Introduction
Breast cancer is the most frequent type of cancer in
women worldwide, and the leading cause of cancer-associated
mortality in women in China (1).
The identification of novel biomarkers involved in breast cancer
progression may provide potential approaches for diagnosis and
treatment.
Hydrogen sulfide (H2S) is the third
gasotransmitter signaling molecule, alongside nitric oxide and
carbon monoxide, which serves important roles in several
physiological processes (2–7). Furthermore, H2S is able to
induce cancer cell proliferation (8). Cystathionine γ-lyase (CSE) is an
endogenous H2S-producing enzyme. A previous study
revealed that endogenous H2S produced by CSE promotes
proliferation of human hepatoma cells (9). Furthermore, our previous study
indicated that CSE is overexpressed in breast cancer and
demonstrated the biological functions of the CSE/H2S
system in promoting breast cancer development and progression
(10); therefore, CSE may be
considered a novel target and marker involved in the progression of
breast cancer. The study of CSE inhibitors may be of great
significance for the treatment of breast cancer.
Signal transducer and activator of transcription 3
(STAT3), which is a transcription factor that regulates critical
cell functions, is constitutively activated in various human cancer
cells and has significant roles in cancer cell growth via the
regulation of gene expression (11–15).
Our previous study revealed that high expression levels of CSE
promote the activation of STAT3 (10); however, it remains unclear as to how
CSE activates STAT3.
The acetylation of STAT3 is crucial for its
activation and stabilization of its dimer (16–20).
Sirtuin 1 (SIRT1) is a histone deacetylase dependent on
NAD+, and SIRT1-induced deacetylation of STAT3 serves a
role in numerous physiological processes (21,22).
Numerous studies have confirmed that SIRT1 has an important role in
breast cancer (23–25). However, a novel finding suggested
that SIRT1 serves a dual role in breast tumors, depending on its
expression rate and the molecular subtype of the cancer (26). The aim of the present study was to
determine the expression levels of SIRT1 in breast cancer tissues
and cells with high CSE expression, and to investigate whether the
CSE/H2S system is involved in STAT3 activation via SIRT1
in breast cancer.
The present study used virtual screening to identify
inhibitors of CSE, and confirmed the inhibitory function of I157172
on CSE and the anticancer effects of the CSE inhibitor I157172 in
breast cancer. This study also investigated whether inhibition of
the CSE/H2S system may mediate inactivation of STAT3 via
SIRT1. Furthermore, the effects of I157172 on SIRT1/acetylated
(acetyl)-STAT3 were detected to confirm the downstream pathway of
the CSE/H2S system. The present study may provide a
novel target for the treatment of breast cancer, and CSE inhibitors
may be considered potential candidates for the treatment of breast
cancer.
Materials and methods
Virtual screening of CSE
inhibitors
Initially, the crystal structure of CSE protein
[Protein Data Bank (PDB) ID: 3COG] was downloaded from the PDB
(http://www.rcsb.org/pdb). The binding pocket was
identified by the Site Finder module with empirical confirmation.
The target library (the SPECS compound library) was prepared with
the Wash module in Molecular Operating Environment (MOE) 2014.09
(Chemical Computing Group ULC, Montreal, QC, Canada). High
throughput rigid docking, followed by flexible docking with force
field refinement, was used to rank the compounds, in order to
achieve a balance between accuracy and efficiency.
MOE Dock was used for docking simulations of the
ligands and predicting their binding affinity with the homology
model. The 2D structures of the ligands were drawn in ChemOffice
(2013; PerkinElmer, Inc., Waltham, MA, USA) and converted to 3D in
MOE through energy minimization. The flexible docking protocol
followed the ‘induced fit’ protocol, in which the side chains of
the receptor pocket were allowed to move according to ligand
conformations, with a constraint on their positions. The weight
used for tethering side chain atoms to their initial positions was
10. Prior to docking, the force field of AMBER12:EHT and the
implicit solvation model of Reaction Field (R-field) were selected.
The protonation state of the protein and the orientation of the
hydrogens were optimized by LigX, at a pH of 7.0 and temperature of
300 K. For rigid docking, the docked poses of the ligands were
ranked by London dG scoring. For flexible docking, a force field
refinement was conducted on the top 30 poses followed by rescoring
of GBVI/WSA dG. MOE Dock, AMBER12:EHT, R-field and GBVI/WSA dG are
programs belonging to the MOE 2014.09 software package.
Compound I157172 and positive
controls
I157172, also known as
2-[(4-(2,5-dimethoxyanilino)-6-{3-nitroanilino}-1,3,5-triazin-2-yl)
sulfanyl]-6-ethoxy-1,3-benzothiazole, was purchased from Specs
(Zoetermeer, The Netherlands), L-propargylglycine (PAG) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and
doxorubicin (DOX) was obtained from Beijing Solarbio Science &
Technology Co., Ltd. (Beijing, China). PAG is an existing CSE
inhibitor and DOX is a common clinical antitumor drug; therefore,
PAG and DOX were used as positive controls.
Patient samples and cell lines
A total of 16 breast cancer tumor samples and
adjacent non-tumor samples were collected from patients. All of the
patients (age, 37–60 years; no smoking history available) had a
non-specific type of invasive breast cancer (4 cases of grade II
and 12 cases of grade III breast cancer) and were recruited from
the Huaihe Hospital (Kaifeng, China). The present study was
approved by the ethics committee of the Medical School, Henan
University (Kaifeng, China), and patients provided written informed
consent. MCF7 and MDA-MB-231 human breast cancer cell lines, and
the Hs578Bst mammary epithelial cell line were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Zeta Life, Inc., San Francisco, CA, USA)
at 37°C in an atmosphere containing 5% CO2.
Determination of H2S
production
H2S determination was performed using the
methylene blue method. Briefly, MCF7 cells (1×106/ml)
were seeded into a 6-well tissue culture plate. After 24 h, the
cells were treated with 20 µM I157172, alongside 2 mM L-cysteine
and 0.5 mM pyridoxal phosphate for 24 h at 37°C. Concurrently, 1%
(w/v) zinc acetate (500 µl) was added to filter papers adhered to
the lid of the 6-well tissue culture plate for 24 h, in order to
absorb H2S. Then the filter papers were placed into
tubes containing 0.2% (w/v) N,
N-dimethyl-p-phenylenediamine-dihydrochloride dye (500 µl), 10%
(w/v) ammonium ferric sulfate (50 µl) and 3 ml H2O, and
were incubated for 20 min at room temperature. Absorbance at 670 nm
was subsequently measured. The production of H2S was
determined using a standard curve of NaHS (0–1 mM;
R2=0.9998) and was presented as nmol·min−1
per 1×106 cells. The assay was conducted in triplicate
for three independent experiments.
Cell viability and proliferation
assays
MTS and 5-ethynyl-2′-deoxyuridine (EdU) assays were
used to assess cell viability and proliferation, respectively. MCF7
and Hs578Bst cells were plated into 96-well plates at a density of
1×106/ml, and were treated with 0, 2.5, 5, 10, 20, 30
and 40 µM I157172 and 0, 5, 10, 20 and 40 µM PAG or DOX for 24 or
48 h at 37°C. Cell viability was evaluated by determining the
number of cells using the MTS assay (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's protocol, and images were captured
with an inverted microscope (IX53, Olympus Corporation, Tokyo,
Japan). The assay was conducted in triplicate for three independent
experiments. PAG and DOX served as positive controls.
