Introduction
According to the World Health Organization, cancer
is the second leading cause of mortality worldwide, with 8,800,000
individuals succumbing to mortality in 2015. Statistically, this
means that one in six individuals succumbed to cancer-associated
mortality worldwide in 2015. The incidence of cancer is increasing
due to changes in life patterns, economic development,
urbanization, population growth, and aging (1).
Oral cavity cancer and pharyngeal cancer represent
the sixth most common types of cancer in the world, and occur more
frequently in men than in women (2). According to data from the Korea
Central Cancer Registry, a high number of individuals aged between
50 and 70 years have oral cancer; the incidence of oral cancer has
also been increasing in the younger generation in recent years.
Oral squamous cell carcinoma (OSCC) is a malignant tumor, which
accounts for >90% of all cases of oral cancer (3). In terms of the distribution of oral
cancer sites, tongue cancer accounts for the highest proportion,
~45% (4). In general, the 5-year
survival rate of individuals with tongue, oral cavity, and
tonsillar cancer is between 50 and 55%, which is comparatively
lower than that of other types of cancer (5). Since 2016, 3,236 cases of oral cancer
have been reported in Korea, 1,059 cases of which resulted in
patient mortality (6). Various
types of oral cancer treatment, including surgery, radiation
therapy and chemotherapy, are typically used to treat patients.
However, the majority of these methods are accompanied by
side-effects and recurrences (7).
Natural products have been used in medicine since
early human history (8). Generally,
they are known to have low toxicity, are readily accessible, and
have a fairly wide range of application methods (9). Although the development of natural
drugs has been continuously expanding, few cases have been reported
in terms of their development as anticancer drugs (10). Therefore, natural drugs require
consideration for cancer therapy (11). For example, the medicinal herb
licorice has been commonly used in China for a number of centuries
and has various pharmacological effects, including anticancer,
anti-inflammatory, anti-ulcer, and antibacterial effects (12). To date, seven retrochalcones, which
are structurally unique and distinguished from ordinary chalcones
at the C-2′ and C-6′ positions, comprising licochalcone (LC) A-E,
LCG and echinatin, have been isolated and characterized from the
roots of Glycyrrhiza inflata, with the exception of the
artificial compounds LCF and LCH (13). LCH was first reported following the
concise synthesis of LCC as a by-product during synthesis. During
the synthesis of LCC, its regioismer LCH was also synthesized using
the acid-mediated Claisen-Schmidt condensation reaction as a key
step (14).
Matrin 3 (Matr3) is a highly conserved nuclear
matrix protein, which can bind to DNA and RNA through zinc finger
domains (ZF1 and ZF2) and RNA recognition motifs (RRM1 and RRM2)
(15). Matr3 is widely expressed in
a variety of tissues and is known to be involved in DNA
replication, apoptosis, transcription, translation, RNA processing,
chromatin remodeling (16), and the
stabilization of RNA and mRNA (17). The Ca2+/calmodulin
binding motif partially overlaps the RNA recognition motifs domain
of Matr3, and Matr3 is also known to be degraded by various
caspases, including caspase-3, −5, −6, −7 and −10. The function of
Matr3 can be regulated by calmodulin- and Ca2+-dependent
interactions and caspase-mediated cleavage during apoptosis
(18).
The present study investigated the anticancer effect
of LCH on OSCCs, which induced cell apoptosis, cell cycle arrest
and anchorage-independent cell transformation in OSCCs. The aim of
the present study was to determine the efficacy of LCH, which
remains to be elucidated in oral cancer, and to suggest the
potential for its development as a potential anticancer drug.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin and streptomycin (P/S), and 0.05%
trypsin-EDTA were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). CNBr-activated Sepharose™ 4B was purchased from
GE Healthcare Life Sciences (Uppsala, Sweden).
4′-6-diamidino-2-phenylindole (DAPI) was purchased from
Sigma-Aldrich; EMD Millipore (Billerica, MD, USA). The antibodies
used included Matr3 (C-20; cat. no. sc-55723), cyclin D1 (M-20;
cat. no. sc-718), p27 (C-19; cat. no. sc-528), B-cell lymphoma 2
(Bcl-2; C-2; cat. no. sc-7382), Bcl-2-extra large (Bcl-xL; H-5;
cat. no. sc-8392), Bcl-2-associated X protein (Bax; N-20; cat. no.
sc-493), and Bcl-2-associated death promotor (Bad; C-7; cat. no.
sc-8044) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA)΄
poly (ADP-ribose) polymerase (PARP; cat. no. 9542), survivin
(71G4B7; cat. no. 2808) and caspase-3 (cat. no. 9662) from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and GAPDH (LF-PA0018)
from Abcam (Cambridge, UK). LCH was synthesized using the same
method as that used in a previous study (14).
Cell culture
The HSC2 and HSC3 human OSCC cells were obtained
from Hokkaido University (Hokkaido, Japan). The cells were cultured
in DMEM containing 10% heat-inactivated FBS, and 100 U/ml each P/S,
and were incubated at 37°C with 5% CO2 in humidified
air.
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay
To investigate the effect of LCH on cell viability,
the CellTiter 96™ Assay kit (Promega Corp., Madison, WI, USA) was
used, according to the manufacturer's protocol. The HSC2 and HSC3
cells (3×103 cells/well) were cultured n 96-well plates
for 24 h, and treated with LCH for 24 and 48 h at 37°C.
Subsequently, 20 µl of MTS solution was added to each well, and the
plates were reacted at 37°C for 4 h. The optical density was
measured at 490 nm using the Epoch™ microplate spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA). Cell viability was
calculated using the following equation: Optical density ratio of
LCH-treated sample/untreated sample ×100 (%).
DAPI staining
The HSC2 and HSC3 cells treated with LCH were
collected, washed with cold 1X phosphate-buffered saline (PBS) and
fixed with 100% methanol for 30 min at room temperature. Following
fixation, the cells on the slide were stained with 2 µg/ml of DAPI
solution. The stained cells were observed using a Nikon C2 Plus
microscope (Nikon Corp., Tokyo, Japan).
