Introduction
Lung cancer (LC) is currently the second most
frequent malignant tumor and the leading cause of mortality in both
genders worldwide, with over 1.5 million cancer-related mortalities
reported each year (1,2). Based on histopathological differences,
LC can be divided into two main types: Small cell lung cancer
(SCLC; 15% of all lung cancers) and non-small cell lung cancer
(NSCLC; 85% of all lung cancers) (3). Despite the significant improvements
that have been made in early diagnosis and therapeutic approaches,
the survival rate of patients with LC is still far from
satisfactory. LC is a highly heterogeneous malignant tumor, and
thus clinical treatment strategy should be precise and personalized
according to each patient's clinical features. In recent years, a
number of systematic studies on novel molecular and gene markers
involved in the pathogenesis of LC have been conducted (4–6).
However, evidence for the underlying mechanisms involved in the
carcinogenesis and development of LC remains limited.
MicroRNAs (miRNA/miRs) are small, non-coding RNA
molecules of approximately 22 nucleotides in length, which bind to
specific complimentary recognition sequences in the 3′-untranslated
regions (UTRs) of target mRNAs, to thereby function in RNA
silencing and post-transcriptional regulation of gene expression
(7,8). While the majority of miRNAs are
located within the cell, certain miRNAs, commonly known as
circulating miRNAs or extracellular miRNAs, have also been found in
extracellular environments, including in various biological fluids
and cell culture media. miRNAs have been proven to play important
roles in many biological behaviors of tumor cells, including cell
survival, proliferation, mobility, apoptosis, metabolism and other
pathological features of various tumors (9). Abnormal expression of miRNAs is
relevant to almost all types of tumor, and miRNAs may function as
both oncogenes and tumor suppressors during tumor progression
(10,11). For instance, miR-224 may promote the
malignant progression of NSCLC by partially antagonizing the
functions of SMAD4 and TNFAIP1 (12). miR-31 is reported to be relevant to
prognosis, survival time and distant metastasis in patients with
lung adenocarcinoma (13). miR-150
has been proven to locate on chromosome 19q13 and participate in
hematopoiesis (14). Further
evidence has demonstrated that miR-150 is a crucial regulatory
factor in tumor progression. In a previous study, miR-150 promoted
gastric cancer cell proliferation by negatively regulating the
proapoptotic gene EGR2 (15).
miR-150 increased the viability and mobility of LC cells by
targeting SRC kinase signal inhibitor 1 or p53 (16,17).
These studies revealed that miRNAs have the potential to be used as
targets in the treatment of different cancers. In particular,
miR-150 may be crucial in the progression of various cancers,
including LC. However, to date, the exact molecular mechanism of
miR-150 in regulating the tumorigenesis of LC has remained poorly
understood.
In the present study, our aim was to investigate the
function of miR-150 in NSCLC cells, and to elucidate the possible
underlying mechanisms. The present data identified that the
expression of miR-150 was dramatically upregulated in NSCLC cells.
Furthermore, miR-150 was found to regulate the expression levels of
SIRT2 and JMJD2A. Moreover, miR-150 was also identified to function
in the viability and mobility of NSCLC cells via regulating
SIRT2/JMJD2A expression. Notably, in vivo assays revealed
that reduced miR-150 expression and/or re-expression of SIRT2
inhibited NSCLC cell growth. Finally, results revealed that miR-150
regulated SIRT2/JMJD2A expression by activating the AKT signaling
pathway. Therefore, the miR-150-SIRT2/JMJD2A axis could be a
promising molecular target in therapeutic strategies aimed at
preventing the malignant progression of NSCLC.
Materials and methods
Cell lines and cell culture
A human normal lung cell line (MRC-5) and four human
NSCLC cell lines (A549, H460, H1299 and H520) were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
These cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories; GE Healthcare Life Sciences, Logan, UT, USA), 100
U/ml penicillin, 100 µg/ml streptomycin and 1% glutamine
(Invitrogen; Thermo Fisher Scientific, Inc.). All cells were
maintained at 37°C with 5% CO2.
Cell infection and transfection
For miR-150 downregulation or overexpression,
miR-150 inhibitor (LNA-anti-miR-150) (Exiqon, Vedbæk, Denmark) or
miR-150 mimics (Invitrogen; Thermo Fisher Scientific, Inc.) were
respectively added to the culture medium as in a previous study
(18). The transfection medium was
replaced at 4 h post-transfection with regular culture medium.
For SIRT2 overexpression or knockdown, A549 and
H1299 cell lines were infected with adenovirus expressing SIRT2
(Ad-SIRT2) or retrovirus expressing sh-STRT2, respectively, as in
our previous study (19).
Reverse transcription-quantitative PCR
(RT-qPCR)
Real-time RT-qPCR was used to detect the expression
level of miR-150 by a MiniOpticon™ Two-Color Real-Time PCR
Detection system (Bio-Rad Laboratories, Hercules, CA, USA). miRNA
was extracted from cultured cells by using an Applied Biosystems
mirVana miRNA Isolation kit (Thermo Fisher Scientific, Inc.). U6
was used as the internal control.
Western blotting
Total protein isolated from 105 A549 and
H1299 cells was lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and detected by a western blot assay
as previously described (18).
Antibodies against the following target proteins were used:
Phosphorylated-AKTSer473 (dilution 1:1,000; cat. no.
sc-7985-R), AKT (dilution 1:1,000; cat. no. sc-8312), JMJD2A
(dilution 1:500; cat. no. sc-81302), SIRT2 (dilution 1:1,000; cat.
no. sc-135793; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
mouse PCNA (dilution 1:1,000; cat. no. ab-18197; Abcam, Cambridge,
MA, USA) and GAPDH (dilution 1:5,000; Kangchen Bio-tech, Shanghai,
China); the secondary antibodies were sheep anti-mouse IgG and
anti-rabbit IgG (dilution 1:6,000; R&D Systems China, Shanghai,
China). GAPDH was used as the internal control. All protein bands
were semi-quantitatively detected with ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
MTT assay
A549 and H1299 cell viability was monitored using a
Cell Proliferation kit I (MTT) (cat. no. 11465007001;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's instructions.