Cell proliferation was assessed using the EdU assay
kit (Guangzhou Ribobio Co., Ltd., Guangzhou, China). The results of
a pre-experiment revealed that treatment of cells with I157172 for
>24 h cannot accurately reflect cell proliferation. Therefore,
in this study, cells were exposed to I157172 for 24 h for the cell
proliferation assay. Briefly, MCF7 cells (1×106/ml) were
cultured in 96-well plates and were exposed to 0, 10, 20 and 30 µM
I157172 for 24 h at 37°C. Subsequently, cells were incubated with
50 µm EdU for 2 h at room temperature, fixed with 4% formaldehyde
for 30 min at room temperature, incubated with glycine (2 mg/ml)
for 5 min and treated with 0.5% Triton X-100 for 10 min to
permeabilize the cells. After being washed with PBS for 5 min, the
cells were incubated with 1X Apollo® 567 (Guangzhou
Ribobio Co., Ltd.) for 30 min and treated twice with 0.5% Triton
X-100. DNA was stained with Hoechst 33342 for 30 min and visualized
with fluorescence microscopy. Five groups of cells in the images
were randomly selected.
Cell migration and invasion
assays
The scratch wound assay was used to determine cell
migration. MCF7 cells were seeded into a 6-well plate and were
scraped with a 10-µl pipette tip once they reached ~90% confluence
to generate a wound, and were rinsed twice with PBS. Subsequently,
the cells were treated with 0, 10, 20 and 30 µM I157172 and were
cultured in medium containing 5% FBS for 24 h at 37°C. The distance
of wound closure was measured at the beginning of the experiment
and after 24 h using an inverted microscope (IX53; Olympus
Corporation). The assay was conducted in triplicate for three
independent experiments.
A Transwell assay was conducted to analyze invasion.
Briefly, 24-well Transwell chambers (pore size, 8 µm; Corning
Incorporated, Corning, NY, USA) were coated with Matrigel matrix
(BD Biosciences, San Joes, CA, USA) for 30 min at 37°C. MCF7 cells
(5×104) suspended in 200 µl DMEM with 1% FBS were seeded
into the top chambers, whereas DMEM with 15% FBS was placed into
the bottom chambers. MCF7 cells in the top chambers were treated
with 0, 10, 20 and 30 µM I157172 for 24 h at 37°C, and the cells on
the upper surface of the membrane were removed. The invasive cells
attaching to the lower surface of membrane were fixed with 4%
polyoxymethylene for 30 min and were stained with 0.1% crystal
violet for 15–20 min at room temperature. Images of the stained
cells were captured with an inverted microscope (IX53; Olympus
Corporation). Subsequently, cells were dissolved in 10% acetic acid
and the OD570 absorbance was measure at 570 nm using a
microplate spectrofluorometer (EnSpire; PerkinElmer, Inc.).
siRNA transfection
MCF7 cells in 6-well plates (30–40% cell confluence)
were transfected with 10 nM scramble siRNA (Sc siRNA), or specific
siRNA against human CSE (Invitrogen; Thermo Fisher Scientific,
Inc.) at a final concentration of 50 nM, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. The medium was replaced 6 h
post-transfection, and silencing efficiency was determined by
western blotting 48 h post-transfection. The sequences for the
CSE-specific siRNA were as follows: Sense,
5′-GGUUUAGCAGCCACUGUAAdTdT-3′; antisense,
5′-UUACAGUGGCUGCUAAACCdTdT-3′, which were designed to target the
open reading frame region of CSE mRNA. The sequences for the Sc
siRNA were as follows: Sense, 5′-GTTCCCTATGCGTGAGAAAdTdT-3′;
antisense, 5′-GCTTACAAGGGTCTTCACAdTdT-3′.
Western blot analysis
Proteins were extracted from human breast cancer and
paracancerous tissues, human breast cancer cells (MCF7 and
MDA-MB-231; MCF7 cells were transfected with CSE siRNA for 48 h or
were treated with 10, 20 and 30 µM I157172 for 24 h) and normal
mammary epithelial Hs578Bst cells using radioimmunoprecipitation
assay buffer (50 mM Tris-HCl, pH 8.0; 150 mM sodium chloride; 1.0%
NP-40; 0.5% sodium deoxycholate; and 0.1% SDS) supplemented with 10
µg/ml phenylmethylsulfonyl fluoride (Sigma-Aldrich; Merck KGaA).
The samples were then centrifuged at 12,000 × g for 10 min, and
protein concentration was determined using the bicinchoninic acid
protein quantitative kit (Solarbio Science & Technology Co.,
Ltd.). Protein samples (40 µg) were separated by 10% SDS-PAGE and
were transferred to polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA) at 70 mA for 2 h at 4°C. The
membrane was then blocked in 5% fat-free milk for 2 h at room
temperature, and probed with specific primary antibodies against
CSE, SIRT1, STAT3, phosphorylated (p)-STAT3, acetyl-STAT3, B-cell
lymphoma 2 (BCL-2), matrix metalloproteinase (MMP)-2, MMP-9,
protein kinase B (Akt) and p-Akt at 4°C overnight. After incubation
with the secondary antibodies for 2 h at room temperature, the
proteins were visualized using an EasyBlot Enhanced
Chemiluminescence kit (Sangon Biotech Co., Ltd., Shanghai, China)
and were detected using a FluorChem Q Multifluor system
(ProteinSimple, San Jose, CA, USA) and semi-quantified using
ImageJ2× (National Institutes of Health, Bethesda, MD, USAs). GAPDH
was used as a loading control. Primary antibodies were as follows:
CSE mouse monoclonal antibody (1:1,000, cat. no. ab54573; Abcam,
Cambridge, UK) SIRT1 mouse monoclonal antibody (1:1,000, cat. no.
#8469), STAT3 mouse monoclonal antibody (1:1,000, cat. no. #9139),
p-STAT3 (Tyr705) rabbit polyclonal antibody (1:1,000, cat. no.
#9131), acetyl-STAT3 rabbit polyclonal antibody (1:1,000, cat. no.
#2523), BCL-2 rabbit monoclonal antibody (1:1,000, cat. no. #2870),
MMP-2 rabbit polyclonal antibody (1:1,000, cat. no. #4022), MMP-9
rabbit polyclonal antibody (1:1,000, cat. no. #3852), Akt rabbit
monoclonal antibody (1:1,000, cat. no. #4685) and p-Akt rabbit
monoclonal antibody (1:1,000, cat. no. #4060) (Cell Signaling
Technology, Inc., Danvers, MA, USA). GAPDH mouse monoclonal
antibody (1:1,000, cat. no. AG019) was obtained from Beyotime
Institute of Biotechnology (Shanghai, China). Secondary antibodies
were as follows: Horseradish peroxidase-conjugated goat anti-rabbit
(1:10,000, cat. no. SA00001-2) and horseradish
peroxidase-conjugated goat anti-mouse (1:10,000, cat. no.
SA00001-1) (Proteintech Group, Inc., Chicago, IL, USA).
Statistical analysis
Each experiment was repeated at least three times.
Statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Data are expressed as the means ± standard
deviation. Differences between multiple groups were analyzed using
one-way analysis of variance followed by Bonferroni post hoc test.
