Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in men and the second in women, with ~1.4 million
cases and a mortality rate of 693,900 in 2012 (1). In patients with CRC, ~25% present with
metastases at the time of diagnosis (2), and ~50% of patients with CRC develop
liver metastases (3,4). Metastatic CRC is associated with a
particularly poor prognosis. Despite advances in treatment over
previous decades (5), the
5-year-survival rate for patients with metastatic CRC remains
<10% (6). Extensive
investigation of the underlying molecular networks of metastasis is
important for the development of effective targeted therapy for
patients with metastatic CRC.
Although the overarching focus of cancer research in
the last four decades has been on the malignant cancer cell, it has
become well established that stromal cells in the tumor
microenvironment are important in tumor progression (7,8). A
variety of stromal cells in the tumor microenvironment are
recruited to tumors, and crosstalk between cancer cells and stromal
cells is critical for tumor progression and the development of
metastases (9). Secreted proteins,
including cytokines, chemokines and growth factors, have been
considered to occupy the main role in this crosstalk. However,
tumor-derived exosomes, which contain various proteins and RNAs,
have also been shown to be involved in this crosstalk (10). In tumor microenvironments,
extracellular microRNAs (miRNAs), including miRNAs in exosomes,
have been suggested to influence tumor progression via
bidirectional tumor-to-stromal and stromal-to-tumor communication
(11). Therefore, miRNA expression
analysis of stromal cells is important for elucidation of the role
of miRNAs in cancer progression in the tumor microenvironment.
Although there have been a number of reports of comprehensive miRNA
expression analysis of stromal cells (12,13),
all have involved the analyses of the differences in miRNA
expression between cancer stromal cells and normal stromal cells.
In order to understand how stromal miRNAs are involved in cancer
metastasis, it is necessary to compare the miRNA profile of the
cancer stroma in cancer with metastasis to that of the cancer
stroma in cancer without metastasis. Comprehensive miRNA expression
analysis comparing cancer stroma in cancer with metastasis with
that in cancers without metastasis is lacking. The purpose of the
present study was to identify the miRNAs whose expression in the
CRC stroma is involved in metastatic ability.
Materials and methods
Patients and tissue samples
The specimens of 113 patients with primary CRC, who
had undergone surgical resection in Yamaguchi University Medical
Hospital (Yamaguchi, Japan) between January 2008 and December 2013,
were used in the present study. All patients had undergone
resection of the primary tumor, and among the 31 patients with
distant metastases, three patients had undergone hepatectomy. None
of the patients had received preoperative treatments, for example,
chemotherapy and/or radiation.
For screening in the miRNA array analysis, the
frozen specimens of primary CRC from 12 patients were used. For
RT-qPCR analysis, the formalin-fixed paraffin embedded tissue
(FFPE) specimens of 101 cases of primary CRC were used. Of these
101 CRC FFPE samples, 40 samples (20 CRCwLM and 20 CRCwoLM) were
used for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) validation of the array analysis (Fig. 1). Survival analysis was performed
for all 101 of the cases from which samples were used for RT-qPCR
analysis. All samples were obtained with informed consent from the
patients. The study protocol was approved by the Institutional
Review Board for the Use of Human Subjects at the Yamaguchi
University School of Medicine (H17-83).
Tissue preparation: Laser capture
microdissection and RNA extraction
For the miRNA array analysis, frozen cancer tissue
sections were immediately cut into 5-mm cubes and embedded in
Tissue-Tek OCT compound medium (Sakura Finetech, Co., Ltd., Tokyo,
Japan) following resection. The sections then were fixed in liquid
nitrogen and stored at −80°C. Frozen specimens were cut into
10-µm-thick slices using a cryostat, and these sections were
mounted onto foil-coated glass membrane slides (Leica Microsystems
GmbH, Wezlar, Germany). The tissue sections were stained with
toluidine blue prior to air drying. The FFPE specimens were cut
into 10-µm-thick sections using a microtome, and these sections
were mounted onto foil-coated glass membrane slides. The tissue
sections were fixed in 70% ethanol for 30 sec and stained with
hematoxylin and eosin prior to dehydration (5 min each in 70, 95
and 100% ethanol). The stained frozen or FFPE sections were
microdissected using an LMD7000 Laser Microdissection system (Leica
Microsystems GmbH). The cancer cells and cancer stromal tissues
were visualized under a bright-field microscope (magnification,
×100) and selectively separated by activation of the laser. From
each slide, 1×107−2×107 µm2
epithelial or stromal cells were captured.
RNA extraction and miRNA
microarray
Total RNA was extracted from dissected tissue using
the miRNeasy FFPE kit (Qiagen GmbH, Hilden, Germany) according to
the manufacturer's protocol. The extracted total RNA (250 ng) was
labeled with Cy5 using the 3D-Gene miRNA labeling kit (Toray
Industries, Inc., Kanagawa, Japan). The labeled RNAs were
hybridized onto 3D-Gene Human miRNA Oligo chips containing 2,555
miRNAs (Human_miRNA_Ver20; Toray Industries, Inc.). The annotation
and oligonucleotide sequences of the probes conformed to the
miRBase miRNA database (http://microrna.sanger.ac.uk/sequences/). Following
stringent washes (1st wash: 0.5X saline-sodium citrate (SSC)/0.1%
sodium dodecyl sulfate (SDS) solution; 2nd wash: 0.2X SSC/0.1%SDS
solution; 3rd wash: 0.05X SSC solution), fluorescent signals were
scanned with the 3D-Gene Scanner (Toray Industries, Inc.) and
analyzed using 3D-Gene Extraction software (Ver 2.0.0.7; Toray
Industries, Inc.). The raw data of each spot were normalized by
substitution with a mean intensity of the background signal
determined by all blank spots' signal intensities of 95% confidence
intervals. Measurements of spots with signal intensities differing
from the background signal intensity by greater than two standard
deviations (SDs) were considered to be valid. The relative
expression level of a given miRNA was calculated by comparing the
signal intensities of the valid spots throughout the microarray
experiments. The normalized data were globally normalized per
array, such that the median of the signal intensity was adjusted to
100.
RT-qPCR analysis
miR-221, miR-222, miR-659, miR-4470, miR-4669,
miR-5703 and RNU6B (internal control)-specific cDNA synthesis was
performed using 10 ng of total RNA and TaqMan miRNA primers
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). RT-qPCR analyses were performed using the following TaqMan
miRNA assays: miR-221-3p (ID000524), miR-222-3p (ID002276), miR-659
(ID001514), miR-4470 (ID464583_mat), miR-4669 (ID464125_mat),
miR5703 (ID472811_mat) and RNU6B (ID001093) with specific primers
(Applied Biosystems; Thermo Fisher Scientific, Inc.) as previously
described (14). In brief, cDNA was
synthesized from total RNA (10 ng) in a 7.5-µl reaction volume (RT
primer 1.5 µl, total RNA lysate 2.5 µl, RT mix 3.5 µl) using the
TaqMan® MicroRNA Reverse Transcription kit. The
reactions were performed at 16°C for 30 min and then at 42°C for 30
min, and were inactivated at 85°C for 5 min. RT-qPCR analysis was
performed using the LightCycler®480 System II (Roche
Diagnostics, Tokyo, Japan). The reactions were performed for 10 min
at 95°C, and then for 55 cycles with denaturation for 15 sec at
95°C and annealing/extension for 60 sec at 60°C. The relative
expression of miRNA to RNU6B RNA was calculated using the
2−ΔΔCq method (15). The
CRC samples were categorized into miR-221 and miR-222 weak and
strong expression groups according to the expression levels of
miR-221 and miR-222 as determined by RT-qPCR analysis, using RNU6B
as the control. High expression was defined as an expression level
above the median value of the 101 CRC samples for each miRNA.