Plate colony formation assay
NSCLC cell lines A549 and H1299 were collected
following transfection, and 1×103 cells were mixed with
high-glucose DMEM supplemented with 10% fetal bovine serum (FBS)
and cultured in 6-well tissue culture plates. The cells were
continuously cultured for 2 weeks. Following culture, all cell
colonies were stained with 0.005% crystal violet dye liquor. The
stained cells were observed by microscopy (Leica DM2500; Leica
Microsystems, Wetzlar, Germany) and 10 random fields were selected
for cell colony counting. The experiment was conducted three
times.
Cell mobility assays
Cell mobility assays were performed with a Transwell
chamber (EMD Millipore, Billerica, MA, USA) according to the
manufacturer's guidelines. For an invasion assay, a total of
5×104 A549 and H1299 cells at 24 h post-transfection
were plated onto an 8-µm pore size Transwell insert pre-coated with
extracellular matrix (ECM) (1:6 mix with DMEM) (BD Biosciences, San
Jose, CA, USA). A cell migration assay was performed using the
Transwell insert without the pre-coating with ECM. After
conventional cell culture for 48 h, all cells which had adhered to
the upper surface of the Transwell insert were wiped away gently
with a cotton swab. The remaining cells which had passed through
the Transwell insert were stained with 0.005% crystal violet dye
liquor for 15 min. The stained cells were observed by microscopy
(Leica DM2500; Leica Microsystems) and 10 random fields were
selected for cell counting. The experiment was conducted three
times.
Tumor xenograft experiments
Xenograft mouse experiments were performed as
previously described (18).
Twenty-four male BALB/c nude mice (20 g weight) at 8 weeks of age
(Shanghai SLAC Laboratory Animal Center of Chinese Academy of
Sciences, Shanghai, China) were randomly divided into four groups
(n=6/group), subcutaneously injected the parental,
LNA-anti-miR150-transfected, Ad-SIRT2-transfected and both
transfected A549 cells, respectively, into the right and left
flanks of male nu/nu mice. Mice were maintained in pathogen-free
conditions and cared for according to the Laboratory Animal Care
guidelines. They were given radiation-sterilized food pellets and
distilled water. Tumor size was measured regularly, and tumor
volume was estimated with the formula: a × b2 × 0.5, in
which a and b represent the maximal and minimal diameters. Mice
were sacrificed by anesthetization at the end of the observation
period and cancer tissues were harvested and the tumor weights were
measured. Then, the tumors were fixed in 10% neutral formaldehyde
for 6 h, and consecutive paraffin-embedded sections were cut to
examine the expression of PCNA, JMJD2A and p-AKTSer473
by immunohistochemistry. The animal welfare guidelines for the care
and use of laboratory animals were followed and the experimental
protocol was approved by the Animal Care Committee of the Second
Affiliated Hospital of Soochow University.
Statistical analysis
The experimental data from three independent
experiments were presented as the mean ± standard deviation (SD)
and analyzed using one-way analysis of variance (ANOVA) test with
post hoc contrasts by the Student-Newman-Keuls test. The software
package PASW Statistics 18.0 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis. A P<0.05 was considered to
indicate statistical significance.
Results
miR-150 is overexpressed in NSCLC cell
lines and is accompanied by suppression of SIRT2
Our previous studies revealed that miR-150 was
obviously upregulated in NSCLC tissues compared with that noted in
normal tissues. To confirm the expression of miR-150 in NSCLC
cells, we performed RT-qPCR to detect the expression of miR-150 in
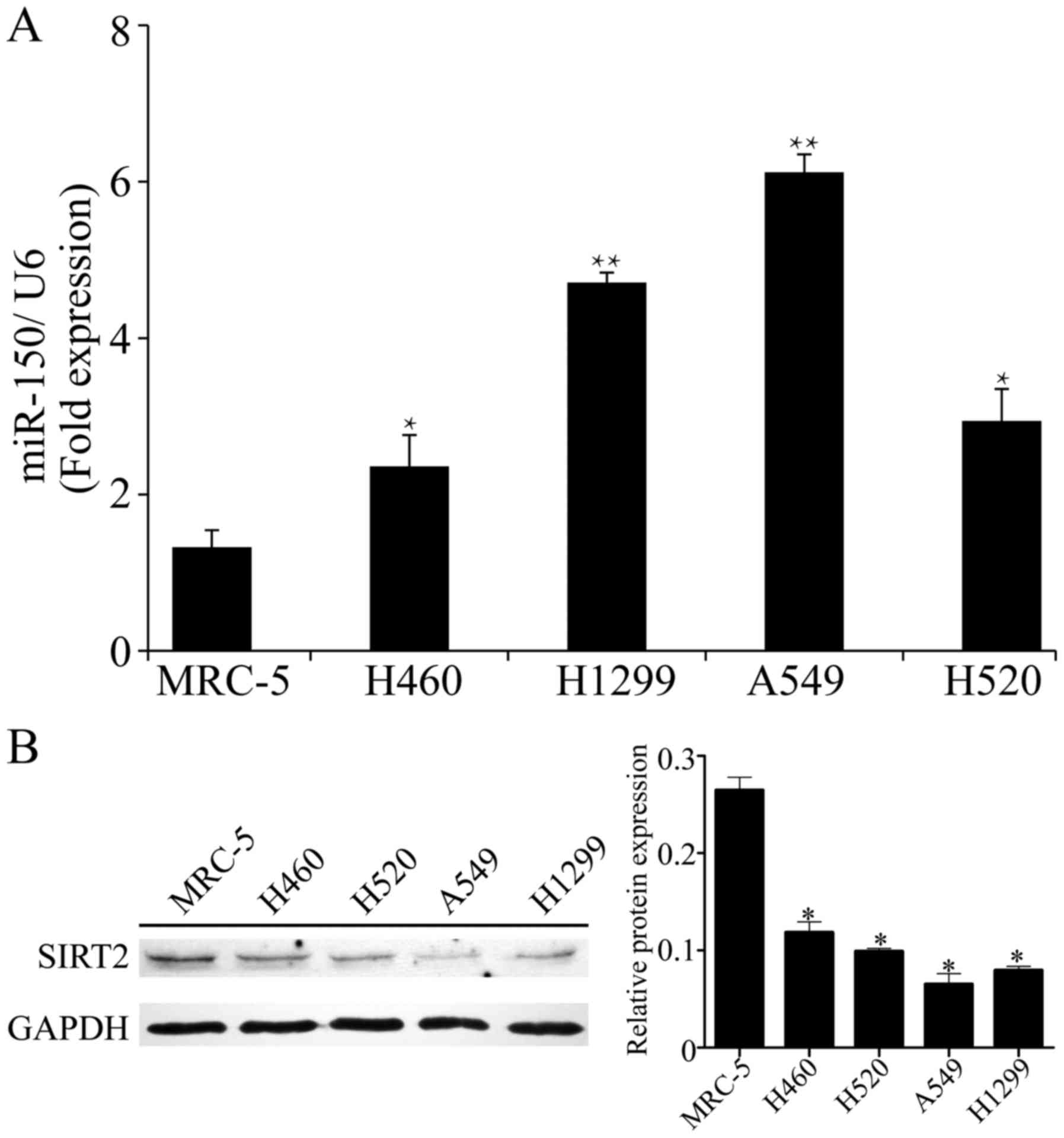
four NSCLC cell lines. As shown in Fig.
