Introduction
Gastric cancer (GC) is one of the leading causes of
cancer-related deaths worldwide in the past decades (1,2).
Despite the improvement of surgical intervention and adjuvant
chemotherapy, the 5-year overall survival rate in GC patients is
less than 30% (3,4). Therefore, a better understanding of
molecular and cellular mechanisms of GC tumorigenesis and
metastasis will assist emergence of preferable therapeutic
strategies.
Solid tumors are composed of tumor cells and tumor
stroma, including the extracellular matrix (ECM), endothelial cells
and a large amount of fibroblasts (5). Following tumorigenesis, local normal
fibroblasts are transformed to cancer-associated fibroblasts (CAFs)
under the influence of cancer cells (6). CAFs are distinguishable from their
normal counterparts with the enhanced expression of alpha-smooth
muscle actin (α-SMA) and fibroblast activation protein (FAP). Other
markers of CAFs reported are fibroblast specific protein-1 (FSP-1),
stromal cell-derived factor-1 (SDF-1) and platelet-derived growth
factor receptor-α (PDGFRα) (7,8).
Accumulating evidence has indicated a significant role of cytokines
secreted by CAFs in mediating tumor growth and metastasis (9–11).
Among these stromal cytokines, HGF is expressed mainly in CAFs and
acts on c-Met-positive cancer cells in the tumor microenvironment
(12,13). Interactions between CAFs and cancer
cells activate the HGF/c-Met signaling pathway and thus trigger a
number of downstream oncogenic signaling cascades, such as PI3K/AKT
and ERK1/2, leading to tumor growth and metastasis (14). Recent studies have reiterated the
promoting effect of fibroblast-derived HGF on tumor progression and
suggest it to be a potential therapeutic target (15–17).
Growth of a solid tumor relies on blood vessels to
transport nutrients to satisfy its metabolic demands (18), particularly when the diameter
extends beyond 2 mm (19).
Endothelium-dependent vessels are the predominant vascularization
in solid tumors and an anti-angiogenesis strategy has been widely
used in the treatment of various types of malignant tumors. In
addition, as an endothelium-independent pattern, vasculogenic
mimicry (VM) tubes formed by cancer cells also participate in
vascularization (20,21). VM can serve as an internal blood
supply network to contribute to tumor progression and has been
revealed to be strongly associated with a poor prognosis in gastric
cancer (18,22). Mosaic vessels, which are formed by
endothelial cells accompanied by tumor cells (23,24),
also reveal their significant involvement in tumor growth and
metastasis (25). It has been
reported that HGF promotes endothelium-dependent angiogenesis in
pancreatic cancer and VM formation in hepatocellular carcinoma
(26,27), while the specific mechanism has not
been well elucidated. In our previous study, we confirmed the
existence of VM and mosaic vessels in gastric cancer (18,28).
In the present study, we further explored the effects of
CAF-derived HGF on angiogenesis, VM and mosaic vessel formation in
gastric cancer and illuminate their underlying mechanisms.
Materials and methods
Cell lines and culture
The human GC cell lines SNU16, MKN74, BGC823, AGS,
SGC7901, MGC803 and NCI-N87, and normal GES1 gastric mucosal cells
were provided by Shanghai Institute of Digestive Surgery (Shanghai,
China). HUVECs were purchased from the Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences (Shanghai, China).
These cells were routinely maintained and cultured. Primary
cancer-associated fibroblasts (CAFs) were isolated from a GC
patient undergoing radical gastrectomy on June 14 in 2017 at the
Department of Surgery, Ruijin Hospital, School of Medicine,
Shanghai Jiaotong University (29).
The patient did not receive preoperative treatment. To maintain the
characteristics of primary cells, CAFs passaged for up to 10
population doublings were used in the subsequent experiments. All
the cells were cultured at 37°C in 5% CO2 with RPMI-1640
medium (Genom, Hangzhou, China) containing 10% fetal bovine serum
(FBS; Gibco-BRL, Grand Island, NY, USA). The study was approved by
the Ruijin Hospital Ethics Committee of Shanghai Jiaotong
University School of Medicine and written informed consent was
provided by the patient.
Survival analysis with an online
database
Survival analysis of 378 GC patients with survival
data from TCGA (The Cancer Genome Atlas) database according to HGF
expression was performed with online website OncoLnc (http://www.oncolnc.org/) and the lower percentile was
set to be equal to the upper percentile. The Kaplan-Meier plotter
(http://www.kmplot.com/analysis/) was
used to assess the effect of HGF (Affymetrix ID: 209961_s_at,
210755_at, 210997_at, 210998_s_at) on the survival of 876 GC
patients from Gene Expression Omnibus (GEO) database and patients
were split by auto select best cutoff.
Gene Set Enrichment Analysis (GSEA)
and correlation analysis
RNA-seq of 415 patients from Stomach Adenocarcinoma
(TCGA, Provisional) was downloaded from cBioPortal platform
(http://www.cbioportal.org/). Microarray
profiles of 300 GC patients were downloaded from the GEO database
(https://www.ncbi.nlm.nih.gov/geo/).
Gene Set Enrichment Analysis was performed with GSEA 3.0 software
(Broad Institute, Cambridge, MA, USA) and the number of
permutations was set to 1,000. Corresponding gene sets were
downloaded from the Molecular Signatures Database v6.1 (http://software.broadinstitute.org/gsea/msigdb/index.jsp).
The mean value of gene (containing different Affymetrix IDs)
expression was used for correlation analysis.
Immunofluorescence
Briefly, CAFs and frozen sections of GC tissues were
fixed in 4% neutralized formaldehyde followed by permeabilization
with 0.5% Triton X-100 (Sigma-Aldrich; Merck KGaA, Taufkirchen,
Germany). Cells and frozen sections were blocked with 3% bovine
serum albumin (BSA) and then incubated at 4°C overnight with
primary antibodies for α-SMA (dilution 1:100; cat. no. ab5694;
Abcam, Cambridge, MA, USA), FAP (dilution 1:100; cat. no. sc-71094;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) and CD31 (dilution
1:100; cat. no. sc-65260; Santa Cruz Biotechnology). Then, the CAFs
and frozen sections were stained with appropriate Alexa
dye-conjugated secondary immune reagents and subjected to Olympus
BX53 microscope (fluorescence; Olympus Corp., Tokyo, Japan)
(magnification, ×200) and EVOS™ FL Color Imaging System
(fluorescence; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
(magnification, ×40), respectively.
ELISA assay
GC cells (1×105), CAFs (1×105)
and HUVECs (1×105) were cultured in 2 ml of RPMI-1640
complete medium for 36 h. The conditioned medium (CM) was collected
and centrifuged at 12,000 × g for 10 min to remove cell debris. The
levels of HGF in supernatants of GC cells, HUVECs and CAFs were
detected by ELISA kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer's instructions.
Cell proliferation
Cell proliferation was performed using Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). MGC803 and
HUVECs were suspended in supernatants with different treatments as
indicated and plated in a 96-well plate at 1,000 cells/well. Cell
proliferation was assessed every 24 h at an absorbance of 450 nm
using spectrophotometry (BioTek Instruments, Winooski, VT,
USA).
