Introduction
An estimated 1.8 million new cases of lung cancer
were diagnosed in 2012 which consisted of 13% of all cancers
diagnosed that year worldwide. Lung cancer contributed to ~1.6
million cancer-related deaths worldwide (1,2). In
China, 4.2 million new cases and >2.8 million cancer-related
deaths were estimated to occur in 2015 (2). Histopathologically, lung cancer can be
classified into four major types, i.e., squamous cell,
adenocarcinoma, large cell and small cell lung cancer. Among them,
lung adenocarcinomas account for ~40% of all lung cancers globally
(3). To date, surgery is still the
most effective treatment option for lung cancer, although recently
developed molecular and targeting therapy represents promising
treatment strategies for certain patients. Most lung cancers are
currently diagnosed at an advanced disease stage, leading to very
poor prognoses (4). Novel
therapeutic or preventive strategies are urgently needed to better
control this deadly disease. In addition, a better understanding of
the molecular mechanisms responsible for lung carcinogenesis and
tumor progression could provide novel biomarkers for diagnosis,
surveillance and novel targets for control of lung cancer.
β-elemene, a member of the class of organic
compounds known as elemane sesquiterpenoids, is found in a variety
of plants and has been demonstrated to possess antitumor activity
in a variety of human cancers (5–15). For
example, β-elemene exhibited activity to induce cell cycle arrest
and apoptosis of non-small cell lung cancer cells (10), and was found to enhance cisplatin or
taxane chemosensitivity or radiation sensitivity of lung cancer
cells (6,8,14). A
previous clinical trial conducted in China revealed that β-elemene
was beneficial for cancer patients (16), but further randomized clinical
trials are required to confirm its therapeutic potential. The human
genome sequencing project and gene profile analyses have further
elucidated the molecular mechanisms underpinning cancer and cancer
chemotherapy, and thus may be useful in revealing the potential
therapeutic mechanism of β-elemene.
In our previous study, we profiled differentially
expressed genes in lung adenocarcinoma A549 cells after β-elemene
treatment, and confirmed that POLE2 expression was altered by
β-elemene. In the present study, we further investigated the
effects of POLE2 knockdown on the regulation of lung cancer cell
proliferation, apoptosis and the underlying molecular events. This
study illuminated the molecular mechanisms responsible for the
antitumor activity of β-elemene, and may aid in the development of
novel therapeutic tools and targets for lung cancer treatment or
prevention.
Materials and methods
Cell lines and culture
Human lung cancer cell line A549, obtained from the
Cell Bank of the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China), was isolated from a patient
with lung adenocarcinoma. This cell line is frequently used as an
in vitro model for lung alveolar basal epithelial cells. Two
other lung cancer cell lines, NCI-H1975 and NCI-H1299, were also
obtained from the Cell Bank of the Shanghai Institute of Cell
Biology, Chinese Academy of Sciences. A549 cells were cultured in
F-12K complete medium (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA), NCI-H1299 cells were cultured in RPMI-1640 complete
medium (Santa Cruz Biotechnology, Inc.) and NCI-H1975 cells were
cultured in complete Dulbecco's modified Eagle's medium (DMEM;
Corning, Inc., Corning, NY, USA). Complete medium was supplemented
with 10% fetal bovine serum (FBS; Vian-Saga Co., Ltd., Shanghai,
China), 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldirch; Merck KGaA, Darmstadt, Germany) and cells were
cultured in a humidified incubator with 5% CO2 at 37°C.
Cells in the exponential growth phase were used for our
experiments. Furthermore, a human embryonic kidney cell line 293T
was obtained from Shangha GeneChem Co., Ltd. (Shanghai, China) and
also cultured in complete DMEM.
Profiling of differentially expressed
genes in A549 cells
We first profiled differentially expressed genes in
A549 cells treated with or without β-elemene (DRUG and NC groups,
respectively) using the GeneChip® PrimeView™
Human Gene Expression Array (Affymetrix; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). In brief, total RNA was isolated from A549
cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The RNA concentration
of samples was measured using a NanoDrop spectrophotometer (Thermo
Fisher Scientific, Inc.). The RNA integrity was assessed using
Agilent 2100 Bioanalyzer (1.7 < A260/A280 < 2.2 and RIN ≥ 7.0
and 28S/18S > 0.7, respectively).
Next, 100 ng of each RNA sample was mixed with a
poly(A) RNA to form double-stranded cDNA (complementary RNA, cRNA)
and these cDNA samples were used to produce aRNA (amplified RNA,
aRNA) by using the in vitro transcription (IVT) primers with
the GeneChip 3′-IVT Express kit (Affymetrix; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions. After
that, the aRNA samples were purified and fragmented, and then
hybridized to human cDNA microarrays with hybridization reaction
mixtures for 16 h at 45°C in a GeneChip Hybridization Oven 645
(Affymetrix; Thermo Fisher Scientific, Inc.). On the next day, the
arrays were washed in the GeneChip Fluidics Station 450
(Affymetrix; Thermo Fisher Scientific, Inc.) with the GeneChip
Hybridization Wash and Stain Kit (Affymetrix; Thermo Fisher
Scientific, Inc.) and scanned with the GeneChip Scanner 3000
(Affymetrix; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
We then utilized the Affymetrix GeneChip Analysis
Software v1.3 for data acquisition, the first-level data analysis,
and desktop data management for the entire GeneChip System, and the
Robust multichip analysis (RMA) to normalize gene expression levels
against the level of background variability between different
hybridizations.
We then performed differential gene expression
analysis in R environment using the Limma (linear models for
microarray data) package (http://www.bioconductor.org/packages/release/bioc/html/limma.html).
The fold change was calculated relative to baseline controls. Three
independent replicate experimental data were used to perform a
paired two-sample t-test for each differentially expressed gene.
Dysregulated genes were defined as fold change of ≥2 or ≤-2
(P<0.05) between the DRUG and NC cells. Such data were then
compared to those from lung cancer in large sample data and Gene
Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and further analyzed
using volcano and scatter plots and cluster diagrams.
Construction of the shPOLE2 lentiviral
vector, lentivirus production and cell infection
We first selected and designed a short hairpin RNA
(shRNA) to knock down POLE2 expression in A549 and NCI-H1299 cells
using the following sequences (5′-GATTGTTCTTGGAATGATA-3′). The
negative control (NC) shRNA sequences were
5′-TTCTCCGAACGTGTCACGT-3′. The synthesized DNA oligonucleotides
were annealed to form double-stranded DNA and inserted into the
AgeI/EcoRI sites of linearized vector GV115 carrying
a Green Fuorescent Protein (GFP) gene (Shanghai GeneChem Co.,
Ltd.). After amplification and DNA sequence confirmation, these
lenti-shRNA vectors were co-transfected into 293T cells with two
packaging plasmids pHelper 1.0 and pHelper 2.0 (Shanghai GeneChem
Co., Ltd.) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h according to the manufacturer's
instructions. Lentiviral particles were purified from the culture
supernatant. A549 and NCI-H1299 cells were infected in 6-well
plates at the density of 2×105 cells/well at a
multiplicity of infection (MOI) of 10 and 5, respectively.
Infection efficiency was evaluated under a florescence microscope
by detection of GFP-positive cells vs. total cells in the plate.