1A, compared with the normal cell line MRC-5, miR-150 was
significantly upregulated in the four NSCLC cells lines. In
particular, the relative expression of miR-150 was highly increased
in the A549 and H1299 cells (6.12±0.23 in A549, 4.71±0.13 in H1299;
P<0.01 vs. normal cell line). Meanwhile, significant
suppression of SIRT2 was observed in the NSCLC cell lines,
particularly in A549 and H1299 cells (P<0.01 vs. normal
cell line; Fig. 1B). Accordingly,
the A549 and H1299 cell lines, with obvious upregulation of miR-150
and downregulation of SIRT2, were selected for the following
assays.
miR-150 effects the expression of
SIRT2 and JMJD2A in NSCLC cell lines
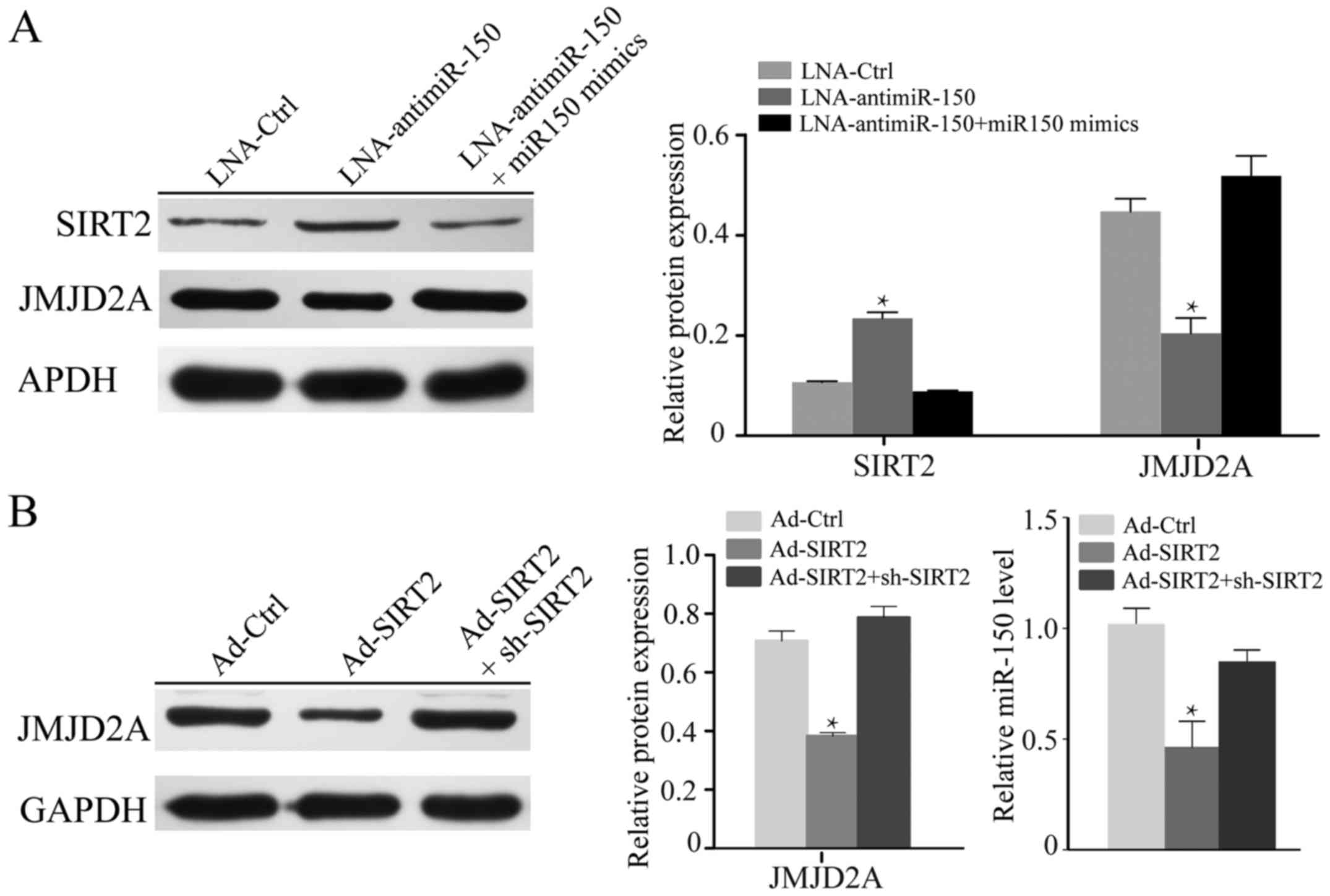
Previous studies have demonstrated that miR-150
expression is markedly positively related to the JMJD2A level,
while being significantly negatively correlated with SIRT2 and
JMJD2A expression in A549 cells (18,19).
To further investigate the effect of miR-150 on H1299 cells,
LNA-anti-miR-150 was used to silence miR-150, and miR-150 mimics
were used to recover the level of miR-150. The knockdown of miR-150
in H1299 cells notably increased the expression of SIRT2 and
reduced the level of JMJD2A (P<0.05; Fig. 2A). In turn, upon transfection with
miR-150 mimics, the normal expression levels of SIRT2 and JMJD2A
were recovered. To further explore the relationship between miR-150
and SIRT2, we knocked down or overexpressed SIRT2 in H1299 cells.
