Introduction
Thyroid cancer is one of the most common malignant
tumors of the endocrine system, and its incidence has steadily
increased since 1980 (1). Papillary
thyroid carcinoma (PTC) accounts for 80% of all thyroid cancers
(2); however, little is known
concerning the genetic mechanisms underlying the development of
PTC, and no ideal genetic markers for detection have been
identified. The primary causes of mortality in PTC patients include
local recurrence and distant metastases. Recent evidence indicates
that these events occur due to the presence of a specific
population of cancer stem cells (CSCs), which exist within various
tumor types (3); these are
considered the root cause of tumorigenesis, metastasis and
recurrence (4,5). Recently, studies utilizing cancer stem
cell markers, CD133, CD44, EpCAM, LGR5 and ALDH1, have confirmed
the presence of CSCs in lung, breast, pancreatic and prostate
cancers (6–9). In our own research, we demonstrated
that the self-renewal capacity of CSCs in thyroid carcinoma is
associated with the poor prognosis of thyroid cancer patients.
Long non-coding RNAs (lncRNAs) are RNA transcripts
approximately 200 nucleotides in length that do not encode
proteins, but do function to regulate the expression of related
genes (10). lncRNAs are also
recognized as regulators of tumorigenesis and tumor progression
(11,12). BRAF mutations are the most common
genetic lesion found in PTCs, occurring in 45% of PTCs (13). BRAF-activated lncRNA (BANCR) is a
693-bp transcript found on chromosome 9 and is frequently
overexpressed in cancer cells, and has been shown to play a
functional role in the migration of melanoma cells (14), and in non-small cell lung cancer
where it was shown to promote the migration and invasion of cancer
cells through the mitogen activated protein kinase (MAPK) signaling
pathway (15). Due to the close
association between BANCR expression and the proliferation of PTC,
the present study investigated the molecular mechanisms underlying
the regulation of CSC expression by BANCR in PTC, and the potential
regulation through the MAPK signaling pathway.
Materials and methods
Patients and tissue samples
Tumor samples and paired tissues from 30 patients
(20–60 years old, the median age was 35 years and the ratio of
females to males was 2:1) who received surgical resection for PTC
between 2015 and 2016 at the Department of General Surgery,
Zhongshan Hospital, Xiamen University, Xiamen, China were analyzed.
All tissue samples were frozen in liquid nitrogen following surgery
and then stored at −80°C for future experiments. The study was
approved by the Ethics Committee on Human Research of Zhongshan
Hospital, Xiamen University, Xiamen and written informed consent
was obtained from all patients.
Cell lines and cell culture
The BCPAP human papillary thyroid carcinoma cell
line was purchased from the Chinese Academy of Sciences. The normal
human thyroid epithelial cell line Nthy-ori 3–1 was purchased from
the Chinese Academy of Sciences. Human undifferentiated thyroid
carcinoma cell line CAL-62 was purchased from the Chinese Academy
of Sciences. The WRO and FTC-133 human follicular thyroid carcinoma
cell lines were purchased from Honsun Biological Technology Co.,
Ltd. (Shanghai, China). All cells were cultured in PRMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin, and 100 mg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2.
Total RNA extraction and quantitative
real-time PCR (qRT-PCR)
Total RNA from tissues and cells was extracted using
RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China). RNA
was reverse transcribed into cDNA using the PrimeScript RT kit
(Takara Biotechnology). The cDNA was amplified using the SYBR
Premix Ex Taq II (Takara Biotechnology) control. Relative
expression values were calculated by the 2−∆∆Cq method
to analyze the relative changes in gene expression from real-time
quantitative PCR experiments. Primers for qRT-PCR were synthesized
by Sangon Biotech (Shanghai, China). The sequences are listed in
Table I. The thermocycling
conditions for pre-incubation were: 95°C for 600 sec; for 2 step
amplification: 95°C for 10 sec, 60°C for 30 sec (45X); and for
melting: 95°C for 10 sec, 65°C for 60 sec, 97°C for 1 sec.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequences |
|---|
| β-actin | F:
5′-ACTGGAACTGTGAAGGTGAC-3′ |
|
| R:
5′-GTGGACTTGGGCGAGGACTG-3′ |
| BANCR | F:
5′-CCTTCTTGTAGGGTCTGGATTG-3′ |
|
| R:
5′-CATTGGTGCTGCAGTCTATTTC-3′ |
Creation of stable cell lines
The BCPAP cell line was infected with a lentiviral
vector containing these constructs: BCPAP-NC or BCPAP-BANCR. The
BCPAP cell line was infected for 24 h, using a transfection
enhancer (Polybrene) at a concentration of 6 µg/ml. Transfection
efficiency was evaluated using qRT-PCR.
Western blot analysis
Cells were washed twice with cold PBS, lysed with
RIPA protein extraction reagent (Beyotime Institute of
Biotechnology, Shanghai, China), and supplemented with a protease
inhibitor cocktail (Roche Molecular Diagnostics, Pleasanton, CA,
USA) and phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology, Beijing, China). Proteins were denatured at 100°C
for 10 min and an equal amount of sample (30 µg) was loaded and
separated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE). Proteins were transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA,
USA). The membranes were blocked with 5% (ECL) chromogenic
substrate. The densitometric analysis for the quantification of the
bands was performed using enhanced chemiluminescence chromogenic
substrate (WesternBright ECL; Advansta, Inc., Menlo Park, CA, USA)
and ChemiDoc™ XRS System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). β-actin bands were used as an internal reference. The
antibodies used in these experiments were purchased from Abcam:
E-cadherin (1:1,000; cat. no. 3195S), N-cadherin (1:1,000; cat. no.
