Introduction
Osteosarcoma (OS) is a primary marrow cavity solid
tumor with high malignant degree, the morbidity of which ranks in
the second place among all primary bone tumors, second only second
to plasma cell myeloma (1). OS
develops from mesenchymal cell lines, which is characterized by the
production of osteoid tissue of fusiform stromal cells, as well as
the osteoid tissue or immature bone produced by tumor cells
(2). OS is the most common primary
malignant bone tumor in clinic, which frequently occurs in children
and adolescent (3). OS accounts for
approximately 30% of malignant bone tumors and 3–4% of all tumors
in children; in addition, it is associated with poor prognosis and
low 5-year survival rate. With the increasing deepening of
understanding towards tumor genesis and development, as well as the
rapid development of treatment technologies such as surgical
technique, imaging diagnostics, adjuvant chemotherapy and
radiotherapy, especially the rapid development of individualized
treatment, cell therapy, monoclonal therapy and tumor vaccine
therapy, the 5-year survival rate of patients has increased from
10–20% to 50–70% by adopting the comprehensive treatment mode of
neoadjuvant therapy with limb-sparing radical surgery, which has
become the successful model in tumor treatment field (3,4).
Research on miRNA in recent years has been
transferred from lower biological regulation like nematode to all
physiological processes of mammals (5). Research concerning correlation of
tumor with miRNA expression is extremely active, which facilitates
the identification of multiple miRNAs that exert important
regulatory effects in tumor progression (6). Currently, a part of miRNA profiles
have been applied in tumor screening and metastasis as tumor
markers (6). Transgenic mice with
over-expression and deletion of specific miRNAs have also been
extensively applied in investigating the roles of miRNAs in
multiple malignant tumors. The present study provided experimental
evidence for the function of microRNA-152 for a cohort of patients
with osteosarcoma and the influence on cell growth of
osteosarcoma.
Materials and methods
Ethics statement, patients, and
samples
We collected serum of OS patients and normal
volunteers at The Second Hospital of Jilin University, (Changchun,
China). This study was approved by the ethics committees on The
Second Hospital of Jilin University.
Total RNA was extracted from serum using the QIAzol
lysis reagent and miRNeasy kit following the protocol of the
manufacturer (Qiagen Inc., Valencia, CA, USA). Total RNA (200 ng)
was used to compound cDNA synthesis using the QuantiTect Reverse
Transcription kit (Qiagen Inc.). The real-time PCR (qPCR) was
detected using SYBR Green Mix (Life Technologies). The PCR
amplification conditions were as follows: 5 min at 94°C, for 40
cycles at 95°C for 30 sec and at 60°C for 30 sec.
Cell cultures and transfection
Human Osteosarcoma F5M2 cell line was acquired from
the American Type Culture Collection (Manassas, VA, USA) and was
cultured with RPMI-1640 medium (Gibco Life Technologies,
Gaitherburg, MD, USA), 10% fetal bovine serum (FBS, Gibco Life
Technologies) and penicillin (100 U/ml)/streptomycin (100 µg/ml) in
a humidified atmosphere with 5% CO2 at 37°C.
MicroRNA-152, anti-microRNA-152, si-DKK1 and negative control
mimics were acquired from Sangon (Shanghai, China). Cell were
transfected by using Lipofectamine 2000 (Gibco Life Technologies)
according to the manufacturer's instructions.
Cell proliferation and flow cytometry
based cell apoptosis assay
After transfection, cells were seeded onto 96-well
plates and treated with methylthiazolyldiphenyl-tetrazolium bromide
(MTT) assay (Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C.
DMSO was added into cells and shaked for 20 min. The absorbance was
determined on a microplate spectrophoto-meter (SpectraMax,
Molecular Devices, Sunnyvale, CA, USA) at 490 nm.
After transfection, cells were seeded onto 6-well
plates and stained with Annexin V-FITC/PI Apoptosis Assay (BD
Biosciences Pharmingen, San Diego, CA, USA) for 20 min in the dark
at 4°C. Apoptotic cells were recorded by applying CellQuest
Software (BD Biosciences Pharmingen).
Caspase-9/3 activation
Cell protein was extracted using RIPA buffer
containing protease inhibitors and phosphatase inhibitors.
Caspase-3/9 activities was analyzed by caspase-3 and caspase-9
activity kits. The absorbance was determined using a microplate
spectrophotometer (SpectraMax, Molecular Devices) at 405 nm.
Western blotting
Cell protein was extracted using RIPA buffer
containing protease inhibitors and phosphatase inhibitors. Total
protein (30 µg) were separated by 8–10% SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to PVDF membranes
(Millipore). Then, membranes were blocked with 5% bovine serum
albumin (BSA), and incubated with Bax, Bcl-2, p53, Wnt, β-catenin,
DKK1 and GAPDH (all, Cell Signaling Technology) at 4°C overnight.
Membranes were incubated with HRP secondary antibodies (Cell
Signaling Technology) for 1 h at 37°C. The protein expression was
analyzed by the ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA,
USA) with Quantity One Program (version 4.6, Bio-Rad).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). ANOVA (one-way analysis of variance) was used to determine
the difference between or among groups. P<0.05 was considered
statistically significant.
Results
The expression of microRNA-152 in OS
patients
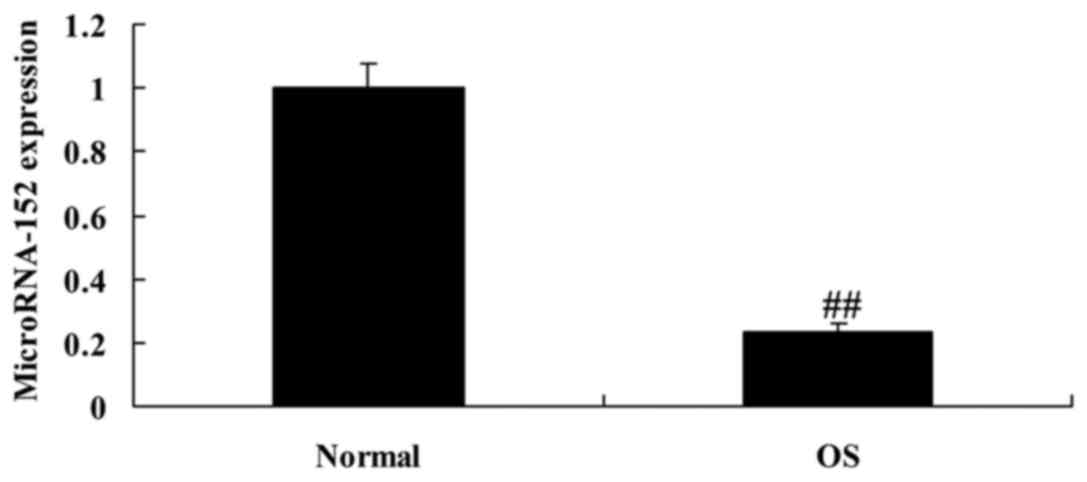
We validated the expression of microRNA-152 in OS
patients using real-time PCR (qPCR). Fig. 1 showed that the expression of
microRNA-152 was downregulated in patients with osteosarcoma. These
results indicate that microRNA-152 may regulate osteosarcoma
occurrence.