miRNA in situ hybridization (ISH)
Of the 101 CRC FFPE samples, 20 samples, (CRCwLM,
n=12; CRCwoLM, n=8) were used for ISH analysis. The locked nucleic
acid (LNA)-ISH system was used to investigate the localization of
miR-221 and miR-222. LNA-ISH was performed according to the
manufacturer's protocol and relevant LNA-ISH literature (16). Briefly, the FFPE advanced colon
cancer tissues were cut into 5-µm-thick sections and
deparaffinized. Following treatment with proteinase K and
post-fixation with 4% paraformaldehyde (Wako Pure Chemical
Industries, Osaka, Japan), the slides were hybridized with the 5′
(digoxigenin-UTP) DIG-labeled miRCURY LNA™ detection probe,
miR-221, and miR-222 (18115-01 and 38499–01; Qiagen GmbH), for 8 h
at 52°C using the Ventana Discovery Ultra instrument (Ventana
Medical Systems, Inc. Tucson, AZ, USA). The digoxigenin was
detected with a polyclonal anti-DIG antibody and an alkaline
phosphatase-conjugated secondary antibody (Ventana Medical Systems,
Inc.), using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl
phosphate as the substrate. The LNA U6 snRNA probe was used as a
positive control for every specimen. The 5′ DIG-labeled miRCURY
LNA™ detection probe for human mature miR-221-3p
(5′-GAAAGCCAGCAGACAATGTAGCT-3′), miR-222-3p
(5′-ACCCAGTAGCCAGATGTAGCT-3′), U6-positive control, and scrambled
negative control were all purchased from Exiqon A/S (Vedbaek,
Denmark). Signals from the tumor cells and stromal cells were
classified as negative (−) or positive (+), respectively.
Statistical analysis
All calculations were performed using the SPSS
software package version 19.0 (SPSS, Inc., Chicago, IL, USA).
Differences between groups were estimated using the Mann-Whitney U
test. Categorical variables were compared using Fisher's exact
test. Patient outcome survival curves were calculated using the
Kaplan-Meier method. Factors shown to be of prognostic significance
in univariate models were evaluated in a multivariate Cox
proportional hazard model. To validate the association between
survival rates and expression of the candidate miRNAs, the
following approach was used to divide the patients into two groups
according to relative expression levels of candidate miRNAs. For
miRNAs, the median values of the expression data were used as the
cut-off. Survival was defined as the interval from the date of
diagnosis until the date of CRC-associated mortality, date of
mortality from other cause, or the end of follow-up (June 30,
2017), whichever came first. Patients lost to follow-up were
censored at the date of the final follow-up contact. All
statistical analyses assumed a two-sided alternative with a 5%
level of significance.
Results
miRNA array analysis of primary CRCwLM
or CRCwoLM
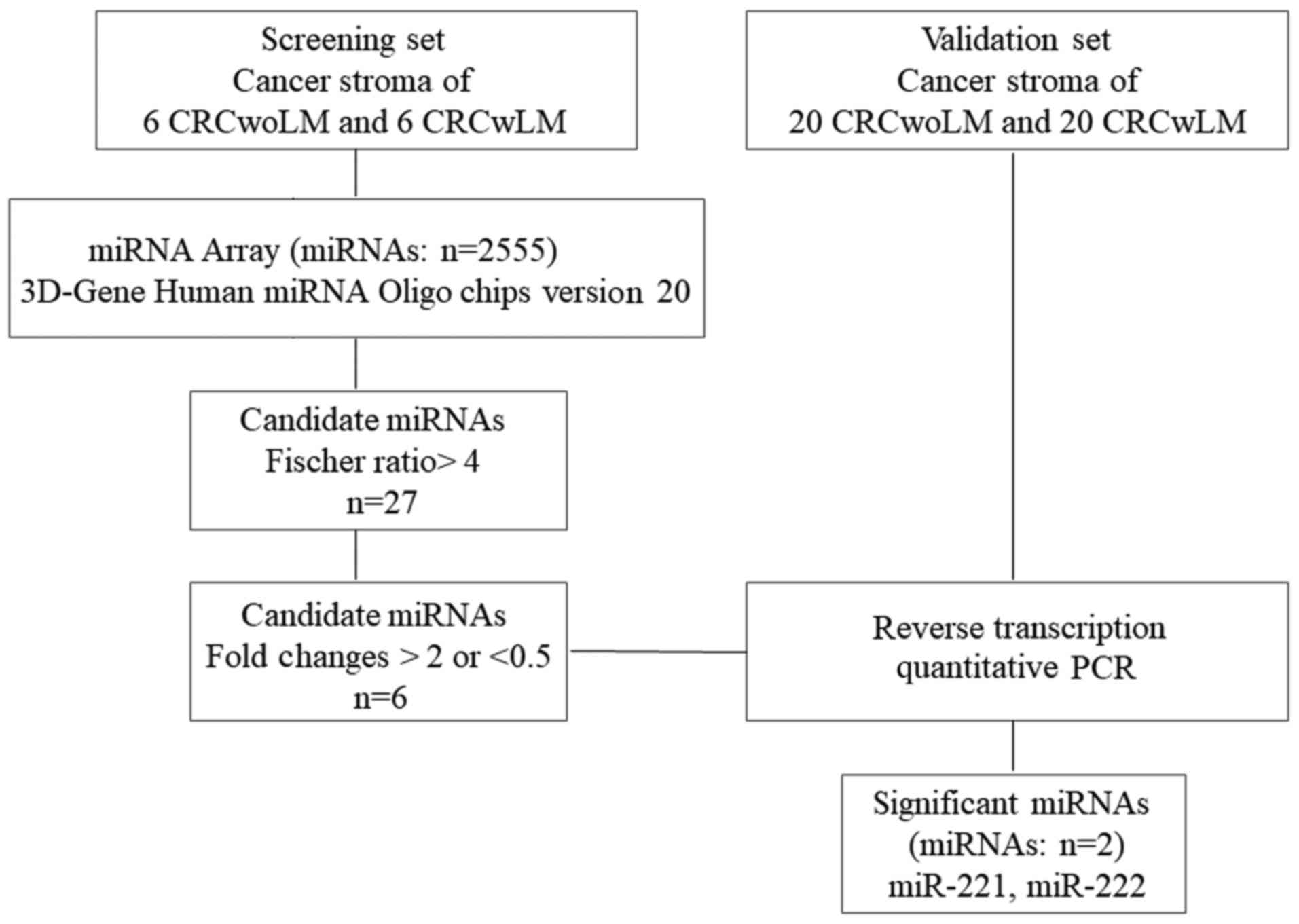
An overview of the experimental design and selection
of candidate miRNAs is shown in Fig.