Cell migration
MGC803 cells (5×104) and HUVECs
(5×104) suspended in 200 µl serum-free RPMI-1640 medium
were cultured in the upper chamber with or without CAFs
(2×104) suspended in 600 µl RPMI-1640 medium containing
10% FBS in the lower chamber for 15 h using Transwell chambers (8
µm; Corning Costar, Corning, NY, USA). Then GC cells and HUVECs
were fixed using 4% neutralized formaldehyde and stained with 0.5%
crystal violet. The migrated cells in the lower chambers were
photographed using Olympus BX50 light microscope (Olympus Corp.;
magnification, ×200) and counted.
Quantitative real-time PCR
(qRT-PCR)
Total RNA extracted from cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was reversely
transcribed to cDNA using a reverse transcription kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Gene expression was quantified by qRT-PCR with
SYBR-Green (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
ABI Prism 7900HT sequence detection system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The relative mRNA levels were
evaluated based on the Ct values and GAPDH was used as an internal
control. The PCR primers used for the genes in the present study
are listed in Table I.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Genes | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| HGF |
GGGCTGAAAAGATTGGATCA |
TTGTATTGGTGGGTGCTTCA |
| MET |
GGTTTTTCCTGTGGCTGAAA |
GGCATGAACCGTTCTGAGAT |
| MMP1 |
GGGGCTTTGATGTACCCTAGC |
TGTCACACGCTTTTGGGGTTT |
| MMP2 |
GATACCCCTTTGACGGTAAGGA |
CCTTCTCCCAAGGTCCATAGC |
| CDH5 |
AAGCGTGAGTCGCAAGAATG |
TCTCCAGGTTTTCGCCAGTG |
| TFPI2 |
TCCTGCCCCTAGACTACGG |
CTCCCAGGTGTAGAAATTGTTGG |
| VEGFR2 |
GTGATCGGAAATGACACTGGAG |
CATGTTGGTCACTAACAGAAGCA |
| GAPDH |
ACAACTTTGGTATCGTGGAAGG |
GCCATCACGCCACAGTTTC |
Western blotting
Western blotting was performed as previously
described (18). In brief, HUVECs
and MGC803 cells were lysed using the RIPA buffer (Thermo Fisher
Scientific, Inc.) after stimulation with recombinant human HGF (50
ng/ml; cat. no. ab105061; Abcam) for 1 h. In groups of inhibition,
HUVECs and MGC803 cells were pretreated with LY294002 (50 µM; cat.
no. 9901) and U0126 (20 µM; cat. no. 9903; both from Cell Signaling
Technology, Inc., Danvers, MA, USA) for 6 h before they were
co-cultured with primary CAFs for 36 h. Human HGF antibody (300
ng/ml; cat. no. 24612; R&D Systems) for neutralization and
LY294002 (50 µM) U0126 (20 µM) for inhibition were added into the
co-culture system. BCA protein assay kit (Thermo Fisher Scientific,
Inc.) was used to calculate the protein concentrations according to
the manufacturer's instructions. Protein samples (20 µg) were
resolved by 10% SDS-PAGE and then transferred to polyvinylidene
fluoride membrane (PVDF; Millipore, Billerica, MA, USA). After
blocking with 5% BSA for 2 h, the membranes were incubated with the
primary antibodies for c-Met (1:2,000; cat. no. 8198), ERK1/2
(1:1,000; cat. no. 9102), p-ERK1/2 (1:1,000; cat. no. 9106), AKT
(1:1,000; cat. no. 4691) and p-AKT (1:1,000; cat. no. 4060; all
from Cell Signaling Technology) and GAPDH (1:1,000; cat. no.
sc-47724; Santa Cruz Biotechnology). The protein bands were
visualized using chemiluminescence with Pierce ECL Western Blotting
Substrate reagents (Thermo Fisher Scientific, Inc.).
Endothelial tube and vasculogenic
mimicry formation
A total of 100 µl Matrigel (BD Biosciences, San
Jose, CA, USA) was added into a 48-well plate and cultured at 37°C
in 5% CO2 to polymerize. HUVECs (2×104) and
MGC803 cells (4×104) were added into plates and cultured
in supernatants with different treatments. In groups of inhibition,
HUVECs and MGC803 cells were pretreated with LY294002 (50 µM) or
U0126 (20 µM) for 6 h. Following 8 h of incubation for HUVECs and
24 h of incubation for MGC803 cells, tubules were photographed
using EVOS™ FL Color Imaging System (light; Thermo Fisher
Scientific, Inc.; magnification, ×40) and evaluated using Image-Pro
Plus software (Media Cybernetics, Rockville, MD, USA).
Mosaic vessel assay
MGC803 cells (2×104) labeled with Dil and
HUVECs (2×104) labeled with DiO in conditioned medium
(CM) with different treatments were added into a 48-well plate
coated with 100 µl Matrigel (BD Biosciences). In groups of
inhibition, HUVECs and MGC803 cells were pretreated with LY294002
(50 µM) or U0126 (20 µM) for 6 h. Following 24 h of incubation at
37°C with 5% CO2, tubules were photographed using EVOS™
FL Color Imaging System (fluorescent; Thermo Fisher Scientific,
Inc.; magnification, ×40) and evaluated using Image-Pro Plus
software (Media Cybernetics).
Statistical analysis
The statistical differences between two groups were
analyzed using Student's t-test. Correlation of gene expression
between two groups was analyzed using Pearson's correlation
coefficient test. All analyses were performed using IBM SPSS 19.0
software (SPSS, Inc., Armonk, NY, USA). All the experiments were
performed in triplicate and results were expressed as the mean ±
standard deviation (SD). A two-tailed P-value ≤0.05 was considered
to indicate a statistically significant result.
