Introduction
Colon cancer is one of the most frequently diagnosed
cancers worldwide and has a particularly poor prognosis for
patients (1). Despite the
development of some novel-targeted therapies, chemotherapy to
inhibit and damage cancer cells using chemicals such as cisplatinum
remains one of the most common treatment options for colon cancer.
However, chemotherapy is often indiscriminate, with many
off-targeted toxic effects and is typically only used to treat
colon cancers in the early stages of the disease. During this
period, cancer cells often acquire drug resistance due to increased
exposure to stressful conditions and cytotoxic drugs (2). This resistance is an important
obstacle to treatment that can result in chemotherapy failure in
many cancers, including colon cancer (3). Therefore, the identification of safe
and effective agents that specifically target drug-resistant
cancers, is vital for the future treatment of colon cancer
(4–6).
Due to the inevitable and often rapid development of
drug resistance to colon cancer chemotherapies, there has been some
research investigating the possible underlying mechanisms. For
example, the aberrant activation of the Mst/Yap-signaling pathway
has been revealed to correlate with drug tolerance acquired during
the treatment of colon cancer patients using cisplatin (DDP) and
erlotinib, an epidermal growth factor receptor (EGFR) inhibitor
(7). However, the role of the
Mst/Yap pathway in the development of drug resistance is poorly
understood in human colon cancers. Functionally, the Mst/Yap
pathway is highly conserved in both humans and Drosophila
and has a key role in regulating growth (8,9). The
tumor suppressor mercaptopyruvate sulfurtransferase (MPST or MST)
and a subsequent kinase cascade, act to negatively regulate YAP, an
oncoprotein involved in cell growth and survival that functions by
transcriptionally regulating various downstream target genes
(10). MST is also one of the core
suppressor molecules in the Hippo signaling pathway and is
phosphorylated and activated by various upstream signaling
proteins. Salvador family WW domain-containing protein 1 (SAV1 or
WW-45) is another core component of the Hippo signaling pathway and
activated MST combines with SAV1 to phosphorylate and activate the
large tumor suppressor 1 (LATS1) kinase. Activated LATS1 binds with
the MOB kinase activator MOB1 to phosphorylate YAP and this
phosphorylated protein is retained in the cytoplasm through
interactions with the 14–3–3 family of proteins. By preventing
movement to the nucleus, YAP is prevented from combining with other
transcription factors to inactivate target promoters (11–14).
However, without the suppressive functions of MST, unphosphorylated
YAP gathers in the nucleus and interacts with transcriptional
enhancer factor domain (TEAD) transcription factors. This in turn
regulates the Mst/Yap pathway via downstream genes that include
cysteine rich angiogenic inducer 61 (CYR61), connective tissue
growth factor (CTGF), survivin (BIRC5) and cyclin D1 (CCND1)
(15–18).
The chemotherapeutic agent DDP is one of the most
extensively used agents for the treatment of cancer. In 1972, it
became the first metal-based drug to enter clinical trials and was
initially applied in a clinical setting in 1979 (19). DDP is now a gold standard drug used
for the treatment of testicular cancer (for which it has a 90% cure
rate) and also for the treatment of head and neck, cervical,
breast, lung, ovarian, gastric and bladder cancers, among many
others (20,21). DDP exerts its antitumor activity
through its alkylating properties. Once the drug enters the
cytoplasm of a cell, chloride ligands are spontaneously and
sequentially replaced with water molecules due to the fact that the
chloride concentration of the cytoplasm is much lower than that of
the blood. This results in the formation of positively charged
bis-aquated platinum complexes that bind to DNA (22–25).
DDP predominantly forms intra-strand adducts between two adjacent
guanines that are followed by an adjacent guanine and adenine.
These adducts cause the DNA helix to bend by up to 60% towards the
major groove and unwind, inhibiting further DNA replication and
transcription. This ultimately leads to cell death (21,26,27).
However, the continuing clinical success of DDP is hindered by two
major limitations, the development of DDP-resistant cancer cells
and the toxic side-effects of the drug. These mechanisms act in
tandem, so that when cells become resistant to DDP, the subsequent
dose must be increased. This in turn increases the severity of
toxic side-effects. These side-effects are primarily due to the
dose-limiting effects of the drug on neurotoxicity and ototoxicity,
although other common side-effects include severe nausea, vomiting,
gastrotoxicity and myelosuppression (28–31).
To further investigate the role of YAP in cancer
drug resistance and to validate DDP as a colon cancer therapy, we
examined how DDP suppresses the Mst/Yap signaling pathway and the
mechanism through which this leads to the inhibition of colon
carcinoma progression and metastasis. Our data demonstrated that
DDP specifically suppressed the expression of YAP and had various
downstream effects on transcription. In addition, we confirmed that
DDP has the potential to be used as a treatment for colon cancer.
These data improved our understanding of the Mst/Yap pathway and
its role in cancer progression and may lead to improved outcomes
for patients.
Materials and methods
Molecular biology
Flag-tagged YAP constructs were made using
the pcDNA 3.1 vector (Invitrogen, Carlsbad, CA, USA). Sequences
encoding the Flag epitope (DYKDDDDK) were added by PCR through the
replacement of the first Met-encoding codon in the respective cDNA
clones.
Cell culture and transient
transfection
Human colon cancer cells SW620, LoVo and NCM460
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Hyclone
Laboratories, San Angelo, TX, USA) in an incubator, at 37°C under a
mixture of 95% air and 5% CO2. Plasmids were transfected
using polyethylenimine (PEI) reagent, following the manufacturer's
instructions.
Western blot analysis
SW620 and LoVo colon cancer cells were transfected
with the relevant plasmids and cultured for 36 h. For western blot
analysis, the cells were lysed in NP-40 buffer (10 mM Tris pH 7.4,
150 mM NaCl, 1% Triton X-100, 1 mM EDTA pH 8.0, 1 mM EGTA pH 8.0, 1
mM PMSF and 0.5% NP-40) at 25°C for 40 min. The lysates were added
to 5X loading dye and then separated by electrophoresis.
Antibodies
The primary antibodies used in the present study
were rabbit anti-Flag (1:1,000, cat. no. sc-166355; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-pYap (cat. no. 14074)
and anti-Yap (cat. no. 13008) (1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-vimentin (cat. no. ab92547),
anti-E-cadherin (cat. no. ab1416) and anti-cleaved capase-3
(1:1,000, cat. no. ab2302; all from Abcam, Cambridge, UK).
Immunofluorescent staining
To examine the subcellular localization of YAP, the
SW620 cells were exposed to DDP for 36 h, seeded onto coverslips in
a 24-well plate, and left overnight. The cells were then fixed
using 4% formaldehyde for 30 min at 25°C and treated with 2% bovine
serum albumin (BSA) in phosphate-buffered saline (PBS) for 30 min.
The coverslips were incubated with rabbit anti-YAP monoclonal
antibody (Cell Signaling Technology, Inc.) at 1:200 dilution in 3%
BSA. The coverslips were then incubated with an Alexa Fluor 594
(red, cat. no. R37117; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) tagged anti-rabbit monoclonal secondary antibody
at 1:1,000 dilution in 3% BSA. DAPI (3 µg/ml) was added for nuclear
counterstaining. Images were captured with a Zeiss Axio Imager Z1
fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany).
Cell flow cytometry assays
DDP-treated and control cells at 80% confluency were
harvested and fixed with 70% ethanol. These were then stained using
propidium iodide (PI) and the cell cycle stage was assessed by flow
cytometry. To evaluate apoptosis, the cells were cultured at 80%
confluency, trypsinized and stained with a PI/Annexin V Apoptosis
Detection kit (Vazyme, Jiangsu Sheng, China). The data were
collected and analyzed on a BD FACScan flow cytometer using FACS
software (BD Biosciences, San Jose, CA, USA).
RNA isolation and real-time reverse
transcription (RT)-PCR assay
We used TRIzol reagent (TransGen Biotech, Beijing,
China) to isolate total RNA from the samples. RNA was reverse
transcribed into first-strand cDNA using a TransScript All-in-One
First-Strand cDNA Synthesis kit (TransGen Biotech). cDNAs were used
in a real-time RT-PCR assay with the human GAPDH gene as an
internal control. The RT-PCR primers were: E-cadherin forward,
ACCATTAACAGGAACACAGG and reverse, CAGTCACTTTCAGTGTGGTG; YAP
forward, GGACCCCAGACGACTTCCTCAACAG and reverse,
CCTTCCAGTGTGCCAAGGTCCACAT; CYR61 forward, GGTCAAAGTTACCGGGCAGT and
reverse, GGAGGCATCGAATCCCAGC; CTGF forward, AATGCTGCGAGGAGTGGGT and
reverse, CGGCTCTAATCATAGTTGGGTCT; vimentin forward,
CGCCAACTACATCGACAAGGTGC and reverse, CTGGTCCACCTGCCGGCGCAG.
