Introduction
Increasing evidence indicates that interactions
between environmental factors and genetic predisposition play a
critical role in cancer development and progression (1–4). In
particular, polycyclic aromatic hydrocarbon (PAH) compounds are a
group of common environmental pollutants with carcinogenic effects
(5–7). 7,12-dimethylbenz[a]anthracene (DMBA),
a well-studied carcinogen, is a prototypical member of the PAH
family that has been extensively used to induce the development of
mammary and other tumor types in preclinical animal models
(8–10). Although PAHs have been studied in
multiple cancer models, the general understanding of the specific
genetic factors that contribute to PAH-induced cancer
susceptibility remains limited. Therefore, clinically relevant
mechanistic studies will provide insight into cancer-promoting
gene-environment interactions, which will be of pivotal
significance in breast cancer etiology and prevention.
Previous studies suggest that DMBA-mediated
carcinogenesis involves DNA damage and the deregulation of genes
critical for cell proliferation and survival (11). It has also been reported that DMBA
activates the aryl hydrocarbon receptor (AhR), a transcription
factor that regulates a number of genes involved in cellular
metabolism (9). AhR-dependent
upregulation of cytochrome P450 enzymes metabolizes DMBA into a
mutagenic intermediate that causes DNA damage and subsequently
contributes to the initiation of tumorigenesis (12,13).
DMBA-mediated upregulation of Cyclin D1 and c-Myc, possibly through
nuclear factor-κB and Wnt pathways, has also been revealed to play
a critical role in carcinogenesis (11,14).
Results from microarray gene expression profiling of mammary
tissues from DMBA-treated rats indicated that DMBA alters genes
involved in major cellular processes, including cellular
differentiation, proliferation/cell cycle regulation, and
microtubule dynamics (14),
suggesting that DMBA-mediated carcinogenesis has broad-reaching
effects on signaling transduction and gene expression. Therefore,
it is of vital importance to study the impact of DMBA exposure on
the specific mechanisms that modulate these pathways. In
particular, the association of DMBA with clinically relevant
genetic predisposition may be a critical factor that influences
DMBA- and other PAH-associated breast cancer risk.
In regards to established models of genetic
predisposition for breast cancer, ErbB2 is an oncogene
overexpressed/amplified in approximately one-third of invasive
breast cancers (15,16). ErbB2 overexpression is also
associated with poor prognosis and therapeutic resistance (17). As a member of the epidermal growth
factor receptor (EGFR) family of receptor tyrosine kinases (RTKs),
ErbB2 activates downstream pathways, such as the
phosphoinositide-3-kinase (PI3K)/RAC-alpha serine/threonine-protein
kinase (Akt) and mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (Erk) pathways that
promote cell proliferation and survival. Deregulation of these
signaling pathways can induce malignant transformation and tumor
progression (18). Although ErbB2
overexpression alone is oncogenic, ErbB2-mediated carcinogenesis
can be modulated by various environmental factors. Therefore,
animal models of ErbB2-overexpressing human breast cancers have
been developed as clinically relevant models for studying
gene-environment interactions that influence breast cancer risk. Of
particular importance to the present study, MMTV-ErbB2 transgenic
mice are a well-established model used for testing the impact of
various factors on breast cancer risk (19–22).
Previous studies demonstrate that mammary tumor development in
MMTV-ErbB2 mice is significantly modulated by various etiological
factors (23–26). As such, MMTV-ErbB2 mice are ideal
for studying breast cancer etiology due to long tumor latency with
the development of spontaneous, nodular mammary tumors around 36
weeks of age. Given the well-defined genetic background and
pathology of mammary tumor development in MMTV-ErbB2 mice (14,27,28),
this animal model has significant advantages for mechanistic
studies involving gene-environment interactions.
In the present study, the effects of DMBA on mammary
tumor development, as well as the underlying mechanisms, were
investigated in MMTV-ErbB2 mice. It was demonstrated that DMBA
promoted ErbB2-mediated carcinogenesis through the enhanced
activation of ErbB2 and estrogen receptor (ER) signaling and the
induction of further genomic instability. Overall, the study
further supports the use of the MMTV-ErbB2 transgenic mouse model
for investigating gene-environment interactions that are associated
with breast carcinogenesis.
Materials and methods
Animals and treatment
Female FVB/N-Tg/MMTV-ErbB2 (MMTV-ErbB2) transgenic
mice (weighing 15–20 g) were purchased from Jackson Laboratory (Bar
Harbor, ME, USA). Mice were fed an estrogen-free AIN-93G
semi-purified diet (Bio-Serv; Flemington, NJ, USA) and provided
water ad libitum. Mice were housed in an environment with an
ambient temperature of 22±2°C, relative humidity of 50±10%, and a
12 h light/dark cycle. A total of 52 female mice were randomly
assigned to either control or DMBA groups (n=26 mice per group).
Beginning at 6 weeks of age, mice were treated weekly by oral
gavage with 1 mg DMBA in 0.1 ml peanut oil or an equal volume of
peanut oil alone (vehicle) for 6 weeks. The DMBA dose was based on
previous studies using the parental FVB/N mouse strain (11). All animal procedures were approved
by the University of Oklahoma Health Sciences Center Institutional
Animal Care and Use Committee (Oklahoma City, OK, USA).
Starting at 10 weeks of age, mice were examined for
mammary tumors twice a week. The dates of the first palpable
mammary tumors were recorded for each mouse. When palpable mammary
tumors reached a volume of 1.5 cm3, mice were euthanized
by carbon dioxide asphyxiation followed by cervical dislocation.
Then, the tumor and lung tissues were harvested for molecular and
histological analyses. For histopathological analysis, the
collected tissues were fixed overnight (~18 h) in 10% neutral
formalin at room temperature and paraffin-embedded (FFPE). Tumor
and lung tissue sections (5 µm thickness) were deparaffinized in
xylene and hydrated in 5 min washes of 100, 95 and 70% ethanol.
Then, sections were hematoxylin and eosin (H&E)-stained with
hematoxylin for 5 min and eosin Y for 1 min at room temperature.
Finally, the sections were dehydrated with 5 min washes of 70%,
95%, and 100% ethanol, cleared with xylene, and mounted with
Permount. Tumor latency and tumor multiplicity were calculated at
the endpoint of the experiment (when all mice had developed
tumors).
Mammary whole mounts
At 15 weeks of age, 6 mice from each group were
euthanized and the abdominal mammary glands were removed and spread
onto glass slides for overnight fixation (~18 h) in Carnoy's
solution (6:3:1 ratio of 100% ethanol:chloroform:glacial acetic
acid) at room temperature. Then, the slides were washed in 70%
ethanol, rehydrated in a series of 50% and 30% ethanol solutions
(in distilled water), and followed by staining for 12 h in carmine
alum at room temperature. Mammary glands were then dehydrated with
70%, 95%, and 100% ethanol and cleared in xylene before mounting
with Permount. Whole mounts were imaged using a Nikon Eclipse
inverted microscope (Nikon DXM1200c; Nikon Corporation, Tokyo,
Japan) with Nikon Elements Imaging System (Nikon Corporation). The
ductal extension beyond the lymph node was measured.
