Introduction
Lung cancer (LC) ranks highly on the list of lethal
cancers, with 222,500 estimated new cases and 155,870 estimated
deaths in the USA in 2017. Despite improvements in diagnosis and
standard therapy, the prognosis of LC patients remains poor, with a
5-year survival rate of only 18% (1). Non-small cell lung cancer (NSCLC)
accounts for a large proportion of all LC cases, and lung
adenocarcinoma (LUAD) is the predominant histological type of NSCLC
(2–4). An early diagnosis and effective
therapy of LUAD is beneficial for the improved survival of LUAD
patients. Thus, an understanding of the molecular mechanisms of
LUAD and a valid biomarker for LUAD are urgently needed.
MicroRNAs (miRNAs) are small non-coding RNAs that
suppress the translation or initiate the degradation of target
mRNAs by perfectly or imperfectly binding to their 3′-untranslated
regions (5). Currently, there is
mounting evidence that miRNAs have vital functions in the
occurrence and progression of a wide variety of cancers, with
essential roles in diverse biological processes such as the
proliferation, apoptosis, differentiation, drug resistance and
metastasis of cancer cells (6–9).
miR-183-5p, which belongs to the miR-183 family, is located at
chromosome 7q32 with a high level of homogeneity (10). Previous studies have shown that
aberrantly expressed miR-183-5p is involved in the progression of a
wide variety of human cancers, including epithelial ovarian cancer,
breast cancer and cervical cancer (11–13).
Therefore, miR-183-5p has the potential to be a promising target
for the effective diagnosis and therapy of cancers.
Several studies have also focused on miR-183-5p in
LUAD. miR-183-5p was reported to be overexpressed in LUAD and to
correlate with the tumor progression as well as poor prognosis of
LUAD (14). In a study of Zhu et
al, miR-183-5p exhibited a significant ability to distinguish
between non-invasive and invasive LUAD (15). An in vitro study by Zhu et
al declared that miR-183-5p targets PTPN4 to promote the
migratory and invasive capacity of LUAD cells (16). Despite these previous findings,
there is an overall lack of evaluation of the clinicopathological
significance of miR-183-5p in LUAD, and the molecular mechanisms
underlying its role remain unclear. Therefore, the present study
aimed to explore miR-183-5p expression in LUAD and the diagnostic
as well as prognostic significance of miR-183-5p in LUAD using the
combined methods of Gene Expression Omnibus (GEO) meta-analysis,
data retrieval from The Cancer Genome Atlas (TCGA) and real-time
quantitative polymerase chain reaction (qPCR). We also endeavored
to clarify the molecular function of miR-183-5p in LUAD using in
vitro experiments and bioinformatic analysis of the target
genes.
Materials and methods
Investigation of miR-183-5p expression in
LUAD based on GEO data
Searching strategies and inclusion or
exclusion criteria
miR-183-5p expression in LUAD and non-cancer tissue
data from GEO microarray chips were acquired from GEO (http://www.ncbi.nlm.nih.gov/geo/) using the
following search strategies: (miRNA OR miR) AND (lung OR pulmonary)
AND (cancer OR neoplasm).
The preliminary retrieval results were first
selected by scanning the title and abstract. The reserved studies
after the initial selection were further screened according to the
established inclusion and exclusion criteria. Studies that met the
following characteristics were eligible for the meta-analysis: i)
the study included LUAD and non-cancer tissue samples; ⅱ) the study
provided expression values for miR-183-5p in LUAD and non-cancer
tissues; and ⅲ) the tissue samples in the study originated from
humans. Studies were excluded if i) the study included tissue
samples of only either LUAD or non-cancer tissues; ⅱ) the study
contained insufficient data on miR-183 expression in the tissue
samples; and ⅲ) the tissue samples in the study were not from
humans.
Data extraction
The following information was extracted from the
included GEO datasets to calculate an overall standardized mean
difference (SMD): GSE ID, first author, publication year, country,
experimental type, sample type, platform, number (N) of cases in
cancer group, mean (M) ± standard deviation (SD) of miR-183-5p
expression in cancer group, N of cases in the non-cancer group, and
M±SD of miR-183-5p expression in the non-cancer group. To obtain
the sensitivity, specificity and Youden index for the data
elements, a summary receiver operating characteristic (SROC) curve
was created, and a receiver operating characteristic (ROC) curve
was created for each GSE dataset by SPSS v.22.0. True positivity
(TP), false positivity (FP), false negativity (FN) and true
negativity (TN) of each GSE dataset were calculated according to
the maximum Youden index and the corresponding cut-off value.
GEO meta-analysis
The effect sizes of the selected studies were
aggregated as the SMD with 95% confidence intervals (95% CI).
Chi-square tests of Q and the I2 statistic were employed
for the evaluation of heterogeneity between included studies. A
P<0.05 or an I2>50% was considered as significant
heterogeneity, which implied that a random-effect model should be
applied to pool the effect sizes. Otherwise, a fixed-effect model
was utilized to pool the effect sizes when P>0.05 or
I2<50% (17). Then,
subgroup analysis was used to detect the source of heterogeneity
based on the characteristics of the studies. The impact of a single
study on the overall pooling results was evaluated by sensitivity
analysis through omission of each study one at a time.
Additionally, Begg's and Egger's tests were carried out to confirm
whether publication bias existed in the studies.
To assess the overall diagnostic value of all the
included GSE datasets as well as the diagnostic value of plasma
miRNA, Meta-DiSc v.1.4 was employed to plot SROC curves based on
the TP, FP, FN and TN value of all studies. Value for area under
curve (AUC) value ranging from 0.5 to 1 was indicative of a
diagnostic capacity from poor to superior, respectively (18).
TCGA data excavation
The clinicopathological significance of miR-183-5p
expression in LUAD was further analyzed with expression data of
miR-183-5p precursor miR-183 in LUAD downloaded from TCGA
(https://cancergenome.nih.gov/). All
statistical analysis of TCGA data was performed in SPSS v.22.0 and
the expression value of miR-183 was presented in the form of M±SD.
The difference of miR-183 expression in two different groups of
clinicopathological parameters was evaluated by independent samples
t-test. When there were three or more groups of clinicopathological
parameters, the distribution difference of miR-183 expression was
assessed by Kruskal-Wallis (K-W) test. The diagnostic significance
of miR-183 in LUAD was estimated by ROC curves and the implications
of AUC for diagnostic ability of miR-183 were the same as stated
above. Additionally, all the LUAD patients were divided based on
the average of miR-183 expression value, and Kaplan-Meier survival
curves were utilized to measure the influence of miR-183 on the
prognosis of LUAD patients. P<0.05 was considered
significant.
Validation of the clinicopathological
significance of miR-183-5p in LUAD using qPCR
Patients
A total of 101 LUAD tissues and paired non-cancer
tissues (56 males and 45 females) processed with formalin fixation
and paraffin embedding were collected from the First Affiliated
Hospital of Guangxi Medical University (Nanning, China) during the
period from January 2012 to February 2014. The study was approved
by the Research Ethics Committee of the First Affiliated Hospital
of Guangxi Medical University. Signed informed consents were
acquired from all the LUAD patients prior to their involvement in
this study.
qPCR
The extraction and normalization of RNA as well as
qPCR was carried out as described in previous studies (19,20).
The coding sequence of miR-183-5p as identified through
TaqMan® MicroRNA Assays (cat. no. 4427975-000416;
Applied Biosystems: Thermo Fisher Scientifi, Inc., Grand Island,
NY, USA) was 000417, UGUAAACAUCCUCGA CUGGAAG. A TaqMan®
MicroRNA Reverse Transcription kit (4366596; Applied Biosystems:
Thermo Fisher Scientifi, Inc.) was applied to perform the RT
reactions in a 10-µl volume. An Applied Biosystems PCR 7900
instrument was utilized to conduct the PCR. All the experiments
including blank controls were carried out in triplicate. The
difference in miR-183-5p expression between LUAD and peripheral
non-cancer tissues was evaluated with the method of
2−ΔCq.
Statistical analysis for qPCR
The statistical analysis for qPCR data was conducted
in SPSS v.22.0. miR-183-5p expression in LUAD and non-cancer
tissues was compared by paired samples t-tests and the subsequent
analysis of the clinicopathological significance of miR-183-5p
expression in LUAD was carried out as described in the TCGA data
excavation section.
Integrated meta-analysis
To achieve an overall assessment of the
clinicopathological significance of miR-183-5p in LUAD, we pooled
all the expression and diagnostic data of miR-183-5p from included
GSE datasets, extracted TCGA data and qPCR results to conduct an
integrated meta-analysis. SMD and SROC were calculated from all the
pooled studies. Then, subgroup analysis, sensitivity analysis and
detection of publication bias were performed to identify the source
of heterogeneity, as described above.
In vitro experiment
Cell transfections and qPCR
Three human NSCLC cell lines: H460, A-549 and H1299
were acquired from American Type Culture Collection (ATCC,
Manassas, VA, USA) and were cultured in Dulbecco's modified Eagle's
medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc., Grand Island,
NY, USA) containing 10% fetal bovine serum and
penicillin-streptomycin at 37°C under a humidified atmosphere of 5%
CO2. Each of the in vitro experiments was
performed 3 times. Before transfection, LUAD cells were plated in
96-well plates at 2.5×103 cells/well and maintained at
37°C for 24 h. Blank control, negative mimic control, miR-183-5p
mimic, negative inhibitor control and miR-183-5p inhibitor (Ambion;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) were transfected
in LUAD cell lines at a final concentration of 60 nmol/l with
Lipofectamine 2000 following the manufacturer's instructions. The
concentration for transfections was determined based on previous
studies (21,22). miR-183-5p expression was detected
with qPCR in Applied Biosystems PCR 7900 system as stated
previously (23–26).
Effect of miR-183-5p on the biological
behaviors of LUAD cells
The impact of miR-183-5p on the proliferation,
viability and apoptosis of LUAD cells was measured by fluorometric
resorufin viability assays, MTS assays and Apo-ONE Homogeneous
Caspase-3/7 assays, as described in previous studies (25,26).
Statistical analysis of in vitro
experiments
Statistical analysis was carried out in SPSS v.22.0.
All data are expressed in the form of M±SD. Two-way analysis of
variance (ANOVA) and Bonferroni post-tests were used for the
comparisons among groups. We defined P<0.05 as statistically
significant.
Network analysis of the target genes
Acquisition of target genes
Target genes of miR-183-5p came from two sources:
TCGA data and miRWalk v.2.0. Downregulated genes in LUAD were
downloaded from TCGA and subsequently processed with an edgeR
software package. Genes with a false discovery rate (FDR) value
<0.05 were selected as potential target genes. Apart from TCGA,
miRWalk v.2.0 was also applied to the data to predict the target
genes. Genes that appeared in at least four of the 12 software
programs from miRWalk v.2.0 were considered as the possible target
genes. Then, overlapping genes in the recorded lists of potential
target genes from TCGA and miRWalk v.2.0 were regarded as the
reliable target genes of miR-183-5p in LUAD.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis
GO analysis and KEGG pathway analysis from the
online tool Database for Annotation, Visualization and Integration
Discovery (DAVID) were applied to analyze functional enrichment of
the target genes as stated previously (27).
Disease Ontology (DO) analysis
The clustering of the target genes in human diseases
was investigated by DO analysis, which was performed by R package:
clusterProfiler. DO terms with both P and Q-value <0.05 were
selected as the significant terms enriched by target genes.
Protein-protein interaction (PPI)
networks
PPI networks for the key KEGG pathways of LUAD were
created by Search Tool for the Retrieval of Interacting Genes
(STRING)/Proteins v.10.0 with all the component genes. The
relationships between proteins were established from the following
four channels: i) protein interactions documented in the
literature; ⅱ) high-throughput experiments; ⅲ) genomic analysis and
prediction; and ⅳ) studies of co-expression. Hub genes of each PPI
network were confirmed by comparing the connectivity degrees of the
nodes from each PPI network.
Verification of genes in key KEGG
pathways as directly targeted by miR-183-5p
To further verify genes from key KEGG pathways as
directly targeted by miR-183-5p, an independent samples t-test was
conducted by SPSS v.22.0 to examine the differential expression of
these genes in LUAD and non-cancer tissues from TCGA. GraphPad
Prism v.5 was employed to perform a Pearson's correlation test for
evaluating the correlation between miR-183 expression and
expression of the genes from TCGA. Moreover, we downloaded
immunohistochemistry results for these genes in LUAD and normal
tissues from the Human Protein Atlas (HPA), a database integrating
immunohistochemistry analysis of proteins in multiple human tissues
and cancers (28), to confirm
whether these genes were downregulated in LUAD tissues or not.
