine. Science. 294:2155–2158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sansom OJ, Reed KR, Hayes AJ, Ireland H,
Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H,
Nathke IS, et al: Loss of Apc in vivo immediately perturbs Wnt
signaling, differentiation, and migration. Genes Dev. 18:1385–1390.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Giel-Moloney M, Rindi G and Leiter
AB: Enteroendocrine precursors differentiate independently of Wnt
and form serotonin expressing adenomas in response to active
beta-catenin. Proc Natl Acad Sci USA. 104:pp. 11328–11333. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suwa S, Kasubata A, Kato M, Iida M,
Watanabe K, Miura O and Fukuda T: The tryptophan derivative,
tranilast, and conditioned medium with indoleamine
2,3-dioxygenase-expressing cells inhibit the proliferation of
lymphoid malignancies. Int J Oncol. 46:1369–1376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohtake F, Baba A, Takada I, Okada M,
Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, et
al: Dioxin receptor is a ligand-dependent E3 ubiquitin ligase.
Nature. 446:562–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kawajiri K, Kobayashi Y, Ohtake F, Ikuta
T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T,
Hirokawa T, et al: Aryl hydrocarbon receptor suppresses intestinal
carcinogenesis in ApcMin/+ mice with natural ligands.
Proc Natl Acad Sci USA. 106:pp. 13481–13486. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Walczak K, Dąbrowski W, Langner E, Zgrajka
W, Piłat J, Kocki T, Rzeski W and Turski WA: Kynurenic acid
synthesis and kynurenine aminotransferases expression in colon
derived normal and cancer cells. Scand J Gastroenterol. 46:903–912.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang B, Butler R, Miao Y, Dai Y, Wu W, Su
W, Fujii-Kuriyama Y, Warner M and Gustafsson JÅ: Dysregulation of
Notch and ERα signaling in AhR−/− male mice. Proc Natl
Acad Sci USA. 113:pp. 11883–11888. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee JS, Cella M, McDonald KG, Garlanda C,
Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry
RD, et al: AHR drives the development of gut ILC22 cells and
postnatal lymphoid tissues via pathways dependent on and
independent of Notch. Nat Immunol. 13:144–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Badawy AA: Kynurenine pathway of
tryptophan metabolism: Regulatory and functional aspects. Int J
Tryptophan Res. 10:11786469176919382017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Poon AM, Mak AS and Luk HT: Melatonin and
2[125I]iodomelatonin binding sites in the human colon. Endocr Res.
22:77–94. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vician M, Zeman M, Herichová I, Juráni M,
Blazícek P and Matis P: Melatonin content in plasma and large
intestine of patients with colorectal carcinoma before and after
surgery. J Pineal Res. 27:164–169. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng X, Pang B, Gu G, Gao T, Zhang R,
Pang Q and Liu Q: Melatonin inhibits glioblastoma stem-like cells
through suppression of EZH2-NOTCH1 signaling axis. Int J Biol Sci.
13:245–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
García-Navarro A, González-Puga C, Escames
G, López LC, López A, López-Cantarero M, Camacho E, Espinosa A,
Gallo MA and Acuña-Castroviejo D: Cellular mechanisms involved in
the melatonin inhibition of HT-29 human colon cancer cell
proliferation in culture. J Pineal Res. 43:195–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F
and Wei W: Melatonin sensitizes human hepatoma cells to endoplasmic
reticulum stress-induced apoptosis. J Pineal Res. 52:322–331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
León J, Casado J, Jiménez Ruiz SM, Zurita
MS, González-Puga C, Rejón JD, Gila A, Muñoz de Rueda P, Pavón EJ,
Reiter RJ, et al: Melatonin reduces endothelin-1 expression and
secretion in colon cancer cells through the inactivation of FoxO-1
and NF-κβ. J Pineal Res. 56:415–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zou DB, Wei X, Hu RL, Yang XP, Zuo L,
Zhang SM, Zhu HQ, Zhou Q, Gui SY and Wang Y: Melatonin inhibits the
migration of colon cancer RKO cells by down-regulating myosin light
chain kinase expression through cross-talk with p38 MAPK. Asian Pac
J Cancer Prev. 16:5835–5842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yamamizu K, Matsunaga T, Uosaki H,
Fukushima H, Katayama S, Hiraoka-Kanie M, Mitani K and Yamashita
JK: Convergence of Notch and beta-catenin signaling induces
arterial fate in vascular progenitors. J Cell Biol. 189:325–338.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Collu GM, Hidalgo-Sastre A, Acar A,
Bayston L, Gildea C, Leverentz MK, Mills CG, Owens TW, Meurette O,
Dorey K, et al: Dishevelled limits Notch signalling through
inhibition of CSL. Development. 139:4405–4415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sato T, van Es JH, Snippert HJ, Stange DE,
Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M
and Clevers H: Paneth cells constitute the niche for Lgr5 stem
cells in intestinal crypts. Nature. 469:415–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ, et al: Identification of stem cells in small intestine
and colon by marker gene Lgr5. Nature. 449:1003–1007. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schuijers J and Clevers H: Adult mammalian
stem cells: The role of Wnt, Lgr5 and R-spondins. EMBO J.
31:2685–2696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Burbach KM, Poland A and Bradfield CA:
Cloning of the Ah-receptor cDNA reveals a distinctive
ligand-activated transcription factor. Proc Natl Acad Sci USA.
89:pp. 8185–8189. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Denison MS and Nagy SR: Activation of the
aryl hydrocarbon receptor by structurally diverse exogenous and
endogenous chemicals. Annu Rev Pharmacol Toxicol. 43:309–334. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gasiewicz TA, Singh KP and Bennett JA: The
Ah receptor in stem cell cycling, regulation, and quiescence. Ann
NY Acad Sci. 1310:44–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bunaciu RP and Yen A: 6-Formylindolo
(3,2-b)carbazole (FICZ) enhances retinoic acid (RA)-induced
differentiation of HL-60 myeloblastic leukemia cells. Mol Cancer.
12:392013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Guo H, Zhang L, Wei K, Zhao J, Wang Y, Jin
F and Xuan K: Exposure to a continuous low dose of
tetrachlorodibenzo-p-dioxin impairs the development of the tooth
root in lactational rats and alters the function of apical
papilla-derived stem cells. Arch Oral Biol. 60:199–207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Harrill JA, Parks BB, Wauthier E, Rowlands
JC, Reid LM and Thomas RS: Lineage-dependent effects of aryl
hydrocarbon receptor agonists contribute to liver tumorigenesis.
Hepatology. 61:548–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Park JH, Choi AJ, Kim SJ, Cheong SW and
Jeong SY: AhR activation by 6-formylindolo[3,2-b]carbazole and
2,3,7,8-tetrachlorodibenzo-p-dioxin inhibit the development of
mouse intestinal epithelial cells. Environ Toxicol Pharmacol.
43:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kilberg MS, Terada N and Shan J: Influence
of amino acid metabolism on embryonic stem cell function and
differentiation. Adv Nutr. 7:780S–789S. 2016. View Article : Google Scholar : PubMed/NCBI
|