Introduction
Epithelial follicular cell-derived thyroid cancer is
the most commonly diagnosed endocrine malignancy and its incidence
has increased 3-fold over the past 30 years (1,2).
Papillary thyroid cancer (PTC) accounts for >80% of all cases of
thyroid cancer. PTC is usually not aggressive and has a 5-year
survival rate of over 95% (3).
However, ~10% of cases develop into more aggressive and
undifferentiated forms of thyroid cancer; such cases are
characterized by metastasis and resistance to conventional therapy,
leading to recurrent disease and mortality (4). Genetic alterations in pathways,
including the mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (Erk) and phosphatidylinositol-3-kinase
(PI3K)/protein kinase B (Akt) pathways, are the driving force
behind thyroid tumorigenesis and progression (5). Previous studies have reported that
over 70% of activating somatic alterations of gene-encoding
effectors occur within the MAPK/Erk signaling pathway; mutations in
BRAF are the most common cause of aberrant MAPK/Erk signaling
(6,7).
In addition to aberrant signaling pathways, other
mechanisms such as alterations in E26 transformation (ETS) family
members of transcription factors have been implicated in all steps
of tumor progression (8).
ETS-specific related transcription factor-3 (ELF3) (ELF3, also
known as ESE-1) belongs to the epithelial-specific subfamily of ETS
transcription factors and has been reported to be involved in
various pathophysiological processes, including cancer and
inflammatory disorders (8,9). ELF3 was reported to serve an oncogenic
role in prostate cancer via constitutive activation of the NF-κB
pathway and the formation of a feedback loop with interleukin
(IL)-1β (10). However, other
studies have reported a contrary role of ELF3; for example,
suppression of E2-dependent MCF7 cell proliferation via inhibition
of the transcription of the estrogen receptor, and increase in the
transcriptional activity of tumor growth- and invasion-promoting
genes (early growth response protein 1 and TGFBR2) in squamous
cancer types by directly binding to the promoter region (10,11).
This controversial role of ELF3 suggests that it serves multiple
roles in tumorigenesis. Nevertheless, its exact function in thyroid
tumorigenesis has not yet been examined.
The present study aimed to examine the exact role of
ELF3 in thyroid cancer and the mechanism underlying the
ELF3-associated promotion of thyroid cancer progression. Firstly,
it was revealed that ELF3 was overexpressed in PTC compared to
normal tissue, and an even higher level of ELF3 was demonstrated in
BRAF-mutant PTC compared with BRAF wild-type PTC. High levels of
ELF3 were found to be associated with a poor prognosis in patients
with PTC. ELF3 silencing dramatically inhibited cell growth and
invasiveness in BRAF-mutant thyroid cancer cell lines. To the best
of our knowledge, the present study was the first to reveal
positive feedback loops between ELF3 and the MAPK/Erk signaling
pathway, contributing to thyroid tumorigenesis. The results of the
present study support the hypothesis that ELF3 functions as an
oncogene in thyroid cancer.
Materials and methods
TCGA data analysis
All the TCGA data were downloaded from Cancer
Broswer in UCSC database (https://genome.ucsc.edu/index.html). Normalized mRNA
expression (ELF3, EGFR, HER2, HER3 and HER4) of PTC and
corresponding clinical data were included in files. Each tumor
sample has its own identify number with ‘01’ at the end, while
paired normal thyroid sample with ‘11’ at the end.
Samples
Paraffin-embedded samples [24 papillary thyroid
cancer (PTC) specimens and 9 non-neoplastic thyroid specimens] and
fresh tissues (17 PTC tissues with paired non-neoplastic thyroid
tissues) were obtained by surgery at Ankang Central Hospital and
Zhoukou Central Hospital, following institutional review board
approval. Written informed consent was obtained from each patient
before surgery. All the samples were collected from 30 female and
11 male patients between January 2006 and December 2017. The age of
the participants ranging from 30 to 65 years with median age of 53
years. The fresh tissues were cut into cubes and put into sterile
freezing tubes. Samples were stored at −80°C.
Reagents
The RAF inhibitor PLX4032 was purchased from Selleck
Chemicals (Houston, TX, USA) and dissolved in dimethyl sulfoxide
(DMSO) for use. BCPAP and 8505C cells were treated with 1 µM
PLX4032 for 10 h, and a western blot analysis was performed to
evaluate the effects of this inhibitor on the MAPK/Erk pathway.
Immunohistochemistry (IHC)
Specimens were cut into 5-µm sections. The sections
were deparaffinized and rehydrated in a graded series of ethanol,
and washed in PBS. The sections were incubated with the anti-ELF3
antibody at a dilution of 1:150 (cat. no. 5715-1; Epitomics-an
Abcam Company, Burlingame, CA, USA) at 4°C overnight and with a
secondary antibody (cat. no. sp9001; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) for 30 min.
Diaminobenzidine (DAB) (Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd.) was used for visualization. A light microscope (Olympus
Corp., Tokyo, Japan) was used to observe and photograph the
staining. For expression analysis, the staining was categorized as
negative, weak, medium and strong and was confirmed by an in-house
pathologist.
Cell culture and siRNA
transfection
All human thyroid cancer cell lines (BCPAP, 8505c
and TPC-1) and immortalized thyroid epithelial cell line (HTori-3)
were obtained from the Shanghai Cell Bank (Type Culture Collection
Committee, Chinese Academy of Sciences, Shangai). Cells were
cultured in RPMI-1640 medium (cat. no. 1937557; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% FBS (Biological
Industries, Shangai, China) and maintained in an incubator with 5%
CO2 at 37°C. For transient siRNA transfection, cells
were seeded into normal growth medium at 30% confluence in 6-well
tissue plates 24 h prior to transfection with 5 nM siRNA (siELF3-1,
5′-GCUACCAAGUGGAGAAGAATT-3′ and siELF3-2,
5′-GCCAUGAGGUACUACUACATT-3′; Shanghai GenePharma Co., Ltd.,
Shanghai, China) using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), in accordance with the
manufacturer's instructions. Non-specific siRNA (siControl;
Shanghai GenePharma Co., Ltd.) was used as a negative control.