13116S), vimentin (1:1,000; cat. no. 5741S), c-Raf (1:2,000; cat.
no. 53745S), MEK1/2 (1:2,000; cat. no. 4694S), ERK1/2 (1:2,000;
cat. no. 4370S), p-c-Raf (1:2,000; cat. no. 9421S), p-MEK1/2
(1:2,000; cat. no. 2338S), p-ERK1/2 (1:2,000; cat. no. 4370S),
GAPDH (1:2,000; cat. no. 5174S) and β-actin (1:1,000; cat. no.
3700S) (all were from Cell Signaling Technology, Inc., Danvers, MA,
USA); secondary antibodies were HRP-goat anti-mouse and HRP-goat
anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA).
Single-clone formation experiment
Cells were digested, suspended, and counted. Cells
were uniformly vaccinated to 6-orifice plates containing 2 ml of
DMEM culture medium per well, at the following concentrations: 0
cells/ml, 500 cells/ml, 800 cells/ml and 1,500 cells/ml, and then
incubated at 37°C and 5% volume fraction of CO2. Medium
was replaced every 3 to 4 days for approximately 10 days or until
clone formation became visible. Clones were fixed using 4%
paraformaldehyde, stained using crystal violet dye solution,
scanned (Epson Perfection V370; Epson, Tokyo, Japan) and counted
(five random fields were captured and quantified).
Microsphere formation experiment
Cells were suspended in serum-free culture medium at
a concentration of 104 cells/ml. Microsphere culture
medium [DMEM/F12, B27 (1:50), EGF 20 ng/ml, bFGF 20 ng/ml, insulin
4 µg/ml and 1% methyl cellulose] was added to 6-wells of an
ultra-low adsorption culture plate; no well was placed in the 2 ml
medium, and no well was added in the same amount of double
resistance. The cell suspension (200 µl) was added to each well of
the culture plate, and incubated for approximately 12 days. The
cells were observed and photographed using a light microscope
(AxioVert.A1; Carl Zeiss GmbH, Jena, Germany), then collected by
centrifugation, and the microsphere formation rate was
calculated.
Flow cytometry
Cells were suspended using pancreatic enzyme,
centrifuged at 200 × g for 3 min, and re-suspended in PBS. Cells
were then centrifuged at 300 × g for 4 min and re-suspended to a
concentration of 1×106 cells/ml in ice cold PBS. Cells
were stained using 5 µl CD133 and EPCAM antibody, 2.5 µl CD44
antibody, and isotype antibody and incubated in the dark at 4°C for
30 min, with oscillation at 15 min. Cells were then washed three
times with PBS, re-suspended in PBS, and 400 µl of the suspension
was added and detection and analysis was carried out using the
Beckman FACS Gallios flow cytometer (Beckman Coulter, Inc., Brea,
CA, USA).
Tumor formation in mice
Four- to 5-week-old non-pathogenic (SPF) female
BALB/c nude mice (18–22 g) were provided by the Shanghai SLAC
laboratory Animal Co., Ltd. (SLAC; Shanghai, China). Animal
experiments were supported by Xiamen University Laboratory Animal
Center (Xiamen, China). The mice were breed under specific
pathogen-free conditions with a controlled temperature range from
18 to 29°C and a relative humidity of 40–70% on a 12-h light/dark
cycle. The fodder was sterile and the mice were fed 3 to 4
times/day. Four- to 5-week-old non-pathogenic (SPF) male BALB/c
nude mice were divided into one of two groups: the BCPAP NC group
and the BANCR-BANCR group. BCPAP stably transfected cell line was
harvested, centrifuged and suspended at a concentration of
1.5×107 and 2.0×107 cells. Each mouse was
injected with 100 µl of cell suspension via subcutaneous injection
and regularly observed. Beginning at day 14 post-injection, tumor
length and width were measured by a vernier caliper (PD-151;
PRO'SKIT, Shanghai, China) once a week for 5 weeks. At 5 weeks, the
mice were sacrificed by cervical dislocation, tumors were measured
and photographed, and tumor volumes were calculated (V = (length ×
width2)/2) and plotted on a growth chart. All animal
experiments were approved by the Animal Care and Use Committee of
the Xiamen University. The ethics code for the approval granted for
the animal experiment was xmulac20170375, which was under the
supervision of Xiamen University Laboratory Animal Center.
Statistical analysis
All data were analyzed using GraphPad Prism 5.0
statistical software (GraphPad Software, Inc., La Jolla, CA, USA),
and the results are expressed as the mean ± standard error of the
mean (SEM). Differences among the groups were assessed by Student's
t-test, ANOVA and a post-hoc test (Student-Newman-Keuls test).
Findings of P<0.05 were considered statistically
significant.