5-Bromo-2′-deoxyuridine (BrdU)
incorporation and immunohistochemistry (IHC)
For the BrdU incorporation assay, 15-week-old
control and DMBA-exposed mice were intraperitoneally injected with
BrdU (3 mg/ml in 0.1 ml) 2 h prior to euthanization. Then, the
inguinal mammary gland tissues were collected. The FFPE tissue
sections were deparaffinized with xylene and hydrated in 5 min
washes of 100%, 95%, and 70% ethanol. For antigen retrieval, the
tissue sections were boiled in citrate buffer (pH 6.0) at 100°C for
30 min, then DNA was denatured with HCl at 37°C for 30 min.
Endogenous peroxidases were blocked by 3% hydrogen peroxide at room
temperature for 10 min. Non-specific binding was blocked by 10%
horse serum (Vector Laboratories; Burlingame, CA, USA) for 1 h at
room temperature, followed by incubation with the primary antibody
against BrdU (1:1,000 dilution; Bu20a Mouse mAb; Cat. #5292; Cell
Signaling Technology, Danvers, MA, USA) at 4°C overnight and
biotinylated anti-mouse secondary antibody (1:200 dilution;
Vectastain ABC kit; Cat. #PK-6102; Vector Laboratories) for 30 min
at room temperature. The sections were exposed to ABC reagent
(Vectastain ABC kit, Vector Laboratories) for 30 min at room
temperature prior to staining with diaminobenzidine (DAB) for 1 min
at room temperature and counterstaining with hematoxylin for about
1.5 min at room temperature. Subsequently, the sections were
dehydrated with 5 min washes at room temperature of 70%, 95%, and
100% ethanol, cleared with xylene, and mounted with Permount for
observation. Side branch ductal structures were analyzed in each
sample using a Nikon Eclipse inverted microscope (×400 total
magnification; Nikon Corporation) to determine the percentage of
epithelial cells that exhibited BrdU+ staining.
Western blotting
Mammary gland tissues were collected at 15 weeks of
age and snap-frozen in liquid nitrogen prior to lysate preparation.
The tissues were homogenized at 4°C in NP40-based cell lysis buffer
(50 mM Tris•HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS) supplemented with protease and phosphatase
inhibitor cocktail (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and centrifuged at 18,000 × g for 20 min at 4°C. The proteins
in the supernatant were collected and quantified using a BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). Total protein
(50 µg) from each sample was separated by 10–12% SDS-PAGE and
transferred onto nitrocellulose membranes. Following blocking in 5%
non-fat milk in Tris-buffered saline/Tween-20 (TBST) for 1 h at
room temperature, membranes were incubated with the indicated
primary antibodies (Table I)
overnight at 4°C, followed by washing with TBST. After incubation
with horseradish peroxidase-linked secondary antibodies (1:5,000
dilution; anti-mouse IgG or anti-rabbit IgG; Cat. #7076 or #7074;
Cell Signaling Technology) for 1 h at room temperature and washing
with TBST, specific protein bands were visualized with SuperSignal
West Pico Chemiluminescent solution (Thermo Fisher Scientific,
Inc.). Protein bands were imaged using the FluorChemE imaging
system.
 | Table I.Primary antibodies used for western
blotting. |
Table I.
Primary antibodies used for western
blotting.
| Antibody name | Dilution | Vendor | Catalog # |
|---|
| EGF Receptor
(D38B1) XP Rabbit mAb | 1:1,000 | Cell Signaling
Technology | 4267 |
| Phospho-EGF
Receptor (Tyr1068) (D7A5) XP Rabbit mAb | 1:1,000 | Cell Signaling
Technology | 3777 |
| HER2/ErbB2 (29D8)
Rabbit mAb | 1:1,000 | Cell Signaling
Technology | 2165 |
| Phospho-HER2/ErbB2
(Tyr877) Antibody | 1:1,000 | Cell Signaling
Technology | 2241 |
| HER3/ErbB3 (D22C5)
XP Rabbit mAb | 1:1,000 | Cell Signaling
Technology | 12708 |
| Akt1 (C73H10)
Rabbit mAb | 1:2,000 | Cell Signaling
Technology | 2938 |
| Phospho-Akt
(Ser473) (D9E) XP Rabbit mAb | 1:2,000 | Cell Signaling
Technology | 4060 |
| ERK2 Antibody
(C-14) | 1:2,000 | Santa Cruz
Biotechnology | sc-154 |
| Phospho-p44/42 MAPK
(Erk1/2) (Thr202/Tyr204) Antibody | 1:2,000 | Cell Signaling
Technology | 9101 |
| β-Actin Antibody
(C4) | 1:3,000 | Santa Cruz
Biotechnology | sc-47778 |
| ERα Antibody
(MC-20) | 1:2,000 | Santa Cruz
Biotechnology | sc-542 |
| Phospho-Estrogen
Receptor α (Ser167) (D5W3Z) Rabbit mAb | 1:1,000 | Cell Signaling
Technology | 64508 |
| ERβ Antibody
(H-150) | 1:1,000 | Santa Cruz
Biotechnology | sc-8974 |
| c-Myc Antibody
(9E10) | 1:2,000 | Santa Cruz
Biotechnology | sc-40 |
| Cyclin D1 Antibody
(HD11) | 1:2,000 | Santa Cruz
Biotechnology | sc-246 |
| Bcl-2 Antibody
(C-2) | 1:2,000 | Santa Cruz
Biotechnology | sc-7382 |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the inguinal mammary
glands using an RNeasy Mini kit (Qiagen, Inc.; Valencia, CA, USA)
according to the manufacturer's protocol. cDNA was synthesized
using 1 µg of RNA and TaqMan Reverse Transcription Reagents
(Applied Biosystems; Thermo Fisher Scientific, Inc.) at 25°C for 10
min, 37°C for 30 min and 95°C for 5 min. qPCR was performed using a
Bio-Rad CFX1000 Real-Time PCR System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). PCR amplification was carried out in a 20 µl
reaction volume containing 50 ng cDNA, 0.4 µM each forward and
reverse primers (Table II), and 10
µl SsoFast EvaGreen Supermix (Bio-Rad Laboratories). PCR reactions
were initiated with denaturation at 95°C for 30 sec, followed by 39
amplification cycles at 95°C for 5 sec and 60°C for 5 sec. Relative
mRNA levels were quantified using the 2−ΔΔCq method
(29) based on the cycle threshold
values and were normalized to β-actin gene expression.