After 48 h of transfection, the cells were harvest for subsequent
experimentation.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from fresh samples and cell
lines using TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian,
China). cDNA was synthesized from 1 µg total RNA with a PrimeScript
RT reagent kit (Takara). RT-qPCR was performed on a CFX96 Thermal
Cycler Dice™ system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using SYBR Green (BioTools Pty. Ltd.,
Queensland, Australia) under the following cycling conditions: 3
min at 95°C, followed by 35 cycles of 10 sec at 95°C and 45 sec at
58°C. The mRNA expression of ELF3 was normalized to 18SrRNA.
Relative mRNA expression was calculated by using 2−ΔΔCq
method (12). Each sample was run
in triplicate. Primer:18s F: 5′-CGCCGCTAGAGGTGAAATTC-3′ R:
5′-CTTTCGCTCTGGTCCGTCTT-3′, ELF3 F: 5′-CATGACCTACGAGAAGCTGAGC-3′ R:
5′-GACTCTGGAGAACCTCTTCCTC-3′, EGFR 5′-AACACCCTGGTCTGGAAGTACG-3′ R:
5′-TCGTTGGACAGCCTTCAAGACC-3′, HER2 F: 5′-GGAAGTACACGATGCGGAGACT-3′
R: 5′-ACCTTCCTCAGCTCCGTCTCTT-3′, HER3 F:
5′-CTATGAGGCGATACTTGGAACGG-3′ R: 5′-GCACAGTTCCAAAGACACCCGA-3′.
Western blot analysis
Cells were lysed in pre-chilled
radioimmunoprecipitation assay buffer containing protease
inhibitors (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Supernatants were collected and loaded onto 10% SDS-PAGE gels and
transferred onto PVDF membranes (Roche). Membranes were blocked
with 5% BSA for 1.5 h at room temperature. The membranes were then
incubated with primary antibodies: Anti-ELF3 (1:750; cat. no.
5715-1; Epitomics), anti-phospho-Akt (dilution 1:1,000; cat. no.
BS4007; Bioworld Technology, Inc., St. Louis Park, MN, USA),
anti-phospho-Erk1/2 (dilution 1:1,000; cat. no. 4370; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-total-Akt
(dilution 1:1,000; cat. no. BS1379; Bioworld), anti-total-Erk1/2
(dilution 1:1,000; cat. no. 9102; Cell Signaling Technology) and
anti-tubulin (dilution 1:200; cat. no. sc-9104; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). This was followed by
incubation with species-specific HRP-conjugated secondary
antibodies (cat. nos. 130004 and 130023) from OriGene Technologies,
Inc. (Rockville, MD, USA). Samples were visualized using the
Western Bright ECL detection system (Millipore Corp., Billerica,
MA, USA). The densitometry were analyzed with Tanon Gis 1D 4.2
(Tanon, Shanghai, China).
Cell proliferation assay and colony
formation
Cells were seeded at a concentration of 800
cells/well and cultured in 96-well plates for 1, 3, 5 and 7 days
following treatment with si-ELF3 or control siRNA for 48 h. At the
indicated times, 20 µl of 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA)
was added into the medium and incubated for 4 h, followed by 150 µl
DMSO for additional 15 min. A microplate reader (Dynatech
Laboratories, El Paso, TX, USA) was used to measure the absorbance
using a test wavelength of 570 nm. For clone formation, transfected
cells were seeded at a concentration of 1,000 cells/well and
cultured in 12-well plates. The medium was refreshed every 3 days.
Following 7–10 days of culture, surviving colonies (≥50
cells/colony) were fixed with methanol and stained with 0.5%
crystal violet, and the colonies were counted with Image-Pro Plus
6.0 (Media Cybernetics, Rockville, MD, USA). Each experiment was
performed in triplicate.
Transwell assays
Cell migration and invasion assays were assessed
with Transwell chambers (8.0-µm pore size; Corning Inc., Corning,
NY, USA). For the cell invasion assay, Transwell chambers were
coated with Matrigel (4X dilution; 15 µl/well; BD Biosciences,
Franklin Lakes, NJ, USA). Cells were seeded in the upper chamber at
a density of 1×104 cells/ml for the migration assay and
1×104 cells/ml for the invasion assay in 200 µl medium
containing 0.5% FBS. Medium with 20% FBS (1 ml) was added to the
lower chamber. After a 12 or 24 h incubation,
non-migrating/non-invading cells in the upper chamber were removed
with a cotton swab, and migrating/invading cells were fixed in 100%
methanol and stained with crystal violet solution (0.5% crystal
violet in 2% ethanol). Images of five fields of view chosen at
random were taken for each membrane. The number of
migrating/invading cells was expressed as the average number of
cells per microscopic field over five fields of view. Images were
captured with an inverted Olympus IX71 microscope (Olympus Corp.,
Tokyo, Japan).
Statistical analysis
The gene expression analysis used data from The
Cancer Genome Atlas (TCGA) (URL: http://genome-cancer.soe.ucsc.edu). The median signal
intensity was set as the cutoff value of ELF3 overexpression. The
linear correlation between the expression of ELF3 and HER family
members were calculated with the Pearson's correlation coefficient.