1. To investigate the miRNAs associated with liver metastasis
in cancer stromal tissue, array-based miRNA profiling was
performed. The stromal tissue of six cases of primary CRCwoLM and
that of six cases of primary CRCwLM were profiled on the Toray
3D-Gene Human miRNA Oligo chips ver. 19. The clinicopathological
data of the patients are summarized in Table I. miRNAs with intensity levels
higher than the negative control +2 SD were selected in all cases.
This filtering resulted in the identification of 1,024 miRNAs among
a total of 2,555. The Fischer ratio was used to evaluate the
potential of the selected miRNAs to discriminate between CRCwLM and
CRCwoLM (17). The 1,024 miRNAs
were ranked in order of decreasing Fischer ratio. The 27 miRNAs
that were significantly dysregulated in cancer stromal tissues,
comparing between CRCwoLM and CRCwLM, are listed in Table II (Fischer ratio >4). For the
selection of candidate miRNAs among these 27 miRNAs, to permit
comparison between CRCwLM and CRCwoLM, a fold change of 2.0 or 0.5
was used as the cut-off. Using this cut-off led to the
identification of four miRNAs (miR-659, −4470, −4669 and −5703)
that were downregulated and two miRNAs (miR-221 and miR-222) that
were upregulated in CRCwLM compared with CRCwoLM.
 | Table I.Clinicopathological data for the 12
cases of CRC analyzed in the miRNA microarray. |
Table I.
Clinicopathological data for the 12
cases of CRC analyzed in the miRNA microarray.
|
| CRC without liver
metastasis | CRC with liver
metastasis |
|---|
|
|
|
|
|---|
| Features Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|
| Age (years) | 87 | 69 | 61 | 75 | 83 | 80 | 48 | 65 | 66 | 64 | 53 | 69 |
| Sex | F | F | M | F | M | M | F | M | F | M | M | F |
| Size (mm) | 90 | 75 | 92 | 70 | 50 | 40 | 30 | 60 | 40 | 120 | 80 | 28 |
| Depth | T3 | T3 | T4 | T4 | T2 | T4 | T3 | T3 | T3 | T3 | T4 | T4 |
| Histological
type | tu 2 | tu 2 | tu 2 | tu 2 | tu 1 | tu 2 | tu 2 | tu 2 | tu 2 | tu 2 | tu 2 | tu 2 |
| Lymph node
metastasis | (−) | (−) | (−) | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (−) |
| Liver
metastasis | (−) | (−) | (−) | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) |
| Stage | IIA | IIA | IIB | IIB | I | IIB | IV | IV | IV | IV | IV | IV |
 | Table II.Dysregulated miRNAs in cancer stromal
tissue on comparison of colorectal cancer with and without liver
metastasis. |
Table II.
Dysregulated miRNAs in cancer stromal
tissue on comparison of colorectal cancer with and without liver
metastasis.
| miRNA | Signal intensity in
cancer stroma without liver metastasis | Signal intensity in
cancer stroma with liver metastasis | Fold change | Regulation | Fischer ratio |
|---|
| hsa-miR-4698 | 84.3853 | 59.9454 | 0.7104 | Down | 8.6870 |
|
hsa-miR-6862-5p | 43.2356 | 23.2163 | 0.5370 | Down | 7.7798 |
| hsa-miR-491-5p | 621.6555 | 358.3432 | 0.5764 | Down | 7.2940 |
|
hsa-miR-7855-5p | 45.1489 | 23.2163 | 0.5142 | Down | 6.5106 |
| hsa-miR-602 | 79.6585 | 43.7653 | 0.5494 | Down | 6.0467 |
|
hsa-miR-4726-5p | 282.4948 | 203.2291 | 0.7194 | Down | 5.9314 |
|
hsa-miR-6845-5p | 690.9057 | 480.6378 | 0.6957 | Down | 5.6611 |
|
hsa-miR-371b-5p | 141.0927 | 84.1496 | 0.5964 | Down | 5.6109 |
|
hsa-miR-6790-3p | 209.2740 | 140.5168 | 0.6714 | Down | 5.3770 |
| hsa-miR-4533 | 59.9515 | 32.3644 | 0.5398 | Down | 5.2842 |
| hsa-miR-663a | 3,729.6112 | 2,507.9348 | 0.6724 | Down | 5.2337 |
|
hsa-miR-1237-5p | 9,092.0858 | 6,981.4697 | 0.7679 | Down | 5.0164 |
|
hsa-miR-6742-5p | 91.7006 | 53.0911 | 0.5790 | Down | 5.0012 |
| hsa-miR-4470 | 71.7076 | 33.4636 | 0.4667 | Down | 4.9666 |
|
hsa-miR-4750-3p | 182.9980 | 149.9362 | 0.8193 | Down | 4.9492 |
| hsa-miR-665 | 622.2152 | 380.7198 | 0.6119 | Down | 4.9448 |
| hsa-miR-221-3p | 172.4139 | 384.7967 | 2.2318 | Up | 4.8359 |
|
hsa-miR-1238-5p | 326.3151 | 238.1282 | 0.7297 | Down | 4.7928 |
| hsa-miR-659-3p | 109.3211 | 51.4299 | 0.4704 | Down | 4.7122 |
| hsa-miR-4669 | 123.4231 | 58.7635 | 0.4761 | Down | 4.5689 |
| hsa-miR-4513 | 333.8607 | 248.4870 | 0.7443 | Down | 4.5341 |
|
hsa-miR-3180-3p | 657.2006 | 471.0584 | 0.7168 | Down | 4.5194 |