Results
Included GEO datasets
A total of 340 GEO datasets appeared after initial
search and 84 GEO datasets were retained after scanning titles and
abstracts (29–37). According to the selection criteria,
a total of 13 GEO datasets with 469 LUAD tissues and 272 non-cancer
tissues were eligible for the meta-analysis. The summarization of
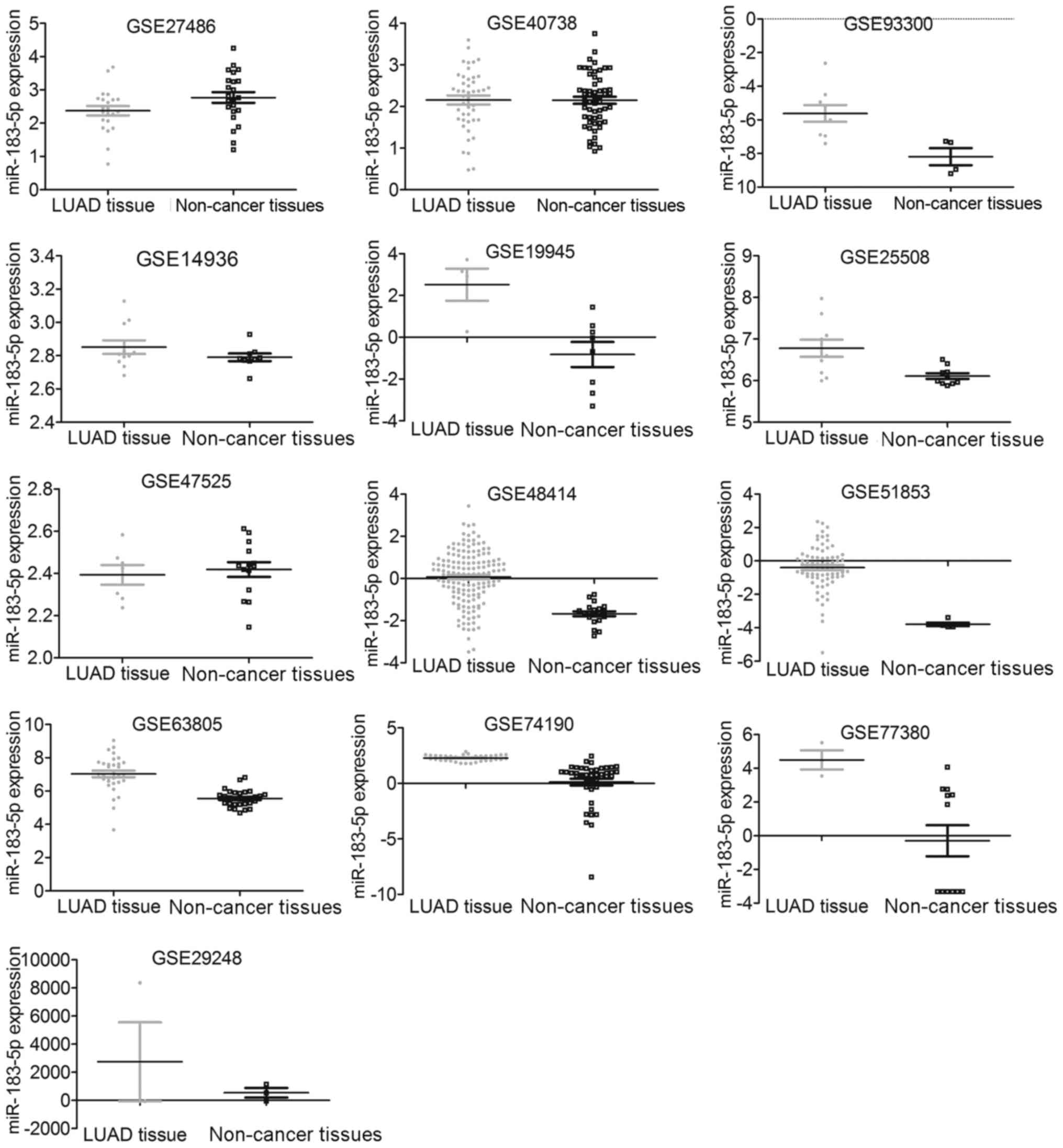
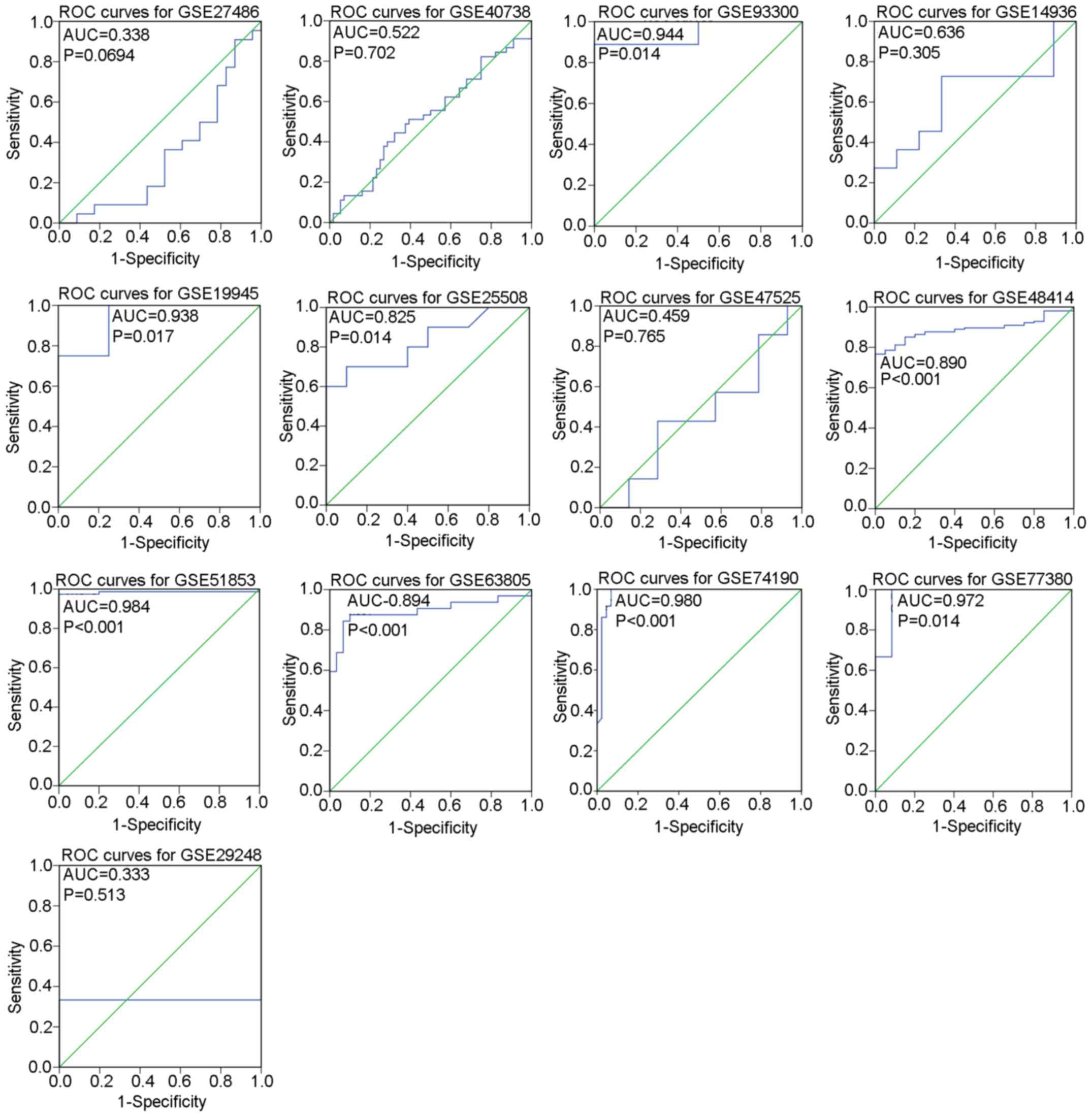
the included studies is displayed in Tables I and II. Distribution of miR-183-5p between
LUAD tissues and non-cancer tissues as well as the diagnostic value
of miR-183-5p for LUAD in each GSE dataset are illustrated by
scatter plots (Fig. 1) and ROC
curves (Fig. 2).
 | Table I.Basic information of all included GSE
datasets. |
Table I.
Basic information of all included GSE
datasets.
| ID | First author | Year of
publication | Country | Experiment
type | Sample type | Platform | Cancer (N) | Cancer (M) | Cancer (SD) | Non-cancer (N) | Non-cancer (M) | Non-cancer
(SD) |
|---|
| GSE27486 | Patnaik (29) | 2011 | USA | Non-coding RNA
profiling by array | Plasma | GPL11432 | 66 | 2.368 | 0.313 | 44 | 0.121 | 2.011 |
| GSE40738 | Patnaik (30) | 2012 | USA | Non-coding RNA
profiling by array | Plasma | GPL16016 | 45 | 2.156 | 0.726 | 56 | 2.150 | 0.636 |
| GSE93300 | Qua | 2017 | China | Non-coding RNA
profiling by array | Plasma | GPL21576 | 9 | −5.618 | 1.490 | 4 | −8.187 | 1.022 |
| GSE14936 | Seike (31) | 2009 | USA | Non-coding RNA
profiling by array | Tissue | GPL8879 | 24 | 2.851 | 0.135 | 22 | 2.790 | 0.069 |
| GSE19945 | Yuichia | 2013 | Japan | Non-coding RNA
profiling by array | Tissue | GPL9948 | 4 | 2.515 | 1.535 | 8 | −0.823 | 1.697 |
| GSE25508 | Nymark (32) | 2011 | Finland | Non-coding RNA
profiling by array | Tissue | GPL7731 | 10 | 6.808 | 0.600 | 10 | 6.249 | 0.181 |
| GSE47525 | van Jaarsveld
(33) | 2013 | Netherlands | Non-coding RNA
profiling by array | Tissue | GPL17222 | 7 | 2.394 | 0.123 | 14 | 2.419 | 0.132 |
| GSE48414 | Bjaanaes (34) | 2014 | Norway | Non-coding RNA
profiling by array | Tissue | GPL16770 | 154 | 0.069 | 1.268 | 20 | −1.678 | 0.508 |
| GSE51853 | Arima (35) | 2014 | Japan | Non-coding RNA
profiling by array | Tissue | GPL7341 | 76 | −0.398 | 1.311 | 5 | −3.789 | 0.234 |
| GSE63805 | Robles (36) | 2015 | USA | Non-coding RNA
profiling by array | Tissue | GPL18410 | 32 | 7.026 | 1.095 | 30 | 5.557 | 0.498 |
| GSE74190 | Jina | 2015 | China | Non-coding RNA
profiling by array | Tissue | GPL19622 | 36 | 2.284 | 0.272 | 44 | 0.121 | 2.011 |
| GSE77380 |
Yoshimotoa | 2016 | Japan | Non-coding RNA
profiling by array | Tissue | GPL16770 | 3 | 4.497 | 0.990 | 12 | −0.303 | 3.193 |
| GSE29248 | Ma (37) | 2012 | China | Non-coding RNA
profiling by array | Tissue | GPL8179 | 3 | 1,647.510 | 3,669.657 | 3 | 303.461 | 534.985 |
 | Table II.Diagnostic data of all included GSE
datasets. |
Table II.