Variance analysis was performed to compare the differences between
independent groups. P<0.05 was considered to be indicative of a
statistically significant result. Results are shown as the mean ±
standard deviation. Kaplan-Meier survival curve analysis was used
to assess the survival of PTC patients. Linear regression analysis
was used to evaluate the relationship of the ELF3 expression with
the expression of HER/ErbB family of receptors. Data analysis was
performed with GraphPad Prism (version 5.01; GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Increased expression of ELF3 is a
potential prognostic marker for patients with PTC
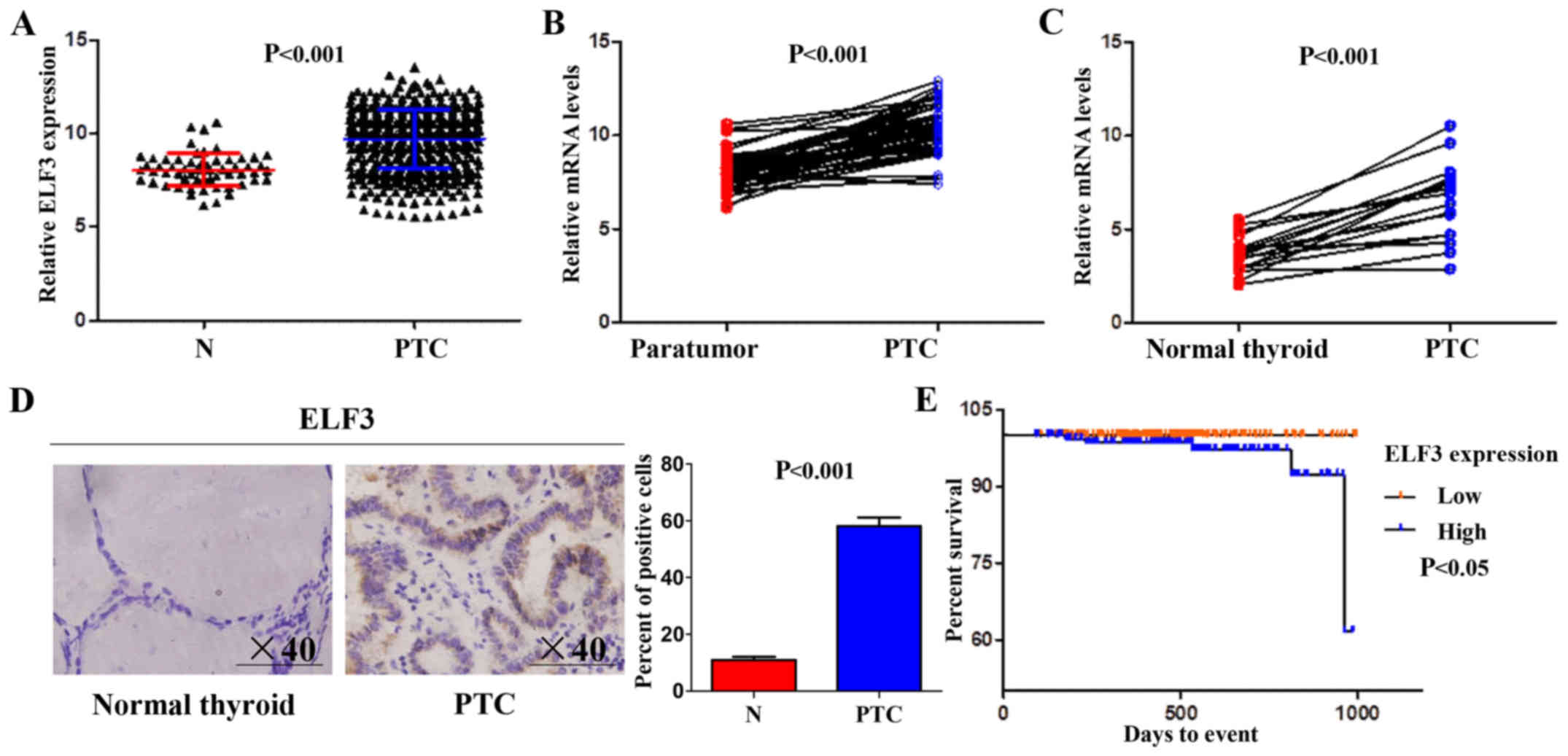
The expression of ELF3 in normal thyroid and PTC
tissues in The Cancer Genome Atlas (TCGA) dataset (13) was analyzed. As presented in Fig. 1A, the mRNA expression of ELF3 was
significantly upregulated in primary PTC compared with that noted
in the normal thyroid tissue. This was further supported by the
TCGA dataset, which revealed that ELF3 expression in PTC was
significantly higher compared with that in matched normal thyroid
tissues (Fig. 1B). ELF3 expression
in 17 PTCs and matched non-cancerous thyroid tissues (control
subjects) was analyzed to confirm the results of the database
analysis. Consistent with the TCGA dataset, ELF3 expression was
also higher compared with that observed in the matched normal
thyroid tissues at the mRNA (Fig.
1C) and protein levels (Fig.
1D).
PTC is not usually aggressive and has a 5-year
survival of over 95% (3). The
prognostic capacity of the ELF3 expression level for patients with
PTC in the TCGA dataset was analyzed using Kaplan-Meier survival
curves; median ELF3 expression was set as the cutoff point. The
results revealed that the ELF3 expression level was significantly
associated with poor survival, and mortality occurred earlier
post-diagnosis in the patients with higher ELF3 expression levels
(Fig. 1E). Together, these data
indicated the oncogenic function and the potential value for
prognosis evaluation of ELF3 in thyroid cancer.