Annexin V/propidium iodide (PI)
staining
The HSC2 and HSC3 cells were cultured and treated
with LCH (0, 10, 20 and 30 µM) for 48 h. The adherent cells and
floating cells were collected and washed with cold 1X PBS. The
cells were then stained with the FITC Annexin V Apoptosis Detection
kit (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using a
fluorescence-activated cell sorter (FACS; BD Biosciences).
Cell cycle analysis
The HSC2 and HSC3 cells were cultured and treated
with LCH at concentrations of 10, 20 and 30 µM for 48 h. The cells
were harvested, washed with cold 1X PBS, and fixed with 70% ethanol
at −20°C for 2 h. The cells were then washed with 1X PBS and
stained with RNase A and PI (BD Biosciences). They were then
analyzed using a FACS, according to the manufacturer's
protocol.
Western blot analysis
The HSC2 and HSC3 cells were harvested following
treatment with LCH (10, 20 and 30 µM) for 48 h. The proteins were
then extracted using RIPA buffer (Thermo Fisher Scientific, Inc.)
containing protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland). The concentration of the extracted protein was
determined using a BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.) and the equivalent quantity of proteins were electrophoresed
on 8, 10 and 15% SDS-PAGE gels, and transferred onto a
polyvinylidene fluoride (PVDF) membrane. The membranes were then
reacted with the specific primary antibody (1:1,000 to 1:2,000)
diluted in Tris-buffered saline (TBS) containing 0.1% Tween-20
(TBST) at 4°C overnight. The reacted membranes were washed three
times for 10 min in TBST. Following incubation with a horseradish
peroxidase (HRP)-labeled secondary antibody against anti-rabbit IgG
(LF-SA8002; Ab Frontier, San Diego, CA, USA), anti-goat IgG (cat.
no. sc-2020; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-mouse IgG (cat. no. sc-2005; Santa Cruz Biotechnology) was
diluted in TBST, the PVDF membrane was reacted with enhanced
chemiluminescence reagent (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and then detected using ImageQuant LAS 4000 Mini system
(GE Healthcare Life Sciences, Chalfont, UK) according to the
manufacturer's protocol.
Pull-down assay
LCH was coupled to CNBr-activated Sepharose™ 4B
matrix beads in 0.1 M NaHCO3 (pH 8.3) containing 0.5 M
NaCl overnight at 4°C, according to the manufacturer's protocol.
The HSC2 and HSC3 cell lysates were mixed with LCH-conjugated
Sepharose 4B beads or with Sepharose 4B beads alone as a control.
The binding of LCH and Matr3 was observed using western blot
analysis.
Anchorage-independent cell
transformation assay
The HSC2 and HSC3 cells were treated with various
concentrations of LCH in 1 ml of 0.33% basal medium Eagle's agar
over 3 ml of 0.5% basal medium Eagle's agar containing 10% FBS. The
cultures were maintained in a 37°C, 5% CO2 incubator for
20 days. The colony numbers and sizes were measured under a
microscope (Nikon C2 Plus system; Nikon Corp.). Respective
experiments were performed in triplicate, and the average values
were used.
Matr3 small interfering RNA
(siRNA)
siRNA sequences targeting Matr3 (matrin-3 siRNA;
cat. no. sc-62604) and a non-targeting control (control siRNA; cat.
no. sc-37007) were purchased from Santa Cruz Biotechnology.
Transfection was performed according to the manufacturer's
protocol. The HSC2 and HSC3 cells were cultured in 6-well plates
and transfected with 50 nM siRNA using siRNA transfection reagent
(Lipofectamine 2000; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Following transformation for 24, 48
and 72 h, the HSC2 and HSC3 cells were used for western blot
analysis, reverse transcription-polymerase chain reaction (RT-PCR)
analysis, and the anchorage-independent cell transformation
assay.
RT-PCR analysis
Total RNA was extracted from the cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). A
total 5 µg of RNA was used to synthesize cDNA using the
AMPIGENE® cDNA synthesis kit (Enzo Life Sciences,
Farmingdale, NY, USA). cDNA was obtained by PCR amplication using
β-actin-specific and Matr3-specific primers (as described below)
for 30 cycles under the PCR conditions of 1 min at 95°C, 1 min at
58°C and 1 min at 72°C. The β-actin primers used were as follows:
Forward 5′-GTGGGGCGCCCCAGGCACCA-3′ and reverse
5′-CTCCTTAATGTCACGCACGATTTC-3′. The Matr3 primers used were as
follows: Forward 5′-TGGAGCAAGTCACAGTCGTC-3′ and reverse
5′-TCTGCCTTTCTGCATGTGTC-3′. The PCR products were analyzed by 1%
agarose gel electrophoresis.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analysis of the data was performed using the
Prism 5.0 statistical package (GraphPad Software, Inc., La Jolla,
CA, USA). The statistical significance of differences among groups
were analyzed using one-way analysis of variance and Fisher's least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference. In the present
study, the data are representative of three independent experiments
performed in triplicate.