SA-β-gal staining
Senescent cells were detected using a Senescence
β-Galactosidase Staining kit (Beyotime Institute of Biotechnology,
Jiangsu, China), according to the manufacturer's instructions.
Cell Counting Kit-8 (CCK-8)
analysis
For CCK-8 analysis, 100 µl of SW620 cell suspensions
were added to a 96-well plate (5,000 cells/well). The plates were
pre-incubated for 24 h in a humidified incubator at 37°C under 5%
CO2. Subsequently, 10 µl of the test treatments were
added to the test plates. The plates were then incubated for a
further 12 h under the same incubation conditions. Subsequently, 10
µl of CCK-8 solution was added to each well and then the plates
were incubated for 4 h. Finally, the absorbance at 450 nm was
assessed using a microplate reader.
Human colon cancer specimen
collection
All human colon cancer and normal colon tissue
specimens were collected from the Affiliated Hospital of Binzhou
Medical College. The experimental protocol was approved by the
Research Ethics Committee of Binzhou Medical University (approval
no. 2017-014-01 for the human tissues and 2017-05-02 for the mouse
tissues). Written informed consents were obtained from all
patients. A total of nine human colon tumor samples with matched
normal colon samples were used for RT-PCR analysis. Eight colon
cancer samples were used for western blot analysis.
Analysis of publicly available
datasets
To analyze the correlation between the expression
level of YAP and the prognostic outcome of patients,
Kaplan-Meier survival curves of colon tumor patients with low and
high expression of YAP were generated using Kaplan-Meier plotter
(www.kmplot.com/analysis).
Wound healing assays
To assess the effects of DDP on wound healing,
105 cells were seeded onto 6-well plates and left for 1
day before treatment with DDP. These cells were then incubated in
5% CO2 at 37°C for 24 h. A wound was scraped into the
cells using a plastic 200-µl pipette tip and then washed by PBS.
The cells were incubated in DMEM containing 10% FBS with 15 µg/ml
of DDP and various plasmids at different time-points to assess
wound healing.
Transwell migration assays
Transwell migration assays were performed using a
24-well chamber (Costar 3422; Corning Inc., Corning, NY, USA). The
lower and upper chambers were separated by a polycarbonate membrane
(8-µm pore size). SW620 cells (1×105) were seeded into
DMEM without FBS in the upper chamber. DMEM containing 10% FBS was
added to the lower chamber. The cells were allowed to migrate for
36 h at 37°C in a humidified atmosphere containing 5%
CO2. The cells remaining on the upper side of the
membrane were removed using PBS-soaked cotton swabs. The membrane
was then fixed in 4% paraformaldehyde for 20 min at 37°C and then
stained with crystal violet. The cells on the lower side of the
membrane were counted under an Olympus light microscope (Olympus,
Tokyo, Japan).
MTT and CCK-8 assays
Cell viability was determined using
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT)
and CCK-8 assays in 96-well plates in a manner similar to that
above described for the CCK-8 assays. The cells were incubated with
various concentrations of DDP for 48 h, followed by incubation with
MTT and CCK-8 for 4 h. Subsequently, 100 µl isopropanol (in 0.04 N
HCL) was added to dissolve the formazan crystals for the MTT assay.
Absorbance was read at 570 nm using a spectrophotometer (Tecan
Group Ltd., Männedorf, Switzerland). The cell viability was
assessed as relative absorbance compared to a dimethyl sulfoxide
(DMSO)-only control.
In vivo experiments
To assess the in vivo effects of DDP, 3- to
5-week old female BALB/c athymic (NU/NU) nude mice were housed in a
level 2 biosafety laboratory and raised according to the
Institutional Animal Guidelines of Binzhou Medical University. All
animal experiments were carried out with the prior approval of the
Binzhou Medical University Committee on Animal Care. For the
experiments, the mice were injected with 5×106 SW620
cells and randomly divided into two groups (five mice per group)
after the diameter of the xenograft tumors had reached ~5 mm.
Xenograft mice were then intraperitoneally administered either DDP
or PBS three times a week and tumor volume and body weight were
assessed every second day. Tumor volume was estimated as 0.5 ×
a2 × b (where a and b represent tumor short and long
diameter, respectively). The mice were euthanized after 6 weeks of
treatment and the tumors were assessed a final time. Subsequently,
tumor and organ tissues were collected from xenograft mice and
analyzed by immunohistochemistry.
Immunohistochemical analysis
Tumor tissues were fixed in 4% paraformaldehyde
overnight and then embedded in paraffin wax. Four-micrometer thick
sections were and stained using hematoxylin and eosin (H&E) for
histological analysis.
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad
Software Inc., La Jolla, CA, USA) and are presented as the means ±
SD. Two-tailed Student's t-tests were used to compare two groups
and ANOVA with Tukey's post-hoc test was used to compare multiple
groups. P-value<0.05 was considered to indicate a statistically
significant difference which is highlighted in the figures with an
asterisk, while P-values <0.01 are highlighted using two
asterisks and P-values <0.001 are highlighted using three
asterisks.
Results
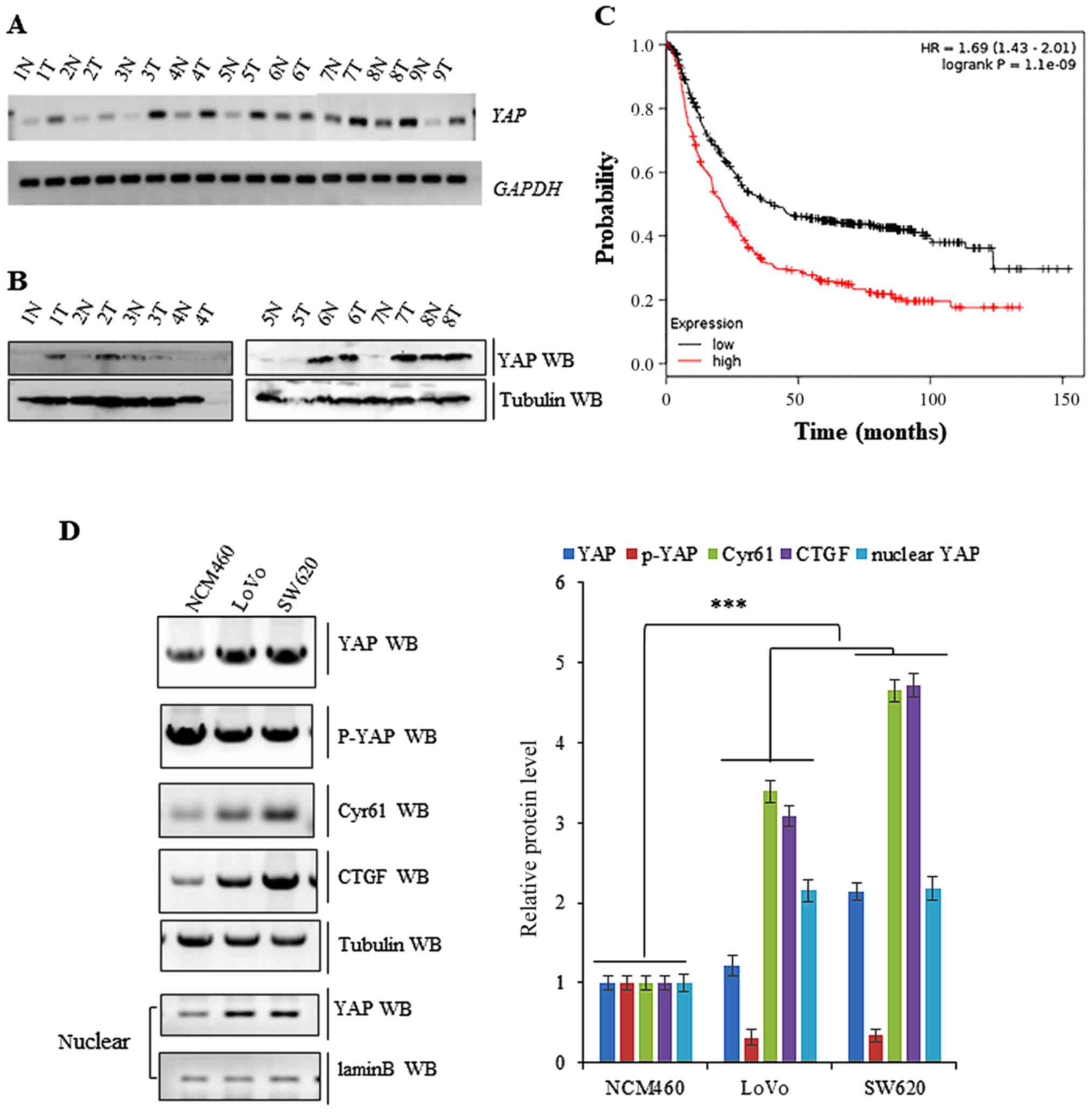
Abnormal activation of YAP in colon
cancer tissues
To determine the expression of YAP in human colon
cancer tissues, we analysed the demographic and tumor
characteristics of 46 patients that met the inclusion criteria for
participating in the study (Table
I). Subsequently, we performed both RT-PCR and western blot
analysis. This demonstrated that YAP mRNA levels were higher
in the nine colon cancer tissues than in matched normal tissue
(Fig. 1A). As displayed in Fig. 1B, the protein levels of YAP were
also higher in eight colon cancer tissues histologically examined
compared to adjacent normal tissue. Publicly available datasets
(http://www.kmplot.com/analysis/index.php?p=service&cancer=gastric
and colon) (32) were filtered and
used to analyze the prognostic correlation between the survival of
colon cancer patients and the expression of YAP. Kaplan-Meier
analyses revealed that YAP protein levels had an inverse
correlation with survival, with high expression associating with
shorter overall survival (OS) (n=1926, P=2.2×10−6)
(Fig. 1C). The expression of YAP
was also higher in SW620 and LoVo colon cancer cell lines relative
to a normal NCM460 cell line. In addition, the amount of activated
YAP found in the nucleus was higher in LoVo and SW620 colon cancer
cells, while inactivated cytoplasmic YAP (p-YAP) was more abundant
in normal NCM460 cells. Furthermore, the activated forms of YAP,
the nuclear localization of YAP, was higher in LoVo and SW620 cells
compared to normal NCM460 cells and the non-activated cytoplasmic
localization form of YAP and p-YAP, was higher in normal NCM460
cells (Fig. 1D).