Results
HGF is mainly derived from CAFs and
negatively correlated with OS (overall survival) in GC
patients
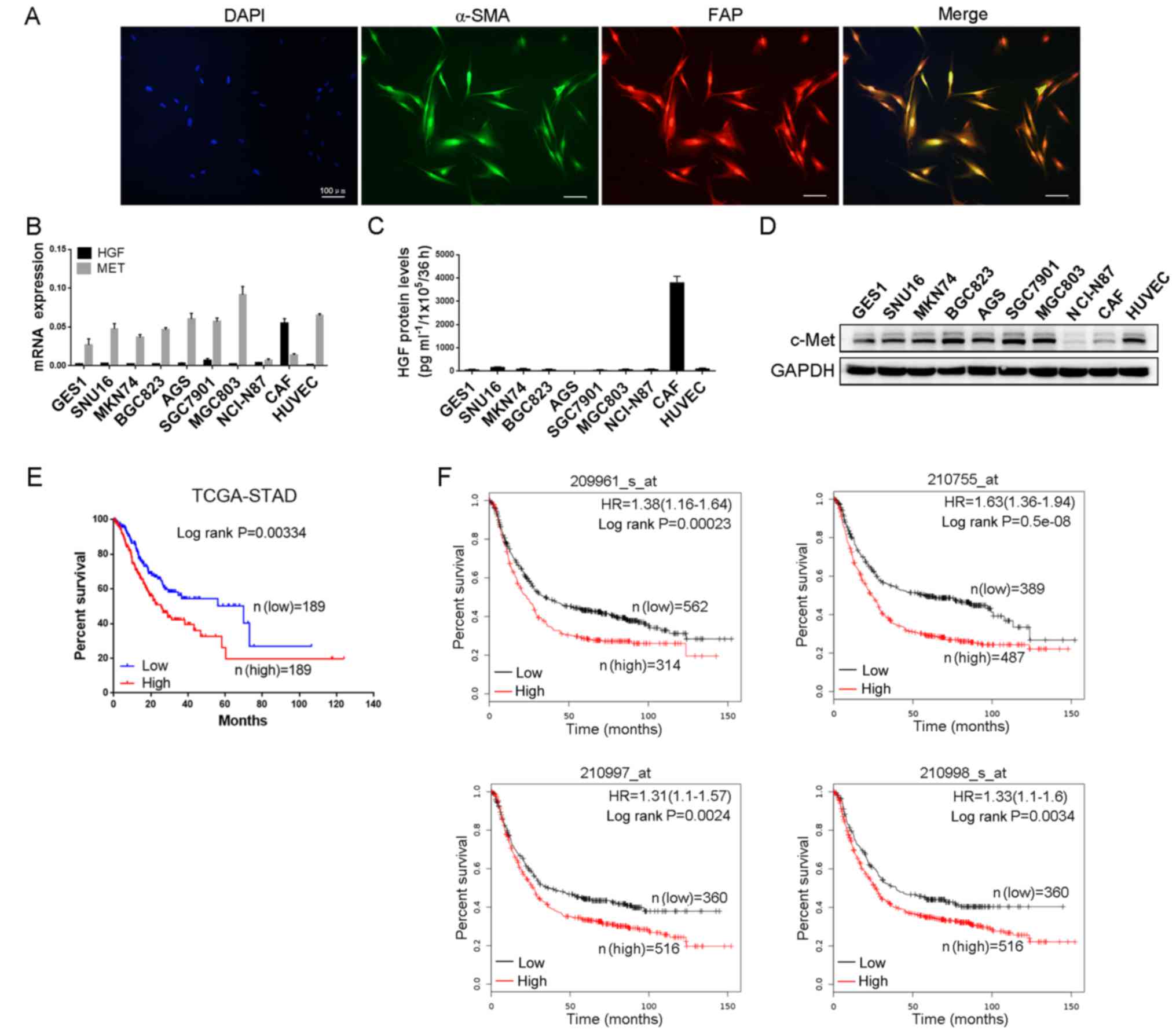
Primary CAFs were isolated from GC tissues and
immunofluorescence staining was performed to identify
spindle-shaped fibroblasts and ascertain the purity of CAFs. As
shown in Fig. 1A, both α-SMA and
FAP proteins were positively expressed and completely overlapped in
CAFs. Subsequently, we detected the expression of HGF in normal
gastric mucosal cells GES1, GC cell lines (SNU16, MKN74, BGC823,
AGS, SGC7901, MGC803 and NCI-N87), primary CAFs and HUVECs. HGF
mRNA expression was significantly higher in CAFs than GC cells and
HUVECs, while the mRNA expression of MET, a gene regulating the
receptor of HGF, was just the opposite (Fig. 1B). The protein levels of HGF and
c-Met were also examined and the results were consistent with their
mRNA expression (Fig. 1C and D).
Therefore, we surmised that HGF mainly originated from CAFs and
acted on GC cells and endothelial cells in GC tissues. To further
explore the clinical influence of HGF on the OS of GC patients, we
analyzed the survival data of 378 GC patients from the TCGA
database and 876 GC patients from the GEO database, respectively.
As shown in Fig. 1E and F, high HGF
expression was found to be associated with a worse OS in GC
patients. Collectively, this indicated that HGF was predominantly
secreted by CAFs and negatively correlated with OS in GC
patients.
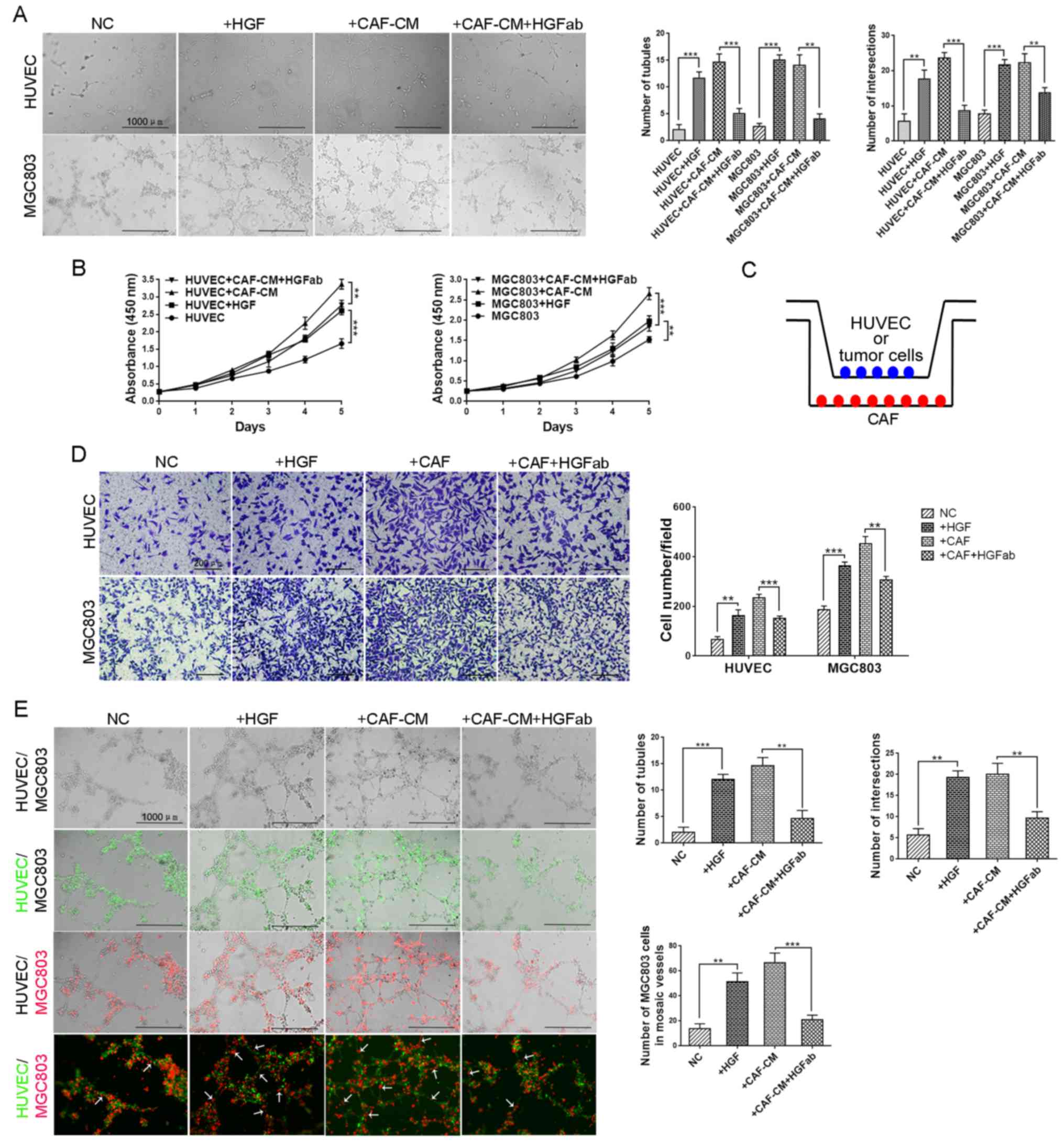
CAF-derived HGF promotes tube
formation of HUVECs, VM formation of GC cells and mosaic vessel
formation in vitro
Angiogenesis and VM tubes are vital to tumor
progression and HGF has exhibited its promoting effects on
angiogenesis in pancreatic cancer and VM formation in
hepatocellular carcinoma (26,27,30).