Overexpression of microRNA-152
inhibits cell proliferation and increases LDH activity
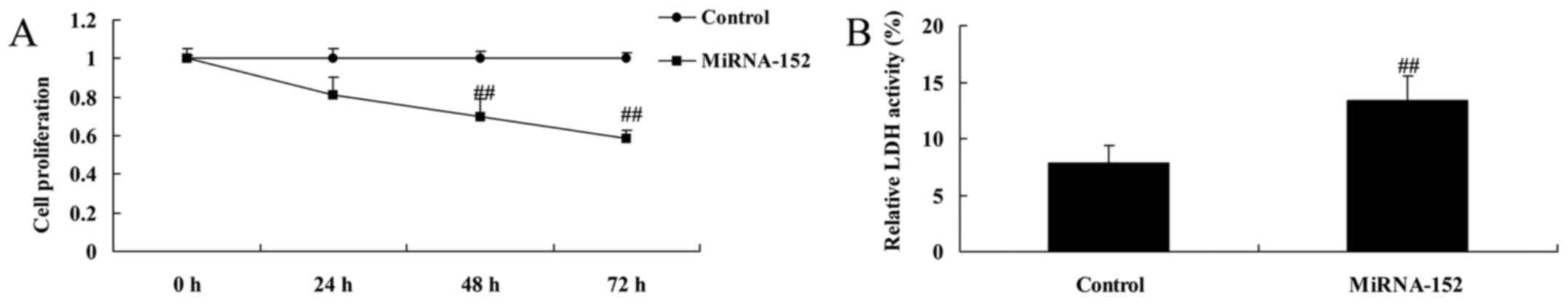
To establish a functional relationship between
microRNA-152 and cell proliferation of osteosarcoma, osteosarcoma
cells were transfected using microRNA-152 mimics. As shown in
Fig. 2, overexpression of
microRNA-152 inhibited cell proliferation and increased LDH
activity of osteosarcoma.
Overexpression of microRNA-152
increases caspase-3/9 activity and apoptosis
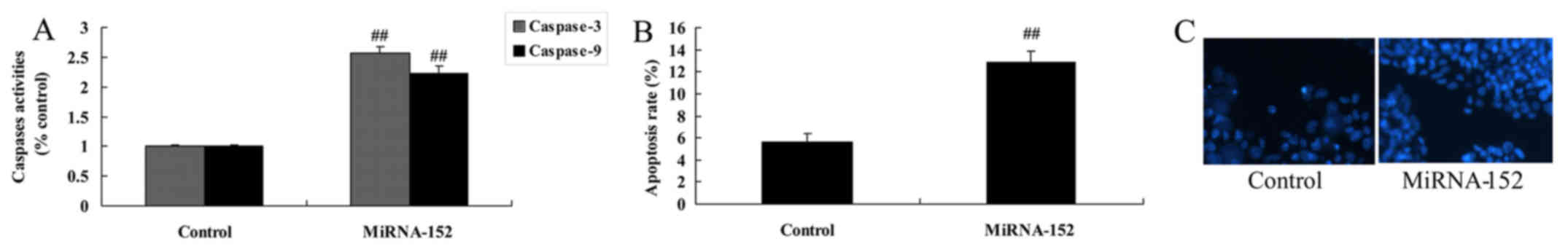
Further, we investigated whether microRNA-152
regulates apoptosis of osteosarcoma. Fig. 3A and B, indicate that overexpression
of microRNA-152 significantly decrease caspase-3/9 activities and
apoptosis of osteosarcoma. Whereas, the results of DAPI staining
showed that overexpression of microRNA-152 remarkably promoted cell
nucleus apoptosis of osteosarcoma (Fig.
3C).
Overexpression of microRNA-152
increases Bax/Bcl-2 and p53 protein expression levels
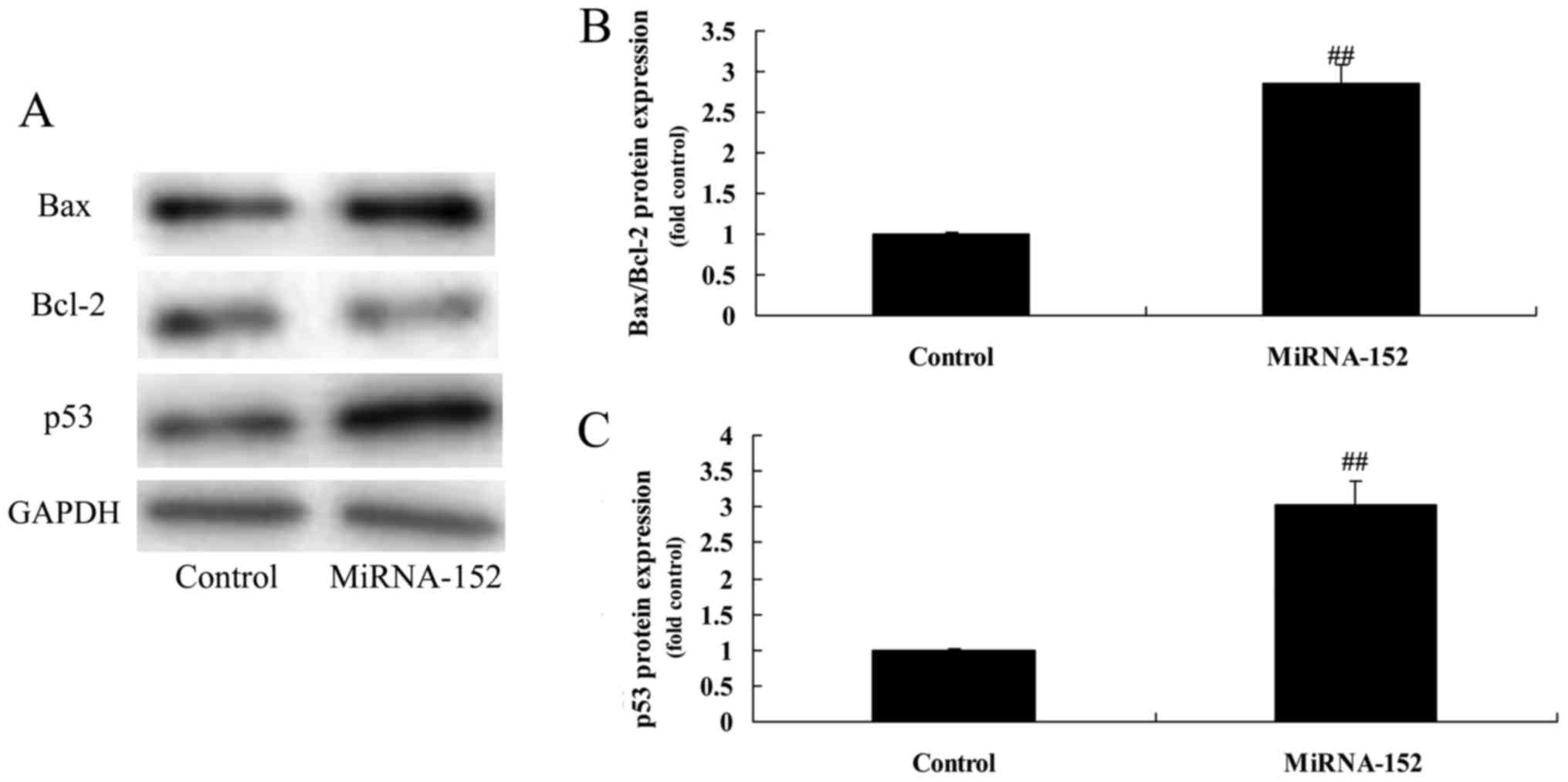
As showed in Fig. 4,
overexpression of microRNA-152 significantly induced Bax/Bcl-2 and
p53 protein expression levels of osteosarcoma, which showed that
microRNA-152 may adjust Bax/Bcl-2 and p53 protein expression levels
to promote apoptosis of osteosarcoma.