| hsa-miR-3185 | 444.7393 | 294.6611 | 0.6625 | Down | 4.5179 |
| hsa-miR-222-3p | 86.9045 | 193.2362 | 2.2235 | Up | 4.4753 |
| hsa-miR-4257 | 1,156.5877 | 1,437.9049 | 1.2432 | Up | 4.3621 |
|
hsa-miR-5196-5p | 154.3040 | 111.5328 | 0.7228 | Down | 4.3175 |
| hsa-miR-5703 | 68.1390 | 33.4572 | 0.4910 | Down | 4.2515 |
Validation of the miRNA array
profiling by RT-qPCR analysis
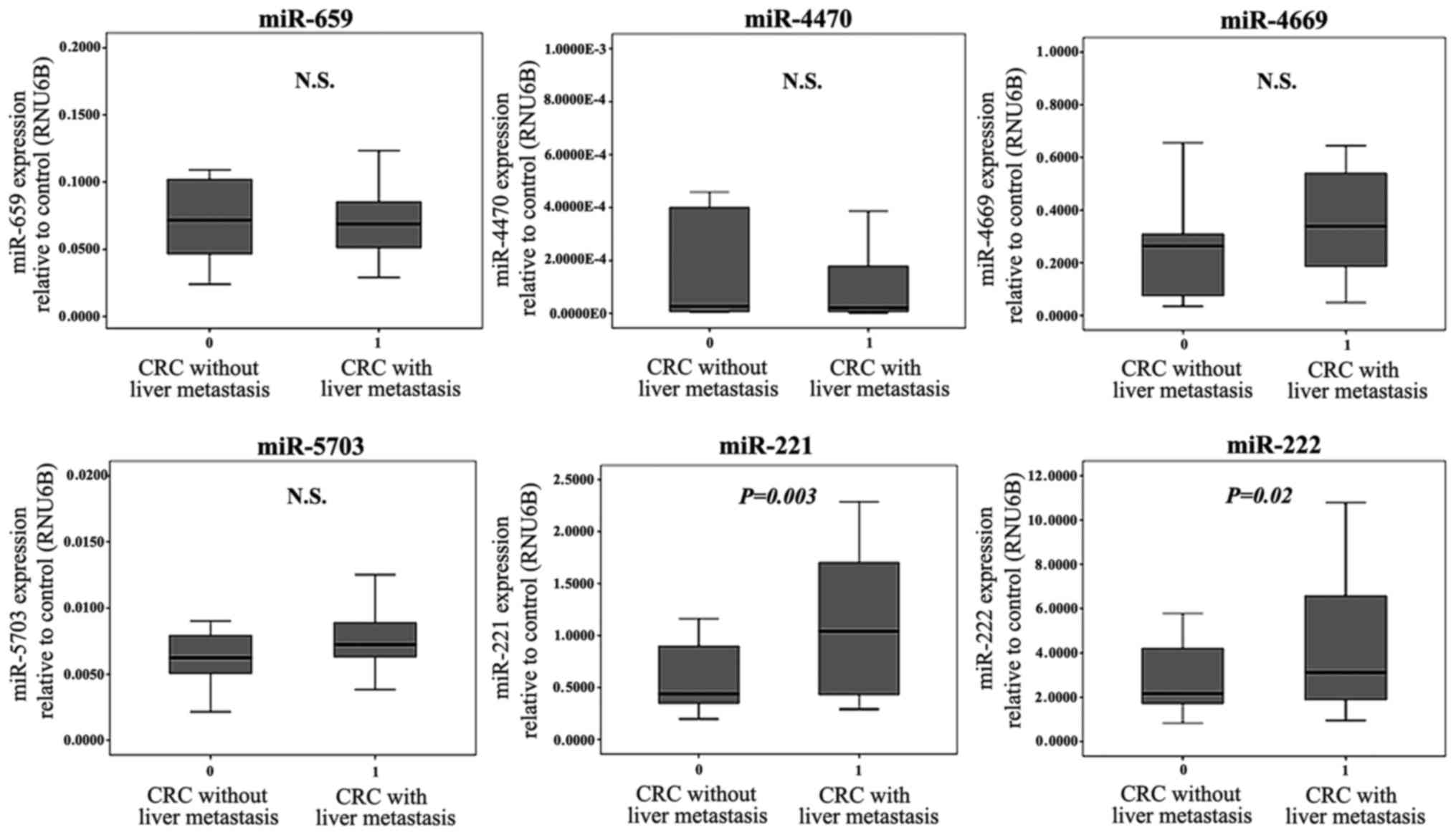
To confirm the microarray findings, RT-qPCR analysis
was used to measure the expression level of these six miRNAs,
described as above, in the stromal tissues of 20 cases CRCwLM and
20 cases of CRCwoLM. The expression levels of miR-221 and miR-222
in the cancer stromal tissues were significantly higher in CRCwLM
than in CRCwoLM (P=0.003 and P=0.02, respectively), whereas no
significant difference was found in the expression of the four
downregulated miRNAs (miR-659, −4470, −4669 and −5703) between
CRCwLM and CRCwoLM (Fig. 2).
Association of the expression of
miR-221 and miR-222 in cancer stromal tissues with
clinicopathological factors
To evaluate whether the expression of miR-221 or
miR-222 was associated with clinicopathological factors and
prognosis, RT-qPCR analysis was used to analyze the expression of
miR-221 and miR-222 in 101 CRC FFPE sample sets of cancer stromal
tissues and corresponding cancer cells; RNU6B was used as the
control. The clinicopathological analysis showed that the group
with a high expression of miR-221 in cancer stromal tissues, that
is, cases with specimens in which the expression of miR-221 was
above the median value, had more advanced venous invasion
(P=0.023), liver metastasis (P=0.028), and distant metastasis
(P=0.003) than the low-expression group (Table III). The group with a high
expression of miR-222 in cancer stromal tissues had more advanced
depth of tumor invasion (P=0.045), liver metastasis (P=0.009), and
distant metastasis (P=0.003) than the low-expression group
(Table IV). By contrast, unlike in
the stromal tissue, the clinicopathological factors and the
expression of miR-221 and miR-222 in cancer cells only showed a
tendency to be associated with distant metastasis (Tables III and IV).
 | Table III.Expression of miR-221 in the cancer
stroma and its association with clinicopathological factors. |
Table III.
Expression of miR-221 in the cancer
stroma and its association with clinicopathological factors.
|
| miR221 expression
in stroma |
| miR221 expression
in cancer cells |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
factor | High (n=50) | Low (n=51) | P-value | High (n=50) | Low (n=51) | P-value |
|---|
| Age (years) | 68.3±11.7 | 67.8±11.6 | P=0.851 | 67.4±12.2 | 69.0±11.0 | P=0.394 |
| Sex |
|
| P=0.356 |
|
| P=0.767 |
|
Male | 23 | 26 |
| 25 | 27 |
|
|
Female | 27 | 25 |
| 25 | 24 |
|
| Degree of
differentiation |
|
| P=0.358 |
|
| P=0.383 |
|
Well/moderate | 44 | 47 |
| 46 | 45 |
|
|
Poor | 6 | 4 |
| 4 | 6 |
|
| Tumor size
(mm) |
|
| P=0.187 |
|
| P=0.378 |
|
<50 | 25 | 31 |
| 29 | 27 |
|
|
≥50 | 25 | 20 |
| 21 | 24 |
|
| Depth |
|
| P=0.098 |
|
| P=0.098 |
|
T2/T3 | 25 | 33 |
| 25 | 33 |
|
| T4 | 25 | 18 |
| 25 | 18 |
|
| Lymph node
metastasis |
|
| P=0.227 |
|
| P=0.227 |
|
Absent | 16 | 21 |
| 16 | 21 |
|
|
Present | 34 | 30 |
| 34 | 30 |
|
| Lymphatic
invasion |
|
| P=0.346 |
|
| P=0.369 |
|
Absent | 5 | 3 |
| 3 | 5 |
|
|
Present | 45 | 48 |
| 47 | 46 |
|
| Venous
invasion |
|
| P=0.023 |
|
| P=0.062 |
|
Absent | 8 | 18 |
| 9 | 17 |
|
|
Present | 42 | 33 |
| 41 | 34 |
|
| Liver
metastasis |
|
| P=0.028 |
|
| P=0.164 |
|
Absent | 33 | 43 |
| 35 | 41 |
|
|
Present | 17 | 8 |
| 15 | 10 |
|
| Distant
metastasis |
|
| P=0.003 |
|
| P=0.083 |
|
Absent | 29 | 43 |
| 32 | 40 |
|
|
Present | 21 | 8 |
| 18 | 11 |
|
| Stage |
|
| P=0.163 |
|
| P=0.288 |
|
I/II | 14 | 20 |
| 15 | 19 |
|
|
III/IV | 36 | 31 |
| 35 | 32 |
|
| Recurrence within 3
years | High (n=30) | Low (n=42) | P=0.087 | High (n=33) | Low (n=39) | P=0.563 |
|
Absent | 20 | 35 |
| 25 | 30 |
|
|
Present | 10 | 7 |
| 8 | 9 |
|
 | Table IV.Expression level of miR-222 in the
cancer stroma and its association with clinicopathological
factors. |
Table IV.