Results
BANCR levels in PTC tissues and cell
lines
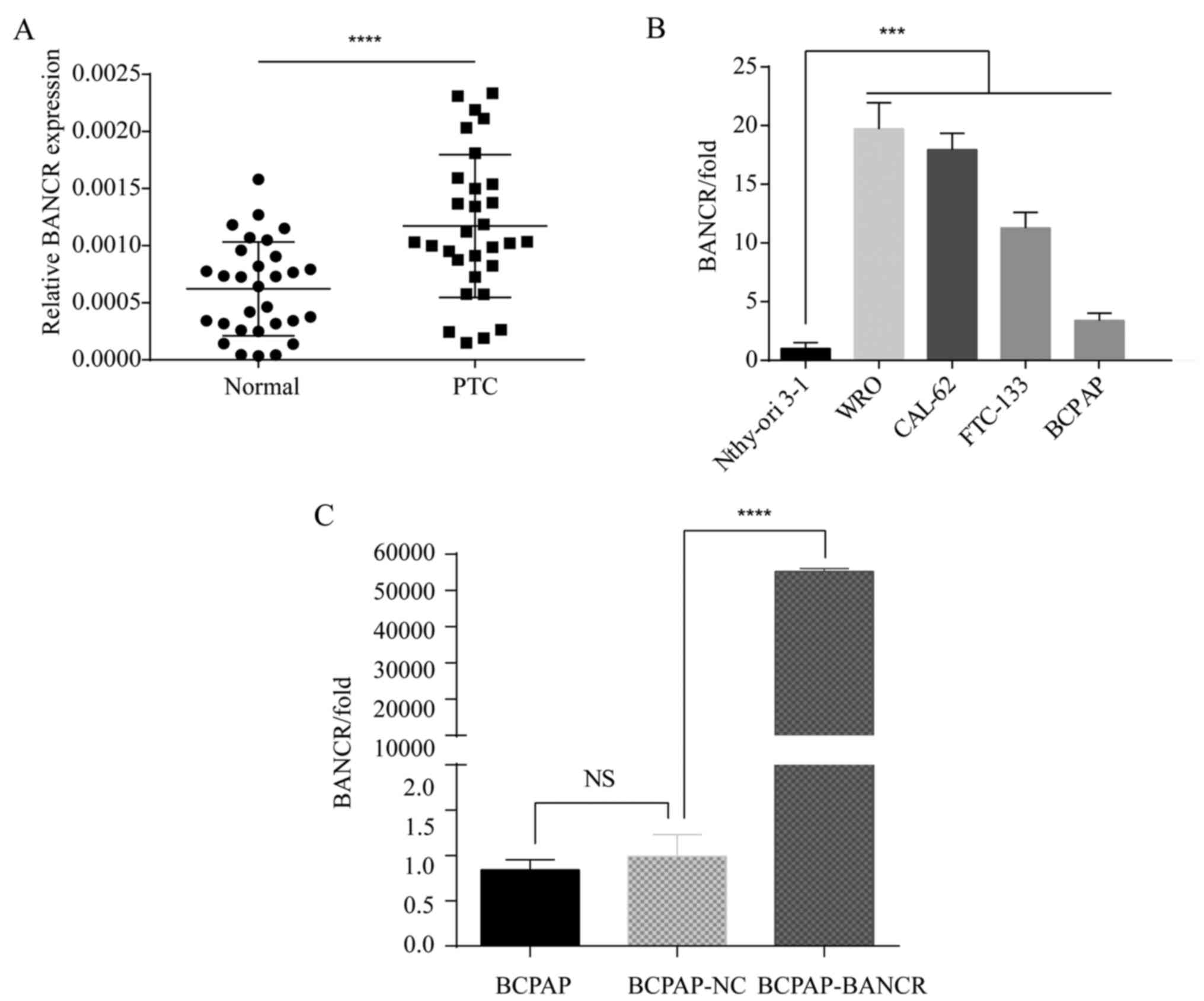
The expression level of BANCR in 30 paired tissue
samples from PTC patients was determined. BANCR expression was
significantly higher in thyroid cancer tissues compared to that
observed in the adjacent normal tissues (P<0.0001) (Fig. 1A). In addition, one normal human
thyroid epithelial cell line Nthy-ori 3–1 and four human thyroid
cancer cell lines that were evaluated exhibited varying expression
levels of BANCR (P<0.001) (Fig.
1B). Therefore, the BCPAP cell line which differed
significantly from the other thyroid cancer cell lines with low
expression of BANCR was chosen for further experiments. Following
transfection with the lentivirus, BCPAP cells (BCPAP-BANCR) showed
significant upregulation of BANCR compared with those cells
transfected with the empty vector (BCPAP-NC) (P<0.0001).
(Fig. 1C).