Overexpression of microRNA-152
suppresses DKK1 and decreases Wnt/β-catenin
To further investigate the correlation between
microRNA-152 and DKK1/Wnt/β-catenin signaling pathway, western
blotting was used to compare the DKK1 protein expression after
overexpression of microRNA-152. Our data suggest that
overexpression of microRNA-152 significantly suppressed DKK1
protein expression, and induced Wnt and β-catenin protein
expression in osteosarcoma (Fig.
5).
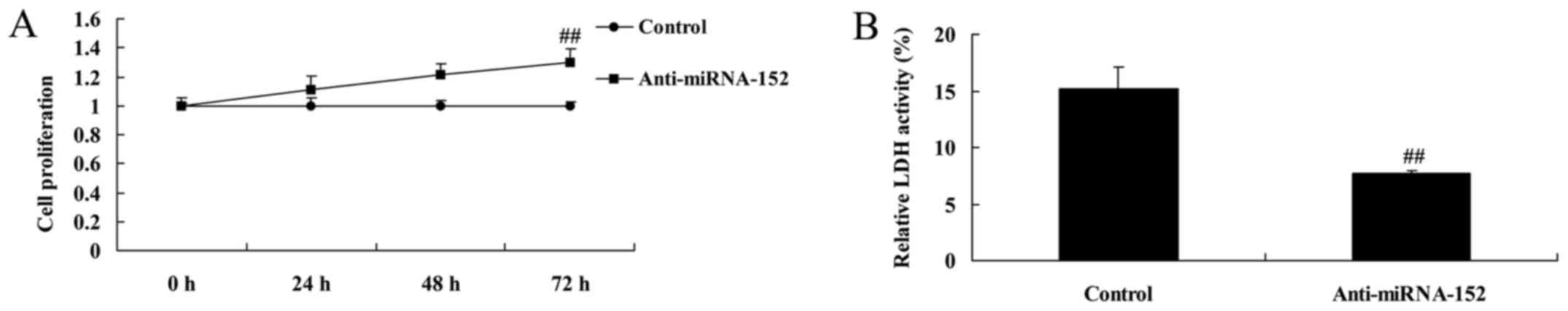
Downregulation of microRNA-152
expression promotes cell proliferation and decreases LDH
activity
We determined whether the reversal of cell
proliferation of osteosarcoma was adjusted by downregulation of
microRNA-152 expression. As shown in Fig. 6, downregulation of microRNA-152
expression significantly promoted cell proliferation and decreased
LDH activity of osteosarcoma.
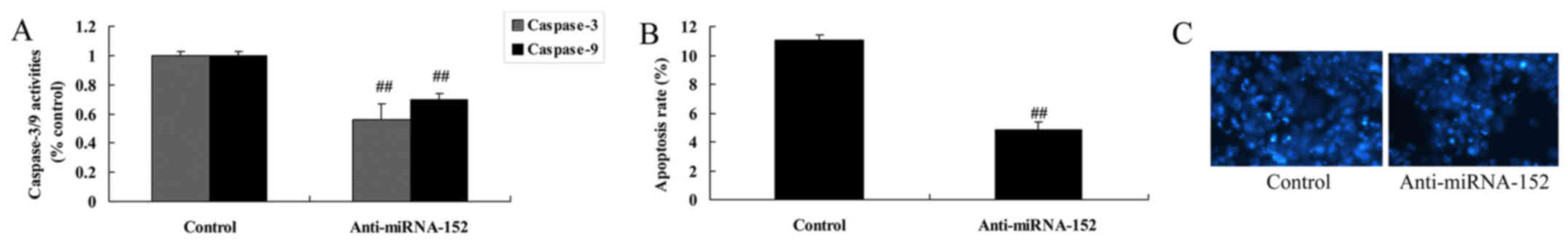
Downregulation of microRNA-152
expression decreases caspase-3/9 activity and apoptosis
In order to investigate more detailed apoptotic
mechanisms of microRNA-152 in osteosarcoma, the caspase-3/9
activity and apoptosis of osteosarcoma cells by microRNA-152
downregulation were measured. As expected, downregulation of
microRNA-152 expression significantly decreased caspase-3/9
activity and apoptosis, and inhibited cell nucleus apoptosis of
osteosarcoma (Fig. 7).