Results
Anticancer effect of LCH on OSCC cell
lines
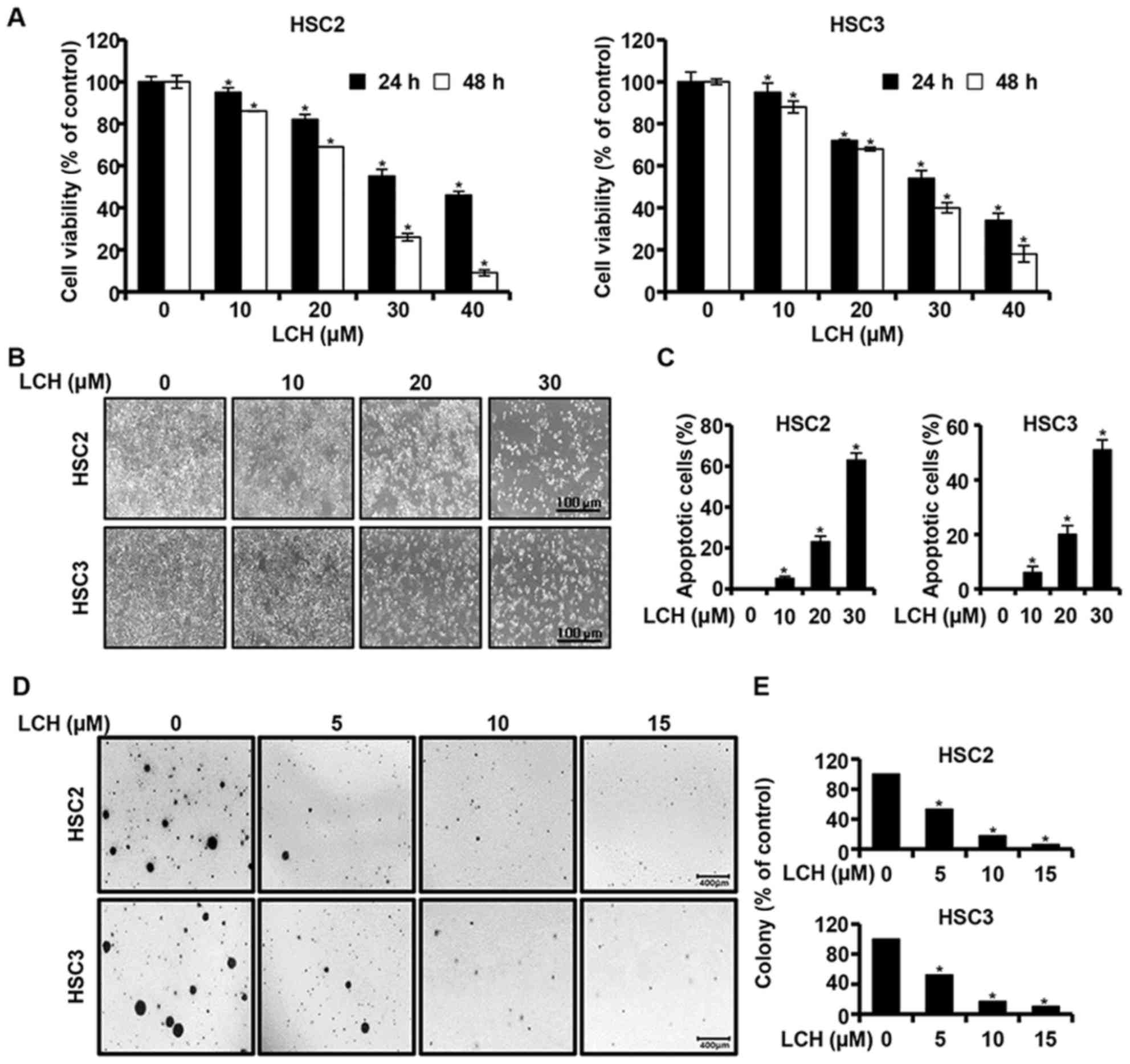
An MTS assay was performed to investigate the effect
of LCH on the viability of OSCC cells (HSC2 and HSC3). The HSC2 and
HSC3 cells were cultured in 96-well plates for 24 h and then
treated with LCH (0, 10, 20, 30 and 40 µM) for 24 and 48 h,
respectively. The results of MTS assay showed that the viability of
the HSC2 and HSC3 cells decreased, in a dose- and time-dependent
manner, compared with that in the untreated group (Fig. 1A). The concentrations of 10, 20 and
30 µM of LCH were selected following confirmation of the
significant viability inhibition of LCH on the HSC2 and HSC3 cells.
Following treatment with LCH, the morphologies of the HSC2 and HSC3
were altered and the cell confluency decreased (Fig. 1B). The HSC2 and HSC3 cells were
treated with LCH, and DAPI staining was performed to determine the
characteristics of apoptosis, including nuclear fragmentation and
chromatin condensation. The percentage of apoptotic cells with
respect to the total cell count is shown in Fig. 1C, showing an increase in the number
of apoptotic cells in the cells treated with LCH compared with the
DMSO-treated group. To investigate the changes of
anchorage-independent colony formation by treatment with LCH in
OSCC cells, a colony formation assay was performed. LCH markedly
suppressed anchorage-independent colony formation in the HSC2 and
HSC3 cells in a concentration-dependent manner (Fig. 1D and E).