Differences between two groups were analyzed by Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Virtual screening of CSE
inhibitors
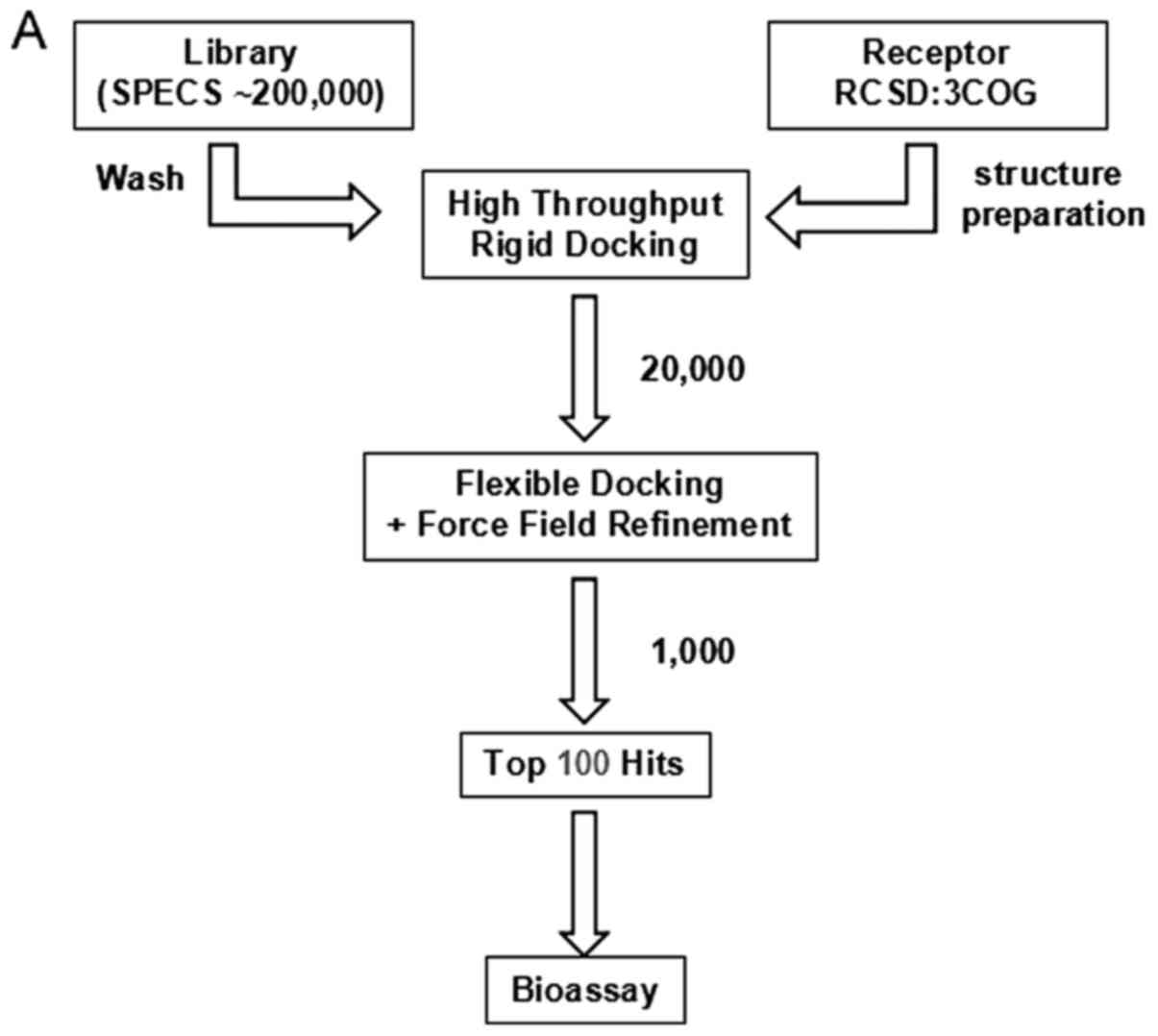
The flowchart of the virtual screening workflow is
depicted in Fig. 1A. MOE Dock was
used for docking simulations of the ligands and for predicting
their binding affinity with the homology model. The final top 100
hits were selected and the top 12 hits are shown as Fig. 1B. I157172 with the lowest binding
score (S:-7.9215) possessed the highest binding affinity with the
homology model. The 3D and 2D binding mode of a compound (S:-7.9042
in Fig. 1B) are predicted and shown
in Fig. 1C.
Confirmation of the inhibitory
activity of I157172 on CSE
I157172 was the top hit, as identified by virtual
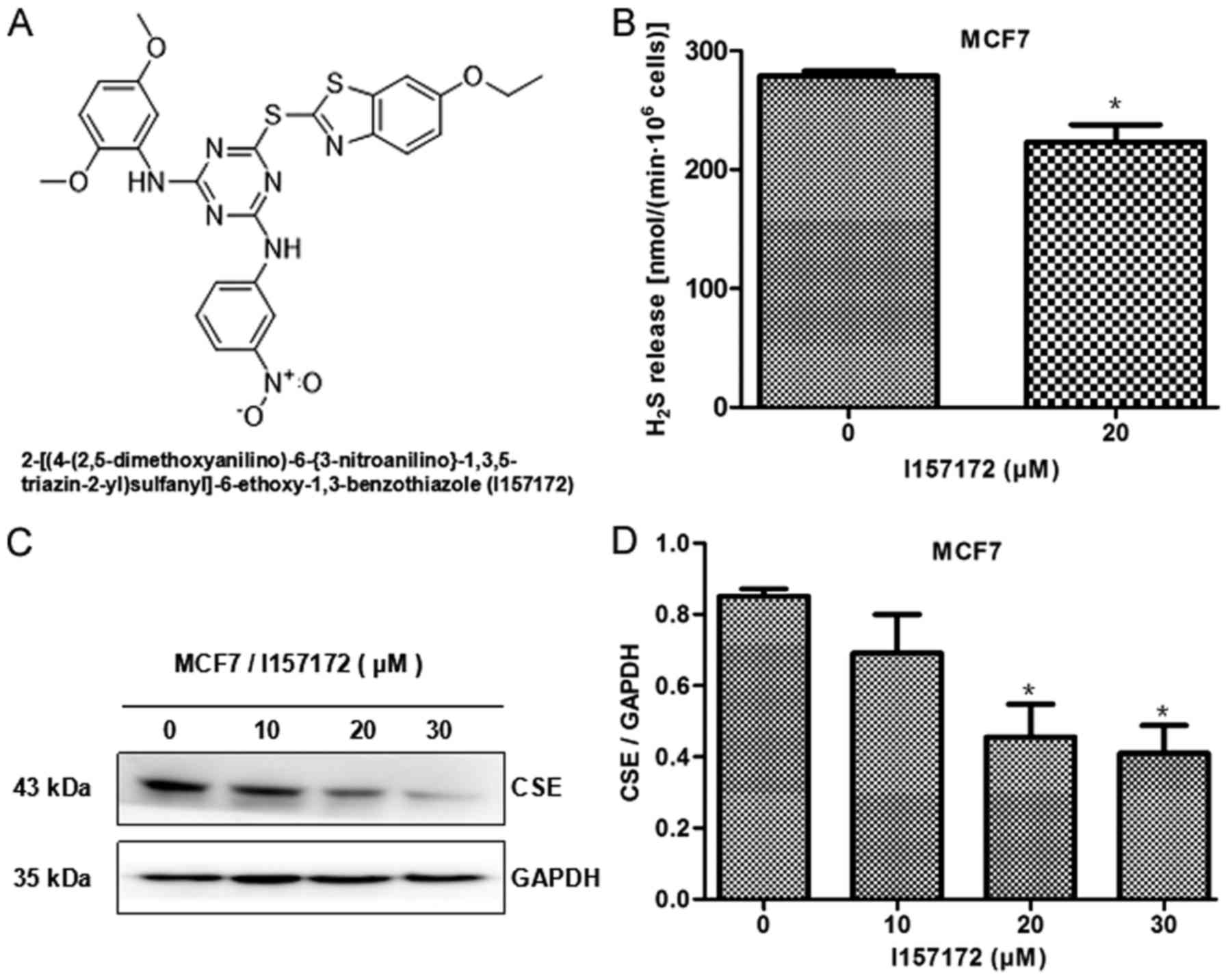
screening, the structure and naming of which are shown in Fig. 2A. To confirm the inhibitory activity
of I157172 on CSE, the present study detected the effects of
I157172 on H2S production and CSE protein expression.