 | Table II.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Sequence
(5′-3′) |
|---|
| EGFR |
| F |
TGGTAAGTCAGGGGCAAGTC |
| R |
ACATGGCACTTCCTGGTGAT |
| ERBB2 |
| F |
CAGCCCCAGAGGATTACAGA |
| R |
TCAGTCCTAGTGGGGTGTCC |
| ERBB3 |
| F |
GAGCTTCCAGACTCCGTTTG |
| R |
AAATGGCCTGCAGCTTACAC |
| ESR1 |
| F |
TCTCTGGAAGAGAAGGACCACATC |
| R |
TGCAGAGTCAGGCCAGCTTT |
| MYC |
| F |
TGAGCCCCTAGTGCTGCAT |
| R |
AGCCCGACTCCGACCTCTT |
| CCND1 |
| F |
GGGCACCTGGATTGTTCT |
| R |
CACCGGAGACTCAGAGCA |
| JUN |
| F |
AAAACCTTGAAAGCGCAAAA |
| R | GTT
TGCAACTGCTGCGTTAG |
| ACTB |
| F |
TGGAATCCTGTGGCATCCATGAAA C |
| R | TAA
AACGCAGCTCAGTAACAGTCCC |
Chromosome preparation and
karyotyping
Chromosomal isolation and karyotyping were performed
according to the authors' previous study (30). Briefly, tumors were removed from
MMTV-ErbB2 mice (n=6 mice per treatment) when tumor volumes reached
1.5 cm3. Then, tumor tissues were processed and cultured
in DMEM/F12 media (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Atlanta Biologicals;
Flowery Branch, GA, USA) and penicillin/streptomycin. After the
second passage, the tumor cells were collected following cell cycle
arrest at metaphase, which was induced using Colcemid (final
concentration: 0.02 µg/ml; Gibco; Thermo Fisher Scientific, Inc.)
for 1 h. Trypsin-Giemsa banding (GTG banding) was used to treat and
stain the chromosomes in the tumor cells. Tumor cells were exposed
to Giemsa stain for 5 min at room temperature. At least 18 cells in
metaphase were karyotyped for each tumor sample.
Statistical analysis
The Kaplan-Meier tumor-free survival curve was
analyzed using a log-rank test to compare tumor latency between the
control and DMBA-treated groups (GraphPad Prism; GraphPad Software,
Inc., La Jolla, CA, USA) (24,31).
For all other analyses, significant differences between the means
of two groups were examined using two-sample Student's t-tests
(GraphPad Prism) (32,33). All samples were analyzed in at least
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
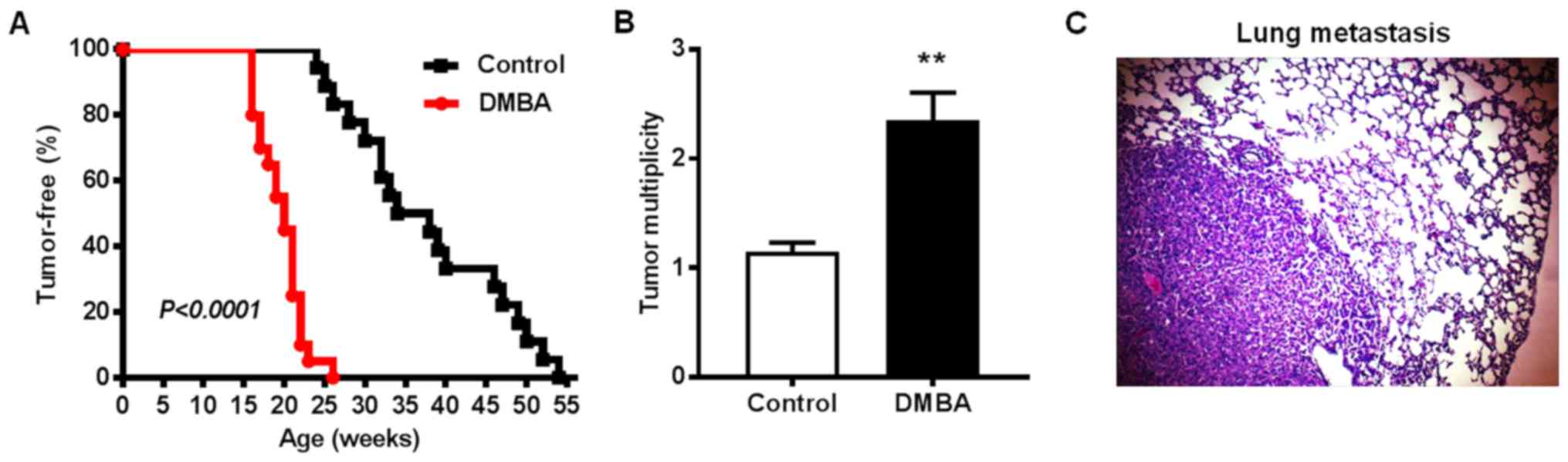
DMBA promotes mammary tumorigenesis in
MMTV-ErbB2 transgenic mice
To test the effects of DMBA on ErbB2-mediated
mammary tumorigenesis, MMTV-ErbB2 transgenic mice were exposed to
DMBA for 6 weeks. In the control group, palpable mammary tumors
first appeared at 24 weeks of age with an average latency of
37.7±2.3 weeks (Fig. 1A). In
contrast, all mice in the DMBA-exposed group developed palpable
mammary tumors between 16 and 26 weeks of age with an average
latency of 19.7±0.6 weeks. By the experimental endpoint (when
tumors reached 1.5 cm3), the average number of palpable
tumors per mouse in the control group was 1.15±0.08 tumors, while
the average tumor multiplicity in the DMBA-exposed mice was
2.35±0.3 tumors (Fig. 1B). As such,
the total number of tumors was 23 in control-treated mice and 47
tumors in DMBA-exposed mice. Since the lungs are a relatively
common metastatic site in MMTV-ErbB2 mice, the present study
examined the frequency of mice with lung metastasis when mammary
tumors reached 1.5 cm3. Metastases in lung tissues
(Fig. 1C) were detected in 6 of 15
DMBA-treated mice (40%) compared with only one mouse with lung
metastasis (5%) in the control group. IHC analysis confirmed that
the lung metastases were ErbB2-overexpressing and derived from the
primary mammary tumors rather than primary lung tumors (data not
shown). Taken together, these results demonstrated that DMBA
exposure significantly accelerated mammary tumor development,
increased tumor multiplicity, and promoted tumor metastasis to the
lungs in MMTV-ErbB2 transgenic mice.
