Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor worldwide, predominantly affecting children
and adolescents (1). OS typically
presents between the ages of 10 and 20 years or in elderly patients
(2,3) and generally develops from primitive
mesenchymal bone-forming cells in the long bones (4). At present, the therapeutic options for
OS are surgery and intensive multi-agent chemotherapy, including
cisplatin, doxorubicin, ifosfamide and methotrexate (5). The 5-year survival rate of patients
with OS without metastases is 60–70%, depending on the use of
chemotherapy (6). However, the
5-year survival rate for OS patients with metastases, especially
pulmonary metastases, is only 20% (7). The current treatment strategies for
metastatic OS typically result in poor prognoses and relapse
(8). It would, therefore, be
beneficial to develop novel therapeutic agents and innovative
treatment approaches to improve survival rates in patients with
OS.
The ubiquitin-proteasome system (UPS) is highly
regulated and plays major roles in several biological processes.
UPS controls different protein functions and important processes
associated with the cell cycle, proliferation, metabolism,
apoptosis and survival by targeting substrates for ubiquitination
and degradation (9). The anaphase
promoting complex/cyclosome (APC/C) is an unusually large
multimeric cullin-RING ubiquitin ligase that plays a role in the
cell cycle. RING finger E3 ubiquitin ligase cell division cycle 20
homolog (Cdc20) serves as an activator of APC/C during the
metaphase-anaphase transition (10–12).
High levels of Cdc20 have been reported in a number of malignancies
and are associated with tumorigenesis and tumor progression
(13,14). It has previously been reported that
Cdc20 overexpression is associated with the occurrence of
glioblastomas, while Cdc20 downregulation occurs in patients with
low-grade tumors (15). Recently,
Mao et al (16) demonstrated
that Cdc20 overexpression may serve as an independent predictor of
biochemical recurrence in patients with clinically localized
prostate cancer undergoing laparoscopic radical prostatectomy
without neoadjuvant therapy. Furthermore, Cdc20 upregulation was
reported to be associated with poor prognosis in urothelial bladder
(17), uterine cervix (18), colorectal cancer (19), pancreatic ductal adenocarcinoma
(20), oral squamous cell carcinoma
(21), gastric (22) and lung cancer (23). These findings revealed that Cdc20
may be a promising novel therapeutic target for cancer treatment
(13). One study revealed that
small interfering (si)RNA-mediated Cdc20 knockdown suppressed
metastatic castration-resistant prostate cancer growth and enhanced
chemosensitivity to docetaxel (24). Recently, Zhang et al
(25) revealed that polyphenol
curcumin inhibited pancreatic cancer cells via downregulation of
the expression of Cdc20.
The APC/C inhibitor apcin is a small novel
cell-permeable molecule that blocks the interaction between APC/C
and Cdc20 or Cdh1 (26). It is
accepted that apcin prevents substrate recognition by binding to
Cdc20. Furthermore, apcin has been reported to induce metaphase
arrest and apoptotic cell death in multiple myeloma (27). de Lange et al (28) demonstrated that several cancer cell
lines with cohesion fatigues exhibited an increased response to
apcin, indicating that APC/C-Cdc20 inhibitors may be effective
therapeutic agents targeting cohesion defective cancers. However,
the antitumor properties of apcin in OS have not been previously
investigated. The aim of the present study was to determine whether
apcin exhibited its antitumor properties in a human OS cell line.
The possible molecular target that regulated cell death was also
investigated.
Materials and methods
Cell culture
Human osteosarcoma cell lines MG63 and U2OS were
purchased from the Chinese Academy of Sciences (Shanghai, China)
and maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Grand Island, NY, USA),
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (HyClone™; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C with 5%
CO2.
Reagents and antibodies
MTT
(3–4,5-dimethyl-2-thiazolyl-2,5-diphenyl-2-H-tetrazolium bromide)
and Calcein-AM were purchased from Sigma-Aldrich; Merck KGaA (St.
Louis, MO, USA). MTT and Calcein-AM were diluted in dimethyl
sulfoxide (DMSO) and stored at −20°C, respectively. Primary
antibodies for Bim [B-cell lymphoma 2 (Bcl-2) interacting mediator
of cell death] (dilution 1:1,000; cat. no. 2933) and p21 (dilution
1:1,500; cat. no. 2947) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Monoclonal anti-tubulin
(dilution 1:3,000; cat. no. T9028) was purchased from
Sigma-Aldrich. Secondary antibodies (dilution 1:2,500; cat. no.
A-11031; dilution 1:3,000; cat. no. A-11034) were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
MTT assay
MG63 and U2OS (2.5×103 cells/well) were
seeded in 96-well plates and cultured overnight. Different
concentrations of apcin were added to the medium and cultured for
24, 48 and 72 h, respectively. An MTT assay was then carried out to
assess the cell viability according to the manufacturer's
protocols. Then, 10 µl of MTT solution (0.5 mg/ml) was added to
each well followed by incubation for 4 h. The liquid supernatant
was then drained off and 100 µl of DMSO was added to each well to
dissolve the crystals. The absorbance of each well at 490 nm was
determined using a Multimode Reader of SpectraMax M5 (Molecular
Devices, Sunnyvale, CA, USA).
Cell apoptosis assay
MG63 and U2OS cells (2×105 cells/well)
were seeded in 6-well plates and treated with different
concentrations of apcin for 48 h. Then, the cells were harvested
and washed with phosphate-buffered saline (PBS). Subsequently, the
cells were resuspended in 500 µl binding buffer with 5 µl propidium
iodide (PI) and 5 µl FITC-conjugated anti-Annexin V antibody. The
apoptosis was analyzed by a flow cytometer (FACScalibur; BD
Biosciences, San Jose, CA, USA).
Transwell assays
MG63 and U2OS cells were suspended at a density of
3×104 cells/well in 200 µl of DMEM. The cell suspension
was placed on the upper layer of a cell permeable membrane in
Matrigel-precoated Transwell inserts (24-well insert; Corning Inc.,
Corning, NY, USA) or inserts that were not coated with Matrigel. A
total of 500 µl of DMEM containing apcin was added in the
lower-chamber below the cell permeable membrane. Following
incubation for ~16 h, the cells that had migrated and invaded,
respectively through the membrane were stained with crystal violet
or Calcein-AM, and then images were captured using an inverted
microscope (Olympus IX71; Olympus Corp., Tokyo, Japan).
Wound healing assay
MG63 and U2OS cells (1×105 cells/ml) were
seeded on a 6-well plate and incubated till the cell monolayer grew
to almost 90% confluence. Directional cell migration was determined
by creating a rectangular wound in a monolayer using a sterile
100-µl pipette tip. The open gap was inspected microscopically over
a period of time as cells migrated and filled the wound.
