Introduction
As one of the most common gastrointestinal diseases,
acute pancreatitis (AP) frequently occurs due to gallstones and
alcohol abuse, with an annual incidence rate of 13–45/100,000 in
USA in 2011 (1). Although 80% of
patients with AP exhibit mild symptoms, including abdominal pain,
fever, nausea and vomiting, ~20% of patients with AP progress to
severe AP (SAP), which has a mortality rate of 20–40% in USA in
2005 (2,3). Furthermore, patients with SAP
frequently develop specific chronic diseases following being
discharged from hospital, including prediabetes, diabetes, chronic
pancreatitis and pancreatic cancer (4,5). At
present, the primary treatment for SAP is limited to supportive
care and treatment of complications (2). However, there are only a limited
number of drugs available, and the efficiency is not satisfactory
(6,7). Therefore, the investigation of an
effective drug or other therapeutic options is critical for the
treatment of SAP in clinic.
SAP begins with premature activation of digestive
enzymes within the pancreatic acinar cell, which can cause acinar
cell necrosis and subsequent activation of the pro-inflammatory
cascade, resulting in systemic inflammatory response syndrome as
well as multiple organ dysfunctions (1,8).
Numerous studies demonstrated that the extent of inflammatory
response positively associates with disease severity; however, the
detailed molecular mechanisms remain unclear (9,10).
Proinflammatory cytokines from activated monocytes and macrophages,
including interleukin (IL)-1β and IL-18, are hypothesized to have
an important function in this cascade (11). Thus, an improved understanding of
IL-1β and IL-18 synthesis and secretion is beneficial for the
development of novel anti-SAP drugs. Recent studies have been
directed to the purinergic receptor P2X ligand-gated ion channel 7
(P2X7), which serves a key role in the maturation of IL-1β and
IL-18 through the recruitment of the NOD-like receptor protein 3
(NLRP3) inflammasome-caspase-1 complex (12–14).
The positive association between the severity of SAP and
pro-inflammatory cascade indicates that the P2X7/NLRP3 signaling
pathway could be a reliable therapeutic target for SAP (9).
Emodin is a natural anthraquinone compound from the
oriental herb Rheum officinale Baill, which has various
physiological effects, particularly its anti-inflammatory
properties (15). It is also the
primary active ingredient of Dachengqi decoction and Qingyi
decoction that have been frequently used for SAP treatment
(16–19). However, the potential therapeutic
mechanism is not fully understood. Previous studies provide
evidence for a novel role of emodin as an antagonist of P2X7R,
which can inhibit ATP-induced IL-1β secretion from rat peritoneal
macrophages through the inhibition of P2X7R activation (20–22).
Han et al (23) determined
the effects of emodin on inflammation-associated disorders,
including endotoxemia, Alzheimer's disease, obesity and
fibromyalgia through the regulation of NLRP3 inflammasome
activation (25,26). In the present study, the effects of
emodin on regulating the P2X7/NLRP3 signaling pathway whilst the
SAP rat model was induced by intraductal infusion of 5.0% sodium
taurocholate, and the functions and mechanisms for its protective
effects were investigated.
Materials and methods
Reagents and materials
Emodin (cat. no. IE0070) and sodium taurocholate
(cat. no. T8510) was obtained from Solarbio Science &
Technology Co., Ltd. (Beijing, China). Rat IL-18 ELISA kit (cat.
no. ab213909), rat IL-1β ELISA kit (cat. no. ab100768), rat
Pancreatic Amylase ELISA kit (cat. no. ab137969) and rat Lipase
ELISA kit (cat. no. ab102524) were obtained from Shanghai Lengton
Bioscience Co., Ltd. (Shanghai, China). The Power Vision Two-Step
histo-staining reagent (cat. no. I003-1) was purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). A BCA protein
assay kit (cat. no. P0010S) was purchased from Beyotime Institute
of Biotechnology (Shanghai, China). Rabbit anti-P2X7 (1:1,000, cat.
no. 1114-1-AP), caspase-1 (1:1,000, cat. no. 22915-1-AP) and
myeloperoxidase (MPO; 1:100, cat. no. 22225-1-AP), GAPDH-conjugated
Affinipure IgG (1:800, cat. no. 10494-1-AP), horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:300, cat. no.
SA00001-2) and tetramethylrhodamine (TRITC)-conjugated goat
anti-rabbit IgG (H+L) (1:300, cat. no. SA00007-2) were purchased
from Proteintech Group, Inc. (Chicago, IL, USA). Rabbit anti-NLRP3
(1:1,000, cat. no. bs-6655R) and rabbit anti-apoptosis-associated
speck-like protein containing a C-terminal caspase recruitment
domain (ASC) (1:1,000, cat. no. bs-6741R) were purchased from
Biosynthesis Biotechnology Co., Ltd. (Beijing, China). All
antibodies were diluted in TBST buffer (20 mM Tris-HCl, 500 mM NaCl
and 0.05% Tween-20; pH 7.5).
Experimental animals
A total of 48 male Sprague-Dawley (SD) rats with
body weight 250±20 g were obtained from the Experimental Animal
Center of Dalian Medical University (Dalian, China). SD rats were
kept at 21±2°C with 50±10% relative humidity and a 12/12 h
light/dark cycle, with free access to standard laboratory feed and
water. The experimental protocol was approved by the Ethical
Committee for Laboratory Animal Care and Use of Dalian Medical
University.
Animal model
SD rats were randomly divided into 4 groups (n=12),
including: Sham operation (SO); SAP model (SAP); and low-dose (30
mg/kg) and high-dose (60 mg/kg) emodin-treated groups. SAP was
induced according to our previously described method (19). Briefly, rats were anesthetized with
2.5% sevoflurane in an induction chamber following fasting for 12
h. Subsequently, the pancreas was exposed along a midline incision.
The biliopancreatic duct was cannulated through the duodenum, and
the hepatic duct was closed by a microvascular clamp, temporarily.
Following this, SAP was induced by a standard retrograde infusion
of 5.0% sodium taurocholate (0.1 ml/100 g body weight) into the
biliopancreatic duct. Finally, the pancreas was carefully replaced
and the abdomen was closed. The SO group was administered with
sterile saline. Additionally, emodin was suspended in 0.5% sodium
carboxymethylcellulose (CMC-Na). Emodin was intra-gastrically
applied to the rats immediately and at 6 h after the administration
of sodium taurocholate. The SO and SAP groups were administered
with 0.5% CMC-Na of equivalent volume. Samples were obtained at 12
h after the model was established. Blood samples were obtained for
biochemical analyses. The pancreatic head was fixed with 10%
formalin for 24 h at room temperature and embedded in paraffin for
histopathological examination. For western blot analyses, pancreas
samples were immediately frozen and maintained at −80°C.