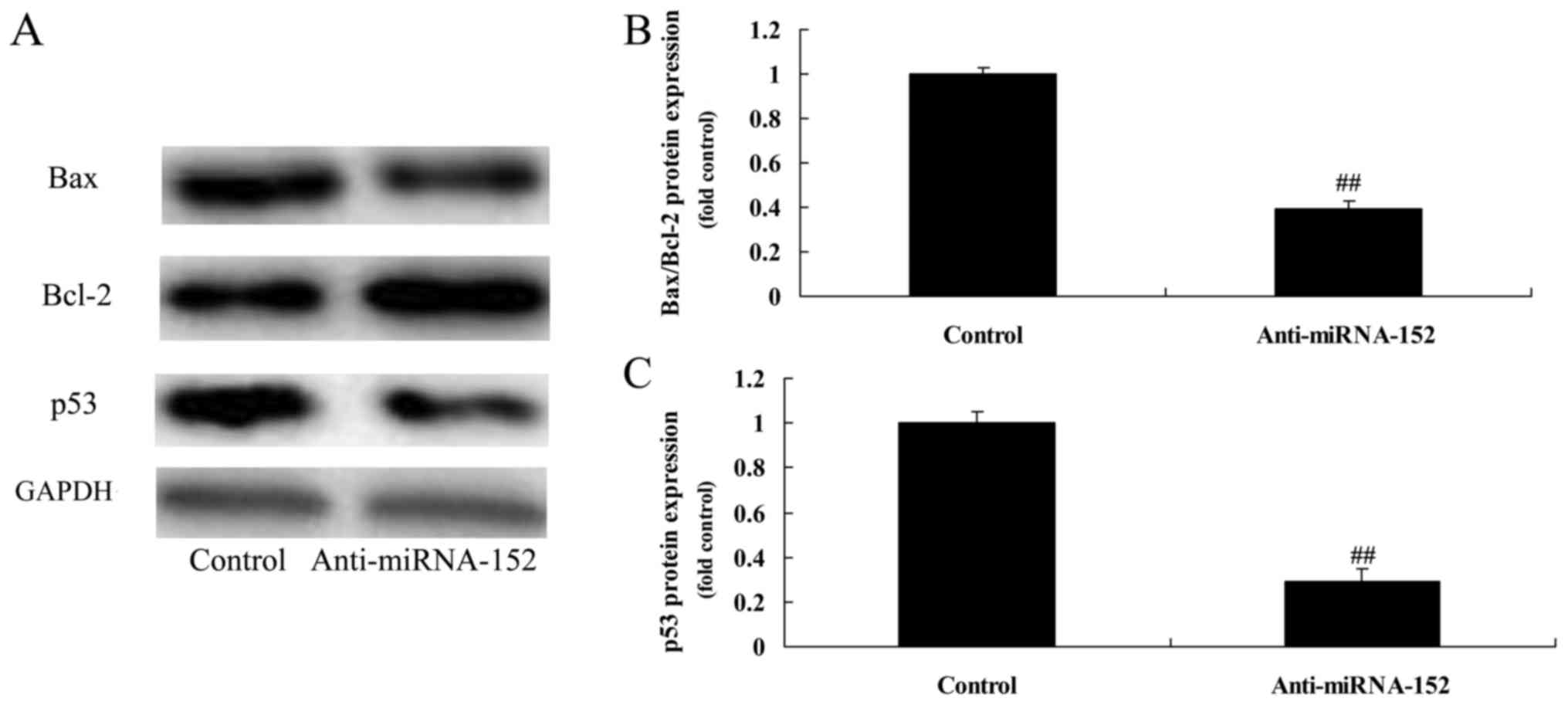
Downregulation of microRNA-152
expression decreases Bax/Bcl-2 and p53 protein expression
levels
As shown in Fig. 8,
downregulation of microRNA-152 expression significantly decreased
Bax/Bcl-2 and p53 protein expression levels of osteosarcoma, which
showed that microRNA-152 downregulation also adjusts cell growth of
osteosarcoma.
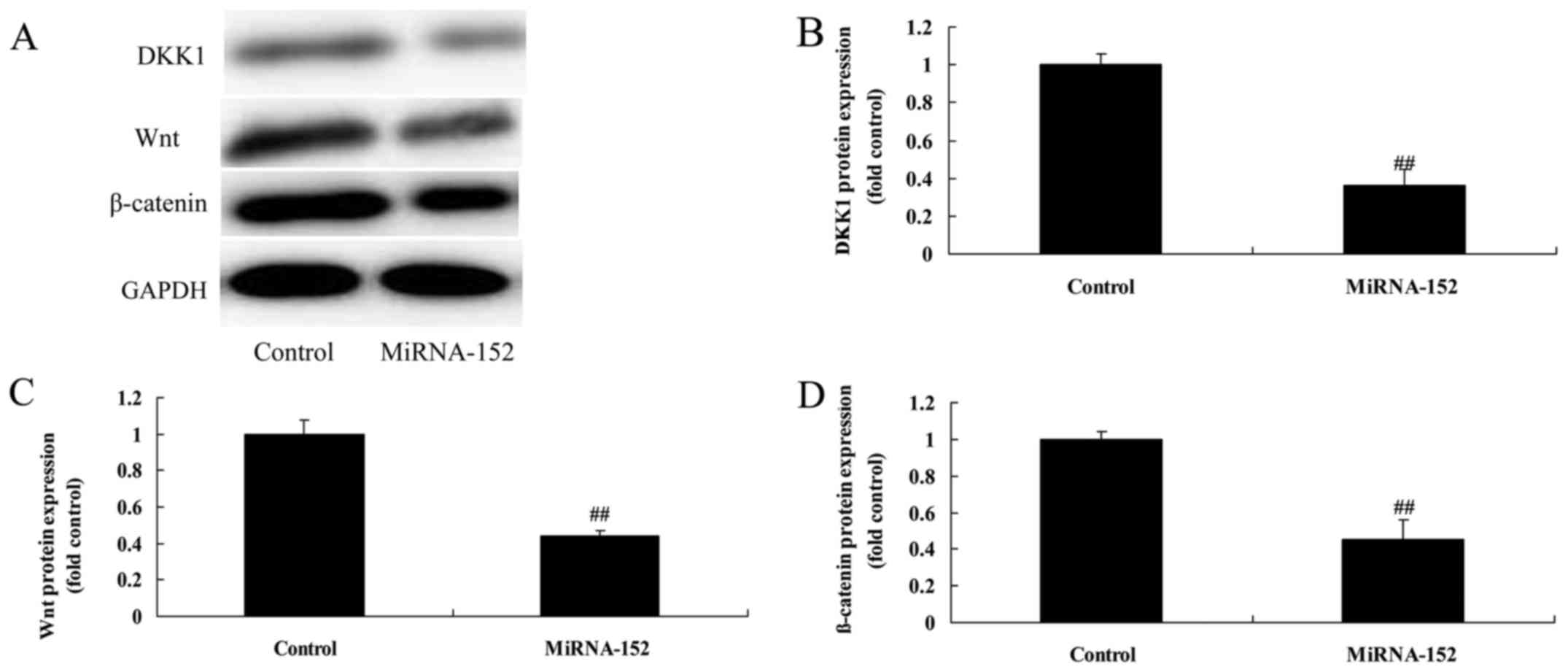
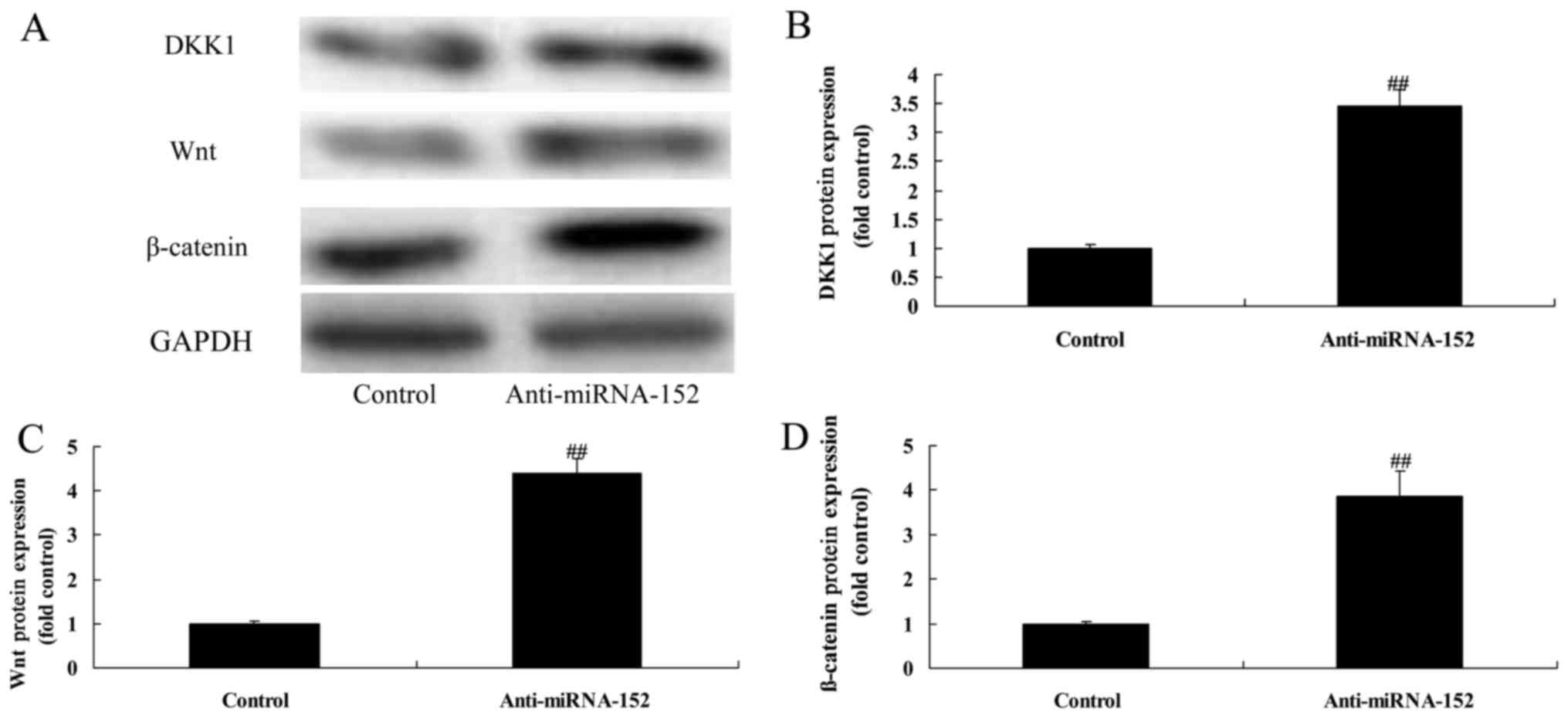
Downregulation of microRNA-152
expression induces DKK1 and increases Wnt/β-catenin
We determined if microRNA-152 downregulation
regulated DKK1/Wnt/β-catenin signaling pathway. Downregulation of
microRNA-152 expression significantly decreased DKK1 protein