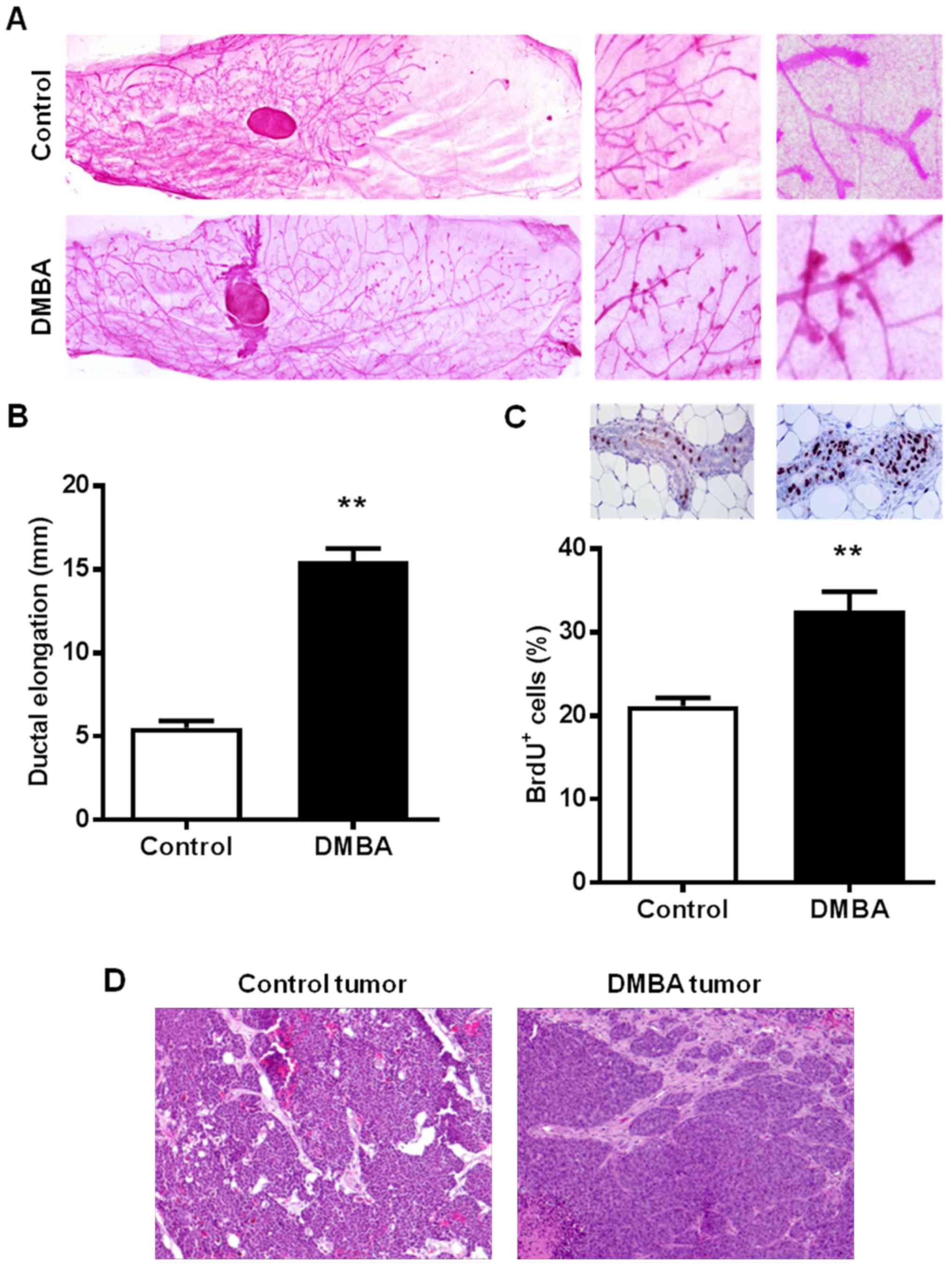
DMBA alters mammary morphogenesis in
MMTV-ErbB2 mice
To study the effects of DMBA on mammary tissues
prior to malignant transformation, the present study examined the
morphology and histopathology of the premalignant mammary glands of
15-week-old control and DMBA-treated mice. It was observed that
DMBA markedly modified mammary morphogenesis as characterized by
distinct ductal growth and lateral branching patterns (Fig. 2A). Mammary glands from 15-week-old
control mice displayed shorter ductal elongation compared with
DMBA-exposed mice (5.5±0.4 vs. 15.5±0.8 mm, respectively), but
relatively more complex ductal branching (Fig. 2B). Notably, DMBA exposure increased
the presence of terminal end bud (TEB)-like structures, which were
not evident in the 15-week-old control mice. IHC analysis of BrdU
nuclear incorporation further demonstrated that DMBA significantly
increased the percentage of proliferating cells as compared to the
mammary tissues from control MMTV-ErbB2 mice (Fig. 2C). Despite the observed differences
in mammary morphogenesis, histopathological analysis of
H&E-stained mammary tumors indicated single nodular tumors in
both control and DMBA-exposed mice (Fig. 2D), which was consistent with
previous reports in MMTV-ErbB2 mice (11,34).
Together, these data suggest that the promotion of ErbB2-mediated
mammary tumorigenesis by DMBA is associated with mammary gland
developmental changes in MMTV-ErbB2 mice.