Diagnostic data of all included GSE
datasets.
| Author | ID | TP | FP | FN | TN |
|---|
| Patnaik (29) | GSE27486 | 20 | 20 | 2 | 3 |
| Patnaik (30) | GSE40738 | 20 | 18 | 15 | 38 |
| Qua | GSE93300 | 8 | 0 | 1 | 4 |
| Seike (31) | GSE14936 | 7 | 3 | 4 | 6 |
| Yuichia | GSE19945 | 4 | 2 | 0 | 6 |
| Nymark (32) | GSE25508 | 7 | 1 | 3 | 9 |
| van Jaarsveld
(33) | GSE47525 | 3 | 5 | 4 | 9 |
| Bjaanaes (34) | GSE48414 | 118 | 0 | 36 | 20 |
| Arima (35) | GSE51853 | 74 | 0 | 2 | 5 |
| Robles (36) | GSE63805 | 27 | 2 | 5 | 28 |
| Jina | GSE74190 | 36 | 3 | 0 | 41 |
|
Yoshimotoa | GSE77380 | 3 | 1 | 0 | 11 |
| Ma (37) | GSE29248 | 1 | 0 | 2 | 3 |
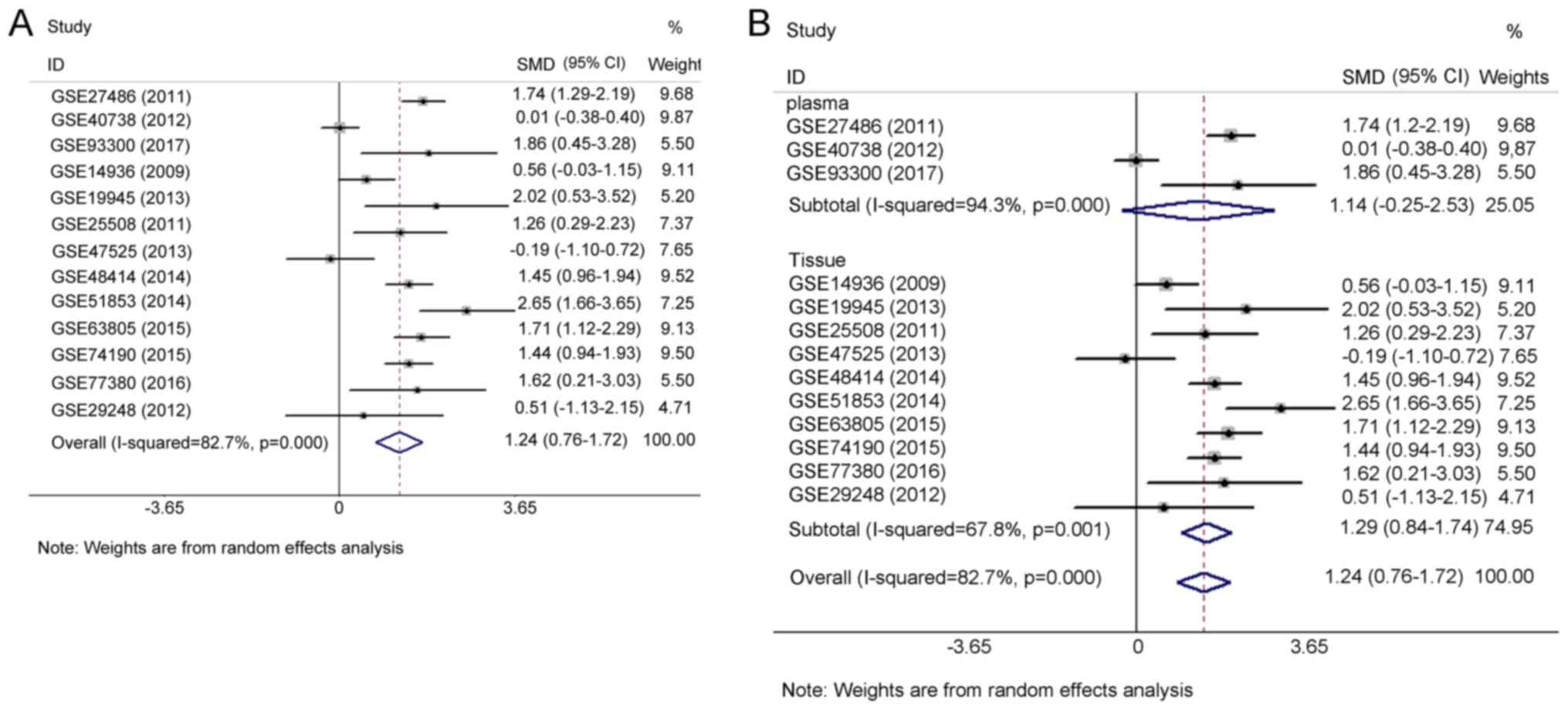
Meta-analysis of GEO datasets
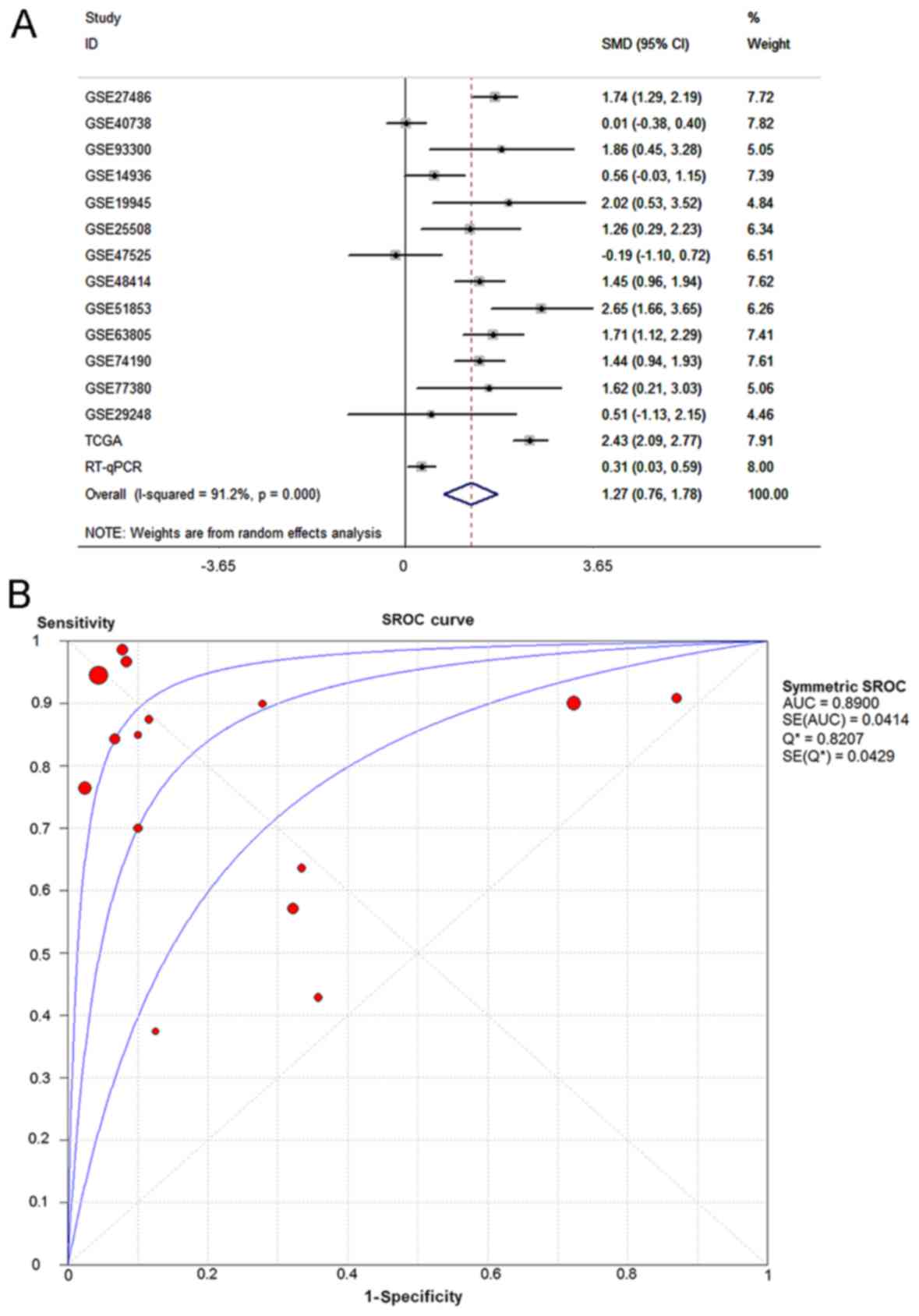
The pooled effect sizes from forest plots (Fig. 3A) indicated that miR-183-5p
expression was significantly higher in LUAD tissues than in
non-cancer tissues (SMD=1.24, 95% CI=0.76–1.72,
I2=82.7%, P<0.001) with considerable study
heterogeneity. To trace the origin of this heterogeneity, subgroup
and sensitivity analyses were performed. Subgroup analysis based on
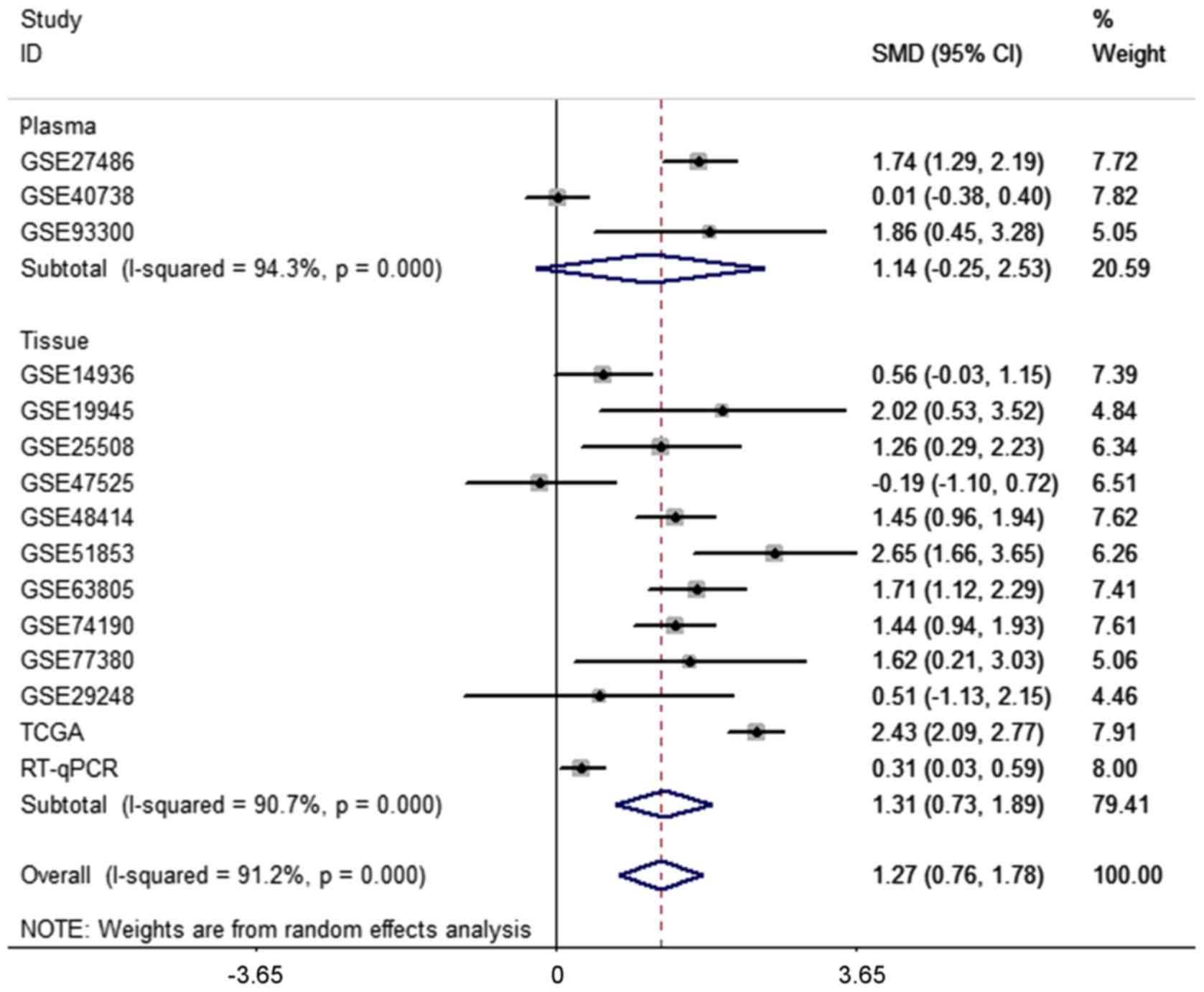
sample types failed to locate the source of heterogeneity due to
the insignificant 95% CI for the plasma subgroup (95%
CI=−0.25–2.53) (Fig. 3B). The
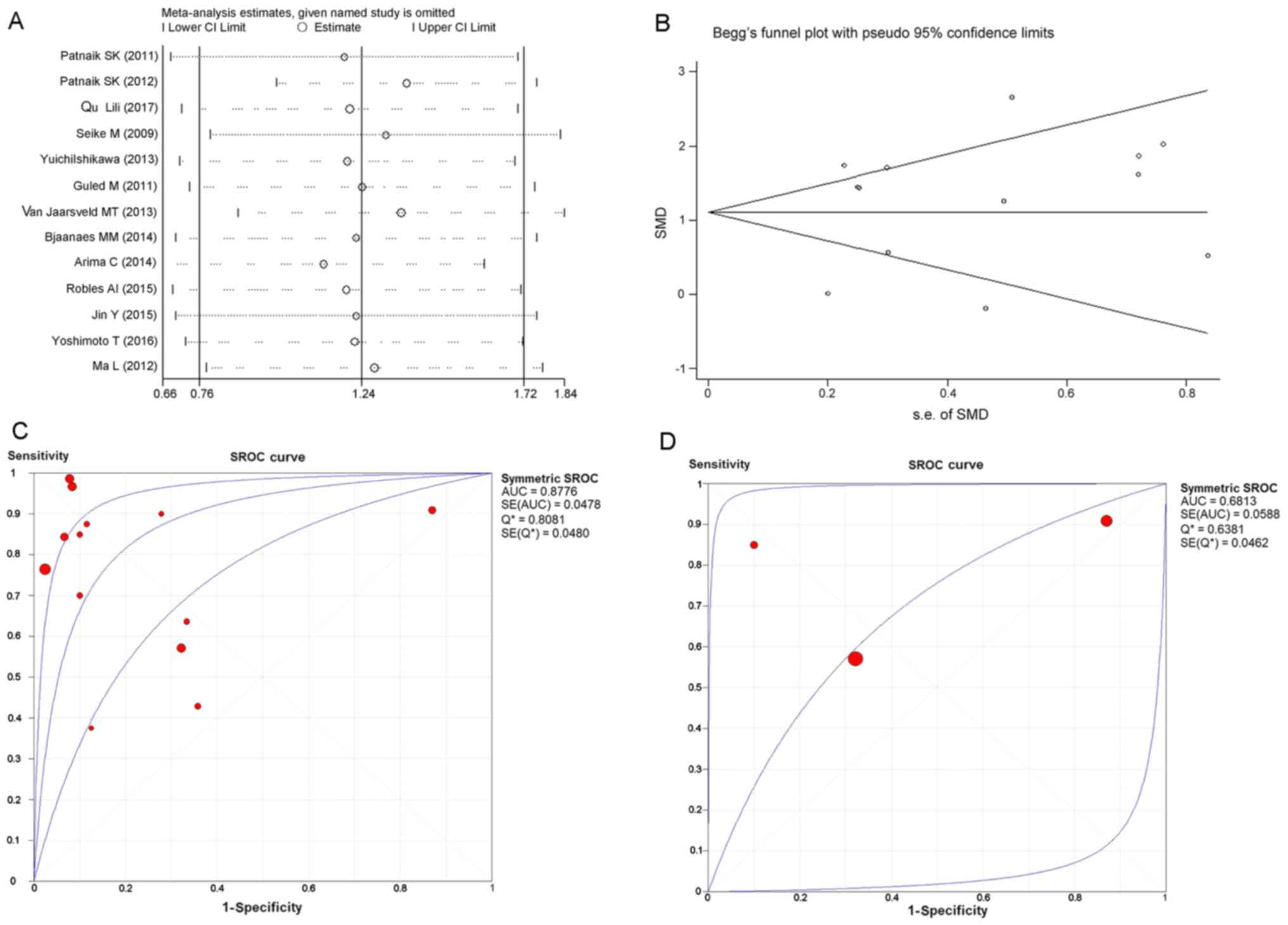
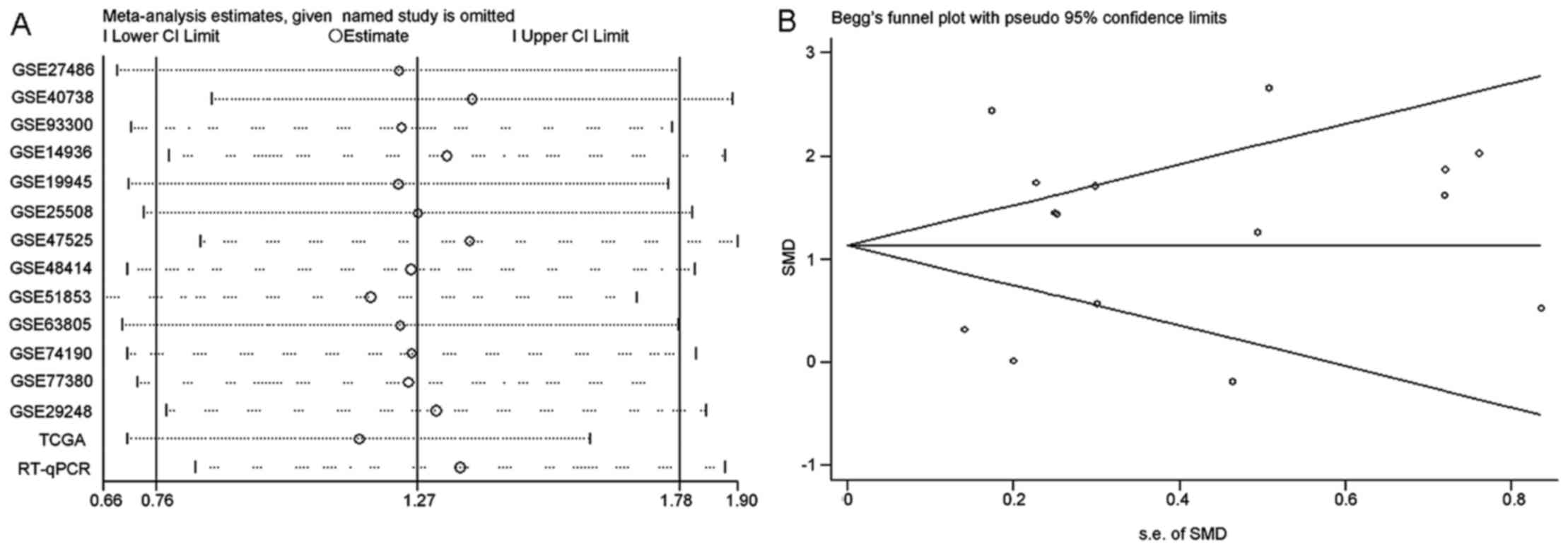
results of sensitivity analysis shown in Fig. 4A revealed that no study caused
obvious deviation from the overall results. Moreover, Begg's and
Egger's tests detected no publication bias (P=0.807) (Fig. 4B). With regard to the diagnostic
ability of miR-183-5p for LUAD, SROC curves generated from all the
included GSE datasets reported an AUC value of 0.8776 (Fig. 4C). Separating GSE datasets sampling
plasma miR-183-5p from all the included GSE datasets, we obtained a
SROC curve with an AUC value of 0.6813 (Fig. 4D).
Clinicopathological significance of
miR-183 expression in TCGA data
As shown in Table
III, miR-183 expression was significantly higher in LUAD
tissues (P<0.001) and patients <60 years of age (P=0.012)
compared with the matched control groups. Nevertheless, no
significant relationships could be established between miR-183
expression and other clinicopathological variables of LUAD
including sex, T stage, number of nodes, number of metastases,
pathological stage, anatomic-organ subdivision and tumor location.
In addition, the results from ROC curves indicated that miR-183
expression showed high diagnostic value for LUAD (AUC=0.985,
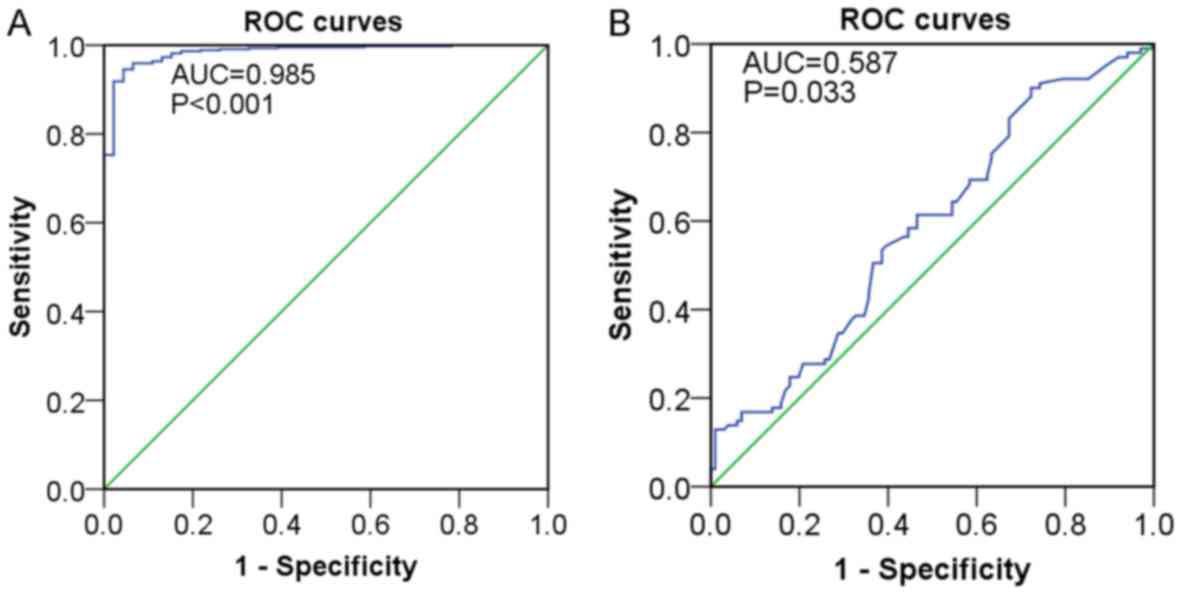
P<0.001) (Fig. 5A). According to
the log-rank test from Kaplan-Meier survival analysis, the
influence of miR-183 on the prognosis of LUAD patients was not
distinct (P>0.05) (data not shown).
 | Table III.Relationship between miR-183
expression and clinicopathological parameters of LUAD from
TCGA. |
Table III.