As shown in Fig. 2B, re-expression
of SIRT2 suppressed the expression of JMJD2A and miR-150
(P<0.05).
Downregulation of miR-150 combined
with upregulation of SIRT2 significantly inhibits the proliferation
and neoplastic capacity of NSCLC cell lines
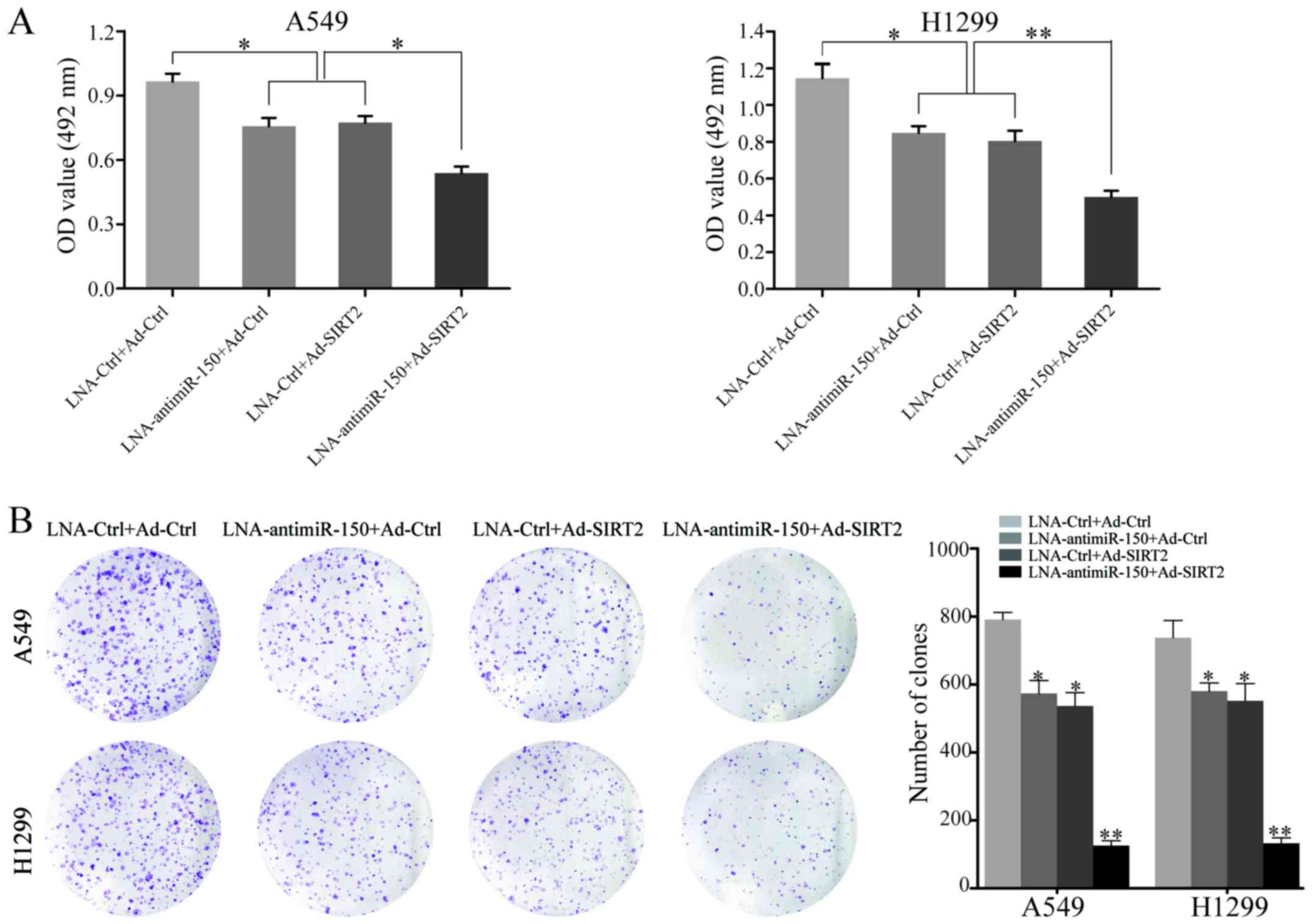
To explore the effect of miR-150 downregulation and
SIRT2 re-expression on the proliferation of NSCLC cells, A549 and
H1299 cell proliferation were measured after transfection with
LNA-anti-miR-150 or Ad-SIRT2. At 48 h after transfection, the
proliferation rate of cells transfected with LNA-anti-miR-150 or
Ad-SIRT2 was decreased, relative to that of the negative controls.
Moreover, combined treatment with LNA-anti-miR-150 and Ad-SIRT2
showed the most significant inhibitory effect on cell proliferation
in A549 and H1299 cells (P<0.05; Fig. 3A). Next, the neoplastic capacity of
NSCLC cells was examined by plate colony formation assay. The
results demonstrated that the cells transfected with
LNA-anti-miR-150 or Ad-SIRT2 formed less colonies than the
corresponding control groups (P<0.05; Fig. 3B). Similarly, the A549 and H1299
groups co-transfected with LNA-anti-miR-150 and Ad-SIRT2 exhibited
the most obvious suppression of neoplastic capacity (P<0.001;
Fig. 3B). In addition, western blot
analysis was used to test the ability of miR-150 downregulation and
SIRT2 re-expression to regulate the expression of PCNA
(proliferating cell nuclear antigen), which serves an important
role in the proliferation of cells. The data indicated that PCNA
protein expression was reduced in the LNA-anti-miR-150 or
Ad-SIRT2-transfected cells, but remained to be expressed to a high
level in the respective negative control groups (P<0.05;
Fig. 5). Furthermore, significant
reduction of PCNA expression was observed in the NSCLC cells
co-transfected with LNA-anti-miR-150 and Ad-SIRT2 (P<0.001;
Fig. 5). These results demonstrated
that the combination of downregulated miR-150 and upregulated SIRT2
could strongly inhibit the proliferation and neoplastic capacity of
NSCLC cells.