Plasma amylase and lipase assays
Plasma amylase and lipase levels were assayed with
commercially available rat Pancreatic Amylase ELISA kit and rat
Lipase ELISA kit, according to the manufacturer's protocols. The
absorbance at 450 nm representing the relative level of amylase and
lipase in each well was measured by a microplate reader purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Histopathological examination
Formalin-fixed tissue samples from each group were
sliced (5 µm), dewaxed and stained with hematoxylin and eosin
(H&E) for histological examination (hematoxylin for 5 min and
0.5% eosin for 3 min) at 37°C. Images were captured using a light
microscope (Leica DM4000B; Leica Microsystems GmbH, Wetzlar,
Germany) at ×200 magnification. The pathological scores were
evaluated based on the scoring system detailed in Table I (16).
 | Table I.Histological scoring for acute
pancreatitis. |
Table I.
Histological scoring for acute
pancreatitis.
| Condition | Score | Description |
|---|
|
| 0 | Absent |
|
| 1 | Focally increased
between lobules (localized enlargement of the pancreas secondary
tointerstitial or inflammatory edema without necrosis) |
| Edema | 2 | Diffusely increased
between lobules |
|
| 3 | Acini disrupted and
separated |
|
| 4 | 3 + diffuse
expansion in intercellular septas |
|
| 0 | Absent |
|
| 1 | Around ductal
margin |
| Inflammation | 2 | In parenchyma
(<50% of lobules) |
|
| 3 | In parenchyma
(51–75% of lobules) |
|
| 4 | In parenchyma
(>75% of lobules) |
| Vacuolization | 0 | Absent |
|
| 1 | Periductal
(<5%) |
|
| 2 | Focal (5–20%) |
|
| 3 | Diffuse
(21–50%) |
|
| 4 | Severe
(>50%) |
Transmission electron microscope
assay
A small portion of pancreas (<1 mm3)
were fixed overnight at 4°C in 2% glutaraldehyde, followed by
postfixed with 1% osmium tetroxide for 2 h at 4°C after washing 3
times (15 min/time) in 0.1 M sodium cacodylate buffer (cat. no.
97068, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Subsequently, tissues were dehydrated in 50, 70, 95 and 100%
ethanol solutions (15 min per concentration) and embedded in
epoxyresin. Ultrathin sections were sliced (70 nm) and collected on
copper grids. The obtained sections were stained with uranyl
acetate (2 h at room temperature) and lead citrate (5 min at room
temperature), and then observed using a JE-2000EX transmission
electron microscope (JEOL Ltd, Tokyo, Japan) at ×12,000
magnification.
Immunohistochemical detection of
MPO
The Power Vision Two-Step Histo-staining Reagent was
used to determine the MPO expression in the pancreas. Briefly, at
room temperature, tissue sections (5 µm) of 10% formalin-fixed
samples in each group were dewaxed in xylene (10 min) and 50, 70,
95 and 100% ethanol (5 min each) and washed 3 times in tap water.
Endogenous peroxidase activity was blocked with 3% (v/v)
H2O2, and the sections were incubated with 5%
bovine serum albumin for 10 min at room temperature. Rabbit
polyclonal anti-MPO antibody (1:100) was incubated overnight at
4°C. Subsequently, sections were washed with PBS 3 times for 15 min
and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:300) for 1 h at 37°C. Images were captured with
a light microscope (Leica DM4000B) at ×200 magnification. Integral
optic density (IOD) of the images was counted using Image Pro Plus
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Immunofluorescence detection of
MPO
Immunofluorescence staining of MPO was performed
with pancreas sections as described by Liu et al (20). The sections were dewaxed in a
gradient of ethanol (50, 70, 95 and 100%; 5 min per concentration)
at room temperature, and blocked with 5% bovine serum albumin for
20 min at room temperature following washing 3 times with PBS (5
min/time). Subsequently, the slides were treated with rabbit
polyclonal anti-MPO antibodies (1:100) at 37°C for 2 h, and then
incubated with TRITC-conjugated goat anti-rabbit IgG (H+L) (1:300)
for 1 h at room temperature in the dark. Digital images were
captured with an Olympus BX63 fluorescent microscope (Olympus BX63;
Olympus Corporation, Tokyo, Japan) at ×200 magnification.
Fluorescent intensity of the images was calculated using Image Pro
Plus 6.0 software.
Western blotting assay
Total protein was extracted from the pancreas
tissues in different groups using a whole protein extraction kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), and the protein
concentration was assayed using a BCA protein assay kit. Samples
(50 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. The blots were blocked with 5%
non-fat milk at room temperature for 3 h, and then incubated
overnight at 4°C with the primary antibodies against P2X7, NLRP3,
ASC and caspase-1 (1:1,000 dilution). Subsequently, the blots were
incubated with the horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:1,000) for 4 h at room temperature. Proteins
were detected by BeyoECL Plus enhanced chemiluminescence substrate
(cat. no. P0018; from Beyotime Institute of Biotechnology). Protein
bands were visualized with a ChemiDoc XRS bioimaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Bands were
normalized with GAPDH (1:800 dilution with TBST buffer) as an
internal control.
Plasma IL-1β and IL-18 assay
Plasma IL-1β and IL-18 levels were detected by Rat
IL-18 ELISA kit and rat IL-1β ELISA kit (cat. no. ab100768),
according to the manufacturer's protocols. Briefly, the plasma in
each group was transferred into a 96-well plate with a coating
layer of IL-1β and IL-18 antibodies, and incubated at 37°C for 60
min. Following this, the plate was washed 5 times with diluted
washing solution. Subsequently, the chromogen solution and stop
solution was sequentially added into each well for incubation of 10
min at room temperature in the dark. Finally, the absorbance at 450
nm in each well was measured by a microplate reader (Thermo Fisher
Scientific Inc.). The standard curve was established to calculate
the concentration of IL-1β and IL-18.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. The differences between the groups
were analyzed using one-way analysis of variance with the least
significance difference test. P<0.05 was considered to indicate
a statistically significant difference.