expression and increased Wnt/β-catenin protein expression of
osteosarcoma (Fig. 9). Taken
together, these data suggested microRNA-152 could be a potential
regulator for Wnt/β-catenin protein expression via targeting
DKK1.
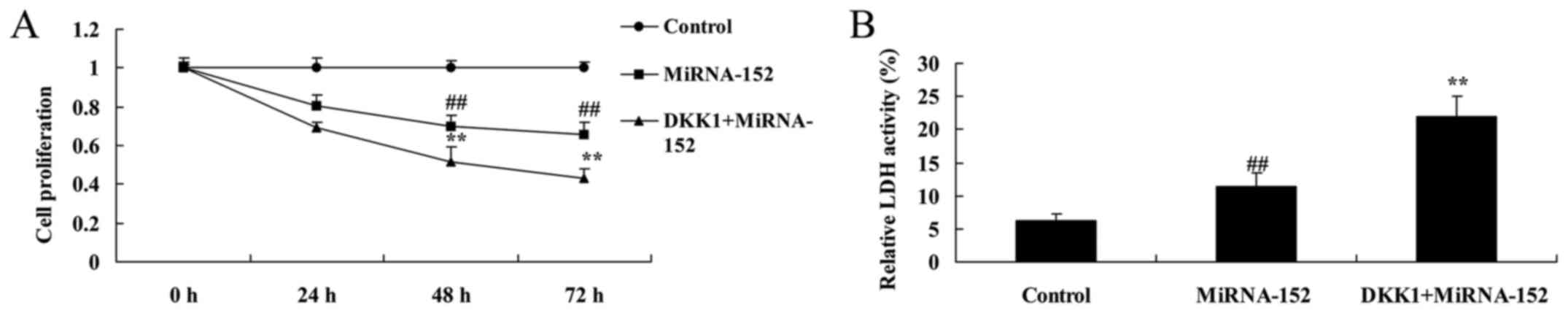
The inhibition of DKK1 promotes
anticancer function of microRNA-152 on cell proliferation and LDH
activity
To further confirm the above findings, we also used
DKK1 protein to increase DKK1 expression of osteosarcoma by
microRNA-152. As shown in Fig. 10,
compared with osteosarcoma-microRNA-152, the promotion of DKK1
promoted the anticancer function of microRNA-152 on cell
proliferation inhibition and LDH activity induction of
osteosarcoma.