The results of methylene blue detection indicated that I157172
significantly decreased H2S release from MCF7 cells
(Fig. 2B), thus suggesting that
I157172 may inhibit the activity of CSE. Western blot analysis
revealed that I157172 markedly inhibited the protein expression
levels of CSE in MCF7 cells (Fig. 2C
and D). These results confirmed the function of I157172 as a
CSE inhibitor.
I157172 inhibits MCF7 cell
proliferation, migration and invasion
The results of an MTS assay revealed that I157172
significantly reduced the viability of MCF7 cells in a
dose-dependent manner and the half maximal inhibitory concentration
(IC50) was 18.51 µM after 48 h (Fig. 3A and B). The inhibitory effects of
I157172 on MCF7 cells were significantly stronger than those of the
CSE inhibitor PAG and were equivalent to those of the positive
control DOX at 40 µM (Fig. 3C).
Furthermore, the damaging effects of I157172 on Hs578Bst normal
mammary cells (IC50=26.32 µM) were weaker than those of
the positive control DOX (IC50=1.62μM) (Fig. 3D). In addition, an EdU assay was
performed to further evaluate the inhibitory effects of I157172 on
cell proliferation. As shown in Fig. 3E
and F, I157172 markedly decreased the number of EdU+
MCF7 cells in a dose-dependent manner. In addition, the inhibitory
activities of I157172 on cell migration and invasion were observed
in MCF7 cells (Fig. 3G-J). Taken
together, the novel CSE inhibitor I157172 may effectively inhibit
the proliferation, migration and invasion in MCF7 cells.
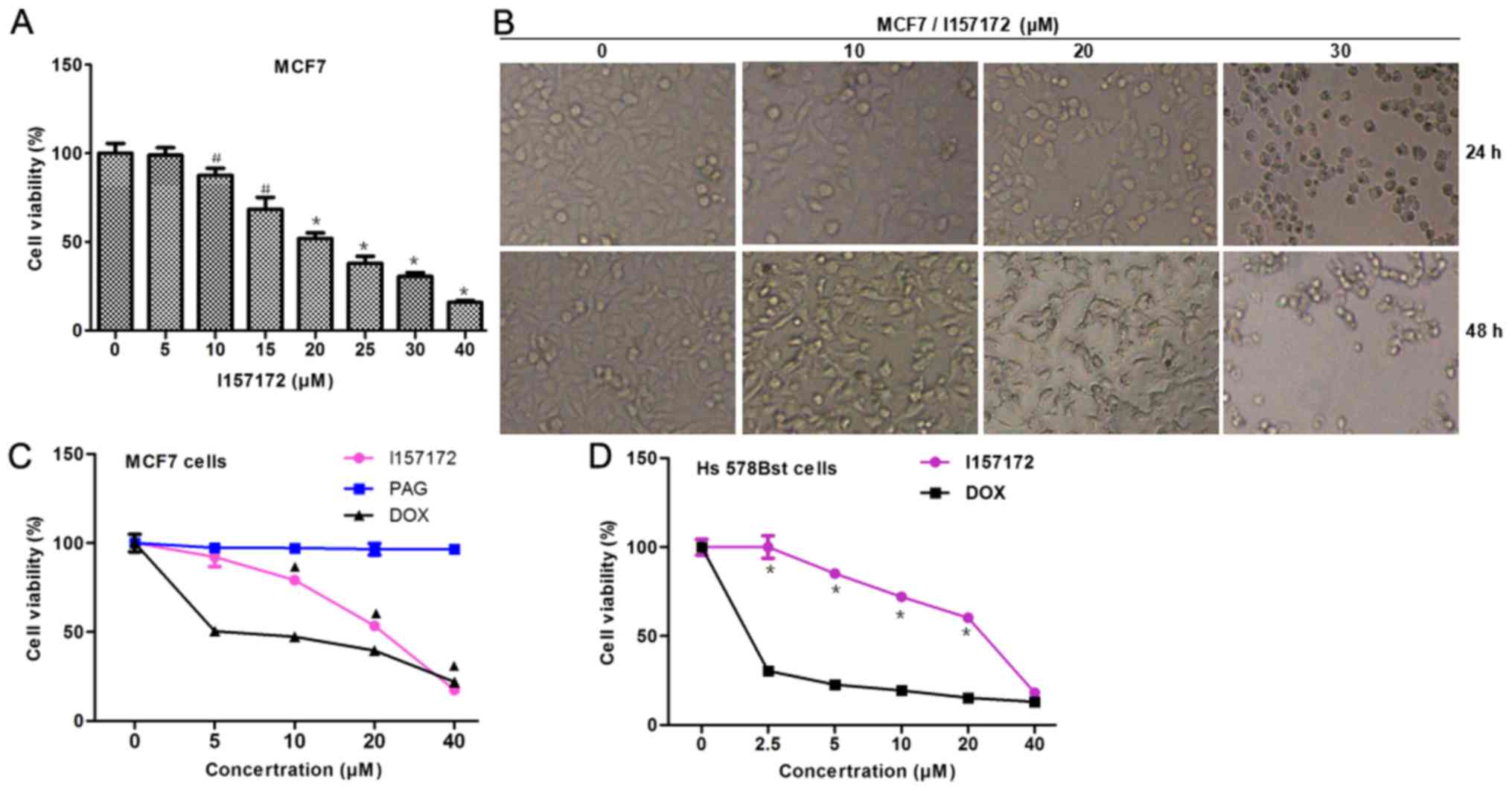 | Figure 3.Antiproliferative effects of I157172
on breast cancer cells. (A and B) Effects of I157172 on MCF7 cell
growth. Cells were exposed to I157172 for 24 and 48 h prior to the
MTS assay. Data are presented as the means ± standard deviation,
obtained from three independent experiments. #P<0.05
vs. the untreated group, *P<0.01 vs. the untreated group. Image
magnification, ×400. (C) Comparison of the antiproliferative
activity of I157172 and the positive controls (DOX and the CSE
inhibitor PAG) in MCF7 cells. ▲P<0.05 vs. the PAG