 | Table I.Patients' demographics and tumor
characteristics. |
Table I.
Patients' demographics and tumor
characteristics.
|
Characteristics | No. of patients
(N=46, %) | P-value |
|---|
| Patients
parameter |
|
|
| Age
(average range, years) | 55 (30–81) | 0.102 |
|
<55 | 20 (43.5) |
|
|
≥55 | 26 (46.5) |
|
|
Sex |
| 0.0781 |
|
Male | 30 (65.2) |
|
|
Female | 16 (34.8) |
|
| Tumor
characteristics |
|
|
| Tumor
size (cm) |
| 0.007 |
|
<4 | 12 (26.1) |
|
|
≥4 | 34 (73.9) |
|
|
Differentiation |
| 0.206 |
|
Poor | 21 (45.7) |
|
|
Well-moderate | 25 (54.3) |
|
| Lymph
node metastasis |
| 0.124 |
|
N− | 36 (78.3) |
|
|
N+ | 10 (21.7) |
|
| Distant
metastasis |
| 0.204 |
|
M− | 28 (60.9) |
|
|
M+ | 18 (39.1) |
|
| Level of YAP |
|
|
| Protein
level | N=20 (Fig. 1A) |
|
|
High | 9 (45.0) | 0.001 |
|
Median | 7 (35.0) | 0.021 |
|
Low | 4 (20.0) | 0.102 |
| mRNA
level | N=15 (Fig. 1B) |
|
|
High | 8 (53.3) | 0.001 |
|
Median | 5 (33.3) | 0.043 |
|
Low | 2 (13.4) | 0.137 |
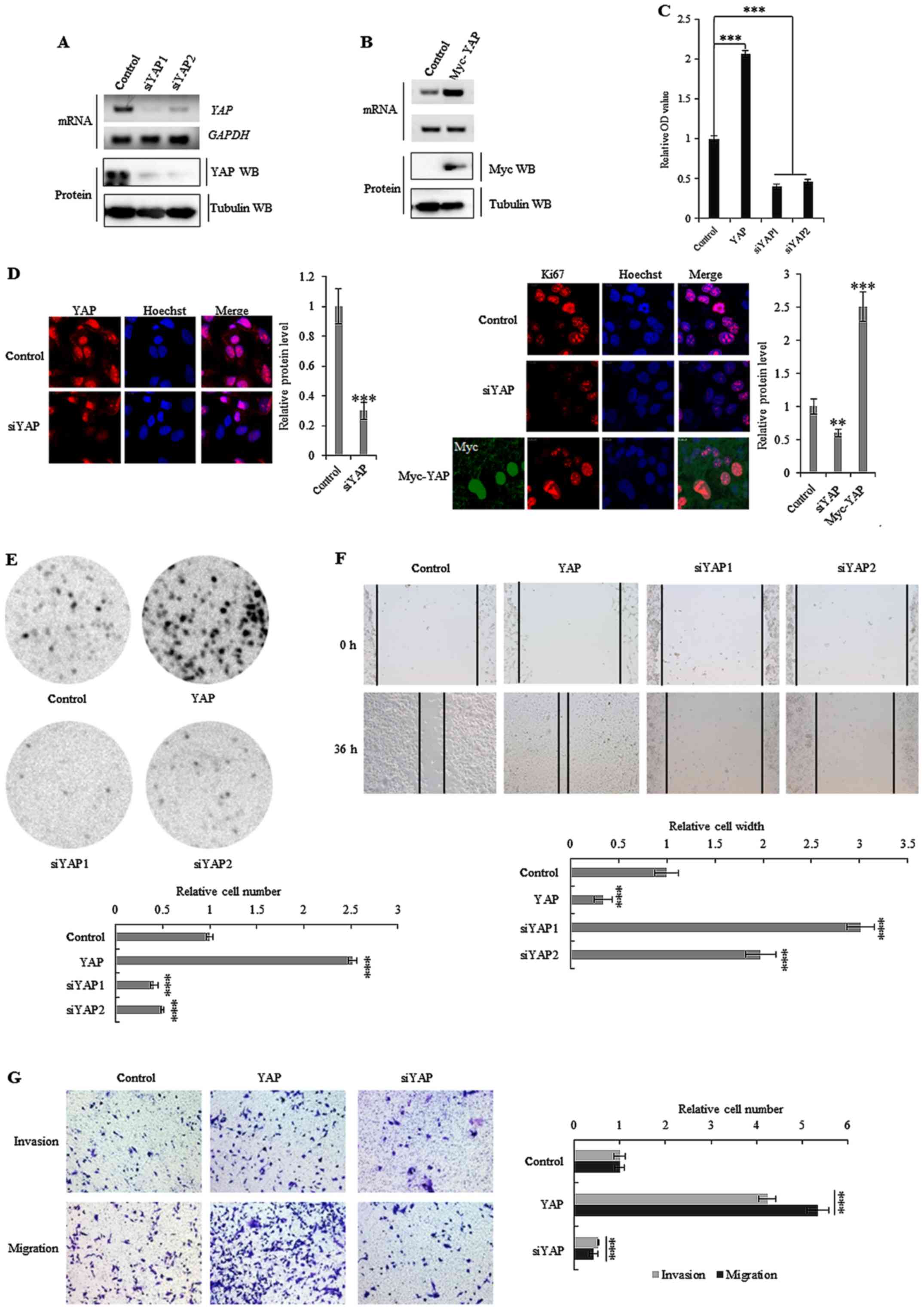
Downregulation of YAP inhibits cell
growth and invasion
Both YAP specific silencing by siRNAs, and stable
ectopic overexpression of YAP, were used to establish whether the
activation of the oncogene affected the initiation, progression and
metastasis of colon cancer (Fig. 2A and
B). Knockdown of YAP was found to decrease cell growth
(Fig. 2C), Ki-67 protein levels
(Fig. 2D), clonal formation
(Fig. 2E) and cell migration
(Fig. 2F) in SW620 cells. The
opposite effect was observed for each of these factors in SW620
cells that overexpressed ectopic YAP (Fig. 2C-F). Notably, depletion of YAP
decreased the invasiveness of SW620 colon cancer cells (Fig. 2G), whereas overexpression increased
cell invasion. These data demonstrated that YAP played an important
role in colon cancer cell growth and invasion.