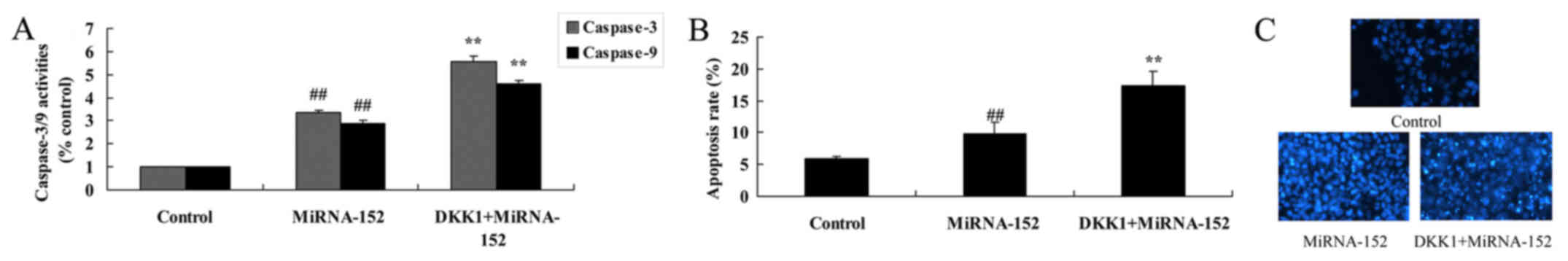
The inhibition of DKK1 reverses the
anticancer function of microRNA-152 on caspase-3/9 activity and
apoptosis
Based on the above, we expected the promotion of
DKK1 on the anticancer function of microRNA-152 apoptosis of
osteosarcoma. As shown in Fig. 11,
the promotion of DKK1 increased the anticancer function of
microRNA-152 on caspase-3/9 activity and apoptosis, and cell
nucleus apoptosis of osteosarcoma.
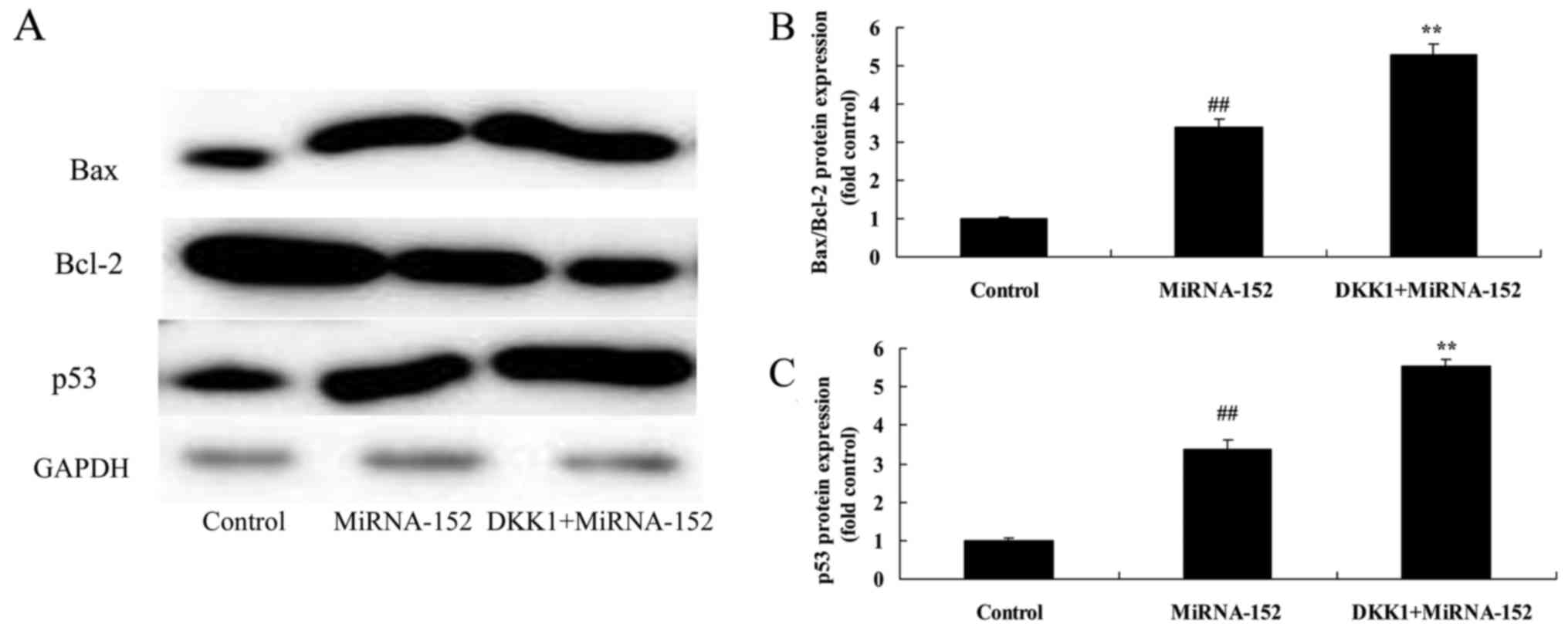
The inhibition of DKK1 reverses the
anticancer function of microRNA-152 on Bax/Bcl-2 and p53 protein
expression levels
Next, we found that the inhibition of DKK1
significantly promoted the anticancer function of microRNA-152 on
Bax/Bcl-2 and p53 protein expression levels of osteosarcoma
(Fig. 12).