Relationship between miR-183
expression and clinicopathological parameters of LUAD from
TCGA.
|
|
| miR-183 relevant
expression |
|---|
|
|
|
|
|---|
| Clinicopathological
feature | N | M±SD | t | P-value |
|---|
| Tissue |
|
|
|
|
|
Normal | 46 | 10.661±0.572 | 25.544 | <0.001 |
| Lung
cancer | 441 | 13.180±1.072 |
|
|
| Age (years) |
|
|
|
|
|
≤60 | 199 | 13.317±1.150 | 2.531 | 0.012 |
|
>60 | 232 | 13.054±1.005 |
|
|
| Sex |
|
|
|
|
|
Female | 234 | 13.166±1.082 | 0.288 | 0.774 |
|
Male | 207 | 13.196±1.063 |
|
|
| T stage |
|
|
|
|
|
T1+T2 | 383 | 13.209±1.084 | 1.433 | 0.153 |
|
T3+T4 | 58 | 12.992±0.980 |
|
|
| Node |
|
|
|
|
| No | 288 | 13.155±1.092 | −0.711 | 0.478 |
|
Yes | 152 | 13.231±1.037 |
|
|
| Metastasis |
|
|
|
|
| No | 278 | 13.198±1.012 | 0.591 | 0.555 |
|
Yes | 159 | 13.133±1.173 |
|
|
| Pathological
stage |
|
|
|
|
|
I+II | 346 | 13.155±1.084 | −0.69 | 0.49 |
|
III+IV | 90 | 13.242±1.023 |
|
|
| Anatomic-organ
subdivision |
|
|
|
|
|
L_lower | 69 | 13.042±0.990 | 3.159a | 0.532 |
|
L_upper | 105 | 13.170±1.085 |
|
|
|
R_lower | 84 | 13.129±1.057 |
|
|
|
R_middle | 18 | 13.226±0.913 |
|
|
|
R_upper | 154 | 13.268±1.132 |
|
|
| Location |
|
|
|
|
|
Peripheral | 106 | 13.264±1.031 | 0.09 | 0.928 |
|
Central | 54 | 13.248±1.082 |
|
|
Relationship between miR-183-5p
expression and the clinicopathological features of LUAD from
qPCR
A total of 101 LUAD and paired non-cancer tissues
were taken from patients enrolled in our study. General information
of the collected samples is listed in Table IV. From the results of the paired
samples t-test, miR-183-5p expression was obviously higher in LUAD
tissues (6.579±3.737) than in non-cancer tissues (5.489±3.230)
(P=0.038) (Table IV). Analysis
from independent samples t-test suggested that patients with lymph
node metastasis, vascular invasion and advanced TNM stage (III–IV)
exhibited higher miR-183-5p expression than patients without those
features (P=0.014, 0.038, and 0.014). With respect to the
diagnostic and prognostic value of miR-183-5p for LUAD, ROC curves
revealed the potential diagnostic significance of miR-183-5p for
LUAD (AUC=0.587, P=0.033) (Fig. 5B)
while Kaplan-Meier survival analysis yielded no significant results
supporting the role of miR-183-5p as an important prognostic factor
for LUAD (log-rank P>0.05).
 | Table IV.Relationship between miR-183-5p
expression and clinicopathological parameters of LUAD from qPCR
data. |
Table IV.
Relationship between miR-183-5p
expression and clinicopathological parameters of LUAD from qPCR
data.
|
|
| miR-183-5p relevant
expression |
|---|
|
|
|
|
|---|
| Clinical
variable | N | M±SD | t | P-value |
|---|
| Tissue type |
|
|
|
|
|
LUAD | 101 | 6.579±3.737 | −2.103 | 0.038 |
|
Non-cancer | 101 | 5.489±3.230 |
|
|
| Age (years) |
|
|
|
|
|
<60 | 41 | 6.556±3.613 | −0.050 | 0.960 |
|
≥60 | 60 | 6.594±3.849 |
|
|
| Sex |
|
|
|
|
|
Female | 45 | 7.063±3.865 | −1.169 | 0.245 |
|
Male | 56 | 6.190±3.618 |
|
|
| Smoke |
|
|
|
|
| No | 26 | 5.648±2.473 | 1.346 | 0.186 |
|
Yes | 18 | 4.840±1.501 |
|
|
| Tumor size
(cm) |
|
|
|
|
| ≤3 | 53 | 6.649±3.717 | 0.198 | 0.843 |
|
>3 | 48 | 6.501±3.796 |
|
|
| Lymph node
metastasis |
|
|
|
|
| No | 45 | 5.605±2.746 | −2.513 | 0.014 |
|
Yes | 56 | 7.361±4.238 |
|
|
| Vascular
invasion |
|
|
|
|
| No | 70 | 5.949±2.867 | −2.151 | 0.038 |
|
Yes | 31 | 8.002±4.962 |
|
|
| TNM |
|
|
|
|
|
I–II | 45 | 5.605±2.746 | −2.513 | 0.014 |
|
III–IV | 56 | 7.361±4.238 |
|
|
| Pathological
grading |
|
|
|
|
| I | 17 | 6.988±2.666 | 1.526a | 0.466 |
| II | 61 | 6.305±3.767 |
|
|
|
III | 23 | 7.004±4.367 |
|
|
| EGFR
amplification |
|
|
|
|
| No | 21 | 5.191±2.162 | 0.284 | 0.778 |
|
Yes | 12 | 4.972±2.067 |
|
|
| EGFR protein
expression |
|
|
|
|
|
Low | 22 | 5.336±2.275 | 0.866 | 0.393 |
|
High | 11 | 4.662±1.697 |
|
|
| EGFR mutation |
|
|
|
|
|
Wild-type | 20 | 5.275±2.152 | 0.551 | 0.585 |
|
Mutation c | 13 | 4.859±2.071 |
|
|
Integrated meta-analysis
The results of the integrated meta-analysis which
contained a large sample with 1,011 LUAD tissues and 419 non-cancer
tissues echoed with those from the GEO meta-analysis. miR-183-5p
showed higher expression in LUAD tissues than in non-cancer tissues
(SMD=1.27, 95% CI=0.76–1.78) (Fig.
6A). SROC curves with an AUC value of 0.890 demonstrated the
significant diagnostic value of miR-183-5p for LUAD (Fig. 6B). Nevertheless, the results
contained significant heterogeneity (I2=91.2%,
P<0.001). Further subgroup analysis and sensitivity analysis
still failed to identify the origin of the heterogeneity (Figs. 7 and 8A). Apart from that, Begg's and Egger's
test revealed that no publication bias existed among all the
studies analyzed (P=0.882) (Fig.
8B).
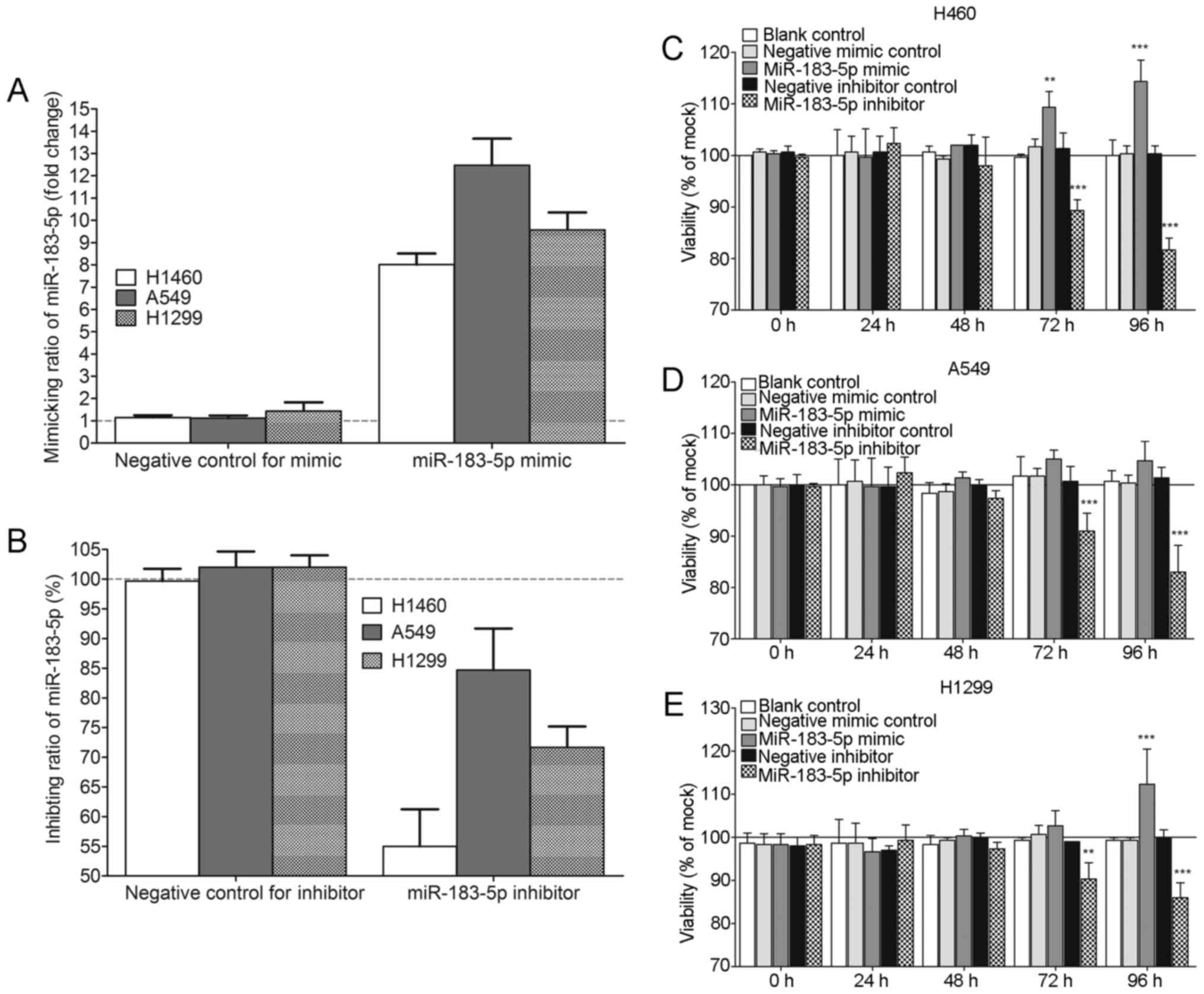
Results from in vitro experiments
Transfection efficiency of miR-183 mimic and
inhibitor was monitored by qPCR. miR-183-5p expression level
increased 8- to 12-fold in all the three cell lines at 96 h
post-transfection with the miR-183 mimic. At 96 h after
transfection with the miR-183 inhibitor, 55–85% miR-183 knockdown
was observed in all the three cell lines (Fig. 9A and B). As illustrated in Fig. 9C-E, cell viability evaluated by
fluorometric resorufin viability assay increased slightly at 48 h
in the H460, A459 and H1299 cells transfected with the miR-183-5p
mimic. The influence of miR-183-5p mimic on cell viability was
observed to be most significant in H460 cells, in which cell
viability was clearly increased compared to that of the blank
control and negative mimic control at 72 h (P<0.01) and 96 h
(P<0.001) (Fig. 9C). In A549
cell lines, cell viability in the miR-183-5p mimic group was higher
than in the blank control and the negative mimic control at 72 and
96 h, although without statistical significance (P>0.05)
(Fig. 9D). In the H1299 cell line,
there was a sharp rise in the cell viability of the miR-183-5p
mimic group at 96 h (P<0.001) (Fig.
9E). In contrast, all the column diagrams in Fig. 9 reflect a downward trend in cell
viability after 48 h post-transfection with miR-183-5p inhibitor.
In all three tested cell lines, cell viability decreased
substantially in the miR-183-5p inhibitor group at 72 h (All
P<0.01) and 96 h (All P<0.001) compared with in the blank
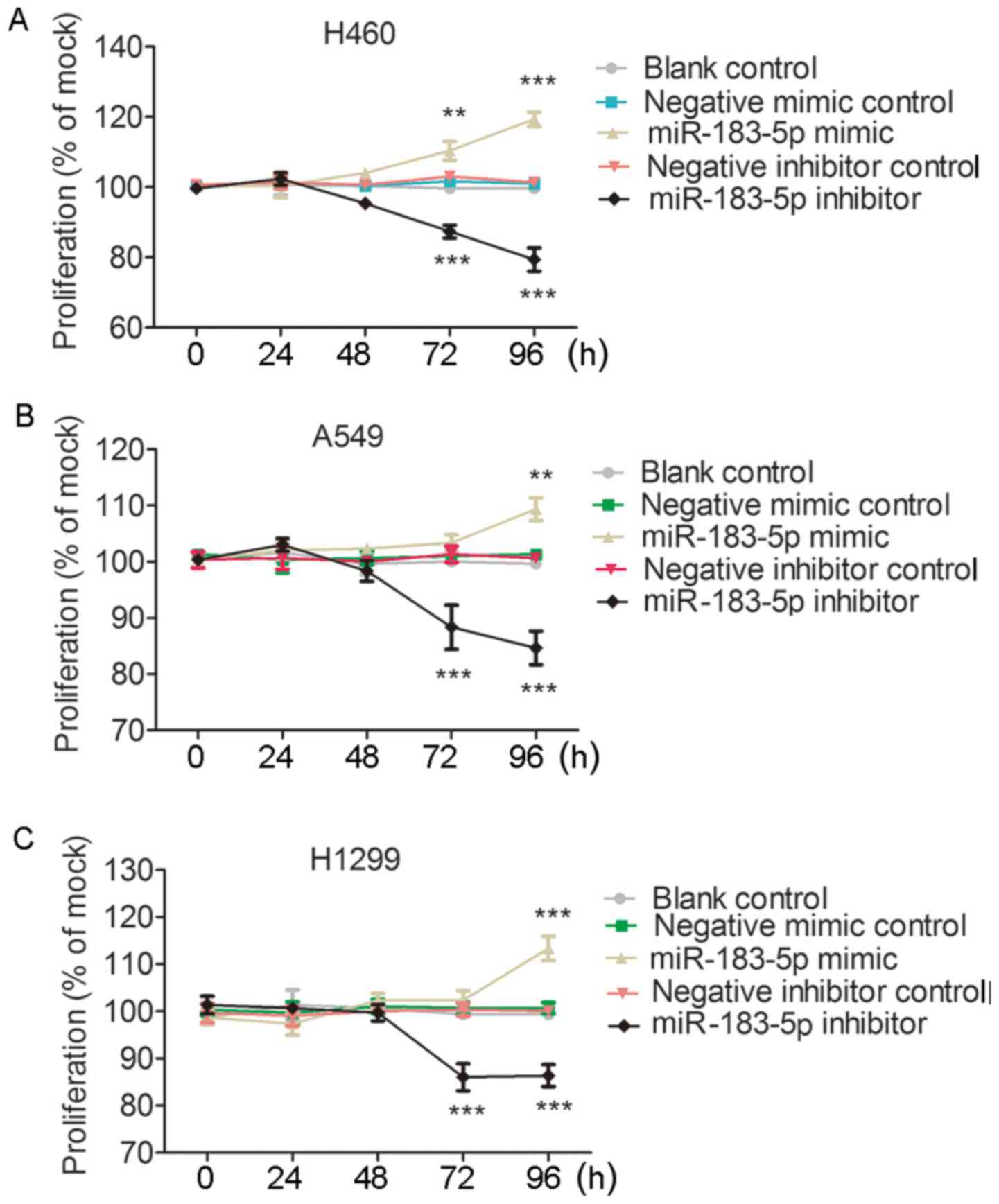
control and the negative inhibitor control (Fig. 9). For the influence of miR-183-5p on
cell proliferation, MTS assays reflected almost the same effect of
miR-183-5p on cell growth as the fluorometric resorufin viability
assay (Fig. 10). With regard to
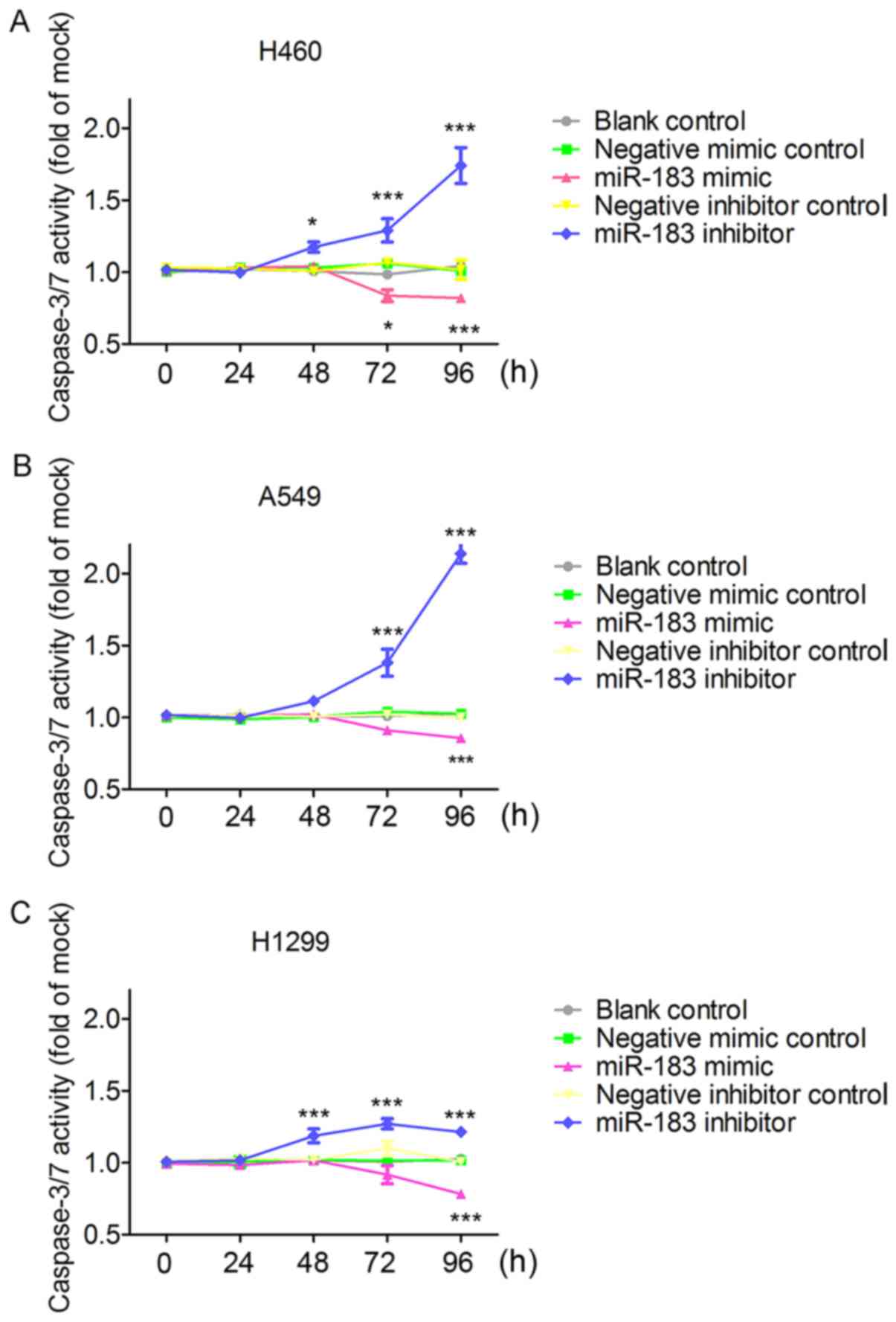
the effect of miR-183-5p on cell apoptosis, results from Apo-ONE
Homogeneous Caspase-3/7 assays showed that the increase in
caspase-3/7 activity started at 48 h in the miR-183 inhibitor group
and the decline of caspase-3/7 activity started at 72 h in the
miR-183 mimic group. Particularly, caspase-3/7 activity was
significantly enhanced (P<0.001) at 72 and 96 h in miR-183-5p
inhibitor group on the basis of the blank control and negative
inhibitor group (Fig. 11), which
was unanimously observed in all the three tested cells. Conversely,
in the miR-183-5p mimic group, caspase-3/7 activity was
significantly reduced on the basis of the blank control
(P<0.001) and negative mimic control (P<0.05) in all the
three tested cell lines (Fig. 11).