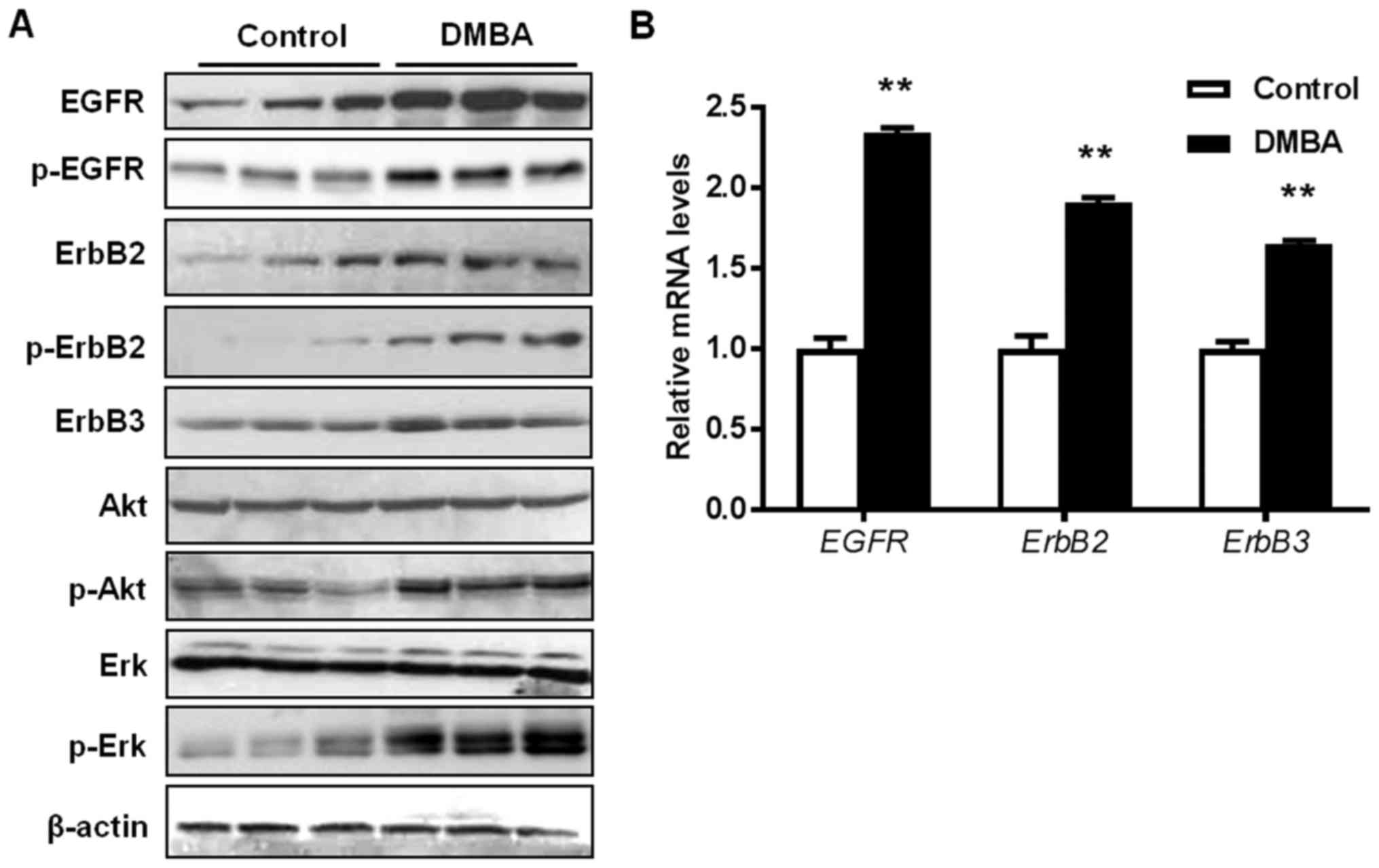
DMBA enhances the activation of RTK
signaling in mammary tissues from MMTV-ErbB2 mice
To understand the tumor-promoting molecular
mechanisms of DMBA in MMTV-ErbB2 mice, the present study examined
the effects of DMBA on RTK signaling and downstream targets in the
premalignant mammary glands. As presented in Fig. 3A, DMBA significantly increased the
activation/phosphorylation of EGFR, ErbB2, Akt, and Erk in the
premalignant mammary tissues from 15-week-old DMBA-treated mice,
compared with control mice. This indicated that DMBA amplified the
activation of ErbB2/MAPK/PI3K/Akt signaling, which is the driving
force of ErbB2-mediated carcinogenesis. It was also revealed that
the mRNA levels of EGFR, ERBB2, and ERBB3 were
increased by DMBA (Fig. 3B). To
note, the mRNA expression of the ERBB2 transgene was not
significantly affected by DMBA exposure (data not shown). Together,
these data suggest that the augmented upregulation of RTKs is an
important mechanism that contributes to DMBA-promoted mammary tumor
development in MMTV-ErbB2 mice.
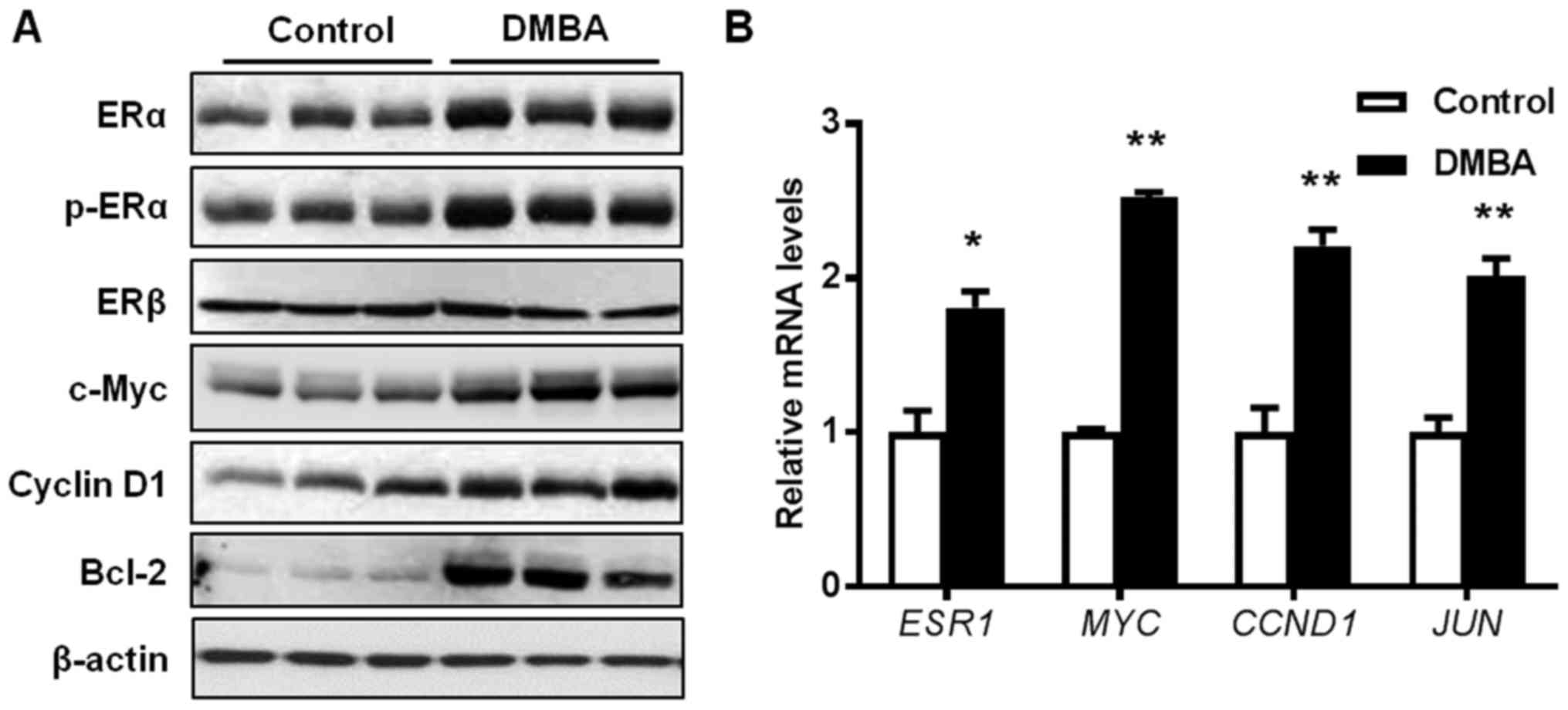
DMBA exposure promotes ER signaling in
premalignant mammary tissues from MMTV-ErbB2 mice
Since DMBA is reported to induce estrogen-dependent
tumors (35), the present study
examined the activation of ERα and ER target genes in mammary
tissues from 15-week-old mice. As presented in Fig. 4A, protein levels of both total ERα
and phosphorylated ERα were significantly increased in the
premalignant mammary glands from DMBA-treated mice, whereas protein
levels of ERβ remained unaltered. Consistently, the protein
expression levels of c-Myc, Cyclin D1, and B cell lymphoma-2
(Bcl-2), which are downstream targets of the ER pathway, were also
increased in DMBA-exposed mammary gland tissues. To further verify
the activation of the ER pathway, mRNA levels of several ER target
genes were detected. Results indicated that the transcription of
ESR1 (ERα), MYC (c-Myc), CCND1 (Cyclin D1),
and JUN (c-Jun) were significantly increased in the mammary
tissues from the DMBA-treated mice, compared with control mice
(Fig. 4B). These data substantiate
that DMBA stimulates ER-mediated gene transcription in the
premalignant mammary tissues.