The POLE2 knockdown efficiency was calculated using quantitative
reverse transcriptase-polymerase chain reaction (qRT-PCR; Roche
Diagnostics, Inc., Basel, Switzerland) and western blot analysis,
respectively.
qRT-PCR
Total RNA was isolated from A549 and NCI-H1299 cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed into cDNA using the M-MLV reverse transcriptase
(Promega, Madison, WI, USA) according to the manufacturers'
instructions. The qPCR was then carried out in the LightCycler 480
II Real-time PCR instruments (Roche Diagnostics) with the SYBR
Premix Ex Taq (Takara Bio Inc., Otsu, Japan). The qPCR conditions
were as follows: an initial cycle at 95°C for 30 sec and 40 cycles
of 95°C for 5 sec and 60°C for 30 sec with the primers (POLE2,
5′-TGAGAAGCAACCCTTGTCATC-3′ and 5′-TCATCAACAGACTGACTGCATTC-3′; and
GAPDH, 5′-TGACTTCAACAGCGACACCCA-3′ and
5′-CACCCTGTTGCTGTAGCCAAA-3′). GAPDH was used as an internal
control. The qPCR products were 84 and 121 bp, respectively for
POLE2 and GAPDH. The level of POLE2 mRNA was calculated using the
2−ΔΔCq method (17). The
experiment was in duplicate and repeated at least once.
Western blotting
An expression vector carrying POLE2-flag tag fusion
cDNA was purchased from Shanghai GeneChem Co., Ltd., and
co-transfected into 293T cells with shPOLE2 or shCtrl expression
vector for 48 h using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Total cellular protein was then
extracted using a lysis buffer from Beyotime Institute of
Biotechnology (Shanghai, China) according to the manufacturer's
protocol. The protein concentration was assessed using BCA Protein
Assay kit (Beyotime Institute of Biotechnology). Equal amount of
protein samples (20 µg for each loading) was separated in 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gels and transferred onto polyvinylidene difluoride
(PVDF) membranes (EMD Millipore, Billerica, MA, USA). The membranes
were then incubated in 5% skim milk-Tris-based saline (TBS) at room
temperature for 1 h, and then further incubated with a mouse
anti-FLAG antibody (cat. no. F1804; Sigma-Aldrich, Merck KGaA)
diluted at 1:2,000 at 4°C overnight. On the next day, the membranes
were washed with TBS-Tween 20 (TBS-T) and then incubated with a
secondary antibody (cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) at a dilution of 1:2,000 at room temperature for 2 h.
Afterwards, the membrane was washed with TBST three times for 10
min each. Protein expression was developed on X-ray film after
brief incubation with Pierce™ ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc.). The experiment was
conducted in duplicate and repeated at least twice. The gray
analysis was scanned using an Epson Perfection V370 Photo scanner
(Epson America Inc., Long Beach, CA, USA) and ImageJ 1.51w software
(National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Lentivirus-infected A549 and NCI-H1299 cells in the
logarithmic phase were reseeded into 96-well plates (Corning Inc.)
at a density of 2,000 cells/well and the plates were incubated for
up to 4 days. At the end of each experiment, cells were incubated
with 10 µl of the CCK-8 reagent (Sigma-Aldrich; Merck KGaA) at 37°C
for 3 h. The optical density (OD) at 450 nm was measured by a
microplate reader (Tecan Infinite; Tecan Austria GmbH, Groedig,
Austria) according to the manufacturer's instructions. The data are
expressed as % of the control. The experiments were carried out in
triplicate and repeated at least twice.
Colony formation assay
Lentivirus-infected A549 and NCI-H1299 cells in the
logarithmic phase were reseeded into 6-well plates at a density of
500 cells/well and incubated for 14 days. The cell culture medium
was refreshed every three days. At the end of each experiment, the
cells were fixed with 4% paraformaldehyde (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for 30 min and then washed with
phosphate-buffered saline (PBS) and stained with 500 µl of Giemsa
(Shanghai Dingguo Biotechnology Co., Ltd., Shanghai, China) for 10
min. Cell colonies were photographed under an inverted microscope
and cell colonies with >50 cells or more were counted. The
experiment was in duplicate and repeated at least twice.
Caspase-3/-7 activity assay
The A549 cells were seeded into 96-well plates at a
density of 1.5×104 cells/well and NCI-H1299 cells at a
density of 1.0×104 cells/well, grown in a 5%
CO2 incubator at 37°C and infected with shPOLE2 and
shCtrl lentiviruses. After 3–5 days, cells were detached using 0.5%
trypsin and counted. A549 and NCI-H1299 cell suspensions with
1×104 cells/well were added into a new 96-well plate.
Cells were then lysed with a mixture of 1:1 Caspase-Glo3/7
substrate buffer (Promega) and cell growth medium on a shaker (43 ×
g) for 30 min according to the manufacturer's protocol. The plate
was further incubated at room temperature for 2 h. The activity of
caspase-3/-7 was then measured using a microplate reader (Tecan
Infinite; Tecan Austria GmbH). The experiment was carried out in
triplicate and repeated twice.
PathScan stress and apoptosis
signaling antibody array
A PathScan® Stress and Apoptosis
Signaling Antibody Array kit was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and used to detect changes in
vital signaling molecules after knockdown of POLE2 expression in
A549 cells. In brief, cells were grown and infected with shPOLE2
and shCtrl lentiviruses for 48 h and then lysed using 1X cell lysis
buffer (Cell Signaling Technology, Inc.). The concentration of cell
lysate was measured and adjusted to 0.2–1.0 mg/ml and these protein
samples were subjected to analysis by a PathScan®
Antibody Array kit according to the manufacturer's protocols. Each
experiment was repeated twice.
Statistical analysis
The data are expressed as the mean ± standard
deviation (SD) and all statistical analyses were performed using
SPSS 22.0 statistical software (IBM Corp., Armonk, NY, USA). The
statistical significance of differences between the two groups was
determined by a two-sided Student's t-test, and one-way analysis of
variance (ANOVA) followed by the Student-Newman-Keuls multiple
comparisons test was used for comparison between multiple groups.
P<0.05 was considered to indicate a statistically significant
result.
Results
Identification of differentially
expressed genes in lung adenocarcinoma cells after β-elemene
treatment
We compiled data from a large lung cancer sample
data set in the GEO database with our Affymetrix whole-profile gene
expression chip data. We performed gene expression profiling
analysis to identify differentially expressed genes in lung
adenocarcinoma cells after incubation with or without β-elemene.