Results
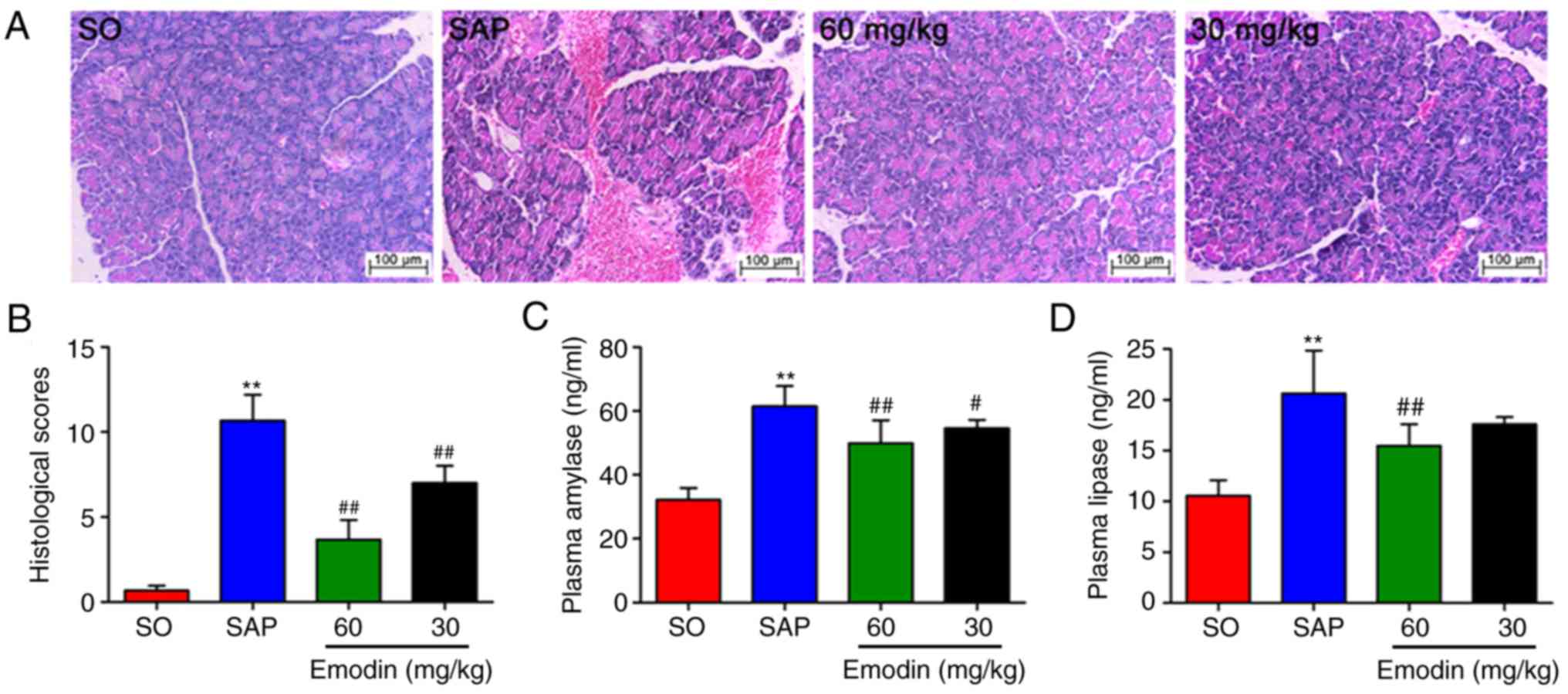
Emodin alleviates pancreatic injury in
SAP rats
As depicted in Fig. 1A
and B, there were no distinct pathological changes in the SO
group, whereas H&E staining results revealed that pancreatic
injury in SAP rats contained large areas of tissue edema, together
with leukocyte infiltration, acinar cell vacuolization, necrosis
and hemorrhage, and the histological scores were significantly
increased (P<0.01, SAP vs. SO). However, alleviated pancreatic
injury and decreased pancreatic histological scores were observed
in the emodin-treated groups, particularly in the high-dose (60
mg/kg) group (P<0.01; Emodin 60 mg/kg vs. SAP).
To examine the effect of emodin on the severity of
SAP, the activities of plasma amylase and lipase were examined
using ELISA kits. The results confirmed that the plasma amylase and
lipase levels in the SAP group were 2-fold increased, compared with
the SO group (P<0.01). However, the application of emodin
notably downregulated these elevations in a dose-dependent manner
(Fig. 1C and D; P<0.01, Emodin
60 mg/kg vs. SAP). These results indicated the beneficial effects
of emodin on pancreatic injury in SAP rats.
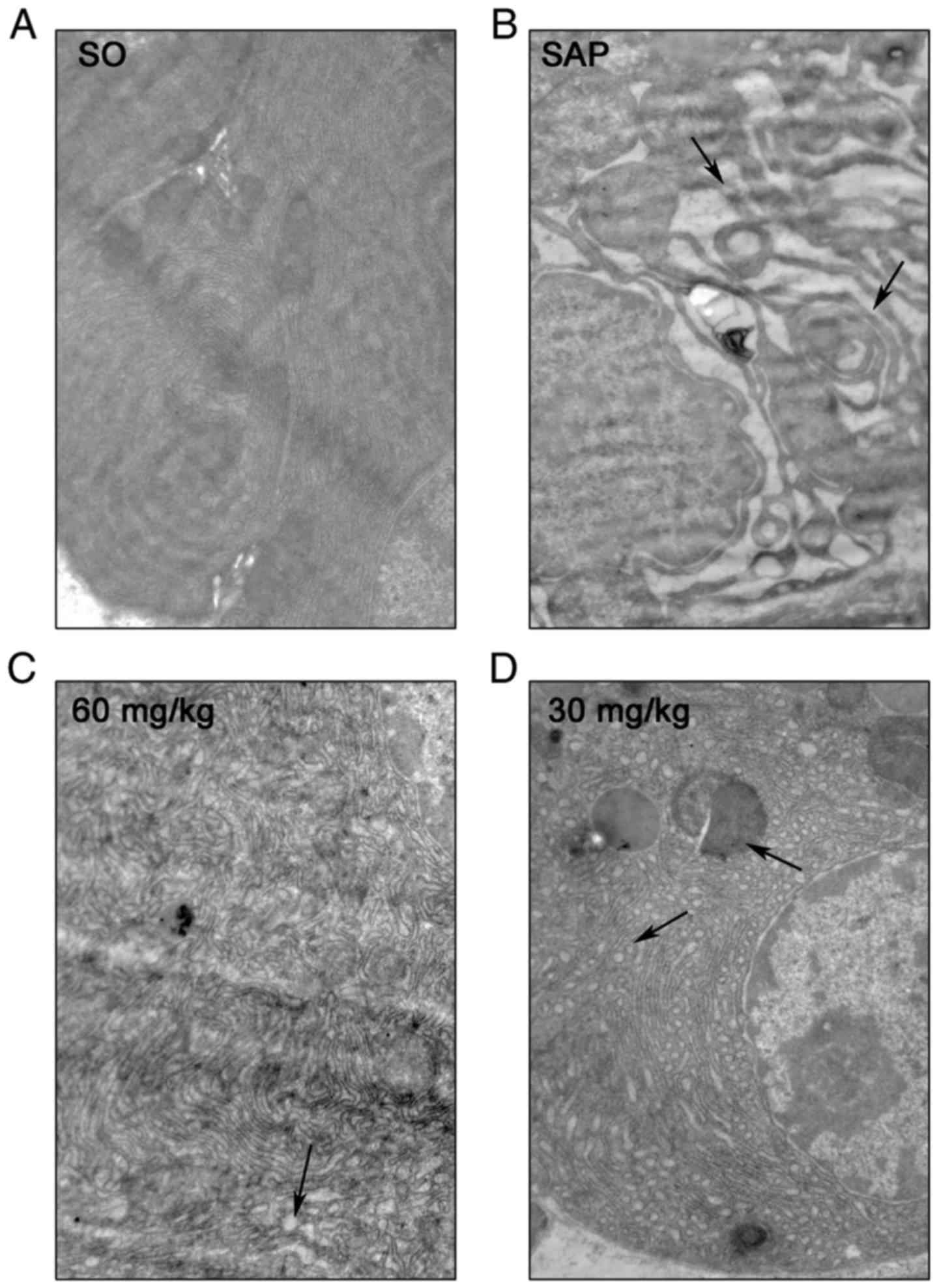
Emodin attenuates acinar cellular
structure injury in SAP rats
The detailed characterization of ultrastructural
changes in rat pancreatic acinar cells following SAP injury was
observed with transmission electron microscope technology at
×12,000 magnification. Electron micrography of acinar cells in the
SO group demonstrated a regular cellular structure with a rough
endoplasmic reticulum (RER)-rich basal region (Fig. 2A). However, in the SAP group, focal
dilation and degranulation of RER was observed in acinar cells,
with an accumulation of autophagic vacuoles and dense materials.