In all the three assays, miR-183-5p stimulated cell growth and
suppressed cell apoptosis in a time-dependent (Figs. 9–11) and dose-dependent (data not shown)
manner.
Network analysis of target genes
According to the TCGA data analysis, a total of
2,609 downregulated genes with an FDR value <0.05 were
identified as potential target genes of miR-183-5p. From the
predictions of miRWalk v.2.0, a total of 5,065 genes occurred in at
least four of the 12 online software programs. Taking genes that
were found from both analyses, we obtained 432 reliable target
genes of miR-183-5p in LUAD.
The selected target genes were further refined by GO
and KEGG pathway analysis in DAVID. As shown in Table V, these target genes were most
significantly enriched in the following terms of biological
processes (BP): positive regulation of gene expression, cell
adhesion and actin filament organization. With respect to cellular
component (CC), the top three terms gathered by these target genes
were plasma membrane, integral component of plasma membrane and
receptor complex. These target genes were also significantly
clustered in terms of molecular function (MF), such as Ras
guanyl-nucleotide exchange factor activity, glycosaminoglycan
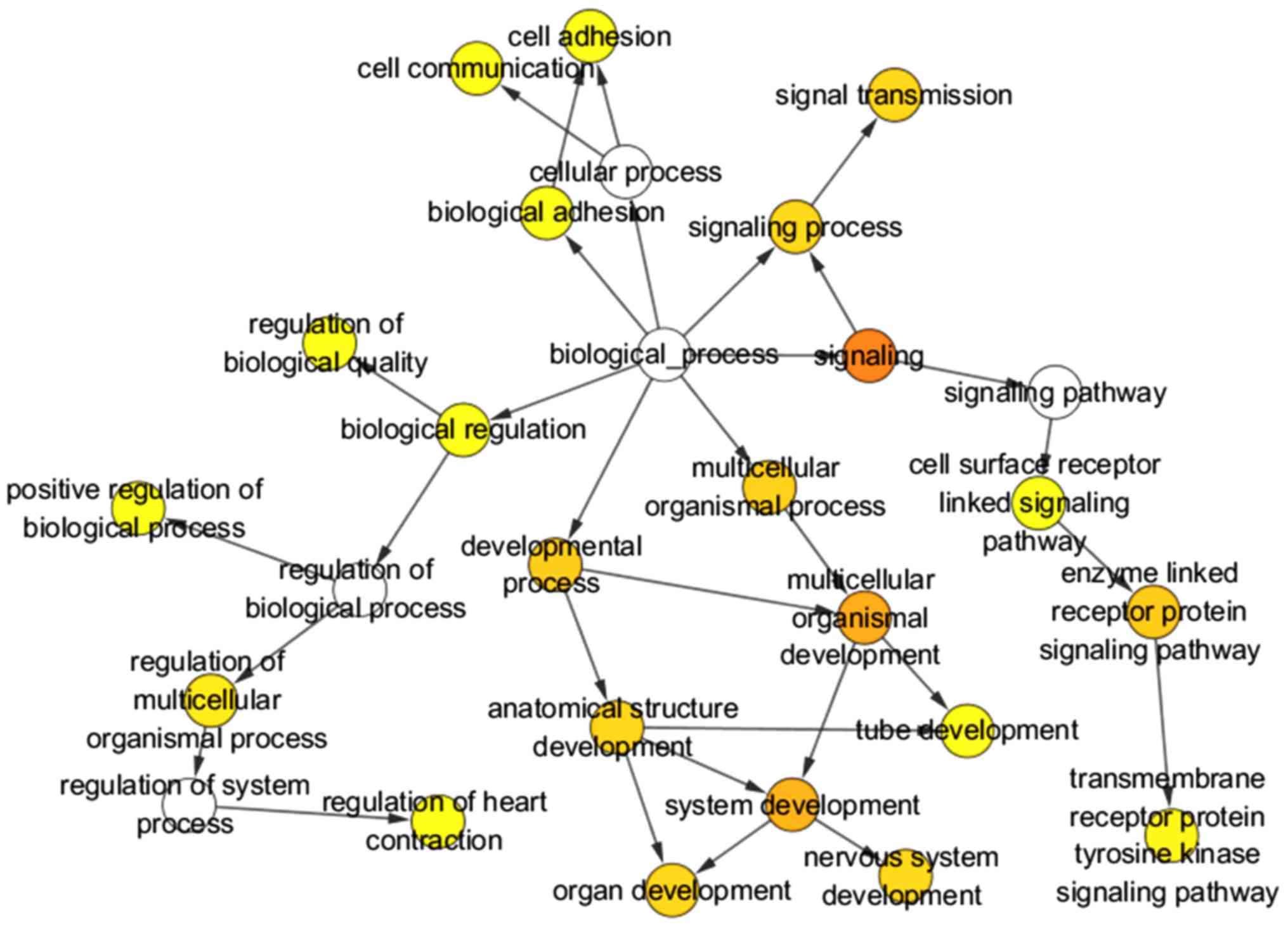
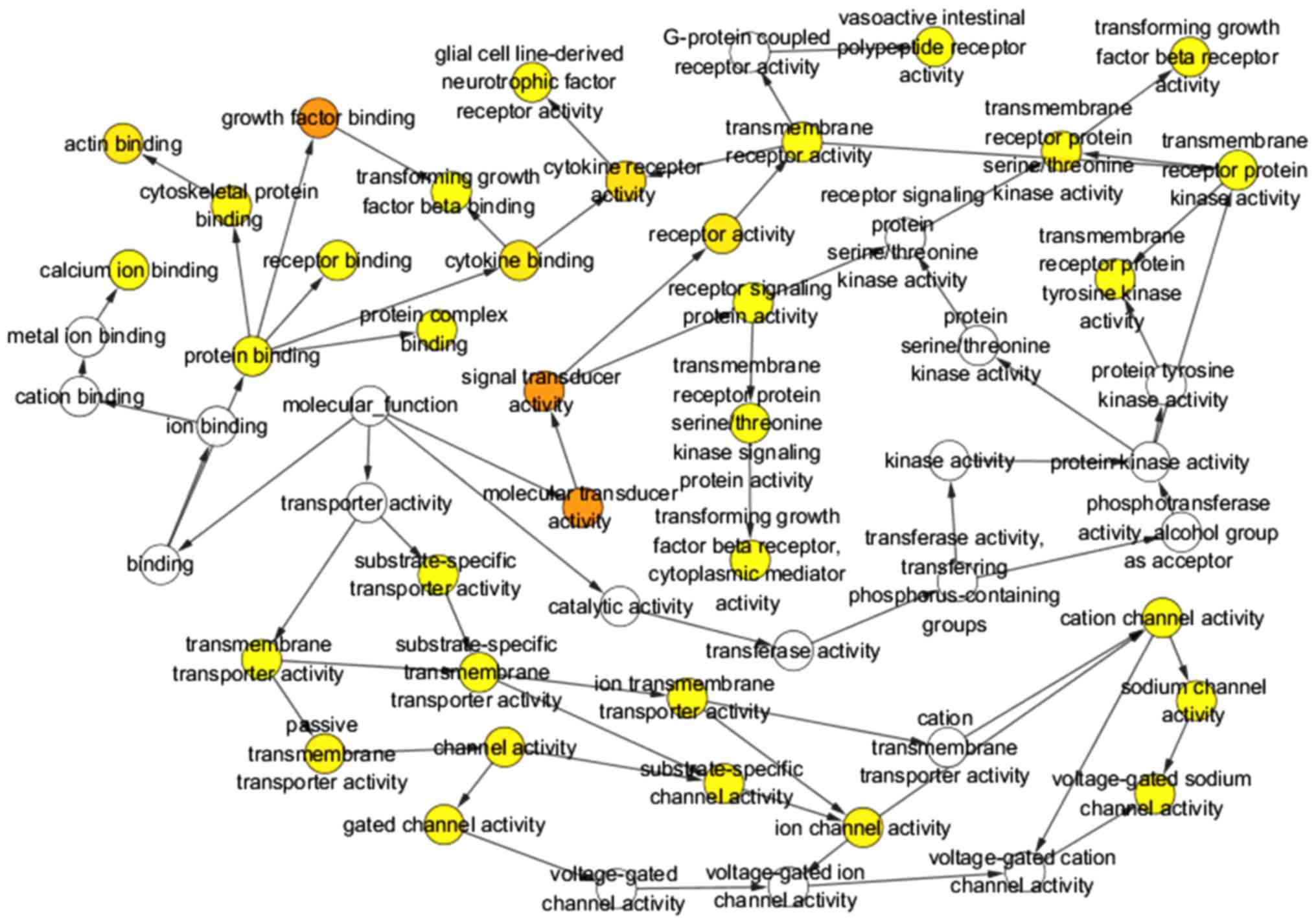
binding and calcium ion binding. Three GO maps (Figs. 12–14) illustrate the functional interactions
of the target genes. With regard to KEGG pathway analysis, the four
most significant signaling pathways for the target genes of
miR-183-5p were cell adhesion molecules (CAMs) and pathways in
cancer, endocytosis and axon guidance (Table VI). The interactions between
component genes of the four most significant pathways are displayed
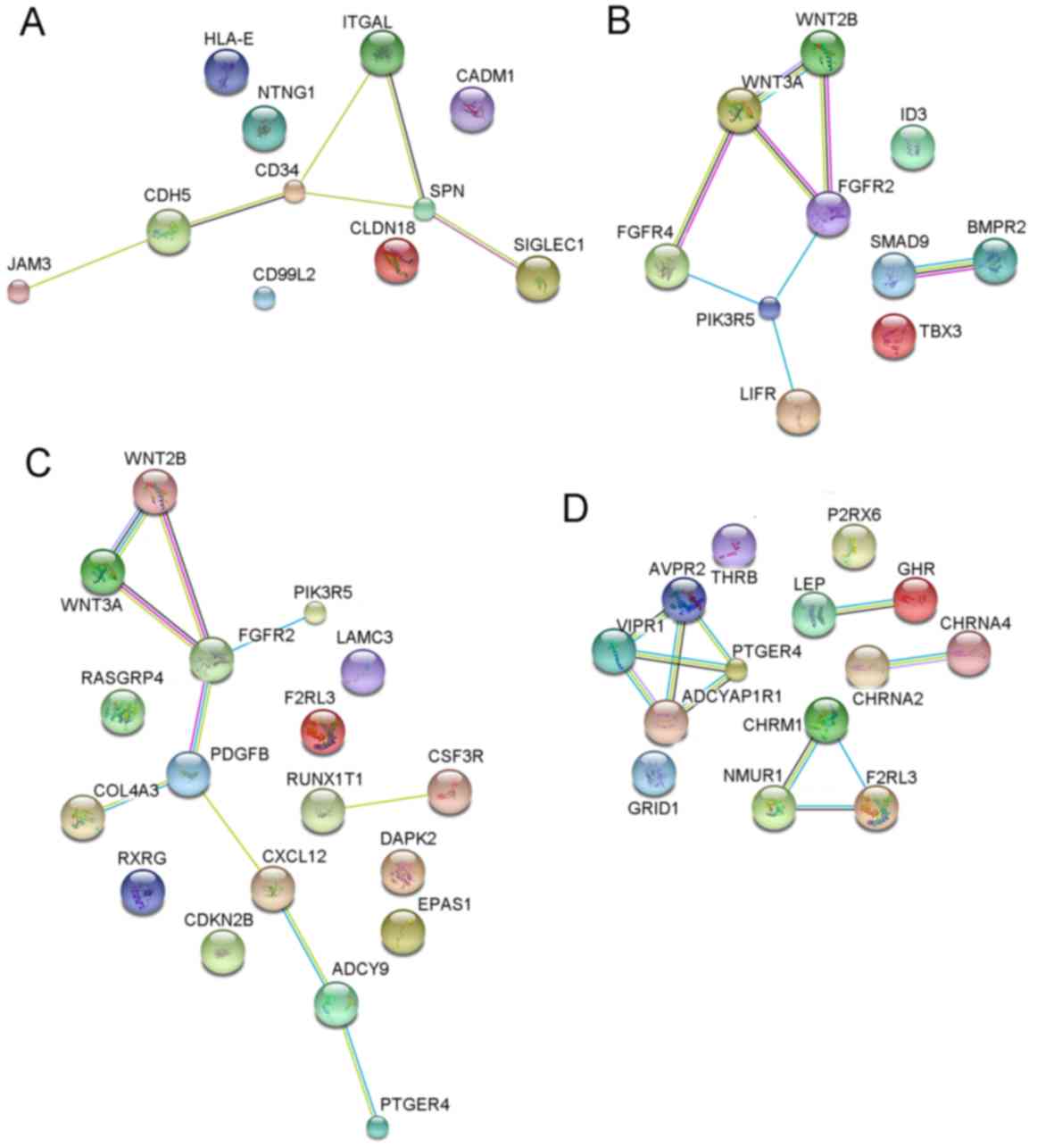
in four PPI networks (Fig. 15). A
total of 10 genes including CD34, sialophorin (SPN),
WNT3A, FGFR2, WNT2B, PDGFB, AVPR2, VIPR1, PTGER4 and
ADCYAP1R1 were selected as the hub genes based on their
degrees of connectivity in corresponding PPI networks.
 | Table V.GO analysis of the target genes. |
Table V.