DMBA induces chromosomal imbalance in
mammary tumors from MMTV-ErbB2 mice
Previous reports have revealed that ErbB2-mediated
carcinogenesis in MMTV-ErbB2 mice involves chromosome instability
(36,37). DMBA-induced DNA adducts and damage
are critical factors in tumor initiation (38,39).
Therefore, the present study karyotyped chromosomes from the tumors
to determine whether DMBA induces enhanced chromosomal alterations
in MMTV-ErbB2 mice. As presented in Table III, one of the six analyzed tumors
(16.7%) from control mice displayed chromosomal abnormalities,
which were associated with an addition in chromosome 4 and trisomy
5. Conversely, 50% of the analyzed tumors from DMBA-treated mice
exhibited abnormal chromosomes. These genetic lesions included
trisomy 2, trisomy 3, and a deletion in chromosome 4. Overall,
these results indicated that DMBA promotes chromosomal instability
in the mammary tumors from MMTV-ErbB2 mice.
 | Table III.DMBA induces chromosomal imbalance in
mammary tumors from MMTV-ErbB2 mice. |
Table III.
DMBA induces chromosomal imbalance in
mammary tumors from MMTV-ErbB2 mice.
| A, Control
tumors |
|---|
|
|---|
| Sample | Karyotype | Chromosomal
abnormality | Pattern |
|---|
| 08-MT-DO11T | 40, XX | Normal |
|
| 08-MT-DO12T | 40, XX | Normal |
|
| 08-MT-DO13T | 40, XX | Normal |
|
| 08-MT-DO14T | 40, XX | Normal |
|
| 08-MT-DO15T | 40, XX,
add(4)(E2)[3]/41, XX, +5[2]/40, XX[15] | Addition,
trisomy | Mosaic |
| 08-MT-DO18T | 40, XX | Normal |
|
|
| B, DMBA
tumors |
|
| Sample |
Karyotype | Chromosomal
abnormality | Pattern |
|
| 08-MT-CT-1 | 41, XX, +2[9]/40,
XX[9] | Trisomy | Mosaic |
| 08-MT-CT-2 | 40, XX | Normal |
|
| 08-MT-CT-3 | 40, XX | Normal |
|
| 08-MT-CT-4 | 41, XX, +3[14]/40,
XX[4] | Trisomy | Mosaic |
| 08-MT-CT-5 | 39, XX,
del(4)(C2D1)[2]/40, XX[15] | Deletion | Mosaic |
| 08-MT-CT-6 | 40, XX | Normal |
|
Discussion
Numerous studies have reported that breast
carcinogenesis can be enhanced by environmental factors that have
pro-estrogenic effects and increase gene susceptibility to
chromosomal alterations, such as bisphenol A and DMBA (4,40–42).
Understanding the molecular mechanisms of gene-environment
interactions in breast carcinogenesis remains a significant
challenge (2,43). The present study investigated the
effects of DMBA on ErbB2-mediated mammary tumor development in
MMTV-ErbB2 transgenic mice. DMBA promotes mammary tumor development
and the incidence of pulmonary metastasis (44,45) in
MMTV-ErbB2 mice. Prior to malignant transformation, DMBA also
induces distinct mammary morphogenic changes as compared with
control tissues (14,46). The present study demonstrated that
DMBA promoted ErbB2-mediated carcinogenesis through the enhanced
activation of RTK and ER signaling. The synergistic effect of DMBA
and ErbB2 deregulation on genomic instability also contributed to
tumor development in this model. Since DMBA is a typical
environmental carcinogen and ErbB2 is a clinically relevant
oncogene associated with genetic predisposition to breast cancer,
the present study provides important insight towards understanding
gene-environment interactions associated with breast
carcinogenesis.
In the present study, distinct changes in mammary
morphogenesis were associated with DMBA exposure in MMTV-ErbB2
mice. In particular, DMBA exposure promoted ductal elongation
beyond the lymph node, however resulted in mammary glands with less
complex ductal structures than what is often induced by estrogenic
factors (47). To potentially
explain these morphological differences, previous reports have
demonstrated that DMBA can upregulate genes involved in microtubule
dynamics, while β-casein and transferrin, two key genes involved in
mammary gland differentiation, are inhibited by DMBA (14). This evidence of DMBA-associated gene
regulation may partially account for the increased ductal
elongation and reduced ductal complexity observed in the study,
which warrants further investigation.
Molecular analysis of premalignant mammary tissues
from DMBA-exposed MMTV-ErbB2 mice provided mechanistic insight into
DMBA-mediated carcinogenesis. It is known that the oncogenic
driving force in MMTV-ErbB2 transgenic mice is increased activation
of RTK signaling and its downstream pathways, such as PI3K/Akt and
MAPK/Erk pathways. The results demonstrated that DMBA exposure
enhanced the activation of EGFR, ErbB2, Akt, and Erk in the
premalignant mammary tissues. These data indicated that DMBA has
specific synergistic effects on ErbB2 activation and consequential
tumorigenesis.
Consistent with previous reports indicating that
DMBA induces estrogen-dependent mammary tumors in rats (35), it was revealed that DMBA
significantly increased ERα protein phosphorylation/activation and
ESR1, MYC, CCND1, and JUN mRNA levels in the
premalignant mammary glands. Previous reports also corroborate that
CCND1 upregulation is associated with carcinogen-induced
mammary tumor development (14).