Additionally, notable damage to the solitary mitochondria and
widespread blebbing of the cytoplasm were observed in the basal
zones of acinar cells (Fig. 2B;
black arrow). Treatment with emodin notably ameliorated acinar
cellular structure injury in a dose-dependent manner (Fig. 2D and C). These results further
demonstrated that emodin possessed a potent protective effect on
the ultrastructure of acinar cells following inducing SAP
damage.
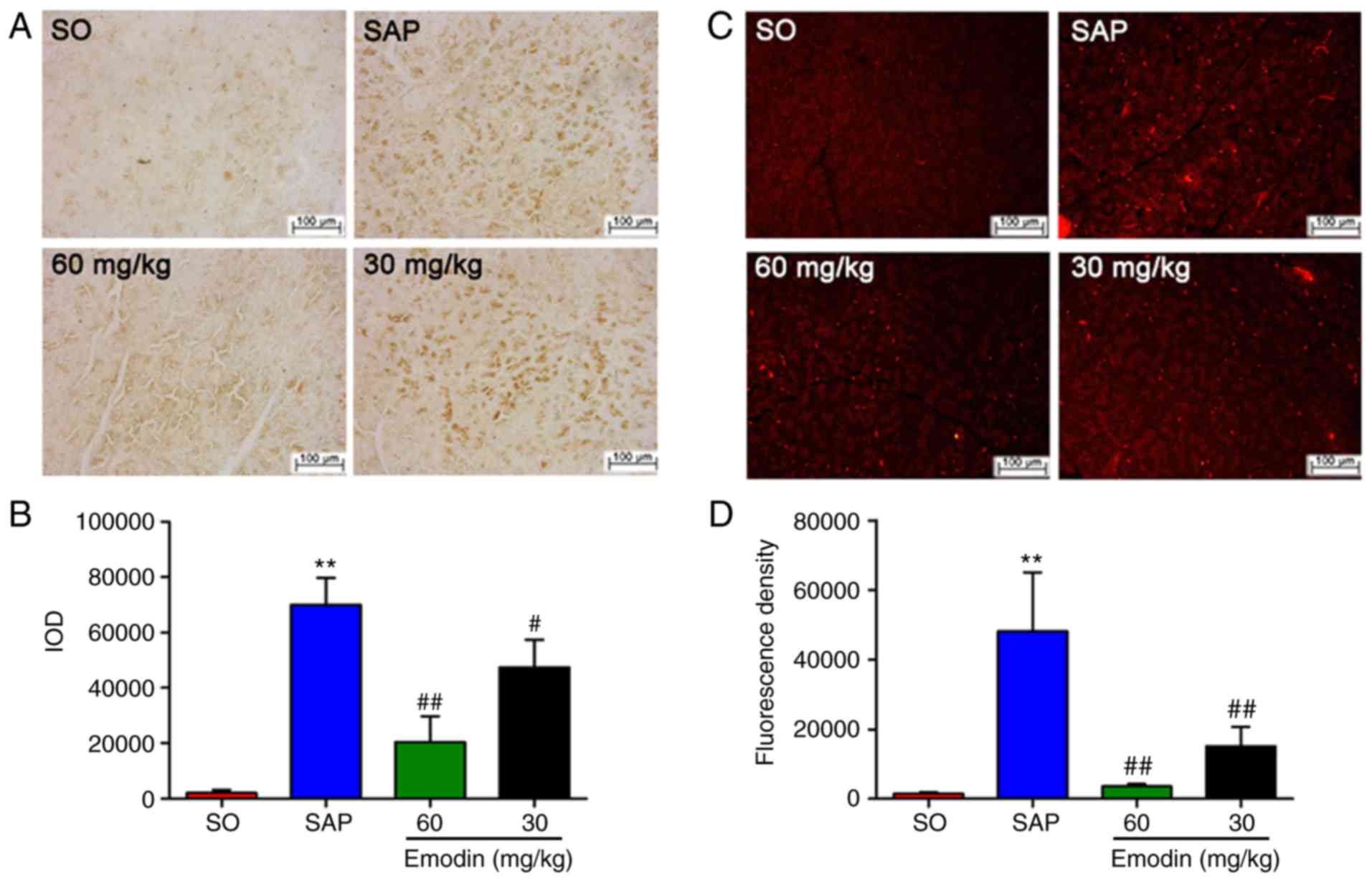
Emodin downregulates the expression
levels of MPO in the pancreatic tissue of SAP rats
MPO content is considered as the indicator of local
neutrophil sequestration in the pancreas following inducing SAP
(21). In the present study, the
images of immunohistochemical analysis indicated that the rats in
the SAP group exhibited an increased MPO-positive area in the
pancreas, compared with the SO group. Following emodin treatment,
the protein expression of MPO was significantly decreased
[P<0.01, Emodin (60 or 30 mg/kg) vs. SAP; P<0.05, Emodin 30
mg/kg vs. SAP], thereby downregulating IOD in a dose-dependent
manner, compared with the SAP group (Fig. 3A and B).
Furthermore, immunofluorescence indicated that MPO
activity was significantly increased following the induction of
SAP. Emodin administration could notably downregulate pancreatic
MPO expression, as manifested by the reduced fluorescence
intensity, compared with the SAP group [Fig. 3C and D; P<0.01, Emodin (60 or 30
mg/kg) vs. SAP]. These data indicated that emodin could decrease
neutrophil infiltration into the pancreas and block the
inflammation cascade.