Therefore, we next examined the effects of HGF on angiogenesis and
VM formation in gastric cancer. Conditioned medium (CM) of CAFs
with or without HGF neutralization and recombinant human HGF were
subjected to HUVECs and MGC803 cells. Both CAF-CM and recombinant
human HGF promoted angiogenesis of HUVECs and VM formation of
MGC803 cells, while HGF neutralization significantly suppressed the
stimulatory effect of CAF-CM on HUVECs and MGC803 cells (Fig. 2A). These results indicated that
CAF-derived HGF promoted angiogenesis and VM formation in gastric
cancer.
Tube formation is associated with cell proliferation
and migration, thus we evaluated the tumor-promoting ability of
HGF. The cell proliferation of HUVECs and MGC803 cells were
increased in the CAF-CM groups and decreased in the HGF
neutralization groups (Fig. 2B). To
better mimic in vivo environments, we build an in
vitro co-culture system (Fig.
2C). Migration assays were performed in a co-culture system and
the results revealed that both stimulation of recombinant human HGF
and co-culture with CAFs increased the ability of cell migration,
which was reversed by HGF neutralization (Fig. 2D). The results aforementioned
indicated that CAF-derived HGF promoted endothelium-dependent
angiogenesis and VM formation by increasing cell proliferation and
migration.
Mosaic vessels, which are composed of endothelia and
cancer cells, serve as a bridge to transfer nutrition during tumor
growth. We ascertained the existence of mosaic vessels in gastric
cancer in our previous study (28).
Since we determined the increase in tubule-forming ability of both
HUVECs and GC cells induced by HGF from CAFs, we wondered whether
HGF promoted the formation of mosaic vessels. As shown in Fig. 2E, more mosaic vessel structures were
observed in groups with treatment of recombinant human HGF and
CAF-CM, and the promoting effects were reversed by neutralizing the
antibody against HGF. The differences among groups were
statistically analyzed and displayed as the number of tubules,
number of intersections and number of MGC803 cells in mosaic
vessels. These findings indicated that CAFs not only promoted
angiogenesis of HUVECs and VM formation of gastric cancer cells,
but also increased the number of mosaic vessels in gastric
cancer.
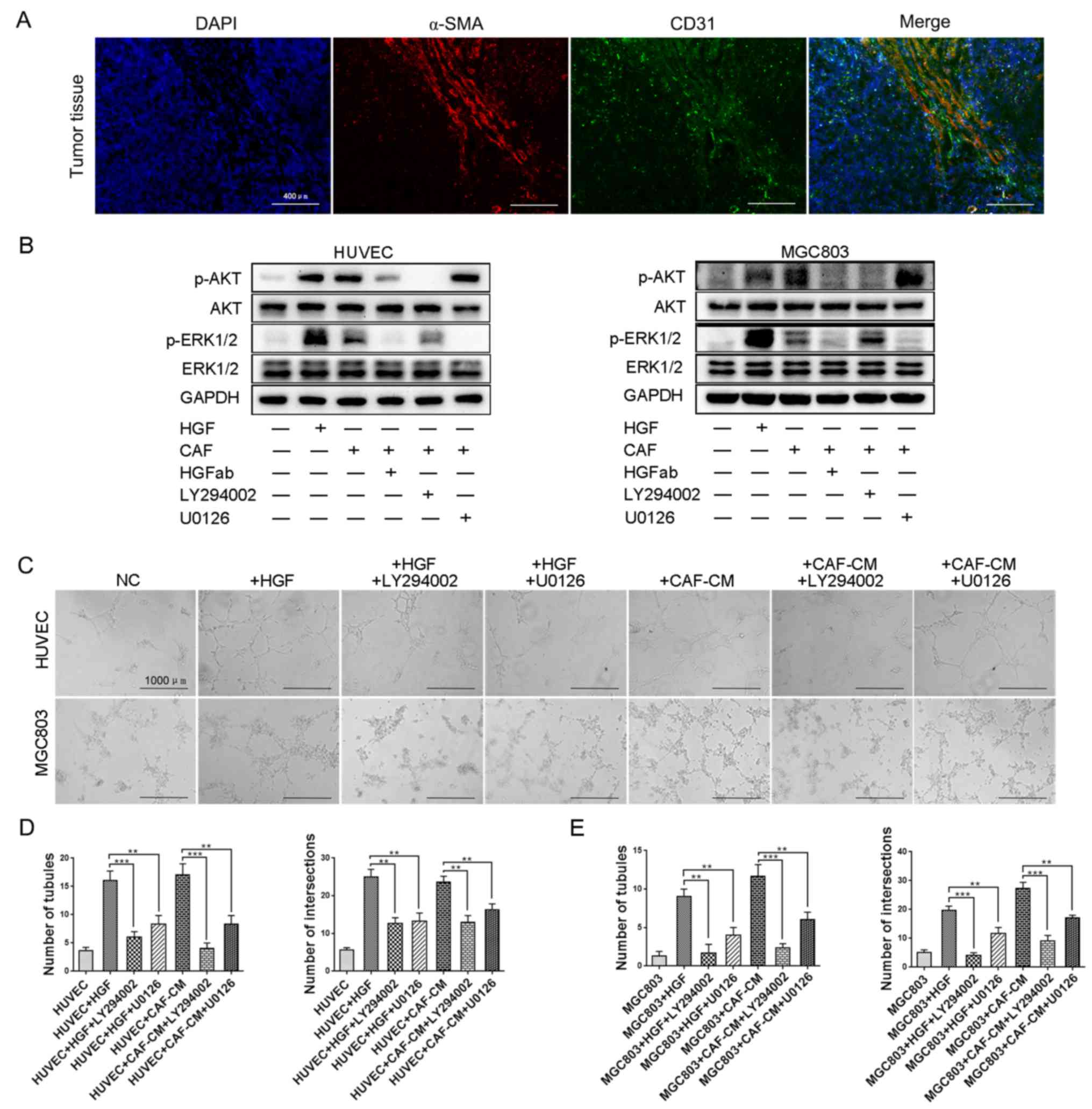
HGF from CAFs promotes angiogenesis of
HUVECs and VM formation of GC cells via PI3K/AKT and ERK1/2
signaling
HGF expression was revealed to be positively
correlated with microvessel density (MVD) quantified in gastric and
colorectal cancer (31,32). As aforementioned, we demonstrated
that HGF was mainly derived from CAFs compared with GC cells and
HUVECs. To elucidate the correlation between CAFs infiltration and
MVD, co-localization of α-SMA and CD31 was performed using frozen
sections of GC tissues. As shown in Fig. 3A, CAFs (represented by α-SMA) were
accompanied by endothelial cells (represented by CD31) to a great
degree. This indicated that CAFs infiltration resulted in
angiogenesis.