Photographic images were captured at the beginning and at 20 h
using an inverted microscope (Olympus IX71; Olympus Corp.). The
images were compared to quantify the migration rate of the
cells.
Western blot analysis
Apcin-treated MG63 and U2OS cell samples were
dissociated in cell lysis buffer supplemented with protease
inhibitors. A BCA assay (Beijing Solarbio Science and Technology,
Co., Ltd., Beijing, China) was performed to quantify the protein
concentrations. Equal protein samples were boiled for 5 min in 1X
loading buffer containing sodium dodecyl sulfate (SDS). The
negatively charged protein samples were then separated by 10%
SDS-PAGE gel. Following electrophoretic separation, separated
proteins were transferred to a polyvinylidene difluoride membrane
(PVDF; EMD Millipore, Billerica, MA, USA). The membrane was blocked
with 5% non-fat milk for 1 h to prevent non-specific antibody
binding and subsequently probed with appropriate primary antibodies
specific to the target proteins overnight at 4°C. The membrane was
washed with TBST and incubated with a secondary antibody for 1 h at
room temperature. Finally, an electrochemiluminescence (ECL;
Sigma-Aldrich, St. Louis, MO, USA) assay was performed to visualize
the protein bands. Quantitative analysis was carried out using
QuantiOne imaging software with gel imaging equipment (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Three replications were performed for each
experiment. All of the data was analyzed by one-way analysis of
variance (ANOVA) and Dunnett's post hoc test using GraphPad Prism
4.0 (Graph Pad Software, Inc., La Jolla, CA, USA). All statistical
data were presented as the mean ± SD of triplicate determinants.
P<0.05 was considered to indicate a statistically significant
result.
Results
Apcin suppresses cell
proliferation
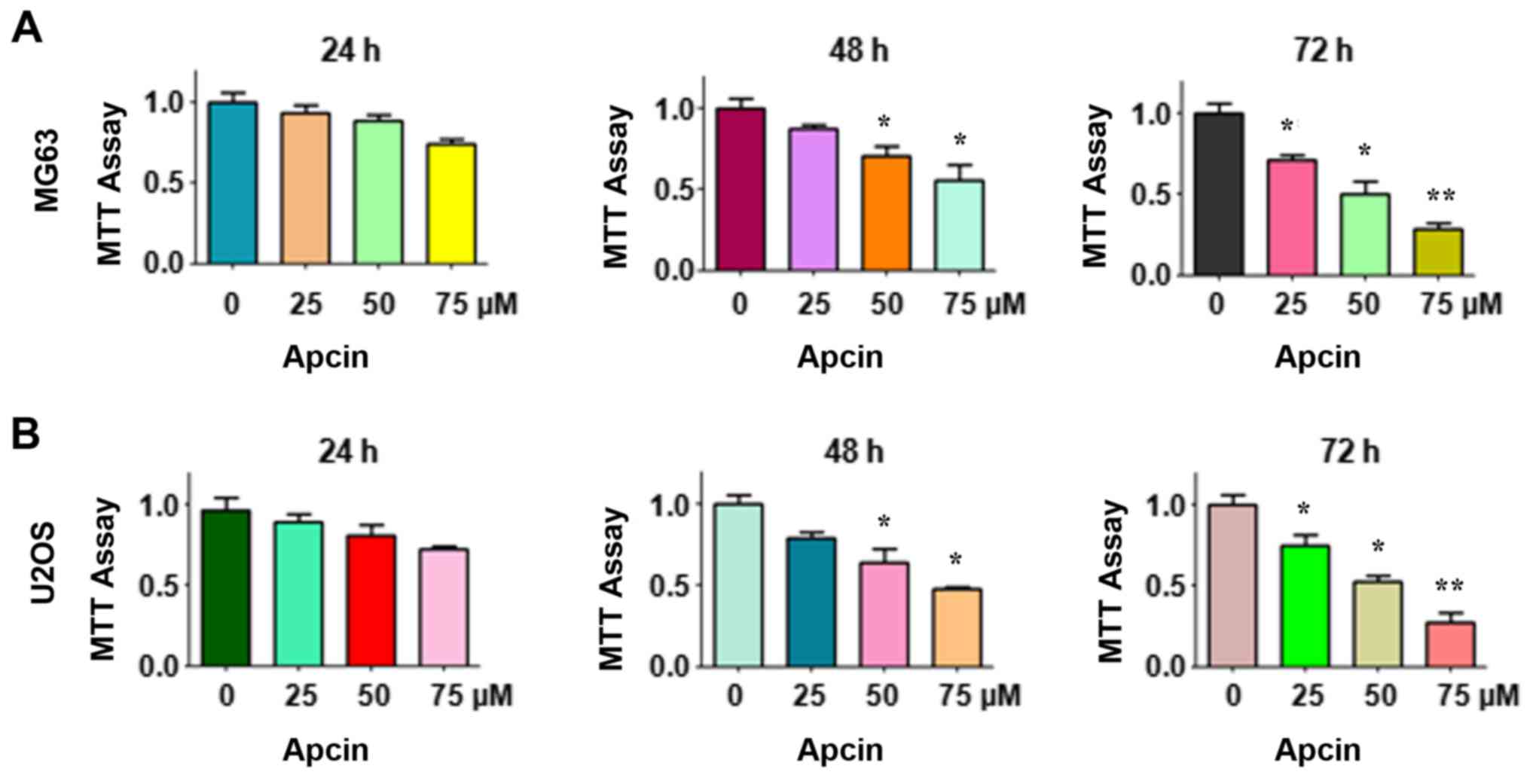
We first determined whether the Cdc20 inhibitor
apcin suppressed human OS cell proliferation. An MTT assay was
performed for MG63 and U2OS cells following treatment with the
designated concentrations of apcin for 24, 48 and 72 h,
respectively. The results revealed that OS cell proliferation was
significantly suppressed by apcin in a time- and dose-dependent
manner (Fig. 1). Treatment with 25
µM apcin caused slight OS cell growth inhibition at 24 and 48 h,
but 25% inhibition at 72 h. However, treatment with 50 or 75 µM
apcin led to ~50 and ~75% cell growth inhibition, respectively, at
72 h in both MG63 and U2OS cells. These results demonstrated that
apcin exhibited its antitumor characteristics in human OS
cells.