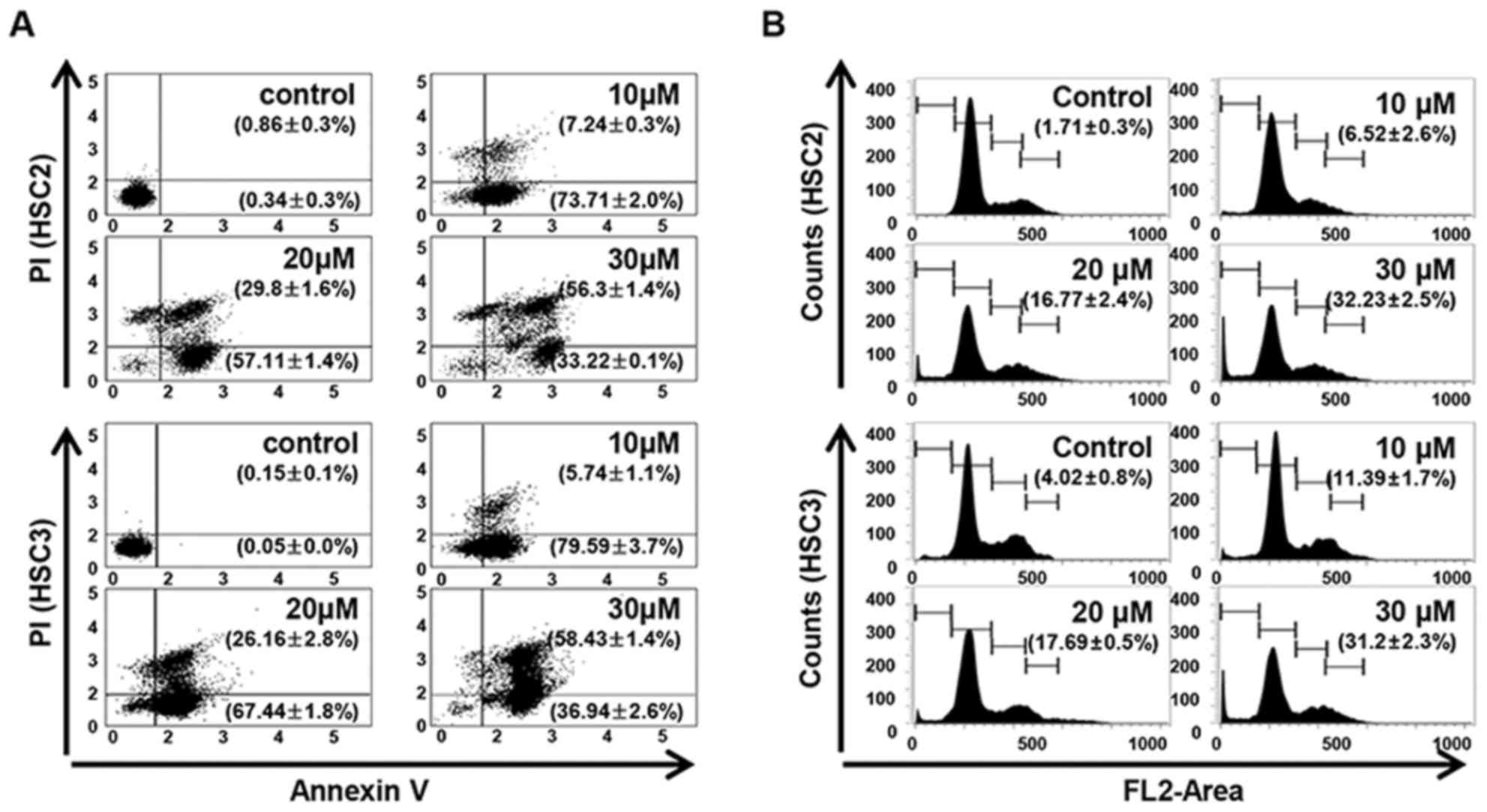
LCH induces apoptosis in OSCC cell
lines
The LCH-induced apoptosis and the Sub-G1 population
of LCH-treated OSCC cells were increased compared with that in the
untreated OSCC cells. Annexin V/PI double staining was performed to
investigate whether LCH affects the induction of apoptosis in HSC2
and HSC3 cells. LCH induced the apoptosis of HSC2 and HSC3 cells in
a concentration-dependent manner. The percentages of total
apoptotic HSC2 cells were 80.96, 86.92, and 89.52% following
treatment with 10, 20 and 30 µM LCH, respectively, and the
percentages of total apoptotic HSC3 cells were 85.34, 93.61 and
95.38% following treatment with 10, 20 and 30 µM, respectively
(Fig. 2A). To investigate the
effect of LCH on apoptosis, FACS analysis was performed by PI
staining. In the on HSC2 cells, the percentage of the Sub-G1
population was 1.71% in the control cells, whereas treatment with
10, 20, and 30 µM of LCH for 48 h led to values of 6.52, 16.77 and
32.23%. In the HSC3 cells, the percentage of the Sub-G1 population
was 4.02% in the control, whereas treatment with LCH led to values
of 11.39, 17.69 and 31.2% (Fig.
2B).
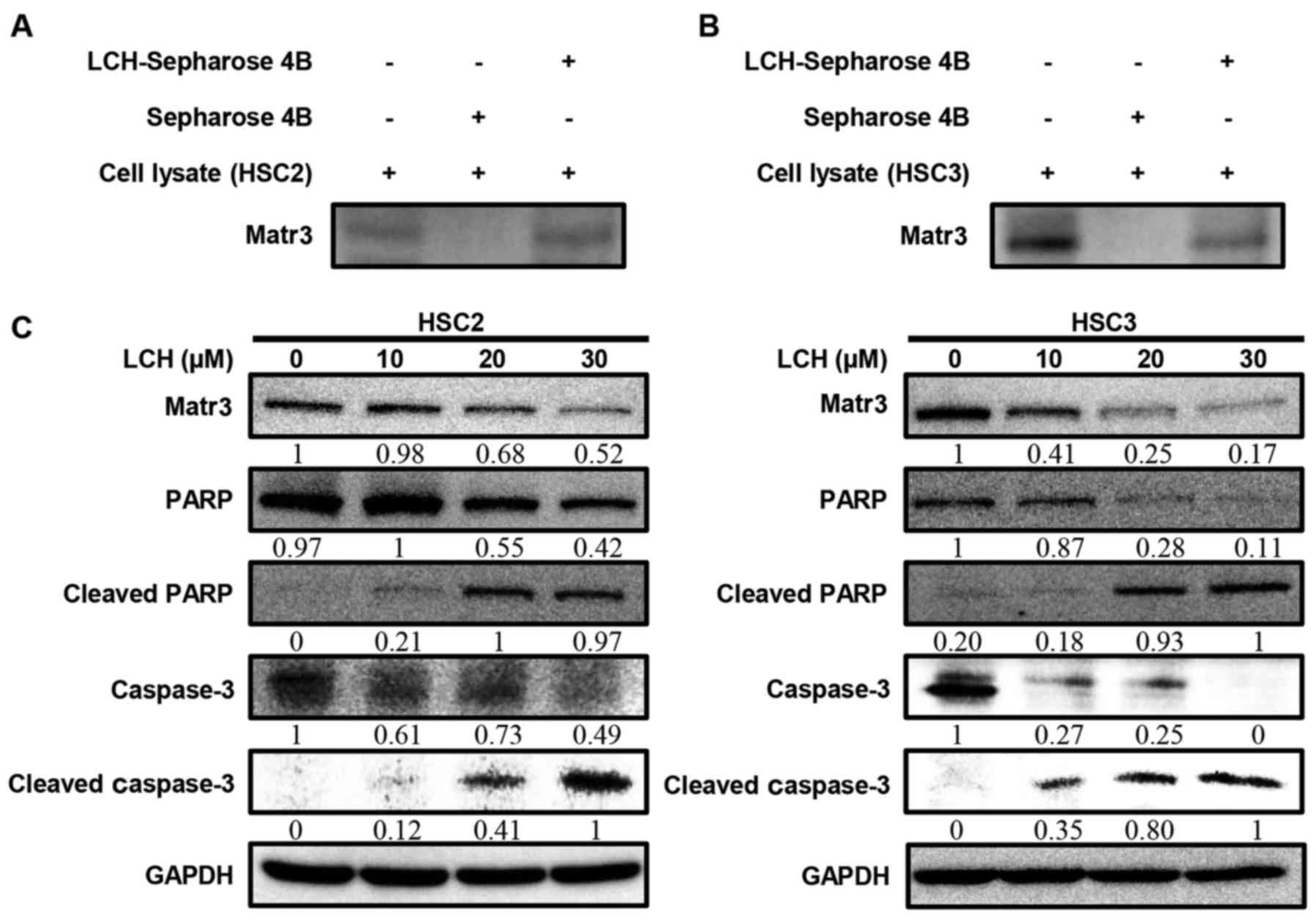
