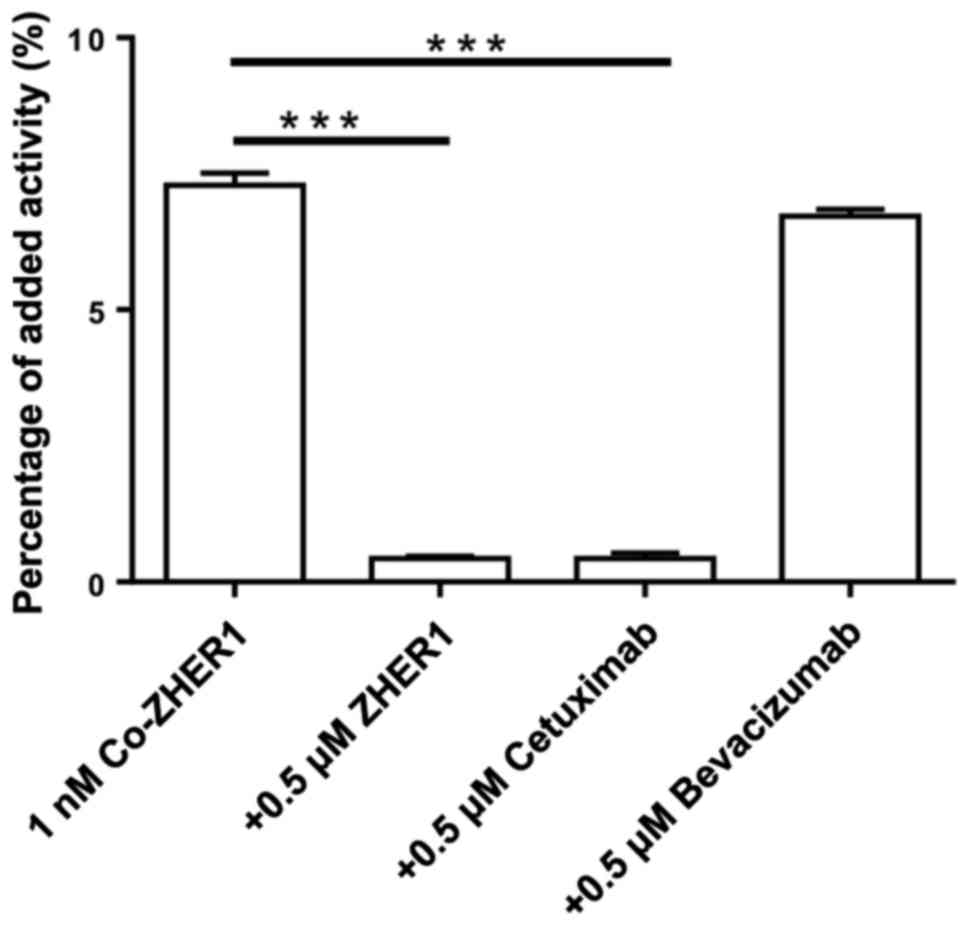
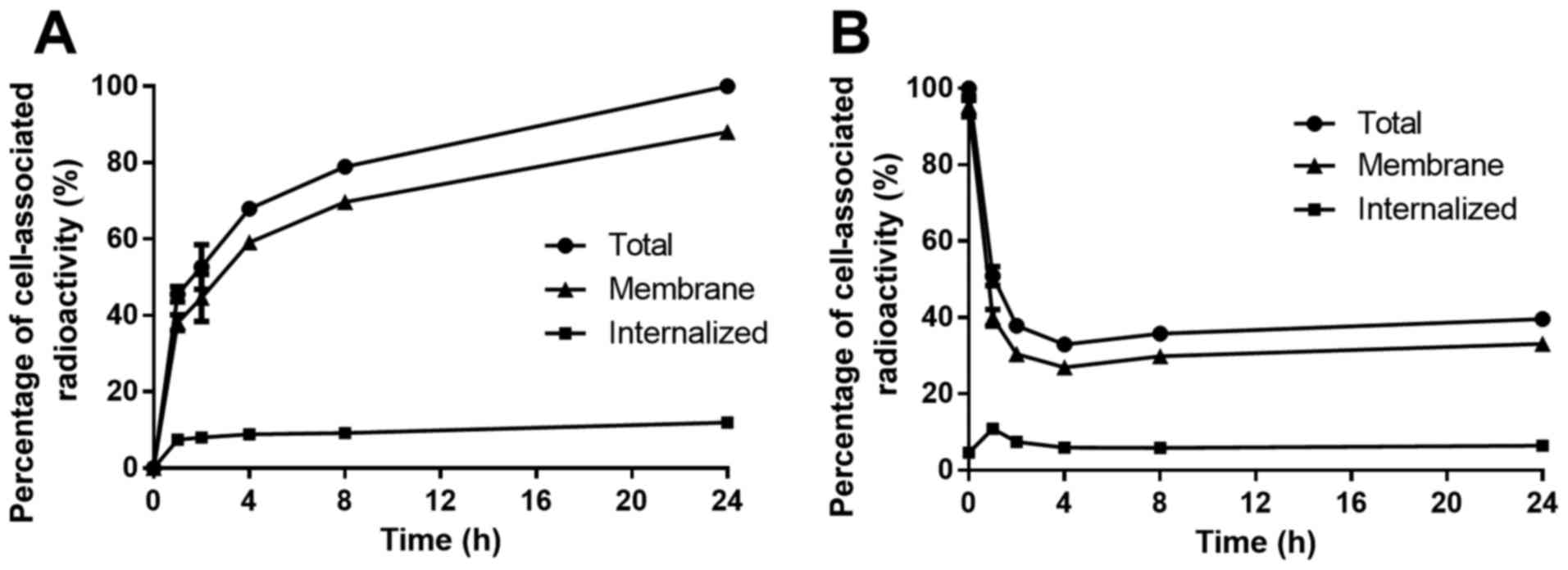
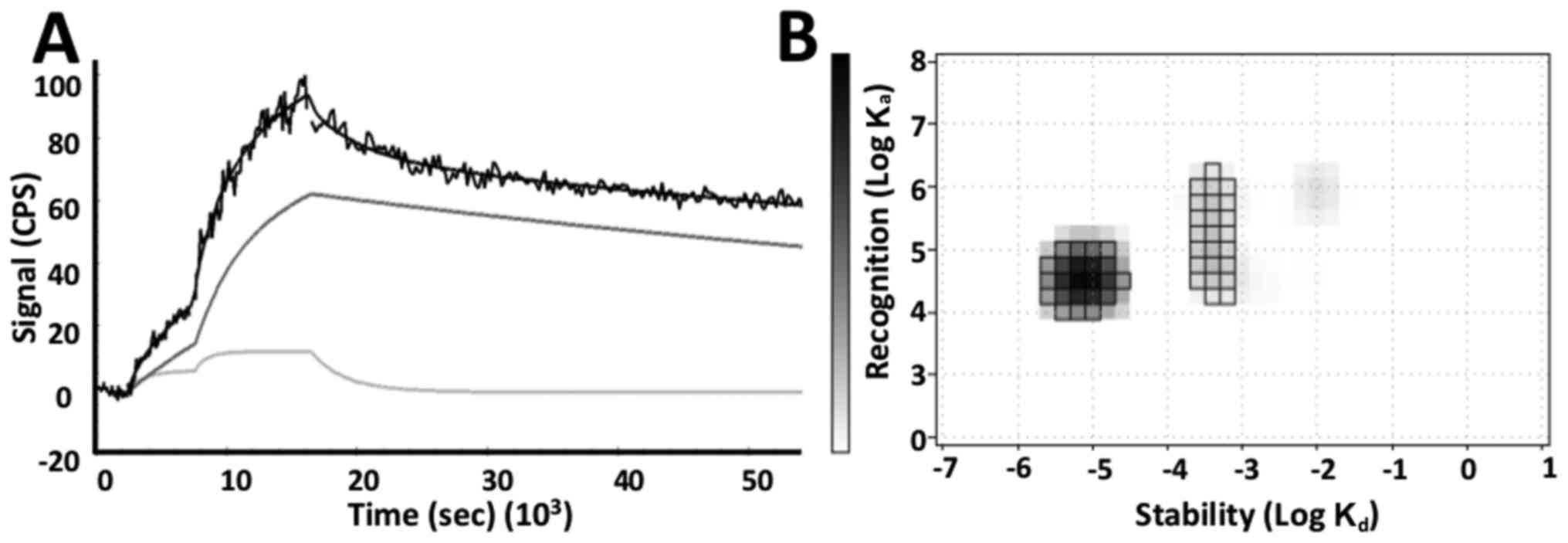
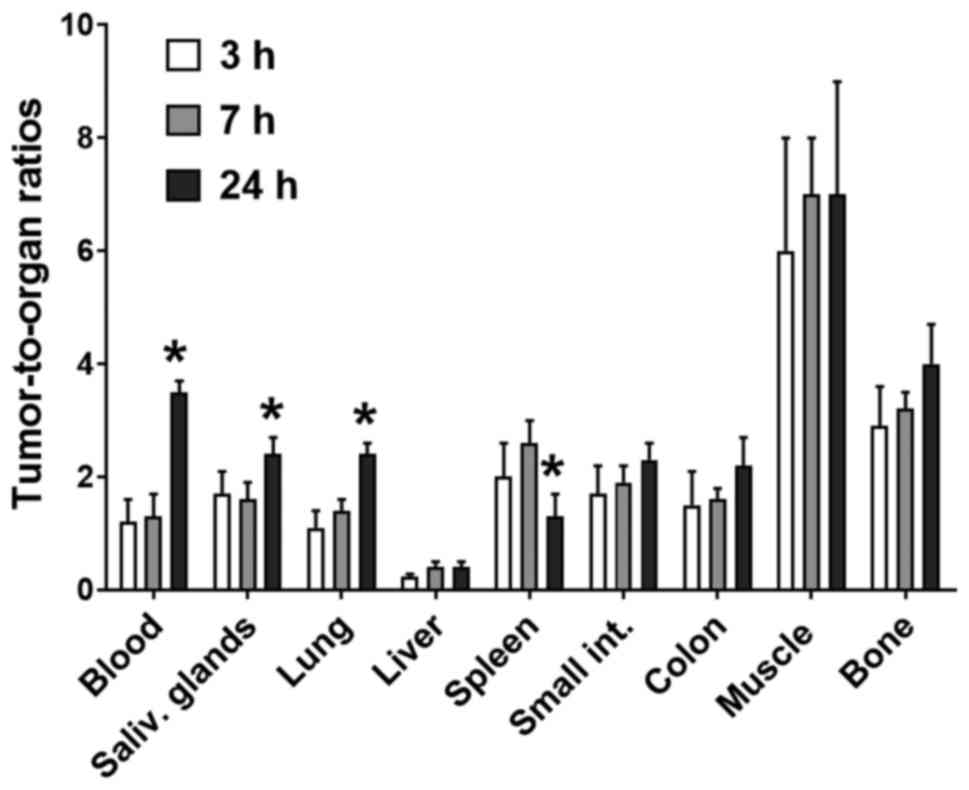
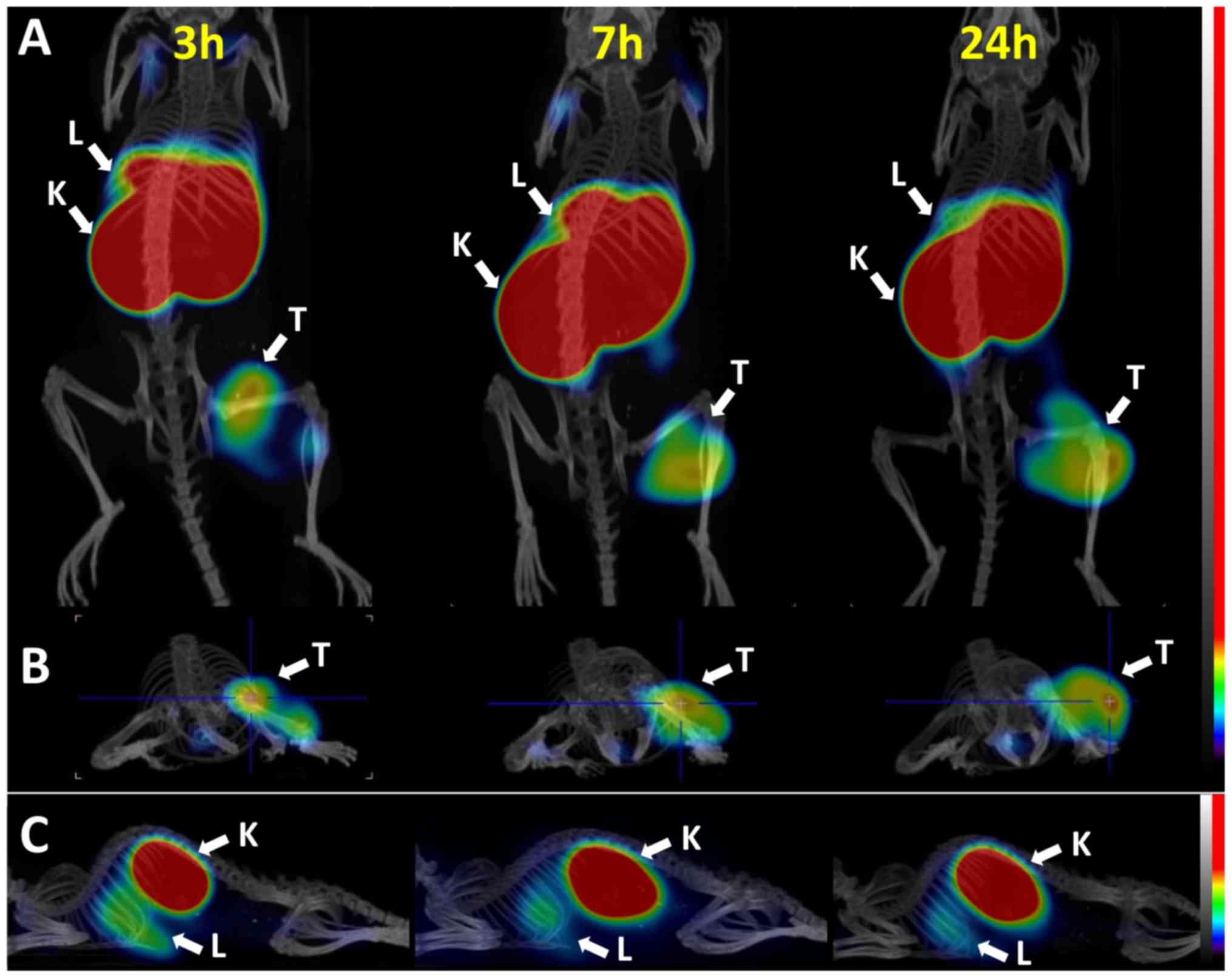
|
1
|
Huggins C and Hodges CV: Studies on
prostate cancer. I. The effect of castration, of estrogen and of
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. CA Cancer J Clin. 22:232–240. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pienta KJ and Bradley F: Mechanisms
underlying the development of androgen-independent PC. Clin Cancer
Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hoffman P and Djavan B: Androgen
deprivation therapy. Rev Urol. 10:305–306. 2008.PubMed/NCBI
|
|
4
|
Yagoda A and Petrylak D: Cytotoxic
chemotherapy for advanced hormone-resistant prostate cancer.