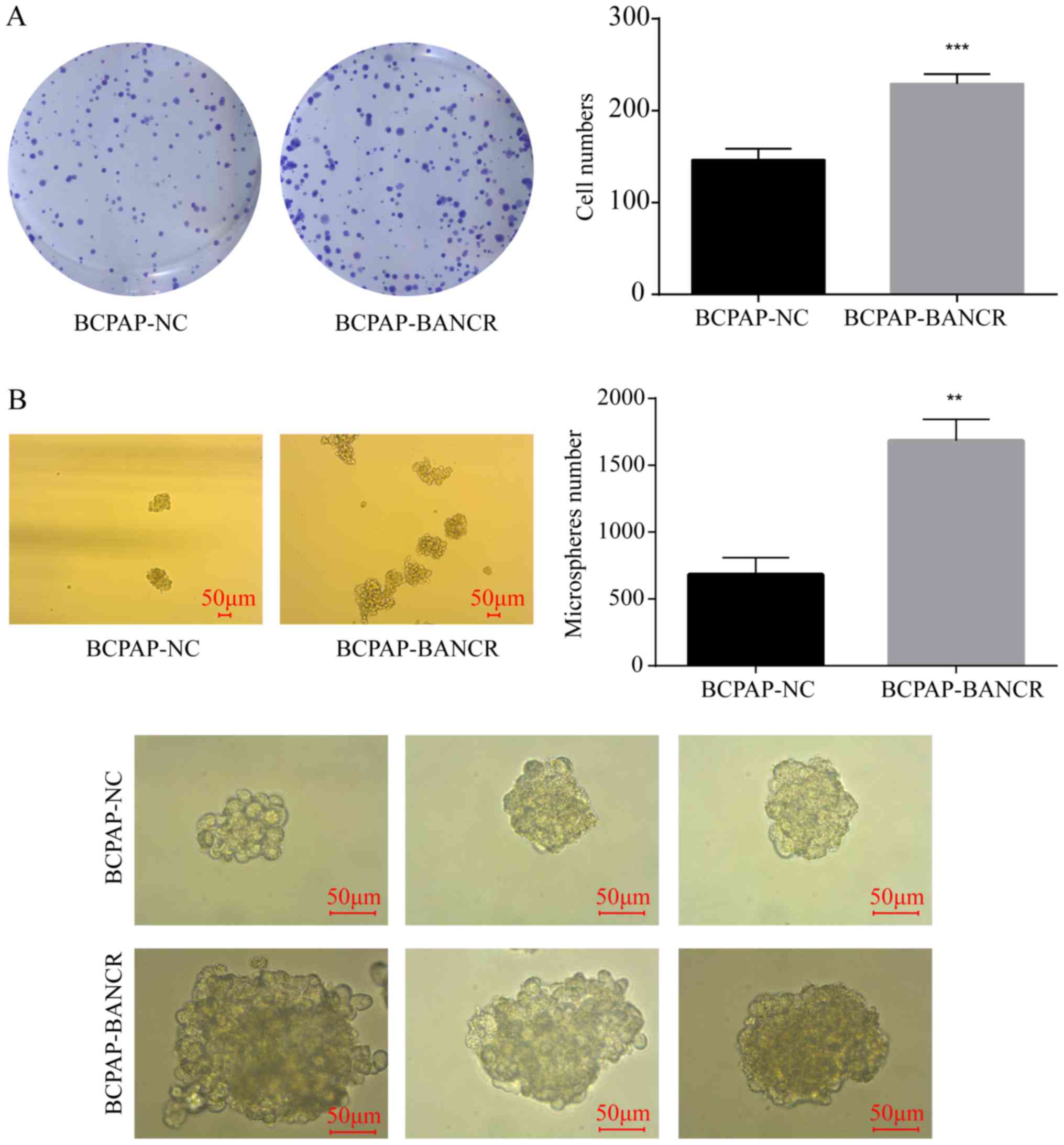
BANCR regulates single-clone formation
and microsphere formation
The overexpression of BANCR in the BCPAP-BANCR cell
line significantly increased the colony formation ability compared
with the BCPAP-NC group (P<0.001) (Fig. 2A), indicating that cloning ability
was significantly promoted by the overexpression of BANCR.
Similarly, compared with the control group, upregulation of BANCR
expression in BCPAP-BANCR cells significantly increased microsphere
number and size (P<0.01) (Fig.
2B).
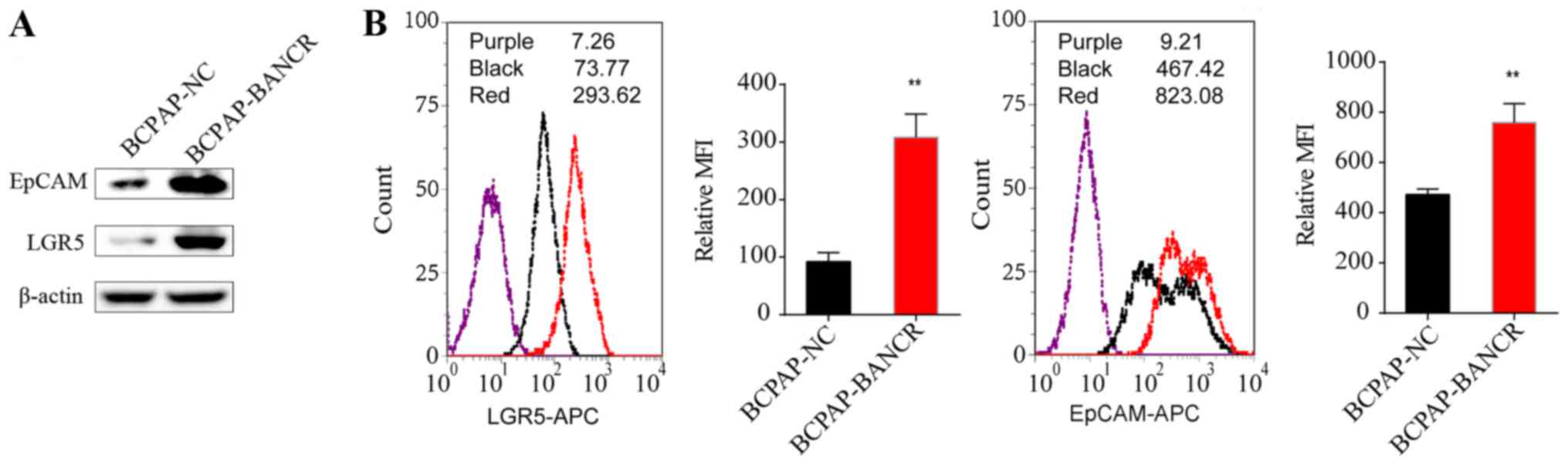
BANCR regulates the expression of
cancer stem cell markers LGR5 and EpCAM in PTC cells
Upregulation of BANCR in BCPAP-BANCR cells
significantly increased expression of the stem cell markers LGR5
and EpCAM, as shown by western blotting (Fig. 3A) and flow cytometry (P<0.01)
(Fig. 3B).
