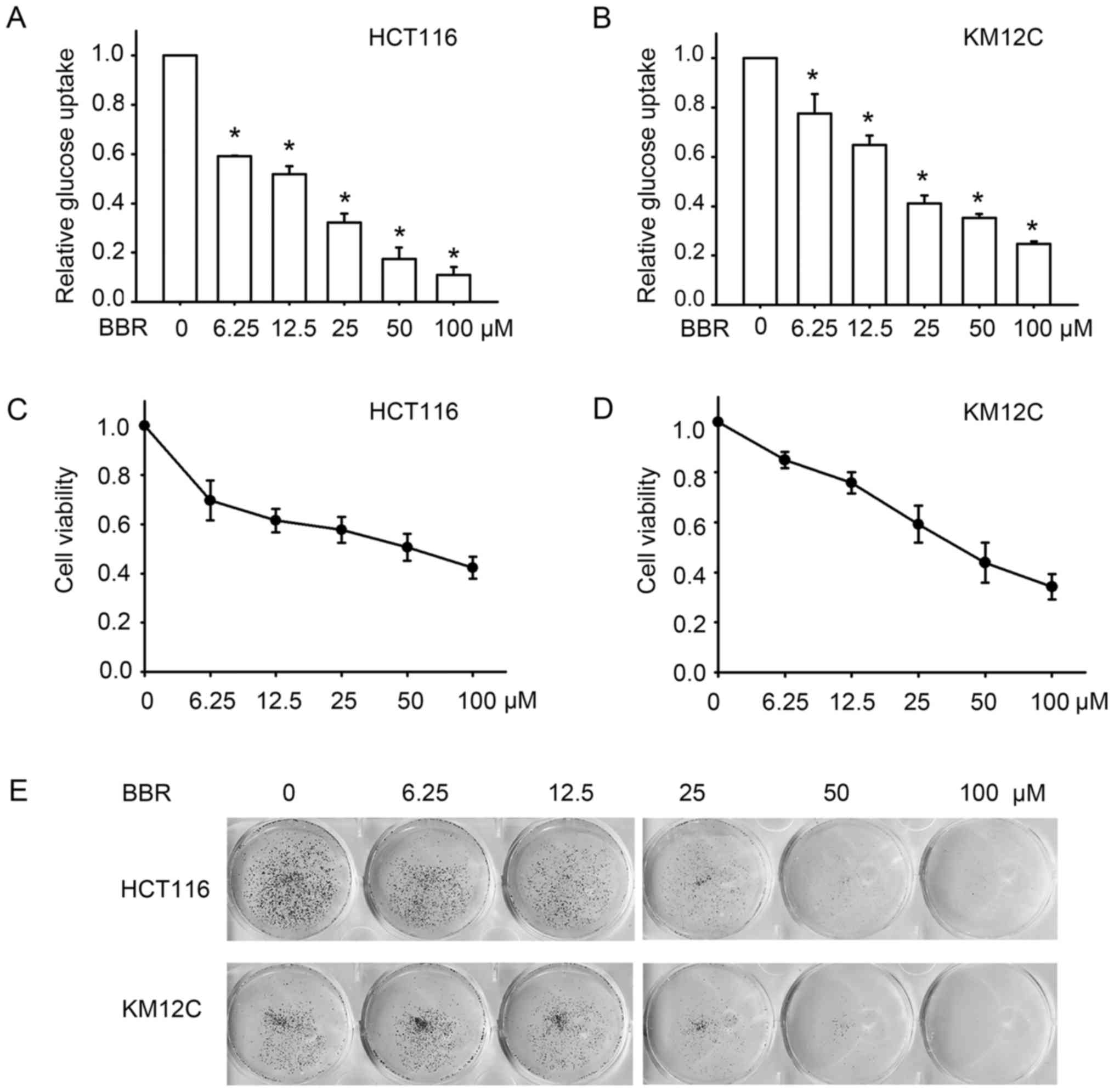
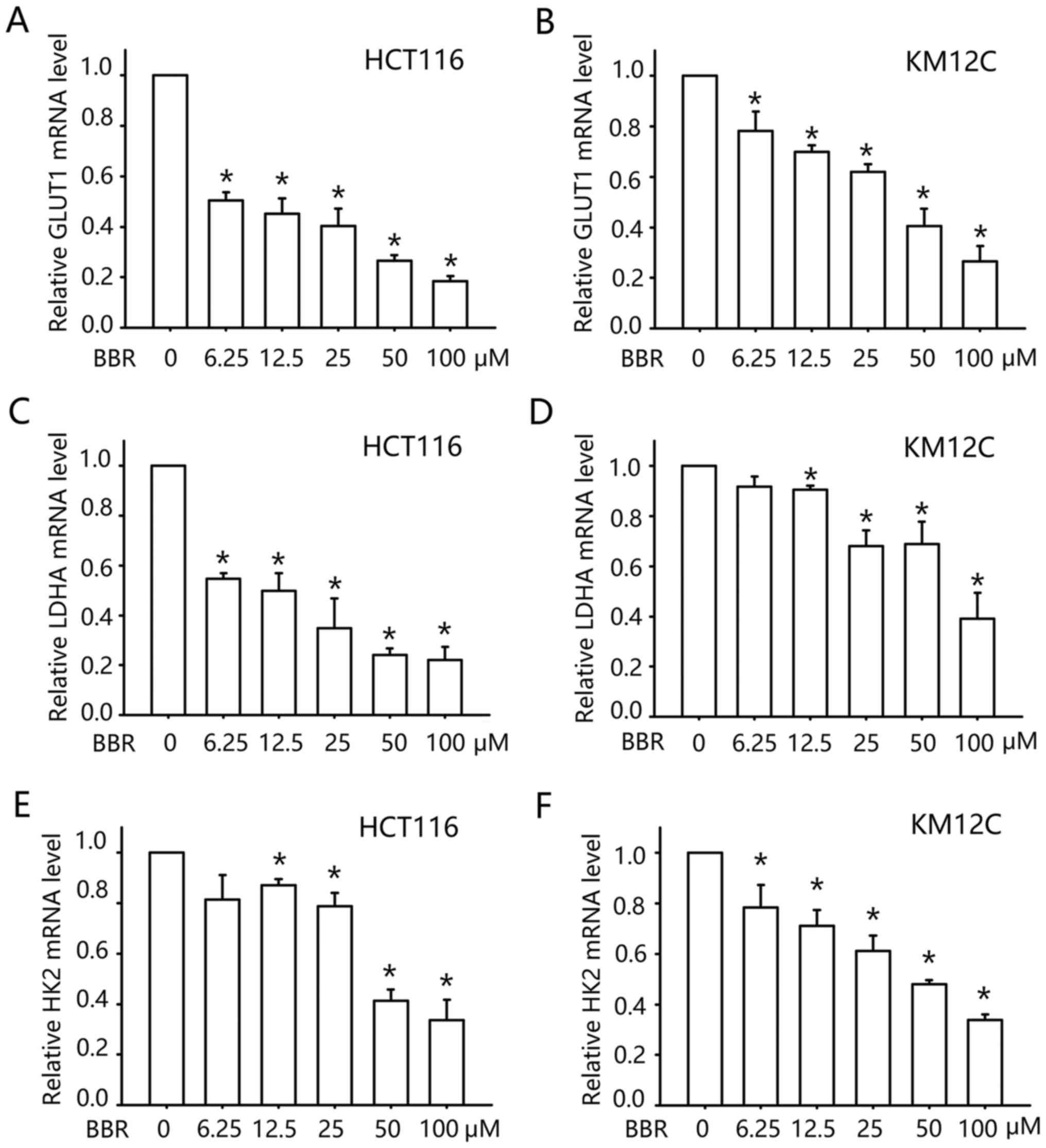
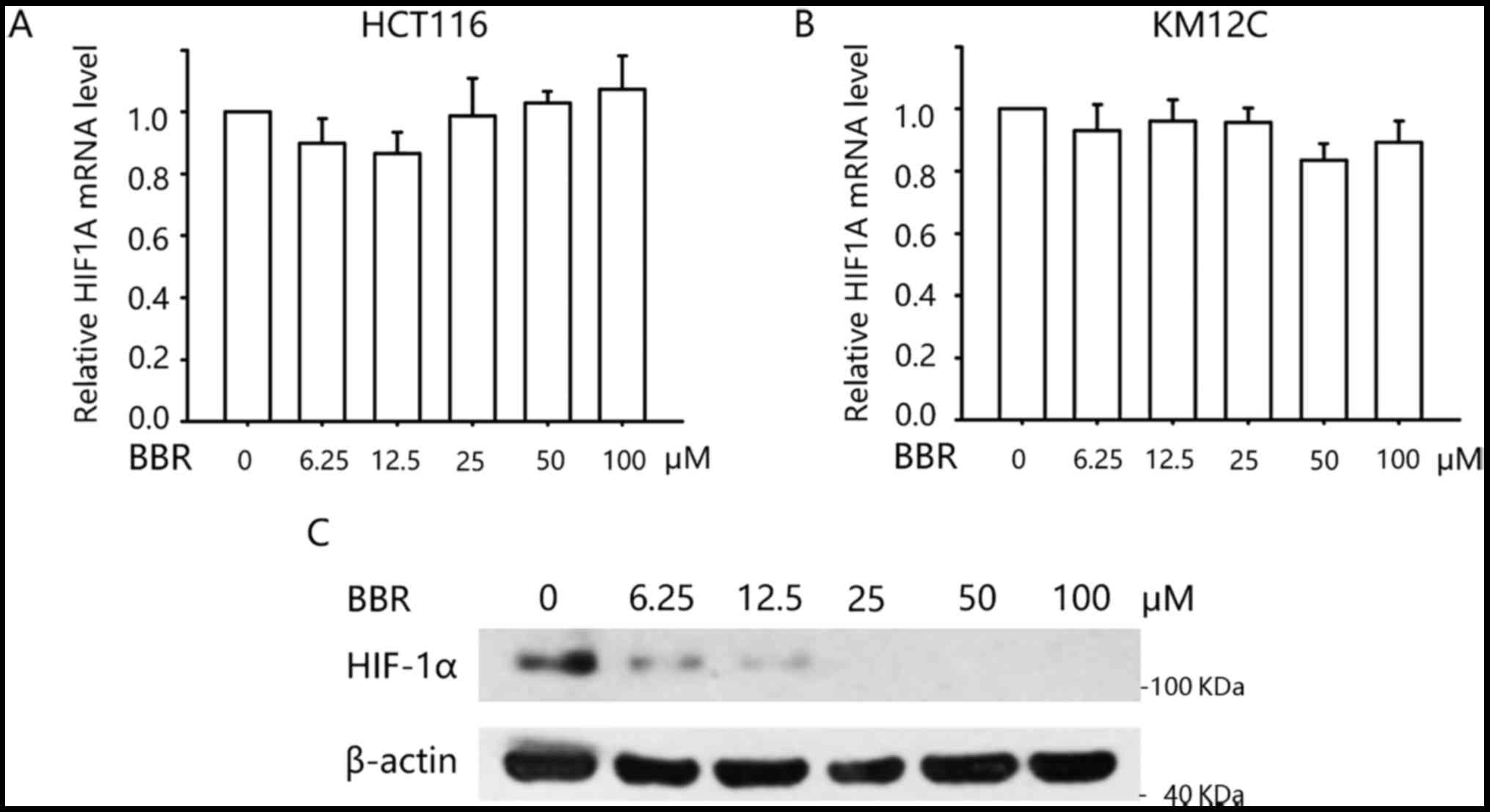
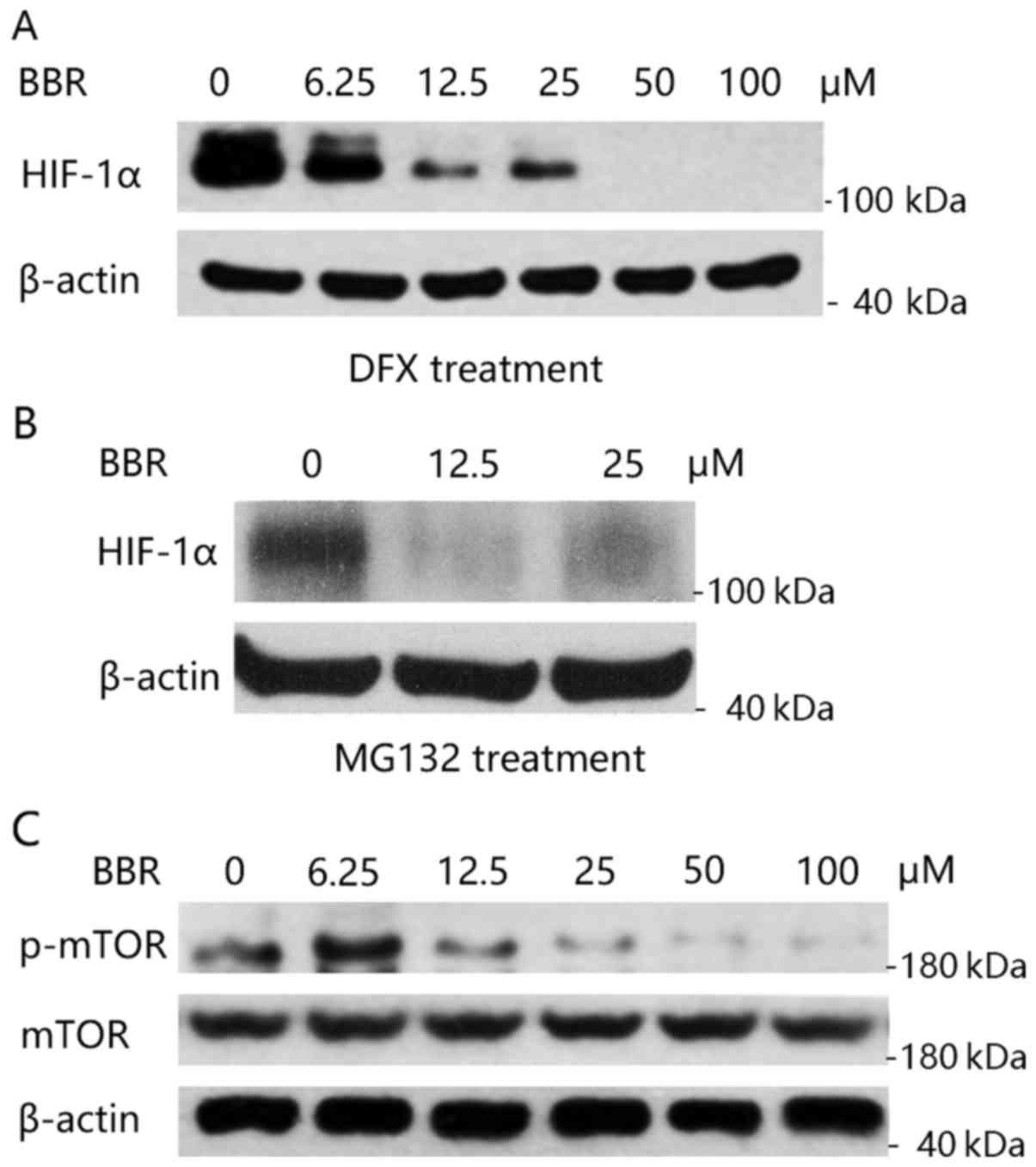
|
1
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cicero AF and Tartagni E: Antidiabetic
properties of berberine: From cellular pharmacology to clinical
effects. Hosp Pract. 40:56–63. 2012. View Article : Google Scholar
|
|
4
|
Pang B, Zhao LH, Zhou Q, Zhao TY, Wang H,
Gu CJ and Tong XL: Application of berberine on treating type 2
diabetes mellitus. Int J Endocrinol. 15:9057492015.
|
|
5
|
Cok A, Plaisier C, Salie MJ, Oram DS,
Chenge J and Louters LL: Berberine acutely activates the glucose
transport activity of GLUT1. Biochimie. 93:1187–1192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou L, Yang Y, Wang X, Liu S, Shang W,