Specifically, we used the Limma package in R to identify genes
expressed in A549 cells at levels differing by 2-fold between
β-elemene and NC groups with a false discovery rate (FDR) of
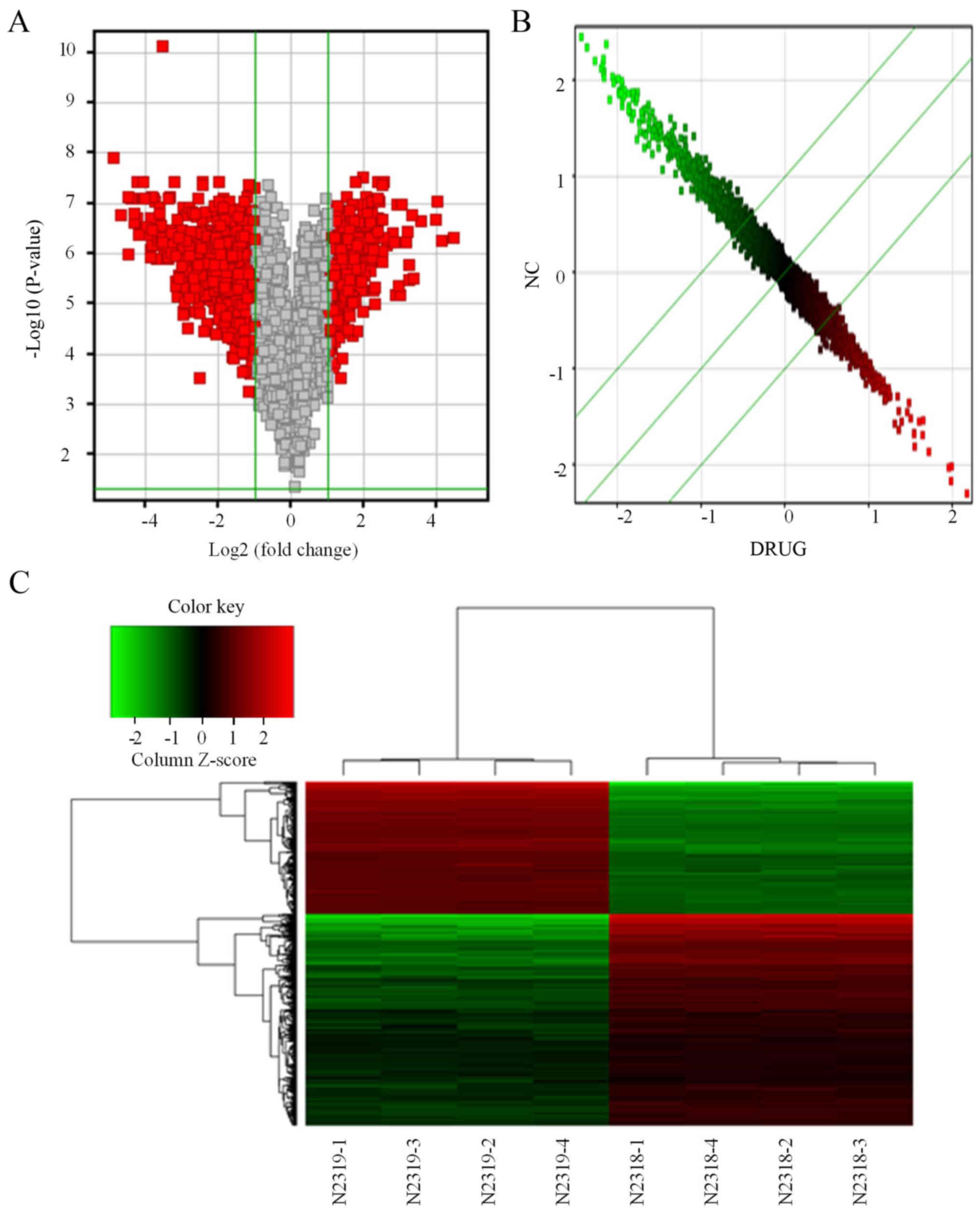
P-value <0.05. We identified 721 differentially expressed genes
in lung cancer cells after treatment with β-elemene, of which 273
were upregulated and 448 were downregulated. The Volcano plot
showed that the distribution of the differential genes between the
treatment and NC groups (Fig. 1A).
The scatter plot data showed the distribution of signal values
between the treatment and NC groups on the plane of the rectangular
coordinate system (Fig. 1B). The
Cluster diagram analysis shows the aggregation of all samples and
differentially expressed genes (Fig.
1C).
Knockdown of POLE2 expression inhibits
A549 and NCI-H1299 cell proliferation and colony formation
We then assessed the effects of POLE2 knockdown on
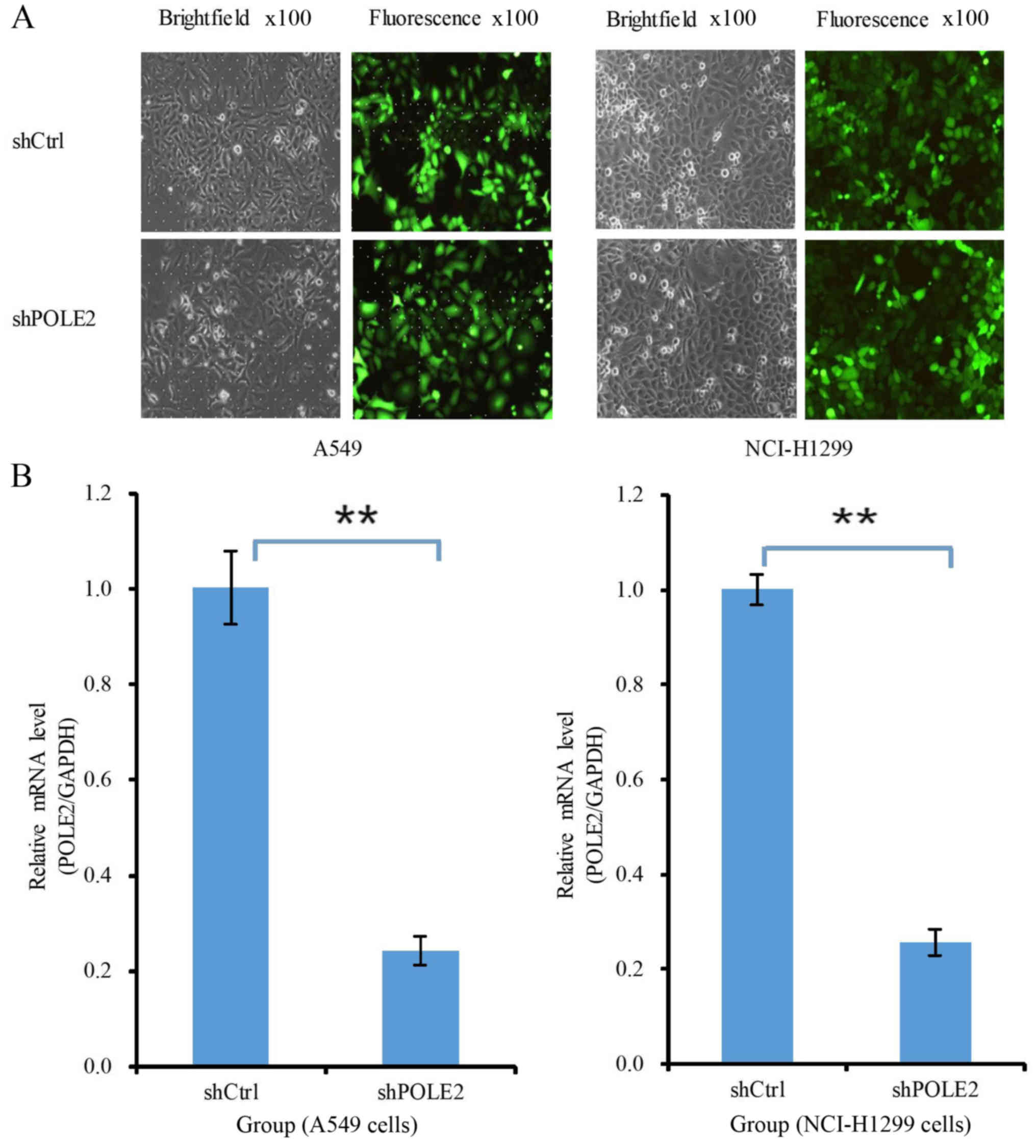
lung cancer cell proliferation. The fluorescence microscopy data
showed that lentiviruses carrying shCtrl and shPOLE2 at MOI of 10
and 5, respectively, to infect A549 and NCI-H1299 cells had an 80%
transfection efficacy at 72 h (Fig.