Increased expression levels of Cyclin D1 and c-Myc are crucial in
the carcinogenic role of DMBA (11). The present study additionally
demonstrated that CCND1 mRNA levels were significantly
upregulated in mammary tumors from the DMBA-exposed mice (data not
shown), which is consistent with previous reports regarding Cyclin
D1 regulation as a major contributor to DMBA-mediated tumor
development in other model systems. Furthermore, concurrent
activation of EGFR/ErbB2 and ER pathways accompanied by increased
ER-targeted gene transcription suggests the activation of crosstalk
between the RTK and ER signaling pathways. In the context of
DMBA-induced oncogenic signaling activation, the findings
underscore the role of EGFR/ErbB2-ER signaling crosstalk in
DMBA-promoted mammary tumor development in MMTV-ErbB2 mice, which
also sheds light on potential mechanisms of DMBA-mediated tumor
development in other model systems.
DNA damage and genomic instability are essential
components of DMBA-associated carcinogenic activities (38,39).
Previous reports have also demonstrated that genomic instability is
involved in mammary tumor development in MMTV-ErbB2 mice (30). In the present study, mammary tumors
from both control and DMBA-exposed MMTV-ErbB2 mice displayed
chromosomal imbalance. Notably, it was revealed that the occurrence
of genetic lesions/abnormalities in ErbB2-overexpressing mammary
tumors from DMBA-treated mice was significantly increased compared
with tumors from control mice, suggesting that enhanced genomic
instability contributes to the accelerated tumor development in
this model.
Results from the present study support the use of
the MMTV-ErbB2 transgenic mouse model to investigate the
interactions of environmental factors and genetic predisposition
that contribute to breast carcinogenesis. Previous reports
exploring the effects of DMBA on mammary tumor development in other
transgenic models, such as MMTV-TGFα transgenic mice, provide
fundamental support regarding the synergistic effects of genetic
and environmental factors on carcinogenesis (48). Since ErbB2 overexpression is
detected in approximately one-third of invasive breast cancers and
more than half of ductal carcinoma in situ (15,16),
studies focusing on the effects of environmental carcinogens,
including DMBA and other PAHs, on ErbB2-mediated carcinogenesis are
of significant clinical relevance.
In conclusion, the establishment of this model
system will facilitate future studies investigating the association
between ErbB2-mediated breast cancer risk and environmental
exposures, which will ultimately lead to effective strategies for
the prevention of ErbB2-overexpressing breast cancers.
Acknowledgements
Not applicable.
Funding
This work was supported in part by the American
Cancer Society (grant no. RSG-08-138-01-CNE), the National
Institute of Environmental Health Sciences (grant no. R21ES025337),
the National Cancer Institute (grant no. 5U54CA156735), and the
National Institute on Alcohol Abuse and Alcoholism (grant no. U54
AA019765) to XY.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
ZM, YMK, SY, YJ, ABP, and XC performed the
experiments and animal care; SDK performed histopathology; EWH, XC,
and XY wrote the manuscript; EWH, SL, and XY performed data
analysis and interpretation; XF, SL, and XY conceived and designed
the experiments.
Ethics approval and consent to
participate
All animal procedures were approved by the
University of Oklahoma Health Sciences Center Institutional Animal
Care and Use Committee (Oklahoma City, OK, USA).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nowell SA, Ahn J and Ambrosone CB:
Gene-nutrient interactions in cancer etiology. Nutr Rev.
62:427–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song M, Lee KM and Kang D: Breast cancer
prevention based on gene-environment interaction. Mol Carcinog.
50:280–290. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chia KS: Gene-environment interactions in
breast cancer. Novartis Found Symp. 293:143–150; discussion
150–155, 181–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubin BS: Bisphenol A: An endocrine
disruptor with widespread exposure and multiple effects. J Steroid
Biochem Mol Biol. 127:27–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belpomme D, Irigaray P, Hardell L, Clapp
R, Montagnier L, Epstein S and Sasco AJ: The multitude and
diversity of environmental carcinogens. Environ Res. 105:414–429.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schell LM, Burnitz KK and Lathrop PW:
Pollution and human biology. Ann Hum Biol. 37:347–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majkova Z, Toborek M and Hennig B: The
role of caveolae in endothelial cell dysfunction with a focus on
nutrition and environmental toxicants. J Cell Mol Med.
14:2359–2370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rybicki BA, Nock NL, Savera AT, Tang D and
Rundle A: Polycyclic aromatic hydrocarbon-DNA adduct formation in
prostate carcinogenesis. Cancer Lett. 239:157–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singhal R, Shankar K, Badger TM and Ronis
MJ: Estrogenic status modulates aryl hydrocarbon receptor-mediated
hepatic gene expression and carcinogenicity. Carcinogenesis.
29:227–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mehta RG, Naithani R, Huma L, Hawthorne M,
Moriarty RM, McCormick DL, Steele VE and Kopelovich L: Efficacy of
chemopreventive agents in mouse mammary gland organ culture (MMOC)
model: A comprehensive review. Curr Med Chem. 15:2785–2825. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Currier N, Solomon SE, Demicco EG, Chang
DL, Farago M, Ying H, Dominguez I, Sonenshein GE, Cardiff RD, Xiao
ZX, et al: Oncogenic signaling pathways activated in DMBA-induced
mouse mammary tumors. Toxicol Pathol. 33:726–737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nebert DW, Petersen DD and Fornace AJ Jr:
Cellular responses to oxidative stress: The [Ah] gene battery as a
paradigm. Environ Health Perspect. 88:13–25. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rundle A, Tang D, Hibshoosh H, Estabrook
A, Schnabel F, Cao W, Grumet S and Perera FP: The relationship
between genetic damage from polycyclic aromatic hydrocarbons in
breast tissue and breast cancer. Carcinogenesis. 21:1281–1289.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papaconstantinou AD, Shanmugam I, Shan L,
Schroeder IS, Qiu C, Yu M and Snyderwine EG: Gene expression
profiling in the mammary gland of rats treated with
7,12-dimethylbenz[a]anthracene. Int J Cancer. 118:17–24. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu E, Thor A, He M, Barcos M, Ljung BM
and Benz C: The HER2 (c-erbB-2) oncogene is frequently amplified in
in situ carcinomas of the breast. Oncogene. 7:1027–1032.
1992.PubMed/NCBI
|
|
16
|
Jardines L, Weiss M and Fowble B: Greene
Mneu(c-erbB-2/HER2) and the epidermal growth factor receptor (EGFR)
in breast cancer. Pathobiology. 61:268–282. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hynes NE and Stern DF: The biology of
erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta.