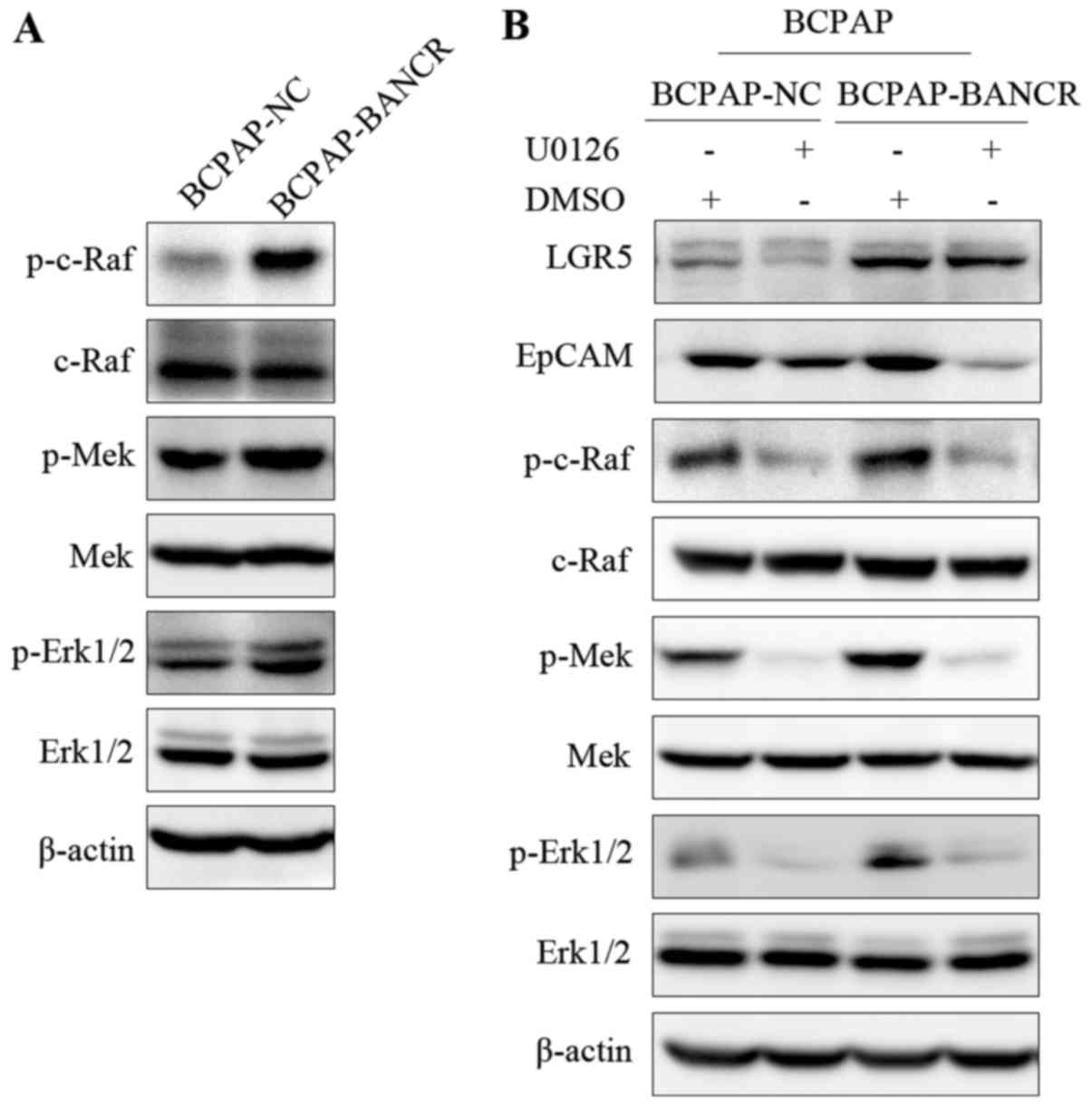
BANCR regulates the expression of
cancer stem cell markers, LGR5 and EPCAM, in PTC cells via the
Raf/MEK/ERK signaling pathway
Expression levels of c-Raf, MEK1/2, and ERK1/2 were
evaluated in the BANCR-upregulated (BCPAP-BANCR) cell line compared
to the control BCPAP-NC cell line via western blotting. Expression
levels of phospho (p)-c-Raf, p-MEK1/2, and p-ERK1/2 were higher in
the BCPAP-BANCR cells than levels noted in the BCPAP-NC cells
(Fig. 4A). These results suggest a
relationship between BANCR and the Raf/MEK/ERK signaling pathway.
