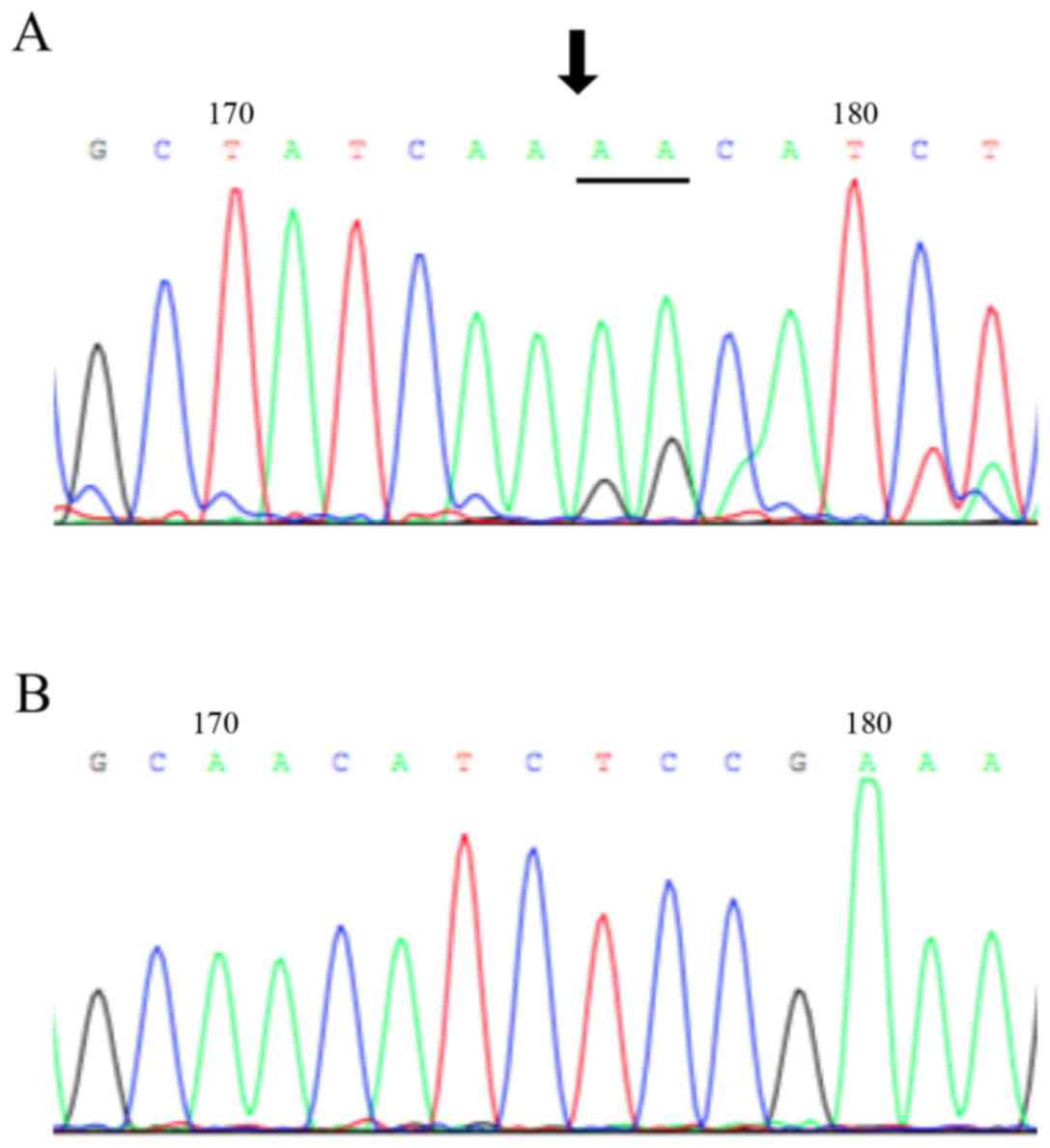
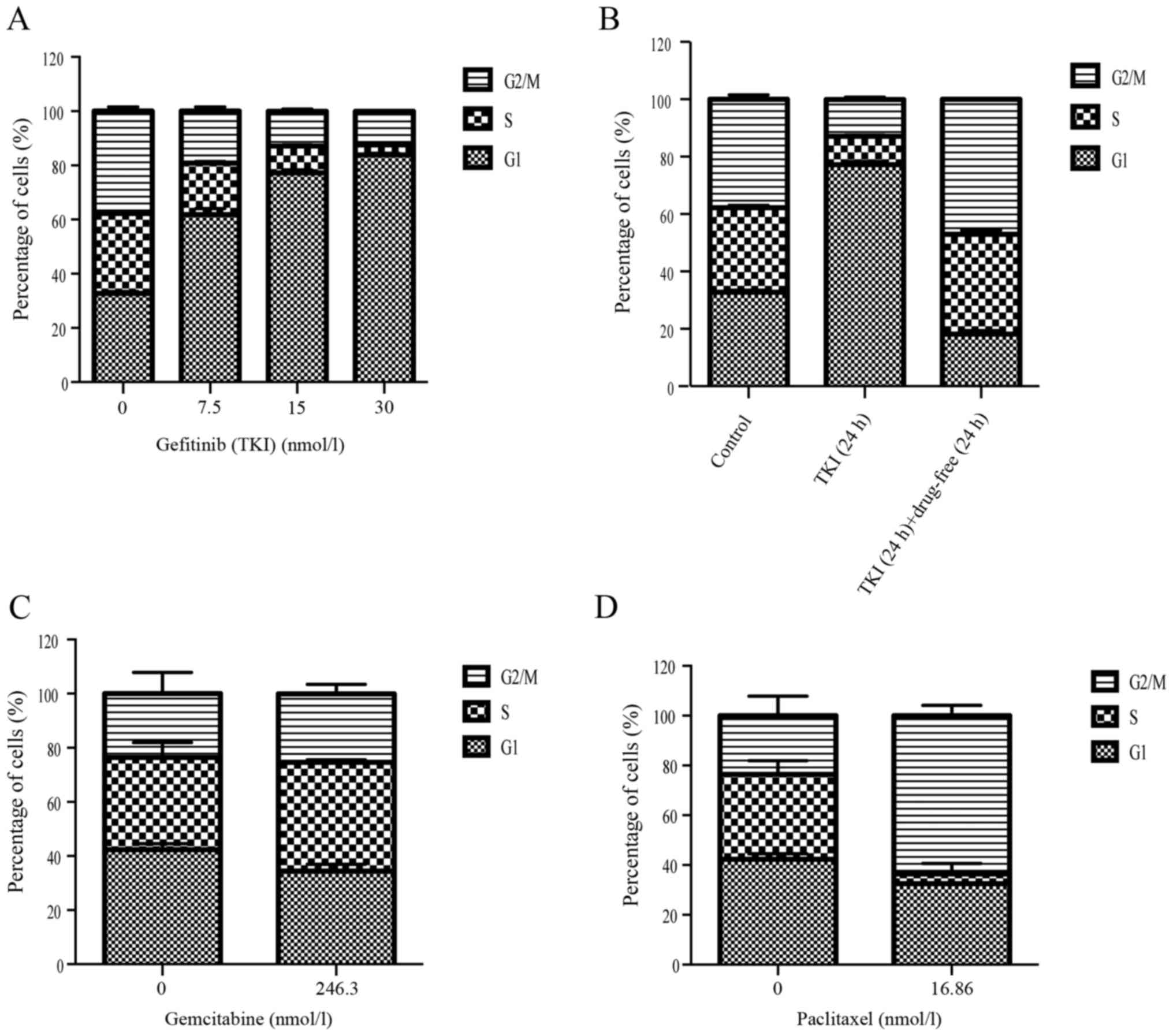
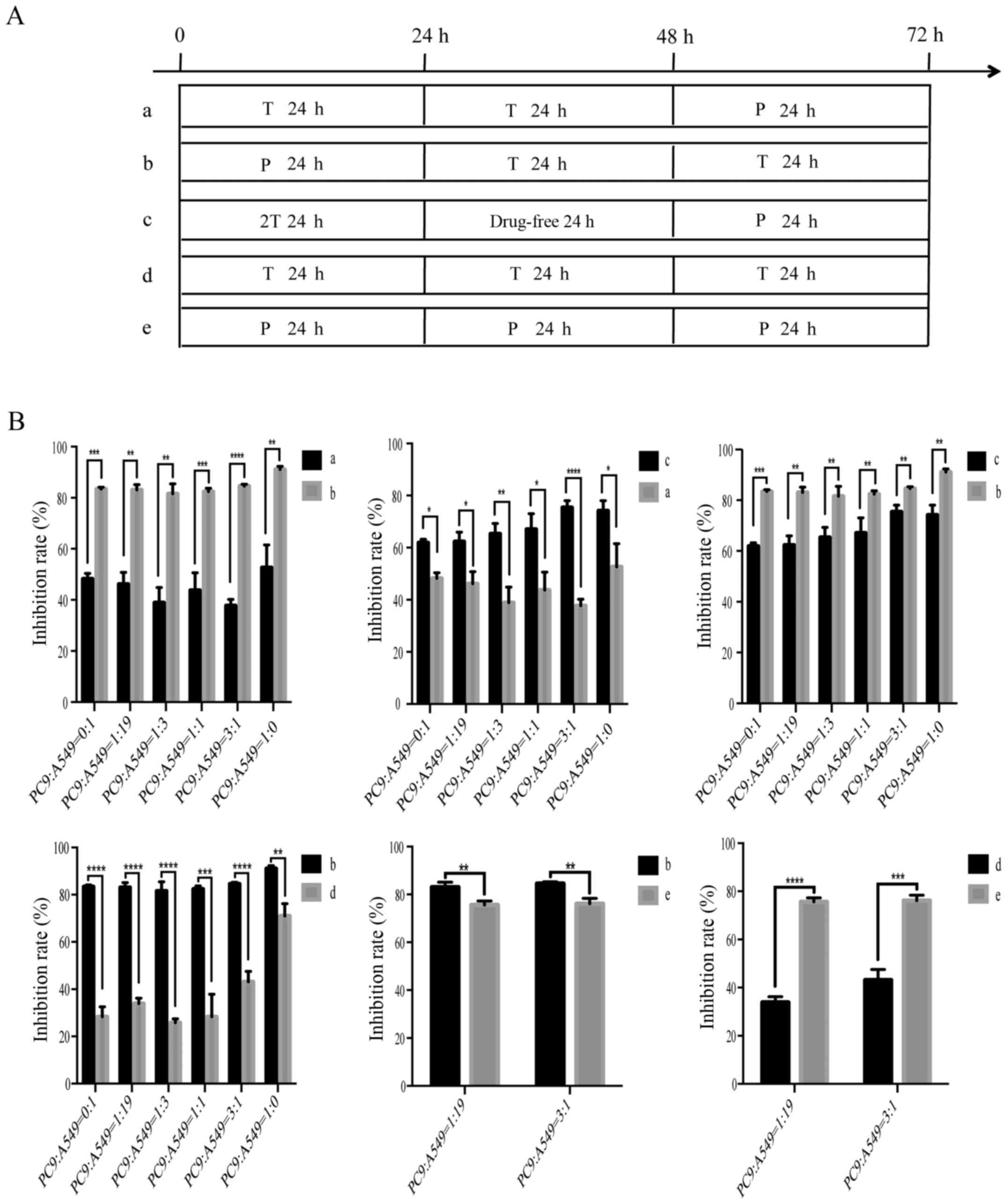
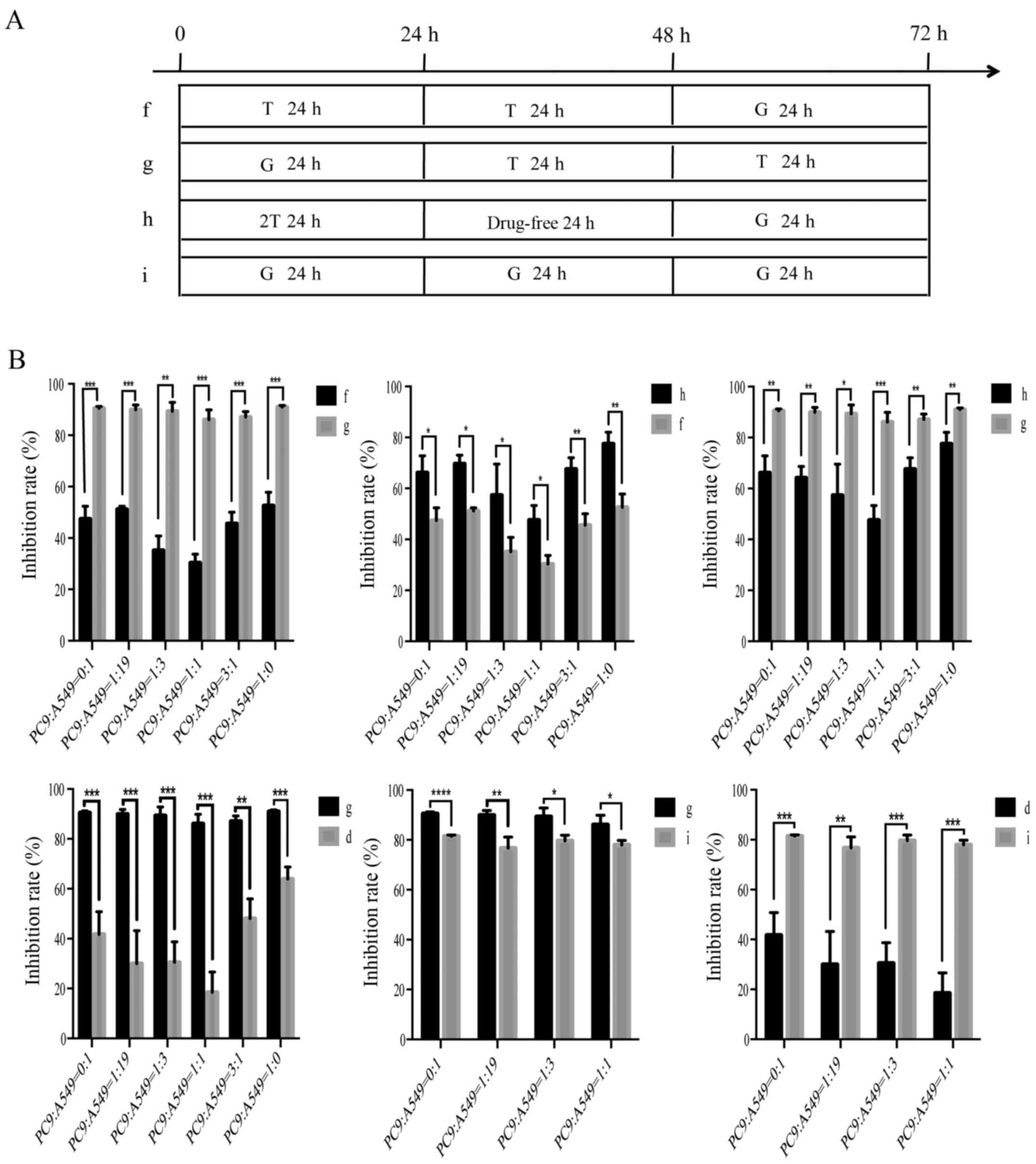
|
1
|
Pignata M, Chouaid C, Le Lay K, Luciani L,
McConnachie C, Gordon J and Roze S: Evaluating the
cost-effectiveness of afatinib after platinum-based therapy for the
treatment of squamous non-small-cell lung cancer in France.
Clinicoecon Outcomes Res. 9:655–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novello S, Barlesi F, Califano R, Cufer T,
Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al:
Metastatic non-small-cell lung cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 27
(Suppl 5):V1–V27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu YL, Saijo N, Thongprasert S, Yang JC,
Han B, Margono B, Chewaskulyong B, Sunpaweravong P, Ohe Y, Ichinose
Y, et al: Efficacy according to blind independent central review:
Post-hoc analyses from the phase III, randomized, multicenter,
IPASS study of first-line gefitinib versus carboplatin/paclitaxel
in Asian patients with EGFR mutation-positive advanced NSCLC. Lung
Cancer. 104:119–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patil VM, Noronha V, Joshi A, Choughule
AB, Bhattacharjee A, Kumar R, Goud S, More S, Ramaswamy A, Karpe A,
et al: phase III study of gefitinib or pemetrexed with carboplatin
in EGFR-mutated advanced lung adenocarcinoma. ESMO Open.