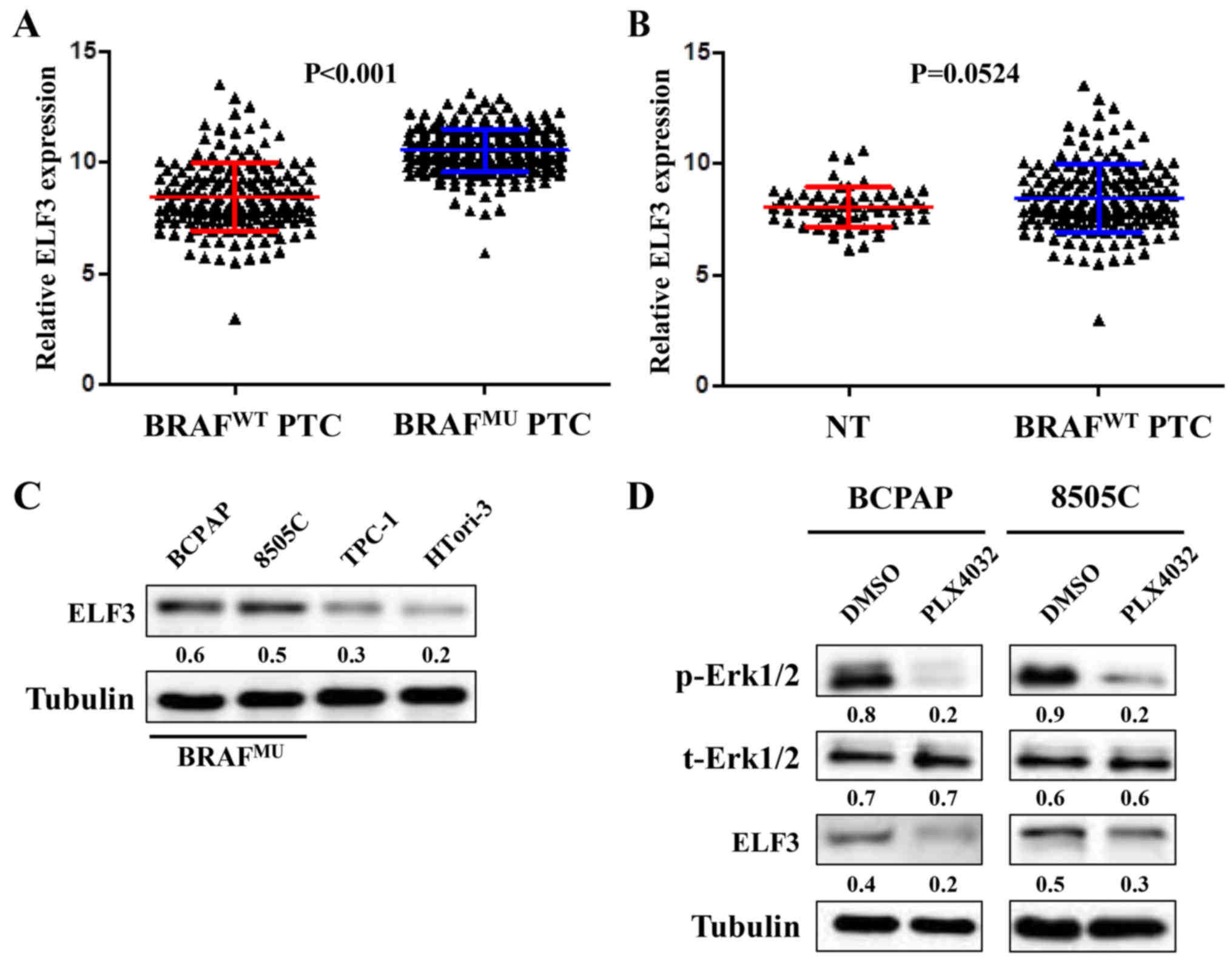
BRAF mutation-induced overactivation
of the MAPK signaling pathway results in upregulation of ELF3 in
thyroid cancer
Mutant BRAF has previously been demonstrated to
induce overactivation of the MAPK/Erk pathway in thyroid
tumorigenesis (5). Therefore, the
association between BRAF mutation and ELF3 expression in the TCGA
dataset was investigated. As presented in Fig. 2A, ELF3 expression was significantly
higher in BRAF-mutant (BRAFMU) PTC compared with
wild-type BRAF (BRAFWT) PTC tissues. Further analysis
revealed a statistical difference between BRAFWT PTC and
normal thyroid tissues (Fig. 2B).
Western blot analysis was performed to investigate the ELF3
expression pattern in 3 different thyroid cancer cell lines (BCPAP,
8505c and TPC-1) and 1 immortalized thyroid epithelial cell line
(HTori-3). The BRAF mutant thyroid cancer cell lines (BCPAP, 8505c)
revealed high basal levels of ELF3 compared with the BRAF wild-type
thyroid cancer cell line (TPC-1) and non-cancer thyroid epithelial
cell line (Fig. 2C). To evaluate
the impact of MAPK/Erk signaling on ELF3 expression, two
BRAF-mutant cell lines with higher ELF3 mRNA expression were
selected and treated with RAF inhibitor PLX4032 at a final
concentration of 1 µM for 6–8 h. As presented in Fig. 2D, inhibition of MAPK/Erk signaling
markedly attenuated ELF3 expression in BRAF-mutant cells. These
observations support the hypothesis that ELF3 expression is
upregulated by the MAPK/Erk signaling pathway in BRAF-mutant
thyroid cancer.