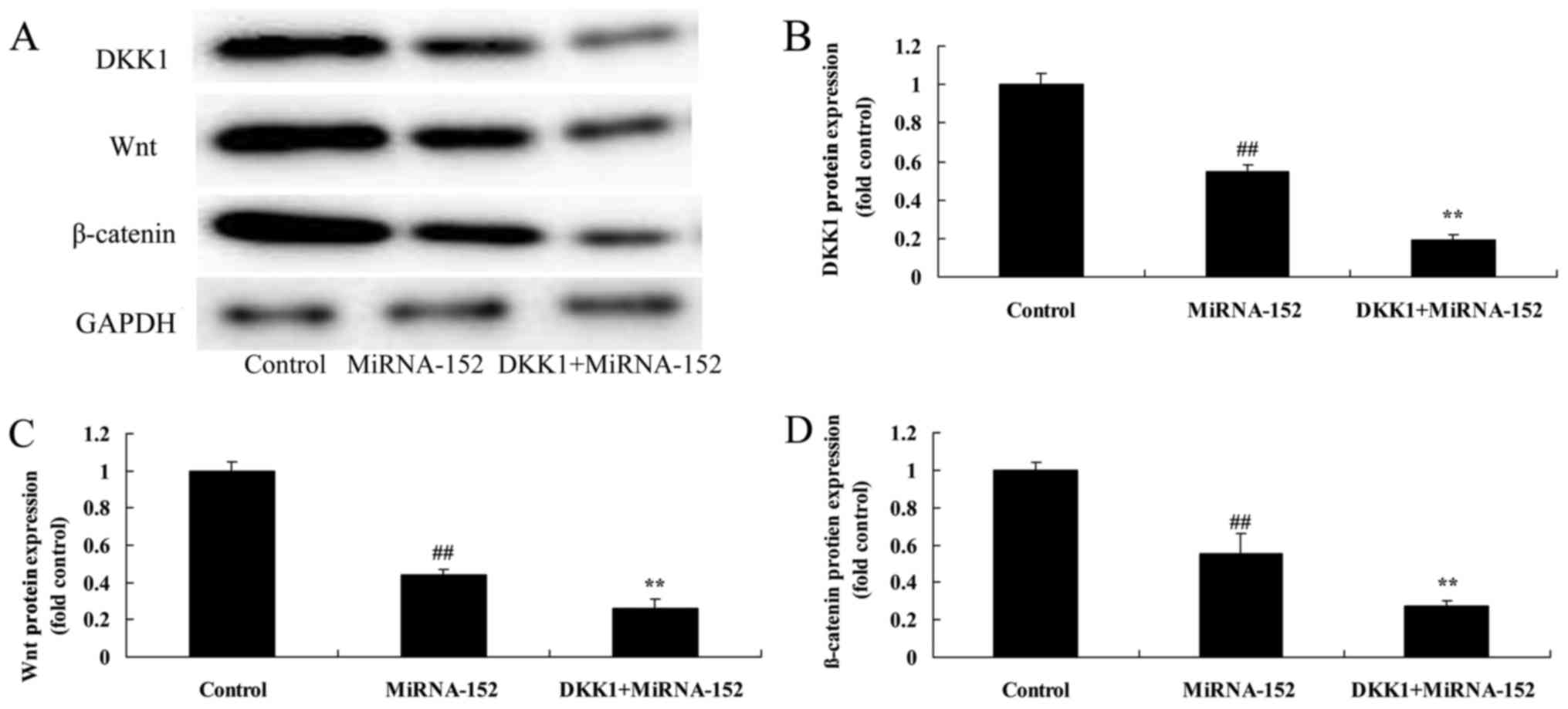
The inhibition of DKK1 reverses the
anticancer function of microRNA-152 on DKK1 and Wnt/β-catenin
Moreover, western blot analysis for DKK1 protein
also demonstrated a significant promotion of DKK1 protein
expression in osteosarcoma by microRNA-152 (Fig. 13A and B). Moreover, the promotion
of DKK1 reversed the anticancer function of microRNA-152 on
Wnt/β-catenin protein expression in osteosarcoma (Fig. 13A, C and D).
Discussion
Morbidity of OS accounts for approximately 0.2% of
human solid malignant tumors, with the age of onset being dominant
at 15–25 years (2). The number of
male patients is greater than that of female patients. It mostly
occurs in metaphysis of long bone, with distal femur and proximal
embryonic bone being dominant, followed by fibula and proximal limb
bone. Besides, it can also occur in bone tissues in other sites,
such as vertebral column, skeleton and upper femur. OS is a primary
malignant bone tumor that is frequently seen in children and
adolescent, and lung metastasis is responsible for the major cause
of death (7). OS cell invasion and
metastasis is the interaction between cells and extracellular
matrix, which is a complicated and multi-step process involving the
effects of multiple oncogenes (8).
In our study, we presume that the expression of microRNA-152 was
downregulated in patients with osteosarcoma. Zhou et al
identified that microRNA-152 inhibited tumor cell growth of
hepatocellular carcinoma by targeting RTKN (9).
miRNAs have become novel research focus since more
and more miRNA molecules have been discovered in human cells in
succession, especially after their extensive regulatory effects on
development, proliferation and death have been revealed (10). Increasing research in recent years
has verified that miRNAs play important regulatory roles in genesis
and development of tumor cells. miRNAs have their own expression
profiles in different malignant tumor tissues, as is demonstrated
(11). Furthermore, it is also
revealed that multiple miRNA molecules participate in regulating
important biological processes of cancer cells through silencing
expression of cancer cell growth-related genes, thus indirectly
exerting tumor-promoting and tumor-suppressing functions (8). We found that the downregulation of
microRNA-152 expression targets DKK1 to promote cell proliferation,
decrease apoptosis, and suppress LDH activity, caspase-3/9
activities of osteosarcoma; overexpression of microRNA-152 targets
DKK1 to inhibit cell proliferation, induce apoptosis, and promote
LDH activity, caspase-3/9 activities of osteosarcoma. Kindrat et
al suggests that microRNA-152 mediated cell growth of liver
carcinogenesis (12).
Bax/Bcl-2 pathway is also known as the mitochondrial
apoptosis pathway. Bcl-2 is widely present in normal tissue and
embryonic tissue cells, such as nerve cells, skin cells, and
embryonic kidney cells and cartilage (13). With the further study, the
understanding of the bcl-2 family members and their roles have
become deeper. It is known that bcl-2 family plays a key role in
apoptosis (13). Bcl-2 itself can
block or delay the apoptosis induced by a variety of
chemotherapeutic drugs by blocking apoptosis signal transduction
system (14). The present study has
confirmed that the downregulation of microRNA-152 expression
suppress Bax/Bcl-2 and p53 protein expression levels of
osteosarcoma; overexpression of microRNA-152 promotes Bax/Bcl-2 and
p53 protein expression levels of osteosarcoma. Cao et al
indicated that miR-152 induced cell apoptosis in human brain
microvascular endothelial cells (15).
DKK1 is a secretory protein that was first verified
to have regulatory effect on the development of embryonic head in
xenopus laevis. Subsequently, DKK1 was supposed to have regulatory
effect on Wnt/β-catenin signal pathway in human (16). Later, it is confirmed in numerous
studies that DKK1 plays an important role in the genesis and
metastasis mechanisms of tumor, and it is verified in some tumors
to have value in predicting prognosis (17). As a kind of secretory protein, its
expression quantity in serum can be treated as an indicator in
diagnosing certain tumors. However, in our study, we found that the
overexpression of microRNA-152 suppressed DKK1 and decreased
Wnt/β-catenin in osteosarcoma, and downregulation of microRNA-152
expression to induced DKK1/Wnt/β-catenin in osteosarcoma. Xu et
al showed that microRNA-152 contributes DKK1 expression in
multiple myeloma (18). Thus it was
shown that microRNA-152 could suppressed DKK1 Wnt/β-catenin in
osteosarcoma, which may be an important signaling pathways for
treatment of osteosarcoma.