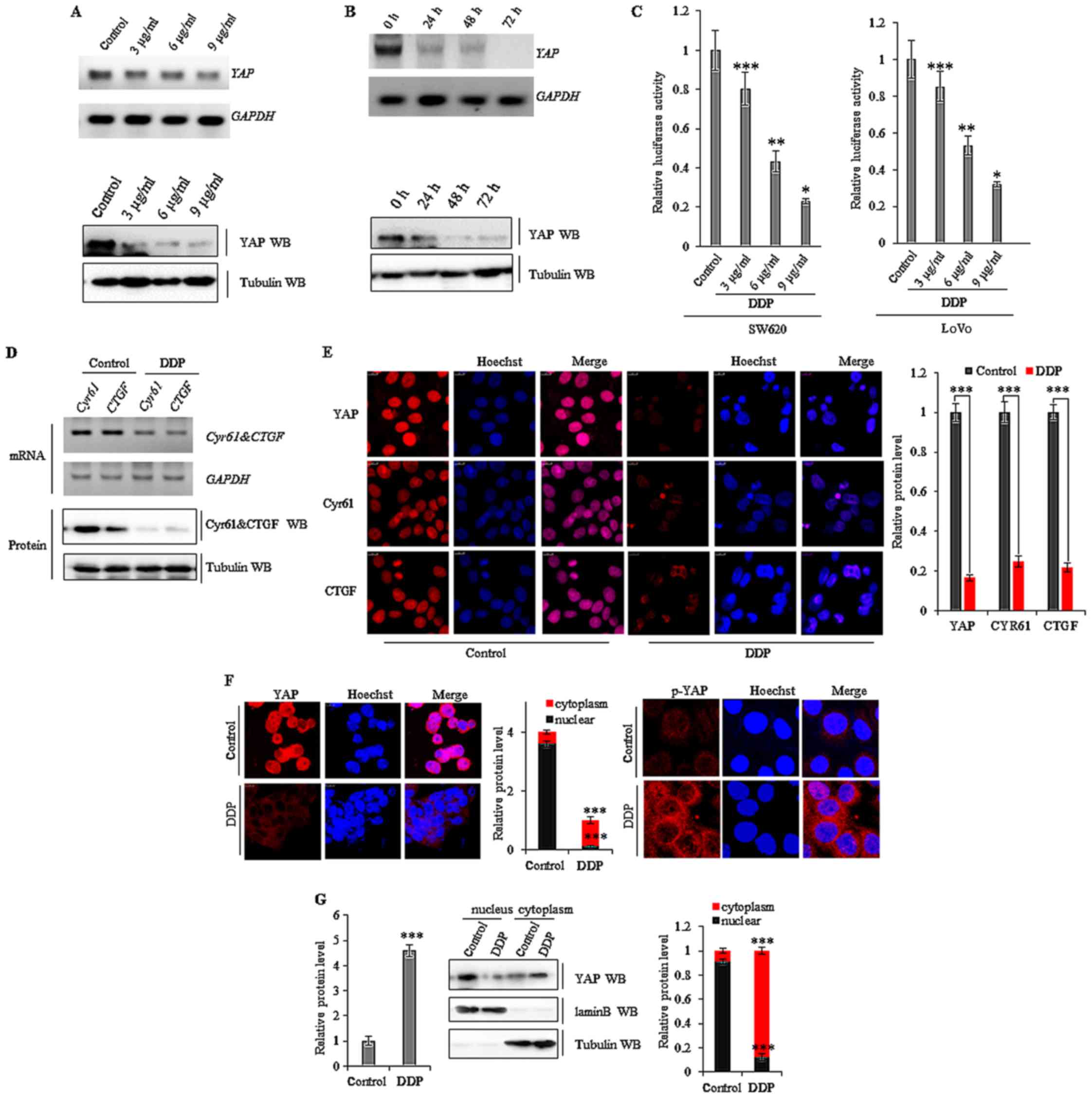
DDP suppresses YAP activity in colon
cancer cells
To further investigate the mechanisms of DDP, we
explored whether the drug affected the Mst/Yap signaling pathway in
colon cancer cells. Our data revealed that DDP dose-dependently
(Fig. 3A) and time-dependently
(Fig. 3B) reduced YAP mRNA
and YAP protein levels in SW620 cells. Subsequently, we assessed if
this was due to a direct effect of DDP on the promoter region of
YAP. A luciferase reporter gene assay using the promoter region of
YAP (−1420/+115, pGL3-Yap) demonstrated that DDP decreased
the luciferase activity of the promoter in a dose-dependent manner
in both SW620 and LoVo cancer cells (Fig. 3C). This indicated that DDP
downregulated the expression of YAP at the transcriptional
level in colon cancer cells through a direct interaction. As DDP
reduced the expression of YAP in colon cancer cells, we
subsequently explored the effects on downstream target genes in the
Mst/Yap signaling pathway. Both RT-PCR and western blot analysis
revealed that treatment with 10 µg/ml of DDP for 72 h suppressed
YAP and the downstream target genes CYR61 and
CTGF mRNA in SW620 cells (Fig.
3D). Furthermore, semi-quantitative analysis of CYR61 and CTGF
immunofluorescent staining indicated that treatment with DDP at 10
µg/ml for 72 h also decreased the expression of these proteins in
SW620 cells (Fig. 3E). Our data
also revealed that the levels of YAP protein were decreased in the
nucleus after DDP treatment. Exposure to 6 µg/ml DDP for 36 h
increased the levels of phosphorylated YAP (p-YAP) in the cytoplasm
of SW620 cells, leading to the translocation of YAP from the
nucleus to the cytoplasm (Fig. 3F and
G).
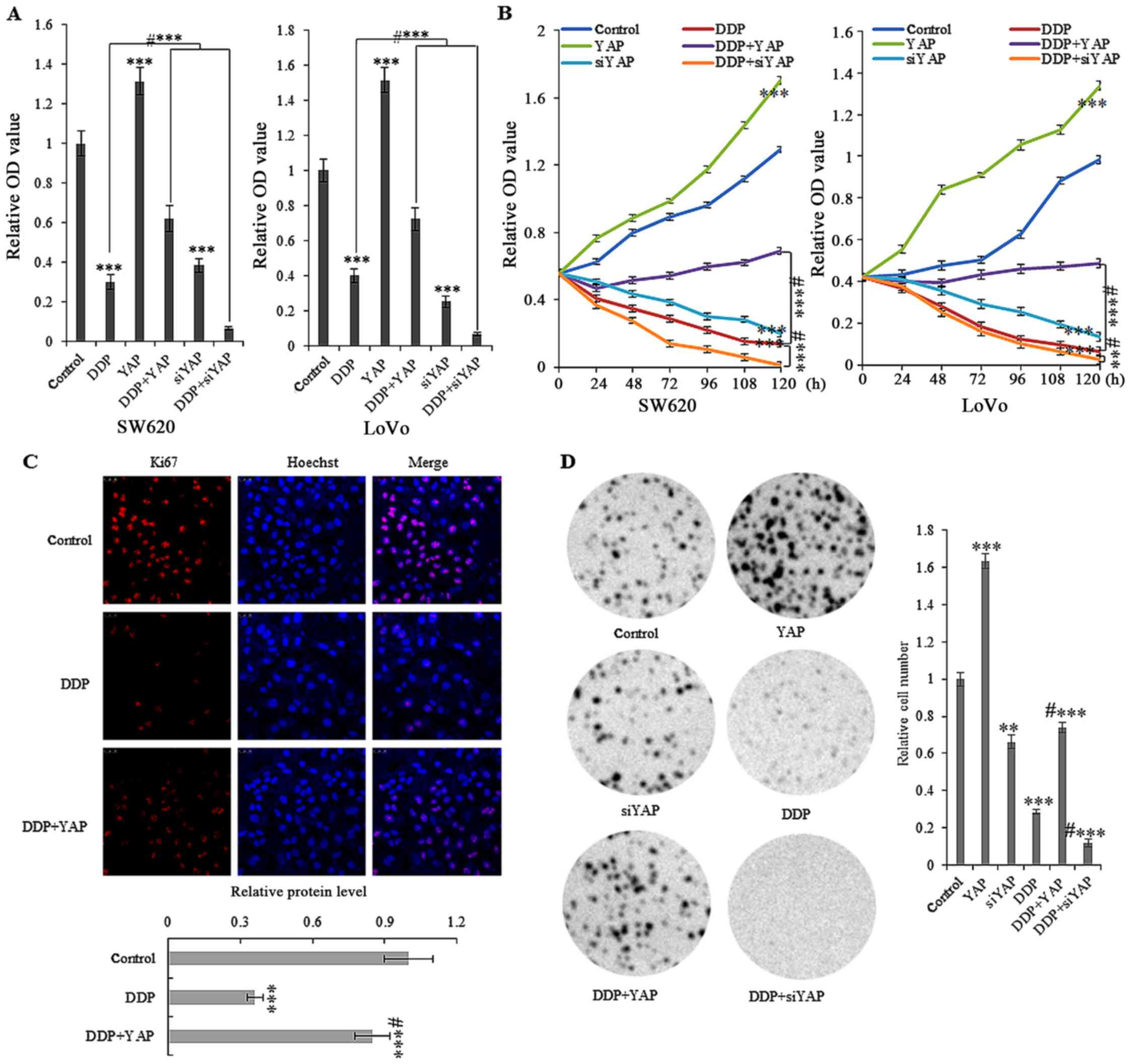
DDP affects YAP-mediated colon cell
proliferation
Cell proliferation assays indicated that abnormally
high expression of YAP increased the in vitro growth of
SW620 and LoVo cells (Fig. 4A and
B). Treatment with 10 µg/ml DDP showed a time-dependent arrest
in the cellular growth of SW620 and LoVo cells, with or without the
stable overexpression of YAP. Furthermore, semi-quantitative
analysis of Ki-67 immunofluorescent staining indicated that 10
µg/ml DDP treatment for 72 h significantly reduced Ki-67 expression
in SW620 cells (Fig. 4C). Colony
formation assays using SW620 cells also revealed that ectopic
overexpression of YAP reversed colony formation after treatment
with DDP at 10 µg/ml for 72 h (Fig.