To test this hypothesis, we incubated BCPAP cells with the c-Raf
inhibitor U0126, which decreased p-c-Raf, p-MEK1/2 and p-ERK1/2 in
the BCPAP cell line (Fig. 4B). From
this, we concluded that U0126 inhibits the effect of BANCR on the
RAF/MEK/ERK signaling pathway. To determine whether the effects of
BANCR on the cancer stem cell markers LGR5 and EPCAM are mediated
via the Raf/MEK/ERK signaling pathway, the cell line was treated
with U0126 and LGR5 and EpCAM protein levels were measured between
the two groups using western blot analysis. The results showed that
in BCPAP cell lines, the expression of LGR5 and EPCAM was
downregulated after the use of U0126, compared to the DMSO controls
(Fig. 4B). This result suggests
that BANCR regulated LGR5 and EpCAM expression in PTC cells via the
Raf/MEK/ERK signaling pathway.
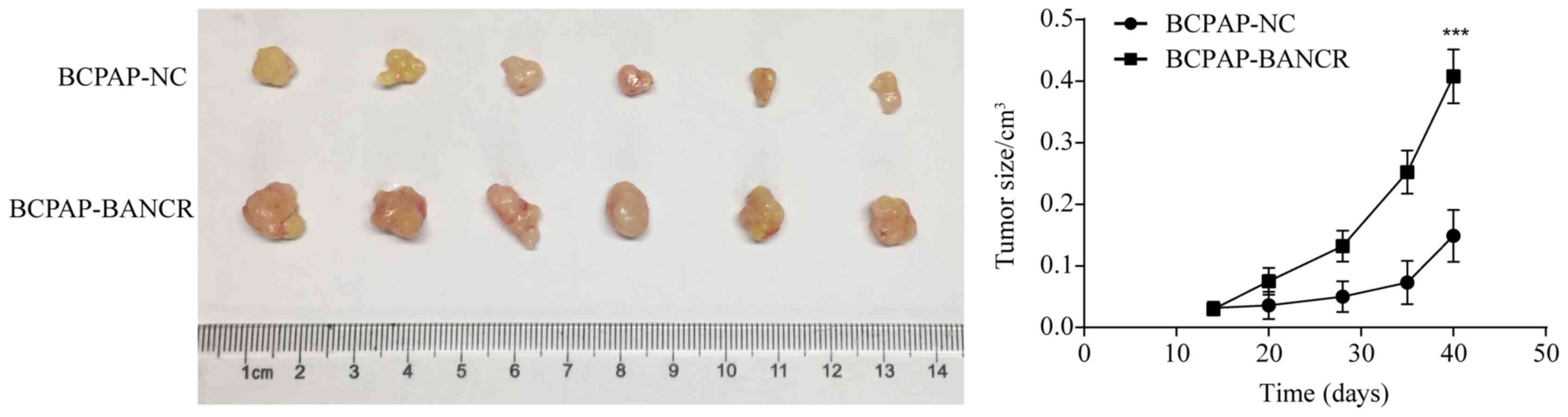
lncRNA BANCR regulates tumor growth in
vivo
To investigate the effects of BANCR on PTC cell
growth in vivo, we utilized a subcutaneous xenograft mouse
model injected with either BANCR-upregulated (BCPAP-BANCR) or
BCPAP-NC cells. Results showed that the average tumor size was
larger in the BCPAP-BANCR group than that in the BCPAP-NC control
group (Fig. 5). The longest
diameter exhibited by a single subcutaneous tumor was 11 mm and no
animal presented with multiple tumors. This indicated that tumor
formation and growth was promoted by the overexpression of BANCR,
and that there is a connection between the expression of BANCR and
CSCs.
Discussion
lncRNAs were long considered to have no function in
cells as they do not encode any proteins. However, recent studies
have confirmed that lncRNAs play an essential role in normal
biological processes such as the regulation of gene expression, and
in pathological processes such as tumorigenesis (16,17).
Studies have shown that lncRNAs affect many cellular processes in
tumor cells, such as cell cycle progression, cell survival,
proliferation and migration (18–21).
In thyroid cancer, many lncRNAs are differentially
expressed between carcinoma and para-cancer tissue (22). For example, MEG3 (maternal expressed
gene 3) was the first lncRNA identified as a tumor suppressor in
melanoma cells (23). In PTC, MEG3
is upregulated in carcinoma tissues as compared with normal
tissues, and it suppresses cell migration and invasion by targeting
Rac1 (24). In addition, PTCSC3
(papillary thyroid carcinoma susceptibility candidate 3) is a tumor
suppressor in thyroid cancer cells which causes significant growth
inhibition, cell cycle arrest and increased apoptosis (25). The lncRNA ANRIL has also been shown
to promote the invasion and metastasis of thyroid cancer cells
through the TGF-β/Smad signaling pathway (26), and nc886 exerts an oncogenic
function in thyroid cancer by suppressing RNA-activated protein
kinase (PKR) (27).
BRAF-activated non-coding RNA (BANCR) promotes the
proliferation of malignant melanoma (14), and was shown to regulate lung
carcinoma proliferation and migration via p38 MAPK and JNK
inactivation (15). In lung cancer
cells, BANCR is associated with poor prognosis and promotes
metastasis by inducing EMT (28).
Finally, BANCR has been shown to promote cell proliferation in PTC
(29).
The characteristics of cancer stem cells include
multi-directional differentiation potential, self-renewal ability,
multiple drug resistance, and the ability to influence tumor
development (5). Therefore, there
is increasing evidence that cancer stem cells in tumor tissues may
contribute to recurrence in many cancers. Reports have shown that
the CSC marker, EpCAM, may be successfully used as a tumor marker
in gastric, ovarian, and breast cancer because its high expression
in these cancers is closely related to tumor growth, metastasis and
advanced tumor stage (30–32). Similarly, increased expression of
LGR5 in esophageal cancer and neuroblastoma can enhance tumor cell
invasion and migration (33,34).