Expression level of miR-222 in the
cancer stroma and its association with clinicopathological
factors.
|
| miR222 expression
in stroma |
| miR222 expression
in cancer cells |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
factor | High (n=50) | Low (n=51) | P-value | High (n=50) | Low (n=51) | P-value |
|---|
| Age (years) | 68.7±11.0 | 67.4±12.2 | P=0.562 | 68.8±10.4 | 67.3±12.7 | P=0.494 |
|
Sex |
|
| P=0.617 |
|
| P=0.207 |
|
Male | 18 | 31 |
| 22 | 27 |
|
|
Female | 32 | 20 |
| 28 | 24 |
|
| Degree of
differentiation |
|
| P=0.383 |
|
| P=0.358 |
|
Well/moderate | 46 | 45 |
| 44 | 47 |
|
|
Poor | 4 | 6 |
| 6 | 4 |
|
| Tumor size
(mm) |
|
| P=0.073 |
|
| P=0.312 |
|
<50 | 23 | 33 |
| 26 | 30 |
|
|
≥50 | 27 | 18 |
| 24 | 21 |
|
| Depth |
|
| P=0.045 |
|
| P=0.187 |
|
T2/T3 | 24 | 34 |
| 26 | 32 |
|
| T4 | 26 | 17 |
| 24 | 19 |
|
| Lymph node
metastasis |
|
| P=0.184 |
|
| P=0.227 |
|
Absent | 21 | 16 |
| 16 | 21 |
|
|
Present | 29 | 35 |
| 34 | 30 |
|
| Lymphatic
invasion |
|
| P=0.141 |
|
| P=0.631 |
|
Absent | 2 | 6 |
| 4 | 4 |
|
|
Present | 48 | 45 |
| 46 | 47 |
|
| Venous
invasion |
|
| P=0.267 |
|
| P=0.433 |
|
Absent | 15 | 11 |
| 12 | 14 |
|
|
Present | 36 | 39 |
| 38 | 37 |
|
| Liver
metastasis |
|
| P=0.009 |
|
| P=0.164 |
|
Absent | 32 | 44 |
| 35 | 41 |
|
|
Present | 18 | 7 |
| 15 | 10 |
|
| Distant
metastasis |
|
| P=0.003 |
|
| P=0.083 |
|
Absent | 29 | 43 |
| 32 | 40 |
|
|
Present | 21 | 8 |
| 18 | 11 |
|
| Stage |
|
| P=0.241 |
|
| P=0.288 |
|
I/II | 19 | 15 |
| 15 | 19 |
|
|
III/IV | 31 | 36 |
| 35 | 32 |
|
| Recurrence within 3
years | High (n=30) | Low (n=42) | P=0.404 | High (n=33) | Low (n=39) | P=0.237 |
|
Absent | 22 | 33 |
| 27 | 28 |
|
|
Present | 8 | 9 |
| 6 | 11 |
|
Association of the expression of
miR-221 and miR-222 and survival rates
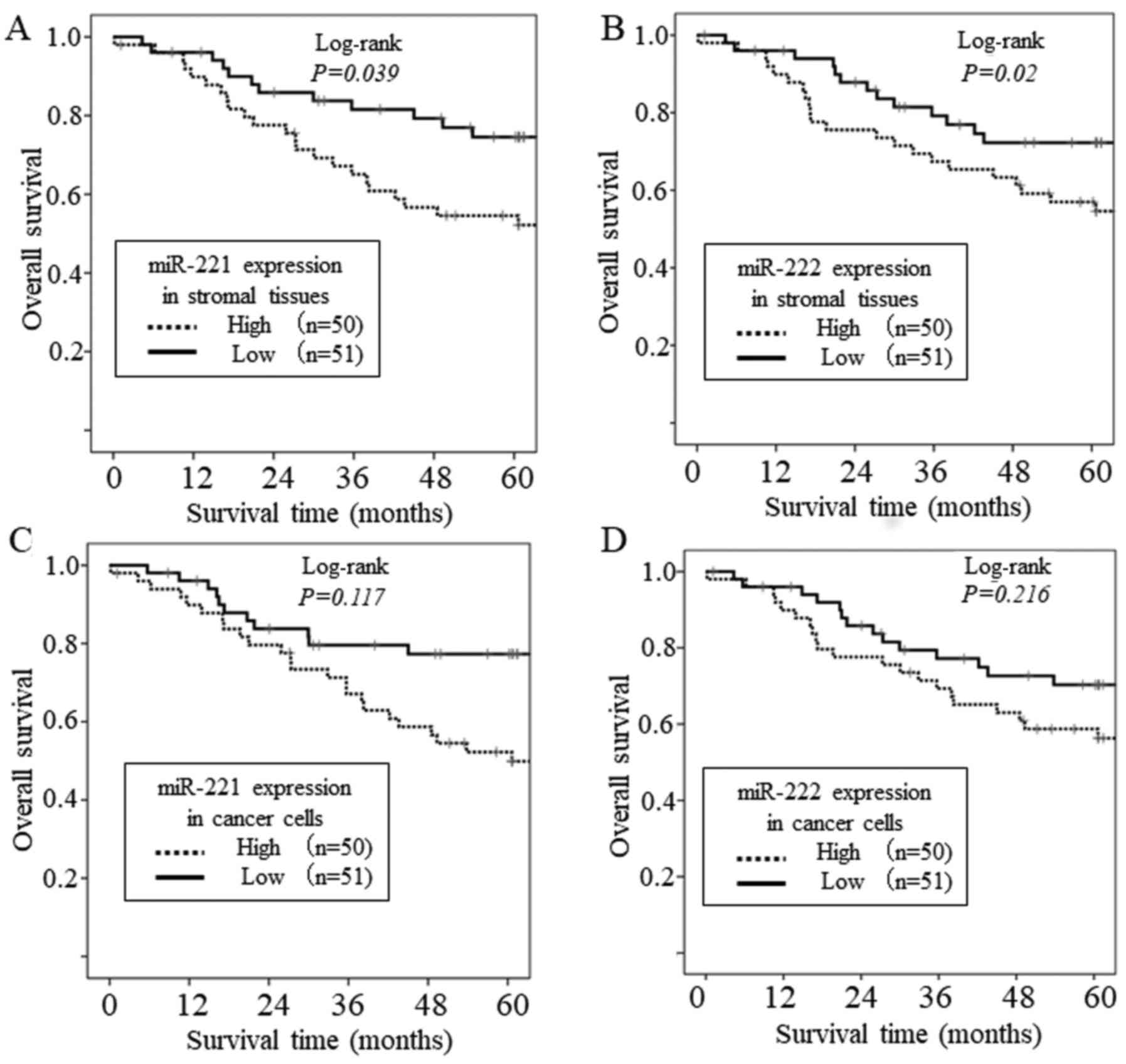
The overall survival rate of the patients according
to the miR-221 and miR-222 expression status of the cancer stroma
are shown in Fig. 3A and B. The
survival analysis showed that the group with a high expression of
miR-221 in cancer stromal tissues had significantly shorter overall
survival rates than the group with a low expression (P=0.039).