4D).
To fully explore the putative biological functions
of DDP, cell cycle analysis in colon cancer cells under DDP
treatment was performed. This revealed that treatment with 10 µg/ml
DDP for 72 h induced cell cycle arrest in G2 phase and also blocked
YAP-induced progression into S phase in SW620 cells. This was
demonstrated using both quantitative analysis and representative
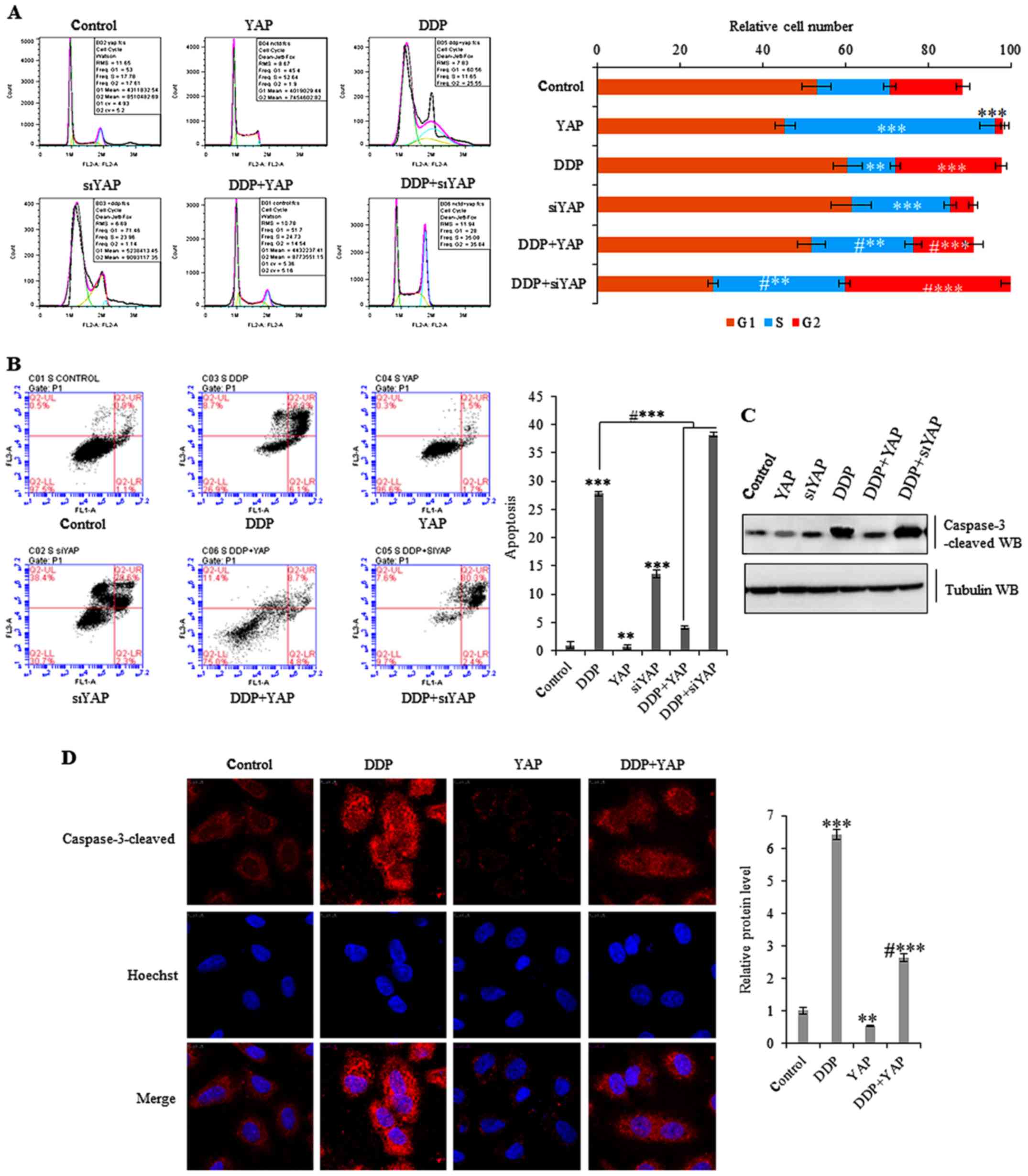
histograms summarizing cell cycle distribution (Fig. 5A). Cell cycle and Annexin V flow
cytometry also revealed that treatment with 10 µg/ml DDP for 72 h
induced increased apoptosis in SW620 cells, whereas ectopic
overexpression of YAP partially reduced DDP-induced apoptosis
(Fig. 5B). Additional
immunofluorescent staining of cleaved caspase-3 (a marker of
apoptosis) confirmed that DDP increased the levels of this protein
product. In addition, ectopic overexpression of YAP partially
reduced DDP-induced apoptosis in SW620 cells over the 72-h period
(Fig. 5C and D).