2A). qRT-PCR data showed that the level of POLE2 mRNA was 75.6%
lower in the shPOLE2-infected A549 cells than that noted in the
shCtrl cells 5 days after infection, and the level of POLE2 mRNA
was 74.3% lower in the shPOLE2-infected NCI-H1299 cells than that
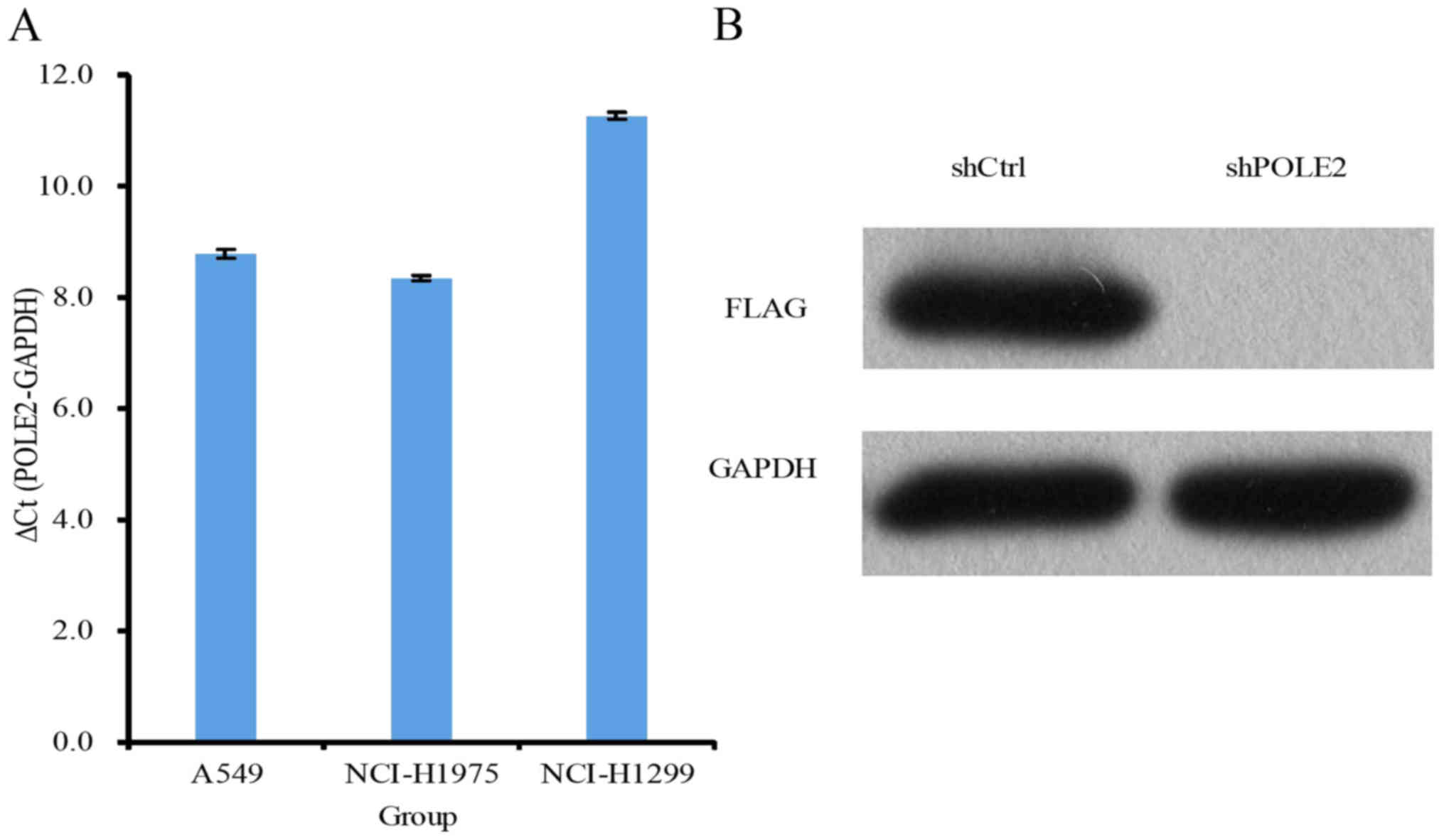
noted in the shCtrl cells 5 days after infection (Fig. 2B). The level of POLE2 expression in
the NCI-H1299 and A549 cells was higher than that in the NCI-H1975
cells (Fig. 3A). We further
assessed the level of POLE2 protein using western blotting and
found significantly lower levels of protein in the shPOLE2
lentivirus-infected A549 cells (Fig.
3B).
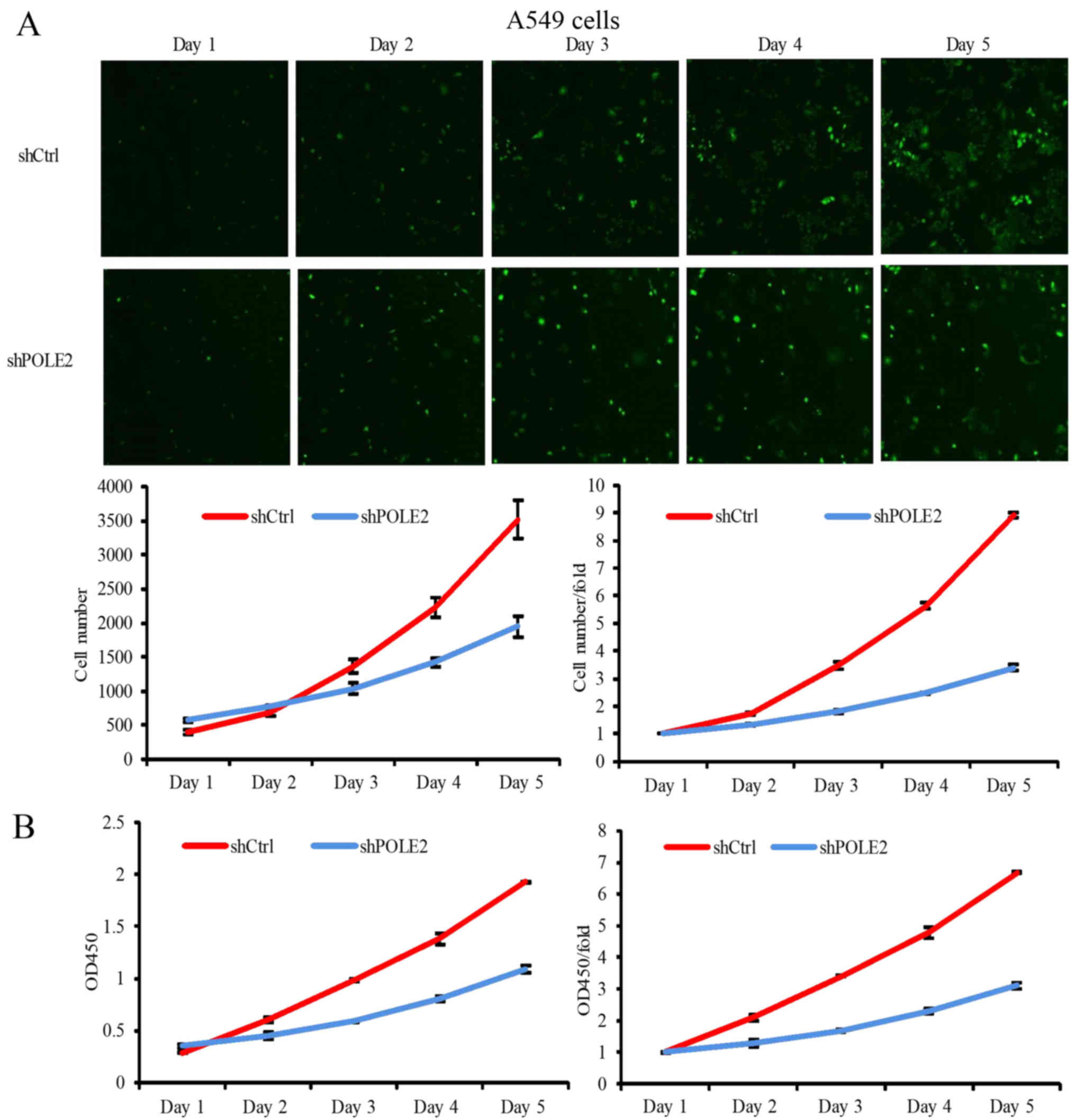
We then assessed proliferation of these infected
A549 and NCI-H1299 cells using the Celigo (Nexcelom Bioscience,
Lawrence, MA, USA) cell count method and found that shCtrl-infected
A549 cells proliferated normally and had an increase in number by
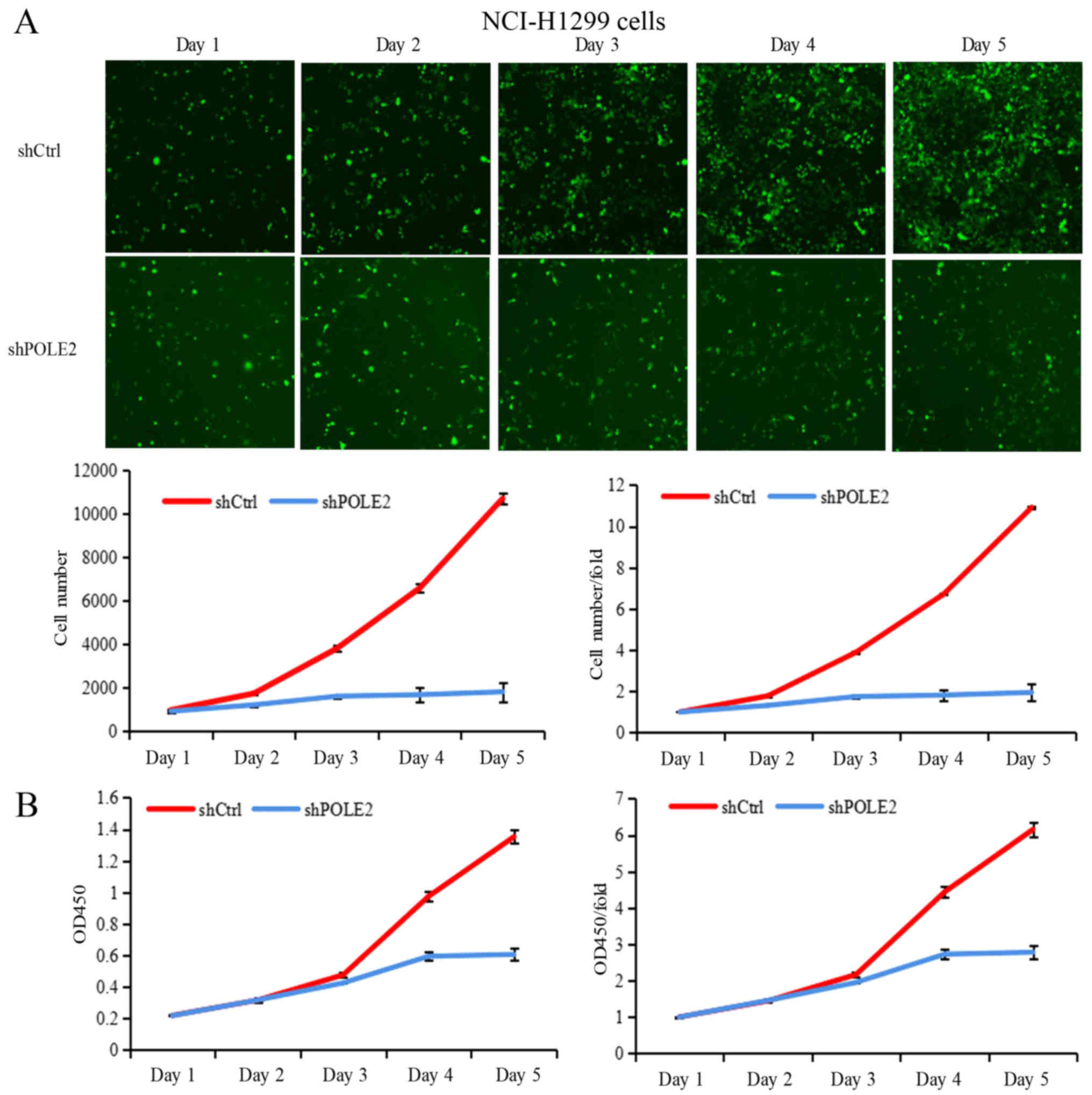