GO analysis of the target genes.
| Category | ID | Term | Count | % | P-value |
|---|
|
GOTERM_BP_DIRECT | GO:0010628 | Positive regulation
of gene expression | 21 | 4.872 | 1.66E-06 |
|
GOTERM_BP_DIRECT | GO:0007155 | Cell adhesion | 27 | 6.265 | 1.25E-05 |
|
GOTERM_BP_DIRECT | GO:0007015 | Actin filament
organization | 10 | 2.320 | 2.90E-05 |
|
GOTERM_BP_DIRECT | GO:0007165 | Signal
transduction | 47 | 10.905 | 9.06E-05 |
|
GOTERM_BP_DIRECT | GO:0010629 | Negative regulation
of gene expression | 12 | 2.784 | 2.39E-04 |
|
GOTERM_BP_DIRECT | GO:0032870 | Cellular response
to hormone stimulus | 7 | 1.624 | 4.46E-04 |
|
GOTERM_BP_DIRECT | GO:0009611 | Response to
wounding | 8 | 1.856 | 4.71E-04 |
|
GOTERM_BP_DIRECT | GO:0001934 | Positive regulation
of protein phosphorylation | 11 | 2.552 | 5.30E-04 |
|
GOTERM_BP_DIRECT | GO:0070374 | Positive regulation
of ERK1 and ERK2 cascade | 13 | 3.016 | 5.39E-04 |
|
GOTERM_BP_DIRECT | GO:0050665 | Hydrogen peroxide
biosynthetic process | 4 | 0.928 | 8.20E-04 |
|
GOTERM_CC_DIRECT | GO:0005886 | Plasma
membrane | 146 | 33.875 | 2.38E-10 |
|
GOTERM_CC_DIRECT | GO:0005887 | Integral component
of plasma membrane | 64 | 14.849 | 4.73E-08 |
|
GOTERM_CC_DIRECT | GO:0043235 | Receptor
complex | 15 | 3.480 | 7.61E-07 |
|
GOTERM_CC_DIRECT | GO:0009986 | Cell surface | 32 | 7.425 | 1.20E-06 |
|
GOTERM_CC_DIRECT | GO:0005578 | Proteinaceous
extracellular matrix | 19 | 4.408 | 2.74E-05 |
|
GOTERM_CC_DIRECT | GO:0030054 | Cell junction | 26 | 6.032 | 2.97E-05 |
|
GOTERM_CC_DIRECT | GO:0016021 | Integral component
of membrane | 151 | 35.035 | 3.25E-05 |
|
GOTERM_CC_DIRECT | GO:0009897 | External side of
plasma membrane | 16 | 3.712 | 7.45E-05 |
|
GOTERM_CC_DIRECT | GO:0001725 | Stress fiber | 7 | 1.624 | 0.001128084 |
|
GOTERM_CC_DIRECT | GO:0031674 | I band | 5 | 1.160 | 0.001440976 |
|
GOTERM_MF_DIRECT | GO:0005088 | Ras
guanyl-nucleotide exchange factor activity | 11 | 2.552 | 2.01E-04 |
|
GOTERM_MF_DIRECT | GO:0005539 | Glycosaminoglycan
binding | 5 | 1.160 | 5.23E-04 |
|
GOTERM_MF_DIRECT | GO:0005509 | Calcium ion
binding | 30 | 6.961 | 9.67E-04 |
|
GOTERM_MF_DIRECT | GO:0004714 | Transmembrane
receptor protein tyrosine kinase activity | 6 | 1.392 | 0.001299161 |
|
GOTERM_MF_DIRECT | GO:0008289 | Lipid binding | 11 | 2.552 | 0.001702358 |
|
GOTERM_MF_DIRECT | GO:0050431 | Transforming growth
factor β binding | 4 | 0.928 | 0.004589053 |
|
GOTERM_MF_DIRECT | GO:0004896 | Cytokine receptor
activity | 5 | 1.160 | 0.007406533 |
|
GOTERM_MF_DIRECT | GO:0008201 | Heparin
binding | 10 | 2.320 | 0.008250718 |
|
GOTERM_MF_DIRECT | GO:0005248 | Voltage-gated
sodium channel activity | 4 | 0.928 | 0.008762612 |
|
GOTERM_MF_DIRECT | GO:0005024 | Transforming growth
factor β-activated receptor activity | 3 | 0.696 | 0.009159889 |
 | Table VI.KEGG pathway analysis of the target
genes. |
Table VI.
KEGG pathway analysis of the target
genes.
| Category | ID | Terms | Count | % | P-value |
|---|
| KEGG_PATHWAY | hsa04514 | CAMs | 11 | 2.552204176 | 0.001466545 |
| KEGG_PATHWAY | hsa04550 | Signaling pathways
regulating pluripotency of stem cells | 10 | 2.320185615 | 0.004641057 |
| KEGG_PATHWAY | hsa05200 | Pathways in
cancer | 18 | 4.176334107 | 0.008133914 |
| KEGG_PATHWAY | hsa04080 | Neuroactive
ligand-receptor interaction | 14 | 3.248259861 | 0.010639115 |
| KEGG_PATHWAY | hsa00380 | Tryptophan
metabolism | 5 | 1.160092807 | 0.012685346 |
| KEGG_PATHWAY | hsa04924 | Renin
secretion | 6 | 1.392111369 | 0.015130907 |
| KEGG_PATHWAY | hsa04670 | Leukocyte
transendothelial migration | 8 | 1.856148492 | 0.018169594 |
| KEGG_PATHWAY | hsa04360 | Axon guidance | 8 | 1.856148492 | 0.026015978 |
| KEGG_PATHWAY | hsa04144 | Endocytosis | 12 | 2.784222738 | 0.033890911 |
| KEGG_PATHWAY | hsa04024 | cAMP signaling
pathway | 10 | 2.320185615 | 0.037334507 |
| KEGG_PATHWAY | hsa04060 | Cytokine-cytokine
receptor interaction | 11 | 2.552204176 | 0.037626628 |
| KEGG_PATHWAY | hsa04725 | Cholinergic
synapse | 7 | 1.62412993 | 0.041290871 |
| KEGG_PATHWAY | hsa04911 | Insulin
secretion | 6 | 1.392111369 | 0.044616235 |
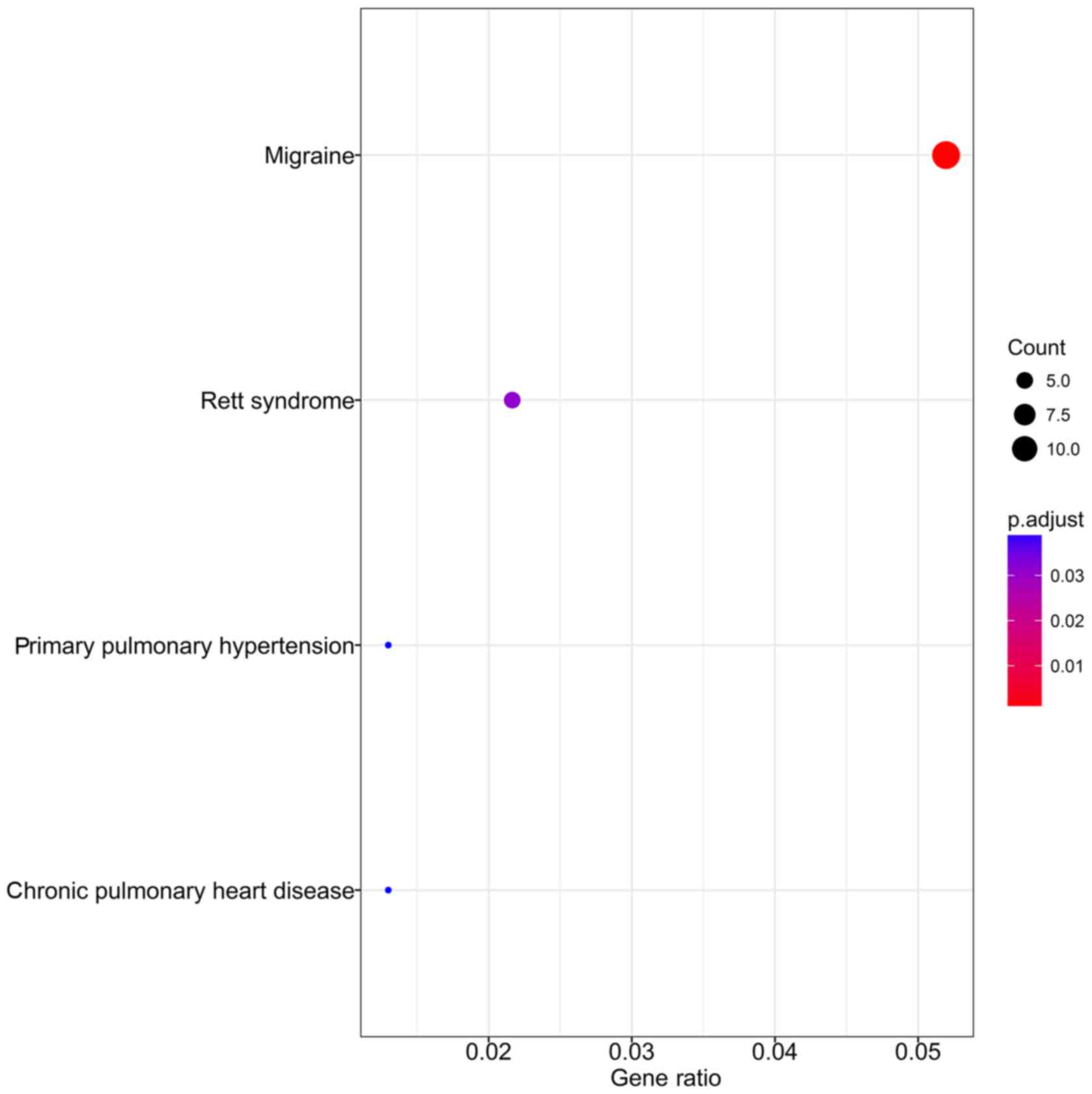
DO analysis by clusterProfiler revealed that the
target genes were significantly associated with the following
diseases: migraine, Rett syndrome, chronic pulmonary heart disease
and primary pulmonary hypertension (all P<0.05 and Q<0.05).
(Table VII) (Fig. 16).
 | Table VII.DO analysis of the target genes. |
Table VII.
DO analysis of the target genes.
| ID | Description | Gene ratio | Bg ratio | P-value | P-adjusted | Q-value | Gene ID | Count |
|---|
| DOID:6364 | Migraine | 12/231 | 73/8,007 | 9.69E-07 | 0.000671237 | 0.000583196 |
477/5241/3683/6532/1636/4842/1524/3949/3952/6323/627/358 | 12 |
| DOID:1206 | Rett syndrome |
5/231 | 17/8,007 | 8.92E-05 | 0.030893672 | 0.026841619 |
3399/1959/3952/22854/627 | 5 |
| DOID:12326 | Chronic pulmonary
heart disease |
3/231 | 5/8,007 | 0.000227082 | 0.039342028 | 0.034181879 | 659/6532/1636 | 3 |
| DOID:14557 | Primary pulmonary
hypertension |
3/231 | 5/8,007 | 0.000227082 | 0.039342028 | 0.034181879 | 659/6532/1636 | 3 |
Verification of genes in key KEGG
pathways as directly targeted by miR-183-5p
We conducted an independent samples t-test and
Pearson's correlation test to validate the capabilities of
miR-183-5p to target all the component genes in the CAM pathway.
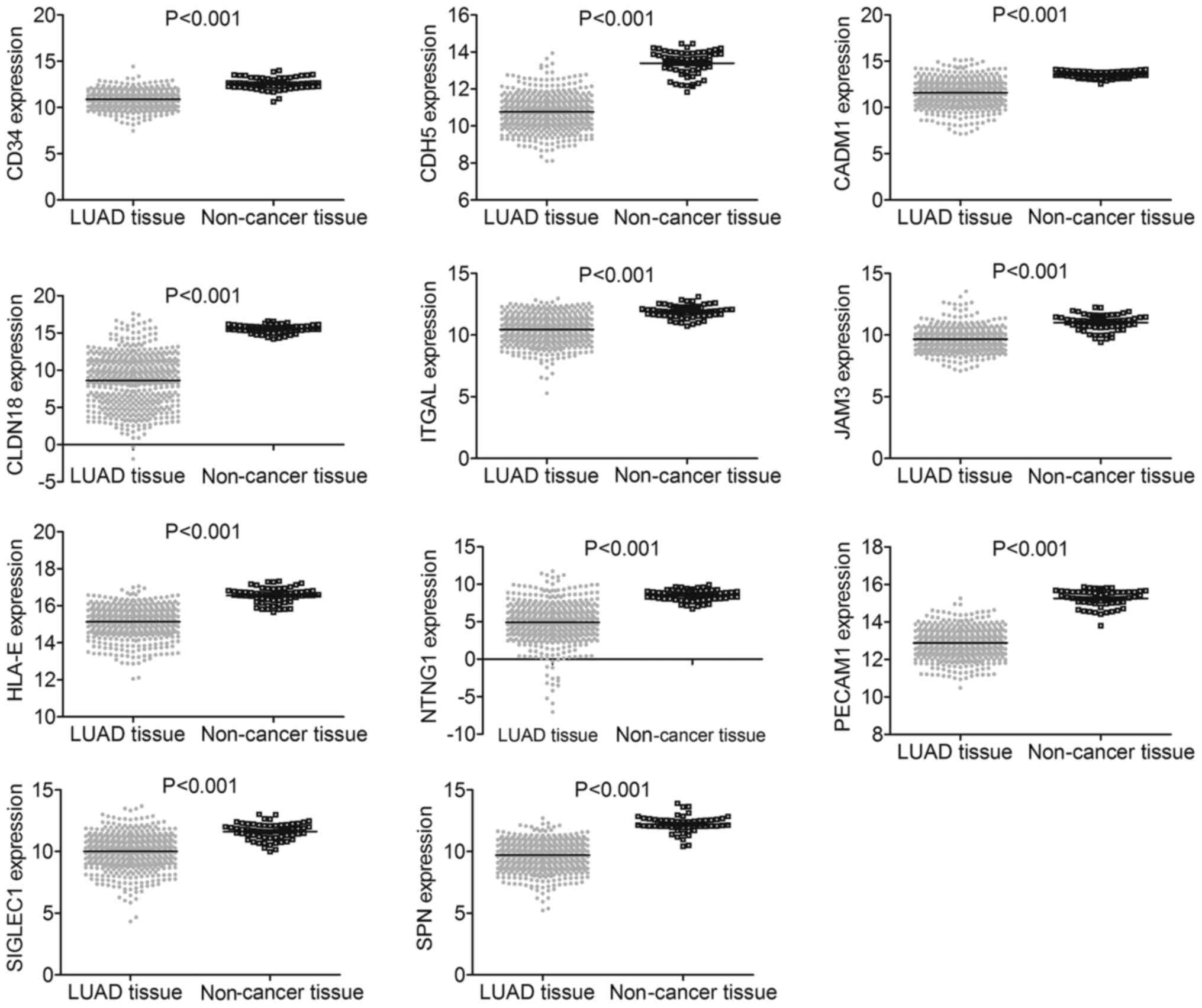
The results from the independent samples t-test suggested that
expression of all these target genes was significantly lower in 535
LUAD tissues than in 59 non-cancer tissues (all P<0.001)
(Fig. 17). Among the 11 component
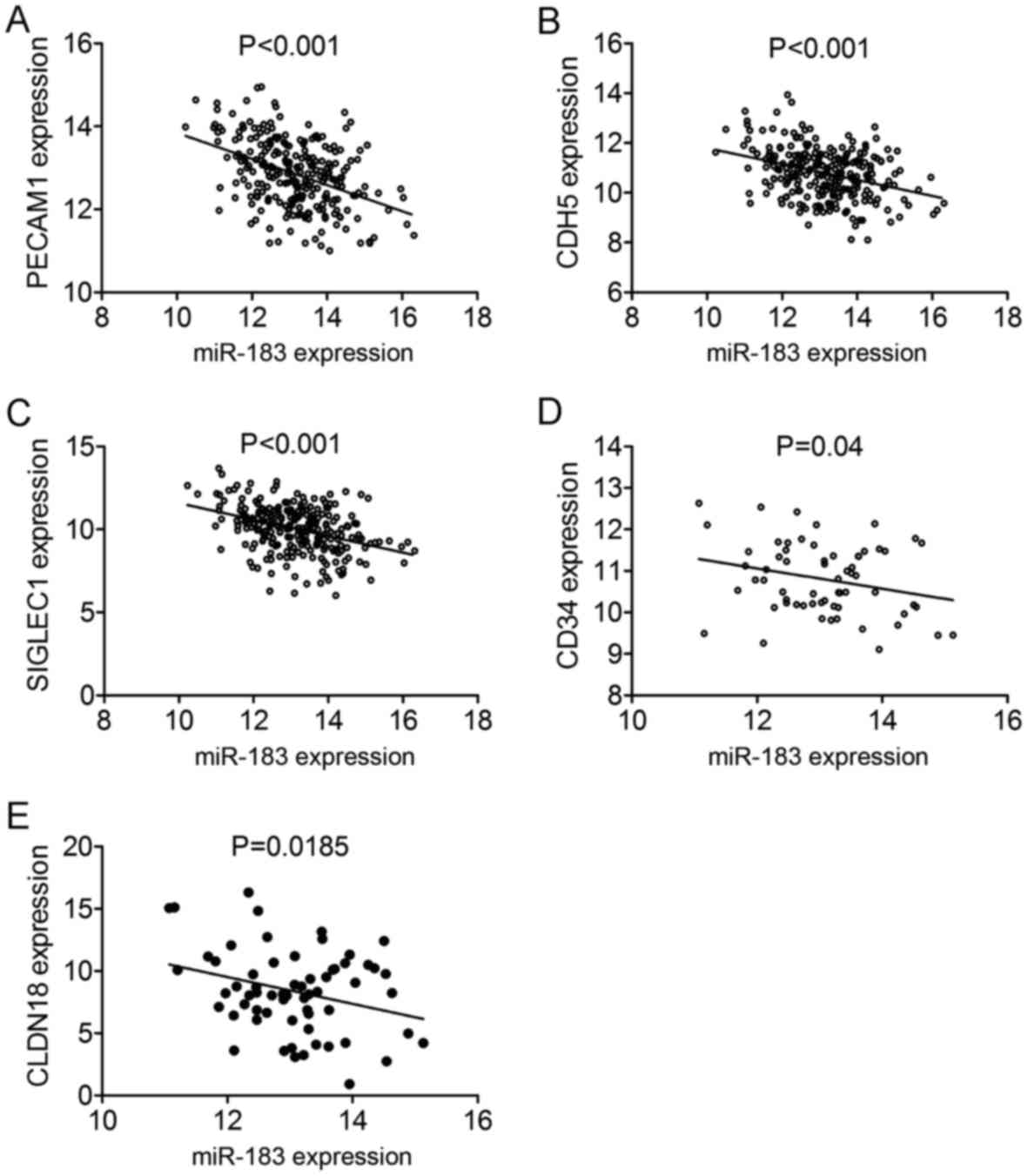
genes, five genes including PECAM1, CDH5, SIGLEC1, CD34 and
CLDN18 showed significant correlation with miR-183-5p in
LUAD (all P<0.05) (Fig. 18).