Cancer. 71((Suppl 3)): S1098–S1109. 1993. View Article : Google Scholar
|
|
5
|
Raghavan D, Koczwara B and Javle M:
Evolving strategies of cytotoxic chemotherapy for advanced prostate
cancer. Eur J Cancer. 33:566–574. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mimeault M and Batra SK: Recent advances
on multiple tumorigenic cascades involved in prostatic cancer
progression and targeting therapies. Carcinogenesis. 27:1–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Djakiew D: Dysregulated expression of
growth factors and their receptors in the development of prostate
cancer. Prostate. 42:150–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hernes E, Fosså SD, Berner AA, Otnes B and
Nesland JM: Expression of the epidermal growth factor receptor
family in prostate carcinoma before and during
androgen-independence. Br J Cancer. 90:449–454. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schlomm T, Kirstein P, Iwers L, Daniel B,
Steuber T, Walz J, Chun FH, Haese A, Kollermann J, Graefen M, et
al: Clinical significance of epidermal growth factor receptor
protein overexpression and gene copy number gains in prostate
cancer. Clin Cancer Res. 13:6579–6584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaw G and Prowse DM: Inhibition of
androgen-independent PC cell growth is enhanced by combination
therapy targeting Hedgehog and ErbB signaling. Cancer Cell Int.
8:32008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marmor MD, Skaria KB and Yarden Y: Signal
transduction and oncogenesis by ErbB/HER receptors. Int J Radiat
Oncol Biol Phys. 58:903–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oda K, Matsuoka Y, Funahashi A and Kitano
H: A comprehensive pathway map of epidermal growth factor receptor
signalling. Mol Syst Biol. 1(2005.0010)2005.PubMed/NCBI
|
|
13
|
Baselga J: The EGFR as a target for
anticancer therapy-focus on cetuximab. Eur J Cancer. 37((Suppl 4)):
S16–S22. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst RS, Kim ES and Harari PM: IMC-
C225, an anti-epidermal growth factor receptor monoclonal antibody,
for treatment of head and neck cancer. Expert Opin Biol Ther.
1:719–732. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonomi P: Erlotinib: A new therapeutic
approach for non-small cell lung cancer. Expert Opin Investig
Drugs. 12:1395–1401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herbst RS: Erlotinib (Tarceva): An update
on the clinical trial program. Semin Oncol. 30((3 Suppl 7)):
S34–S46. 2003. View Article : Google Scholar
|
|
17
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol.
21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ranson M, Hammond LA, Ferry D, Kris M,
Tullo A, Murray PI, Miller V, Averbuch S, Ochs J, Morris C, et al:
A selective oral epidermal growth factor receptor-tyrosine kinase
inhibitor, is well tolerated and active in patients with solid,
malignant tumors: Results of a phase I trial. J Clin Oncol.
2:2240–2250. 2002. View Article : Google Scholar
|
|
19
|
Guérin O, Fischel JL, Ferrero JM, Bozec A
and Milano G: EGFR targeting in hormone-refractory prostate cancer:
Current appraisal and prospects for treatment. Pharmaceuticals
(Basel). 3:2238–2247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vignot S, Besse B, André F, Spano JP and
Soria JC: Discrepancies between primary tumor and metastasis: A
literature review on clinically established biomarkers. Crit Rev
Oncol Hematol. 84:301–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cuartero-Plaza A, Martínez-Miralles E,
Rosell R, Vadell-Nadal C, Farré M and Real FX: Radiolocalization of
squamous lung carcinoma with 131I-labeled epidermal growth factor.