6.07- and 9.53-fold by day 4 and 5, respectively (Fig. 4A). The shCtrl-infected NCI-H1299
cells proliferated normally and had an increase in number by 6.72-
and 10.92-fold by day 4 and 5, respectively (Fig. 5A). In contrast, proliferation of
shPOLE2-infected A549 cells was significantly slower, increasing in
number by only 4.01- and 5.86-fold by day 4 and 5, and
proliferation of shPOLE2-infected NCI-H1299 cells was also
significantly slower, increasing in number by only 1.82- and
1.95-fold by day 4 and 5, respectively (Figs. 4A and 5A). Furthermore, the CCK-8 assay showed
that the proliferation of shPOLE2 A549 and NCI-H1299 cells were
also significantly reduced on day 4 (P<0.001; Figs. 4B and 5B).
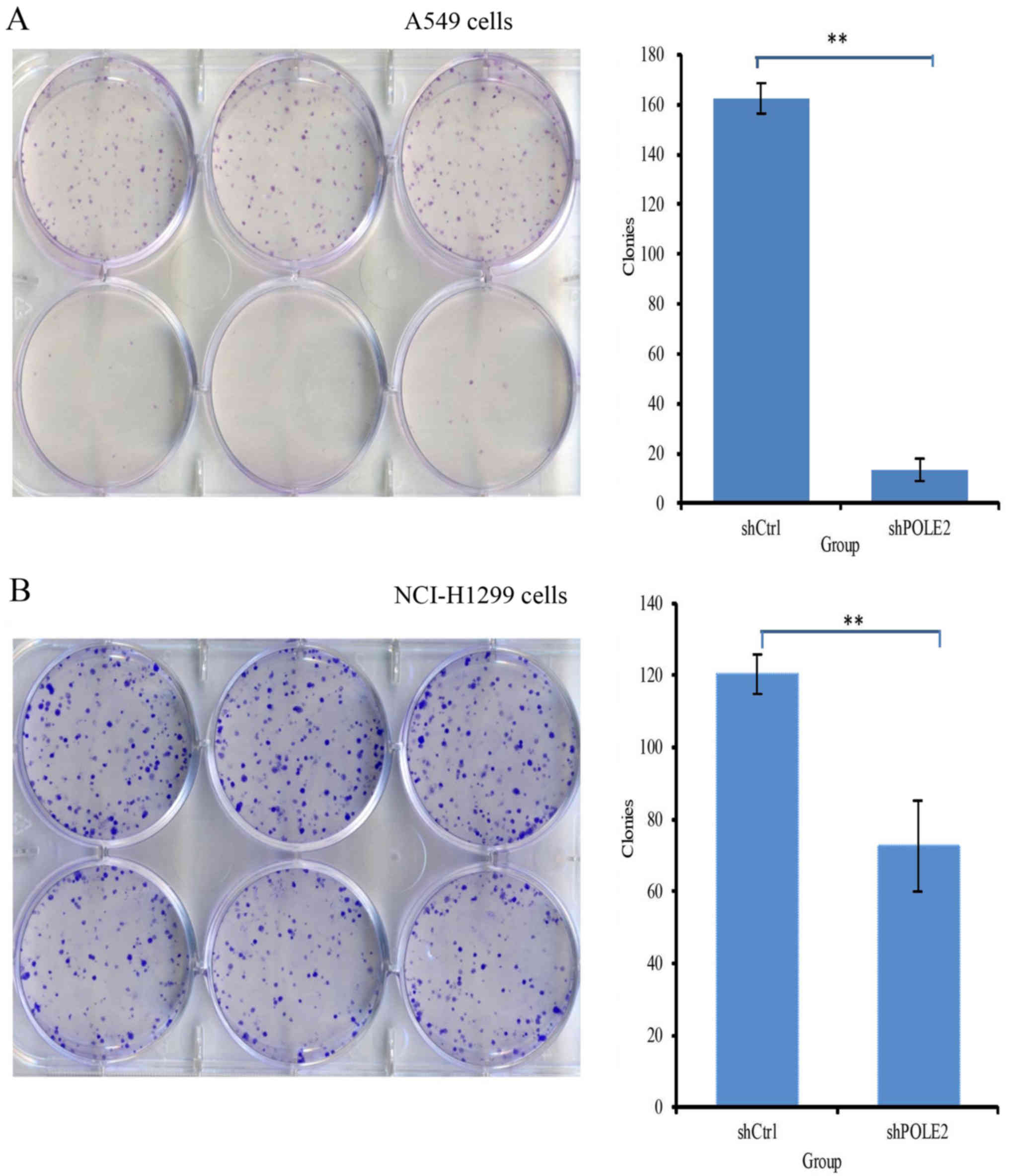
Colony formation measures cell tumorigenic ability
in vitro. After shPOLE2 and shCtrl infection in A549 cells,
the colony forming capacity was 163±6 and 13±5 (P<0.05; Fig. 6A). In NCI-H1299 cells, the colony
forming capacity was 120±6 and 73±13, respectively (P<0.05;
Fig. 6B).