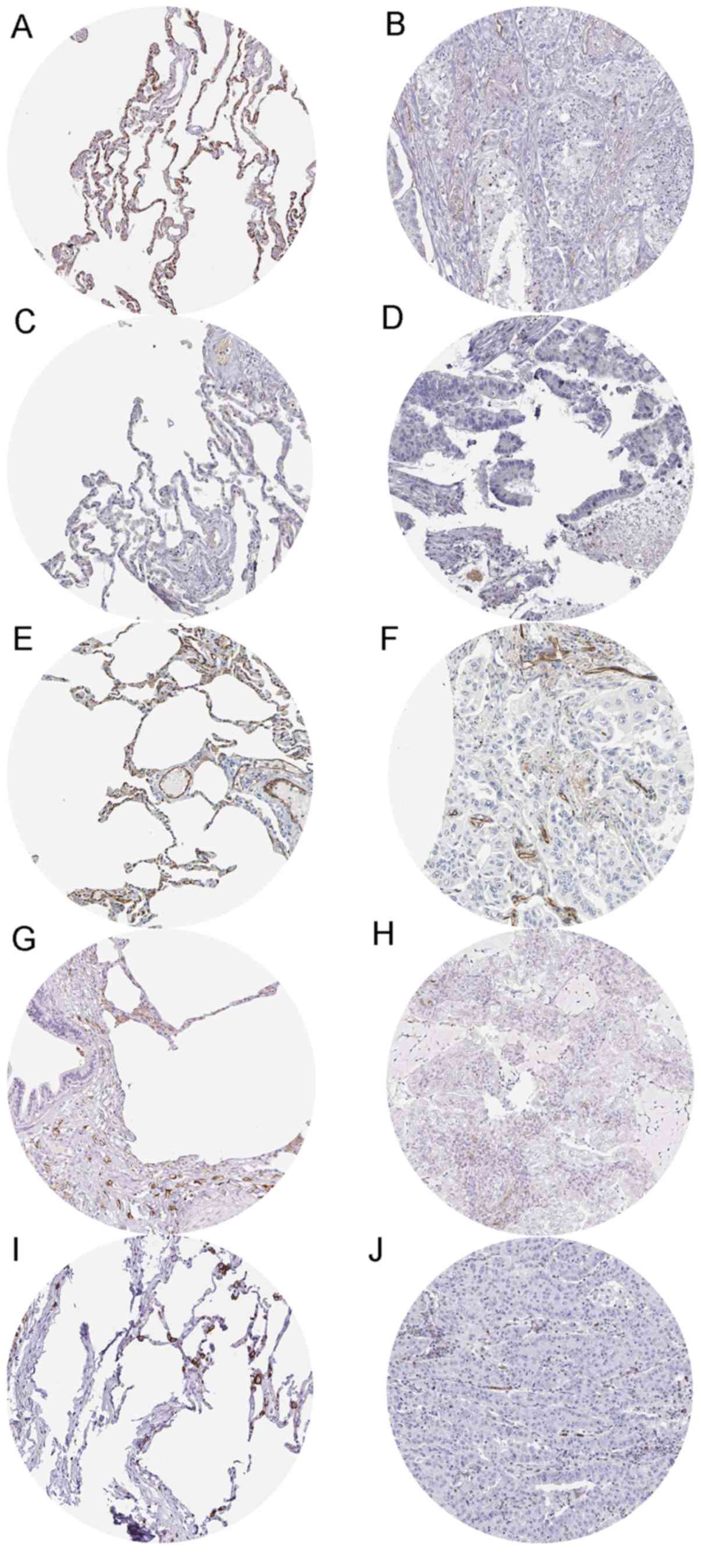
Moreover, immunostaining evidence from the HPA database supported
the downregulation of CD34 (antibody HPA036722), CADM1 (antibody
CAB037266), PECAM1 (antibody HPA004690), SIGLEC1 (antibody
HPA053457) and SPN (antibody HPA055244) in LUAD tissues. Fig. 19 exhibits the immunostaining
results of the five genes in normal and LUAD tissues. The staining
intensity of the five genes was medium or strong in normal tissues,
while low to non-existent in LUAD tissues.
Discussion
In this report, we used a combined method of GEO
meta-analysis, TCGA data mining, qPCR, integrated meta-analysis,
in vitro experiments and bioinformatic analysis of the
target genes to comprehensively investigate the clinicopathological
significance of miR-183-5p in LUAD and its underlying molecular
basis. The results from this report revealed that overexpressed
miR-183-5p had considerable diagnostic value in LUAD and was
associated with the malignant progression of LUAD. Further in
vitro experiments and bioinformatic analysis of the target
genes of miR-183-5p showed the positive effect of miR-183-5p on
cell growth in LUAD and revealed the specific biological processes
and gene pathways common to miR-183-5p target genes, which may
provide new insights into the oncogenesis of LUAD.
GEO meta-analysis, TCGA data mining, qPCR and the
integrated meta-analysis in our study concordantly reported
overexpression of miR-183-5p in LUAD tissues, which was in
agreement with previous studies (38,39).
The diagnostic value of miR-183-5p in LUAD was also studied. We
found that miR-183-5p possessed strong diagnostic capacity for
distinguishing LUAD tissues from non-cancer tissues via ROC curves
derived from all the included GSE datasets, TCGA data mining and
the integrated meta-analysis. ROC curves from qPCR also
demonstrated the diagnostic potential of miR-183-5p for LUAD,
although with weak statistical significance. The difference between
ROC curves from all the included GSE datasets, TCGA data mining,
qPCR and integrated meta-analysis might be explained by the type
and number of samples. It should be noted that all the non-cancer
tissues from qPCR were matched with the corresponding LUAD tissues,
which is not the case in the GEO meta-analysis, the TCGA data
mining or the integrated meta-analysis. Now that sensitive, less
non-invasive biomarker was necessary for the early detection of
LUAD, we singled out GSE datasets sampling plasma miR-183-5p to
assess the diagnostic capacity of plasma miR-183-5p. Nevertheless,
the results indicated a poorer diagnostic ability of plasma
miR-183-5p in our study compared with miR-183-5p from both tissues
and plasma. We conjectured that the less significant diagnostic
ability of plasma miR-183-5p might be attributed to the lower
levels of miR-183-5p in blood samples than in tissues, which is
related to the phenomenon that LUAD cells in lung tissues
assimilating exosomes containing miRNAs from the blood as a
supplement of transcribed essential miRNAs (40–42).
Considering the results of all the diagnostic evaluations, we are
optimistic about the prospect of miR-183-5p as a diagnostic target
for LUAD.
To investigate the role of miR-183-5p in the
development of LUAD, we analyzed the relationship between
miR-183-5p and the clinicopathological characteristics of LUAD.
Given the positive correlation between miR-183-5p overexpression
and lymph node metastasis, vascular invasion and advanced TNM stage
in LUAD, it can be inferred that miR-183-5p may enhance the
malignant properties of LUAD. To answer the question of how
miR-183-5p affects the pathogenesis of LUAD, we conducted in
vitro experiments to determine the influence of miR-183-5p on
cell growth in LUAD.
As suggested by fluorometric resorufin viability
assay and MTS assay, the proliferative ability of LUAD cells was
augmented by miR-183-5p. The promotion of tumor cell growth by
miR-183-5p has also been shown in in vitro experiments with
pediatric acute myeloid leukemia, tongue squamous cell carcinoma
and endometrial cancer (43–46).
To ascertain whether the knockdown of miR-183-5p caused apoptosis
or just inhibited the proliferation of the cancer cells, we
performed Apo-ONE Homogeneous Caspase-3/7 assays. Caspase-3 and −7
are effector caspases that execute cell apoptosis via cleaving
relevant cellular substrates (46).
The negative effect of miR-183-5p on caspase-3/7 activity indicated
that the knockdown of miR-183-5p caused apoptosis of LUAD cells.
Therefore, we hypothesized that miR-183-5p may accelerate the
malignant progression of LUAD by enhancing tumor growth and
inhibiting tumor apoptosis. It was discovered in previous studies
that miR-183-5p modulated the expression of tumor-suppressor genes
such as PDCD4 and SOCS-6 to accelerate the proliferation of cancer
cells (47,48). In an in vitro experiment by
Yan et al, the reduced cell growth and increased cell
apoptosis by miR-183-5p inhibitor was correlated with upregulated
caspase-3 and downregulated anti-apoptotic protein BCL-xl (43). These findings might provide
interpretation of how miR-183-5p contributed to cell growth and
suppressed cell apoptosis in LUAD.
In order to develop effective therapeutic and
diagnostic targets for LUAD, it is not sufficient to merely
identify the relationship between miR-183-5p expression and the
malignant properties of LUAD. Therefore, we further attempted to
explore the underlying molecular basis of the cell growth-promoting
effects of miR-183-5p in LUAD via in silico analysis of
miR-183-5p target genes. The significant terms identified from the
GO analysis including positive regulation of gene expression, cell
adhesion, actin filament organization, Ras guanyl-nucleotide
exchange factor activity, glycosaminoglycan binding and calcium ion
binding imply that target genes regulated by miR-183-5p may
participate in these biological functions to cause the occurrence
and development of LUAD. We can also draw conclusions from the KEGG
pathway analysis. Among all the listed KEGG pathways significantly
identified by analysis of the target genes of miR-183-5p, we noted
that the top two pathways, CAMs and pathways in cancer, were
closely associated with the formation of human cancers. CAMs are
cell surface proteins that mediate the adhesion between cells and
the extracellular matrix. This feature allows CAMs to stimulate the
motility, invasion and angiogenesis of tumor cells (49,50).
We speculate that miR-183-5p may target downstream CAMs to
contribute to the progression of LUAD. Apart from CAMs and pathways
in cancer, other pathways, such as endocytosis and axon guidance,
were also identified as significantly enriched by analysis of the
target genes of miR-183-5p. Involvement of target genes in these
additional pathways may have an impact on LUAD; this awaits further
study. In addition to the above results, we noted from DO analysis
that the target genes were significantly assembled in chronic
pulmonary heart disease and primary pulmonary hypertension, the
function of target genes in chronic pulmonary heart disease and
primary pulmonary hypertension may constitute part of the
pathogenesis of LUAD.
Although the regulatory network of target genes was
intricate, we focused on hub genes to shed light on the molecular
mechanism of miR-183-5p in LUAD. Ten genes were screened out as hub
genes from the four most significant KEGG pathways. However,
several of the hub genes including CD34, PDGFB, FGFR2 and
WNT3A were described in previous studies to relate to the
tumorigenesis or poor prognosis of LUAD (51–54).
Although there have not been studies investigating the expression
of SPN, WNT2B and PTGER4 in LUAD, all the three genes
were reported to act as an oncogene in other cancers. SPN could
induce cell adhesion and migration as well as suppress apoptosis
when secreted by activated leukocytes (55). WNT2B controlled multiple biological
events, including cell proliferation, differentiation and migration
to serve as an oncogene in numerous human cancers (56–58).
PTGER4 could transduce a series of signaling pathways such as Akt,
ERK1/2 and early growth response factor-1 to mediate cancer cell
survival and tumor development with its overexpression in a wide
variety of cancers (59). Thus, we
are skeptical about the regulatory targeting relationship between
miR-183-5p and these genes. Whether these genes were the direct
targets of miR-183-5p needs to be verified in future study. Apart
from these genes, we found evidence that VIPR1 was downregulated in
LUAD and served as a tumor-suppressor gene in the study of Mlakar
et al (60), which lend
credence to the assumption that miR-183-5p directly targets VIPR1.
In addition to the hub genes, component genes in CAMs pathway
validated by Pearson's correlation test and immunostaining results
from HPA were also worthy for attention. Despite that we found no
literature evidence of the downregulation of expression in LUAD for
all component genes in the CAMs pathway, the tumor suppressor roles
of SIGLEC1 and CADM1 human cancers were explored in
previous research. The study conducted by Strömvall et al
confirmed that reduced expression of SIGLEC1 in metastasis-free
regional lymph nodes was responsible for the inhibited antitumor
immune response (61). CADM1, a
membrane-spanning glycoprotein that participates in the process of
attenuating cell proliferation and activating cell apoptosis, was
downregulated in various cancers such as breast, prostate,
pancreatic and hepatocellular cancer (62). We believe that miR-183-5p-regulated
downexpression of genes such as VIPR1, SIGLEC1 and
CADM1 may help explain the oncogenic function of miR-183-5p
in LUAD. Future experiments are warranted to identify the
interactions between miR-183-5p and these genes in LUAD.