Yuan G, Li F, Tang J, Chen M and Chen J: Berberine stimulates
glucose transport through a mechanism distinct from insulin.
Metabolism. 56:405–412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li
JY, Nan FJ and Li J: Berberine-stimulated glucose uptake in L6
myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta.
1760:1682–1689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ortiz LM, Lombardi P, Tillhon M and
Scovassi AI: Berberine, an epiphany against cancer. Molecules.
19:12349–12367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang N, Tan HY, Li L, Yuen MF and Feng Y:
Berberine and Coptidis Rhizoma as potential anticancer agents:
Recent updates and future perspectives. J Ethnopharmacol.
176:35–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan W, Li Y, Chen M and Wang Y: Berberine
hydrochloride: Anticancer activity and nanoparticulate delivery
system. Int J Nanomedicine. 6:1773–1777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou C, Wang Y and Shen Z: 2-NBDG as a
fluorescent indicator for direct glucose uptake measurement. J
Biochem Biophys Methods. 64:207–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SH, Shin EJ, Kim ED, Bayaraa T, Frost
SC and Hyun CK: Berberine activates GLUT1-mediated glucose uptake
in 3T3-L1 adipocytes. Biol Pharm Bull. 30:2120–2125. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haber RS, Rathan A, Weiser KR, Pritsker A,
Itzkowitz SH, Bodian C, Slater G, Weiss A and Burstein DE: GLUT1
glucose transporter expression in colorectal carcinoma: A marker
for poor prognosis. Cancer. 83:34–40. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saigusa S, Toiyama Y, Tanaka K, Okugawa Y,
Fujikawa H, Matsushita K, Uchida K, Inoue Y and Kusunoki M:
Prognostic significance of glucose transporter-1 (GLUT1) gene
expression in rectal cancer after preoperative chemoradiotherapy.
Surg Today. 42:460–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Regulation of cancer cell
metabolism by hypoxia-inducible factor 1. Semin Cancer Biol.
19:12–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kallio PJ, Wilson WJ, O'Brien S, Makino Y
and Poellinger L: Regulation of the hypoxia-inducible transcription
factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem.