2:e0001682017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Batson S, Mitchell SA, Windisch R, Damonte
E, Munk VC and Reguart N: Tyrosine kinase inhibitor combination
therapy in first-line treatment of non-small-cell lung cancer:
Systematic review and network meta-analysis. Onco Targets Ther.
10:2473–2482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skrzypski M, Szymanowska-Narloch A and
Dziadziuszko R: Osimertinib-Effective treatment of NSCLC with
activating EGFR mutations after progression on EGFR tyrosine kinase
inhibitors. Contemp Oncol. 21:254–258. 2017.
|
|
7
|
Chen YM, Liu JM, Chou TY, Perng RP, Tsai
CM and Whang-Peng J: phase II randomized study of daily gefitinib
treatment alone or with vinorelbine every 2 weeks in patients with
adenocarcinoma of the lung who failed at least 2 regimens of
chemotherapy. Cancer. 109:1821–1828. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng Y, Murakami H, Yang PC, He J,
Nakagawa K, Kang JH, Kim JH, Wang X, Enatsu S, Puri T, et al:
Randomized phase II trial of gefitinib with and without pemetrexed
as first-line therapy in patients with advanced nonsquamous
non-small-cell lung cancer with activating epidermal growth factor
receptor mutations. J Clin Oncol. 34:3258–3266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soria JC, Wu YL, Nakagawa K, Kim SW, Yang
JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S, et al: Gefitinib plus
chemotherapy versus placebo plus chemotherapy in
EGFR-mutation-positive non-small-cell lung cancer after progression
on first-line gefitinib (IMPRESS): A phase 3 randomised trial.
Lancet Oncol. 16:990–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jian H, Li W, Ma Z, Huang J, Feng J, Song
Y, Gao B, Zhu H, Tao M, Bai C, et al: Intercalating and maintenance
gefitinib plus chemotherapy versus chemotherapy alone in selected
advanced non-small cell lung cancer with unknown EGFR status. Sci
Rep. 7:84832017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
La Salvia A, Rossi A, Galetta D, Gobbini
E, De Luca E, Novello S and Di Maio M: Intercalated chemotherapy
and epidermal growth factor receptor inhibitors for patients with
advanced non-small-cell lung cancer: A systematic review and
meta-analysis. Clin Lung Cancer. 18:23–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiao L, Wang J, Long G and Jiang Y:
Sequential treatment of tyrosine kinase inhibitor and
platinum-based doublet chemotherapy on EGFR mutant non-small cell
lung cancer: A meta-analysis of randomized controlled clinical
trials. Onco Targets Ther. 10:1279–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao S, Gao F, Zhang Y, Zhang Z and Zhang
L: Bevacizumab in combination with different platinum-based
doublets in the first-line treatment for advanced nonsquamous
non-small-cell lung cancer: A network meta-analysis. Int J Cancer.
142:1676–1688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Wang S, Su ZF and Yuan Y:
Synergistic effects of sorafenib in combination with gemcitabine or
pemetrexed in lung cancer cell lines with K-ras mutations. Contemp
Oncol. 20:33–38. 2016.
|
|
15
|
Wang MC, Liang X, Liu ZY, Cui J, Liu Y,
Jing L, Jiang LL, Ma JQ, Han LL, Guo QQ, et al: In vitro
synergistic antitumor efficacy of sequentially combined
chemotherapy/icotinib in nonsmall cell lung cancer cell lines.
Oncol Rep. 33:239–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mok TS, Wu YL, Yu CJ, Zhou C, Chen YM,
Zhang L, Ignacio J, Liao M, Srimuninnimit V, Boyer MJ, et al:
Randomized, placebo-controlled, phase II study of sequential
erlotinib and chemotherapy as first-line treatment for advanced
non-small-cell lung cancer. J Clin Oncol. 27:5080–5087. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu YL, Lee JS, Thongprasert S, Yu CJ,
Zhang L, Ladrera G, Srimuninnimit V, Sriuranpong V, Sandoval-Tan J,
Zhu Y, et al: Intercalated combination of chemotherapy and
erlotinib for patients with advanced stage non-small-cell lung
cancer (FASTACT-2): A randomised, double-blind trial. Lancet Oncol.