Clin Cancer Res. 2:13–20. 1996.PubMed/NCBI
|
|
22
|
Goldenberg A, Masui H, Divgi C, Kamrath H,
Pentlow K and Mendelsohn J: Imaging of human tumor xenografts with
an indium-111-labeled anti-epidermal growth factor receptor
monoclonal antibody. J Natl Cancer Inst. 81:1616–1625. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Divgi CR, Welt S, Kris M, Real FX, Yeh SD,
Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, et al:
Phase I and imaging trial of indium 111-labeled anti-epidermal
growth factor receptor monoclonal antibody 225 in patients with
squamous cell lung carcinoma. J Natl Cancer Inst. 83:97–104. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai W, Chen K, He L, Cao Q, Koong A and
Chen X: Quantitative PET of EGFR expression in xenograft-bearing
mice using 64Cu-labeled cetuximab, a chimeric anti-EGFR
monoclonal antibody. Eur J Nucl Med Mol Imaging. 34:850–858. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ping Li W, Meyer LA, Capretto DA, Sherman
CD and Anderson CJ: Receptor-binding, biodistribution, and
metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging
agent for epidermal growth-factor receptor-positive tumors. Cancer
Biother Radiopharm. 23:158–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nayak TK, Garmestani K, Baidoo KE, Milenic
DE and Brechbiel MW: Preparation, biological evaluation, and
pharmacokinetics of the human anti-HER1 monoclonal antibody
panitumumab labeled with 86Y for quantitative PET of carcinoma. J
Nucl Med. 51:942–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nayak TK, Garmestani K, Milenic DE and
Brechbiel MW: PET and MRI of metastatic peritoneal and pulmonary
colorectal cancer in mice with human epidermal growth factor
receptor 1-targeted 89Zr-labeled panitumumab. J Nucl
Med. 53:113–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang AJ, De Silva RA and Lapi SE:
Development and characterization of 89Zr-labeled
panitumumab for immuno-positron emission tomographic imaging of the
epidermal growth factor receptor. Mol Imaging. 12:17–27.
2013.PubMed/NCBI
|
|
29
|
Ahlgren S and Tolmachev V: Radionuclide
molecular imaging using Affibody molecules. Curr Pharm Biotechnol.
11:581–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sörensen J, Sandberg D, Sandström M,
Wennborg A, Feldwisch J, Tolmachev V, Åström G, Lubberink M,
Garske-Román U, Carlsson J and Lindman H: First-in-human molecular
imaging of HER2 expression in breast cancer metastases using the
111In-ABY-025 affibody molecule. J Nucl Med. 55:730–735.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sörensen J, Velikyan I, Sandberg D,
Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M,
Lubberink M, Olofsson H, et al: Measuring HER2-receptor expression
in metastatic breast cancer using [68Ga]ABY-025 affibody
PET/CT. Theranostics. 6:262–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tolmachev V, Rosik D, Wållberg H, Sjöberg
A, Sandström M, Hansson M, Wennborg A and Orlova A: Imaging of EGFR
expression in murine xenografts using site-specifically labelled
anti-EGFR 111In-DOTA-ZEGFR:2377 Affibody molecule:
Aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging.
37:613–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garousi J, Andersson KG, Dam JH, Olsen BB,
Orlova A, Buijs J, Ståhl S, Löfblom J, Thisgaard H and Tolmachev V:
The use of radiocobalt as a label improves imaging of EGFR using
DOTA-conjugated Affibody molecule. Sci Rep. 7:59612017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garousi J, Andersson KG, Mitran B, Pichl
ML, Ståhl S, Orlova A, Löfblom J and Tolmachev V: PET imaging of
epidermal growth factor receptor expression in tumours using
89Zr-labelled ZEGFR:2377 affibody molecules. Int J
Oncol. 48:1325–1332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang D, Kuan CT, Payne J, Kihara A, Murray
A, Wang LM, Alimandi M, Pierce JH, Pastan I and Lippman ME:
Recombinant heregulin-Pseudomonas exotoxin fusion proteins:
Interactions with the heregulin receptors and antitumor activity in
vivo. Clin Cancer Res. 4:993–1004. 1998.PubMed/NCBI
|
|
36
|
Mitran B, Thisgaard H, Rosenström U, Dam
JH, Larhed M, Tolmachev V and Orlova A: High contrast PET imaging
of GRPR expression in prostate cancer using cobalt-labeled Bombesin
antagonist RM26. Contrast Media Mol Imaging 2017. 68736842017.