Moreover, studies are underway to determine the relationship
between LGR5 and PTC (35).
However, little is concerning the regulatory mechanisms involved in
the maintenance of EpCAM and LGR5, or whether BANCR is associated
with these CSC markers. Therefore, the aim of this study was to
investigate these topics.
The present study showed that the expression of
BANCR in 30 paired tissue samples from PTC patients was
significantly increased compared with that noted in the adjacent
normal tissues. Next, utilizing the BCPAP cell line, we found that
upregulation of BANCR promoted increased expression of the cancer
stem cell markers, LGR5 and EpCAM, in PTC cells. BCPAP was once
considered to be a poorly differentiated thyroid gland carcinoma
instead of PTC (36). However, in a
study investigating papillary and anaplastic thyroid cancers, the
author hypothesized that distinct cell phenotypes are governed by
different sets of gene master regulators (GMRs). He next proved
that anaplastic (8505C) and papillary (BCPAP) TC phenotypes have
major differences in cell-cycle pathway and gene networking
(37). The two cell lines have
different malignant degree, as BCPAP exhibits low-grade malignant
in contrast with 8505C. The real origin of the cell line BCPAP is
still unclear, based on recent reports and the analysis of the
results, we conclude that even if BCPAP is considered to be a
poorly differentiated thyroid gland carcinoma instead of PTC, the
phenotype of BCPAP is still closer to PTC and we think it is
unlikely to affect the outcomes of our study. The result of a
single-clone formation experiment showed that upregulated BANCR
expression increased the number of clones in the BCPAP cell line.
In the microsphere forming experiments, we also demonstrated that
overexpression BANCR resulted in an increased number and size of
microspheres compared with the control cell line. Additionally,
overexpression of BANCR increased the rate of tumor growth as well
as tumor size in a xenograft mouse model, suggesting a connection
between BANCR expression and CSCs. Thus, this study confirmed that
BANCR can regulate the expression of cancer stem cell markers.
In thyroid cancer, there are two classical cell
signaling pathways, ERK/MAPK and PI3K/AKT. Studies indicate that
the BRAF (V600E) mutation aberrantly activates the MAPK pathway
(38). Since BANCR is related to
the BRAF V600E mutation, and BANCR regulates lung carcinoma
proliferation and migration via the MAPK pathway (15), we investigated the Raf/MEK/ERK
signaling pathway. The results showed that c-Raf, MEK1/2, and
ERK1/2 were expressed in the BCPAP cell lines, and that
phospho-c-Raf, phospho-MEK1/2, and phospho-ERK1/2 were upregulated
by overexpression of BANCR. In addition, the effects of BANCR on
phospho-c-Raf, phospho-MEK1/2, and phospho-ERK1/2 were inhibited by
the c-Raf inhibitor U0126. Furthermore, in the BCPAP cell line, the
expression of LGR5 and EpCAM was downregulated after the use of
U0126 compared with the DMSO controls. Therefore, we conclude that
in PTC cells, BANCR regulates LGR5 and EpCAM expression via the
Raf/MEK/ERK signaling pathway. In future research, we plan to
expand upon the current study by analyzing CSCs in larger numbers
of tissue samples from PTC patients, with the goal of providing new
insight into the role of BANCR in PTC.
Acknowledgements
The present study was supported by the Department of
General Surgery, Zhongshan Hospital. The authors wish to thank the
Digestive Diseases Center of Xiamen City for equipment support.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YW designed the study and wrote the manuscript. XL,
XF and WY prepared the figures. FL, PK and YL reviewed the
manuscript. EL and XH edited the manuscript. GW agreement to be
accountable for all aspects of the work. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee on
Human Research of Zhongshan Hospital, Xiamen University, Xiamen and
written informed consent was obtained from all patients. The
ethical code for the approval granted for the animal experiment was
xmulac20170375, which was under the supervision of Xiamen
University Laboratory Animal Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interests.
References
|
1
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu W, Wang J, Ma L, Tang X, Qiao Y, Pan Q,
Yu Y and Sun F: CD166 plays a pro-carcinogenic role in liver cancer
cells via inhibition of FOXO proteins through AKT. Oncol Rep.
32:677–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoshino Y, Nishida J, Katsuno Y, Koinuma
D, Aoki T, Kokudo N, Miyazono K and Ehata S: Smad4 decreases the
population of pancreatic cancer-initiating cells through
transcriptional repression of ALDH1A1. Am J Pathol. 185:1457–1470.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao J, Mu J, Liu T-R and Xu H: Dig the
root of cancer: Targeting cancer stem cells therapy. J Med Discov.
2:JMD17003. 2017.doi: 10.24262/jmd.2.2.17003.
|
|
6
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H and Tang DG: Prostate cancer stem
cells and their potential roles in metastasis. J Surg Oncol.
103:558–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pece S, Tosoni D, Confalonieri S, Mazzarol
G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG and Di Fiore
PP: Biological and molecular heterogeneity of breast cancers
correlates with their cancer stem cell content. Cell. 140:62–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sullivan JP and Minna JD: Tumor
oncogenotypes and lung cancer stem cell identity. Cell Stem Cell.