Apcin induces apoptosis
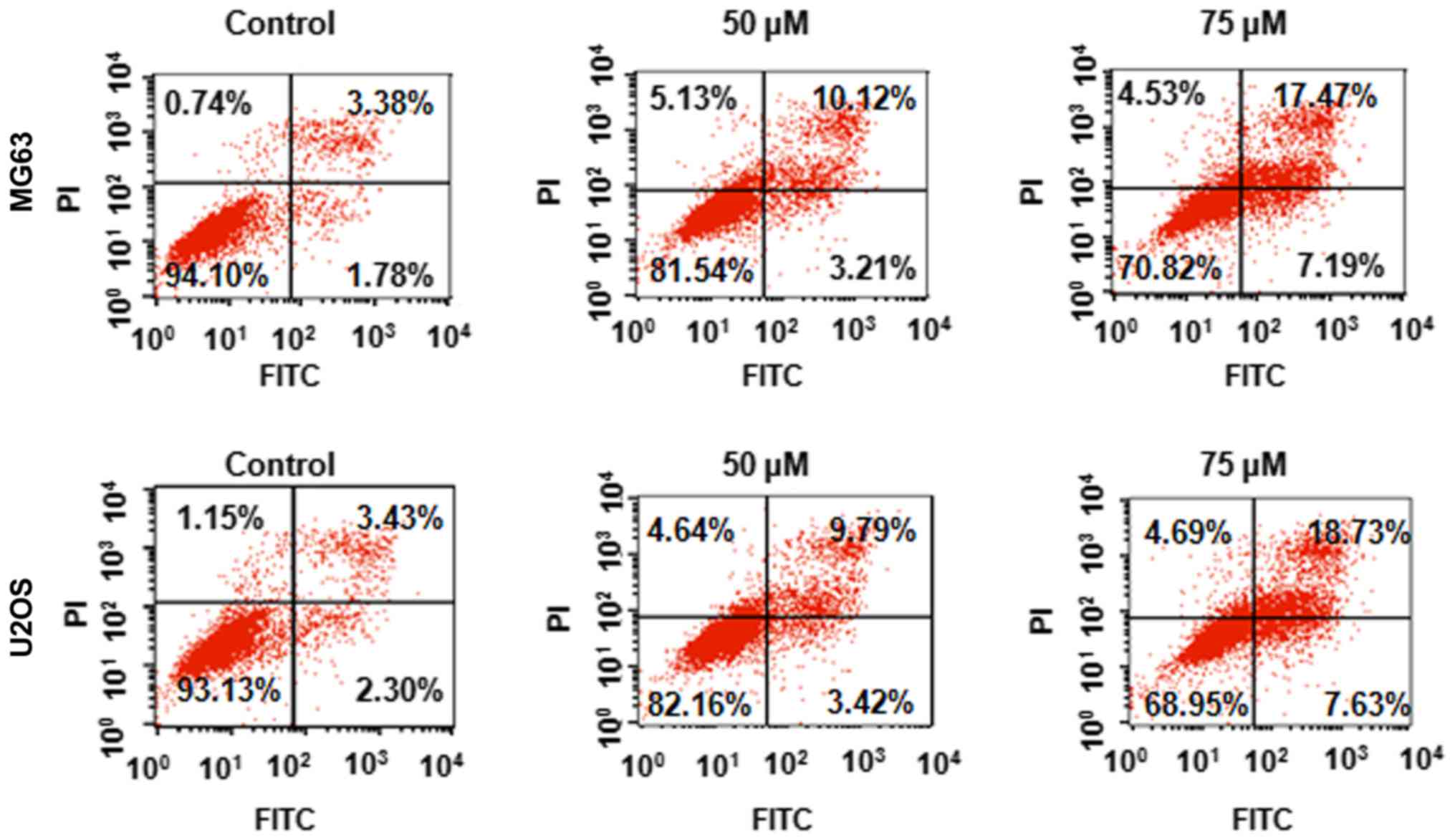
It was further investigated whether apcin affected
apoptosis in human OS cells. MG63 and U2OS cells were treated with
the designated concentrations of apcin for 48 h and cell apoptosis
was assessed using a PI/FITC-Annexin V assay. We revealed that
apcin treatment induced significant cell apoptosis in a
dose-dependent manner (Fig. 2).
These results indicated that apcin inhibited human OS cell
proliferation by inducing apoptosis.
Apcin inhibits Transwell cell
migration and invasion
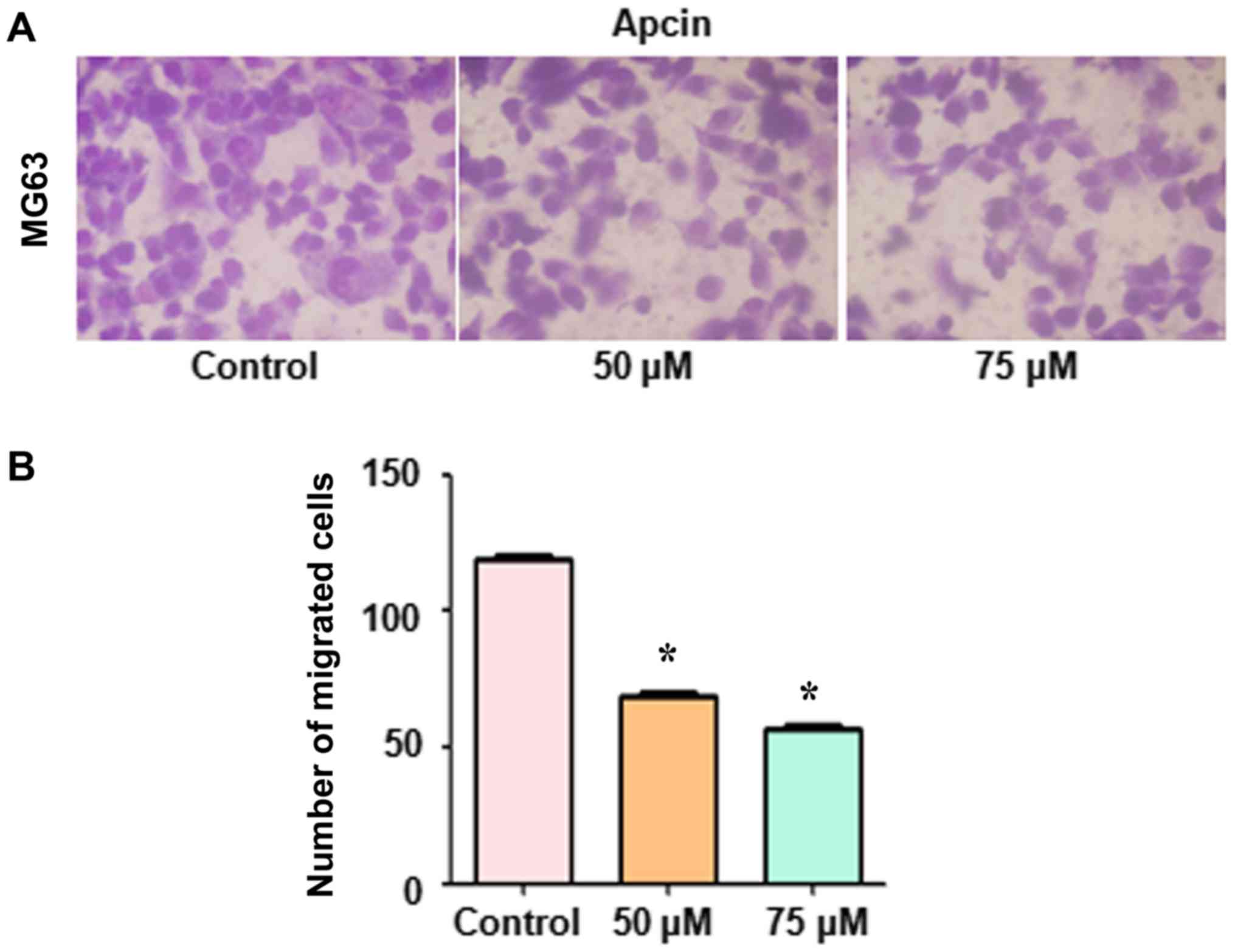