DDP affects YAP-mediated colon tumor
cell senescence, invasiveness and epithelial-mesenchymal transition
(EMT)
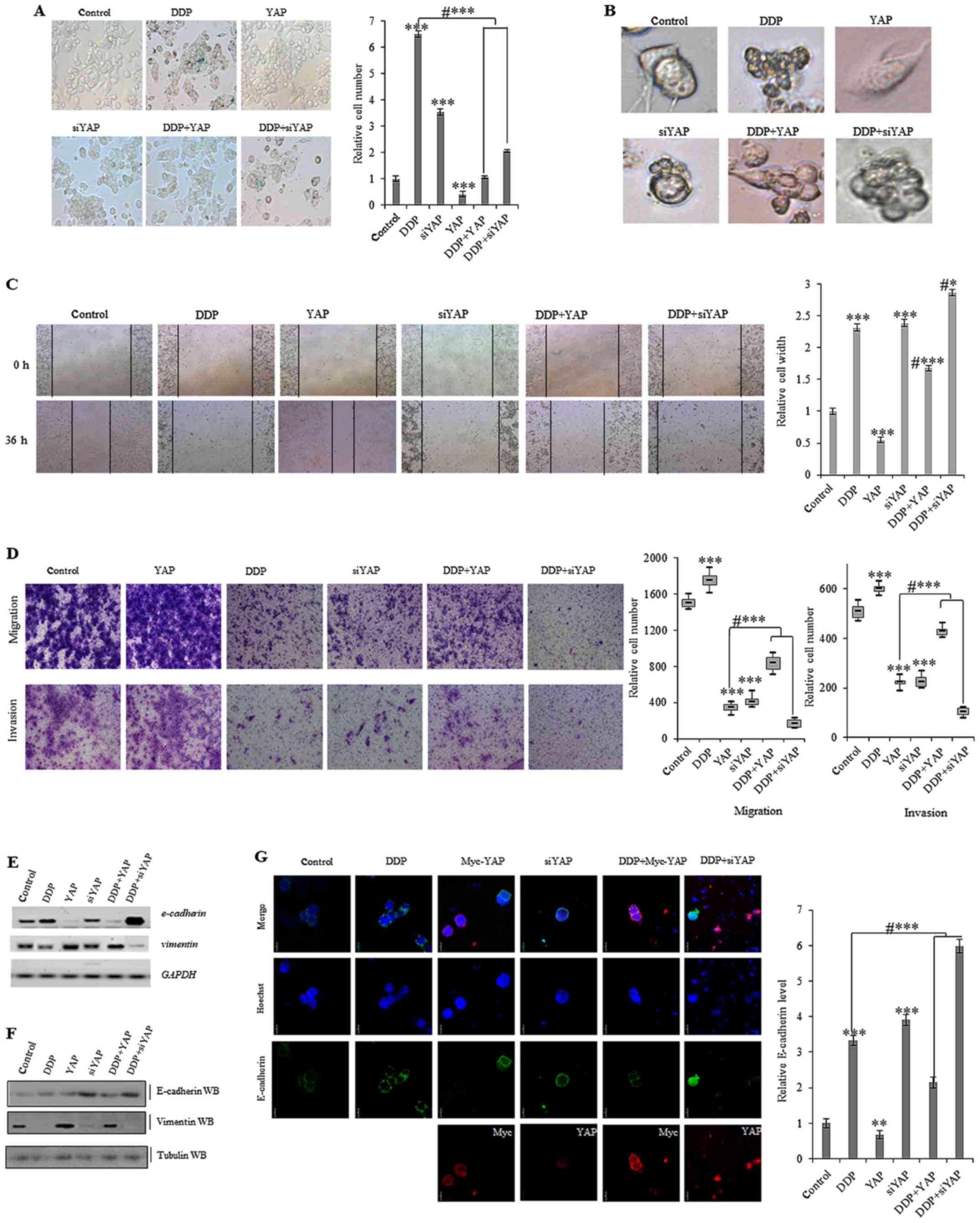
Cellular apoptosis and senescence resistance is a
common mechanism by which cancer cells avoid death. It has also
been previously reported that aberrant activation of YAP inhibits
senescence in human tumor cells (7). To analyze whether DDP influences
YAP-induced cellular senescence in colon tumor cells, we used
β-galactosidase staining to identify cells that were senescent.
This indicated that exposure to 10 µg/ml DDP for 72 h increased the
number of SW620 cells displaying senescence. Ectopic overexpression
of YAP lowered the number of DDP-induced senescent cells, whereas
knockdown of YAP using siRNA promoted senescence (Fig. 6A). Phase contrast microscopy
analysis indicated that cellular morphology was markedly altered
after treatment with 10 µg/ml DDP for 72 h in cells overexpressing
YAP and in YAP-knockdown cells (Fig.
6B).
Cellular invasion is one of the most important
defining features of cancer cells. Using a scratch assay, we found
that treatment with 10 µg/ml DDP for 36 h decreased the YAP-induced
migration in SW620 cells (Fig. 6C).
Additionally, a Matrigel invasion and migration assay was performed
to explore the effects of DDP on these properties in SW620 colon
cancer cells. This indicated that treatment with 10 µg/ml DDP for
72 h decreased cell invasion and migration. DDP-induced inhibition
of migration and growth was also partially reduced by
overexpressing YAP in SW620 cells (Fig.
6D). As YAP-mediated invasion and migration could be inhibited
by treatment with DDP in colon cancer cells, we next determined
whether DDP influenced the YAP-mediated cellular phenotype
transformation in colon cancer cells by examining markers
associated with EMT, such as vimentin and E-cadherin. As dislpayed
in Fig. 6E and F, the mRNA and
protein levels of E-cadherin were increased, whereas vimentin
expression was decreased, after treatment with DDP for 72 h in
SW620 cells. Ectopic overexpression of YAP decreased the
DDP-induced expression changes in E-cadherin and vimentin
expression, while an opposing effect was observed in YAP knockdown
cells. This indicated that DDP interfered with the YAP-mediated EMT
in colon tumor cells. Furthermore, immunofluorescent staining of
E-cadherin confirmed that DDP increased E-cadherin protein
expression under ectopic overexpression of YAP, while knockdown of
YAP using siRNA partially blocked the DDP-induced increase in
E-cadherin expression (Fig.
6G).
DDP inhibits the in vivo growth of in
situ colon xenografts
To definitively address the central question as to
whether DDP treatment can delay tumorigenesis in colon cancer, we
explored the in vivo antitumor activity of DDP in colon
cells using in situ colon xenografts and survival analysis.
Approximately two weeks after the subcutaneous xenografting of
SW620 cells into a concave niche of the ceca of mice, larger tumors
were observed in the control group treated with PBS when compared
to the group treated with DDP (Fig. 7A
and B). Histochemical analysis of the tumors also indicated
that tumor growth was inhibited by treatment with DDP relative to
PBS. Further semi-quantitative immunohistochemical analysis of the
YAP expression in the xenografts revealed that DDP treatment led to
less YAP protein compared to the control group (Fig. 7C). Additional examination of the
in situ xenografts by optical microscopy under H&E
staining indicated that several critical organs outside of the
xenograft site, including the large intestine, small intestine,
stomach, heart, liver, spleen, lungs and kidneys, were also
severely damaged (Fig. 7D). We also
confirmed that there was reduced body weight loss in mice treated
with 1.5 mg/kg DDP during the treatment period compared to those
administered PBS (Fig. 7E).
Finally, it was observed that the survival times of the DDP treated
group of xenograft mice were significantly longer than those of the
control group (Fig. 7F).