group. (D) Comparison of the cytotoxicity of I157172 and the
positive control DOX in Hs578Bst normal mammary cells. *P<0.05
vs. the DOX group. (E and F) Effects of I157172 on cell
proliferation, as determined by the EdU assay. (G and H) Effects of
I157172 on cell migration. (I and J) Effects of I157172 on cell
invasion. *P<0.05 vs. the untreated group, ▲P<0.05
vs. the 10 µM I157172 group, #P<0.05 vs. the 20 µM
I157172 group. Image magnification, ×200. CSE, cystathionine
γ-lyase; DOX, doxorubicin; EdU, 5-ethynyl-2′-deoxyuridine; OD,
optical density; PAG, L-propargylglycine. |
CSE/H2S system may activate
STAT3 via the downregulation of SIRT1
Our previous study demonstrated the biological
functions of the CSE/H2S system in promoting breast
cancer development and progression, and revealed that upregulation
of CSE promotes activation of STAT3 (10). Acetylation of STAT3 is essential for
its activation, whereas SIRT1 can induce deacetylation of STAT3
(16–22). To further explore the mechanism
underlying the effects of the CSE/H2S system on the
promotion of breast cancer development, this study investigated the
effects of the CSE/H2S system on SIRT1 expression and
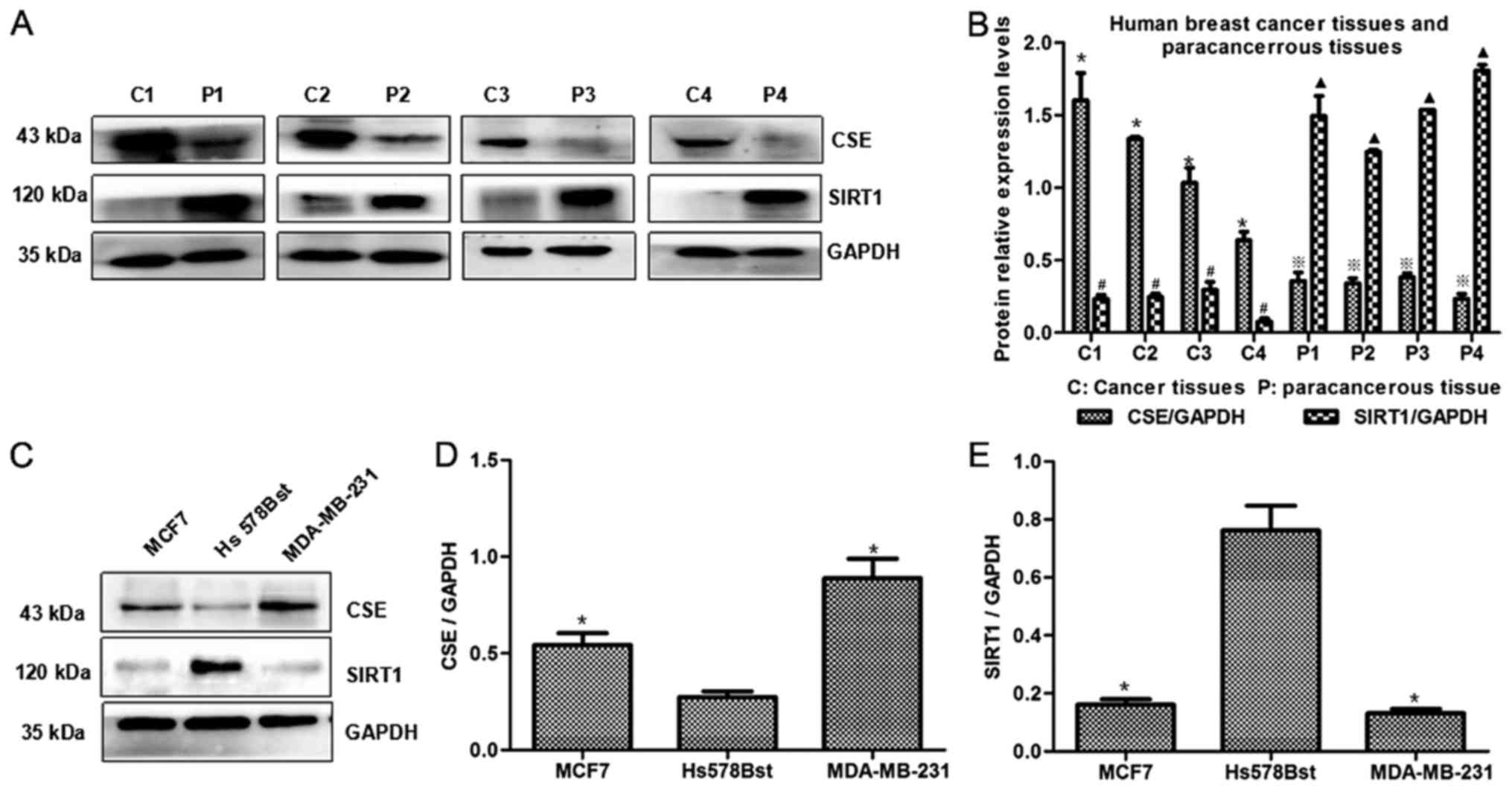
STAT3 acetylation. The association between CSE and SIRT1 expression
in breast cancer tissues and paracancerous tissues was initially
investigated. As shown in Fig. 4A and
B, SIRT1 expression was reduced in breast cancer tissues with
high CSE expression. Furthermore, low expression levels of SIRT1
were detected in MCF7 and MDA-MB-231 cells, which had high CSE
expression levels (Fig. 4C-E).
Furthermore, it was revealed that CSE knockdown induced an increase
in SIRT1 levels (Fig. 5A-C), and
decreased STAT3 (Fig. 5A and D),
acetyl-STAT3 (Fig. A and E), and p-STAT3 (Tyr705) levels (Fig. 5A and F) in MCF7 cells. Subsequently,
the target proteins downstream of STAT3 were analyzed and it was
revealed that knockdown of CSE resulted in downregulation of p-Akt,
BCL-2, MMP-2 and MMP-9 proteins (Fig.
5G-L). These data suggested that CSE/H2S system
might promote the activation of STAT3 via reducing the levels of
SIRT1.