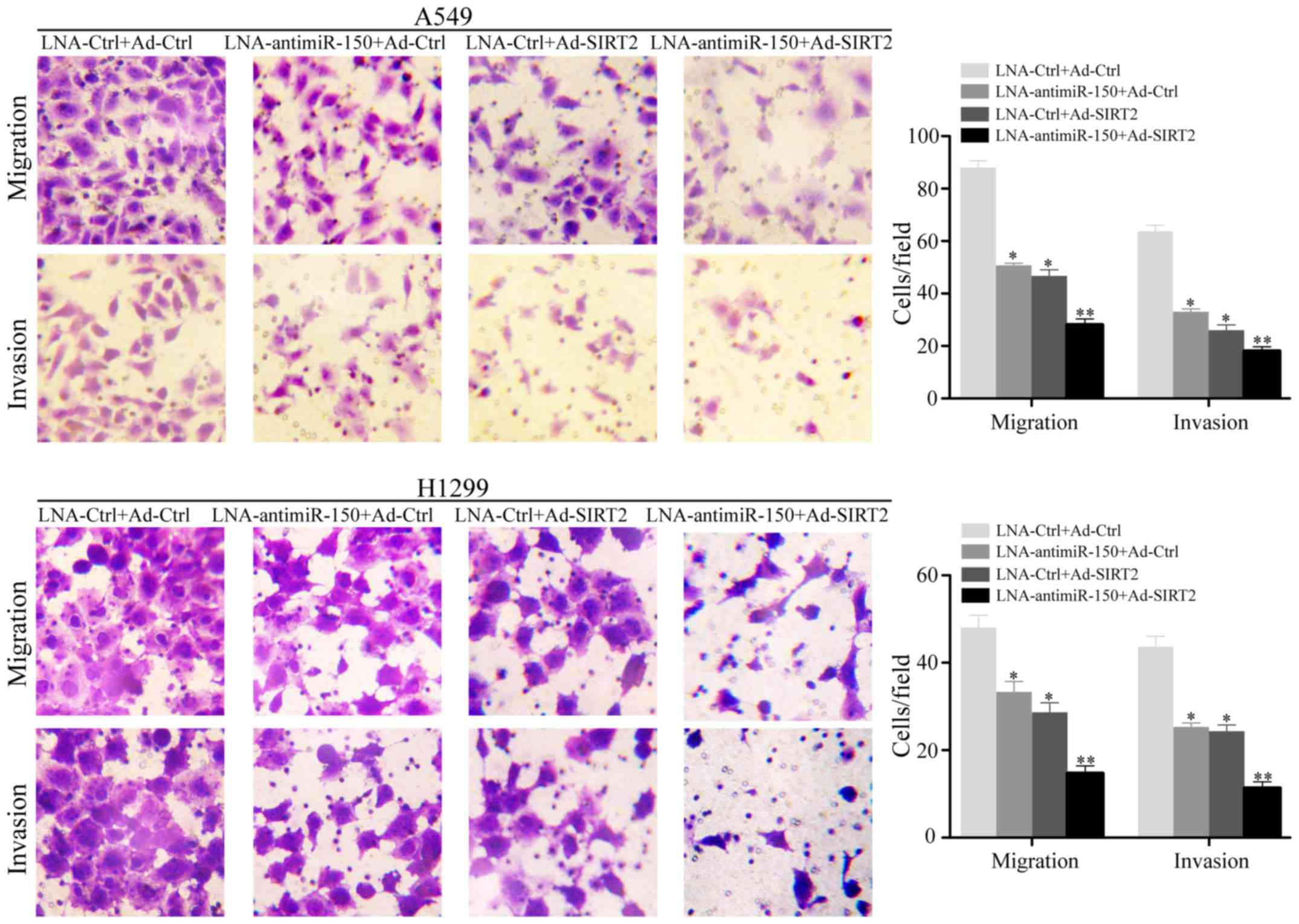
Downregulation of miR-150 combined
with upregulation of SIRT2 obviously reduces cellular motility in
NSCLC cell lines
To investigate the effect of miR-150 downregulation
and SIRT2 re-expression on cellular motility, the NSCLC cell lines
A549 and H1299 were subjected to Transwell cellular mobility assays
after transfection with LNA-anti-miR-150 and/or Ad-SIRT2. The
results indicated that the relative migrated and invaded cell
numbers were obviously decreased in the cell groups transfected
with LNA-anti-miR-150 or Ad-SIRT2, compared with the negative
controls (P<0.05; Fig. 4). In
particular, the group transfected with both LNA-anti-miR-150 and
Ad-SIRT2 showed the lowest numbers of migrated and invaded cells
(P<0.001; Fig. 4).
Reduction of miR-150 and re-expression
of SIRT2 leads to suppression of JMJD2A and inactivation of the AKT
signaling pathway in NSCLC cell lines
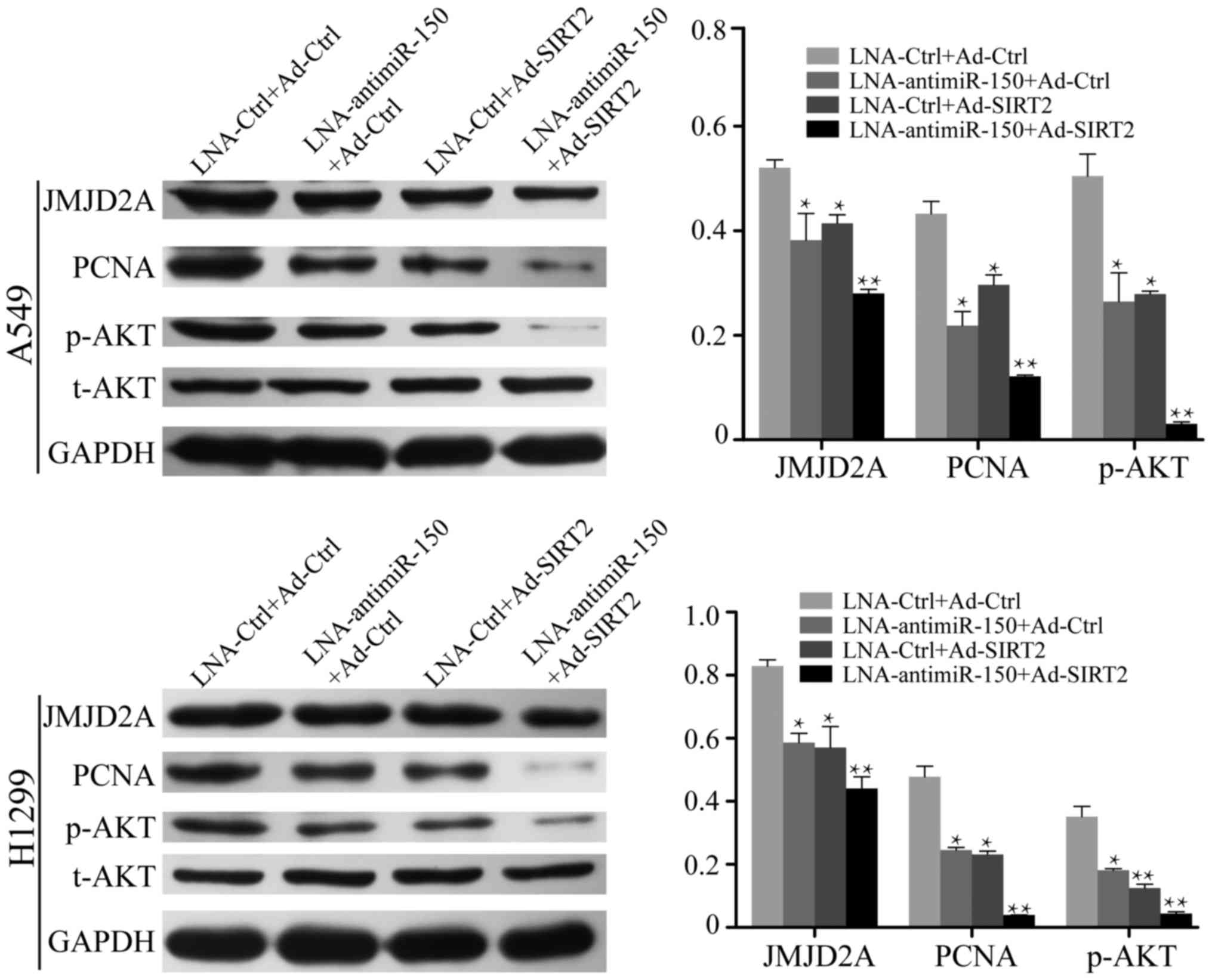
It has been well demonstrated that NSCLC cells
exhibit high expression of miR-150 and low expression of SIRT2. To
further investigate the effect of miR-150 suppression and SIRT2
re-expression on NSCLC cells, LNA-anti-miR-150 and Ad-SIRT2 were