Wnt/β-catenin signal transduction pathway in the
genesis and development of OS has received continuous attention at
present (19). Abnormalities of the
signal pathway mainly manifest as abnormal aggregation of β-catenin
in cytoplasm and nucleus, as well as activation of downstream
target genes such as cyclin D1 and surviving (20). These target genes are mostly
associated with cell cycle, cell apoptosis and cell proliferation,
which influence the normal regulation of cell proliferation and
differentiation, render excessive cell proliferation, and induce
cancer (21). Our results showed
the promotion of DKK1 reversed the anticancer function of
microRNA-152 overexpression through Wnt/β-catenin signaling
pathway. Miao et al revealed that microRNA-152 modulates Wnt
pathway activation in rheumatoid arthritis rat (22). Thus, we thought that microRNA-152
modulates DKK1 to suppress Wnt/β-catenin signaling pathway in
osteosarcoma.
In conclusion, our study for the first time
identified that the expression of microRNA-152 was downregulated in
patients with osteosarcoma. Overexpression of microRNA-152 targets
DKK1 to inhibit cell proliferation, induce apoptosis, and promote
LDH activity, caspase-3/9 activity and Bax/Bcl-2 and p53 protein
expression levels of osteosarcoma through inactivation of the
Wnt/β-catenin signaling pathway, exploration of novel interaction
network of molecules such as microRNA-152 and DKK1 may reveal novel
strategies for the treatment of osteosarcoma. MicroRNA-152 has been
further enhanced to understand occurrence mechanism of
osteosarcoma. microRNA-152 and DKK1 may be developed to treat
osteosarcoma for further clinical applications.
References
|
1
|
Zhou X, Jing J, Peng J, Mao W, Zheng Y,
Wang D, Wang X, Liu Z and Zhang X: Expression and clinical
significance of galectin-3 in osteosarcoma. Gene. 546:403–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou AJ, Gupta R, Bell MD, Riewe KO,
Meyers PA and Gorlick R: Inhaled lipid cisplatin (ILC) in the
treatment of patients with relapsed/progressive osteosarcoma
metastatic to the lung. Pediatr Blood Cancer. 60:580–586. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Italian Sarcoma Group: Sorafenib and
everolimus for patients with unresectable high-grade osteosarcoma
progressing after standard treatment: A non-randomised phase 2
clinical trial. Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujiwara T, Katsuda T, Hagiwara K, Kosaka
N, Yoshioka Y, Takahashi RU, Takeshita F, Kubota D, Kondo T,
Ichikawa H, et al: Clinical relevance and therapeutic significance
of microRNA-133a expression profiles and functions in malignant
osteosarcoma-initiating cells. Stem Cells. 32:959–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH
and Wang WJ: MicroRNAs in osteosarcoma. Clin Chim Acta. 444:9–17.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye Z, Jingzhong L, Yangbo L, Lei C and
Jiandong Y: Propofol inhibits proliferation and invasion of
osteosarcoma cells by regulation of microRNA-143 expression. Oncol
Res. 21:201–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song J, Li Y and An RF: Identification of
early-onset preeclampsia-related genes and microRNAs by
bioinformatics approaches. Reprod Sci. 22:954–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Han S and Sun K: Combined
analysis of gene expression, miRNA expression and DNA methylation
profiles of osteosarcoma. Oncol Rep. 37:1175–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou J, Zhang Y, Qi Y, Yu D, Shao Q and
Liang J: MicroRNA-152 inhibits tumor cell growth by directly
targeting RTKN in hepatocellular carcinoma. Oncol Rep.
37:1227–1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang J, Yao M, Li Y, Zhao D, Hu S, Cui X,
Liu G, Shi Q, Wang Y and Yang Y: MicroRNAs for osteosarcoma in the
mouse: A meta-analysis. Oncotarget. 7:85650–85674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, He N, Dong Y and Jiang C:
MiR-24-BIM-Smac/DIABLO axis controls the sensitivity to doxorubicin
treatment in osteosarcoma. Sci Rep. 6:342382016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kindrat I, Tryndyak V, de Conti A,
Shpyleva S, Mudalige TK, Kobets T, Erstenyuk AM, Beland FA and
Pogribny IP: MicroRNA-152-mediated dysregulation of hepatic
transferrin receptor 1 in liver carcinogenesis. Oncotarget.
7:1276–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding Y, Wang Y, Chen J, Hu Y, Cao Z, Ren P
and Zhang Y: p21 overexpression sensitizes osteosarcoma U2OS cells
to cisplatin via evoking caspase-3 and Bax/Bcl-2 cascade. Tumour
Biol. 35:3119–3123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu G, Kuang G, Jiang W, Jiang R and Jiang
D: Polydatin promotes apoptosis through upregulation the ratio of
Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin
signaling in human osteosarcoma cells. Am J Transl Res. 8:922–931.
2016.PubMed/NCBI
|
|
15
|
Cao YH, Li DG, Xu B, Wang MQ, Zhen N, Man
LX, Zhang YY and Chi M: A microRNA-152 that targets the phosphatase
and tensin homolog to inhibit low oxygen induced-apoptosis in human
brain microvascular endothelial cells. Genet Mol Res. 15:gmr7371.
2016. View Article : Google Scholar
|
|
16
|
Qian L, Cai C, Yuan P, Jeong SY, Yang X,
Dealmeida V, Ernst J, Costa M, Cohen SN and Wei W: Bidirectional
effect of Wnt signaling antagonist DKK1 on the modulation of
anthrax toxin uptake. Sci China Life Sci. 57:469–481. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Duan Y, Zhang M, Wu M and Wang Y:
Expression of Wnt3a, Wnt10b, β-catenin and DKK1 in periodontium
during orthodontic tooth movement in rats. Acta Odontol Scand.
74:217–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Chen B, George SK and Liu B:
Downregulation of MicroRNA-152 contributes to high expression of
DKK1 in multiple myeloma. RNA Biol. 12:1314–1322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ,
Huang J, Wu K, Luo JY, Zuo GW, Chen L, et al: Oridonin inhibits the
proliferation of human osteosarcoma cells by suppressing
Wnt/β-catenin signaling. Int J Oncol. 45:795–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi XJ, Zhao YH, Qiao LX, Jin CL, Tian J
and Li QS: Aberrant Wnt/β-catenin signaling and elevated expression
of stem cell proteins are associated with osteosarcoma side
population cells of high tumorigenicity. Mol Med Rep. 12:5042–5048.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brun J, Dieudonné FX, Marty C, Müller J,
Schüle R, Patiño-García A, Lecanda F, Fromigué O and Marie PJ: FHL2
silencing reduces Wnt signaling and osteosarcoma tumorigenesis in
vitro and in vivo. PLoS One. 8:e550342013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao CG, Yang YY, He X, Huang C, Huang Y,
Qin D, Du CL and Li J: MicroRNA-152 modulates the canonical Wnt
pathway activation by targeting DNA methyltransferase 1 in
arthritic rat model. Biochimie. 106:149–156. 2014. View Article : Google Scholar : PubMed/NCBI
|