|
|
37
|
Malmberg J, Tolmachev V and Orlova A:
Imaging agents for in vivo molecular profiling of disseminated
prostate cancer-targeting EGFR receptors in prostate cancer:
Comparison of cellular processing of [111In]-labeled
affibody molecule Z(EGFR:2377) and cetuximab. Int J Oncol.
38:1137–1143. 2011.PubMed/NCBI
|
|
38
|
Björkelund H, Gedda L and Andersson K:
Comparing the epidermal growth factor interaction with four
different cell lines: Intriguing effects imply strong dependency of
cellular context. PLoS One. 6:e165362011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rocha-Lima CM, Soares HP, Raez LE and
Singal R: EGFR targeting of solid tumors. Cancer Control.
14:295–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Henson E, Chen Y and Gibson S: EGFR family
members regulation of autophagy is at a crossroads of cell survival
and death in cancer. Cancers (Basel). 9:E272017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Slovin SF, Kelly WK, Wilton A, Kattan M,
Myskowski P, Mendelsohn J and Scher HI: Anti-epidermal growth
factor receptor monoclonal antibody cetuximab plus Doxorubicin in
the treatment of metastatic castration-resistant prostate cancer.
Clin Genitourin Cancer. 7:E77–E82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fleming MT, Sonpavde G, Kolodziej M,
Awasthi S, Hutson TE, Martincic D, Rastogi A, Rousey SR, Weinstein
RE, Galsky MD, et al: Association of rash with outcomes in a
randomized phase II trial evaluating cetuximab in combination with
mitoxantrone plus prednisone after docetaxel for metastatic
castration-resistant prostate cancer. Clin Genitourin Cancer.
10:6–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cathomas R, Rothermundt C, Klingbiel D,
Bubendorf L, Jaggi R, Betticher DC, Brauchli P, Cotting D, Droege
C, Winterhalder R, et al: Efficacy of cetuximab in metastatic
castration-resistant prostate cancer might depend on EGFR and PTEN
expression: Results from a phase II trial (SAKK 08/07). Clin Cancer
Res. 18:6049–6057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Özcan F, Klein P, Lemmon MA, Lax I and
Schlessinger J: On the nature of low- and high-affinity EGF
receptors on living cells. Proc Natl Acad Sci USA. 103:5735–5740.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Björkelund H, Gedda L, Malmqvist M and
Andersson K: Resolving the EGF-EGFR interaction characteristics
through a multiple-temperature, multiple-inhibitor, real-time
interaction analysis approach. Mol Clin Oncol. 1:343–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tolmachev V, Friedman M, Sandström M,
Eriksson TL, Rosik D, Hodik M, Ståhl S, Frejd FY and Orlova A:
Affibody molecules for epidermal growth factor receptor targeting
in vivo: Aspects of dimerization and labeling chemistry. J Nucl
Med. 50:274–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Samkoe KS, Gunn JR, Marra K, Hull SM,
Moodie KL, Feldwisch J, Strong TV, Draney DR, Hoopes PJ, Roberts
DW, et al: Toxicity and pharmacokinetic profile for single-dose
injection of ABY-029: A fluorescent anti-EGFR synthetic affibody
molecule for human use. Mol Imaging Biol. 19:512–521. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sandström M, Lindskog K, Velikyan I,
Wennborg A, Feldwisch J, Sandberg D, Tolmachev V, Orlova A,
Sörensen J, Carlsson J, et al: Biodistribution and radiation
dosimetry of the Anti-HER2 affibody molecule
68Ga-ABY-025 in breast cancer patients. J Nucl Med.
57:867–871. 2016. View Article : Google Scholar : PubMed/NCBI
|