Effect of LCH on Matr3 in OSCC cell
lines
To determine whether LCH binds to Matr3, ex
vivo pull-down assays were performed. The HSC2 and HSC3 cell
lysates directly interacted with the LCH-Sepharose 4B beads in
vitro (Fig. 3A and B). The HSC2
and HSC3 cells were used to examine the effect of LCH on the
expression of Matr3 and other apoptosis-related factors. The
protein expression levels of the Matr3 in the HSC2 and HSC3 cells
treated with LCH were decreased in a dose-dependent manner
(Fig. 3C). The cleavage of PARP and
caspase-3, which are involved in the apoptotic pathway, increased
in a dose-dependent manner (Fig.
3C).
LCH regulates apoptosis-related
proteins in OSCC cell lines
The HSC2 and HSC3 cells were treated with LCH for 48
h, and the Matr3 regulatory protein expression levels were analyzed
by western blot analysis. Following treatment, the expression level
of p27, which regulates the cell cycle, increased, and the
expression levels of cyclin D1 and survivin decreased (Fig. 4A). Pro-survival proteins, Bcl-2 and
Bcl-xL decreased and pro-apoptotic proteins Bax and Bad increased
in a dose-dependent manner.
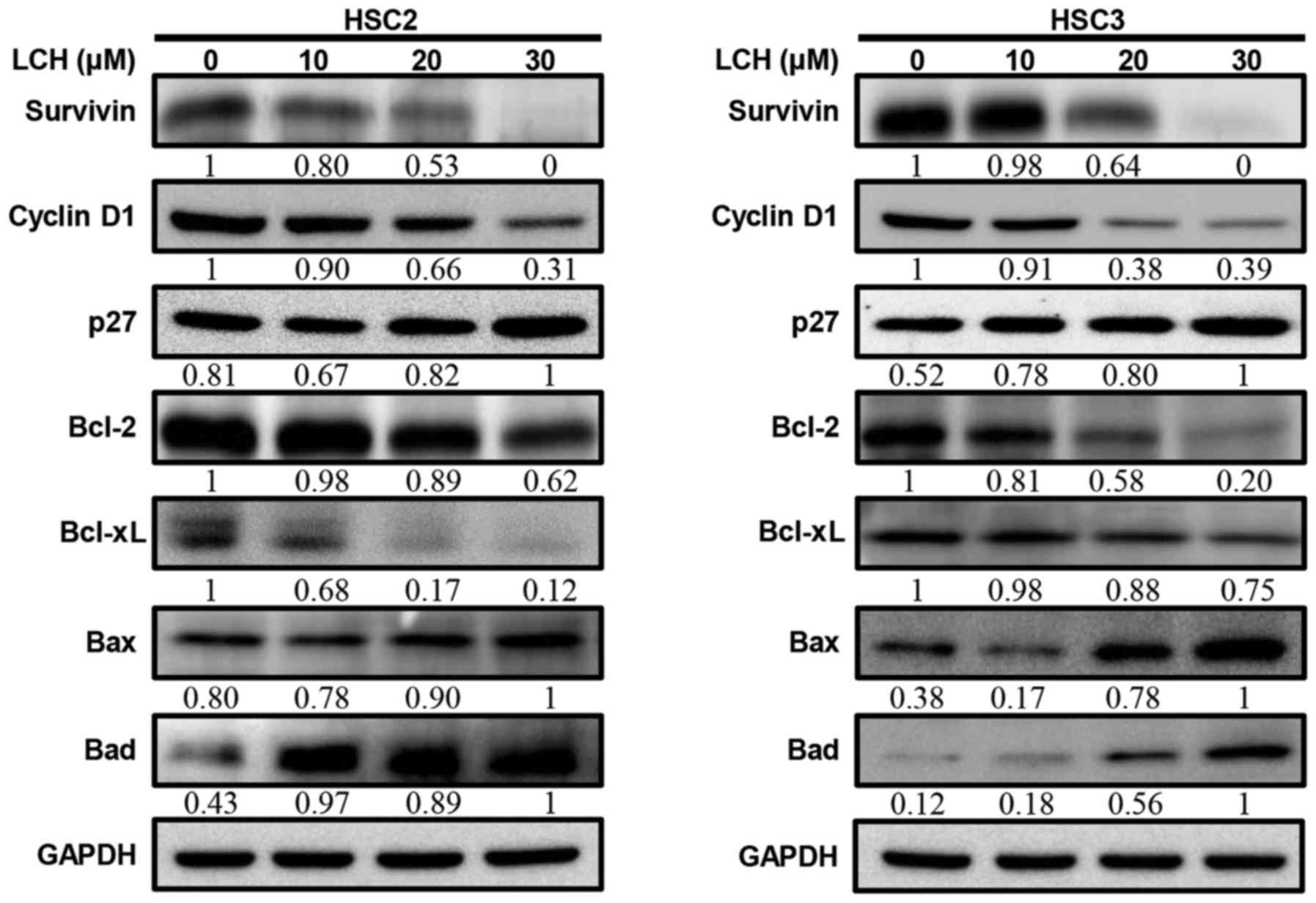 | Figure 4.Effect of LCH on apoptosis regulatory