transfected into A549 and H1299 cells, and the relative expression
levels of AKT and JMJD2A were measured by western blot analysis.
The results demonstrated that the expression of JMJD2A was
decreased in the cell groups transfected with LNA-anti-miR-150 or
Ad-SIRT2 (P<0.05; Fig. 5). The
inhibition of JMJD2A expression was more marked in cells
co-transfected with LNA-anti-miR-150 and Ad-SIRT2 (P<0.001;
Fig. 5). In addition, reduction of
miR-150 or re-expression of SIRT2 decreased the expression of
p-AKTSer473 in A549 and H1299 cells (P<0.05; Fig. 5). Co-treatment to reduce miR-150
expression and re-express SIRT2 further inhibited
p-AKTSer473 expression in the NSCLC cells (P<0.001;
Fig. 5). These results suggested
that reduction of miR-150 and re-expression of SIRT2 leads to
suppression of JMJD2A and effectively inactivates the AKT signaling
pathway.
Reduction of miR-150 and re-expression
of SIRT2 leads to suppression of NSCLC tumor growth in vivo
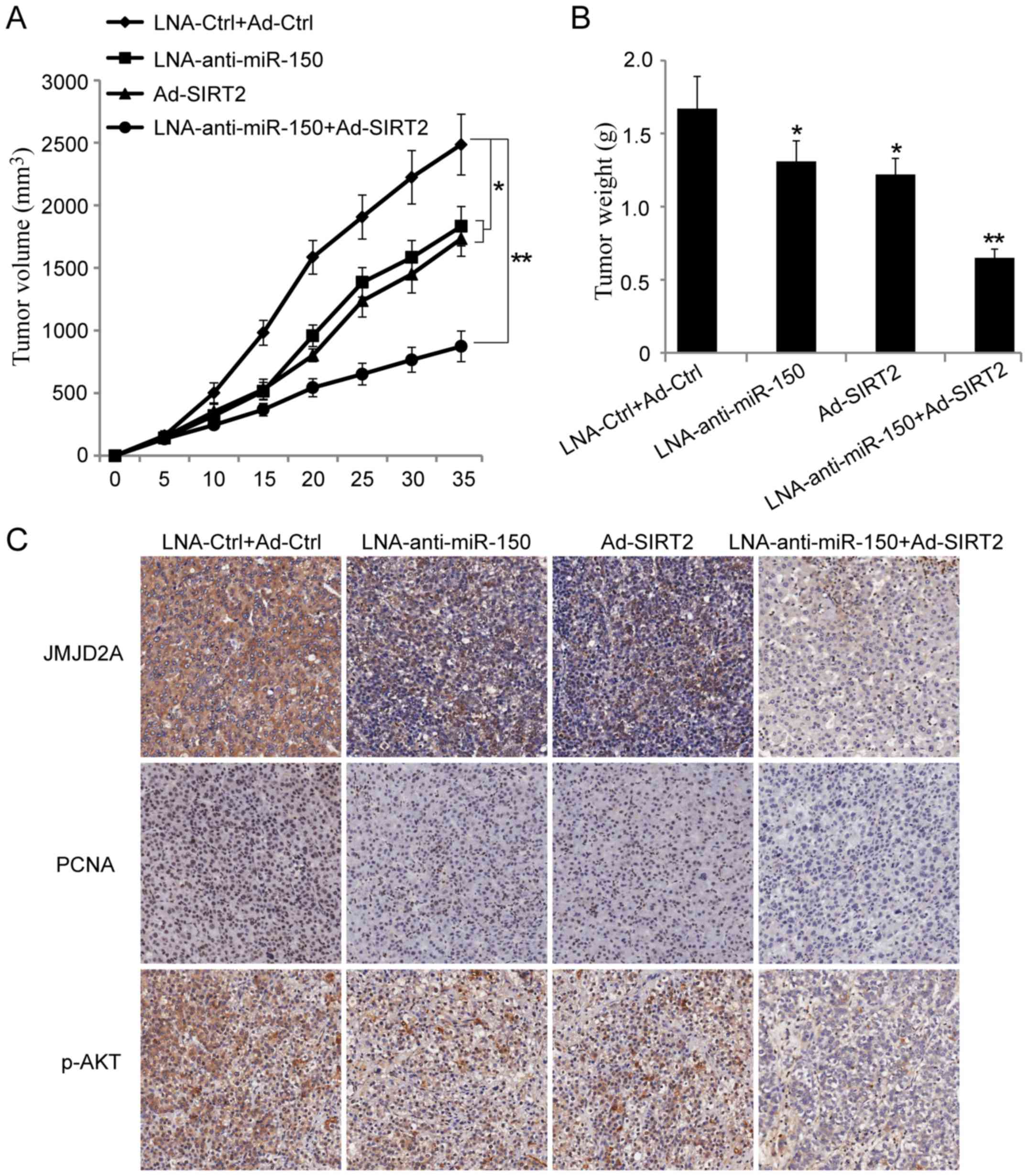
To further explore the effect of miR-150 on the
NSCLC cells, a xenograft tumor growth assay was performed. The
results showed that miR-150 knockdown or SIRT2 overexpression
significantly suppressed NSCLC tumor growth in vivo
(Fig. 6A and B; P<0.05).