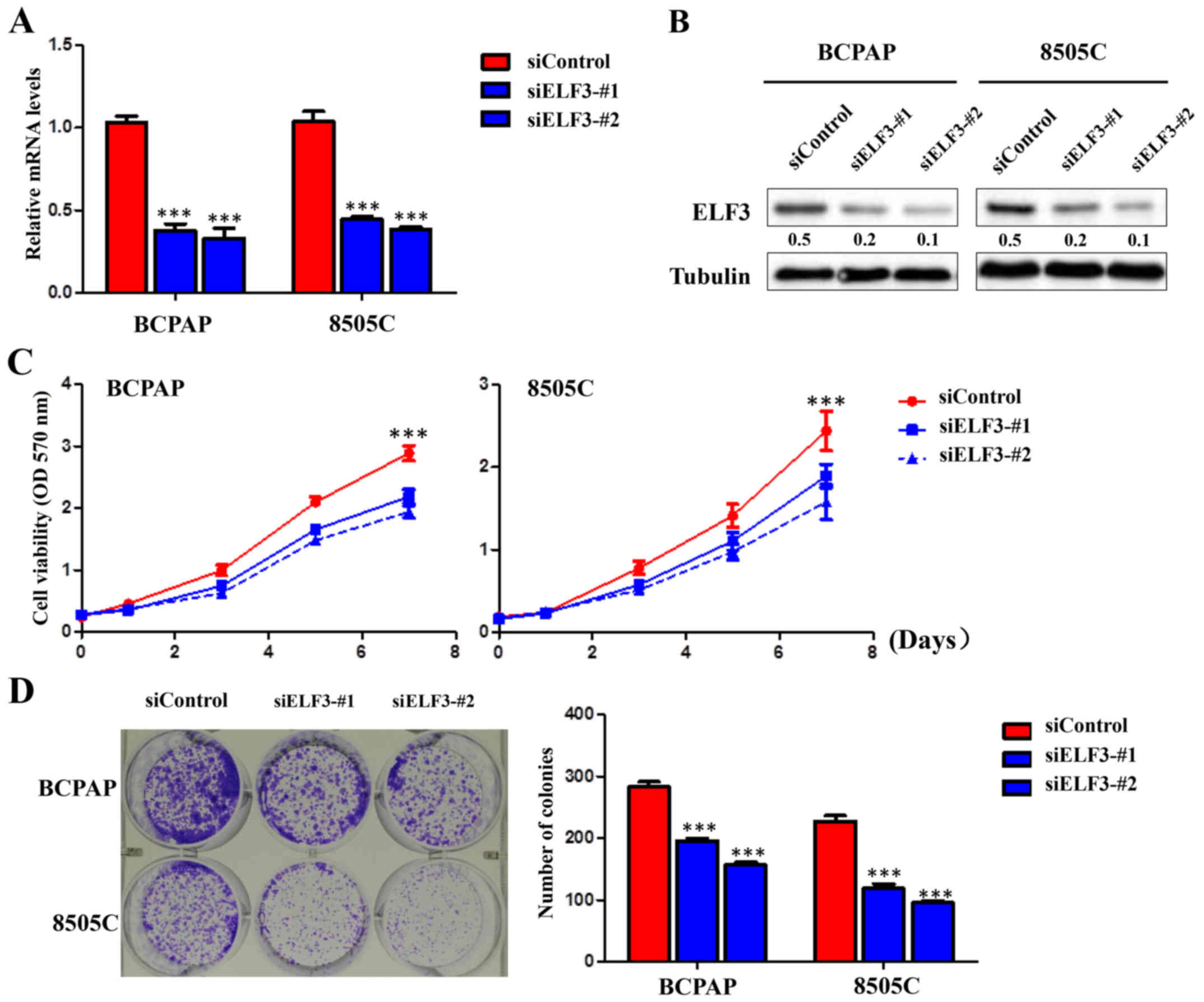
ELF3 knockdown inhibits thyroid cancer
cell growth
To illustrate the biological function of ELF3,
knockdown experiments were performed in BRAF-mutant thyroid cancer
cell lines BCPAP and 8505C. Knockdown of ELF3 by two different
siRNA sequences (siELF3-#1 and #2) was confirmed at the mRNA level
by RT-qPCR (Fig. 3A) and at the
protein level by western blot analysis (Fig. 3B). Knockdown of ELF3 significantly
inhibited the proliferation and clone formation of BCPAP and 8505C
thyroid cancer cell lines compared with the non-sense siRNA control
(siControl) (Fig. 3C and D).
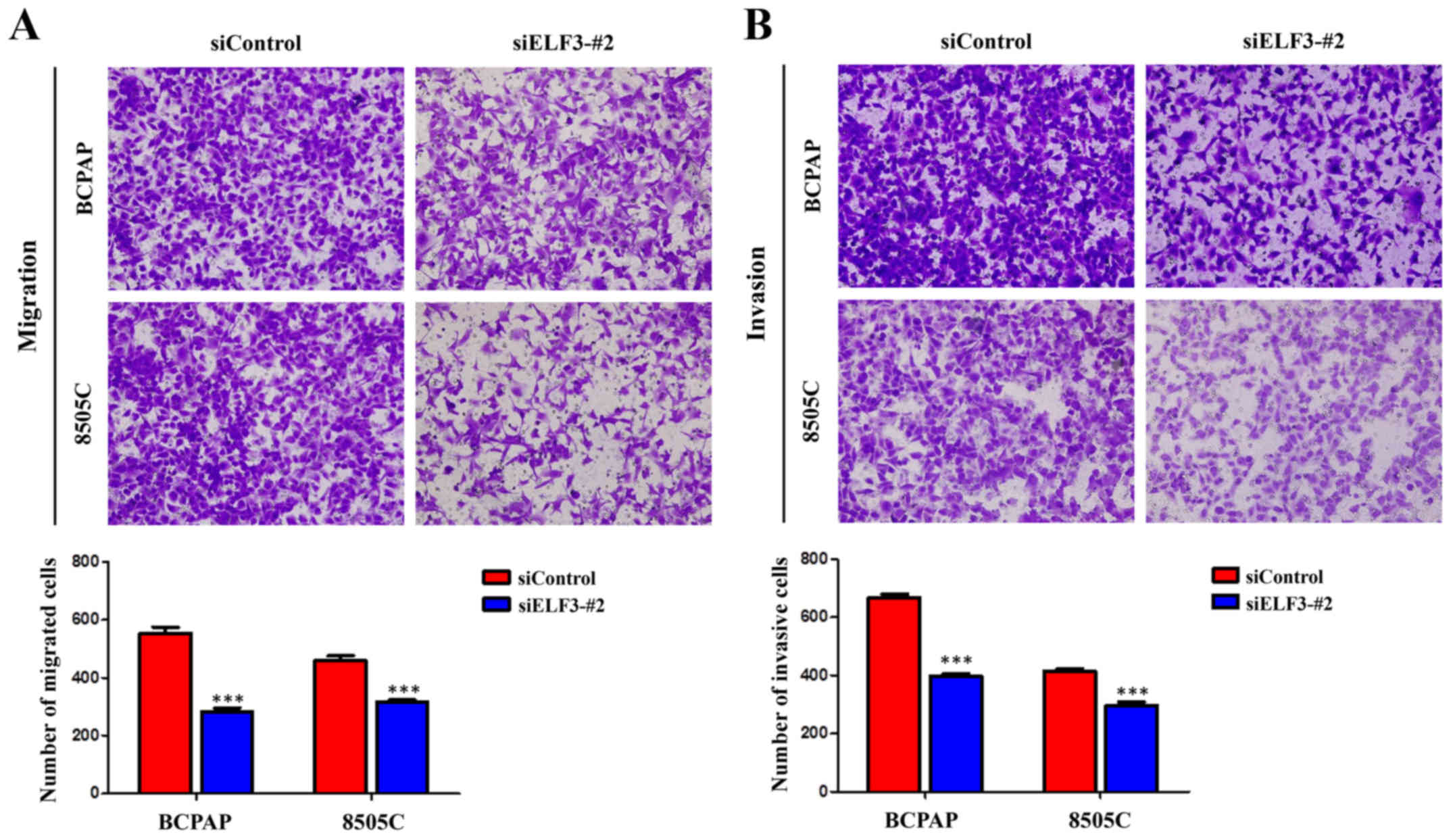
ELF3 knockdown inhibits thyroid cancer
cell migration and invasion
The majority of cancer-associated mortality is
caused by invasion and metastasis (14). Therefore, it was hypothesized that
early mortality was correlated with high ELF3 levels in patients
with thyroid cancer, resulting in the induction of cancer cell
invasion and metastasis. Transwell assays were performed to
investigate this hypothesis. As illustrated in Fig. 4A, the number of migrated cells was
significantly lower in the siELF3-#2 transfected cells compared
with the siControl transfected cells. Furthermore, the invasion
assay demonstrated that ELF3 knockdown significantly decreased the
ability of cells to pass through the Matrigel-coated membrane
(Fig. 4B). These data suggested a
strong link between high expression of ELF3 and metastatic
phenotypes in BRAF-mutant thyroid cancer cells.