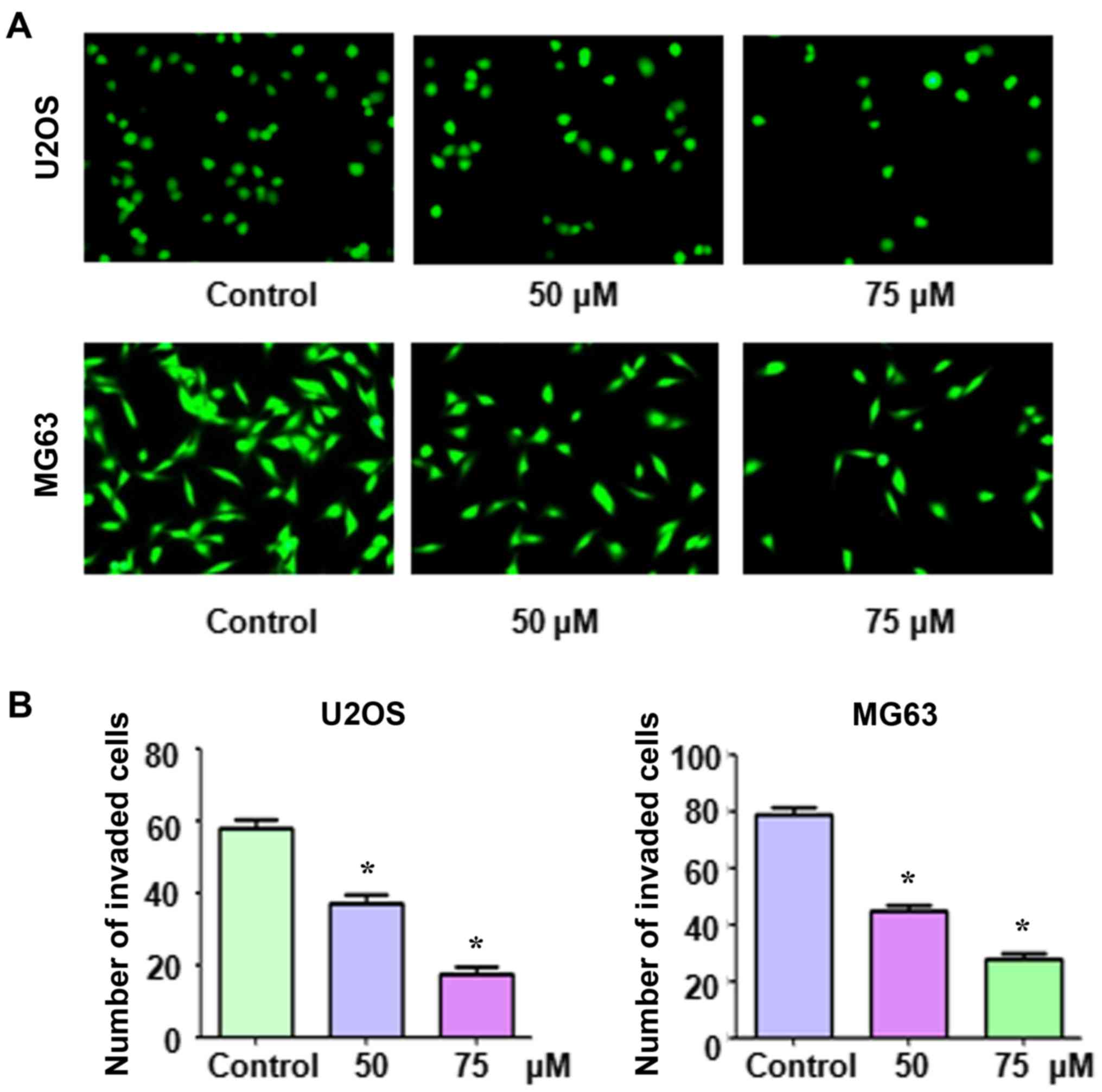
Thereafter, we determined whether apcin reduced OS
cell migration and invasion. Cells were stained with crystal violet
or Calcein-AM and Transwell migration and invasion assays were
performed. The results revealed that apcin treatment suppressed the
migration in MG63 cells (Fig. 3).
In addition, it was revealed that apcin treatment suppressed the
invasion of both MG63 and U2OS cells through the pores of the
Matrigel-coated membrane (Fig. 4).
Furthermore, the inhibition of migration and invasion was
demonstrated to be inhibited in a dose-dependent manner.