7:2–4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L and Xu PC: Downregulated LncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L
and Sha N: Long non-coding RNA BANCR promotes proliferation in
malignant melanoma by regulating MAPK pathway activation. PLoS One.
9:e1008932014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang
L, Tian Y, Han X and Tian D: Long non-coding RNA BANCR promotes
proliferation and migration of lung carcinoma via MAPK pathways.
Biomed Pharmacother. 69:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Cancer Res. 73 (Suppl 8):LB–248. 2013.
View Article : Google Scholar
|
|
17
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia M, Yao L, Zhang Q, Wang F, Mei H, Guo
X and Huang W: Long noncoding RNA HOTAIR promotes metastasis of
renal cell carcinoma by up-regulating histone H3K27 demethylase
JMJD3. Oncotarget. 8:19795–19802. 2017.PubMed/NCBI
|
|
19
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Up-regulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. FEBS J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang C, Li X, Wang Y, Zhao L and Chen W:
Long non-coding RNA UCA1 regulated cell cycle distribution via CREB
through PI3-K dependent pathway in bladder carcinoma cells. Gene.
496:8–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lan X, Zhang H, Wang Z, Dong W, Sun W,
Shao L, Zhang T and Zhang D: Genome-wide analysis of long noncoding
RNA expression profile in papillary thyroid carcinoma. Gene.
569:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Yan G, Zhang Y, Jia X and Bu P:
Long non-coding RNA MEG3 suppresses migration and invasion of
thyroid carcinoma by targeting of Rac1. Neoplasma. 62:541–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan M, Li X, Jiang W, Huang Y, Li J and
Wang Z: A long non-coding RNA, PTCSC3, as a tumor suppressor and a
target of miRNAs in thyroid cancer cells. Exp Ther Med.
5:1143–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao JJ, Hao S, Wang LL, Hu CY, Zhang S,
Guo LJ, Zhang G, Gao B, Jiang Y, Tian WG, et al: Long non-coding
RNA ANRIL promotes the invasion and metastasis of thyroid cancer
cells through TGF-β/Smad signaling pathway. Oncotarget.
7:57903–57918. 2016.PubMed/NCBI
|
|
27
|
Lee EK, Hong SH, Shin S, Lee HS, Lee JS,
Park EJ, Choi SS, Min JW, Park D, Hwang JA, et al: nc886, a
non-coding RNA and suppressor of PKR, exerts an oncogenic function
in thyroid cancer. Oncotarget. 7:75000–75012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun M, Liu XH, Wang KM, Nie FQ, Kong R,
Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, et al: Downregulation of
BRAF activated non-coding RNA is associated with poor prognosis for
non-small cell lung cancer and promotes metastasis by affecting
epithelial-mesenchymal transition. Mol Cancer. 13:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng H, Wang M, Jiang L, Chu H, Hu J,
Ning J, Li B, Wang D and Xu J: BRAF-activated long noncoding RNA
modulates papillary thyroid carcinoma cell proliferation through
regulating thyroid stimulating hormone receptor. Cancer Res Treat.
48:698–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai M, Yuan F, Fu C and Shen G, Hu S and
Shen G: Relationship between epithelial cell adhesion molecule
(EpCAM) overexpression and gastric cancer patients: A systematic
review and meta-analysis. PLoS One. 12:e01753572017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng J, Zhao S, Yu X, Huang S and Liu HY:
Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer
effectively inhibits intraperitoneal ovarian cancer growth.
Theranostics. 7:1373–1388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao S, Sun Y, Liu X, Zhang D and Yang X:
EpCAM and COX-2 expression are positively correlated in human
breast cancer. Mol Med Rep. 15:3755–3760. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vieira GC, Chockalingam S, Melegh Z,
Greenhough A, Malik S, Szemes M, Park JH, Kaidi A, Zhou L,
Catchpoole D, et al: Correction: LGR5 regulates pro-survival
MEK/ERK and proliferative Wnt/β-catenin signalling in
neuroblastoma. Oncotarget. 8:323812017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv Z, Yu JJ, Zhang WJ, Xiong L, Wang F, Li
LF, Zhou XL, Gao XY, Ding XF, Han L, et al: Expression and
functional regulation of stemness gene Lgr5 in esophageal squamous
cell carcinoma. Oncotarget. 8:26492–26504. 2017.PubMed/NCBI
|
|
35
|
Michelotti G, Jiang X, Sosa JA, Diehl AM
and Henderson BB: LGR5 is associated with tumor aggressiveness in
papillary thyroid cancer. Oncotarget. 6:34549–34560. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saiselet M, Floor S, Tarabichi M, Dom G,
Hébrant A, van Staveren WC and Maenhaut C: Thyroid cancer cell
lines: An overview. Front Endocrinol (Lausanne).
3:1332012.PubMed/NCBI
|
|
37
|
Iacobas DA, Tuli NY, Iacobas S, Rasamny
JK, Moscatello A, Geliebter J and Tiwari RK: Gene master regulators
of papillary and anaplastic thyroid cancers. Oncotarget.
9:2410–2424. 2017.PubMed/NCBI
|
|
38
|
Fraser S, Go C, Aniss A, Sidhu S,
Delbridge L, Learoyd D, Clifton-Bligh R, Tacon L, Tsang V, Robinson
B, et al: BRAF(V600E) mutation is associated with decreased
disease-free survival in papillary thyroid cancer. World J Surg.
40:1618–1624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|