proteins. HSC2 and HSC3 cells were seeded onto a cell culture plate
for 24 h, and treated with 10, 20 and 30 µM LCH for 48 h. The
proteins were then separated via SDS-PAGE and western blot analysis
was performed using survivin, cyclin D1, p27, Bcl-2, Bcl-xL, Bax
and Bad antibodies. GAPDH protein was used here as an internal
control. LCH, licochalcone H; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; Bad, Bcl-2-associated death
promotor. |
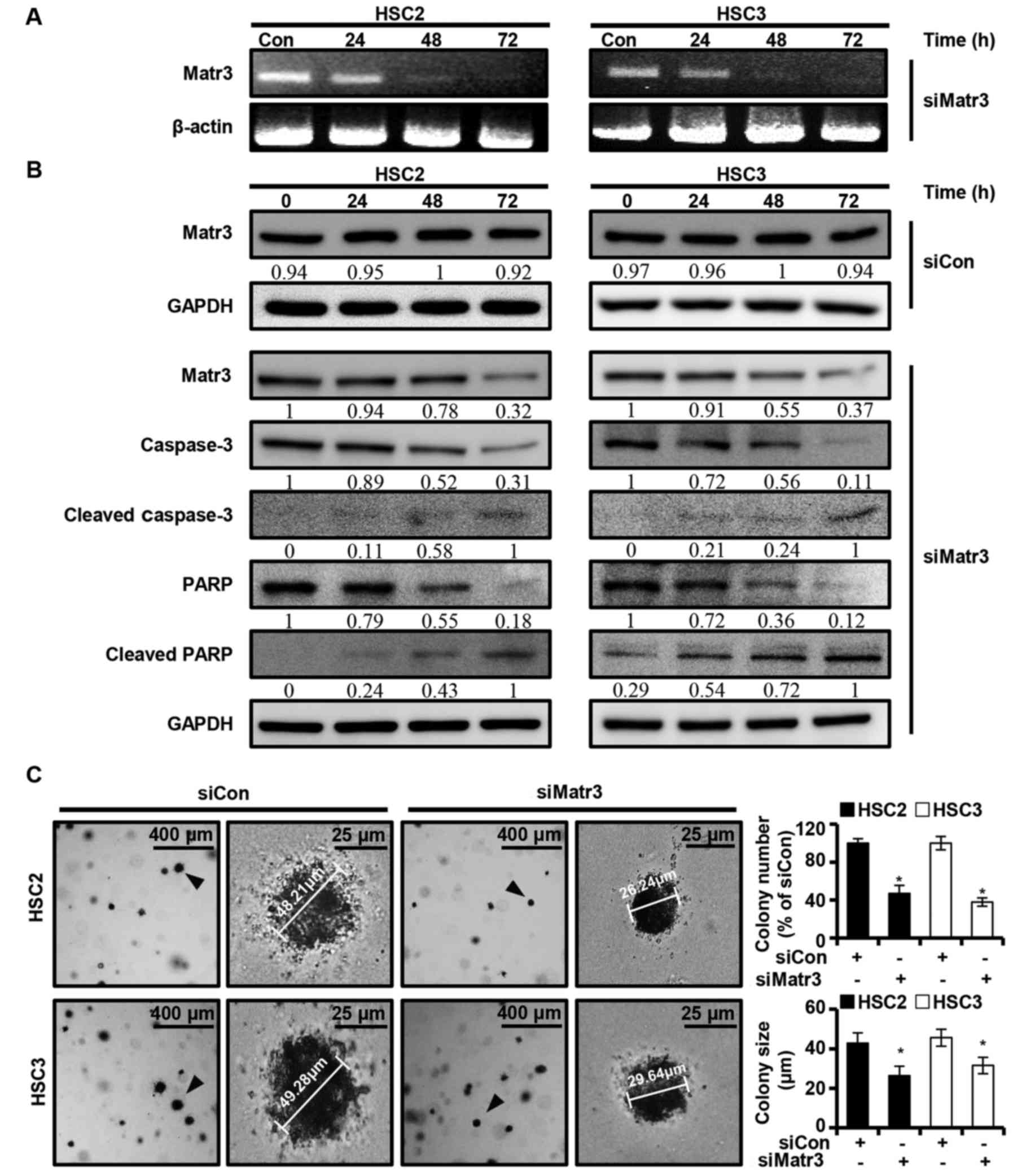
Role of Matr3 in OSCC cell lines
through siRNA transfection
The present study investigated whether the
expression of Matr3 affects the apoptosis of OSCC cells. A
knocked-down version of Matr3 (siMatr3) was transiently transfected
into HSC2 and HSC3 cells, and the expression levels of the Matr3
gene and other apoptosis-related factors were examined at different
post-transfection time-points (24, 48 and 72 h). It was found that
siMatr3 regulated the mRNA and protein levels of Matr3, and the
full length of caspase-3 and PARP in a time-dependent manner
(Fig. 5A and B). Also, no change
was identified in Matr3 protein expression of HSC2 and HSC3 in the
control siMatr3 (siCon) group (Fig.