Moreover, co-transfection of A549 cells with miR-150 knockdown and
SIRT2 re-expression vectors could further inhibit NSCLC tumor
growth in vivo (Fig. 6A and
B; P<0.001). Next, the paraffin-embedded tumor sections were
examined immunohistochemically. The results showed that the
expression of JMJD2A, PCNA and p-AKTSer473 were
decreased in the groups injected with cells transfected with
LNA-anti-miR-150 or Ad-SIRT2. Moreover, the expression of JMJD2A,
PCNA and p-AKTSer473 were more significantly inhibited
in the group injected with cells co-transfected with
LNA-anti-miR-150 and Ad-SIRT2 (Fig.
6C). These results suggested that reduction of miR-150 and
re-expression of SIRT2 could inhibit NSCLC growth in vivo.
It also provided evidence that miR-150 may play a tumor-promoting
role in lung cancer.
Discussion
Abnormal expression of miRNAs is relevant to almost
all types of malignant tumor, and miRNAs can function both as
oncogenes and tumor suppressors during tumor progression. To date,
several miRNAs have been identified to play vital roles in LC
pathogenesis, and could be novel target molecules for early
diagnosis and clinical treatment (20–23).
miR-150 was first identified as a hematopoietic
cell-specific miRNA that participated in hematopoiesis (24). Increasing evidence has demonstrated
that miR-150 may also be involved in the malignant progression of
various human tumors. More notably, miR-150 may function as an
oncogene or a tumor suppressor in different tumors, dependent on
its expression level and target genes in specific tumors. Recently,
the biological functions of miR-150 in relation to the malignant
phenotypes of cancer cells have been reported, and a series of
target genes have been identified. In breast cancer, miR-150
promotes the proliferation and malignant behavior (including
migration, invasion and apoptosis resistance) of tumor cells by
targeting the pro-apoptotic purinergic P2×7 receptor (25). In gastric cancer cells, miR-150
could promote cell growth by negatively regulating the
pro-apoptotic gene EGR2 in vitro (15). Moreover, miR-150 inhibited tumor
cell metastasis in esophageal squamous cell carcinoma and
hepatocellular carcinoma by targeting ZEB1 and GAB1, respectively
(26,27). However, to date, the exact molecular
mechanism of miR-150 in the malignant progression of NSCLC remains
poorly understood.
Our previous studies have revealed that miR-150 was
upregulated in NSCLC tissues and positively related to the
expression of JMJD2A. In turn, JMJD2A was able to regulate cell
growth and apoptosis in an miR-150-dependent manner in NSCLC.
Meanwhile, SIRT2, as an anti-oncogenic protein, exhibited
downregulated expression in NSCLC. Additionally, SIRT2 could
combine to the promoter region of JMJD2A and negatively regulated
the expression of JMJD2A (18,19).
Based on this previous evidence, we speculated that miR-150 may
affect the malignant progression of NSCLC cells by regulating
SIRT2/JMJD2A. Our present data revealed that miR-150 was
overexpressed in NSCLC cells compared with that noted in normal
lung cells. Notably, NSCLC cell lines that highly expressed miR-150
exhibited downregulation of SIRT2. Moreover, silencing of miR-150
in NSCLC cells obviously promoted the expression of SIRT2 and
reduced the level of JMJD2A, which could be recovered by
transfection with miR-150 mimics. To further explore the
relationship between miR-150 and SIRT2, we knocked down or
overexpressed SIRT2 in NSCLC cells. Our results revealed that the
re-expression of SIRT2 could suppress the expression of JMJD2A and
miR-150. To further investigate whether miR-150 regulated NSCLC
viability and mobility via SIRT2/JMJD2A, we knocked down miR-150
and overexpressed SIRT2 respectively or simultaneously in NSCLC
cells. The silencing of miR-150 inhibited cell viability, while
overexpressing-SIRT2 aggravated the inhibitory effect on cell
proliferation, migration and invasion, suggesting that miR-150 was
essential for the function of SIRT2/JMJD2A in NSCLC. These results
suggested that miR-150 regulated NSCLC viability and mobility by
regulating SIRT2/JMJD2A. Additionally, the silencing of miR-150 or
re-expression of SIRT2 decreased the expression of PCNA and p-AKT
Ser473 in NSCLC cells; furthermore, co-treatment to
knockdown miR-150 and re-express SIRT2 could further inhibit PCNA
and p-AKT Ser473 expression in the NSCLC cells.
Meanwhile, in vivo results of a tumor xenograft model
demonstrated that miR-150 suppression and SIRT2 re-expression could
inhibit NSCLC growth, and provided evidence that miR-150 may play a
tumor-supporting role in lung cancer. Finally, our results
suggested that reduction of miR-150 and re-expression of SIRT2 may
lead to suppression of JMJD2A and effectively inactivate the AKT
signaling pathway. Further studies are now needed to elucidate the
signaling pathways involved in more detail.