HGF has been revealed to bind to its receptor and
then trigger a number of downstream signaling cascades, among which
PI3K/AKT and ERK1/2 are associated with tumor angiogenesis and VM
formation (33,34). The expression of p-AKT and p-ERK1/2
in HUVECs and MGC803 cells was upregulated with treatment of
recombinant human HGF as well as in a co-culture system with CAFs,
which was reversed by HGF neutralization (Fig. 3B). To determine whether CAF-derived
HGF promoted angiogenesis and VM formation through PI3K/AKT and
ERK1/2 signaling, the inhibitor of PI3K/AKT signaling, LY294002,
and inhibitor of ERK1/2 signaling, U0126, were used to investigate
the underlying mechanisms. As shown in Fig. 3C, the promoting effects of
recombinant human HGF and CAF-CM on angiogenesis of HUVECs and VM
formation of MGC803 cells were significantly inhibited by LY294002
and U0126, respectively. Statistical analysis of the number of
tubules and number of intersections among these groups confirmed
the results (Fig. 3D and E). This
indicated that both PI3K/AKT and ERK1/2 signaling participated in
angiogenesis and VM formation induced by HGF in gastric cancer.
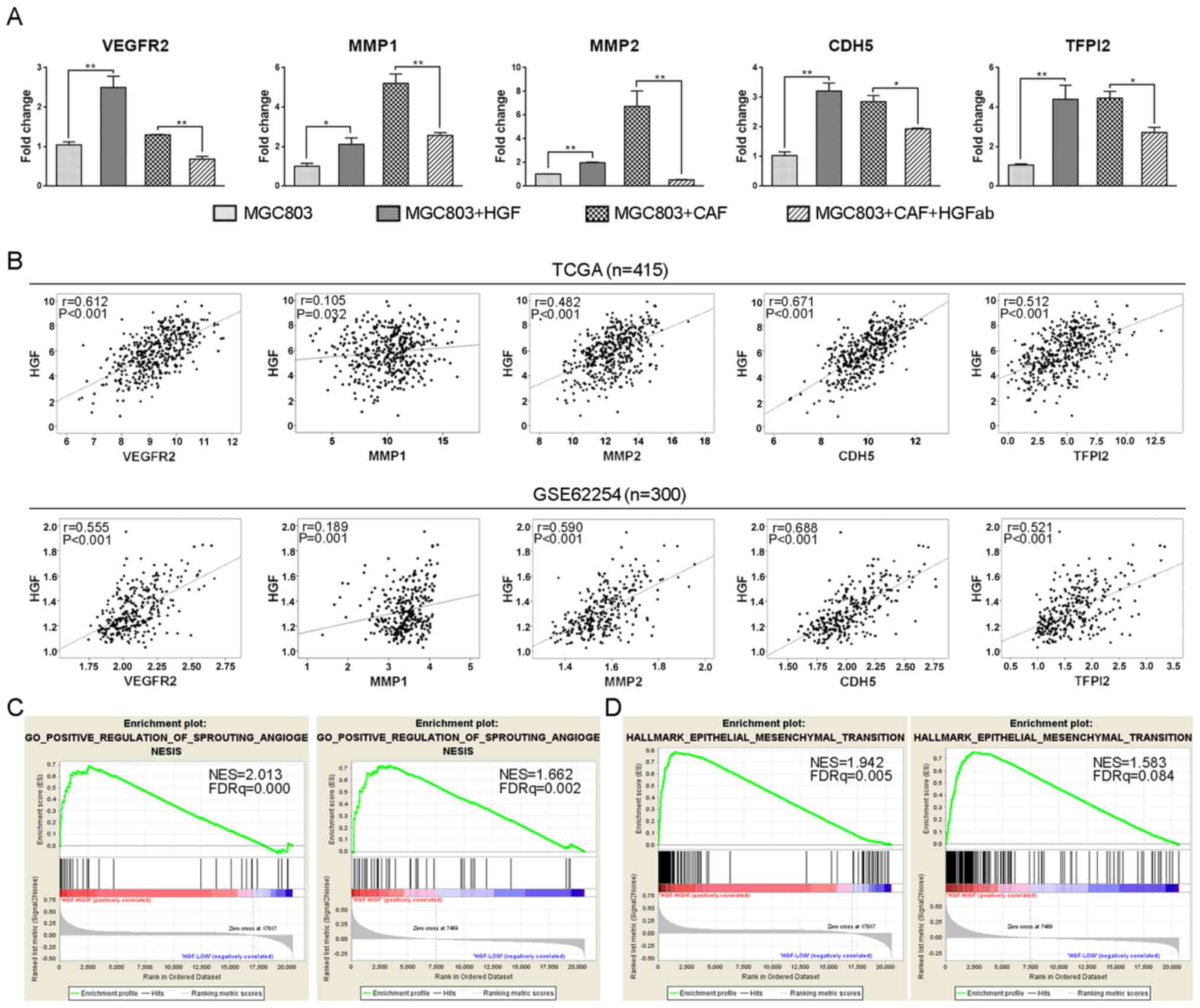
Multiple molecules have been reported to participate
in angiogenesis and VM formation (21,35).
To confirm the promoting effects of HGF on angiogenesis and VM
formation and explore the underlying mechanisms, we investigated
and determined that HGF could regulate the expression of
angiogenesis-related gene, VEGFR2 and VM-promoting related genes,
MMP1, MMP2, CDH5 (VE-cadherin) and TFPI2. As shown in Fig. 4A, these genes were upregulated in
MGC803 cells with treatment of recombinant human HGF and co-culture
with CAFs compared with the negative control and HGF neutralized
groups, respectively. Moreover, we analyzed gene expression
patterns using RNA-seq of 415 GC patients from the TCGA database
and microarray profiles of 300 GC patients from the GSE62254
database, and found that HGF was positively correlated with these
molecules, respectively (Fig. 4B).
The correlation between HGF and angiogenesis was also analyzed by
Gene Set Enrichment Analysis (GSEA) with the TCGA and GSE62254
databases, and the results revealed that genes positively
correlated with angiogenesis were enriched in HGF-high expression
samples (Fig. 4C). Increasing
evidence has indicated that epithelial-mesenchymal transition (EMT)
could induce VM formation (36,37),
thus, we subsequently explored the relationship between HGF and EMT
by GSEA and found that genes positively correlated with EMT were
also enriched in HGF-high expression samples (Fig. 4D). In conclusion, CAF-derived HGF
promoted angiogenesis and VM formation via PI3K/AKT and ERK1/2
signaling and upregulated the expression of these processes-related
genes in gastric cancer.