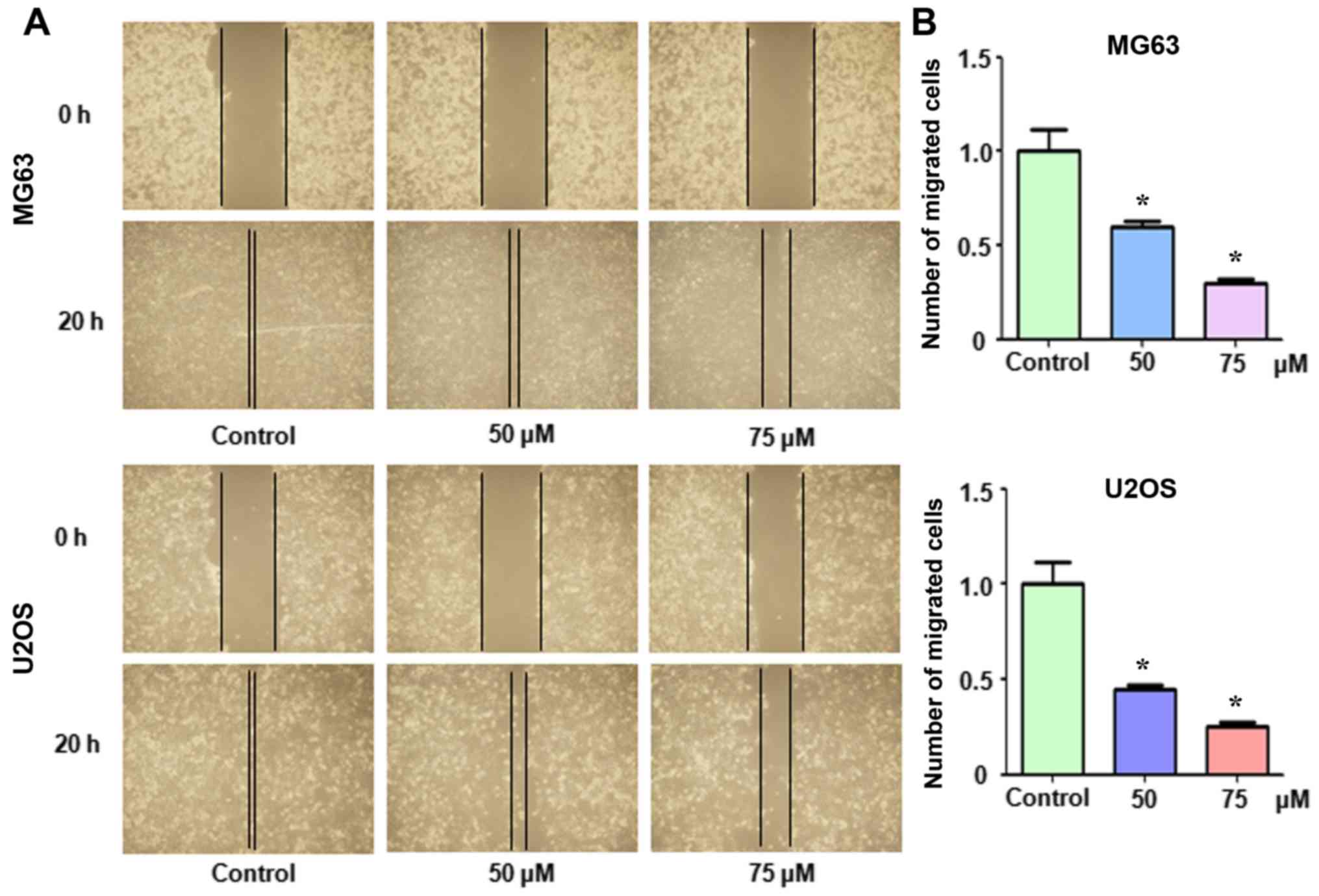
Apcin inhibits cell migration in a
wound healing assay
We also examined whether apcin suppressed OS cell
migration. Both MG63 and U2OS cells were treated with apcin and a
wound healing assay was performed. It was observed that apcin
treatment markedly suppressed OS cell migration in a dose-dependent
manner (Fig. 5).
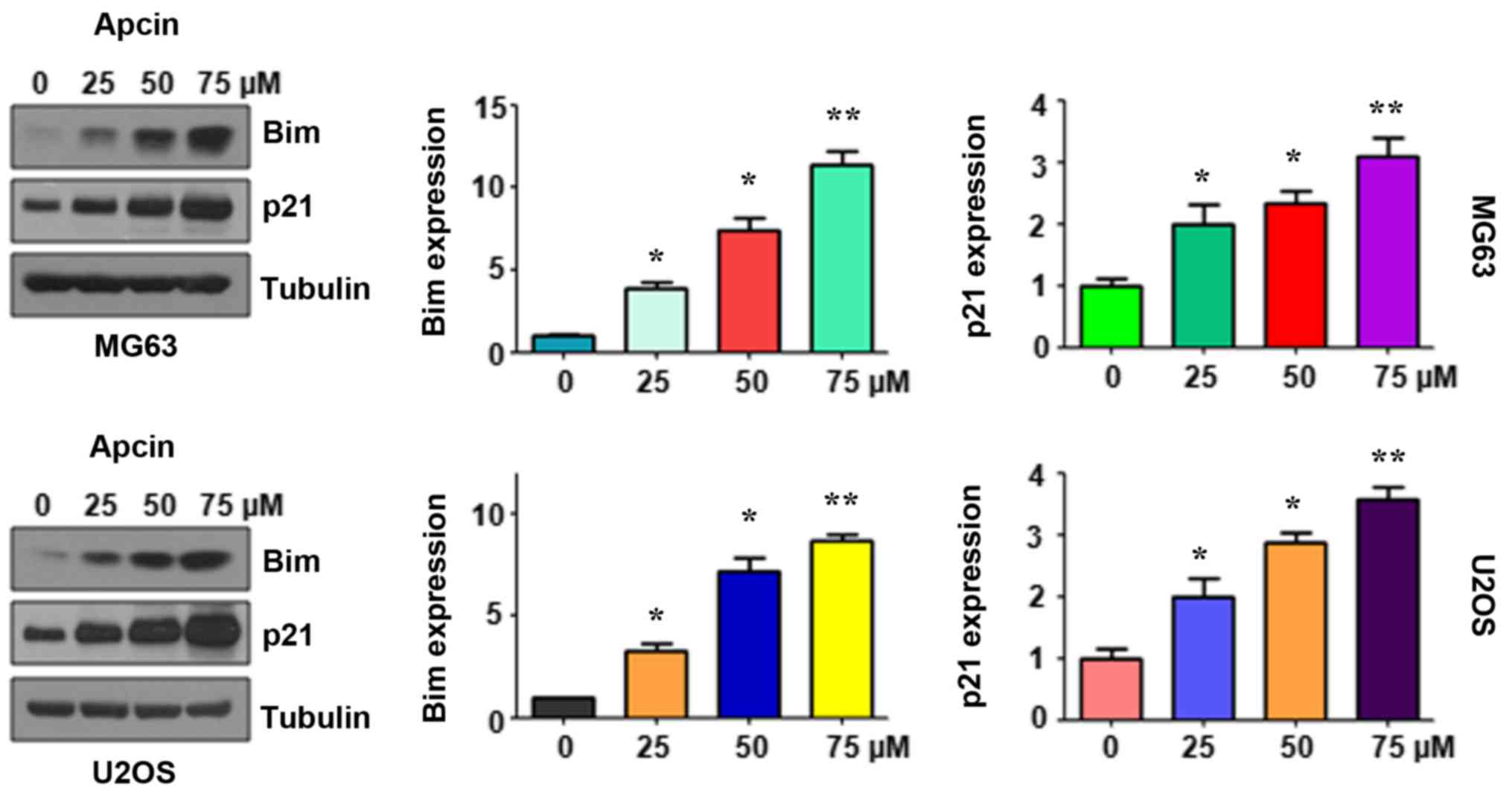
Apcin induces Bim expression
The pro-apoptotic molecule Bim has attracted
increasing attention as a possible target for tumor therapy. In the
present study, we determined whether Bim expression was induced by
apcin treatment and whether it exhibited its antitumor activity in
human OS cells. The results of western blotting demonstrated that
the expression of Bim was increased in MG63 and U2OS cells
following apcin treatment (Fig. 6).