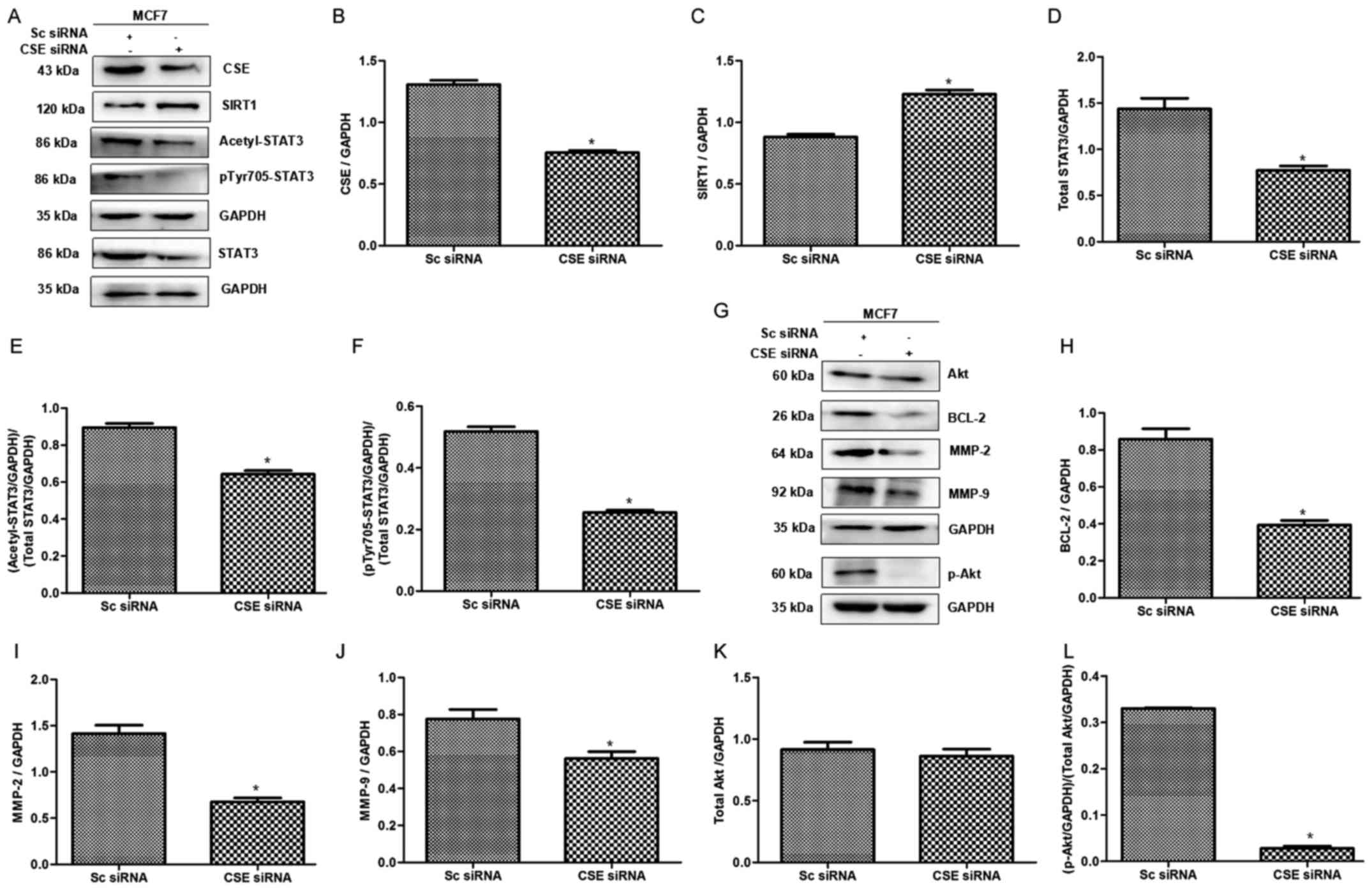 | Figure 5.Effects of CSE knockdown on the
expression levels of SIRT1 and acetyl-STAT3, and the STAT3
downstream pathway in breast cancer cells. (A-F) Effects of CSE
knockdown on SIRT1, acetyl-STAT3 and p-STAT3 expression. CSE
knockdown led to an increase in SIRT1 expression, and a decrease in
acetyl-STAT3 and p-STAT3. (G-L) Effects of CSE knockdown on STAT3
downstream proteins. CSE siRNA inhibited the expression levels of
STAT3 downstream proteins, p-Akt, BCL-2, MMP-2 and MMP-9.
*P<0.05 vs. the Sc siRNA group. Acetyl, acetylated; Akt, protein
kinase B; BCL-2, B-cell lymphoma 2; CSE, cystathionine γ-lyase;
MMP, matrix metalloproteinase; p, phosphorylated; Sc, scramble;
siRNA, small interfering RNA; SIRT1, sirtuin 1; STAT3, signal
transducer and activator of transcription 3. |
I157172 promotes SIRT1-mediated
deacetylation of STAT3 in breast cancer cells
To further confirm the role of SIRT1 in the
activation of STAT3 and explore the mechanism underlying the
effects of the CSE inhibitor I157172 on breast cancer, the effects
of I157172 on SIRT1, acetyl-STAT3 and p-STAT3 expression were
investigated. Western blotting revealed that I157172 upregulated
the expression levels of SIRT1, and downregulated acetyl-STAT3 and
p-STAT3 expression in a dose-dependent manner (Fig. 6A-D). Furthermore, the expression
levels of STAT3 downstream proteins, p-Akt, BCL-2, MMP-2 and MMP-9,
were reduced in a dose-dependent manner in MCF7 cells treated with
I157172 (Fig. 6E-K). These data
further confirmed the roles of the CSE/H2S system in
deacetylation of STAT3 via SIRT1 in promoting breast cancer
progression, and indicated that I157172 promoted SIRT1-mediated
deacetylation of STAT3 in breast cancer cells and consequently
inhibited the growth of breast cancer cells.
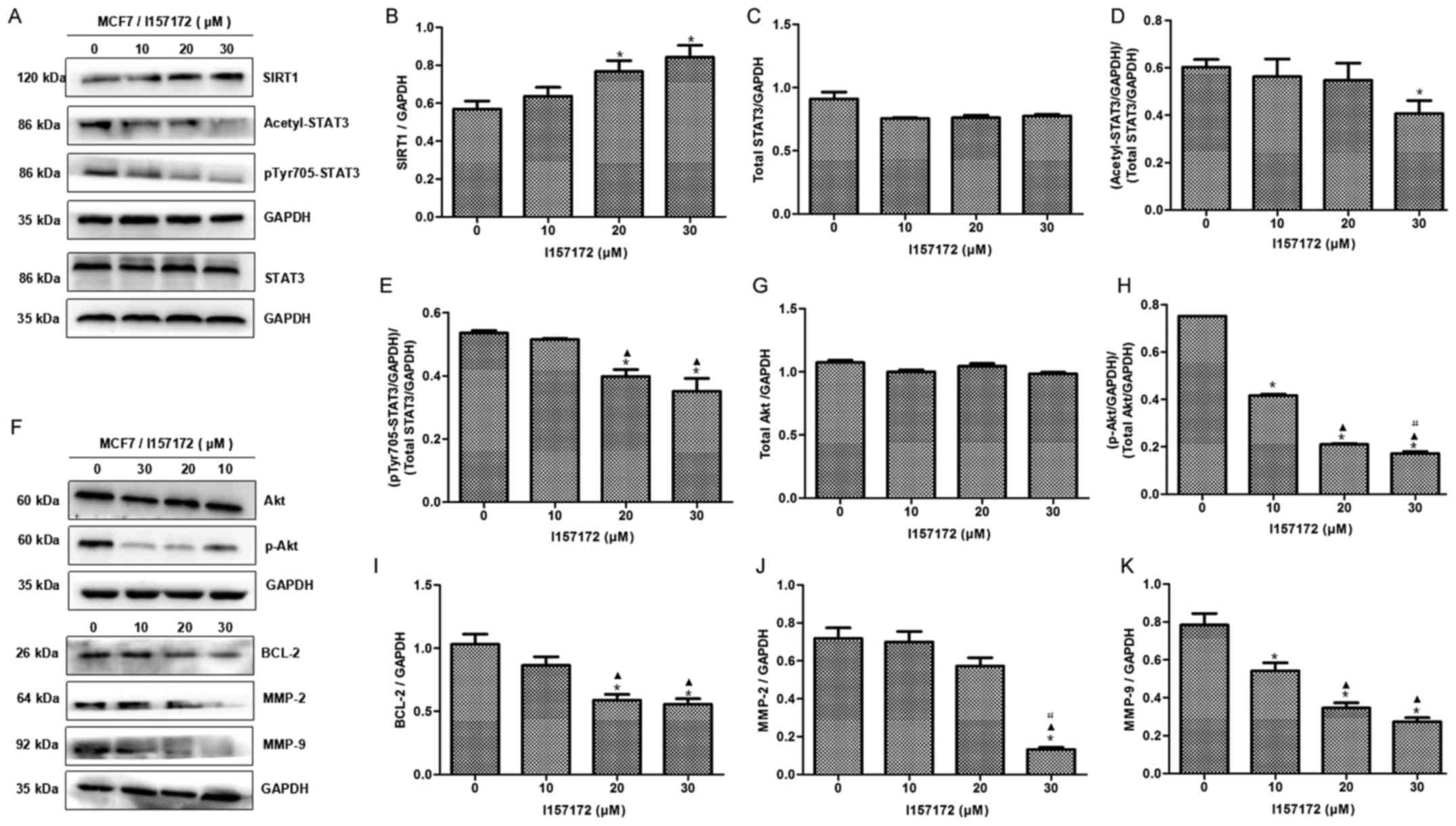 | Figure 6.Effects of I157172 on the expression
levels of SIRT1 and acetyl-STAT3, as well as STAT3 downstream
pathway proteins, in breast cancer cells. (A-E) Effects of I157172
on SIRT1, acetyl-STAT3 and p-STAT3 expression. I157172 upregulated
SIRT1, and decreased acetyl-STAT3 and p-STAT3 expression, in a
dose-dependent manner. (F-K) Effects of I157172 on STAT3 downstream
proteins. I157172 inhibited the expression of STAT3 downstream
proteins, p-Akt, BCL-2, MMP-2 and MMP-9, in a dose-dependent
manner. *P<0.05 vs. the untreated group, ▲P<0.05
vs. the 10 µM I157172 group, #P<0.05 vs. the 20 µM
I157172 group. Acetyl, acetylated; Akt, protein kinase B; BCL-2,
B-cell lymphoma 2; MMP, matrix metalloproteinase; p,
phosphorylated; SIRT1, sirtuin 1; STAT3, signal transducer and
activator of transcription 3. |
Discussion
H2S serves important roles in cancer
cells (8,9) alongside its physiological functions in
normal somatic cells (27). The
effects of exogenous H2S on cancer cells differ from
those of endogenous H2S. Several H2S donors
exert therapeutic effects on cancer cells (27), whereas endogenous H2S may
promote the proliferation of some cancer cells (4,8,9).