5B). Furthermore, siMatr3 significantly inhibited colony
formation and reduced colony size in the HSC2 and HSC3 cells
(Fig. 5C).
Discussion
Globally, cancer is the second leading cause of
mortality in humans, and its incidence at younger ages is
increasing (1). Although oral
cancer has a lower incidence rate than other major types of cancer,
including lung, breast and colorectal cancer, its treatment
requires attention, as its incidence has gradually increased over
the last 20 years worldwide (2).
There have been substantial advances in treatment from drug
development studies, specifically to hallmark signaling of cancer
cell proliferation, growth, death, angiogenesis, invasion and
metastasis (19). Previous studies
have suggested that Matr3 is involved in DNA replication, RNA
metabolism and nuclear retention of hyper-edited RNA, apoptosis,
transcription and translation in HeLa cells, and is known to
stabilize RNA and mRNA in 293T cells (15). It also has been reported that Matr3
regulates cell growth and proliferation by forming complexes with
nucleoproteins that regulate pro- and anti-apoptotic signaling
pathways (19). Matr3 is an RNA-
and DNA-binding nuclear matrix protein found to be associated with
degenerative diseases (21) and
cancer (22).
The results of the present study showed that the
chemotherapeutic agent LCH inhibited the carcinogenesis of oral
cancer through the suppression of Matr3 protein. A previous study
showed that the knockdown of Matr3 in endothelial cells induced
predominantly necrosis but not apoptosis, indicating that Matr3 is
involved in the control of cell proliferation and viability of
endothelial cells, with Matr3 being a key regulator of endothelial
cell survival (23). The results of
the present study demonstrated that decreased Matr3 in OSCC cells
induced apoptosis. Using a combination of siRNA techniques, it was
found that Matr3 regulated apoptosis and colony formation in OSCC
cells (Fig. 5). The expression
levels of apoptotic factors PARP and caspase-3 were found to
decrease, in contrast to the cleaved forms of PARP and caspase-3,
which were increased in siMatr3 transfected cells (Fig. 5B). These results showed that Matr3
protein is important in the process of carcinogenesis in OSCC. The
present study also established OSCC lines with downregulated Matr3
to identify the role of Matrin3 in OSCCs. However, it was not
possible to establish Matr3-knockdown OSCC lines, as survival rate
was low in Matr3-knockdown OSCC cells (data not shown).
A wide variety of LCs isolated from the roots of
Glycyrrhiza inflate have shown various biological effects
and/or chemopreventive potential (12). LCH has a 3,3-dimethylallyl group at
C-5 in the B ring, unlike LCC at C-3 in the B ring, and its
structure is similar to that of LCA, with the exception of an allyl
group. Studies have revealed that compounds with substituents at
C-5 in the B ring exhibit more beneficial biological effects
(24,25). To date, LCs have shown to exhibit
various biological activities, and the anticancer effect of LCH is
anticipated. The present study demonstrated that LCH inhibited the
cell growth of HSC2 and HSC3 human OSCC cells through the induction
of apoptotic cell death and suppression of anchorage-independent
colony formation via a decrease in the expression of Matr3. The
half-maximal inhibitory concentration values were 36 and 23 µM in
HSC2 cells following treatment for 24 and 48 h, respectively, and
were 33 and 19 µM in the HSC3 cells following treatment for 24 and
48 h, respectively. In order to clarify the association between LCH
and Matr3, pull-down analysis was performed using LCH-Sepharose-4B
beads with OSCC cell lysates. As shown in Fig. 3A and B, LCH directly bound with
Matr3 protein in the OSCC cells. LCH also significantly decreased
the protein expression of Matr3 in HSC2 and HSC3 cells (Fig. 3C). This result suggested that LCH
directly targeted Matr3 in OSCC cells. LCH led to time-dependent
and dose-dependent OSCC cell growth inhibition (Fig. 1A), which appeared to be due to its
ability to induce the Sub-G1 population (Fig. 2B). The association between the cell
cycle and apoptosis provides evidence that manipulation of the cell
cycle may either prevent or induce an apoptotic response (25). LCH inhibited cyclin D1 and increased
p27 in a dose dependent manner (Fig.
4). During the G1 to S progression of the cell cycle, cyclin D1
and cyclin-dependent kinase inhibitor p27kip1 are
involved in growth arrest resulting from DNA damage, cell
senescence, and terminal differentiation or cell cycle entry,
progression, and apoptosis (27).
The present study analyzed LCH-mediated apoptosis using Annexin
V/PI staining. When apoptosis is induced, phosphatidyl serine,
which exists inside the cell membrane, is externally exposed and
Annexin V binds to the released phosphatidyl serine.
Early-apoptosis is positive for Annexin V staining as PI does not
penetrate the cell membrane; however, as apoptosis progresses, the
integrity of the plasma membrane is impaired and PI can pass
through the membrane for staining (28). The present study confirmed that
early-apoptosis and late-apoptosis were increased following
treatment with LCH (Fig. 2A). LCH
exhibited an apoptotic effect on the HSC2 and HSC3 cells.
Anti-apoptotic proteins, including Bcl-2 and Bcl-xL, can directly
or indirectly suppress apoptosis, and apoptosis is induced by the
overexpression of Bax and Bad (29). The present study examined the
protein expression of Bcl-xL, Bcl-2, Bax, and Bad in HSC2 and HSC3
cells (Fig. 4), LCH significantly
downregulated the protein expression of Bcl-2 and Bcl-xL and
upregulated the protein expression of Bax and Bad, compared with
expression levels in the control.
Taken together, these results suggested that LCH
regulated Matr3, and ultimately caused apoptosis in OSCC.