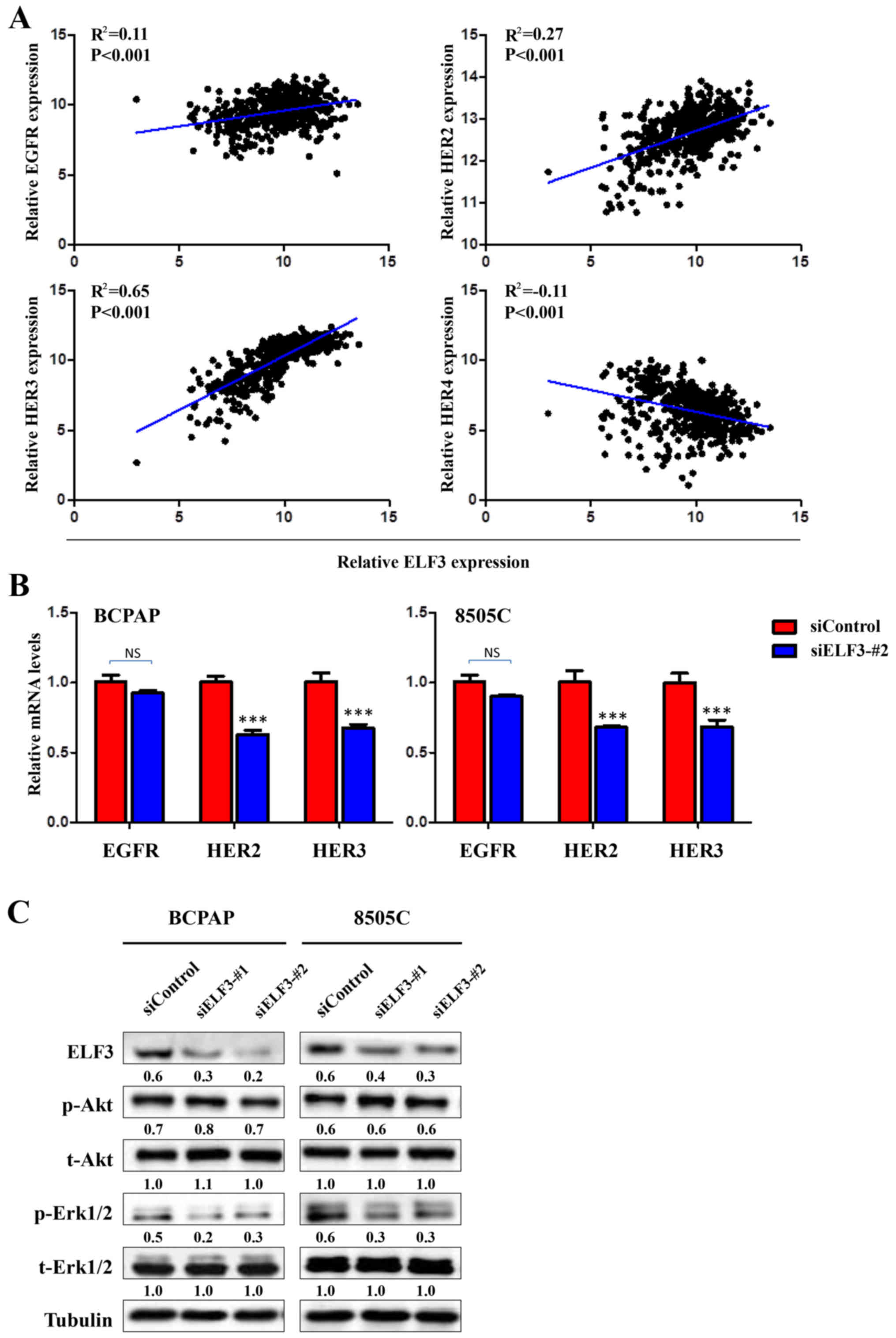
ELF3 transcriptionally regulates the
expression of the HER/ErbB family of receptors to form a positive
feedback loop with MAPK pathways in BRAF-mutant thyroid cancer
It has previously been reported that the HER/ErbB
family of receptors, including EGFR, HER2, HER3 and HER4, serves a
critical role in the tumorigenesis of thyroid cancer (15–17).
Furthermore, certain potential ETS binding sites (EBS,
5′-GGAA/T-3′) were revealed in HER/ErbB family promoter regions
using MatInspector online software (www.genomatix.de/online_help/help_matinspector/matinspector_help).