Impact of POLE2 knockdown on A549 and
NCI-H1299 cell apoptosis
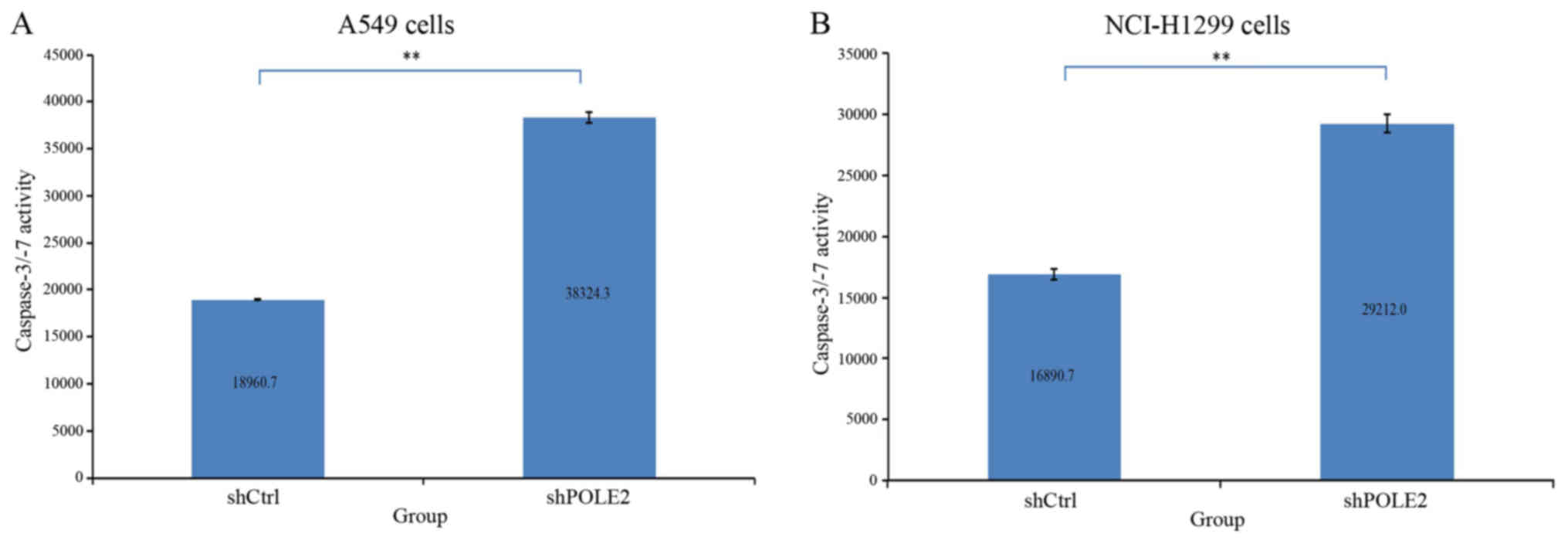
We further assessed the effect of shPOLE2 on A549
and NCI-H1299 cell apoptosis. Forty-eight hours after lentivirus
infection, the activity of caspase-3/-7 was significantly higher in
the shPOLE2-infected A549 and NCI-H1299 cells than the activity
noted in the control cells (P<0.01; Fig. 7). These results indicate that
downregulated POLE2 suppressed A549 and NCI-H1299 apoptosis.
Identification of genes mediating
POLE2 activity in lung cancer cells
To investigate the molecular events that may be
responsible for the observed impact of POLE2, we performed PathScan
stress and apoptosis signaling antibody array analysis in A549
cells infected with shPOLE2 or shCtrl lentiviruses. The
chemiluminescence and gray scale data (Fig. 8A and B) indicated that shPOLE2
altered expression of 19 cell stress and apoptosis-related
signaling molecules. Statistical analysis of the gray values showed
that expression levels of p-Akt, p-Smad2, p-p38 MAPK, p-SAPK/JNK,
cleaved caspase-7, IκBα, p-Chk1, p-IκBα, p-eIF2α, p-TAK1, survivin
and α-tubulin were significantly lower in shPOLE2-transfected cells
and p-p53 was significantly higher in the shPOLE2-transfected cells
when compared with these levels in the shCtrl-infected cells
(P<0.05; Fig. 8C). These data
further indicate that downregulated POLE2 may suppress lung tumor
cell apoptosis.