It was also observed that apcin promoted Bim expression in a
dose-dependent manner. Furthermore, p21, a downstream target of
Bim, was also significantly upregulated in a dose-dependent manner
following apcin treatment (Fig. 6).
These findings revealed that the antitumor activity of apcin in OS
cancer cells may be attributable, at least in part, to the
increased expression of Bim and p21.
Discussion
Osteosarcoma (OS) is a malignant tumor of
mesenchymal origin that primarily affects the long bones in
children and young adults. Typically, it has a bimodal age
distribution in the second decade of life and late adulthood
(3,29). At present, patients with low-grade
OS lesions are typically treated with surgical resection alone.
However, for high-grade lesions, neoadjuvant therapy followed by
wide surgical resection of the tumor and a course of adjuvant
chemotherapy is the typical treatment regimen. The use of adjuvant
chemotherapy improves the 5-year survival rate of patients with OS
from <20 to ~70% (6,30). Neoadjuvant chemotherapy with
high-dose methotrexate, doxorubicin, cisplatin and ifosfamide is
commonly used to treat patients with OS. Other agents, including
vincristine, bleomycin and cyclophosphamide, are sometimes used as
well (31).
Previous studies have revealed that Cdc20 controls
the substrate specificity of APC/C to bind and ubiquitinate its
target proteins for subsequent degradation. Cdc20 is often
upregulated in tumors and is associated with clinicopathological
parameters (13). For instance,
high expression of Cdc20 was associated with higher tumor grades in
bladder, cervical, colonic, endometrial, gastric, liver, ovarian,
prostatic and renal carcinomas (32). There was a significant correlation
between Cdc20 upregulation and advanced tumor stage in breast,
colon, endometrium and prostate cancers (32). Furthermore, Cdc20 overexpression
predicted a poor prognosis in a wide range of human malignant
tumors (17–22,23),
and thus may be used as a biomarker of tumor prognosis. As such,
inhibiting Cdc20 expression or binding may serve as an effective
cancer treatment. Inhibition of Cdc20 by its siRNA reduced the cell
growth and invasion of OS cells (33). A number of Cdc20 inhibitors have
been identified and extracted from natural compounds, including
withaferin A, which was demonstrated to modulate the spindle
assembly checkpoint by degrading the Mad2-Cdc20 complex in
colorectal cancer cells (34).
Compound 331 was demonstrated to selectively induce glioma cell
death by upregulating miR-494 and downregulating Cdc20 (35). Furthermore, rottlerin was
demonstrated to inhibit cell growth and invasion by downregulating
Cdc20 in glioma cells (36). It has
also been reported that curcumin suppressed Cdc20 expression in
pancreatic cancer cells (25). A
number of natural compounds exhibit multiple non-specific targets
and it would be beneficial to discover a specific Cdc20 inhibitor
for cancer treatment. To this end, Zeng et al (37) demonstrated that pharmacologically
disrupting the APC-Cdc20/Cdh1 interaction with tosyl-L-arginine
methyl ester inhibited E3 ligase activity, inducing spindle
checkpoint-dependent mitotic arrest in the absence of spindle
damage. In addition, they identified another Cdc20-specific APC
inhibitor, apcin, that was able to bind with Cdc20, block substrate
recognition and inhibit the ubiquitination of Cdc20 substrates
(26).
Apcin was initially reported as an inhibitor of
cyclin proteolysis in mitotic Xenopus egg extract (38). Recently, Sackton et al
(26) reported that apcin directly
disturbed the binding of Cdc20 to its substrates and subsequently
blocked mitotic exit in human cancer cells. Apcin inhibited the
oncogenic function of Cdc20 by reducing cell viability and inducing
apoptosis in a dose-dependent manner in prostate cancer cells
(39). Furthermore, it was reported
that Speckle-type POZ protein (SPOP)-deficient prostate cancer
cells were resistant to apcin, suggesting that apcin could be
clinically used to treat patients with SPOP-WT prostate cancer, but
not SPOP-deficient prostate cancer. It was reported that Warsaw
breakage syndrome cell lines and several cancer cell lines with
cohesion defects exhibited a significantly increased response to
apcin (28). In the present study,
it was demonstrated that apcin inhibited OS cell growth and induced
significant apoptosis. The mobility of OS cells was also markedly
inhibited by apcin treatment. Our findings revealed that apcin
could have potential application in chemotherapy for OS
patients.
A previous study reported that Cdc20 suppressed cell
apoptosis largely via targeting Bim for polyubiquitination and
degradation (40). Bim belongs to
the Bcl-2-homology 3-only family (41). It has been reported that Bim
interacts with Bcl-2, Bcl2L1 and other Bcl-2 proteins to act as an
apoptotic activator under both physiological and pathological
conditions to initiate the indigenous apoptotic pathway (41–44).
Bim is widely expressed in a variety of tissues and regulates a
number of normal and pathological systems (45). In the past few years, Bim has
attracted more and more attention as a plausible target for cancer
therapy (46). It has been reported
that Bim plays a vital role in the anoikis of a number of cancer
cells, including breast and lung cancer, fibrosarcoma, melanoma and
OS (47–50). It was further demonstrated that
aberrant levels of Bim affected chemotherapy response (51). For instance, Bim overexpression was
revealed to enhance the efficacy of microtubule-targeting
chemotherapy and may be a powerful predictor of tumor response to
microtubule-targeting agents, including paclitaxel and vinorelbine
(52). In contrast, Bim
downregulation following siRNA transfection delayed
paclitaxel-induced apoptosis, suggesting that low expression of Bim
is responsible for paclitaxel-resistance in cancer cells (52). It has also been demonstrated that
Bim deletion polymorphisms are associated with a poor clinical
response to erlotinib and may be an independent prognostic factor
for patients with non-small cell lung cancer with the EGFR mutation
(53,54). Collectively, these previous studies
suggest that many chemotherapeutic agents use Bim to trigger
apoptosis in a variety of cancer cells. Bim-targeting therapies may
offer more effective and individual options for cancer treatment in
the future.
A number of chemotherapeutic agents, including
molecular-targeting agents imatinib, gefitinib and bortezomib, use
Bim as an executioner. These agents could be classified as
‘primitive’ Bim-targeting agents (46). Recently, Gambichler et al
(55) demonstrated that Bim protein
expression was significantly inversely correlated with melanoma
features that are associated with poor prognosis. They further
reported that Bim was an independent predictor of advanced disease,
confirming that this pro-apoptotic BH3-only protein may be a potent
biomarker and promising therapeutic target. High Bim expression is
a potential prognostic marker as well as a chemotherapeutic target
for cervical cancer (56). The Bim
deletion polymorphism was found to be associated with primary
resistance to crizotinib in patients with ALK fusion-positive
non-small cell lung cancer (57).