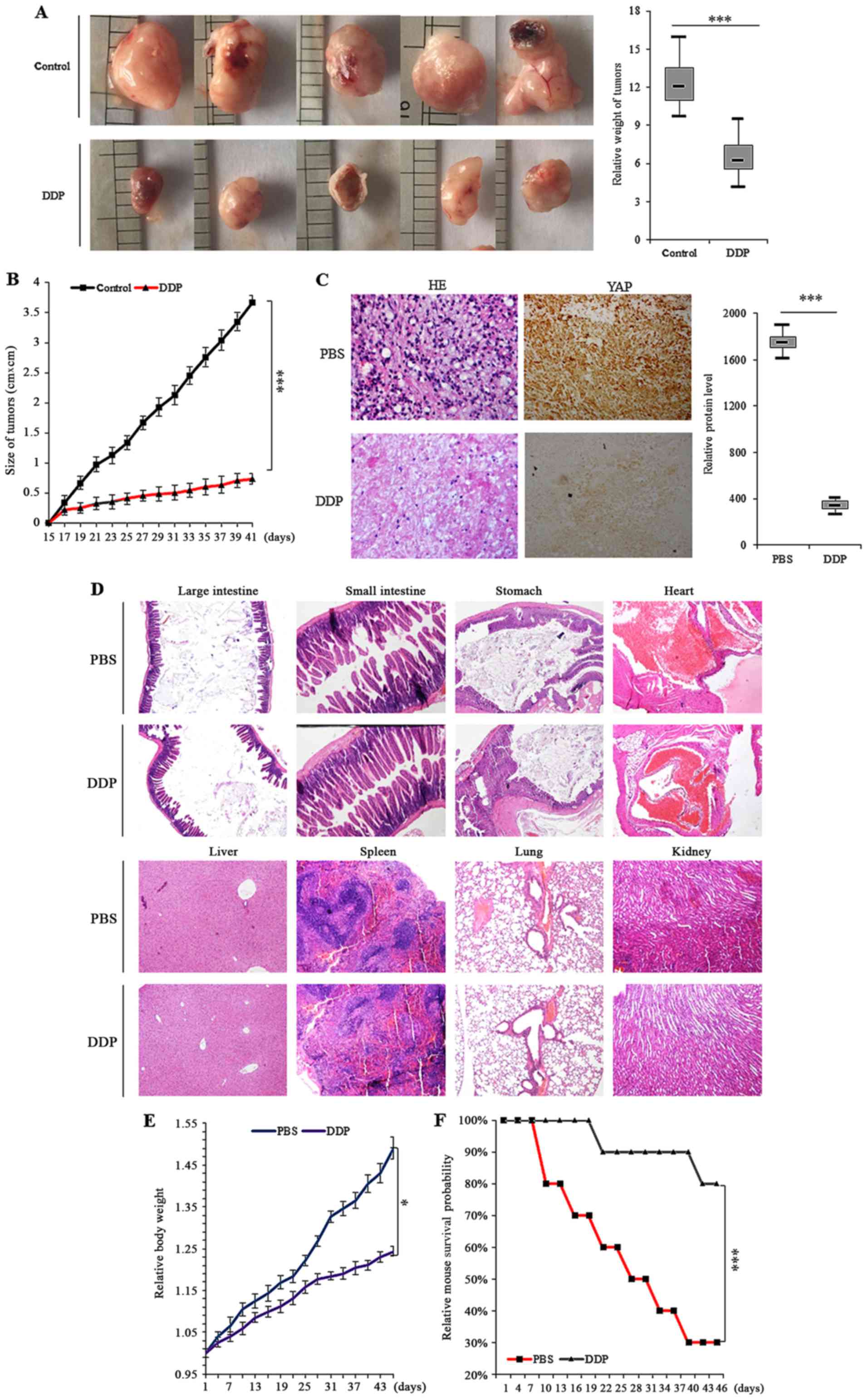 | Figure 7.DDP inhibits the growth of in
situ colon xenografts in vivo. (A) Xenograft mice with
colon cancer cell tumors were treated with control (PBS) or DDP and
the dimensions and weights of tumors were assessed at regular
time-points. (B) Overall tumor sizes and growth curves. (C) H&E
microscopy of colon tumor nodules from primary SW620 cells in the
subcutaneous xenografts of nude mice treated with control (PBS) and
DDP. Immunohistochemical staining revealed that YAP expression was
decreased in xenograft tumor tissues after treatment with DDP
relative to PBS treatment. (D) Representative H&E stained
microscopic images of the large intestine, small intestine,
stomach, heart, liver, spleen, colon and kidneys of nude mice with
xenografted colon cancer cell tumors. There was less obvious organ
damage in DDP-treated mice compared to PBS-treated mice. (E) The
body weights of nude mice after treatment with DDP compared to
PBS-treated mice demonstrated that DDP treatment did not
significantly alter the body weight of animals and could also
alleviate body weight loss. (F) Kaplan-Meier OS curves of mice
treated with PBS and DDP. Results were presented as mean ± SD, and
the error bars represent the SD of three independent experiments.
*P<0.05; ***P<0.001 vs. control group. DDP, cisplatin; OS,
overall survival; H&E, hematoxylin and eosin; PBS,
phosphate-buffered saline. |
Discussion
In the present study we have explored the efficacy
and specificity of DDP in inhibiting colon carcinoma progression.
Our data demonstrated that DDP is a suitable therapeutic drug
candidate for colon cancers which sensitively and specifically
targets the Mst/Yap signaling pathway. Although numerous effective
treatments and detection methods for colon tumors have improved
survival rates for patients, the disease remains one of the most
aggressive malignant cancers and has a particularly high fatality
rate. Due to the pathology of the disease, most patients present in
advanced stages, meaning that the best treatment options are
palliative, such as radiotherapy and chemotherapy. However, normal
tissue cells are often destroyed by these indiscriminate methods of
treatment. Therefore, identifying novel natural compounds that are
highly selective for cancer cells and have low non-specific
toxicity, is important for improving cancer therapy.
The anticancer activity of DDP, a commonly used
chemotherapeutic agent developed in China, has been examined in
several studies. Unlike many other treatments, DDP induces few
side-effects in the digestive system and is also relatively easy to
synthesize. It has been demonstrated to inhibit cell growth in
several cancer cell lines and transplanted tumors, while
simultaneously increasing white blood cell counts by sensitizing
the bone marrow. Due to this effect, the drug has also some
antagonistic effects against leukopenia. In addition, clinical
approaches that apply DDP as a mono-therapeutic agent have reported
that the drug has beneficial effects in patients with several
different types of tumors simultaneously. Our data indicated that
DDP inhibited the in vitro growth of SW620 and LoVo human
colon cancer cell lines in a dose- and time-dependent manner.
Immunoblotting analysis revealed that DDP dose-dependently
downregulated YAP and the expression of downstream target genes
CTGF and CYR61 via an interaction with the promoter region of the
YAP gene, indicating a functional mechanism. This
downregulation of downstream genes led to cell cycle arrest in
colon cancer cells and the observed inhibition of tumor growth.
Notably, DDP also suppressed SW620 cell invasion and migration
without reducing cell viability. These data indicated that DDP may
have wider direct or adjuvant therapeutic applications for the
treatment of human colon tumors and this will require further
examination.
Currently, the efficacy of available colon cancer
therapeutic options is limited by the development of treatment
resistance in cancer cells. YAP in particular is a novel
anticancer drug target gene that has been associated with high
chemoresistance in colon cancer. Aberrant activation of this
oncogene contributes to the initiation, progression and metastasis
of colon tumors and associates with poor prognosis. This would also
act to promote drug resistance against the targeted therapy. YAP
has been found to be highly activated in colon cancer and reducing
its expression in cancer cells may increase the therapeutic effects
of colon cancer treatments. Our results demonstrated that the
Mst/Yap signaling pathway was abnormally activated in colon cancer
tumor tissues and cells. Like other tumors, this would lead to
increased cell growth and invasiveness. However, our functional
data demonstrated that DDP specifically suppressed the Mst/Yap
signaling pathway, affecting YAP-mediated colon cancer progression
and metastasis, by arresting the cell cycle and inducing apoptosis
and cell senescence. We also confirmed that DDP suppression of YAP
activity reduced EMT and decreased the motility, metastatic and
invasive capacities of colon cancer cells in vitro, probably
by enhancing the expression of E-cadherin and decreasing the
expression of fibronectin/vimentin.
In addition, our data demonstrated that DDP not only
regulated YAP transcriptional activity, but also
post-translationally modified YAP, affecting the subcellular
distribution of phosphorylated and unphosphorylated protein between
the cytosol and the nucleus. Markedly, DDP demonstrated no kinase
activity, but some other researches revealed that zyxin formed a
ternary complex with Siah2 and Lats2 responding to TGF-β stimuli,
thus stabilized their interaction and facilitated deactivation of
the YAP signaling pathway, thereby promoting tumor progression
(33,34). Furthermore, DDP could regulate the
activity of TGF-β to suppressed lung cancer progression and
metastasis (35). Therefore, we
hypothesized that DDP may regulate cytoplasmic p-YAP by regulating
the upstream signals of YAP, however we should further explore this
hypothesis. Furthermore, we have shown that DDP dose-dependently
and time-dependently increased p-YAP alongside decreasing
YAP mRNA and YAP protein expression in SW620 colon cancer
cells. This provided further information to elucidate the molecular
mechanisms of DDP and the therapeutic activity of the drug against
colon cancer. However, it is important to note that this study is
prospective and, although these are important results, there are
many issues that require further examination. These include
understanding the mechanism through which the inhibition of YAP
expression by DDP improves the chemotherapeutic treatment of
drug-resistant colon carcinomas.
In conclusion, the present study indicated that the
anticancer drug DDP was an effective therapeutic drug for the
treatment of human colon cancer. DDP enhanced apoptosis, senescence
and cell cycle arrest in cancer cells by suppressing YAP
expression, thereby inhibiting cell proliferation. Notably, we
demonstrated that the effects of DDP were potentially mediated via
an interaction with the promoter region of the YAP gene. Our
results indicated that DDP acted as a chemotherapeutic antitumor
agent that can be used for suppressing tumorigenesis and the
initiation of colon tumor via the downregulation of YAP in human
colon cancer. The inhibition of cancer cell proliferation by DDP
was associated with interference in cell cycle progression in colon
tumor cells, increasing the proportion of cells in the G2/M phase.
These data improved our understanding of colon cancer and the role
that the Mst/Yap pathway plays in cancer progression. In addition,
the present indicated that DDP may serve as an important future
treatment for colon cancer.
Acknowledgements
Not applicable.