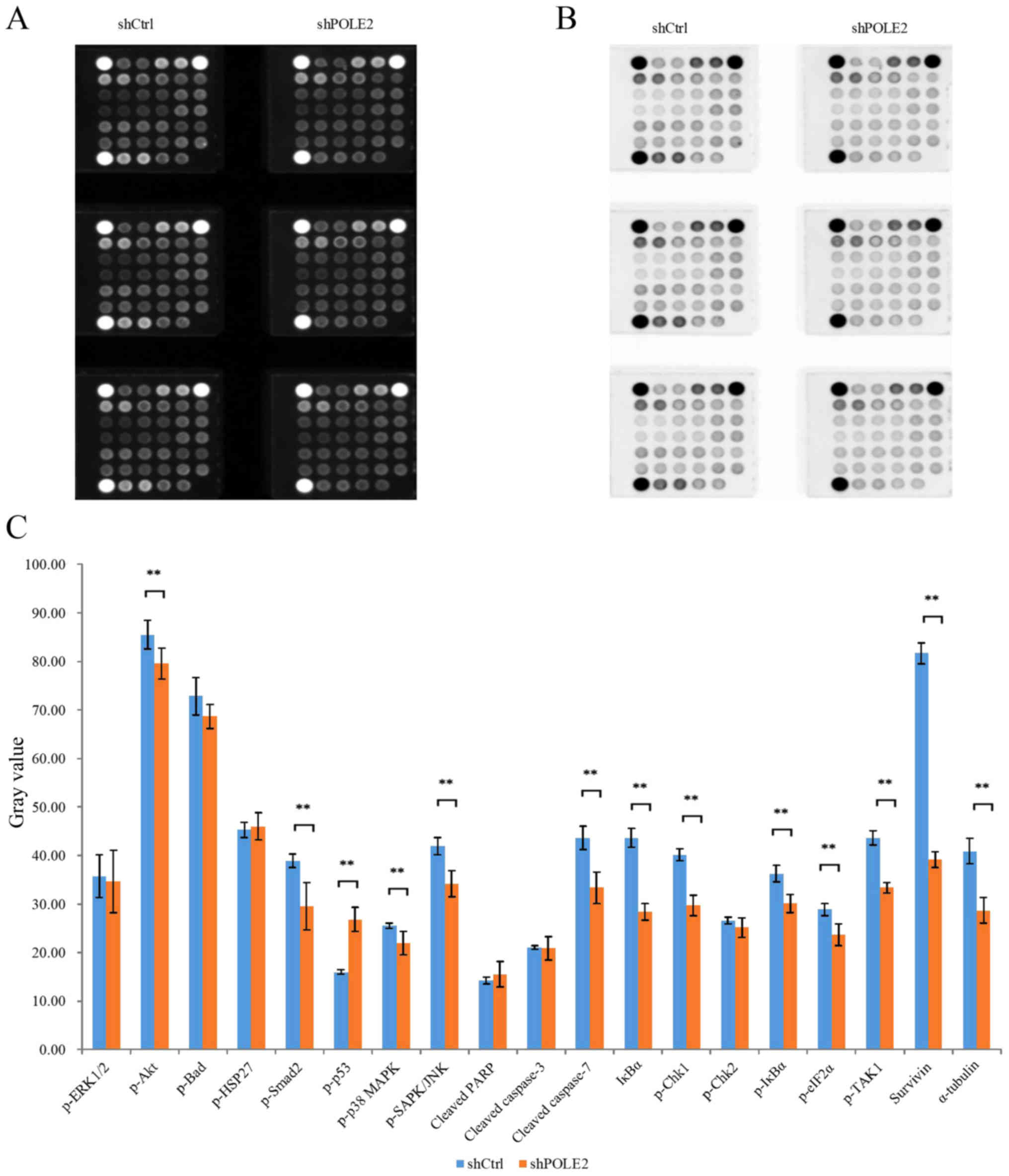 | Figure 8.Identification of shPOLE2 knocked
down-related gene expression. (A and B) A Stress and Apoptosis
Signaling Antibody Array kit was used to generate the
chemiluminescence and gray scale results. (C) Statistical analysis
of the gray values revealed that expression levels of p-Akt,
p-Smad2, p-p38 MAPK, p-SAPK/JNK, cleaved caspase-7, IκBα, p-Chk1,
p-IκBα, p-eIF2α, p-TAK1, survivin and α-tubulin were significantly
decreased in the shPOLE2 compared to shCtrl cells and p-p53 was
significantly increased in the shPOLE2 compared to shCtrl cells
(**P<0.05). |
Discussion
To better understand the antitumor activity of
β-elemene, we profiled differentially expressed genes in A549 cells
after treatment with or without β-elemene. POLE2 was identified as
one of the genes most significantly downregulated by β-elemene. We
then investigated the effects of POLE2 knockdown on lung cancer
cell proliferation and apoptosis. We found that knockdown of POLE2
expression significantly inhibited A549 and NCI-H1299 cell
proliferation and colony formation, but induced tumor cell
apoptosis. Statistical analysis of the gray values revealed that
expression of p-Akt, p-Smad2, p-p38 MAPK, p-SAPK/JNK, cleaved
caspase-7, IkBα, p-Chk1, p-IκBα, p-eIF2α, p-TAK1, survivin and
α-tubulin were significantly lower in the shPOLE2-transfected and
p-p53 was signficantly higher in the shPOLE2-transfected cells than
that noted in the shCtrl-transfected A549 cells (P<0.05).
Further study will be required to explore whether the strategy of
targeting POLE2 expression could control lung adenocarcinoma
clinically.
β-elemene is the active ingredient in Curcuma
wenyujin and is found in other plants (18). β-elemene is one of the second-class
of novel non-cytotoxic broad-spectrum antitumor agents developed in
China as it possesses wide-spectrum antitumor activity and mild
toxicity in various types of human cancers (5–15). To
date, β-elemene has been assessed as a potential complementary,
replacement or alternative medicine for human cancer, including
lung cancer. β-elemene was found to restrain tumor cell synthesis
of DNA, RNA and protein (19) and
mitosis, therefore inhibiting tumor proliferation by arresting
tumor cell cycle and inducing apoptosis (10). Moreover, β-elemene also reversed
resistance of cancer cells to chemotherapy and increased
chemosensitivity (11). β-elemene
was also found to suppress expression of Bcl-2 and Bcl-xL (18,20).
In the present study we revealed that POLE2 was downregulated in
lung cancer cells treated with β-elemene.
The human genome contains at least 15 DNA
polymerases for genome replication and DNA repair (21). The eukaryotic DNA polymerase epsilon
was first isolated from Saccharomyces in 1970, and belongs to the
DNA polymerase B family, which contains four subunits. The largest
subunit is POLE (subunit A), the second largest subunit is POLE2
(subunit B) with a molecular weight of 59 kDa, and the two smaller
subunits POLE3 and POLE4 have molecular weights of 17 and 12 kDa
(22), respectively. POLE2 was
previously reported to be expressed in breast, colorectal cancer,
mantle cell lymphoma, cervical and bladder cancer (21,23–26),
yet to date, alteration of POLE2 expression has not been reported
in lung adenocarcinoma. In the present study, we used Affymetrix
gene expression profile chip and GEO database search to identify a
total of 721 differentially expressed genes, including 273
upregulated and 448 downregulated genes in lung adenocarcinoma A549
cells after incubation with β-elemene.
Our current data show that POLE2 is highly expressed
in three different lung cancer cell lines and we selected A549 and
NCI-H1299 cells as the model in which to knock down POLE2
expression to assess the role of POLE2 in lung cancer. We found
that POLE2 knockdown significantly reduced A549 and NCI-H1299 cell
proliferation and colony formation, but induced tumor cell
apoptosis. These observations are consistent with reports of the
antitumor activity of β-elemene on human cancer cells (9,10). To
date, studies of POLE2 are rare, but a small number of genomic
studies have shown mutations in POLE2 in human diseases such as
colorectal cancer (27,28). Thus, further study is needed to
evaluate and identify the role of POLE2 in lung cancer development
and progression. Furthermore, we also observed that knockdown of
POLE2 expression reduced expression of p-Akt, p-Smad2, p-p38 MAPK,
p-SAPK/JNK, cleaved caspase-7, IκBα, p-Chk1, p-IκBα, p-eIF2α,
p-TAK1, survivin and α-tubulin proteins and the knockdown of POLE2
expression increased the expression of p-p53. These proteins are
involved in cell stress responses, proliferation, and apoptosis,
which further confirmed the tumor-promoting effects of POLE2 in
lung cancer cells. In the present study, caspase-3/-7 activity
assay showed a significant increase in caspase-3/-7 activity after
POLE2 knockdown, but our Stress and Apoptosis Signaling Antibody
Array experiment did not show a significant change in the levels of
cleaved caspase-3, although the change in cleaved caspase-7 was
significant. This discrepancy could be due to the sensitivity of
these two assays, i.e., activity assay is much more sensitive than
that of western blotting. However, the role of POLE2 in apoptosis
needs further investigation.