As such, targeting and manipulating Bim expression or activity may
affect the outcomes of human cancers. Pharmacological inhibition of
APC/C with proTAME induced apoptosis in multiple myeloma cells and
was partially mediated by Bim, as well as the phosphorylation of
Bcl-2 and Bcl-xL (27). It was
reported that Bim expression was upregulated by proTAME treatment,
while the apoptotic consequences of Cdc20 depletion have been
attributed to the accumulation of Bim. The results of the present
study demonstrated that Bim was upregulated in OS cells following
apcin treatment. Furthermore, apcin-induced Bim upregulation was
dose-dependent. These results revealed that apcin exhibited its
antitumor activity in OS cells. Bim overexpression may be due, at
least in part, to disruption of the apcin-induced APC-Cdc20
interaction. As such, apcin may have potential as a treatment for
human OS and Cdc20 may be a promising molecular target for
chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YG and GS were involved in the conceptualization of
the study; YG, BZ, and YW were involved in data curation; YG and GS
were involved in formal analysis; YG, BZ, YW, and GS were involved
in the investigative aspects of the study; GS was involved in
project administration and supervised the study; YG and GS wrote
and edited the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shaikh AB, Li F, Li M, He B, He X, Chen G,
Guo B, Li D, Jiang F, Dang L, et al: Present advances and future
perspectives of molecular targeted therapy for osteosarcoma. Int J
Mol Sci. 17:5062016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
3
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones KB: Osteosarcomagenesis: Modeling
cancer initiation in the mouse. Sarcoma. 2011:6941362011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Jiang H, Xiao L, Wang S and Zheng J:
miR-200bc/429 inhibits osteosarcoma cell proliferation and invasion
by targeting PMP22. Med Sci Monit. 23:1001–1008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
Clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nandi D, Tahiliani P, Kumar A and Chandu
D: The ubiquitin-proteasome system. J Biosci. 31:137–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang LF, Zhang Z, Yang J, McLaughlin SH
and Barford D: Molecular architecture and mechanism of the
anaphase-promoting complex. Nature. 513:388–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Passmore LA, McCormack EA, Au SW, Paul A,
Willison KR, Harper JW and Barford D: Doc1 mediates the activity of
the anaphase-promoting complex by contributing to substrate
recognition. EMBO J. 22:786–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sewart K and Hauf S: Different
functionality of Cdc20 binding sites within the mitotic checkpoint
complex. Curr Biol. 27:1213–1220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Zhang J, Wan L, Zhou X, Wang Z and
Wei W: Targeting Cdc20 as a novel cancer therapeutic strategy.
Pharmacol Ther. 151:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paul D, Ghorai S, Dinesh US, Shetty P,
Chattopadhyay S and Santra MK: Cdc20 directs proteasome-mediated
degradation of the tumor suppressor SMAR1 in higher grades of
cancer through the anaphase promoting complex. Cell Death Dis.
8:e28822017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marucci G, Morandi L, Magrini E, Farnedi
A, Franceschi E, Miglio R, Calò D, Pession A, Foschini MP and
Eusebi V: Gene expression profiling in glioblastoma and
immunohistochemical evaluation of IGFBP-2 and CDC20. Virchows Arch.
453:599–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao Y, Li K, Lu L, Si-Tu J, Lu M and Gao
X: Overexpression of Cdc20 in clinically localized prostate cancer:
Relation to high Gleason score and biochemical recurrence after
laparoscopic radical prostatectomy. Cancer Biomark. 16:351–358.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi JW, Kim Y, Lee JH and Kim YS: High
expression of spindle assembly checkpoint proteins CDC20 and MAD2
is associated with poor prognosis in urothelial bladder cancer.
Virchows Arch. 463:681–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim Y, Choi JW, Lee JH and Kim YS: MAD2
and CDC20 are upregulated in high-grade squamous intraepithelial
lesions and squamous cell carcinomas of the uterine cervix. Int J
Gynecol Pathol. 33:517–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu WJ, Hu KS, Wang DS, Zeng ZL, Zhang DS,
Chen DL, Bai L and Xu RH: CDC20 overexpression predicts a poor
prognosis for patients with colorectal cancer. J Transl Med.
11:1422013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang DZ, Ma Y, Ji B, Liu Y, Hwu P,
Abbruzzese JL, Logsdon C and Wang H: Increased CDC20 expression is
associated with pancreatic ductal adenocarcinoma differentiation
and progression. J Hematol Oncol. 5:152012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moura IM, Delgado ML, Silva PM, Lopes CA,
do Amaral JB, Monteiro LS and Bousbaa H: High CDC20 expression is
associated with poor prognosis in oral squamous cell carcinoma. J
Oral Pathol Med. 43:225–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding ZY, Wu HR, Zhang JM, Huang GR and Ji
DD: Expression characteristics of CDC20 in gastric cancer and its
correlation with poor prognosis. Int J Clin Exp Pathol. 7:722–727.
2014.PubMed/NCBI
|
|
23
|
Shi R, Sun Q, Sun J, Wang X, Xia W, Dong
G, Wang A, Jiang F and Xu L: Cell division cycle 20 overexpression
predicts poor prognosis for patients with lung adenocarcinoma.
Tumour Biol. 39:10104283176922332017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li K, Mao Y, Lu L, Hu C, Wang D, Si-Tu J,
Lu M, Peng S, Qiu J and Gao X: Silencing of CDC20 suppresses
metastatic castration-resistant prostate cancer growth and enhances
chemosensitivity to docetaxel. Int J Oncol. 49:1679–1685. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Xue YB, Li H, Qiu D, Wang ZW and
Tan SS: Inhibition of cell survival by curcumin is associated with
downregulation of cell division cycle 20 (Cdc20) in pancreatic
cancer cells. Nutrients. 9:E1092017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sackton KL, Dimova N, Zeng X, Tian W,
Zhang M, Sackton TB, Meaders J, Pfaff KL, Sigoillot F, Yu H, et al:
Synergistic blockade of mitotic exit by two chemical inhibitors of
the APC/C. Nature. 514:646–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lub S, Maes A, Maes K, De Veirman K, De
Bruyne E, Menu E, Fostier K, Kassambara A, Moreaux J, Hose D, et
al: Inhibiting the anaphase promoting complex/cyclosome induces a
metaphase arrest and cell death in multiple myeloma cells.
Oncotarget. 7:4062–4076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Lange J, Faramarz A, Oostra AB, de
Menezes RX, van der Meulen IH, Rooimans MA, Rockx DA, Brakenhoff
RH, van Beusechem VW, King RW, et al: Defective sister chromatid
cohesion is synthetically lethal with impaired APC/C function. Nat
Commun. 6:83992015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wachtel M and Schäfer BW: Targets for
cancer therapy in childhood sarcomas. Cancer Treat Rev. 36:318–327.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bernthal NM, Federman N, Eilber FR, Nelson
SD, Eckardt JJ, Eilber FC and Tap WD: Long-term results (>25
years) of a randomized, prospective clinical trial evaluating
chemotherapy in patients with high-grade, operable osteosarcoma.