Despite the research progress in our study, there
were also limitations. In our GEO and integrated meta-analysis,
great heterogeneity existed in the pooled studies, and even the
subgroup analysis or sensitivity analysis failed to solve the
problem, which unfortunately impacts the reliability of our
results. This intractable heterogeneity may partly be attributed to
the different proportion of LUAD and non-cancer tissues in the
studies or the varying experimental platforms of studies. The
number of LUAD tissues exceeded non-cancer tissues in GSE27486,
GSE93300, GSE14936, GSE48414, GSE51853, GSE63805, GSE74190 and the
TCGA data; conversely, the cases of non-cancer tissues outnumbered
LUAD tissues in GSE19945, GSE40738, GSE47525 and GSE77380. The
experimental platforms of studies also varied from each other. A
feasible way to prevent this problem may be to enroll larger study
cohorts with a balanced proportion of LUAD and non-cancer tissues
in future studies. Another flaw of this study was that neither
western blotting nor flow cytometry was conducted in in
vitro experiments. Western blotting and flow cytometry should
be conducted after knockdown or overexpression of miR-183-5p in the
LC cell lines. Nevertheless, we failed to perform western blotting
and flow cytometry due to the restriction of the experimental
condition. This imperfect design of the in vitro experiment
should be improved in future research.
In conclusion, our study confirmed that
overexpression of miR-183-5p may play an oncogenic role in LUAD
through involvement in the regulatory networks of its target genes.
miR-183-5p is anticipated to be a novel diagnostic and therapeutic
target for lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
The study was supported by funds from the National
Natural Science Foundation of China (NSFC81560469, NSFC81360327),
the Natural Science Foundation of Guangxi, China
(2015GXNSFCA139009) and teh Guangxi Medical University Training
Program for Distinguished Young Scholars (2017).
Availability of data and materials
The datasets generated and analysed during the
current study are available in Gene Expression omnibus (GEO;
https://www.ncbi.nlm.nih.gov/pubmed)
and TCGA data portal (https://portal.gdc.cancer.gov/).
Authors' contributions
RQH and LG analyzed and interpreted data and drafted
the manuscript. JM, ZYL, XHH and GC provided information from
database. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of the First Affiliated Hospital of Guangxi Medical
University. Signed informed consents were acquired from all the
LUAD patients prior to their involvement in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi YL, Sun JM, Cho J, Rampal S, Han J,
Parasuraman B, Guallar E, Lee G, Lee J and Shim YM: EGFR mutation
testing in patients with advanced non-small cell lung cancer: A
comprehensive evaluation of real-world practice in an East Asian
tertiary hospital. PLoS One. 8:e560112013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cobo M, Gutiérrez V, Villatoro R, Trigo
JM, Ramos I, López O, Ruiz M, Godoy A, López I and Arroyo M:
Spotlight on ramucirumab in the treatment of nonsmall cell lung
cancer: Design, development, and clinical activity. Lung Cancer
(Auckl). 8:57–66. 2017.PubMed/NCBI
|
|
5
|
Yang Q, Zhang RW, Sui PC, He HT and Ding
L: Dysregulation of non-coding RNAs in gastric cancer. World J
Gastroenterol. 21:10956–10981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romero-Cordoba SL, Salido-Guadarrama I,
Rodriguez-Dorantes M and Hidalgo-Miranda A: miRNA biogenesis:
Biological impact in the development of cancer. Cancer Biol Ther.
15:1444–1455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mulrane L, McGee SF, Gallagher WM and
O'Connor DP: miRNA dysregulation in breast cancer. Cancer Res.
73:6554–6562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palanichamy JK and Rao DS: miRNA
dysregulation in cancer: Towards a mechanistic understanding. Front
Genet. 5:542014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Guan X, Tu Y, Zheng S, Long J, Li
S, Qi C, Xie X, Zhang H and Zhang Y: MicroRNA-29b attenuates
non-small cell lung cancer metastasis by targeting matrix
metalloproteinase 2 and PTEN. J Exp Clin Cancer Res. 34:592015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dambal S, Shah M, Mihelich B and Nonn L:
The microRNA-183 cluster: The family that plays together stays
together. Nucleic Acids Res. 43:7173–7188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Zhang L, Zhang L, Du J, Wang H and
Wang B: MicroRNA-183 correlates cancer prognosis, regulates cancer
proliferation and bufalin sensitivity in epithelial ovarian caner.
Am J Transl Res. 8:1748–1755. 2016.PubMed/NCBI
|
|
12
|
Cheng Y, Xiang G, Meng Y and Dong R:
MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in
human breast cancer by targeting the PDCD4. Reprod Biol.
16:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X,
Jin X and Tian X: MicroRNA-183 functions as the tumor suppressor
via inhibiting cellular invasion and metastasis by targeting MMP-9
in cervical cancer. Gynecol Oncol. 141:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu F, Zhang H, Su Y, Kong J, Yu H and Qian
B: Up-regulation of microRNA-183-3p is a potent prognostic marker
for lung adenocarcinoma of female non-smokers. Clin Transl Oncol.
16:980–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu WY, Zhang YK, Chai Z, Hu X, Tan L,
Wang Z, Chen Z and Le H: Identification of factors for the
preoperative prediction of tumour subtype and prognosis in patients
with T1 lung adenocarcinoma. Dis Markers. 2016:93546802016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu C, Deng X, Wu J, Zhang J, Yang H, Fu
S, Zhang Y, Han Y, Zou Y, Chen Z, et al: MicroRNA-183 promotes
migration and invasion of CD133(+)/CD326(+) lung adenocarcinoma
initiating cells via PTPN4 inhibition. Tumour Biol. 37:11289–11297.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harbord RM, Deeks JJ, Egger M, Whiting P
and Sterne JA: A unification of models for meta-analysis of
diagnostic accuracy studies. Biostatistics. 8:239–251. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen G, Kronenberger P, Teugels E and De
Grève J: Influence of RT-qPCR primer position on EGFR interference
efficacy in lung cancer cells. Biol Proced Online. 13:12010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen G, Noor A, Kronenberger P, Teugels E,
Umelo IA and De Grève J: Synergistic effect of afatinib with
su11274 in non-small cell lung cancer cells resistant to gefitinib
or erlotinib. PLoS One. 8:e597082013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang R, Zhong T, Dang Y, Zhang X, Li P and
Chen G: Association between downexpression of MiR-203 and poor
prognosis in non-small cell lung cancer patients. Clin Transl
Oncol. 18:360–368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang S, He R, Rong M, Dang Y and Chen G:
Synergistic effect of MiR-146a mimic and cetuximab on
hepatocellular carcinoma cells. Biomed Res Int.
2014:3841212014.PubMed/NCBI
|
|
24
|
Dang YW, Zeng J, He RQ, Rong MH, Luo DZ
and Chen G: Effects of miR-152 on cell growth inhibition, motility
suppression and apoptosis induction in hepatocellular carcinoma
cells. Asian Pac J Cancer Prev. 15:4969–4976. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dang Y, Luo D, Rong M and Chen G:
Underexpression of miR-34a in hepatocellular carcinoma and its
contribution towards enhancement of proliferating inhibitory
effects of agents targeting c-MET. PLoS One. 8:e610542013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rong M, Chen G and Dang Y: Increased
miR-221 expression in hepatocellular carcinoma tissues and its role
in enhancing cell growth and inhibiting apoptosis in vitro. BMC
Cancer. 13:212013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang HW, Ye ZH, Yin SY, Mo WJ, Wang HL,
Zhao JC, Liang GM, Feng ZB, Chen G and Luo DZ: A comprehensive
insight into the clinicopathologic significance of miR-144-3p in
hepatocellular carcinoma. Onco Targets Ther. 10:3405–3419. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patnaik SK, Yendamuri S, Kannisto E,
Kucharczuk JC, Singhal S and Vachani A: MicroRNA expression
profiles of whole blood in lung adenocarcinoma. PLoS One.
7:e460452012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patnaik SK, Kannisto ED, Mallick R,
Vachani A and Yendamuri S: Whole blood microRNA expression may not
be useful for screening non-small cell lung cancer. PLoS One.
12:e01819262017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et
al: MiR-21 is an EGFR-regulated anti-apoptotic factor in lung
cancer in never-smokers. Proc Natl Acad Sci USA. 106:12085–12090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nymark P, Guled M, Borze I, Faisal A,
Lahti L, Salmenkivi K, Kettunen E, Anttila S and Knuutila S:
Integrative analysis of microRNA, mRNA and aCGH data reveals
asbestos- and histology-related changes in lung cancer. Genes
Chromosomes Cancer. 50:585–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Jaarsveld MT, Wouters MD, Boersma AW,
Smid M, van Ijcken WF, Mathijssen RH, Hoeijmakers JH, Martens JW,
van Laere S, Wiemer EA, et al: DNA damage responsive microRNAs
misexpressed in human cancer modulate therapy sensitivity. Mol
Oncol. 8:458–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bjaanaes MM, Halvorsen AR, Solberg S,
Jørgensen L, Dragani TA, Galvan A, Colombo F, Anderlini M,
Pastorino U, Kure E, et al: Unique microRNA-profiles in
EGFR-mutated lung adenocarcinomas. Int J Cancer. 135:1812–1821.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arima C, Kajino T, Tamada Y, Imoto S,
Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T,
et al: Lung adenocarcinoma subtypes definable by lung
development-related miRNA expression profiles in association with
clinicopathologic features. Carcinogenesis. 35:2224–2231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robles AI, Arai E, Mathé EA, Okayama H,
Schetter AJ, Brown D, Petersen D, Bowman ED, Noro R, Welsh JA, et
al: An integrated prognostic classifier for stage I lung
adenocarcinoma based on mRNA, microRNA, and DNA methylation
biomarkers. J Thorac Oncol. 10:1037–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma L, Huang Y, Zhu W, Zhou S, Zhou J, Zeng
F, Liu X, Zhang Y and Yu J: An integrated analysis of miRNA and
mRNA expressions in non-small cell lung cancers. PLoS One.
6:e265022011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng Z, Pan L, Niu Z, Li W, Dang X, Wan L,
Zhang R and Yang S: Identification of microRNAs as potential
biomarkers for lung adenocarcinoma using integrating genomics
analysis. Oncotarget. 8:64143–64156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pak MG, Lee CH, Lee WJ, Shin DH and Roh
MS: Unique microRNAs in lung adenocarcinoma groups according to
major TKI sensitive EGFR mutation status. Diagn Pathol. 10:992015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka M, Oikawa K, Takanashi M, Kudo M,
Ohyashiki J, Ohyashiki K and Kuroda M: Down-regulation of miR-92 in
human plasma is a novel marker for acute leukemia patients. PLoS
One. 4:e55322009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohyashiki K, Umezu T, Yoshizawa S, Ito Y,
Ohyashiki M, Kawashima H, Tanaka M, Kuroda M and Ohyashiki JH:
Clinical impact of down-regulated plasma miR-92a levels in
non-Hodgkin's lymphoma. PLoS One. 6:e164082011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shigoka M, Tsuchida A, Matsudo T, Nagakawa
Y, Saito H, Suzuki Y, Aoki T, Murakami Y, Toyoda H, Kumada T, et
al: Deregulation of miR-92a expression is implicated in
hepatocellular carcinoma development. Pathol Int. 60:351–357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan D, Cai X and Feng Y: miR-183 modulates
cell apoptosis and proliferation in tongue squamous cell carcinoma
SCC25 cell line. Oncol Res. 24:399–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ruan H, Liang X, Zhao W, Ma L and Zhao Y:
The effects of microRNA-183 promots cell proliferation and invasion
by targeting MMP-9 in endometrial cancer. Biomed Pharmacother.
89:812–818. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X, Zuo D, Yuan Y, Yang X, Hong Z and
Zhang R: MicroRNA-183 promotes cell proliferation via regulating
programmed cell death 6 in pediatric acute myeloid leukemia. J
Cancer Res Clin Oncol. 143:169–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui R, Kim T, Fassan M, Meng W, Sun HL,
Jeon YJ, Vicentini C, Tili E, Peng Y, Scarpa A, et al: MicroRNA-224
is implicated in lung cancer pathogenesis through targeting
caspase-3 and caspase-7. Oncotarget. 6:21802–21815. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ren LH, Chen WX, Li S, He XY, Zhang ZM, Li
M, Cao RS, Hao B, Zhang HJ, Qiu HQ, et al: MicroRNA-183 promotes
proliferation and invasion in oesophageal squamous cell carcinoma
by targeting programmed cell death 4. Br J Cancer. 111:2003–2013.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Makrilia N, Kollias A, Manolopoulos L and
Syrigos K: Cell adhesion molecules: Role and clinical significance
in cancer. Cancer Invest. 27:1023–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Henderson MP, Hirte H, Hotte SJ and Kavsak
PA: Cytokines and cell adhesion molecules exhibit distinct profiles
in health, ovarian cancer, and breast cancer. Heliyon.
2:e000592016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kaseda K, Ishii G, Aokage K, Takahashi A,
Kuwata T, Hishida T, Yoshida J, Kohno M, Nagai K and Ochiai A:
Identification of intravascular tumor microenvironment features
predicting the recurrence of pathological stage I lung
adenocarcinoma. Cancer Sci. 104:1262–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu J, Lv W, Hu Y, Wang L, Wang Y, Cao J
and Hu J: Wnt3a expression is associated with
epithelial-mesenchymal transition and impacts prognosis of lung
adenocarcinoma patients. J Cancer. 8:2523–2531. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Timsah Z, Berrout J, Suraokar M, Behrens
C, Song J, Lee JJ, Ivan C, Gagea M, Shires M, Hu X, et al:
Expression pattern of FGFR2, Grb2 and Plcγ1 acts as a novel
prognostic marker of recurrence recurrence-free survival in lung
adenocarcinoma. Am J Cancer Res. 5:3135–3148. 2015.PubMed/NCBI
|
|
54
|
Neri S, Miyashita T, Hashimoto H, Suda Y,
Ishibashi M, Kii H, Watanabe H, Kuwata T, Tsuboi M, Goto K, et al:
Fibroblast-led cancer cell invasion is activated by
epithelial-mesenchymal transition through platelet-derived growth
factor BB secretion of lung adenocarcinoma. Cancer Lett. 395:20–30.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fu Q, Cash SE, Andersen JJ, Kennedy CR,
Madadi AR, Raghavendra M, Dietrich LL, Agger WA and Shelley CS:
Intracellular patterns of sialophorin expression define a new
molecular classification of breast cancer and represent new targets
for therapy. Br J Cancer. 110:146–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiang H, Li F, He C, Wang X, Li Q and Gao
H: Expression of Gli1 and Wnt2B correlates with progression and
clinical outcome of pancreatic cancer. Int J Clin Exp Pathol.
7:4531–4538. 2014.PubMed/NCBI
|
|
57
|
Liu C, Li G, Ren S, Su Z, Wang Y, Tian Y,
Liu Y and Qiu Y: miR-185-3p regulates the invasion and metastasis
of nasopharyngeal carcinoma by targeting WNT2B in vitro. Oncol
Lett. 13:2631–2636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Catalano RD, Wilson MR, Boddy SC, McKinlay
AT, Sales KJ and Jabbour HN: Hypoxia and prostaglandin E receptor 4
signalling pathways synergise to promote endometrial adenocarcinoma
cell proliferation and tumour growth. PLoS One. 6:e192092011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mlakar V, Strazisar M, Sok M and Glavac D:
Oligonucleotide DNA microarray profiling of lung adenocarcinoma
revealed significant downregulation and deletions of vasoactive
intestinal peptide receptor 1. Cancer Invest. 28:487–494. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Strömvall K, Sundkvist K, Ljungberg B,
Halin Bergström S and Bergh A: Reduced number of CD169+
macrophages in pre-metastatic regional lymph nodes is associated
with subsequent metastatic disease in an animal model and with poor
outcome in prostate cancer patients. Prostate. 77:1468–1477. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wikman H, Westphal L, Schmid F, Pollari S,
Kropidlowski J, Sielaff-Frimpong B, Glatzel M, Matschke J, Westphal
M, Iljin K, et al: Loss of CADM1 expression is associated with poor
prognosis and brain metastasis in breast cancer patients.
Oncotarget. 5:3076–3087. 2014. View Article : Google Scholar : PubMed/NCBI
|