Therefore, LCH offers potential to be developed as a promising
therapeutic agent for OSCC. Additionally, Matr3 was essential for
OSCC proliferation, and the downregulation of Matr3 induced
apoptosis, suggesting that Matr3 may be an effective therapeutic
target for oral cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant (grant
no. 16182MFDS391) from the Korean Ministry of Food and Drug Safety
in 2017 and the ‘Cooperative Research Program for Agriculture
Science and Technology Development (project no. PJ012704012018)’ of
the National Institute of Animal Science, Rural Development
Administration, Republic of Korea. This study was also carried out
with the support of the ‘Cooperative Research Program for
Agriculture Science and Technology Development (project no.
PJ013842)’, Rural Development Administration, Republic of
Korea.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JHSh and JIC conceived the project and designed all
experiments. SHN, GY and JIC designed and performed the cell
experiments, and JHSe, HNO, SSC, HK and HWC performed and analyzed
the biological experiments. JIC, JHSh, SHN and GY wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
LCH
|
licochalcone H
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
DAPI
|
4′-6-diamidino-2-phenylindole
|
|
P/S
|
penicillin and streptomycin
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
propidium iodide
|
|
siRNA
|
small interfering RNA
|
|
siMatrin3
|
matrin 3-specific targeting siRNA
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: Clinical features. Oral Oncol. 46:414–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schantz SP and Yu GP: Head and neck cancer
incidence trends in young Americans, 1973–1997, with a special
analysis for tongue cancer. Arch Otolaryngol Head Neck Surg.
128:268–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feller LL, Khammissa RR, Kramer BB and
Lemmer JJ: Oral squamous cell carcinoma in relation to field
precancerisation: Pathobiology. Cancer Cell Int. 13:312013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung KW, Won YJ, Oh CM, Kong HJ, Cho H,
Lee JK, Lee DH and Lee KH: Prediction of cancer incidence and
mortality in Korea, 2016. Cancer Res Treat. 48:451–457. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho JH, Lee RH, Jeon YJ, Shin JC, Park SM,
Choi NJ, Seo KS, Yoon G, Cho SS, Kim KH, et al: Role of
transcription factor Sp1 in the 4-O-methylhonokiol-mediated
apoptotic effect on oral squamous cancer cells and xenograft. Int J
Biochem Cell Biol. 64:287–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watkins R, Wu L, Zhang C, Davis RM and Xu
B: Natural product-based nanomedicine: Recent advances and issues.
Int J Nanomedicine. 10:6055–6074. 2015.PubMed/NCBI
|
|
10
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZY and Nixon DW: Licorice and cancer.
Nutr Cancer. 39:1–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon G, Jung YD and Cheon SH: Cytotoxic
allyl retrochalcone from the roots of Glycyrrhiza inflata. Chem
Pharm Bull (Tokyo). 53:694–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Cao Y, Paudel S, Yoon G and Cheon
SH: Concise synthesis of licochalcone C and its regioisomer,
licochalcone H. Arch Pharm Res. 36:pp. 1432–1436. 2013, View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura A, Osonoi T and Terauchi Y:
Relationship between urinary sodium excretion and
pioglitazone-induced edema. J Diabetes Investig. 1:208–211. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeitz MJ, Malyavantham KS, Seifert B and
Berezney R: Matrin 3: Chromosomal distribution and protein
interactions. J Cell Biochem. 108:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salton M, Elkon R, Borodina T, Davydov A,
Yaspo ML, Halperin E and Shiloh Y: Matrin 3 binds and stabilizes
mRNA. PLoS One. 6:e238822011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valencia CA, Ju W and Liu R: Matrin 3 is a
Ca2+/calmodulin-binding protein cleaved by caspases.
Biochem Biophys Res Commun. 361:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Przygodzka P, Boncela J and Cierniewski
CS: Matrin 3 as a key regulator of endothelial cell survival. Exp
Cell Res. 317:802–811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coelho MB, Attig J, Bellora N, König J,
Hallegger M, Kayikci M, Eyras E, Ule J and Smith CW: Nuclear matrix
protein Matrin3 regulates alternative splicing and forms
overlapping regulatory networks with PTB. EMBO J. 34:653–668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaudhary R, Gryder B, Woods WS,
Subramanian M, Jones MF, Li XL, Jenkins LM, Shabalina SA, Mo M,
Dasso M, et al: Prosurvival long noncoding RNA PINCR regulates a
subset of p53 targets in human colorectal cancer cells by binding
to Matrin 3. Elife. 6(pii): e232442017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patrycja P, Joanna B and Czeslaw SC:
Matrin 3 as a key regulator of endothelial cell survival. Exp Cell
Res. 317:802–811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon G, Lee W, Kim SN and Cheon SH:
Inhibitory effect of chalcones and their derivatives from
Glycyrrhiza inflata on protein tyrosine phosphatase 1B. Bioorg Med
Chem Lett. 19:5155–5157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Lee W, Kim SN, Yoon G and Cheon SH:
Design, synthesis, and evaluation of bromo-retrochalcone
derivatives as protein tyrosine phosphatase 1B inhibitors. Bioorg
Med Chem Lett. 21:pp. 3755–3758. 2011, View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schafer KA: The cell cycle: A review. Vet
Pathol. 35:461–478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ilyin GP, Glaise D, Gilot D, Baffet G and
Guguen-Guillouzo C: Regulation and role of p21 and p27
cyclin-dependent kinase inhibitors during hepatocyte
differentiation and growth. Am J Physiol Gastrointest Liver
Physiol. 285:G115–G127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh H, Yoon G, Shin JC, Park SM, Cho SS,
Cho JH, Lee MH, Liu K, Cho YS, Chae JI, et al: Licochalcone B
induces apoptosis of human oral squamous cell carcinoma through the
extrinsic- and intrinsic-signaling pathways. Int J Oncol.
48:1749–1757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai D, Jin C, Huang Z, Satterthwait AC
and Reed JC: Differential regulation of Bax and Bak by
anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem.
283:9580–9586. 2008. View Article : Google Scholar : PubMed/NCBI
|