References
|
1
|
Xu Z, Jiang H, Zhu Y, Wang H, Jiang J,
Chen L, Xu W, Hu T and Cho CH: Cryptotanshinone induces
ROS-dependent autophagy in multidrug-resistant colon cancer cells.
Chem Biol Interact. 273:48–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Udawat H, Nunia V, Mathur P, Udawat HP,
Gaur KL, Saxena AK and Mohan MK: Histopathological and
immunohistochemical findings in congenital pouch colon: A
prospective study. Pathobiology. 84:202–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senol S, Ceyran AB, Kösemetin D, Gobanoglu
B, Aydin D, Duran EA and Leblebici M: Immunohistochemical profile
of tumor pathways and prognostic significance in colon
adenocarcinomas. J Environ Pathol Toxicol Oncol. 36:29–41. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hakoda K, Yoshimitsu M, Emi M, Omori I,
Kohashi T, Kaneko M, Ohdan H and Hirabayashi N: Complete
pathological response of multiple huge liver metastases of colon
cancer: A case report. Oxf Med Case Reports. 2017:omx0162017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sloothaak DAM, van der Linden RLA, van de
Velde CJH, Bemelman WA, Lips DJ, van der Linden JC, Doornewaard H,
Tanis PJ, Bosscha K, van der Zaag ES, et al: Prognostic
implications of occult nodal tumour cells in stage I and II colon
cancer: The correlation between micrometastasis and disease
recurrence. Eur J Surg Oncol. 43:1456–1462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura Y, Hattori N, Iida N, Yamashita
S, Mori A, Kimura K, Yoshino T and Ushijima T: Targeting of
super-enhancers and mutant BRAF can suppress growth of BRAF-mutant
colon cancer cells via repression of MAPK signaling pathway. Cancer
Lett. 402:100–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo J, Wu Y, Yang L, Du J, Gong K, Chen W,
Dai J, Li X and Xi S: Repression of YAP by NCTD disrupts NSCLC
progression. Oncotarget. 8:2307–2319. 2017.PubMed/NCBI
|
|
8
|
Azzolin L, Panciera T, Soligo S, Enzo E,
Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V,
et al: YAP/TAZ incorporation in the β-catenin destruction complex
orchestrates the Wnt response. Cell. 158:157–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin K, Pritchett J, Llewellyn J, Mullan
AF, Athwal VS, Dobie R, Harvey E, Zeef L, Farrow S, Streuli C, et
al: PAK proteins and YAP-1 signalling downstream of integrin beta-1
in myofibroblasts promote liver fibrosis. Nat Commun. 7:125022016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moleirinho S, Hoxha S, Mandati V, Curtale
G, Troutman S, Ehmer U and Kissil JL: Regulation of localization
and function of the transcriptional co-activator YAP by angiomotin.
eLife. 6:e239662017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tranchant R, Quetel L, Tallet A, Meiller
C, Renier A, de Koning L, de Reynies A, Le Pimpec-Barthes F,
Zucman-Rossi J, Jaurand MC, et al: Co-occurring mutations of tumor
suppressor genes, LATS2 and NF2, in malignant pleural mesothelioma.
Clin Cancer Res. 23:3191–3202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng G, Zhu Z, Li WJ, Lin Q, Chai Y, Dong
MQ and Ou G: Hippo kinases maintain polarity during directional
cell migration in Caenorhabditis elegans. EMBO J. 36:334–345. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Luo JY, Li B, Tian XY, Chen LJ,
Huang Y, Liu J, Deng D, Lau CW, Wan S, et al: Integrin-YAP/TAZ-JNK
cascade mediates atheroprotective effect of unidirectional shear
flow. Nature. 12:72016.
|
|
14
|
Moroishi T, Hayashi T, Pan WW, Fujita Y,
Holt MV, Qin J, Carson DA and Guan KL: The Hippo pathway kinases
LATS1/2 suppress cancer immunity. Cell. 167:1525–1539.e17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharif GM and Wellstein A: Cell density
regulates cancer metastasis via the Hippo pathway. Future Oncol.
11:3253–3260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim T, Hwang D, Lee D, Kim JH, Kim SY and
Lim DS: MRTF potentiates TEAD-YAP transcriptional activity causing
metastasis. EMBO J. 36:520–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto M, Ohsawa S, Kunimasa K and Igaki
T: The ligand Sas and its receptor PTP10D drive tumour-suppressive
cell competition. Nature. 542:246–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L
and Zhou Z: VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt
and Hippo signalling in colorectal cancer. Nat Commun. 8:140582017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oun R and Rowan E: Cisplatin induced
arrhythmia; electrolyte imbalance or disturbance of the SA node?
Eur J Pharmacol. 811:125–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alterio D, Rocca Cossu M, Russell-Edu W,
Dicuonzo S, Fanetti G, Marvaso G, Preda L, Zorzi S, Verri E, Nole'
F, et al: Med Oncol. 34:862017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F, Yu X, Liu X, Zhou T, Nie T, Cheng
M, Liu H, Dai M and Zhang B: ABT-737 potentiates cisplatin-induced
apoptosis in human osteosarcoma cells via the mitochondrial
apoptotic pathway. Oncol Rep. 38:2301–2308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Kho DH, Zhou JY, Davis RJ and Wu
GS: MKP-1 suppresses PARP-1 degradation to mediate cisplatin
resistance. Oncogene. 36:5939–5947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen LG, Xia YJ and Cui Y: Upregulation of
miR-101 enhances the cytotoxic effect of anticancer drugs through
inhibition of colon cancer cell proliferation. Oncol Rep.
38:100–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu M, Tang X, Guo J, Sun W and Tang F:
Reversal effect of adenovirus-mediated human interleukin 24
transfection on the cisplatin resistance of A549/DDP lung cancer
cells. Oncol Rep. 38:2843–2851. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jacobs J, Deschoolmeester V, Rolfo C,
Zwaenepoel K, Van den Bossche J, Deben C, Silence K, de Haard H,
Hermans C, Rottey S, et al: Preclinical data on the combination of
cisplatin and anti-CD70 therapy in non-small cell lung cancer as an
excellent match in the era of combination therapy. Oncotarget.
8:74058–74067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mukherjee S, Dash S, Lohitesh K and
Chowdhury R: The dynamic role of autophagy and MAPK signaling in
determining cell fate under cisplatin stress in osteosarcoma cells.
PLoS One. 12:e01792032017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Duan W, Zhang L, Li X, Fu X, Wang X,
Wu J, Sun Z, Zhang X, Chang Y, et al: The efficacy and safety of
gemcitabine, cisplatin, prednisone, thalidomide versus CHOP in
patients with newly diagnosed peripheral T-cell lymphoma with
analysis of biomarkers. Br J Haematol. 178:772–780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi BY, Joo JC, Lee YK, Jang IS, Park SJ
and Park YJ: Anti-cancer effect of Scutellaria baicalensis in
combination with cisplatin in human ovarian cancer cell. BMC
Complement Altern Med. 17:2772017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rudolph C, Melau C, Nielsen JE, Jensen
Vile K, Liu D, Pena-Diaz J, Rajpert-De Meyts E, Rasmussen LJ and
Jørgensen A: Involvement of the DNA mismatch repair system in
cisplatin sensitivity of testicular germ cell tumours. Cell Oncol
(Dordr). 40:341–355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ge L, Li DS, Chen F, Feng JD, Li B and
Wang TJ: TAZ overexpression is associated with
epithelial-mesenchymal transition in cisplatin-resistant gastric
cancer cells. Int J Oncol. 51:307–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akdemir Ekinci FN, Albayrak M, Calik M,
Bayir Y and Gulcin I: The protective effects of p-coumaric acid on
acute liver and kidney damages induced by cisplatin. Biomedicines.
5:E182017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma B, Cheng H, Gao R, Mu C, Chen L, Wu S,
Chen Q and Zhu Y: Zyxin-Siah2-Lats2 axis mediates cooperation
between Hippo and TGF-β signalling pathways. Nat Commun.
7:111232016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaspar P, Holder MV, Aerne BL, Janody F
and Tapon N: Zyxin antagonizes the FERM protein expanded to couple
F-actin and Yorkie-dependent organ growth. Curr Biol. 25:679–689.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Chen Y, Xiang F, Li M, Li H, Chi J
and Ren K: Suppression of TGF-β1 enhances chemosensitivity of
cisplatin-resistant lung cancer cells through the inhibition of
drug-resistant proteins. Artif Cells Nanomed Biotechnol. 8:1–8.
2017. View Article : Google Scholar
|