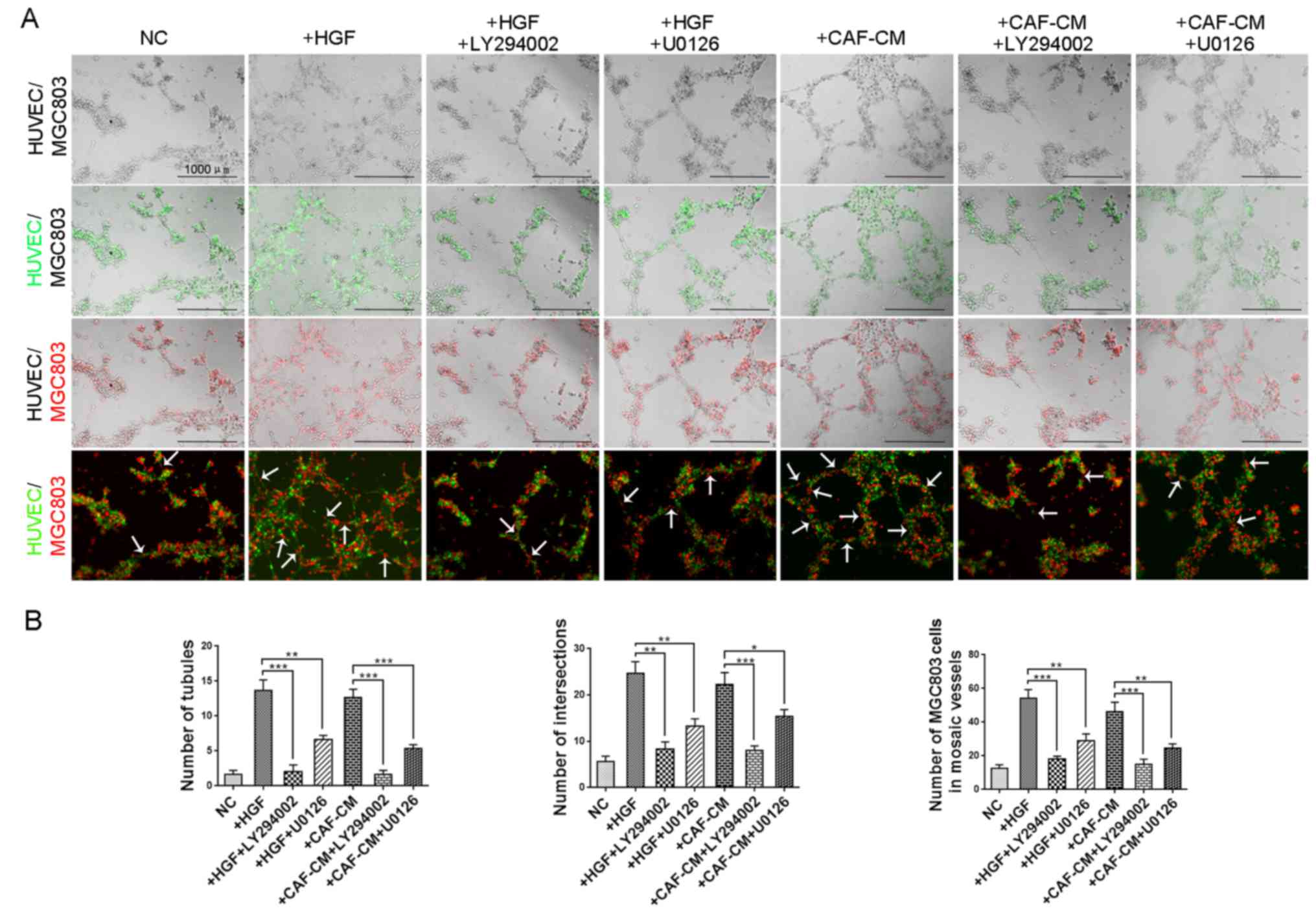
Inhibitors of PI3K/AKT and ERK1/2
signaling reduce mosaic vessels induced by CAF-derived HGF
Mosaic vessels are formed by the cooperation of
endothelia and cancer cells. Since we demonstrated the inhibiting
effects of LY294002 and U0126 on both angiogenesis of HUVECs and VM
formation of GC cells, we next examined whether these inhibitors
had the same influence on mosaic vessel formation. As shown in
Fig. 5A, both recombinant human HGF
and conditioned medium of CAFs increased the number of mosaic
vessels, which was reversed by inhibition of PI3K/AKT and ERK1/2
signaling. The number of tubules, number of intersections and
number of MGC803 cells in mosaic vessels in the CAF-CM group were
significantly increased compared with the control groups and
significantly decreased when treated with LY294002 and U0126
(Fig. 5B). These results indicated
that CAF-derived HGF promotes mosaic vessel formation via both
PI3K/AKT and ERK1/2 signaling.
Discussion
Cancer-associated fibroblasts have revealed their
irreplaceable roles in maintaining malignancy of solid tumors
through secreting various types of cytokines, among which HGF has
been reported to facilitate tumorigenesis and tumor progression
(17,38). HGF overexpression was revealed to be
positively correlated with depth of invasion, lymph node
metastasis, TNM stage and poor survival of patients with gastric
cancer (39). We analyzed the
survival data of GC patients from TCGA and GEO databases and
confirmed the negative correlation between HGF expression and
overall survival. In the present study, we determined that HGF
originating from CAFs accelerated endothelium-dependent
angiogenesis through promotion of HUVEC proliferation and
migration, which was consistent with a previous study in pancreatic
cancer (26). In a co-culture
system, the migration ability of GC cells was enhanced through
reciprocal interactions with CAFs, which, however, was inhibited by
neutralizing antibody against HGF. Thus, we demonstrated that
CAF-derived HGF also facilitated VM formation. Mosaic vessels are
formed by both endothelia and cancer cells. Given that HGF
increased the abilities of both HUVECs and MGC803 cell migration,
we further investigated and confirmed the promoting effect of
CAF-derived HGF on mosaic vessel formation. These results revealed
that HGF promotes vascularization, namely angiogenesis, VM
formation and mosaic vessel formation. Thus, it is reasonable to
hypothesize that HGF derived from CAFs may facilitate tumor
progression through promotion of angiogenesis, VM formation and
mosaic vessel formation in the GC microenvironment.
As one of the hallmarks of cancer, tumor
angiogenesis is positively correlated with tumorigenesis, tumor
growth and metastasis, which has been demonstrated by an increasing
number of studies (30,40). An anti-angiogenesis strategy has
been applied to inhibit tumor progression in multiple types of
cancers (41–43). However, cancer cells could evade
inhibition of angiogenesis after an initial response to therapeutic
strategies that target endothelial cells. Thus, vasculogenic
mimicry, a cancer cell-dependent pattern associated with poor
survival, has become a potential target for anticancer strategy
(44). HGF exhibits its
tumor-promoting effect through binding to its receptor, c-Met, and
then triggering several oncogenic signaling cascades, among which
PI3K/AKT and ERK1/2 signaling have been reported to regulate
angiogenesis and VM formation (21,33,34).