Similarly, the group with a high expression of miR-222 in cancer
stromal tissues had significantly shorter overall survival rate
than the group with a low expression (P=0.02). However, there was
no association between the overall survival rate and the expression
level of miR-221 or miR-222 in cancer cells (Fig. 3C and D). In addition, a Cox
proportional hazard regression model was used to determine whether
the expression of miR-221 and miR-222 in the cancer stroma was an
independent risk factor for overall survival rate (Table V). The univariate analysis revealed
that high levels of stromal miR-221 (P=0.038), high levels of
stromal miR-222 (P=0.024), higher pathological T stage (T4;
P=0.001), and lymph node metastasis (P=0.007) were significantly
associated with poor overall survival rate. The subsequent
multivariate analysis confirmed these results and showed that the
stromal expression of miR-222 [hazard ratio (HR)=2.280, 95%
confidence interval (CI)=1.097–4.742, P=0.027), higher pathological
T stage (T4; HR=2.078, 95% CI, 1.043–4.142, P=0.038), and lymph
node metastasis (HR=3.679, 95% CI, 1.497–9.043, P=0.005) were
independent markers for poor overall survival rates in patients
with CRC (Table V).
 | Table V.Univariate and multivariate Cox
proportional hazard models of overall survival rates. |
Table V.
Univariate and multivariate Cox
proportional hazard models of overall survival rates.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| miR-221
(stroma) |
| Low,
vs. high | 2.021 | P=0.038 | 1.023–3.991 |
|
|
|
| miR-222
(stroma) |
| Low,
vs. high | 2.222 | P=0.024 | 1.110–4.444 | 2.280 | P=0.027 | 1.097–4.742 |
| miR-221 (cancer
cell) |
| Low,
vs. high | 1.699 | P=0.121 | 0.869–3.322 |
|
|
|
| miR-222 (cancer
cell) |
| Low,
vs. high | 1.506 | P=0.22 | 0.780–2.938 |
|
|
|
| Age (years) |
| <60,
vs. ≥60 | 1.406 | P=0.395 | 0.641–3.086 |
|
|
|
| Sex |
| Male,
vs. female | 1.644 | P=0.142 | 0.847–3.192 |
|
|
|
| Tumor size
(mm) |
| <50,
vs. ≥50 | 1.036 | P=0.916 | 0.537–1.999 |
|
|
|
| Depth |
| T2/T3,
vs. T4 | 2.977 | P=0.001 | 1.543–5.746 | 2.078 | P=0.038 | 1.043–4.142 |
| Lymph node
metastasis |
| Absent,
vs. present | 3.369 | P=0.007 | 1.400–8.106 | 3.679 | P=0.005 | 1.497–9.043 |
Localization of miR-221 and miR-222 in
colon cancer
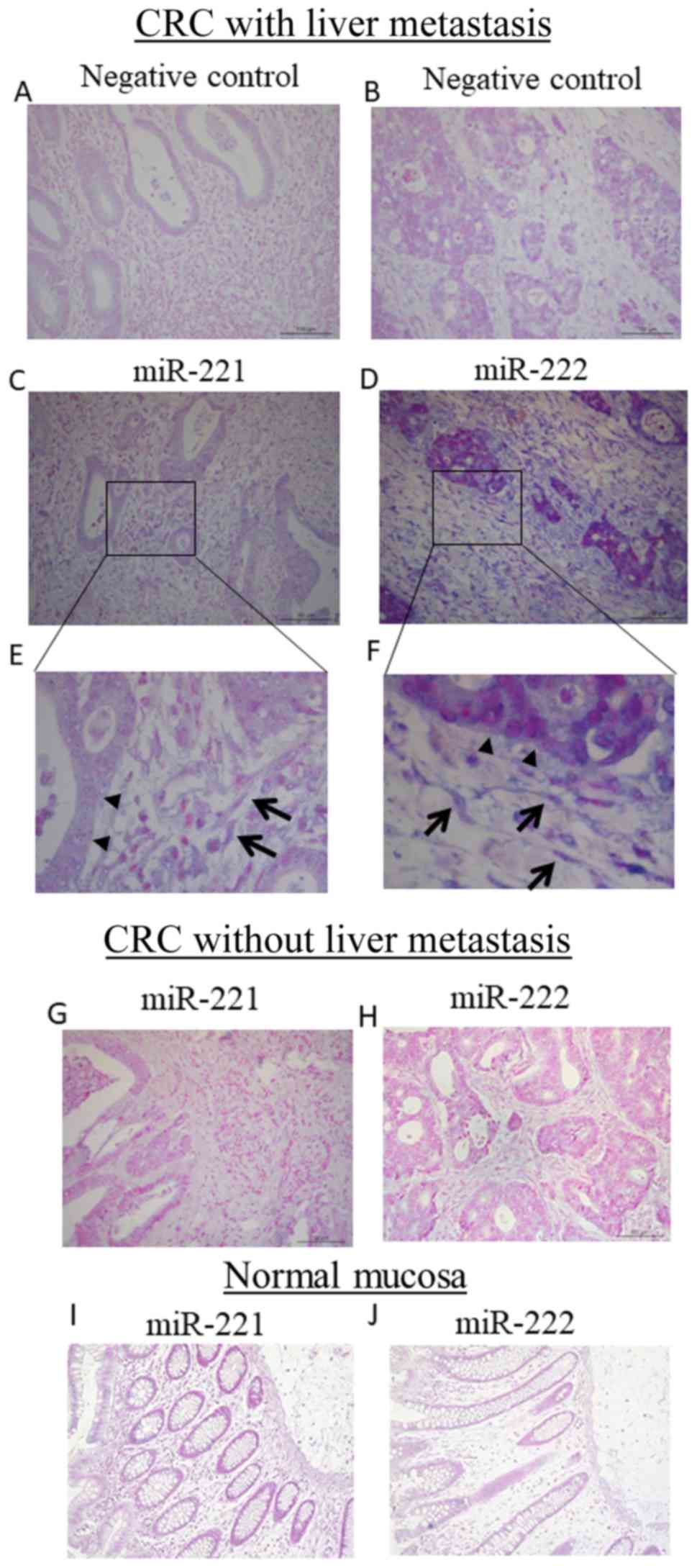
To investigate the localization of miR-221 and
miR-222 in cancer tissue, ISH was performed. The negative control
groups are shown in Fig. 4A and B.
In CRC with liver metastasis, miR-221 and miR-222 were localized in
the cytoplasm of fibroblasts in stromal tissue and cancer cells
(Fig. 4C-F). By contrast, in
primary CRC without liver metastasis, there was low expression of
miR-221 and miR-222 in cancer cells and stromal cells (Fig. 4G and H). In terms of fibroblasts.
9/12 (75%) CRCwLM and 2/8 (25%) CRCwoLM cases (P=0.04) had
miR-222-positive fibroblasts. By contrast, 7/12 (58%) CRCwLM and
3/8 (37.5%) CRCwoLM cases (P=0.36) had miR-221-positive
fibroblasts. The miR-221 or miR-222 signal was observed in <10%
of non-cancerous tissues (Fig. 4I and
J).
Discussion
The dysregulation of miRNA expression is frequently
detected in CRC and has been associated with increased metastatic
potential and poor clinical outcome, suggesting the importance of
miRNAs in cancer progression (18,19).