Cancer. 118:5888–5893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maki RG: Ifosfamide in the neoadjuvant
treatment of osteogenic sarcoma. J Clin Oncol. 30:2033–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gayyed MF, El-Maqsoud NM, Tawfiek ER, El
Gelany SA and Rahman MF: A comprehensive analysis of CDC20
overexpression in common malignant tumors from multiple organs: Its
correlation with tumor grade and stage. Tumour Biol. 37:749–762.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shang G, Ma X and Lv G: Cell division
cycle 20 promotes cell proliferation and invasion and inhibits
apoptosis in osteosarcoma cells. Cell Cycle. 17:43–52. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Das T, Roy KS, Chakrabarti T, Mukhopadhyay
S and Roychoudhury S: Withaferin A modulates the Spindle assembly
checkpoint by degradation of Mad2-Cdc20 complex in colorectal
cancer cell lines. Biochem Pharmacol. 91:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Niu T, Huang Y, Zhu H, Zhong W,
Lin J and Zhang Y: Compound 331 selectively induces glioma cell
death by upregulating miR-494 and downregulating CDC20. Sci Rep.
5:120032015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X,
Zhou X, Zhou L and Wang Z: Rottlerin inhibits cell growth and
invasion via down-regulation of Cdc20 in glioma cells. Oncotarget.
7:69770–69782. 2016.PubMed/NCBI
|
|
37
|
Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff
KL, Oh DC, Hathaway N, Dimova N, Cuny GD and King RW: Pharmacologic
inhibition of the anaphase-promoting complex induces a spindle
checkpoint-dependent mitotic arrest in the absence of spindle
damage. Cancer Cell. 18:382–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Verma R, Peters NR, D'Onofrio M, Tochtrop
GP, Sakamoto KM, Varadan R, Zhang M, Coffino P, Fushman D, Deshaies
RJ and King RW: Ubistatins inhibit proteasome-dependent degradation
by binding the ubiquitin chain. Science. 306:117–120. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu F, Dai X, Gan W, Wan L, Li M, Mitsiades
N, Wei W, Ding Q and Zhang J: Prostate cancer-associated mutation
in SPOP impairs its ability to target Cdc20 for poly-ubiquitination
and degradation. Cancer Lett. 385:207–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wan L, Tan M, Yang J, Inuzuka H, Dai X, Wu
T, Liu J, Shaik S, Chen G, Deng J, et al: APC(Cdc20) suppresses
apoptosis through targeting Bim for ubiquitination and destruction.
Dev Cell. 29:377–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng JH, Viacava Follis A, Kriwacki RW
and Moldoveanu T: Discoveries and controversies in BCL-2
protein-mediated apoptosis. FEBS J. 283:2690–2700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Connor L, Strasser A, O'Reilly LA,
Hausmann G, Adams JM, Cory S and Huang DC: Bim: A novel member of
the Bcl-2 family that promotes apoptosis. EMBO J. 17:384–395. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
U M, Miyashita T, Shikama Y, Tadokoro K
and Yamada M: Molecular cloning and characterization of six novel
isoforms of human Bim, a member of the proapoptotic Bcl-2 family.
FEBS Lett. 509:135–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Correia C, Lee SH, Meng XW, Vincelette ND,
Knorr KL, Ding H, Nowakowski GS, Dai H and Kaufmann SH: Emerging
understanding of Bcl-2 biology: Implications for neoplastic
progression and treatment. Biochim Biophys Acta. 1853:1658–1671.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sionov RV, Vlahopoulos SA and Granot Z:
Regulation of Bim in health and disease. Oncotarget. 6:23058–23134.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Akiyama T, Dass CR and Choong PF:
Bim-targeted cancer therapy: A link between drug action and
underlying molecular changes. Mol Cancer Ther. 8:3173–3180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tan TT, Degenhardt K, Nelson DA, Beaudoin
B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM and White E:
Key roles of BIM-driven apoptosis in epithelial tumors and rational
chemotherapy. Cancer Cell. 7:227–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Woods NT, Yamaguchi H, Lee FY, Bhalla KN
and Wang HG: Anoikis, initiated by Mcl-1 degradation and Bim
induction, is deregulated during oncogenesis. Cancer Res.
67:10744–10752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen K, Tu Y, Zhang Y, Blair HC, Zhang L
and Wu C: PINCH-1 regulates the ERK-Bim pathway and contributes to
apoptosis resistance in cancer cells. J Biol Chem. 283:2508–2517.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Iurlaro R and Muñoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Savry A, Carre M, Berges R, Rovini A,
Pobel I, Chacon C, Braguer D and Bourgarel-Rey V: Bcl-2-enhanced
efficacy of microtubule-targeting chemotherapy through Bim
overexpression: Implications for cancer treatment. Neoplasia.
15:49–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cardona AF, Rojas L, Wills B, Arrieta O,
Carranza H, Vargas C, Otero J, Corrales-Rodriguez L, Martín C,
Reguart N, et al: BIM deletion polymorphisms in Hispanic patients
with non-small cell lung cancer carriers of EGFR mutations.
Oncotarget. 7:68933–68942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Costa C, Molina MA, Drozdowskyj A,
Giménez-Capitán A, Bertran-Alamillo J, Karachaliou N, Gervais R,
Massuti B, Wei J, Moran T, et al: The impact of EGFR T790M
mutations and BIM mRNA expression on outcome in patients with
EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the
randomized phase III EURTAC trial. Clin Cancer Res. 20:2001–2010.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gambichler T, Rooms I, Scholl L,
Stockfleth E, Stucker M and Sand M: BH3-only protein Bim predicts
advanced stage of cutaneous melanoma. J Eur Acad Dermatol Venereol.
30:1926–1929. 2016.PubMed/NCBI
|
|
56
|
Kim BW, Cho H, Ylaya K, Kitano H, Chung
JY, Hewitt SM and Kim JH: Bcl-2-like protein 11 (BIM) expression is
associated with favorable prognosis for patients with cervical
cancer. Anticancer Res. 37:4873–4879. 2017.PubMed/NCBI
|
|
57
|
Zhang L, Jiang T, Li X, Wang Y, Zhao C,
Zhao S, Xi L, Zhang S, Liu X, Jia Y, et al: Clinical features of
Bim deletion polymorphism and its relation with crizotinib primary
resistance in Chinese patients with ALK/ROS1 fusion-positive
non-small cell lung cancer. Cancer. 123:2927–2935. 2017. View Article : Google Scholar : PubMed/NCBI
|