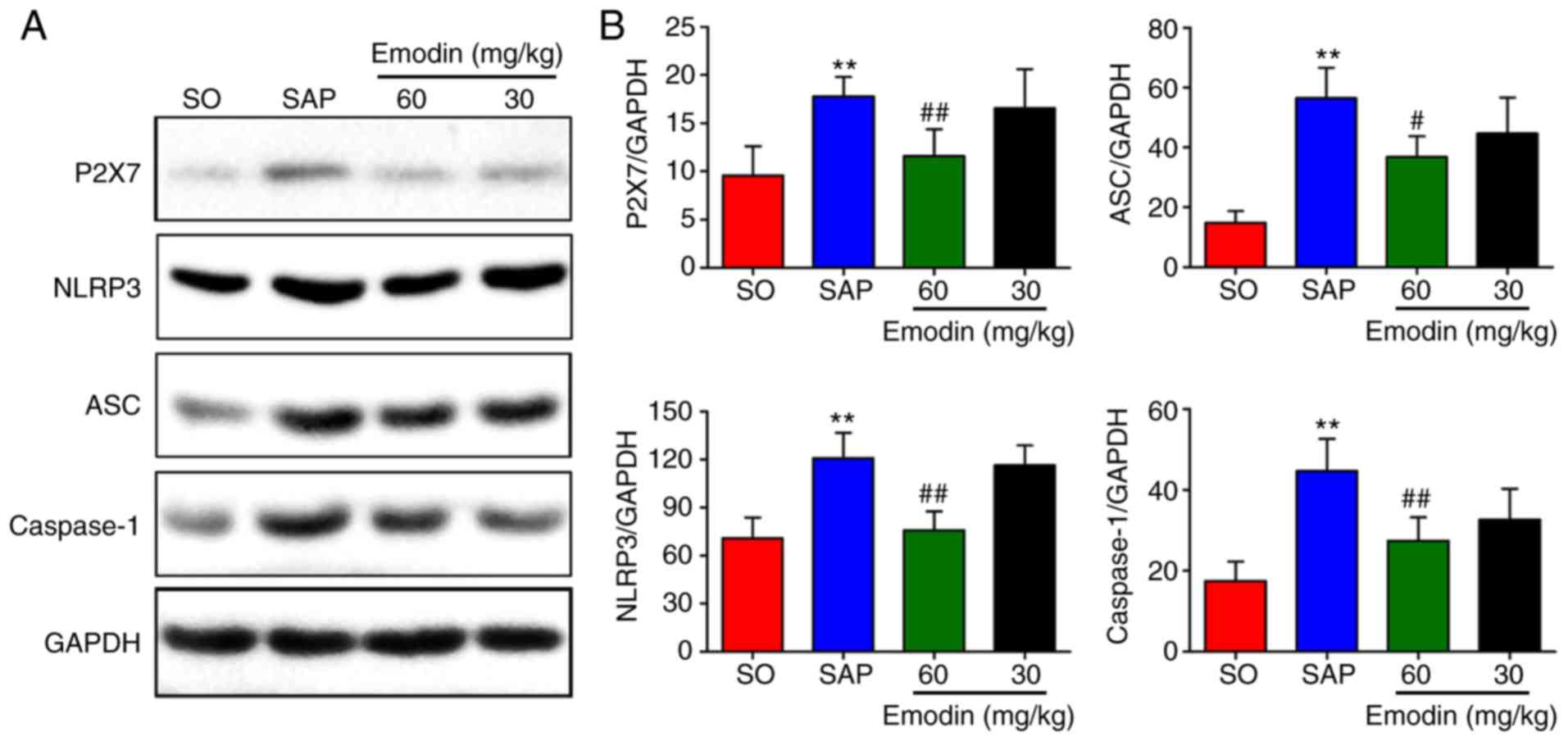
Emodin inhibits the expression of
P2X7/NLRP3 pathway-associated proteins in pancreatic tissues
In the present study, whether the P2X7/NLRP3
signaling pathway is involved in the protective effects of emodin
on SAP in rats was investigated. As depicted in Fig. 4A-D, the expression levels of P2X7,
NLRP3, ASC and caspase-1 in the pancreatic tissue were
significantly increased (P<0.01, SAP vs. SO) Furthermore,
treatment with emodin (30 mg/kg) inhibited the expression levels of
P2X7, NLRP3, ASC and caspase-1, but the difference was not
statistically significant (P>0.05, Emodin 30 mg/kg vs. SAP).
However, these inhibitions were significant in the high-dose
emodin-treated (60 mg/kg) group (P<0.01, Emodin 60 mg/kg vs.
SAP). The western blot results indicated that emodin inhibited the
P2X7/NLRP3-mediated signaling pathway.
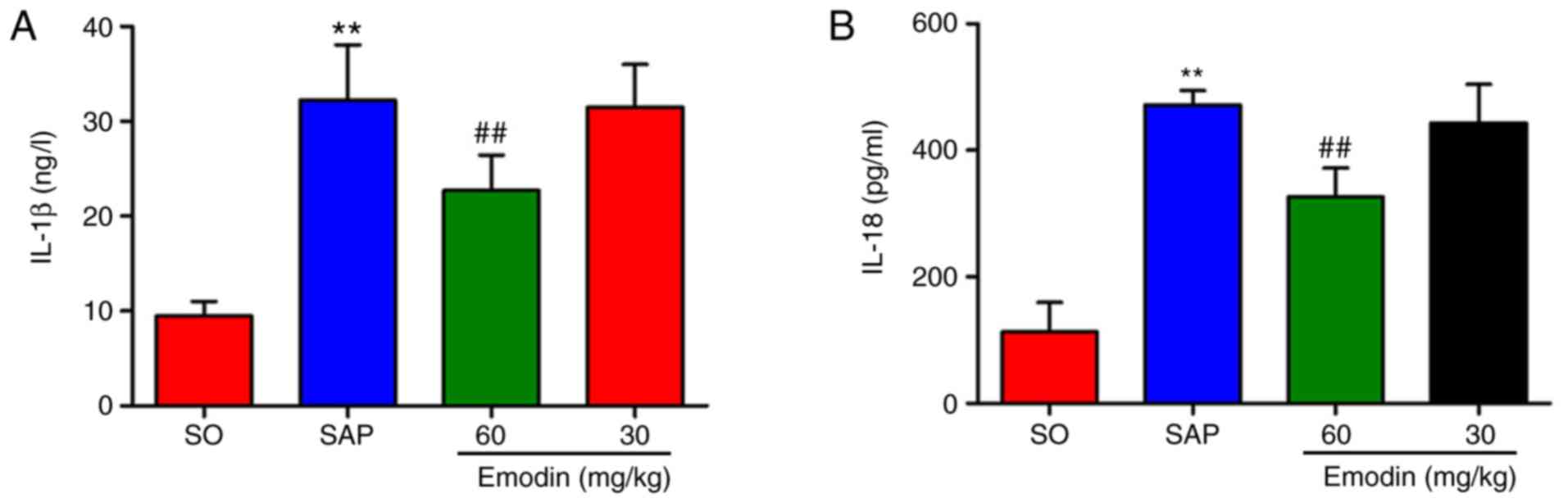
Emodin reduces the plasma
concentrations of IL-1β and IL-18
The in vivo effects of emodin on IL-1β and
IL-18 release was also investigated. As depicted in Fig. 5A and B, compared with the SO group,
the plasma levels of the IL-1β and IL-18 were significantly
increased following the SAP event (P<0.01, SAP vs. SO), which
were reduced when emodin (60 mg/kg) was administered (P<0.01,
Emodin 60 mg/kg vs. SAP). The levels of these 2 pro-inflammatory
cytokines were reduced in the low-dose emodin-treated group, but
this was not statistically significant, while this reduction was
significantly decreased in the high-dose emodin-treated group
(P<0.01, Emodin 60 mg/kg vs. SAP).
Discussion
SAP is a common clinical condition with high
incidence of morbidity and mortality due to the lack of effective
medicine against this disease (1,2).
Therefore, novel drugs with high efficacy and minimal side effects
are vital for SAP treatment. Studies have indicated that emodin is
a potential candidate in the treatment of clinical and experimental
SAP, but the underlying mechanisms by which emodin performs its
pharmacological activities, particularly on anti-inflammatory
effects during SAP, remain unknown (18,19,27).