It was therefore hypothesized that ELF3, as a member of the ETS
transcription factor family, may be associated with the activation
of the HER family of receptors. To verify this hypothesis, the
association between ELF3 and the HER family in the TCGA dataset was
analyzed. The expression of HER family members was observed to have
a significant positive association with ELF3 expression, except for
HER4 (Fig. 5A). Furthermore, ELF3
knockdown significantly decreased the expression of HER2 and HER3
in BCPAP and 8505C thyroid cancer cell lines (Fig. 5B). A growing body of evidence
indicates that overexpression of HER family members leads to the
activation of downstream pathways, including MAPK/Erk and PI3K/Akt,
which serve a fundamental role in thyroid tumorigenesis and cancer
progression (5,18). Therefore, it was hypothesized that
high expression of ELF3 induced by over-activation of the MAPK
signaling pathway may form a positive feedback loop with the MAPK
signaling pathway in BRAF-mutant PTC. As presented in Fig. 5C, ELF3 knockdown attenuated the
phosphorylation of Erk (p-Erk) in the BCPAP and 8505C thyroid
cancer cells. However, the phosphorylation of Akt (p-AKT) was not
affected. Collectively, these data indicated that overexpression of
the oncogene ELF3 is induced by the activated MAPK/Erk pathway, and
ELF3 may, in turn, further activate the MAPK/Erk pathway,
potentially via transcriptional upregulation of the HER/ErbB family
of receptors, in BRAF-mutant PTC.
Discussion
To the best of our knowledge, the present study is
the first to provide evidence to support the oncogenic role of ELF3
in BRAF-mutant thyroid cancer. ELF3 was revealed to be highly
expressed in primary PTCs compared with that noted in thematched
non-tumor tissues. It was also revealed that increased expression
of ELF3 may be used as a potential prognostic marker for PTC
patients. Finally, ELF3 was demonstrated to form positive feedback
loops with the MAPK/Erk signaling pathway, and therefore contribute
to thyroid tumorigenesis.
As a member of the ETS transcription factor family,
ELF3 has been reported to be involved in a number of different
types of cancer, including colon, ovarian and ampullary cancers,
but the role of ELF3 in other types of tumors is still
controversial (19–21). Indeed, a number of studies have
revealed multifunctional roles and different mechanisms of ELF3 in
cancer (10,11,19–22).
It is clear that ETS factors such as ETS-1 and ETS-2 serve a
critical role in thyroid cell transformation (23). However, the role and mechanisms of
ELF3 in thyroid cancer have remained unknown until now. The present
study investigated the biological role of ELF3 in thyroid cancer
cells with a series of in vitro studies. As expected, ELF3
knockdown resulted in a strong inhibition of growth via suppression
of cell proliferative and colony forming capacity. Furthermore,
ELF3 knockdown reduced cell migration/invasion. Collectively, these
results suggested that ELF3 possesses a strong tumorigenic function
in thyroid cancer.
To better understand the mechanism of ELF3 as an
oncogene in thyroid cancer, the association between ELF3 and two
major cascades (MAPK/Erk and PI3K/Akt) in thyroid cancer cells was
investigated. As major therapeutic targets, these two pathways
serve important roles in thyroid tumorigenesis (5). On the one hand, the RAF inhibitor
PLX4032 was revealed to significantly reduce the expression of ELF3
in BRAF-mutant thyroid cancer cell lines; further analysis of the
TCGA dataset indicated a higher expression of ELF3 in BRAF-mutant
thyroid cancer. On the other hand, knockdown of ELF3 strongly
reduced the phosphorylation of Erk, supporting the hypothesis that
ELF3 performs its oncogenic function in thyroid cancer through
modulation of the activity of the MAPK/Erk pathway and the
formation of positive feedback loops with the MAPK/Erk pathway.
However, the mechanism underlying the upregulation of ELF3 by the
MAPK/Erk signaling pathway warramts further investigation.
The proximal HER family member promoter contains a
conserved ETS-responsive element (GAGGAA), which is recognized by
an ETS-immunoreactive factor in breast cancer cells (24). Although >10 different ETS
transcription factors have been revealed in human cancers, only a
few ETS family members including ELF3 (25,26)
have been reported as potential HER family member transactivators.
The HER/ErbB family of receptors are transmembrane receptor
tyrosine kinases that were demonstrated to be widely overexpressed
in a variety of human cancers including thyroid cancer (27,28).
Furthermore, the constitutively activated HER/ErbB family of
receptors promotes cell proliferation and inhibits apoptosis via
the MAPK/Erk pathway (29). Further
analysis of the TGCA database indicated positive associations of
ELF3 expression with the expression of EGFR, HER2 and HER3. In
addition, knockdown of ELF3 in thyroid cancer cells significantly
reduced the expression of EGFR, HER2 and HER3, respectively. These
data suggested that the HER/ErbB family of receptors may be a
potential downstream target of ELF3. It was therefore hypothesized
that ELF3 formed positive feedback loops with MAPK/Erk pathway
through transcriptional regulation of HER2 and HER3 expression.
Although some potential ETS binding sites were found in HER/ErbB
family promoter regions with MatInspector online software, further
investigations are required to investigate the specific ELF3
binding site within the HER/ErbB family promoter regions. Notably,
no influence of ELF3 knockdown on the PI3K/Akt pathway was
revealed; this pathway is located downstream of the HER/ErbB family
and has been revealed to serve an important role in thyroid
tumorigenesis (5,18). This may be due to differences in the
expression of different components in these signaling pathways,
including Smad4, which was proven to serve a crucial role in
connecting ELF3 and the PI3K/Akt pathway (30). Further studies are required to
better understand the mechanism underlying the associations between
ELF3 and the PI3K/Akt pathway in thyroid cancer. In conclusion,
overexpression of ELF3 was demonstrated to be a potential
prognostic marker in patients with thyroid cancer. ELF3
transcriptionally regulates the expression of the HER/ErbB family
of receptors and forms a positive feedback loop with the MAPK
pathway leading to the progression of BRAF-mutant thyroid cancer.
ELF3 may function as a possible therapeutic target against thyroid
cancer.
Acknowledgements
This research was supported by the Department of
Endocrinology, Ankang City Central Hospital.