In the present study, it was shown that the
overexpression of miR-221 and miR-222 in the cancer stromal tissue
of primary tumors was associated with malignant potential in
patients with CRC. To date, several studies have profiled the
expression of miRNAs involved in cancer metastasis in CRC, however,
those studies were performed primarily on cancer cells or cancer
tissue (20,21). In the present study, the stromal
miRNA expression profile of CRCwLM was compared with that of
CRCwoLM using microdissection. Using miRNA array analysis, six
miRNAs were identified that were differentially expressed between
the two patient groups. Following reassessment of these six miRNAs
using RT-qPCR analysis, differential expression between CRCwLM and
CRCwoLM was confirmed for miR-221 and miR-222. To the best of our
knowledge, this is the first comprehensive miRNA array analysis
performed to identify miRNAs in the CRC stroma whose expression is
associated with metastatic ability. A small number of previous
studies have reported differential miRNA expression profiles
between the cancer stroma and normal stroma. Nishida et al
reported oncogenic miRNAs, including miR-21, miR-221, the
miR-17-92a cluster, and the miR-106b-25 cluster, which were
upregulated in the cancer stroma compared with the normal stroma.
In addition, the upregulation of miR-25 and miR-92a in stromal
tissues has been associated with a variety of clinicopathological
factors (12). Bullock et al
observed distinct patterns of stromal miRNA expression in CRC and
paired normal colonic tissue, and reported that stromal levels of
miR-21 and miR-556 predicted short disease-free survival and
overall survival rates in stage II disease (13). Furthermore, Bullock et al
reported the stromal upregulation of miR-214 and downregulation of
miR-192, mir-194, miR-200a, and miR-215 in Dukes C CRC compared
with Dukes A CRC among 95 miRNAs analyzed using RT-qPCR analysis.
However, the results from this particular study were limited as the
study only compared five Dukes C samples with five Dukes A samples
using RT-qPCR analysis. This low number of samples analyzed may
have been insufficient to detect novel stromal miRNAs that are
associated with the malignant potential of CRC.
miR-221 and miR-222 are homologous miRNAs, which are
encoded on the X chromosome as part of a locus designated the
miR-221-222 cluster (22). This
cluster has been found to be overexpressed in various types of
human cancer, including CRC (23).
In previous years, there have been reports on the association
between the metastatic potential of CRC and the expression of
miR-221 or mir-222. However, previous studies have analyzed the
expression of miR-221 or mir-222 in cancer cells or cancer tissues,
or in the blood. To the best of our knowledge, previous studies
have not reported that the expression of miR-221 or mir-222 in the
cancer stroma was associated with the development of CRC. It has
been shown that miR-221 and miR-222 promote oncogenesis by
downregulating the expression of tumor suppressors, including
phosphatase and tensin homolog, reversion-inducing-cysteine-rich
protein with kazal motifs, p53 upregulated modulator of apoptosis,
and p27 (24–28). Qin and Lui reported that the
overexpression of miR-221 enhances CRC cell migration and invasion
in vitro and metastasis in vivo (26). Furthermore, miR-221 and miR-222 have
the same seed sequence. The fact that two oncogenic miRNAs with the
same seed sequence were identified in a comprehensive analysis is
of interest, as these miRNAs share the same targets and may work
coordinately.
To evaluate the clinicopathologic relevance of the
identified miRNAs, the present study analyzed the expression of
miR-221and miR-222 in 101 advanced CRC samples. A high expression
level of miR-221 or miR-222 in patients with CRC was significantly
associated with liver metastasis, distant metastasis, and shorter
overall survival rate. Furthermore, it was found that the
association between the malignant potential of CRC and the level of
miR-221 or miR-222 in the cancer stroma was more marked than that
in the cancer cells. Using ISH for miRNA measurement, Uozaki et
al reported that the level of miR-21 in cancer cells was not
associated with clinicopathological factors, however, the stromal
level of miR-21 was associated with several factors in gastric
cancer, including tumor stage, size, and nodal metastasis (29). These results are similar to the
results of the present study, and the combination of these results
suggests that the overexpression of certain oncogenic miRNAs,
including miR-21, miR-221 and miR-222, in the cancer stroma may be
important for cancer development.
To detect the localization of miR-221 and miR-222 in
CRC, ISH was performed in 20 CRC samples. In this analysis, miR-221
and miR-222 were upregulated in the cancer cells and stromal cells,
particularly in fibroblasts, in metastatic CRC. Furthermore, the
expression of miR-221 and miR-222 in fibroblasts tended to be
higher in fibroblasts surrounding cancer cells strongly positive
for miR-221 and miR-222. A report by Kosaka et al suggested
that, in tumor microenvironments, extracellular miRNAs may
influence tumor progression via bidirectional tumor-to-stroma and
stroma-to-tumor communication (11). These results suggest that the
crosstalk of miR-221 and miR-222 between cancer cells and stromal
cells occurs in the cancer microenvironment. The precise mechanisms
underlying the upregulation of miR-221 and miR-222 in stromal cells
with metastatic CRC remain to be elucidated. One possible mechanism
is that the crosstalk of miR-221 and miR-222 between cancer cells
and stromal cells is involved in cancer progression. A previous
comprehensive array analysis showed that miR-221 is upregulated in
cancer-associated fibroblasts (CAFs) compared with that in normal
fibroblasts (NFs) in breast cancer (30). Furthermore, Shimoda et al
reported that the knockdown of issue inhibitor of metalloproteinase
(TIMP) family genes resulted in acquisition of the properties of
CAFs by NFs (31). TIMP2 and TIMP3,
which belong to the TIMP family, have been reported as being target
genes of miR-221 and miR-222 (32,33).
These results indicate that miR-221 and miR-222 from tumor-derived
exosomes mad modify the cellular phenotype of fibroblasts to that
of CAFs. This change in phenotype can promote the invasion and
metastatic potential of the tumor cells.
A limitation of the present study is that no in
vivo transfection was performed, therefore, it was not possible
to assess whether overexpression of miR-221 or miR-222 in the
cancer stroma facilitates metastasis in vivo. In order to
confirm this detailed mechanism, it is necessary to transfect
miR-221 and/or miR-222 into the cancer stroma rather than into
cancer cells. However, it is difficult to selectively transfect the
cancer stroma only with specific miRNAs, therefore, no such
experiments were performed in the present study. Further functional
studies are required in order to clarify the role of stromal
miR-221/222 expression.
Acknowledgements
The authors would like to thank Mr. Yasutake Yano
and Mr. Naoya Touyama from the Yamaguchi University School of
Medicine for their technical support.
Funding
The present study was performed as a research
program of the Project for Development of Innovative Research on
Cancer Therapeutics (P-DIRECT; grant no. 11039020). This study was
funded by the Japan Agency for Medical Research and Development
(AMED; grant no. 15cm0106085h0005), and was supported in part by a
grant for Leading Advanced Projects for Medical Innovation (LEAP;
grant no. 16am0001006h0003) from AMED.
Availability of data and materials
All data generated or analyzed during the present
study are included within.