1198:165–184. 1994.PubMed/NCBI
|
|
18
|
Citri A and Yarden Y: EGF-ERBB signalling:
Towards the systems level. Nat Rev Mol Cell Biol. 7:505–516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guy CT, Webster MA, Schaller M, Parsons
TJ, Cardiff RD and Muller WJ: Expression of the neu protooncogene
in the mammary epithelium of transgenic mice induces metastatic
disease. Proc Natl Acad Sci USA. 89:10578–10582. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bouchard L, Lamarre L, Tremblay PJ and
Jolicoeur P: Stochastic appearance of mammary tumors in transgenic
mice carrying the MMTV/c-neu oncogene. Cell. 57:931–936. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marcotte R and Muller WJ: Signal
transduction in transgenic mouse models of human breast
cancer-implications for human breast cancer. J Mammary Gland Biol
Neoplasia. 13:323–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hutchinson JN and Muller WJ: Transgenic
mouse models of human breast cancer. Oncogene. 19:6130–6137. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Yang S, McKimmey C, Liu B,
Edgerton SM, Bales W, Archer LT and Thor AD: Genistein induces
enhanced growth promotion in ER-positive/erbB-2-overexpressing
breast cancers by ER-erbB-2 cross talk and p27/kip1 downregulation.
Carcinogenesis. 31:695–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang X, Edgerton SM, Kosanke SD, Mason TL,
Alvarez KM, Liu N, Chatterton RT, Liu B, Wang Q, Kim A, et al:
Hormonal and dietary modulation of mammary carcinogenesis in mouse
mammary tumor virus-c-erbB-2 transgenic mice. Cancer Res.
63:2425–2433. 2003.PubMed/NCBI
|
|
25
|
Warin R, Chambers WH, Potter DM and Singh
SV: Prevention of mammary carcinogenesis in MMTV-neu mice by
cruciferous vegetable constituent benzyl isothiocyanate. Cancer
Res. 69:9473–9480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen K and Novak RF: DDT stimulates
c-erbB2, c-met, and STATS tyrosine phosphorylation, Grb2-Sos
association, MAPK phosphorylation, and proliferation of human
breast epithelial cells. Biochem Biophys Res Commun. 231:17–21.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shan L, Yu M and Snyderwine EG: Global
gene expression profiling of chemically induced rat mammary gland
carcinomas and adenomas. Toxicol Pathol. 33:768–775. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shan L, He M, Yu M, Qiu C, Lee NH, Liu ET
and Snyderwine EG: cDNA microarray profiling of rat mammary gland
carcinomas induced by
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and
7,12-dimethylbenz[a]anthracene. Carcinogenesis. 23:1561–1568. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YM, Ma Z, Lee S, Lee J, Li S and Yang
X: Trisomy chromosome 5 is a recurrent cytogenetic lesion in
mammary tumors from parous MMTV-erbB-2 transgenic mice. Oncol Lett.
2:1077–1081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Oliveira Andrade F, Fontelles CC, Rosim
MP, de Oliveira TF, de Melo Loureiro AP, Mancini-Filho J, Rogero
MM, Moreno FS, de Assis S, Barbisan LF, et al: Exposure to
lard-based high-fat diet during fetal and lactation periods
modifies breast cancer susceptibility in adulthood in rats. J Nutr
Biochem. 25:613–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin LQ, Xu JY, Tezuka H, Wang PY and Hoshi
K: Commercial soy milk enhances the development of
7,12-dimethylbenz(a) anthracene-induced mammary tumors in rats. In
Vivo. 21:667–671. 2007.PubMed/NCBI
|
|
33
|
Qi C, Lan H, Ye J, Li W, Wei P, Yang Y,
Guo S, Lan T, Li J, Zhang Q, et al: Slit2 promotes tumor growth and
invasion in chemically induced skin carcinogenesis. Lab Invest.
94:766–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muller WJ, Sinn E, Pattengale PK, Wallace
R and Leder P: Single-step induction of mammary adenocarcinoma in
transgenic mice bearing the activated c-neu oncogene. Cell.
54:105–115. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohibi S, Mirza S, Band H and Band V:
Mouse models of estrogen receptor-positive breast cancer. J
Carcinog. 10:352011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cool M, Depault F and Jolicoeur P: Fine
allelotyping of Erbb2-induced mammary tumors in mice reveals
multiple discontinuous candidate regions of tumor-suppressor loci.
Genes Chromosomes Cancer. 45:191–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu S, Liu W, Jakubczak JL, Erexson GL,
Tindall KR, Chan R, Muller WJ, Adhya S, Garges S and Merlino G:
Genetic instability favoring transversions associated with
ErbB2-induced mammary tumorigenesis. Proc Natl Acad Sci USA.
99:3770–3775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daniel FB and Joyce NJ: DNA adduct
formation by 7, 12-dimethylbenz[a]anthracene and its
noncarcinogenic 2-fluoro analogue in female Sprague-Dawley rats. J
Natl Cancer Inst. 70:111–118. 1983.PubMed/NCBI
|
|
39
|
Russo IH and Russo J: Mammary gland
neoplasia in long-term rodent studies. Environ Health Perspect.
104:938–967. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Umekita Y, Souda M, Hatanaka K, Hamada T,
Yoshioka T, Kawaguchi H and Tanimoto A: Gene expression profile of
terminal end buds in rat mammary glands exposed to
diethylstilbestrol in neonatal period. Toxicol Lett. 205:15–25.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsu PY, Deatherage DE, Rodriguez BA,
Liyanarachchi S, Weng YI, Zuo T, Liu J, Cheng AS and Huang TH:
Xenoestrogen-induced epigenetic repression of microRNA-9-3 in
breast epithelial cells. Cancer Res. 69:5936–5945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lamartiniere CA, Jenkins S, Betancourt AM,
Wang J and Russo J: Exposure to the endocrine disruptor bisphenol a
alters susceptibility for mammary cancer. Horm Mol Biol Clin
Investig. 5:45–52. 2011.PubMed/NCBI
|
|
43
|
Wogan GN, Hecht SS, Felton JS, Conney AH
and Loeb LA: Environmental and chemical carcinogenesis. Semin
Cancer Biol. 14:473–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Medina D: Chemical carcinogenesis of rat
and mouse mammary glands. Breast Dis. 28:63–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Plante I, Stewart MK, Barr K, Allan AL and
Laird DW: Cx43 suppresses mammary tumor metastasis to the lung in a
Cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Russo J and Russo IH: Influence of
differentiation and cell kinetics on the susceptibility of the rat
mammary gland to carcinogenesis. Cancer Res. 40:2677–2687.
1980.PubMed/NCBI
|
|
47
|
Markey CM, Luque EH, Munoz De Toro M,
Sonnenschein C and Soto AM: In utero exposure to bisphenol A alters
the development and tissue organization of the mouse mammary gland.
Biol Reprod. 65:1215–1223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Coffey RJ Jr, Meise KS, Matsui Y, Hogan
BL, Dempsey PJ and Halter SA: Acceleration of mammary neoplasia in
transforming growth factor alpha transgenic mice by
7,12-dimethylbenzanthracene. Cancer Res. 54:1678–1683.
1994.PubMed/NCBI
|