PI3K/AKT and ERK1/2 signaling pathways have been revealed to
regulate the growth, survival, and migration of endothelial cells
and thus promote angiogenesis (45). Suppressing the phosphorylation of
VEGFR2 could reduce the activation of the PI3K/AKT and ERK1/2
signaling pathways and thus inhibit angiogenesis (46). ERK1/2 was revealed to be positively
involved in hypoxia-induced VM formation (47), and PI3K/AKT inhibition suppressed VM
formation capacity of hepatocellular carcinoma cells (48). HGF has been reported to promote
angiogenesis in pancreatic cancer and VM formation in
hepatocellular carcinoma (26,27),
and in the present study, we found the same effects of HGF in
gastric cancer. To further investigate whether the promoting
effects of HGF on angiogenesis and VM formation rely on PI3K/AKT
and ERK1/2 signaling, specific inhibitors of the two signaling
pathways were subjected to the following experiments. Both
inhibition of PI3K/AKT and ERK1/2 signaling could decrease the
tubule-like structures of angiogenesis induced by HUVECs and VM
induced by MGC803 cells. Many genes have been reported to
facilitate VM formation (33,34,49).
In addition, we found that HGF increased the expression of
VM-promoting genes, such as MMP1, MMP2, CDH5 (VE-cadherin) and
TFPI2 in GC cells, which were confirmed by correlation analysis of
415 samples from the TCGA database and 300 samples from the
GSE62254 database. Other molecules that could increase VM
formation, such as MMP9, MT1-MMP and EphA2 were also positively
correlated with HGF by correlation analysis using these databases.
However, they were not upregulated in groups with stimulation of
HGF (data not shown). Thus, we hypothesized that HGF could
facilitate VM formation through, at least in part, upregulation of
the expression of these VM-promoting genes. Given these results, we
examined the influence of LY294002 and U0126 on mosaic vessel
formation, and found that they both significantly decreased the
number of mosaic vessels. These findings suggest that PI3K/AKT and
ERK1/2 signaling also participate in mosaic vessel formation. In
addition, there are some instructive points that warrant
improvement in our experiments: i) in vivo experiments
investigating the impact of CAF-derived HGF on angiogenesis, VM
formation and mosaic vessel formation should be conducted; and ii)
the characteristics of CAFs may be influenced by clinical features,
like TNM stage, pathological type and status of HP infection, thus
CAFs isolated from different pathological types, tumor stages, and
HP infection status should be analyzed. Fortunately, both these
aforementioned points will be addressed in our ongoing follow-up
studies.
Collectively, CAF-secreted HGF promoted
angiogenesis, VM formation and mosaic vessel formation in gastric
cancer. Crosstalks between CAFs and HUVECs, as well as gastric
cancer cells promoted HUVEC and gastric cancer cell migration, and
thus accelerated the process of vascularization. However, these
effects could be inhibited by suppressing PI3K/AKT and ERK1/2
signaling. These results indicated that CAF-derived HGF promotes
vascularization via PI3K/AKT and ERK1/2 signaling in gastric
cancer, and it may serve as a prognostic indicator and potential
therapeutic target for cancer anti-vascular treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (nos. 81672327, 81502013 and 81602411),
the Program of Shanghai Academic/Technology Research Leader (no.
17XD1402600), the Program for Outstanding Medical Academic Leader
and Shanghai Municipal Education Commission-Gaofeng Clinical
Medicine Grant Support (no. 20161410), the Development Grant for
Clinical Trial (no. SHDC12017×06), the SCORE Foundation (no.
Y-MX2015-078) and the Shanghai Municipal Commission of Health and
Family Planning (no. 20154Y496).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XSD, WQX and JZ conceived and designed the study.
XSD, WQX, JJ and QC performed the experiments. XSD, WQX, JLJ and MS
wrote the manuscript. YYY, ZGZ and JZ reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ruijin Hospital Ethics
Committee of Shanghai Jiaotong University School of Medicine and
written informed consent was provided by the patient.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Son T and Hyung WJ: Laparoscopic gastric
cancer surgery: Current evidence and future perspectives. World J
Gastroenterol. 22:727–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu W, Beeharry MK, Liu W, Yan M and Zhu Z:
Preoperative chemotherapy for gastric cancer: Personal
interventions and precision medicine. Biomed Res Int.
2016:39235852016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KW, Yeo SY, Sung CO and Kim SH: Twist1
is a key regulator of cancer-associated fibroblasts. Cancer Res.
75:73–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Satoyoshi R, Kuriyama S, Aiba N, Yashiro M
and Tanaka M: Asporin activates coordinated invasion of scirrhous
gastric cancer and cancer-associated fibroblasts. Oncogene.
34:650–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhou Q, Yu Z, Wu X, Chen X, Li J,
Li C, Yan M, Zhu Z, Liu B, et al: Cancer-associated
fibroblast-derived Lumican promotes gastric cancer progression via
the integrin β1-FAK signaling pathway. Int J Cancer. 141:998–1010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugihara H, Ishimoto T, Yasuda T, Izumi D,
Eto K, Sawayama H, Miyake K, Kurashige J, Imamura Y, Hiyoshi Y, et
al: Cancer-associated fibroblast-derived CXCL12 causes tumor
progression in adenocarcinoma of the esophagogastric junction. Med
Oncol. 32:6182015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto K and Nakamura T: Hepatocyte
growth factor and the Met system as a mediator of tumor-stromal
interactions. Int J Cancer. 119:477–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon Y, Smith BD, Zhou Y, Kaufman MD and
Godwin AK: Effective inhibition of c-MET-mediated signaling, growth
and migration of ovarian cancer cells is influenced by the ovarian
tissue microenvironment. Oncogene. 34:144–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phan LM, Fuentes-Mattei E, Wu W,
Velazquez-Torres G, Sircar K, Wood CG, Hai T, Jimenez C, Cote GJ,
Ozsari L, et al: Hepatocyte growth factor/cMET pathway activation
enhances cancer hallmarks in adrenocortical carcinoma. Cancer Res.
75:4131–4142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grugan KD, Miller CG, Yao Y, Ohashi S,
Klein-Szanto AJ, Diehl JA, Herlyn M, Han M, Nakagawa H and Rustgi
AK: Fibroblast-secreted hepatocyte growth factor plays a functional
role in esophageal squamous cell carcinoma invasion. Proc Natl Acad
Sci USA. 107:11026–11031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jedeszko C, Victor BC, Podgorski I and
Sloane BF: Fibroblast hepatocyte growth factor promotes invasion of
human mammary ductal carcinoma in situ. Cancer Res. 69:9148–9155.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Chen X, Zhou Q, Li P, Yu B, Li J, Qu
Y, Yan J, Yu Y, Yan M, et al: Hepatocyte growth factor activates
tumor stromal fibroblasts to promote tumorigenesis in gastric
cancer. Cancer Lett. 335:128–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Liu W, Guo X, Zhang R, Zhi Q, Ji
J, Zhang J, Chen X, Li J, Zhang J, et al: IRX1 influences
peritoneal spreading and metastasis via inhibiting BDKRB2-dependent
neovascularization on gastric cancer. Oncogene. 30:4498–4508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Huang J, Yao WY, Ben QW, Chen DF,
He XY, Li L and Yuan YZ: The origins of vacularization in tumors.
Front Biosci. 17:2559–2565. 2012. View
Article : Google Scholar
|
|
21
|
Zhang J, Qiao L, Liang N, Xie J, Luo H,
Deng G and Zhang J: Vasculogenic mimicry and tumor metastasis.