Pancreatic histopathological alterations, including
interstitial edema, inflammatory cell infiltration and acinar cell
vacuolization, are the crucial alterations for evaluating
pancreatic damage (19). In the
present experiments, the classic rat model of SAP induced by
intraductal infusion of 5.0% sodium taurocholate was used and it
was demonstrated that emodin may improve SAP-induced
histopathological and ultrastructural alterations in a
dose-dependent manner. According to the Atlanta classification and
definitions, pancreatic digestive enzymes, including amylase and/or
lipase, are the most common biochemical hallmarks of SAP (8). Additionally, treatment with emodin may
decrease plasma amylase and lipase levels as well as MPO activities
in pancreatic tissues of SAP rats.
SAP is an aseptic inflammation characterized by a
large number of pro-inflammatory cytokines released from damaged
acinar cells (11). P2X7, as a
member of the P2X family of ATP-gated cation channels, is an
important molecule in inflammation (28). It has a long (250 amino acid)
intracellular C-terminal domain, which enables the coupling of
channel activation to a complex and diverse downstream signaling
cascade (12). The activation of
P2X7 stimulates multiple signaling pathways, including reactive
oxygen species (ROS) and mitogen-activated protein kinase, and
nuclear factor (NF)-κB transcription factors, with the latter
producing a large number of inflammatory mediators (29–31).
Recent studies demonstrated that P2X7 activation is one of the most
effective stimuli for NLRP3 inflammasome activation (12,32,33).
The NLRP3 inflammasome is a macromolecular multiprotein complex
with a molecular weight of ~700 kDa, which composes of NLRP3,
adaptor protein ASC and effector protein caspase-1 (34). NLRP3 is one of the members of the
NOD-like receptor family of cytoplasmic recognition receptors that
recognizes intrinsic hazards and external stress signals, including
ATP and ROS (35). As a core
protein, activated NLRP3 recruits downstream proteins ASC and
caspase-1 to form the NLRP3 inflammasome (36). Following the assembly, the inactive
caspase-1 precursor is activated into caspase-1, which promotes
cleavage, maturation and release of inactivated pro-IL-1β and
pro-IL-18, and induces the inflammatory cascade (37). Studies demonstrated that NLRP3 can
also increase the expression of IL-1β precursor by activating the
NF-κB signaling pathway and further enhance the effect of the NLRP3
inflammasome (21,22,38).
To investigate whether the protective functions of emodin
administration derived from the P2X7/NLRP3 signaling pathway, the
expression levels of P2X7, NLRP3, ASC and caspase-1 in pancreatic
tissues were examined. It was determined that all 4 protein
expression levels were significantly enhanced in the SAP group,
compared with the SO group, indicating that the P2X7/NLRP3
signaling pathway-associated proteins participated in SAP, while
the expression levels of these 4 proteins were decreased following
emodin administration (vs. SAP). This indicated that the protective
effects of emodin against SAP may be partially through inhibiting
the activation of P2X7 and NLRP3 inflammasome. Numerous studies
demonstrated that P2X7R is primarily expressed in rodent duct cells
in the pancreas, which regulates calcium signaling and ion
transport (13,14,39).
Notably, Novak et al (40)
demonstrated that the P2X7 receptors are also expressed in exocrine
glands. The acini in the pancreas exhibits low functionality of
purinergic receptor signaling and apparent lack of P2X7 receptors,
but the pancreatic duct cells highly expressed various P2
receptors, particularly P2X7 receptors (40). Additionally, the initial injury in
SAP is characteristically sterile, which results in acinar cells
necrosis (41). Furthermore, the
sterile inflammatory response mediated through damage associated
molecular patterns (DAMPs) released from necrotic acinar cells
serve a vital role in the process of pancreatic injury, which
functions through the plasma membrane P2X7 receptor (42). Further studies will focus on the
regulating effect of emodin on the DAMPs system in SAP.
Additionally, based on the present data, the P2X7/NLRP3 mediated
inflammatory signaling pathway was activated at 12 h after the
event. However, inflammation primarily depends on the time-course,
which indicated that P2X7 induction would be associated with the
severity of pancreatitis. This is an notable expectation for P2X7
research in pancreatitis in the future. IL-1β and IL-18 are
important pro-inflammatory cytokines regulated by the NLRP3
inflammasome, and serve a central role in the regulation of
inflammation and innate immune system during inflammatory diseases,
including Crohn's disease and Blau syndrome (12,43).
IL-1β is considered to be one of the earliest and most potent
pro-inflammatory factors synthesized and released in response to
infectious and injuries, and therefore it is significant to septic
and sterile inflammation (11,44).
Previous studies demonstrated that IL-1β is associated with the
severity of SAP, and inhibition of IL-1β can relieve SAP (45,46).
Mature IL-1β binds to IL-1β receptors to regulate the modification
and activation of proinflammatory cytokines, including tumor
necrosis factor (TNF)-α, IL-8 and IL-17 (47). IL-18 is a novel pro-inflammatory
cytokine with a similar structure and function to IL-1β (48). Recently, numerous studies focused on
the important roles that IL-18 serves in the synthesis and
secretion of IL-1, TNF-α and other chemokines (12,23).
Therefore, blocking the overproduction of these proinflammatory
factors indicates a potential efficient route for SAP therapy.