CSE, which is an endogenous H2S synthase,
is a pyridoxal-5′-phosphate-dependent enzyme that catalyzes
L-cysteine to yield H2S (28,29).
Our previous study focused on the biological functions of the
CSE/H2S system in breast cancer and revealed that CSE
expression may function as a potential tumor promoter (9); therefore, CSE may be considered a
novel target for breast cancer treatment. Further study of CSE
inhibitors may be of great significance for the treatment of breast
cancer.
MCF7 cells retain many characteristics of
differentiated mammary epithelium, and are commonly used in breast
cancer research. The present study investigated the inhibitory
effects of compound I157172, which is a CSE inhibitor identified
using the virtual screening method, on MCF7 cells. The results
revealed that the CSE inhibitor I157172 significantly inhibited the
growth, proliferation and migration of MCF7 cells. Furthermore, the
inhibitory effects of I157172 were significantly stronger than
those of the existing CSE inhibitor PAG, and the damaging effects
of I157172 on Hs578Bst normal mammary cells were weaker than those
of the positive control DOX. These findings indicated that the CSE
inhibitor I157172 may possess significant anti-breast cancer
activity.
STAT3 is a member of the STAT family, which has
important roles in cellular transformation, proliferation,
inflammation and metastasis in cancer (30). Our previous study revealed that high
expression levels of CSE promote the activation of STAT3 (10). Acetylation of STAT3 is essential for
STAT3 activation, whereas SIRT1 can induce deacetylation of STAT3
(16–22). Therefore, it was hypothesized that
the CSE/H2S system may activate STAT3 via reducing SIRT1
levels.
To verify the aforementioned hypothesis, the
expression levels of CSE and SIRT1 were detected in breast cancer
tissues and cells. Notably, a negative association was determined
between CSE and SIRT1 expression in breast cancer tissues and
cells. In addition, SIRT1 protein levels were increased, whereas
acetyl-STAT3 and p-STAT3 (Tyr705) levels were decreased in CSE
knockdown MCF7 cells. Furthermore, the STAT3 downstream proteins,
BCL-2, p-AKT, MMP-2 and MMP-9, were inhibited when CSE was knocked
down in MCF7 breast cancer cells. Taken together, the low
expression of SIRT1 might mediate the effects of the
CSE/H2S system on STAT3 activation, consequently
promoting the progression of breast cancer.
To further confirm the aforementioned hypothesis,
the effects of I157172 were investigated on SIRT1/acetyl-STAT3. The
results revealed that I157172 induced upregulation of SIRT1, and
downregulation of acetyl-STAT3 and p-STAT3 (Tyr705), as well as
inhibition of STAT3 downstream proteins.
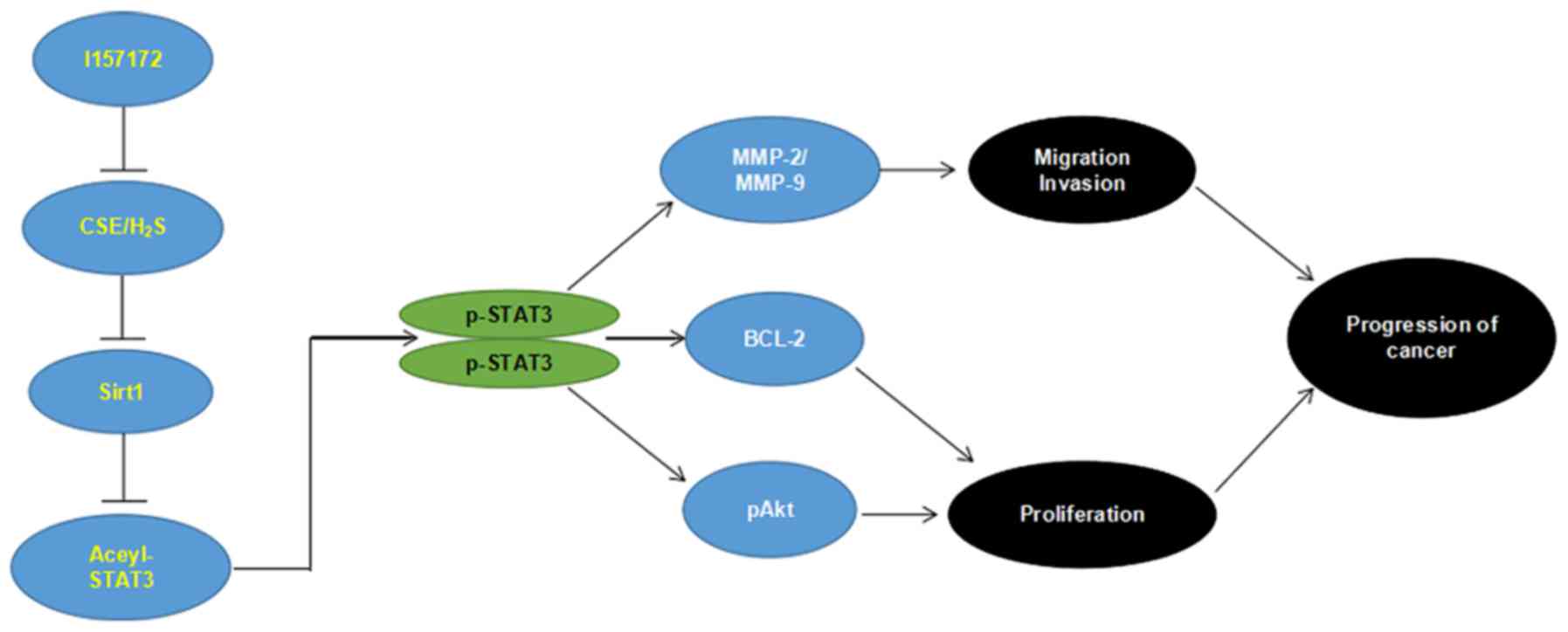
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that reduced expression
of SIRT1 may mediate the effects of the CSE/H2S system
on STAT3 activation, consequently promoting breast cancer
development and progression. In addition, the novel CSE inhibitor
I157172 possessed anti-breast cancer activity via the
SIRT1/acetyl-STAT3 signaling pathway (Fig. 7). The present study provided novel
insights into the function and mechanism of the CSE/H2S
system in cancer cells, and indicated that CSE/H2S
system inhibitors may be potential candidates for the treatment of
breast cancer. In future studies, we aim to further explore the
anticancer effects and mechanism of I157172 in vivo.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by
grants from the Key Science and Technology Fund of Henan Province
in China (grant no.162300410035) and the Henan Province University
Science and Technology Innovation Team (grant no.