The present study is simply a proof-of-principle one
and does have some limitations. For example, only two repeats were
performed for certain experiments; cell proliferation was assessed
by using Celigo Cell Counting, CCK-8 and colony formation assays,
while apoptosis was only measured by caspase-3/-7 activity and
Stress and Apoptosis Signaling Antibody Array. In the future, we
will further utilize more apoptotic assays and precisely
characterize the role of POLE2 in lung cancer. In conclusion, this
study for the first time reports that POLE2 is downregulated by
β-elemene. We assessed the tumor-promoting effects of POLE2 in lung
cancer. These data indicate that POLE2 may represent a useful
therapeutic target for the treatment of lung adenocarcinoma.
Acknowledgements
The authors would like to thank Professor Yili Wang
of the Institute of Cancer Research, School of Basic Medical
Sciences, Xi'an Jiaotong University (Shaanxi, China).
Funding
The present study was supported in part by grants
from the National Natural Science Foundation of China (no.
81372857), the Natural Science Foundation of Shaanxi Province,
China (no. 2012JC2-06) and the Science and Technology Planning
Project of Xi'an, Shaanxi Province, China [no.
2016047SF/YX03(3)].
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contribution
JL and YW conceived and designed the experiments;
JL, JY and YZ have been involved in drafting the manuscript or
revising it critically for important intellectual content; JW
performed the Affymetrix Human Gene Expression Array of the A549
cells; YD performed the experiments of gene knockdown and PathScan
antibody array; YF performed the experiments of CCK-8, colony
formation and caspase-3/-7 activity assays. NL and YZ obtained
data, and analyzed and interpreted the data. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable as human or animal tissues were not
utilized in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: The Correlation between EGFR Gene
Mutation and serum tumor markers in lung adenocarcinoma
(unpublished master's thesis)Zhengzhou University. Zhengzhou: 2014,
View Article : Google Scholar
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Lu Y, Wu J, Gao M, Wang A and Xu
B: Beta-elemene inhibits melanoma growth and metastasis via
suppressing vascular endothelial growth factor-mediated
angiogenesis. Cancer Chemother Pharmacol. 67:799–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang H, Ma S and Feng J: In vitro study
of radiosensitization by beta-Elemene in A549 cell line from
adenocarcinoma of lung. Chinese-German J Clin Oncol. 8:12–15. 2009.
View Article : Google Scholar
|
|
7
|
Li QQ, Wang G, Reed E, Huang L and Cuff
CF: Evaluation of cisplatin in combination with β-elemene as a
regimen for prostate cancer chemotherapy. Basic Clin Pharmacol
Toxicol. 107:868–876. 2010.PubMed/NCBI
|
|
8
|
Li QQ, Wang G, Zhang M, Cuff CF, Huang L
and Reed E: beta-Elemene, a novel plant-derived antineoplastic
agent, increases cisplatin chemosensitivity of lung tumor cells by
triggering apoptosis. Oncol Rep. 22:161–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Wang G, Zhao J, Ding H, Cunningham
C, Chen F, Flynn DC, Reed E and Li QQ: Antiproliferative effect of
beta-elemene in chemoresistant ovarian carcinoma cells is mediated
through arrest of the cell cycle at the G2-M phase. Cell Mol Life
Sci. 62:894–904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao CC, Tu YR, Jiang J, Ye SF, Du HX and
Zhang Y: β-elemene reverses the drug resistance of lung cancer
A549/DDP cells via the mitochondrial apoptosis pathway. Oncol Rep.
31:2131–2138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao YQ, Ding X, Jia YC, Huang CX, Wang YZ
and Xu YH: Anti-tumor effect of beta-elemene in glioblastoma cells
depends on p38 MAPK activation. Cancer Lett. 264:127–134. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Z, Wang R, Xu L, Xie S, Dong J and Jing
Y: β-Elemene piperazine derivatives induce apoptosis in human
leukemia cells through downregulation of c-FLIP and generation of
ROS. PLoS One. 6:e158432011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Li QQ, Zou B, Wang G, Li X, Kim
JE, Cuff CF, Huang L, Reed E and Gardner K: In vitro
combination characterization of the new anticancer plant drug
β-elemene with taxanes against human lung carcinoma. Int J Oncol.
31:241–252. 2007.PubMed/NCBI
|
|
15
|
Zhu T, Xu Y, Dong B, Zhang J, Wei Z, Xu Y
and Yao Y: β-elemene inhibits proliferation of human glioblastoma
cells through the activation of glia maturation factor β and
induces sensitization to cisplatin. Oncol Rep. 26:405–413.
2011.PubMed/NCBI
|
|
16
|
Peng X, Zhao Y, Liang X, Wu L, Cui S, Guo
A and Wang W: Assessing the quality of RCTs on the effect of
beta-elemene, one ingredient of a Chinese herb, against malignant
tumors. Contemp Clin Trials. 27:70–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du XS, Yu J, Li JY, Liu A, Yang SY and
Wang YL: Effect of β-elemene on the proliferation and apoptosis of
human lung cancer A549 and H460 cells. Shaanxi Med J. 9:1107–1108.
2015.(In Chinese).
|
|
19
|
Gao HJ and Qin HF: Elemene for non-small
cell lung cancer. Xiandai Shengwu Yixue Jinzhan. 21:4149–4151.
2010.
|
|
20
|
Li YJ, Yu J and Miao Y: Effects of
β-elemene on the proliferation of lung adenocarcinoma cells.
Shaanxi Med J. 2:136–138+164. 2016.
|
|
21
|
Zhou Q, Effati R, Talvinen K, Pospiech H,
Syväoja JE and Collan Y: Genomic changes of the 55 kDa subunit of
DNA polymerase epsilon in human breast cancer. Cancer Genomics
Proteomics. 5:287–292. 2008.PubMed/NCBI
|
|
22
|
Loeb LA and Monnat RJ Jr: DNA polymerases
and human disease. Nat Rev Genet. 9:594–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chubb D, Broderick P, Dobbins SE, Frampton
M, Kinnersley B, Penegar S, Price A, Ma YP, Sherborne AL, Palles C,
et al: Rare disruptive mutations and their contribution to the
heritable risk of colorectal cancer. Nat Commun. 7:118832016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartmann E, Fernàndez V, Moreno V, Valls
J, Hernández L, Bosch F, Abrisqueta P, Klapper W, Dreyling M,
Hoster E, et al: Five-gene model to predict survival in mantle-cell
lymphoma using frozen or formalin-fixed, paraffin-embedded tissue.
J Clin Oncol. 26:4966–4972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu D: Sine oculis homeobox homolog1
promotes cell proliferation and metastasis of cervical cancer
(doctoral thesis)Huazhong University of Science and Technology.
Wuhan: 2014, (In Chinese). View Article : Google Scholar
|
|
26
|
Zekri AR, Hassan ZK, Bahnassy AA, Khaled
HM, El-Rouby MN, Haggag RM and Abu-Taleb FM: Differentially
expressed genes in metastatic advanced Egyptian bladder cancer.
Asian Pac J Cancer Prev. 16:3543–3549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frugoni F, Dobbs K, Felgentreff K,
Aldhekri H, Al Saud BK, Arnaout R, Ali AA, Abhyankar A, Alroqi F,
Giliani S, et al: A novel mutation in the POLE2 gene causing
combined immunodeficiency. J Allergy Clin Immunol. 137(635–638):
e12016.
|
|
28
|
Spier I, Holzapfel S, Altmüller J, Zhao B,
Horpaopan S, Vogt S, Chen S, Morak M, Raeder S, Kayser K, et al:
Frequency and phenotypic spectrum of germline mutations in
POLE and seven other polymerase genes in 266 patients with
colorectal adenomas and carcinomas. Int J Cancer. 137:320–331.
2015. View Article : Google Scholar : PubMed/NCBI
|