Funding
No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JK designed the research. HC and XZ performed the
research and drafted the manuscript. HC, ZW, WC, XZ, LH, GT and JK
analyzed the data and were also involved in writing the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from all of
the patients before surgery. This study was approved by the Ethics
Committee of Ankang Central Hospital and Zhoukou Central
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988-2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hay ID, Thompson GB, Grant CS, Bergstralh
EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL,
et al: Papillary thyroid carcinoma managed at the Mayo Clinic
during six decades (1940–1999): Temporal trends in initial therapy
and long-term outcome in 2444 consecutively treated patients. World
J Surg. 26:879–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burns WR and Zeiger MA: Differentiated
thyroid cancer. Semin Oncol. 37:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikiforov YE and Nikiforova MN: Molecular
genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol.
7:569–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Research Network:
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oikawa T and Yamada T: Molecular biology
of the Ets family of transcription factors. Gene. 303:11–34. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oliver JR, Kushwah R and Hu J: Multiple
roles of the epithelium-specific ETS transcription factor, ESE-1,
in development and disease. Lab Invest. 92:320–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Longoni N, Sarti M, Albino D, Civenni G,
Malek A, Ortelli E, Pinton S, Mello-Grand M, Ostano P, D'Ambrosio
G, et al: ETS transcription factor ESE1/ELF3 orchestrates a
positive feedback loop that constitutively activates NF-κB and
drives prostate cancer progression. Cancer Res. 73:4533–4547. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gajulapalli VN, Samanthapudi VS, Pulaganti
M, Khumukcham SS, Malisetty VL, Guruprasad L, Chitta SK and
Manavathi B: A transcriptional repressive role for
epithelial-specific ETS factor ELF3 on oestrogen receptor alpha in
breast cancer cells. Biochem J. 473:1047–1061. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas Research Network.
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haugen DR, Akslen LA, Varhaug JE and
Lillehaug JR: Expression of c-erbB-2 protein in papillary thyroid
carcinomas. Br J Cancer. 65:832–837. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haugen DR, Akslen LA, Varhaug JE and
Lillehaug JR: Expression of c-erbB-3 and c-erbB-4 proteins in
papillary thyroid carcinomas. Cancer Res. 56:1184–1188.
1996.PubMed/NCBI
|
|
17
|
Mitsiades CS, Kotoula V, Poulaki V,
Sozopoulos E, Negri J, Charalambous E, Fanourakis G, Voutsinas G,
Tseleni-Balafouta S and Mitsiades N: Epidermal growth factor
receptor as a therapeutic target in human thyroid carcinoma:
Mutational and functional analysis. J Clin Endocrinol Metab.
91:3662–3666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JL, Chen ZF, Chen HM, Wang MY, Kong
X, Wang YC, Sun TT, Hong J, Zou W, Xu J, et al: Elf3 drives
β-catenin transactivation and associates with poor prognosis in
colorectal cancer. Cell Death Dis. 5:e12632014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeung TL, Leung CS, Wong KK,
Gutierrez-Hartmann A, Kwong J, Gershenson DM and Mok SC: ELF3 is a
negative regulator of epithelial-mesenchymal transition in ovarian
cancer cells. Oncotarget. 8:16951–16963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gingras MC, Covington KR, Chang DK,
Donehower LA, Gill AJ, Ittmann MM, Creighton CJ, Johns AL, Shinbrot
E, Dewal N, et al: Ampullary cancers harbor ELF3 tumor suppressor
gene mutations and exhibit frequent WNT dysregulation. Cell Rep.
14:907–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yachida S, Wood LD, Suzuki M, Takai E,
Totoki Y, Kato M, Luchini C, Arai Y, Nakamura H, Hama N, et al:
Genomic sequencing identifies ELF3 as a driver of ampullary
carcinoma. Cancer Cell. 29:229–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Nigris F, Mega T, Berger N, Barone MV,
Santoro M, Viglietto G, Verde P and Fusco A: Induction of ETS-1 and
ETS-2 transcription factors is required for thyroid cell
transformation. Cancer Res. 61:2267–2275. 2001.PubMed/NCBI
|
|
24
|
Scott GK, Daniel JC, Xiong X, Maki RA,
Kabat D and Benz CC: Binding of an ETS-related protein within the
DNase I hypersensitive site of the HER2/neu promoter in human
breast cancer cells. J Biol Chem. 269:19848–19858. 1994.PubMed/NCBI
|
|
25
|
Chang CH, Scott GK, Kuo WL, Xiong X,
Suzdaltseva Y, Park JW, Sayre P, Erny K, Collins C, Gray JW, et al:
ESX: A structurally unique Ets overexpressed early during human
breast tumorigenesis. Oncogene. 14:1617–1622. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eckel KL, Tentler JJ, Cappetta GJ, Diamond
SE and Gutierrez-Hartmann A: The epithelial-specific ETS
transcription factor ESX/ESE-1/Elf-3 modulates breast
cancer-associated gene expression. DNA Cell Biol. 22:79–94. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher KE, Jani JC, Fisher SB, Foulks C,
Hill CE, Weber CJ, Cohen C and Sharma J: Epidermal growth factor
receptor overexpression is a marker for adverse pathologic features
in papillary thyroid carcinoma. J Surg Res. 185:217–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Landriscina M, Pannone G, Piscazzi A, Toti
P, Fabiano A, Tortorella S, Occhini R, Ambrosi A, Bufo P and
Cignarelli M: Epidermal growth factor receptor 1 expression is
upregulated in undifferentiated thyroid carcinomas in humans.
Thyroid. 21:1227–1234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolch W and Pitt A: Functional proteomics
to dissect tyrosine kinase signalling pathways in cancer. Nat Rev
Cancer. 10:618–629. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Cho SN, Akkanti B, Jin N, Mao J,
Long W, Chen T, Zhang Y, Tang X, Wistub II, et al: ErbB2 pathway
activation upon Smad4 loss promotes lung tumor growth and
metastasis. Cell Rep. Mar 3–2015.(Epub ahead of print).
|