16IRTSTHN019).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TW and XS conceived and designed the experiments.
LW, HS and XiaoyuZ performed the experiments. LW, HS, XiaoyuZ,
XiuliZ, YL and WK analyzed the data and made the figures. LW wrote
and proofread the paper. TW and XS revised the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Medical School, Henan University. Patients provided written
informed consent.
Patient consent for publication
Patient consent for publication was received.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi XJ, Au WW, Wu KS, Chen LX and Lin K:
Mortality characteristics and prediction of female breast cancer in
China from 1991 to 2011. Asian Pac J Cancer Prev. 15:2785–2791.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang R: Hydrogen sulfide: The third
gasotransmitter in biology and medicine. Antioxid Redox Signal.
12:1061–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coletta C, Papapetropoulos A, Erdelyi K,
Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I,
Martin E, et al: Hydrogen sulfide and nitric oxide are mutually
dependent in the regulation of angiogenesis and
endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA.
109:9161–9166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimura Y, Goto Y and Kimura H: Hydrogen
sulfide increases glutathione production and suppresses oxidative
stress in mitochondria. Antioxid Redox Signal. 12:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheng J, Shim W, Wei H, Lim SY, Liew R,
Lim TS, Ong BH, Chua YL and Wong P: Hydrogen sulphide suppresses
human atrial fibroblast proliferation and transformation to
myofibroblasts. J Cell Mol Med. 17:1345–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Popov D: An outlook on vascular hydrogen
sulphide effects, signalling, and therapeutic potential. Arch
Physiol Biochem. 119:189–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai WJ, Wang MJ, Ju LH, Wang C and Zhu YC:
Hydrogen sulfide induces human colon cancer cell proliferation:
Role of Akt, ERK and p21. Cell Biol Int. 34:565–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y,
Li N, Qi J, Wang L, Shi Y, et al: Sp1 is involved in regulation of
cystathionine γ-lyase gene expression and biological function by
PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell
Signal. 24:1229–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You J, Shi X, Liang H, Ye J, Wang L, Han
H, Fang H, Kang W and Wang T: Cystathionine-γ-lyase promotes
process of breast cancer in association with STAT3 signaling
pathway. Oncotarget. 8:65677–65686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin L, Hutzen B, Zuo M, Ball S, Deangelis
S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, et al: Novel
STAT3 phosphorylation inhibitors exhibit potent growth-suppressive
activity in pancreatic and breast cancer cells. Cancer Res.
70:2445–2454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hutzen B, Friedman L, Sobo M, Lin L, Cen
L, De Angelis S, Yamakoshi H, Shibata H, Iwabuchi Y and Lin J:
Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth
in breast and pancreatic carcinomas. Int J Oncol. 35:867–872.
2009.PubMed/NCBI
|
|
13
|
Groner B, Lucks P and Borghouts C: The
function of Stat3 in tumor cells and their microenvironment. Semin
Cell Dev Biol. 19:341–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu A, Liu Y, Xu Z, Yu W, Wang H, Li C and
Lin J: Novel small molecule, XZH-5, inhibits constitutive and
interleukin-6-induced STAT3 phosphorylation in human
rhabdomyosarcoma cells. Cancer Sci. 102:1381–1387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan ZL, Guan YJ, Chatterjee D and Chin
YE: Stat3 dimerization regulated by reversible acetylation of a
single lysine residue. Science. 307:269–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang R, Cherukuri P and Luo J: Activation
of Stat3 sequence-specific DNA binding and transcription by
p300/CREB-binding protein-mediated acetylation. J Biol Chem.
280:11528–11534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Zhou C, Gao H, Li C, Li D, Liu P,
Huang M, Shen X and Liu L: Therapeutic effect of Cryptotanshinone
on experimental rheumatoid arthritis through downregulating p300
mediated-STAT3 acetylation. Biochem Pharmacol. 138:119–129. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dasgupta M, Unal H, Willard B, Yang J,
Karnik SS and Stark GR: Critical role for lysine 685 in gene
expression mediated by transcription factor unphosphorylated STAT3.
J Biol Chem. 289:30763–30771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang S: Regulation of STAT signaling by
acetylation. Cell Signal. 25:1924–1931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sestito R, Madonna S, Scarponi C,
Cianfarani F, Failla CM, Cavani A, Girolomoni G and Albanesi C:
STAT3-dependent effects of IL-22 in human keratinocytes are
counterregulated by sirtuin 1 through a direct inhibition of STAT3
acetylation. FASEB J. 25:916–927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nie Y, Erion DM, Yuan Z, Dietrich M,
Shulman GI, Horvath TL and Gao Q: STAT3 inhibition of
gluconeogenesis is downregulated by SirT1. Nat Cell Biol.
11:492–500. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung YR, Kim H, Park SY, Park IA, Jang
JJ, Choe JY, Jung YY, Im SA, Moon HG, Lee KH, et al: Distinctive
role of SIRT1 expression on tumor invasion and metastasis in breast
cancer by molecular subtype. Hum Pathol. 46:1027–1035. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Qin Q, Chen R, Wei C and Mo Q: SIRT1
promotes proliferation, migration, and invasion of breast cancer
cell line MCF-7 by upregulating DNA polymerase delta1 (POLD1).
Biochem Biophys Res Commun. 502:351–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin X, Wei Y, Xu F, Zhao M, Dai K, Shen R,
Yang S and Zhang N: SIRT1 promotes formation of breast cancer
through modulating Akt activity. J Cancer. 9:2012–2023. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rifaï K1: Judes GIdrissou MDaures MBignon
YJPenault-Llorca FBernard-Gallon D Dual SIRT1 expression patterns
strongly suggests its bivalent role in human breast cancer.
Oncotarget. 8:110922–110930. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee ZW and Deng LW: Role of H2S donors in
cancer biology. Handb Exp Pharmacol. 230:243–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura H: Hydrogen sulfide: from brain to
gut. Antioxid Redox Signal. 12:1111–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang R: Hydrogen sulfide: the third
gasotransmitter in biology and medicine. Antioxid Redox Signal.
12:1061–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann NY Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|