In the present study, the levels of plasma IL-1β and
IL-18 following emodin treatment were analyzed. Results
demonstrated that the levels of IL-1β and IL-18 in the SAP group
were significantly increased, compared with the SO group, while
they were slightly reduced in the low-dose emodin-treated group,
but notably decreased in the high-dose emodin-treated group. This
indicated a potential suppression function of emodin on the
P2X7/NLRP3 signaling pathway. The rat model that was used in the
present study is a typical model of SAP (24). According to our previous studies, at
6 h after the induction, the rat exhibited a notable increase of
inflammatory level, which could mimic the clinic characteristics of
patients with SAP. Therefore, using the 6 h time point for the
administration of emodin would provide the highest beneficial
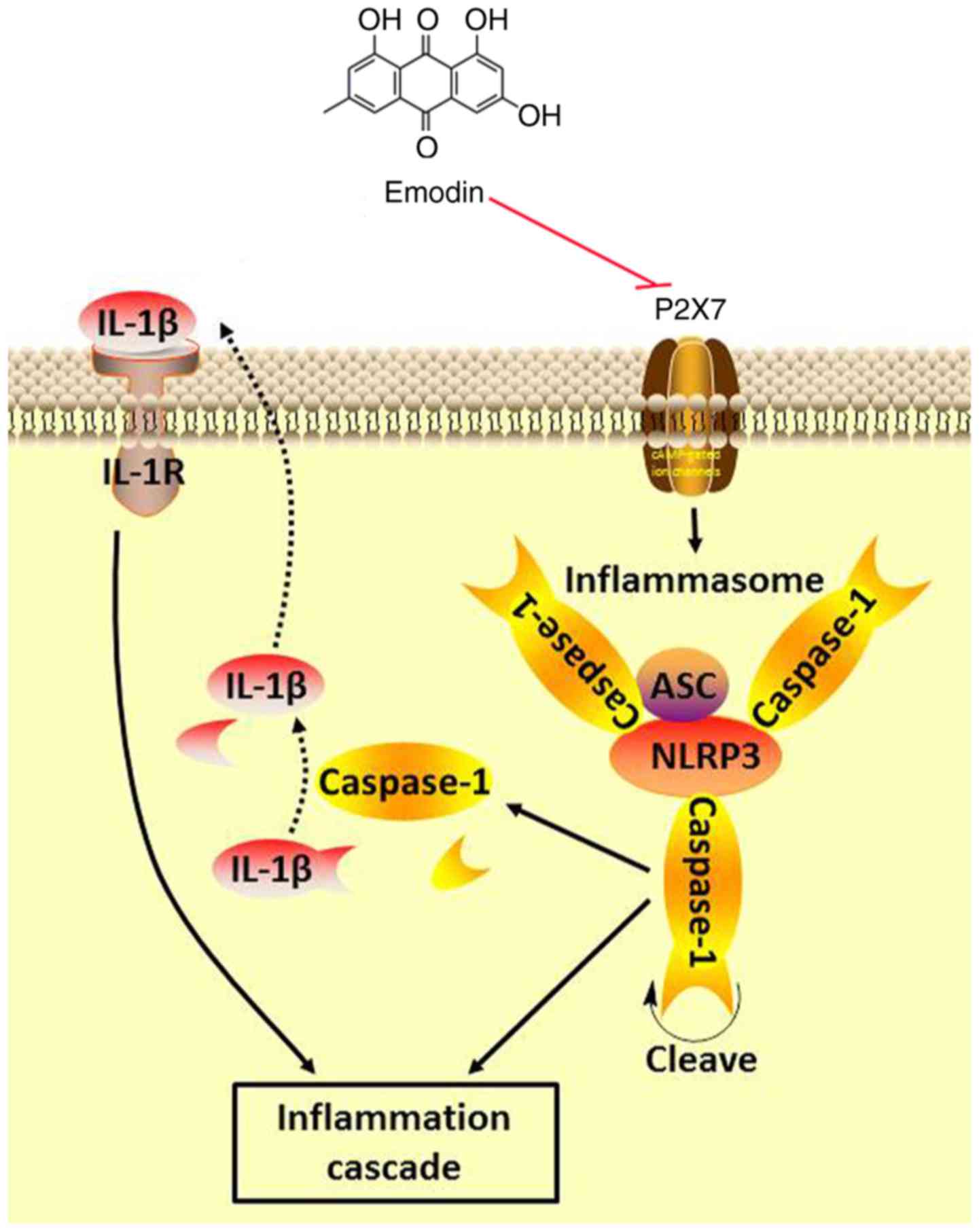
effects (24,49). In conclusion, the anti-inflammatory
effect of emodin on SAP rats may reduce the concentration of IL-1β
and IL-18 in plasma by inhibiting the P2X7/NLRP3 signaling pathway,
thereby delaying the progress of SAP and improving the systemic
inflammatory status (Fig. 6).
Therefore, emodin is expected to be one of the emerging and
efficient candidate natural products for SAP treatment. However, it
requires further clinical investigations on the positive effects of
emodin based on routine treatment and to determine how to inhibit
inflammatory responses in the early stages of SAP.
Acknowledgements
The authors would like to thank Dr Hong Xiang
(Department of Integrative Medicine Surgery, Dalian Medical
University, Dalian, China) for data analysis and Figure
revision.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81373875) and the Key
Project Supported by Clinical Ability Construction of Liaoning
Province (grant no. LNCCC-A03-2015).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and XT performed the experiment and the data
analysis. DS and HL drafted the manuscript, designed the experiment
and revised the manuscript. SX, JQ, HS and JL performed the
immunostaining, transmission electron microscopy and western blot
analysis assay. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Ethical Committee for Laboratory Animal Care and Use of Dalian
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tenner S, Baillie J, DeWitt J and Vege SS:
American College of Gastroenterology: American college of
gastroenterology guideline: Management of acute pancreatitis. Am J
Gastroenterol. 108:1400–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ince AT and Baysal B: Pathophysiology,
classification and available guidelines of acute pancreatitis. Turk
J Gastroenterol. 25:351–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav D, O'Connell M and Papachristou GI:
Natural history following the first attack of acute pancreatitis.
Am J Gastroenterol. 107:1096–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gillies N, Pendharkar SA, Asrani VM,
Mathew J, Windsor JA and Petrov MS: Interleukin-6 is associated
with chronic hyperglycemia and insulin resistance in patients after
acute pancreatitis. Pancreatology. 16:748–755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kambhampati S, Park W and Habtezion A:
Pharmacologic therapy for acute pancreatitis. World J
Gastroenterol. 20:16868–16880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hegazi RA and DeWitt T: Enteral nutrition
and immune modulation of acute pancreatitis. World J Gastroenterol.
20:16101–16105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zerem E: Treatment of severe acute
pancreatitis and its complications. World J Gastroenterol.
20:13879–13892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caserta S, Mengozzi M, Kern F, Newbury SF,
Ghezzi P and Llewelyn MJ: Severity of systemic inflammatory
response syndrome affects the blood levels of circulating
inflammatory-relevant MicroRNAs. Front Immunol. 8:19772018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoque R, Malik AF, Gorelick F and Mehal
WZ: Sterile inflammatory response in acute pancreatitis. Pancreas.
41:353–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giuliani AL, Sarti AC, Falzoni S and Di
Virgilio F: The P2X7 receptor-interleukin-1 liaison. Front
Pharmacol. 8:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang GX, Wang MX, Nie W, Liu DW, Zhang Y
and Liu HB: P2X7R blockade prevents NLRP3 inflammasome activation
and pancreatic fibrosis in a mouse model of chronic pancreatitis.
Pancreas. 46:1327–1335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu PY, Lee IH, Tan PH, Wang YP, Tsai CF,
Lin HC, Lee FY and Lu CL: P2X7 receptor mediates spinal microglia
activation of visceral hyperalgesia in a rat model of chronic
pancreatitis. Cell Mol Gastroenterol Hepatol. 1:710–720.e5. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L,
Huyiligeqi and Ni J: Emodin: A review of its pharmacology, toxicity
and pharmacokinetics. Phytother Res. 30:1207–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang CG: The treatment of acute
pancreatitis with Dachengqi decoction in 32 cases. Zhongguo Wei
Zhong Bing Ji Jiu Yi Xue. 22:2492010.(In Chinese). PubMed/NCBI
|
|
17
|
Yang DY, Duan SB and Aili JT: Effect of
qingyi decoction in treating severe acute pancreatitis and its
impacts on blood level of tumor necrosis factor-alpha,
interleukin-6 and inteleukin-8. Zhongguo Zhong Xi Yi Jie He Za Zhi.
29:1122–1124. 2009.(In Chinese). PubMed/NCBI
|
|
18
|
Wu L, Cai B, Zheng S, Liu X, Cai H and Li
H: Effect of emodin on endoplasmic reticulum stress in rats with
severe acute pancreatitis. Inflammation. 36:1020–1029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia XM, Li BK, Xing SM and Ruan HL: Emodin
promoted pancreatic claudin-5 and occludin expression in
experimental acute pancreatitis rats. World J Gastroenterol.