Authors' contributions
MI, SH, and HN designed the study; MI, HTakenouchi,
SK, YuT, STo, YoT and SYo collected the data; MI, SH, RT, HTanaka,
HTakenouchi, KF, and MK performed the experiments. MI, SH, KS, NS,
STa, TU, and SYa performed the statistical analysis; MI, SH, and HN
wrote the manuscript. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient at the time of enrollment. The study was performed in
accordance with the Declaration of Helsinki on experiments
involving human subjects. The study protocol was approved by the
Institutional Ethics Review Boards of Yamaguchi University.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors state that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CRCwLM
|
colorectal cancer with liver
metastasis
|
|
CRCwoLM
|
colorectal cancer without liver
metastasis
|
|
miRNA
|
microRNA
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
SSC
|
saline-sodium citrate
|
|
SDS
|
sodium dodecyl sulfate
|
|
SD
|
standard deviation
|
|
ISH
|
in situ hybridization
|
|
LNA-ISH
|
locked nucleic acid-in situ
hybridization
|
|
DIG
|
digoxigenin-UTP
|
|
CAF
|
cancer associated fibroblast
|
|
NF
|
normal fibroblast
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rees M, Tekkis PP, Welsh FK, O'Rourke T
and John TG: Evaluation of long-term survival after hepatic
resection for metastatic colorectal cancer: A multifactorial model
of 929 patients. Ann Surg. 247:125–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leporrier J, Maurel J, Chiche L, Bara S,
Segol P and Launoy G: A population-based study of the incidence,
management and prognosis of hepatic metastases from colorectal
cancer. Br J Surg. 93:465–474. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colvin H, Mizushima T, Eguchi H, Takiguchi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterol Surg. 1:5–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giessen C, Laubender RP, Ankerst DP,
Stintzing S, Modest DP, Mansmann U and Heinemann V:
Progression-free survival as a surrogate endpoint for median
overall survival in metastatic colorectal cancer: Literature-based
analysis from 50 randomized first-line trials. Clin Cancer Res.
19:225–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Penfornis P, Vallabhaneni KC, Whitt J and
Pochampally R: Extracellular vesicles as carriers of microRNA,
proteins and lipids in tumor microenvironment. Int J Cancer.
138:14–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valadi H, Ekstrom K, Bossios A, Sjostrand
M, Lee JJ and Lotvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosaka N, Iguchi H, Hagiwara K, Yoshioka
Y, Takeshita F and Ochiya T: Neutral sphingomyelinase 2
(nSMase2)-dependent exosomal transfer of angiogenic microRNAs
regulate cancer cell metastasis. J Biol Chem. 288:10849–10859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishida N, Nagahara M, Sato T, Mimori K,
Sudo T, Tanaka F, Shibata K, Ishii H, Sugihara K, Doki Y, et al:
Microarray analysis of colorectal cancer stromal tissue reveals
upregulation of two oncogenic miRNA clusters. Clin Cancer Res.
18:3054–3070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bullock MD, Pickard K, Mitter R, Sayan AE,
Primrose JN, Ivan C, Calin GA, Thomas GJ, Packham GK and Mirnezami
AH: Stratifying risk of recurrence in stage II colorectal cancer
using deregulated stromal and epithelial microRNAs. Oncotarget.
6:7262–7279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kijima T, Hazama S, Tsunedomi R, Tanaka H,
Takenouchi H, Kanekiyo S, Inoue Y, Nakashima M, Iida M, Sakamoto K,
et al: MicroRNA-6826 and −6875 in plasma are valuable noninvasive
biomarkers that predict the efficacy of vaccine treatment against
metastatic colorectal cancer. Oncol Rep. 37:23–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods (San Diego, Calif).
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Sun Y, Wu H, Yu S, Zhang L, Meng
Y, Liu M, Yang H, Liu P, Mao X, et al: Elevated miR-483-3p
expression is an early event and indicates poor prognosis in
pancreatic ductal adenocarcinoma. Tumour Biol. 36:9447–9456. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iizuka N, Oka M, Yamada-Okabe H, Nishida
M, Maeda Y, Mori N, Takao T, Tamesa T, Tangoku A, Tabuchi H, et al:
Oligonucleotide microarray for prediction of early intrahepatic
recurrence of hepatocellular carcinoma after curative resection.
Lancet. 361:923–929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M and Chen H: The role of microRNAs in
colorectal cancer. J Genet Genomics. 37:347–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vicinus B, Rubie C, Stegmaier N, Frick VO,
Kölsch K, Kauffels A, Ghadjar P, Wagner M and Glanemann M: miR-21
and its target gene CCL20 are both highly overexpressed in the
microenvironment of colorectal tumors: Significance of their
regulation. Oncol Rep. 30:1285–1292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ling H, Pickard K, Ivan C, Isella C, Ikuo
M, Mitter R, Spizzo R, Bullock M, Braicu C, Pileczki V, et al: The
clinical and biological significance of MIR-224 expression in
colorectal cancer metastasis. Gut. 65:977–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Zhang M, Huang Y, Feng L, Chen H,
Hu Y, Chen H, Zhang K, Zheng L and Zheng S: MicroRNA-320b promotes
colorectal cancer proliferation and invasion by competing with its
homologous microRNA-320a. Cancer Lett. 356:669–675. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YK, Yu J, Han TS, Park SY, Namkoong B,
Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK and Kim VN: Functional links
between clustered microRNAs: Suppression of cell-cycle inhibitors
by microRNA clusters in gastric cancer. Nucleic Acids Res.
37:1672–1681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuzaki J and Suzuki H: Role of
MicroRNAs-221/222 in digestive systems. J Clin Med. 4:1566–1577.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F,
Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z and Chun-Sheng
K: MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell
proliferation and radioresistance by targeting PTEN. BMC Cancer.
10:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka R, Tomosugi M, Horinaka M, Sowa Y
and Sakai T: Metformin Causes G1-phase arrest via down-regulation
of MiR-221 and enhances TRAIL sensitivity through DR5 Up-regulation
in pancreatic cancer cells. PLoS One. 10:e01257792015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin J and Luo M: MicroRNA-221 promotes
colorectal cancer cell invasion and metastasis by targeting RECK.
FEBS Lett. 588:99–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Zhang J, Zhang A, Wang Y, Han L,
You Y, Pu P and Kang C: PUMA is a novel target of miR-221/222 in
human epithelial cancers. Int J Oncol. 37:1621–1626.
2010.PubMed/NCBI
|
|
28
|
Zhao JJ, Chu ZB, Hu Y, Lin J, Wang Z,
Jiang M, Chen M, Wang X, Kang Y, Zhou Y, et al: Targeting the
miR-221-222/PUMA/BAK/BAX pathway abrogates dexamethasone resistance
in multiple Myeloma. Cancer Res. 75:4384–4397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uozaki H, Morita S, Kumagai A, Aso T,
Soejima Y, Takahashi Y and Fukusato T: Stromal miR-21 is more
important than miR-21 of tumour cells for the progression of
gastric cancer. Histopathology. 65:775–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Sun Y, Hou Y, Peng Q, Wang L, Luo
H, Tang X, Zeng Z and Liu M: MiRNA expression analysis of
cancer-associated fibroblasts and normal fibroblasts in breast
cancer. Int J Biochem Cell Biol. 44:2051–2059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimoda M, Principe S, Jackson HW, Luga V,
Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas
C, et al: Loss of the Timp gene family is sufficient for the
acquisition of the CAF-like cell state. Nat Cell Biol. 16:889–901.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garofalo M, Di Leva G, Romano G, Nuovo G,
Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P,
et al: miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Q, Li P, Chen X, Zong L, Jiang Z, Nan
L, Lei J, Duan W, Zhang D, Li X, et al: miR-221/222 induces
pancreatic cancer progression through the regulation of matrix
metalloproteinases. Oncotarget. 6:14153–14164. 2015.PubMed/NCBI
|