274:6519–6525. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demidenko ZN, Rapisarda A, Garayoa M,
Giannakakou P, Melillo G and Blagosklonny MV: Accumulation of
hypoxia-inducible factor-1alpha is limited by
transcription-dependent depletion. Oncogene. 24:4829–4838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abraham RT: mTOR as a positive regulator
of tumor cell responses to hypoxia. Curr Top Microbiol Immunol.
279:299–319. 2004.PubMed/NCBI
|
|
23
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yi P, Lu FE, Xu LJ, Chen G, Dong H and
Wang KF: Berberine reverses free-fatty-acid-induced insulin
resistance in 3T3-L1 adipocytes through targeting IKKbeta. World J
Gastroenterol. 14:876–883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang CH, Yu RY, Liu YH, Tu XY, Tu J, Wang
YS and Xu GL: Interaction of baicalin with berberine for glucose
uptake in 3T3-L1 adipocytes and HepG2 hepatocytes. J Ethnopharmaco.
151:864–872. 2014. View Article : Google Scholar
|
|
26
|
Inoue K, Kulsum U, Chowdhury SA, Fujisawa
S, Ishihara M, Yokoe I and Sakagami H: Tumor-specific cytotoxicity
and apoptosis-inducing activity of berberines. Anticancer Res.
25:4053–4059. 2005.PubMed/NCBI
|
|
27
|
Liu B, Wang G, Yang J, Pan X, Yang Z and
Zang L: Berberine inhibits human hepatoma cell invasion without
cytotoxicity in healthy hepatocytes. PLoS One. 6:e214162011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Liu L, Shi Y, Cao H, Chaturvedi R,
Calcutt MW, Hu T, Ren X, Wilson KT, Polk DB and Yan F: Berberine
induces caspase-independent cell death in colon tumor cells through
activation of apoptosis-inducing factor. PLoS One. 7:e364182012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murthy Chidambara KN, Jayaprakasha GK and
Patil BS: The natural alkaloid berberine targets multiple pathways
to induce cell death in cultured human colon cancer cells. Eur J
Pharmacol. 688:14–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu T, Tang B and Sun X: Development of
Inhibitors Targeting hypoxia-inducible factor 1 and 2 for cancer
therapy. Yonsei Med J. 58:489–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Melillo G: Targeting hypoxia cell
signaling for cancer therapy. Cancer Metastasis Rev. 26:341–352.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang X, Yang B, Cai J, Zhang C, Zhang Q,
Xu L, Qin Q, Zhu H, Ma J, Tao G, et al: Berberine enhances
radiosensitivity of esophageal squamous cancer by targeting HIF-1α
in vitro and in vivo. Cancer Biol Ther. 14:1068–1073. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin S, Tsai SC, Lee CC, Wang BW, Liou JY
and Shyu KG: Berberine inhibits HIF-1 alpha expression via enhanced
proteolysis. Mol Pharmacol. 66:612–619. 2004.PubMed/NCBI
|
|
34
|
Zhang C, Yang X, Zhang Q, Yang B, Xu L,
Qin Q, Zhu H, Liu J, Cai J, Tao G, et al: Berberine radiosensitizes
human nasopharyngeal carcinoma by suppressing hypoxia-inducible
factor-1α expression. Acta Otolaryngol. 134:185–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Zhang C, Yang X, Yang B, Wang J,
Kang Y, Wang Z, Li D, Huang G, Ma Z, et al: Berberine inhibits the
expression of hypoxia induction factor-1alpha and increases the
radiosensitivity of prostate cancer. Diagn Pathol. 9:982014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dazert E and Hall MN: mTOR signaling in
disease. Curr Opin Cell Biol. 23:744–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen ZZ: Berberine induced apoptosis of
human osteosarcoma cells by inhibiting phosphoinositide 3
kinase/protein kinase B (PI3K/Akt) signal pathway activation. Iran
J Public Health. 45:578–585. 2016.PubMed/NCBI
|
|
39
|
Kou Y, Li L, Li H, Tan Y, Li B, Wang K and
Du B: Berberine suppressed epithelial mesenchymal transition
through cross-talk regulation of PI3K/AKT and RARα/RARβ in melanoma
cells. Biochem Biophys Res Commun. 479:290–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Milella M, Falcone I, Conciatori F,
Matteoni S, Sacconi A, De Luca T, Bazzichetto C, Corbo V, Simbolo
M, Sperduti I, et al: PTEN status is a crucial determinant of the
functional outcome of combined MEK and mTOR inhibition in cancer.
Sci Rep. 7:430132017. View Article : Google Scholar : PubMed/NCBI
|