18:2132–2139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Zou J, Liu X, Jiang LH and Li J:
Inhibition of ATP-induced macrophage death by emodin via
antagonizing P2X7 receptor. Eur J Pharmacol. 640:15–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu T, Zhang L, Ling S, Duan J, Qian F, Li
Y and Xu JW: Scropolioside B inhibits IL-1β and cytokines
expression through NF-κB and inflammasome NLRP3 pathways. Mediators
Inflamm. 2014:8190532014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodgers MA, Bowman JW, Fujita H, Orazio N,
Shi M, Liang Q, Amatya R, Kelly TJ, Iwai K, Ting J, et al: The
linear ubiquitin assembly complex (LUBAC) is essential for NLRP3
inflammasome activation. J Exp Med. 211:1333–1347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han JW, Shim DW, Shin WY, Heo KH, Kwak SB,
Sim EJ, Jeong JH, Kang TB and Lee KH: Anti-inflammatory effect of
emodin via attenuation of NLRP3 inflammasome activation. Int J Mol
Sci. 16:8102–8109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiang H, Wang G, Qu J, Xia S, Tao X, Qi B,
Zhang Q and Shang D: Yin-chen-hao tang attenuates severe acute
pancreatitis in rat: An experimental verification of in silico
network target prediction. Front Pharmacol. 7:3782016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cen Y, Liu C, Li X, Yan Z, Kuang M, Su Y,
Pan X, Qin R, Liu X, Zheng J, et al: Artesunate ameliorates severe
acute pancreatitis (SAP) in rats by inhibiting expression of
pro-inflammatory cytokines and Toll-like receptor 4. Int
Immunopharmacol. 38:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tukaj C, Olewniak-Adamowska A, Pirski MI
and Woźniak M: Ultrastructural aspects of acute pancreatitis
induced by 2, 2′-azobis (2-amidinopropane) dihydrochloride (AAPH)
in rats. Folia Morphol. 71:136–141. 2012.
|
|
27
|
Zhang XP, Li ZF, Liu XG, Wu YT, Wang JX,
Wang KM and Zhou YF: Effects of emodin and baicalein on rats with
severe acute pancreatitis. World J Gastroenterol. 11:2095–2100.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanz JM, Chiozzi P, Ferrari D, Colaianna
M, Idzko M, Falzoni S, Fellin R, Trabace L and Di Virgilio F:
Activation of microglia by amyloid {beta} requires P2X7 receptor
expression. J Immunol. 7:4378–4385. 2009. View Article : Google Scholar
|
|
29
|
Zhu S, Wang Y, Wang X, Li J and Hu F:
Emodin inhibits ATP-induced IL-1β secretion, ROS production and
phagocytosis attenuation in rat peritoneal macrophages via
antagonizing P2X7 receptor. Pharm Biol. 52:51–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu H, Xiong C, He L, Wu B, Peng L, Cheng
Y, Jiang F, Tan L, Tang L, Tu Y, et al: Trans-resveratrol
attenuates high fatty acid-induced P2X7 receptor expression and
IL-6 release in PC12 cells: Possible role of P38 MAPK pathway.
Inflammation. 38:327–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Q, Wu H, Qin S, Liu C, Chen Y, Yang Y
and Xu C: The P2X7 receptor involved in gp120-induced cell injury
in BV2 Microglia. Inflammation. 39:1814–1826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albalawi F, Lu W, Beckel JM, Lim JC,
McCaughey SA and Mitchell CH: The P2X7 receptor primes IL-1β and
the NLRP3 inflammasome in astrocytes exposed to mechanical strain.
Front Cell Neurosci. 11:2272017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yue N, Huang H, Zhu X, Han Q, Wang Y, Li
B, Liu Q, Wu G, Zhang Y and Yu J: Activation of P2X7 receptor and
NLRP3 inflammasome assembly in hippocampal glial cells mediates
chronic stress-induced depressive-like behaviors. J
Neuroinflammation. 10:1022017. View Article : Google Scholar
|
|
34
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan Y, Jiang W, Liu L, Wang X, Ding C,
Tian Z and Zhou R: Dopamine controls systemic inflammation through
inhibition of NLRP3 inflammasome. Cell. 160:62–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Slowik A, Lammerding L, Zendedel A, Habib
P and Beyer C: Impact of steroid hormones E2 and P on the
NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells
after in vitro hypoxia. J Steroid Biochem Mol Biol. 183:18–26.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li X and Zhong F: Nickel induces
interleukin-1β secretion via the NLRP3-ASC-caspase-1 pathway.
Inflammation. 37:457–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang A, Pan W, Lv J and Wu H: Protective
effect of amygdalin on LPS-induced acute lung injury by inhibiting
NF-κB and NLRP3 signaling pathways. Inflammation. 40:745–751. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hansen MR, Krabbe S and Novak I:
Purinergic receptors and calcium signalling in human pancreatic
duct cell lines. Cell Physiol Biochem. 22:157–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Novak I, Nitschke R and Amstrup J:
Purinergic receptors have different effects in rat exocrine
pancreas. Calcium signals monitored by fura-2 using confocal
microscopy. Cell Physiol Biochem. 12:83–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu G, Tong Z, Ding Y, Liu J, Pan Y, Gao L,
Tu J, Wang Y, Liu G and Li W: Aspirin protects against acinar cells
necrosis in severe acute pancreatitis in mice. Biomed Res Int.
2016:60894302016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Novak I, Jans IM and Wohlfahrt L: Effect
of P2X7 receptor knockout on exocrine secretion of
pancreas, salivary glands and lacrimal glands. J Physiol.
588:3615–3627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Torre-Minguela C, Mesa Del Castillo P
and Pelegrín P: The NLRP3 and pyrin inflammasomes: Implications in
the pathophysiology of autoinflammatory diseases. Front Immunol.
8:432017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gunjaca I, Zunic J, Gunjaca M and Kovac Z:
Circulating cytokine levels in acute pancreatitis-model of
SIRS/CARS can help in the clinical assessment of disease severity.
Inflammation. 35:758–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang XY, Tang QQ, Zhang JL, Fang MY and Li
YX: Effect of SB203580 on pathologic change of pancreatic tissue
and expression of TNF-α and IL-1β in rats with severe acute
pancreatitis. Eur Rev Med Pharmacol Sci. 18:338–343.
2014.PubMed/NCBI
|
|
47
|
Wen H, Miao EA and Ting JP: Mechanisms of
NOD-like receptor-associated inflammasome activation. Immunity.
39:432–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu MH, Yuan FL, Wang SJ, Xu HY, Li CW and
Tong X: Association of interleukin-18 and asthma. Inflammation.
40:324–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiang H, Tao X, Xia S, Qu J, Song H, Liu J
and Shang D: Emodin alleviates sodium taurocholate-induced
pancreatic acinar cell injury via MicroRNA-30a-5p-mediated
inhibition of high-temperature requirement A/Transforming growth
factor beta 1 inflammatory signaling. Front Immunol. 8:14882017.
View Article : Google Scholar : PubMed/NCBI
|