JBUON. 21:533–541. 2016.PubMed/NCBI
|
|
22
|
Guo Q, Yuan Y, Jin Z, Xu T, Gao Y, Wei H,
Li C, Hou W and Hua B: Association between tumor vasculogenic
mimicry and the poor prognosis of gastric cancer in China: An
updated systematic review and meta-analysis. Biomed Res Int.
2016:24086452016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Folkman J: Can mosaic tumor vessels
facilitate molecular diagnosis of cancer? Proc Natl Acad Sci USA.
98:398–400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
di Tomaso E, Capen D, Haskell A, Hart J,
Logie JJ, Jain RK, McDonald DM, Jones R and Munn LL: Mosaic tumor
vessels: Cellular basis and ultrastructure of focal regions lacking
endothelial cell markers. Cancer Res. 65:5740–5749. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang YS, di Tomaso E, McDonald DM, Jones
R, Jain RK and Munn LL: Mosaic blood vessels in tumors: Frequency
of cancer cells in contact with flowing blood. Proc Natl Acad Sci
USA. 97:14608–14613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu D, Matsuo Y, Ma J, Koide S, Ochi N,
Yasuda A, Funahashi H, Okada Y and Takeyama H: Cancer cell-derived
IL-1alpha promotes HGF secretion by stromal cells and enhances
metastatic potential in pancreatic cancer cells. J Surg Oncol.
102:469–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lirdprapamongkol K, Chiablaem K, Sila-Asna
M, Surarit R, Bunyaratvej A and Svasti J: Exploring stemness gene
expression and vasculogenic mimicry capacity in well-and
poorly-differentiated hepatocellular carcinoma cell lines. Biochem
Biophys Res Commun. 422:429–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zang M, Zhang Y, Zhang B, Hu L, Li J, Fan
Z, Wang H, Su L, Zhu Z, Li C, et al: CEACAM6 promotes tumor
angiogenesis and vasculogenic mimicry in gastric cancer via FAK
signaling. Biochim Biophys Acta. 1852:1020–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li P, Shan JX, Chen XH, Zhang D, Su LP,
Huang XY, Yu BQ, Zhi QM, Li CL, Wang YQ, et al: Epigenetic
silencing of microRNA-149 in cancer-associated fibroblasts mediates
prostaglandin E2/interleukin-6 signaling in the tumor
microenvironment. Cell Res. 25:588–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu CC, Chen C, Xu ZQ, Zhao JK, Ou BC, Sun
J, Zheng MH, Zong YP and Lu AG: CCR6 promotes tumor angiogenesis
via the AKT/NF-kappaB/VEGF pathway in colorectal cancer. Biochim
Biophy Acta. 1864:387–397. 2018. View Article : Google Scholar
|
|
31
|
Gao LM, Wang F, Zheng Y, Fu ZZ, Zheng L
and Chen LL: Roles of fibroblast activation protein and hepatocyte
growth factor expressions in angiogenesis and metastasis of gastric
cancer. Pathol Oncol Res. 2017. View Article : Google Scholar
|
|
32
|
Ma TH, Gao CC, Xie R, Yang XZ, Dai WJ,
Zhang JL, Yan W and Wu SN: Predictive values of FAP and HGF for
tumor angiogenesis and metastasis in colorectal cancer. Neoplasma.
64:880–886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Wang JH, Li S, Li LL, Huang M,
Zhang YH, Liu Y, Yang YT, Ding R and Ke YQ: Histone deacetylase 3
expression correlates with vasculogenic mimicry through the
phosphoinositide3-kinase/ERK-MMP-laminin5gamma2 signaling pathway.
Cancer Sci. 106:857–866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen LX, He YJ, Zhao SZ, Wu JG, Wang JT,
Zhu LM, Lin TT, Sun BC and Li XR: Inhibition of tumor growth and
vasculogenic mimicry by curcumin through down-regulation of the
EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer
Biol Ther. 11:229–235. 2014. View Article : Google Scholar
|
|
35
|
Ruf W, Seftor EA, Petrovan RJ, Weiss RM,
Gruman LM, Margaryan NV, Seftor RE, Miyagi Y and Hendrix MJ:
Differential role of tissue factor pathway inhibitors 1 and 2 in
melanoma vasculogenic mimicry. Cancer Res. 63:5381–5389.
2003.PubMed/NCBI
|
|
36
|
Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J,
Sun T, Wang J, Sun R and Liu Y: Hypoxia promotes vasculogenic
mimicry formation by inducing epithelial-mesenchymal transition in
ovarian carcinoma. Gynecol Oncol. 133:575–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Q, Qiao L, Liang N, Xie J and Zhang J,
Deng G, Luo H and Zhang J: The relationship between vasculogenic
mimicry and epithelial-mesenchymal transitions. J Cell Mol Med.
20:1761–1769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Deng T, Liu R, Bai M, Zhou L,
Wang X, Li S, Wang X, Yang H, Li J, et al: Exosome-delivered EGFR
regulates liver microenvironment to promote gastric cancer liver
metastasis. Nat Commun. 8:150162017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hao NB, Tang B, Wang GZ, Xie R, Hu CJ,
Wang SM, Wu YY, Liu E, Xie X and Yang SM: Hepatocyte growth factor
(HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB
signaling pathway for gastric cancer metastasis. Cancer Lett.
361:57–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Borcoman E and Le Tourneau C:
Pembrolizumab in cervical cancer: Latest evidence and clinical
usefulness. Ther Adv Med Oncol. 9:431–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fuchs CS, Marshall J and Barrueco J:
Randomized, controlled trial of irinotecan plus infusional, bolus,
or oral fluoropyrimidines in first-line treatment of metastatic
colorectal cancer: Updated results from the BICC-C study. J Clin
Oncol. 26:689–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye W: The Complexity of translating
anti-angiogenesis therapy from basic science to the clinic. Dev
Cell. 37:114–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang S, Zhang D and Sun B: Vasculogenic
mimicry: Current status and future prospects. Cancer Lett.
254:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin J, Yuan F, Shen MQ, Feng YF and He QL:
Vascular endothelial growth factor regulates primate
choroid-retinal endothelial cell proliferation and tube formation
through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem.
381:267–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim GD: Kaempferol inhibits angiogenesis
by suppressing HIF-1α and VEGFR2 activation via ERK/p38 MAPK and
PI3K/Akt/mTOR signaling pathways in endothelial cells. Prev Nutr
Food Sci. 22:320–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang B, Xiao E and Huang M: MEK/ERK
pathway is positively involved in hypoxia-induced vasculogenic
mimicry formation in hepatocellular carcinoma which is regulated
negatively by protein kinase A. Med Oncol. 32:4082015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chiablaem K, Lirdprapamongkol K,
Keeratichamroen S, Surarit R and Svasti J: Curcumin suppresses
vasculogenic mimicry capacity of hepatocellular carcinoma cells
through STAT3 and PI3K/AKT inhibition. Anticancer Res.
34:1857–1864. 2014.PubMed/NCBI
|
|
49
|
Williamson SC, Metcalf RL, Trapani F,
Mohan S, Antonello J, Abbott B, Leong HS, Chester CPE, Simms N,
Polanski R, et al: Vasculogenic mimicry in small cell lung cancer.
Nat Commun. 7:133222016. View Article : Google Scholar : PubMed/NCBI
|