In conclusion, the present study demonstrates that
miR-150 may contribute to the malignant phenotype in NSCLC by
regulating SIRT2/JMJD2A. miR-150 alone or in combination with SIRT2
may serve as a potential therapeutic target in NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology Development Project of Suzhou Municipality,
Jiangsu Province (grant no. SYSD2016090) and the Science and
Technology Bureau of Suzhou Municipality, Jiangsu Province (grant
no. SYS201719).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YC and WX conceived and designed the experiments; KJ
and MS performed the experiments; KJ analyzed the data; KJ and WX
wrote the study. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal welfare guidelines for the care and use
of laboratory animals were followed and the experimental protocol
was approved by the Animal Care Committee of the Second Affiliated
Hospital of Soochow University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Feng Q, Wei X and Yu Y: MicroRNA-490
regulates lung cancer metastasis by targeting poly r(C)-binding
protein 1. Tumour Biol. 37:15221–15228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ourari-Dhahri B, Ben Slima H, Ben Amar J,
El Gharbi L, Ali M and Baccar Azzabi S: Management of non-small
cell lung cancer. Tunis Med. 90:847–851. 2012.PubMed/NCBI
|
|
4
|
Zamay TN, Zamay GS, Kolovskaya OS, Zukov
RA, Petrova MM, Gargaun A, Berezovski MV and Kichkailo AS: Current
and prospective protein biomarkers of lung cancer. Cancers.
9:E1552017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lazzari C, Spreafico A, Bachi A, Roder H,
Floriani I, Garavaglia D, Cattaneo A, Grigorieva J, Viganò MG,
Sorlini C, et al: Changes in plasma mass-spectral profile in course
of treatment of non-small cell lung cancer patients with epidermal
growth factor receptor tyrosine kinase inhibitors. J Thorac Oncol.
7:40–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Y, Qiao G, Xu E, Xuan Y, Liao M and
Yin G: Biomarkers for early diagnosis, prognosis, prediction, and
recurrence monitoring of non-small cell lung cancer. Onco Targets
Ther. 12:4527–4534. 2017. View Article : Google Scholar
|
|
7
|
Lou W, Liu J, Gao Y, Zhong G, Chen D, Shen
J, Bao C, Xu L, Pan J, Cheng J, et al: MicroRNAs in cancer
metastasis and angiogenesis. Oncotarget. 8:115787–115802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Q, Huang SX, Zhang F, Li SJ, Liu C,
Xi YY, Wang L, Wang X, He QQ, Sun CC and Li DJ: MicroRNAs: A novel
potential biomarker for diagnosis and therapy in patients with
non-small cell lung cancer. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
9
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin PY, Yu SL and Yang PC: MicroRNA in
lung cancer. Br J Cancer. 103:1144–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui R, Meng W, Sun HL, Kim T, Ye Z, Fassan
M, Jeon YJ, Li B, Vicentini C, Peng Y, et al: MicroRNA-224 promotes
tumor progression in non-small cell lung cancer. Proc Natl Acad Sci
USA. 112:E4288–E4297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng W, Ye Z, Cui R, Perry J,
Dedousi-Huebner V, Huebner A, Wang Y, Li B, Volinia S, Nakanishi H,
et al: MicroRNA-31 predicts the presence of lymph node metastases
and survival in patients with lung adenocarcinoma. Clin Cancer Res.
19:5423–5433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams BD, Guo S, Bai H, Guo Y, Megyola CM,
Cheng J, Heydari K, Xiao C, Reddy EP and Lu J: An in vivo
functional screen uncovers miR-150-mediated regulation of
hematopoietic injury response. Cell Rep. 2:1048–1060. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K,
Ren G, Su T, Pan Y, Feng B, et al: MiR-150 promotes gastric cancer
proliferation by negatively regulating the pro-apoptotic gene EGR2.
Biochem. Biophys Res Commun. 392:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Wei X and Xu L: miR-150 promotes
the proliferation of lung cancer cells by targeting P53.
FEBS Lett. 587:2346–2351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao M, Hou D, Liang H, Gong F, Wang Y, Yan
X, Jiang X1, Wang C, Zhang J, Zen K, et al: miR-150 promotes the
proliferation and migration of lung cancer cells by targeting SRC
kinase signaling inhibitor 1. Eur J Cancer. 50:1013–1024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu W, Jiang K, Shen M, Qian Y and Peng Y:
SIRT2 suppresses non-small cell lung cancer growth by targeting
JMJD2A. Biol Chem. 396:929–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu W, Jiang K, Shen M, Chen Y and Huang
HY: Jumonji domain containing 2A predicts prognosis and regulates
cell growth in lung cancer depending on miR-150. Oncol Rep.
35:352–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orellana EA and Kasinski AL: MicroRNAs in
cancer: A historical perspective on the path from discovery to
therapy. Cancers. 7:1388–1405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frixa T, Donzelli S and Blandino G:
Oncogenic MicroRNAs: Key players in malignant transformation.
Cancers. 7:2466–2485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inamura K and Ishikawa Y: MicroRNA in lung
cancer: Novel biomarkers and potential tools for treatment. J Clin
Med. 5:pii: E36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi RU, Miyazaki H and Ochiya T: The
roles of MicroRNAs in breast cancer. Cancers. 7:598–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Y, Jiang X and Chen J: The role of
miR-150 in normal and malignant hematopoiesis. Oncogene.
33:3887–3893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang S, Chen Y, Wu W, Ouyang N, Chen J,
Li H, Liu X, Su F, Lin L and Yao Y: miR-150 promotes human breast
cancer growth and malignant behavior by targeting the pro-apoptotic
purinergic P2X7 receptor. PLoS One. 8:e807072013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yokobori T, Suzuki S, Tanaka N, Inose T,
Sohda M, Sano A, Sakai M, Nakajima M, Miyazaki T, Kato H and Kuwano
H: MiR-150 is associated with poor prognosis in esophageal
squamous cell carcinoma via targeting the EMT inducer ZEB1.
Cancer Sci. 104:48–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun W, Zhang Z, Wang J, Shang R, Zhou L,
Wang X, Duan J, Ruan B, Gao Y, Dai B, et al: MicroRNA-150
suppresses cell proliferation and metastasis in hepatocellular
carcinoma by inhibiting the GAB1-ERK axis. Oncotarget.
7:11595–11608. 2016.PubMed/NCBI
|