14:777–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J,
Gu A, Zhong H, Wang H, Zhang X, et al: Combination of chemotherapy
and gefitinib as first-line treatment for patients with advanced
lung adenocarcinoma and sensitive EGFR mutations: A randomized
controlled trial. Int J Cancer. 141:1249–1256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshimura N, Kudoh S, Mitsuoka S,
Yoshimoto N, Oka T, Nakai T, Suzumira T, Matusura K, Tochino Y,
Asai K, et al: Phase II study of a combination regimen of gefitinib
and pemetrexed as first-line treatment in patients with advanced
non-small cell lung cancer harboring a sensitive EGFR mutation.
Lung cancer. 90:65–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gridelli C, Ciardiello F, Gallo C, Feld R,
Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A,
et al: First-line erlotinib followed by second-line
cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung
cancer: The TORCH randomized trial. J Clin Oncol. 30:3002–3011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubo E, Yamamoto N, Nokihara H, Fujiwara
Y, Horinouchi H, Kanda S, Goto Y and Ohe Y: Randomized phase II
study of sequential carboplatin plus paclitaxel and gefitinib in
chemotherapy-naïve patients with advanced or metastatic
non-small-cell lung cancer: Long-term follow-up results. Mol Clin
Oncol. 6:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu M, Yuan Y, Pan YY and Zhang Y: Combined
gefitinib and pemetrexed overcome the acquired resistance to
epidermal growth factor receptor tyrosine kinase inhibitors in
non-small cell lung cancer. Mol Med Rep. 10:931–938. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu M, Yuan Y, Pan YY and Zhang Y:
Antitumor activity of combination treatment with gefitinib and
docetaxel in EGFR-TKI-sensitive, primary resistant and acquired
resistant human non-small cell lung cancer cells. Mol Med Rep.
9:2417–2422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Shi Y, Zhang X, Xu J, Wang B, Hao
X, Li J and Yan W: Phase II trial of paclitaxel-carboplatin with
intercalated gefitinib for untreated, epidermal growth factor
receptor gene mutation status unknown non-small cell lung cancer.
Thorac cancer. 5:149–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EY, Cho EN, Park HS, Kim A, Hong JY,
Lim S, Youn JP, Hwang SY and Chang YS: Genetic heterogeneity of
actionable genes between primary and metastatic tumor in lung
adenocarcinoma. BMC Cancer. 16:272016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jakobsen JN and Sorensen JB: Intratumor
heterogeneity and chemotherapy-induced changes in EGFR status in
non-small cell lung cancer. Cancer Chemother Pharmacol. 69:289–299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Biase D, Genestreti G, Visani M,
Acquaviva G, DiBattista M, Cavallo G, Paccapelo A, Cancellieri A,
Trisolini R, Degli Esposti R, et al: The percentage of epidermal
growth factor receptor (EGFR)-mutated neoplastic cells correlates
to response to tyrosine kinase inhibitors in lung adenocarcinoma.
PLoS One. 12:e01778222017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoon S, Lee DH and Kim SW: Gefitinib with
pemetrexed as first-line therapy in patients with advanced
nonsquamous non-small cell lung cancer with activating epidermal
growth factor receptor mutations. Ann Transl Med. 5:112017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
La Monica S, Madeddu D, Tiseo M, Vivo V,
Galetti M, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Falco A,
et al: Combination of gefitinib and pemetrexed prevents the
acquisition of TKI resistance in NSCLC cell lines carrying
EGFR-activating mutation. J Thorac Oncol. 11:1051–1063. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu CR, Zhong WZ, Zhou Q, Zhang XC, Yang JJ
and Wu YL: Heterogeneity of the resistance to gefitinib treatment
in a non-small cell lung cancer patient with active epidermal
growth factor receptor mutation. Thorac Cancer. 8:51–53. 2017.
View Article : Google Scholar : PubMed/NCBI
|